Abstract
The prevalence of metabolic syndrome (MetS) is increasing and the relationship between ultra-processed food (UPF) consumption and MetS remains uncertain in Chinese adults. This study aimed to examine the longitudinal association of UPF consumption with the risk of MetS and its components in Chinese adults. Adults aged 18 years and above who participated in at least two waves of the China Health and Nutrition Survey (CHNS) in 2009, 2015, and 2018 were included in this analysis. Dietary intake data were collected by three consecutive 24 h dietary recalls and weighing household foods and condiments. Depending on the purpose and extent of food processing, UPFs were classified using the NOVA food classification system. A multivariate Cox proportional risk model was used to explore the association between UPF consumption (grouped by quartile: quartile 1 (Q1), quartile 2 (Q2), quartile 3 (Q3), and quartile 4 (Q4)) and risk of MetS and its components. A total of 5147 adults were included. During a median (IQR) 6.0 (3.0, 9.0) year follow-up with 31,878 person-years, 1712 MetS cases were identified, with an incidence of 33.26%. After multivariable adjustment, the risk of MetS was increased by 17% in the highest quartile with UPF consumption (HR: 1.17, 95% CI: 1.01–1.35, p trend: 0.047), with the lowest quartile as a reference. For the components of MetS, the risk of central obesity, raised triglycerides (TG), reduced high-density lipoprotein cholesterol (HDL-C), and raised blood pressure (BP) was increased by 33% (HR: 1.33, 95% CI: 1.18–1.51, p trend: <0.001), 26% (HR: 1.26, 95% CI: 1.08–1.48, p trend: 0.003), 25% (HR: 1.25, 95% CI: 1.07–1.46, p trend: 0.007), and 16% (HR: 1.16, 95% CI: 1.03–1.32, p trend: 0.018) in the highest quartile with UPF consumption, respectively. Adults aged 45–59 years and living in urban areas with higher UPF consumption had higher odds of MetS. These results indicate that higher long-term UPF consumption was associated with an increased risk of MetS in Chinese adults. Further studies such as intervention trials are needed to confirm the mechanism of correlation between UPF consumption and health-related outcomes. Nutritional education actions are warranted to promote a balanced diet and improve the overall dietary quality of residents to reduce the risk of MetS effectively.
Keywords: ultra-processed foods, long-term consumption, metabolic syndrome, adults, China Health and Nutrition Survey
1. Introduction
Metabolic syndrome (MetS) is defined as a cluster of cardiovascular risk factors characterized by abdominal obesity, dyslipidemia, hypertension, and high fasting blood glucose [1]. MetS has received increased attention over the past decade and has become a major public health challenge worldwide. MetS is considered to be a risk factor for multiple noncommunicable diseases (NCDs), including cardiovascular disease (CVD), stroke, coronary heart disease, type 2 diabetes (T2D), and all-cause mortality [2]. A nationally representative cross-sectional survey among Chinese adults in 2000–2001 indicated that the standardized prevalence of MetS was 9.8% [3]. About ten years later, the Chinese National Nutrition and Health Surveillance (2010–2012) reported that the prevalence of MetS in Chinese adults was 18.7% and an estimated 189 million adults living with MetS in China [4]. In 2015–2017, the standardized prevalence of MetS increased to 31.1% and nearly a third of adults had MetS in China according to the China Nutrition and Health Surveillance [5].
Previous studies have found many factors associated with MetS, including lifestyle and diet [6,7,8]. A systematic review and meta-analysis of forty observational studies reported that the “Meat/Western” dietary pattern with the characteristics of high fat, processed meat, and sweets was significantly associated with increased MetS risk [9].
NOVA is a new classification considering the nature, extent, and purpose of processing and is commonly used in recent years [10]. NOVA classifies all foods and food products into four groups: unprocessed and minimally processed foods (MPFs), processed culinary ingredients (PCIs), processed foods (PFs), and ultra-processed foods (UPFs) [11]. UPFs are industrial formulations including products made from substances extracted from foods, typically with additives, and flavorings, and commonly high in energy density, salt, added sugars, and trans fats. A growing number of studies have shown that UPF consumption is associated with an increased risk of MetS and its components [12]. Several prospective studies and randomized controlled trials have found associations between UPF consumption and increased risks of overweight/obesity [13,14,15,16]. Some prospective studies found direct significant associations between UPFs and the risk of hypertension [17,18,19]. Several European cohort studies have shown positive associations between UPF consumption and the risk of type 2 diabetes [20,21,22,23]. With regards to MetS, the cross-sectional National Health and Nutrition Examination Survey (NHANES 2009–2014) reported that for adults aged 20 years and above in the fifth quintile of UPF contribution, the risk of MetS increased by 28% (PR: 1.28, 95% CI: 1.09–1.50) compared to the contribution of the first quintile [24]. With the development of the global economy and advances in food processing technology, UPF consumption is increasing rapidly in both high-income and middle-income countries [25,26]. In China, dietary patterns are in transition from traditional dietary patterns to Western dietary patterns [27]. Li et al. showed that the mean daily UPF increased four times between 1997 and 2011 and higher UPF consumption was positively associated with overweight/obesity, diabetes, and hypertension among Chinese adults [28,29,30].
However, less research has focused on the association between UPF consumption and MetS among Chinese residents. Given the increasing prevalence of MetS and higher consumption of UPF in Chinese adults, clarification of the relationship has vital significance for the prevention of MetS through diet. Therefore, we conducted the present study to explore the association between UPF consumption and MetS and its components among Chinese adults using cohort study data from the China Health and Nutrition Survey (CHNS). The aim of our study is to derive a more precise estimation of the association between UPF consumption and MetS and provide targeted suggestions for dietary behavior in Chinese adults.
2. Materials and Methods
2.1. Study Design and Population
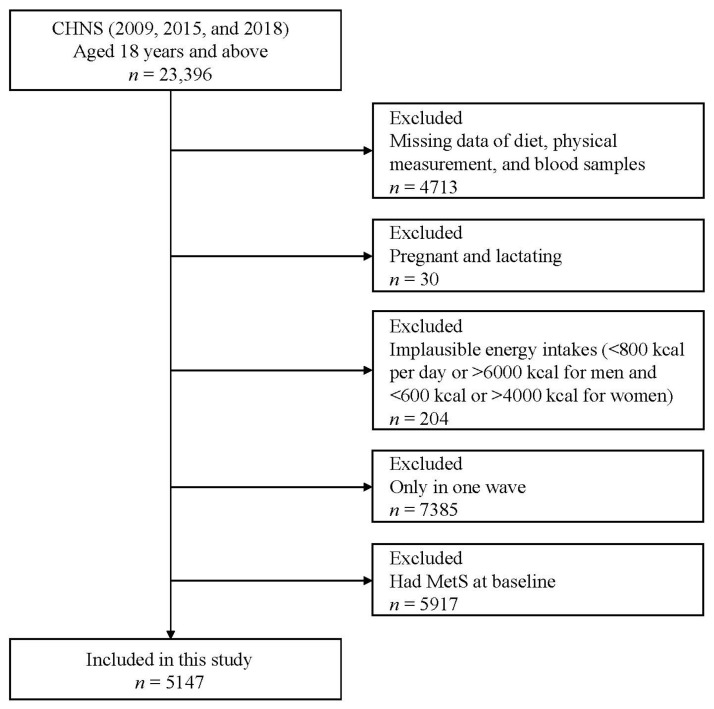
The CHNS is a longitudinal, ongoing, and prospective cohort study in China which initiated in 1989 and completed 11 waves in 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, 2015, and 2018. The CHNS used a multistage random cluster sampling method to collect the sample information including demographic geography, economic activity, community conditions, diet, and health in 15 provinces. The detailed design and procedures have been described elsewhere [31,32]. During the three waves of investigation in 2009, 2015, and 2018, blood samples were added at the same time, so the data from these three waves were used for analysis in this study. We excluded 4713 participants with deficiency of dietary, anthropometric data, and blood samples data; 30 pregnant or lactating women; 204 participants with implausible energy intakes (men: <800 kcal/day or >6000 kcal/day; women: <600 kcal/day or >4000 kcal/day); 7385 participants with only one wave; 5917 participants with MetS at baseline. Finally, a total of 5147 adults aged 18 years and above were included in this study (Figure 1).
Figure 1.
Flowchart of the participants included in this study.
The Institutional Review Board of the University of North Carolina at Chapel Hill and the Institutional Review Committee of the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, approved the survey (No. 201524, 20 August 2015). All participants provided their written, informed consent.
2.2. Dietary Assessment and UPF Consumption
Dietary data were collected using consecutive three-day 24 h dietary recalls (two weekdays and one weekend) for individuals in each wave of the CHNS. Meanwhile, a trained investigator weighed food items and seasonings such as oil and salt in the household inventory. Food consumption at the household level was calculated by the times of eating at home and the ratio of the energy intake of all members. Total energy and nutrients such as protein, fat, carbohydrate, and dietary sodium intake per day were calculated using the Chinese Food Composition Table [33,34].
According to the definition of NOVA classifications, food items were categorized into four groups [11]. UPF mainly includes the following food items, sugar-sweetened beverages (SSBs), packaged snacks, sweet, ice cream, chocolate, mass-produced packaged breads, cakes, desserts, biscuits, pastries, pre-prepared pies, pizza dishes, hot dogs, and sausages and other reconstituted meat products. As for uncertain food items, the presence in the list of ingredients of one or more food substances not used in kitchens including hydrolyzed proteins, “mechanically separated meat”, fructose, inverted sugar, maltodextrin, interesterified, or hydrogenated oil identified a product as UPF.
2.3. Definition of Metabolic Syndrome
MetS is defined using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria in this study. If at least three out of five of the following components were present, the person was determined to have MetS: (1) central obesity: waist circumference (WC) ≥90 cm (men) and ≥80 cm (women); (2) raised triglycerides (TG): ≥150 mg/dL or relevant specific treatment for hyperlipidemia; (3) reduced high-density lipoprotein cholesterol (HDL-C): < 1.0 mmol/L (men) and 1.3 mmol/L (women); (4) raised blood pressure: systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg or specific treatment of previously diagnosed hypertension; (5) raised fasting plasma glucose (FPG): ≥ 6.0 mmol/L or diagnosed type 2 diabetes previously [35].
2.4. Covariates and Physical Measurement
Multiple covariates were involved in this study including gender, age, education level, geographical location, income level, smoking history, alcohol drinking status, physical activity (PA), urbanization levels, body mass index (BMI), total energy intake, dietary protein, dietary fat, dietary carbohydrate, and dietary sodium. Age was divided into three groups (18–44 years, 50–59 years, and 60 years and above). Education level was divided into two groups (junior high school or below and senior high school or above). Residence was separated into two groups (urban and rural areas). In view of the differences between the north and the south, regions were divided into the north (Beijing, Liaoning, Heilongjiang, Shandong, Henan, and Shaanxi) and the south (Shanghai, Jiangsu, Zhejiang, Hubei, Hunan, Guangxi, Chongqing, Guizhou, and Yunnan). Annual per capita household income was divided into three groups (low, medium, and high by the tertiles). Smoking history and drinking past year were divided into two groups (yes and no), respectively. Physical activities included occupational, household chores, leisure time, and transportation, and calculated into a metabolic equivalent of task (METs h/week) based on the American College of Sports Medicine Association’s recommended standard, and were then divided into three groups (low, medium, and high by the tertiles) [36]. Urbanization levels were calculated based on the economic environment of the community and the cultural and social environment and divided into three groups (low, medium, and high by the tertiles). BMI was calculated as body weight (kg) divided by the square of height (m2) and divided into three groups (<18.5 kg/m2, 18.5–23.9 kg/m2, and ≥24.0 kg/m2).
WC was measured using an inelastic flexible ruler, and weight and height were measured using an electronic weight scale and portable SECA206 stadiometer. Cholesterol oxidase-phenol and amino phenazone methods were used to measure TG and HDL-C. Blood pressure was measured using a standard mercury sphygmomanometer (Korotkoff sound). The participants were in a seated position in a quiet room for at least five minutes of rest and with the bladder emptied. The average value of three consecutive standard measurements was taken as the result for each participant. Fasting plasma glucose was measured using the hexokinase method with a Roche 702 instrument. All measurements were performed by trained professional technicians with strict quality control.
2.5. Statistical Analysis
Categorical and continuous variables were described by n, percentage (%) and mean, and standard deviation, respectively. Categorical and continuous variables were compared by the χ2 test and Kruskal–Wallis test given the skewed distribution of the data. A multivariate Cox proportional risk model was used to estimate the association between UPF consumption (grouped by quartile: quartile 1 (Q1), quartile 2 (Q2), quartile 3 (Q3), quartile 4 (Q4)) and risk of MetS and its components. We performed tests for linear trends by entering the median value of each quartile of UPF consumption as a continuous variable in the models. Meanwhile, stratified analysis was performed by covariates and interaction analysis was performed to evaluate the effect of stratification factors on the relationship between UPF consumption and the risk of MetS. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA), and p < 0.05 (two-tailed) was defined as statistical significance.
3. Results
3.1. Baseline Characteristics
Table 1 presents the demographic and baseline characteristics of the quartile of UPF consumption. Compared with those in the bottom quartile of UPF consumption, those with higher UPF consumption were more likely to live in urban areas, have higher education levels, higher income, lower levels of physical activity, higher BMI, higher energy intake, higher protein intake, higher fat intake, higher sodium intake, and lower carbohydrate intake (p < 0.05). Baseline HDL-C and FPG were different between the quartile of groups (p < 0.05) while WC, TG, SBP, DBP as well as gender, age, smoking history, and drinking past year were not significantly different between the quartiles of UPF consumption groups (p > 0.05).
Table 1.
Basic characteristics of participants by quartile of UPF consumption.
| Quartile of UPF (g/Day) | |||||
|---|---|---|---|---|---|
| Q1 (<6.5) | Q2 (6.5–16.3) | Q3 (16.3–36.1) | Q4 (>36.1) | p-Value | |
| Gender | 0.070 | ||||
| Men | 610 (47.4) | 636 (49.4) | 651 (50.6) | 676 (52.5) | |
| Women | 676 (52.6) | 652 (50.6) | 635 (49.4) | 611 (47.5) | |
| Age | 0.566 | ||||
| 18–44 | 435 (33.8) | 455 (35.3) | 437 (34.0) | 477 (37.1) | |
| 45–59 | 511 (39.7) | 501 (38.9) | 525 (40.8) | 483 (37.5) | |
| ≥60 | 340 (26.4) | 332 (25.8) | 324 (25.2) | 327 (25.4) | |
| Education level | <0.001 | ||||
| Junior high school or below | 1050 (81.7) | 984 (76.4) | 954 (74.2) | 777 (60.4) | |
| Senior high school or above | 236 (18.4) | 304 (23.6) | 332 (25.8) | 510 (39.6) | |
| Place of residence | <0.001 | ||||
| Urban areas | 267 (20.8) | 352 (27.3) | 445 (34.6) | 626 (48.6) | |
| Rural areas | 1019 (79.2) | 936 (72.7) | 841 (65.4) | 661 (51.4) | |
| Region of residence | <0.001 | ||||
| Northern regions | 424 (33.0) | 493 (38.3) | 475 (36.9) | 554 (43.1) | |
| Southern regions | 862 (67.0) | 795 (61.7) | 811 (63.1) | 733 (56.9) | |
| Individual annual income | <0.001 | ||||
| Low | 536 (41.7) | 493 (38.3) | 411 (32.0) | 303 (23.5) | |
| Medium | 449 (34.9) | 450 (34.9) | 436 (33.9) | 370 (28.8) | |
| High | 301 (23.4) | 345 (26.8) | 439 (34.1) | 614 (47.7) | |
| Smoking history | 0.340 | ||||
| Yes | 382 (29.7) | 346 (26.9) | 369 (28.7) | 382 (29.7) | |
| No | 904 (70.3) | 942 (73.1) | 917 (71.3) | 905 (70.3) | |
| Drinking past year | 0.549 | ||||
| Yes | 393 (30.6) | 387 (30.1) | 399 (31.0) | 419 (32.6) | |
| No | 893 (69.4) | 901 (70.0) | 887 (69.0) | 868 (67.4) | |
| Physical activity | <0.001 | ||||
| Low | 424 (33.0) | 405 (31.4) | 422 (32.8) | 464 (36.1) | |
| Medium | 406 (31.6) | 427 (33.2) | 410 (31.9) | 475 (36.9) | |
| High | 456 (35.5) | 456 (35.4) | 454 (35.3) | 348 (27.0) | |
| Urbanization | <0.001 | ||||
| Low | 571 (44.4) | 487 (37.8) | 402 (31.3) | 321 (24.9) | |
| Medium | 414 (32.2) | 424 (32.9) | 422 (32.8) | 417 (32.4) | |
| High | 301 (23.4) | 377 (29.3) | 462 (35.9) | 549 (42.7) | |
| BMI (kg/m2) | 0.014 | ||||
| <18.5 | 82 (6.4) | 97 (7.5) | 84 (6.5) | 70 (5.4) | |
| 18.5–23.9 | 812 (63.1) | 758 (58.9) | 761 (59.2) | 742 (57.7) | |
| ≥24.0 | 392 (30.5) | 433 (33.6) | 441 (34.3) | 475 (36.9) | |
| Energy (kcal/day) | 2165.5 ± 703.2 | 2188.5 ± 677.1 | 2217.8 ± 709.9 | 2259.2 ± 743.4 | 0.025 |
| Protein (g/day) | 66.1 ± 24.1 | 69.9 ± 24.8 | 72.2 ± 26.9 | 76.4 ± 30.4 | <0.001 |
| Fat (g/day) | 74.5 ± 38.4 | 79.1 ± 38.5 | 83.3 ± 41.3 | 88.8 ± 43.7 | <0.001 |
| Carbohydrate (g/day) | 299.1 ± 117.2 | 286.6 ± 108.4 | 282.2 ± 106.7 | 278.1 ± 109.6 | <0.001 |
| Sodium (mg/day) | 4380.3 ± 4188.8 | 4778.0 ± 3575.4 | 5439.8 ± 6152.9 | 5859.4 ± 5939.3 | <0.001 |
| WC (cm) | 79.95 ± 9.33 | 80.35 ± 9.34 | 80.43 ± 10.50 | 80.68 ± 10.73 | 0.052 |
| TG (mmol/L) | 1.21 ± 0.77 | 1.23 ± 0.80 | 1.21 ± 0.83 | 1.18 ± 0.73 | 0.284 |
| HDL-C (mmol/L) | 1.49 ± 0.37 | 1.49 ± 0.44 | 1.47 ± 0.42 | 1.45 ± 0.35 | 0.045 |
| SBP (mmHg) | 121.54 ± 17.06 | 122.17 ± 17.09 | 122.47 ± 17.05 | 121.32 ± 15.94 | 0.272 |
| DBP (mmHg) | 78.58 ± 10.72 | 79.24 ± 10.90 | 79.18 ± 10.31 | 78.59 ± 9.75 | 0.199 |
| FPG (mmol/L) | 5.12 ± 1.02 | 5.11 ± 0.99 | 5.09 ± 0.90 | 5.03 ± 0.97 | 0.011 |
Values are given as the number of subjects, the percentage for categorical variables, and mean ± SD for continuous variables.
3.2. Associations of UPF Consumption with MetS and Its Components
Table 2 explores the associations of UPF consumption with MetS and its components in diverse groups. During a median (IQR) 6.0 (3.0, 9.0) year follow-up with 31,878 person-years, 1712 MetS cases were identified, with an incidence of 33.26%. After adjustment for confounding factors, such as gender, age, education level, place of residence, region, income level, smoking history, drinking status, metabolic equivalents, urbanicity, BMI, total energy, protein, fat, carbohydrate, and sodium intake, the risk of MetS was increased by 17% in the highest quartile with UPF consumption (HR: 1.17, 95% CI: 1.01–1.35, p trend: 0.047), with the lowest quartile as a reference.
Table 2.
Associations of UPF consumption with MetS and its components.
| Quartile of UPF (g/Day) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p Trend | |
| MetS a | |||||
| Median | 3.3 | 10.9 | 23.5 | 60.8 | |
| Model 1 | 1.00 (ref) | 1.08 (0.95, 1.24) | 1.08 (0.94, 1.24) | 1.14 (0.99, 1.31) | 0.126 |
| Model 2 | 1.00 (ref) | 1.08 (0.95, 1.24) | 1.09 (0.95, 1.25) | 1.16 (1.00, 1.33) * | 0.075 |
| Model 3 | 1.00 (ref) | 1.08 (0.94, 1.23) | 1.08 (0.94, 1.24) | 1.17 (1.01, 1.35) * | 0.047 |
| Central obesity b | |||||
| Median | 3.3 | 11.6 | 26.5 | 67.0 | |
| Model 1 | 1.00 (ref) | 1.07 (0.95, 1.20) | 1.12 (0.99, 1.26) | 1.28 (1.13, 1.44) *** | <0.001 |
| Model 2 | 1.00 (ref) | 1.08 (0.96, 1.21) | 1.13 (1.01, 1.28) * | 1.30 (1.15, 1.47) *** | <0.001 |
| Model 3 | 1.00 (ref) | 1.07 (0.95, 1.20) | 1.13 (1.01, 1.28) * | 1.33 (1.18, 1.51) *** | <0.001 |
| Raised TG c | |||||
| Median | 3.4 | 11.4 | 25.0 | 63.5 | |
| Model 1 | 1.00 (ref) | 1.06 (0.91, 1.23) | 1.07 (0.92, 1.25) | 1.25 (1.07, 1.46) ** | 0.003 |
| Model 2 | 1.00 (ref) | 1.06 (0.91, 1.24) | 1.08 (0.92, 1.25) | 1.26 (1.08, 1.48) ** | 0.002 |
| Model 3 | 1.00 (ref) | 1.06 (0.91, 1.23) | 1.08 (0.92, 1.26) | 1.26 (1.08, 1.48) ** | 0.003 |
| Reduced HDL-C d | |||||
| Median | 3.4 | 11.0 | 23.7 | 60.3 | |
| Model 1 | 1.00 (ref) | 1.07 (0.92, 1.24) | 1.21 (1.04, 1.40) * | 1.18 (1.01, 1.38) * | 0.044 |
| Model 2 | 1.00 (ref) | 1.08 (0.93, 1.25) | 1.22 (1.05, 1.41) ** | 1.21 (1.04, 1.41) * | 0.023 |
| Model 3 | 1.00 (ref) | 1.08 (0.93, 1.26) | 1.24 (1.07, 1.44) ** | 1.25 (1.07, 1.46) ** | 0.007 |
| Raised BP e | |||||
| Median | 3.3 | 11.6 | 26.9 | 66.9 | |
| Model 1 | 1.00 (ref) | 1.05 (0.94, 1.17) | 1.05 (0.93, 1.17) | 1.14 (1.02, 1.29) * | 0.033 |
| Model 2 | 1.00 (ref) | 1.06 (0.95, 1.18) | 1.05 (0.94, 1.18) | 1.16 (1.03, 1.31) * | 0.022 |
| Model 3 | 1.00 (ref) | 1.05 (0.94, 1.18) | 1.04 (0.93, 1.16) | 1.16 (1.03, 1.32) * | 0.018 |
| Raised FPG f | |||||
| Median | 3.4 | 11.3 | 25.2 | 64.4 | |
| Model 1 | 1.00 (ref) | 1.04 (0.92, 1.18) | 1.04 (0.92, 1.17) | 1.07 (0.94, 1.22) | 0.404 |
| Model 2 | 1.00 (ref) | 1.06 (0.94, 1.20) | 1.05 (0.93, 1.19) | 1.09 (0.96, 1.24) | 0.287 |
| Model 3 | 1.00 (ref) | 1.05 (0.93, 1.19) | 1.07 (0.94, 1.21) | 1.11 (0.98, 1.27) | 0.141 |
a n = 5147. b n = 5558. c n = 5412. d n = 5411. e n = 5695. f n = 5585. * p < 0.05, ** p < 0.01, *** p < 0.001. Model 1 adjusted gender, age, education level, place of residence, regions, and income level; Model 2 further adjusted smoking history, drinking status, metabolic equivalents, and urbanicity based on Model 1; Model 3 further adjusted BMI, total energy intake, protein intake, fat intake, carbohydrate intake, and sodium intake based on Model 2.
For the associations of UPF consumption with components of MetS, after adjusting for all covariates, the risk of central obesity, raised TG, reduced HDL-C, and raised BP was increased by 33% (HR: 1.33, 95% CI: 1.18–1.51, p trend: <0.001), 26% (HR: 1.26, 95% CI: 1.08–1.48, p trend: 0.003), 25% (HR: 1.25, 95% CI: 1.07–1.46, p trend: 0.007), and 16% (HR: 1.16, 95% CI: 1.03–1.32, p trend: 0.018) in the highest quartile with UPF consumption, respectively. No correlation was observed between UPF consumption and raised FPG (HR: 1.11, 95% CI: 0.98–1.27, p trend: 0.141).
3.3. Stratified Analyses of MetS Risk and UPF Consumption
Table 3 presents the sensitivity analysis of MetS risk and UPF consumption. The results showed that the positive association of UPF consumption with risk of MetS was consistent in women, 45–59 years age group, subjects in urban areas, and southern regions. In addition, place of residence and urbanization had an interactive effect on the association between UPF consumption and risk of MetS (p < 0.05).
Table 3.
Stratified analyses of MetS risk and UPF consumption.
| Quartile of UPF (g/Day) | |||||
|---|---|---|---|---|---|
| Q1 (<6.5) | Q2 (6.5–16.3) | Q3 (16.3–36.1) | Q4 (>36.1) | p for Interaction | |
| Gender | 0.208 | ||||
| Men | 1.00 (ref) | 1.12 (0.93, 1.36) | 1.06 (0.87, 1.28) | 1.09 (0.89, 1.33) | |
| Women | 1.00 (ref) | 1.00 (0.82, 1.22) | 1.10 (0.90, 1.34) | 1.26 (1.02, 1.55) * | |
| Age | 0.093 | ||||
| 18–44 | 1.00 (ref) | 0.98 (0.76, 1.26) | 0.91 (0.70, 1.18) | 0.97 (0.74, 1.27) | |
| 45–59 | 1.00 (ref) | 1.16 (0.94, 1.44) | 1.27 (0.91, 1.40) | 1.29 (1.03, 1.61) * | |
| ≥60 | 1.00 (ref) | 1.02 (0.80, 1.32) | 1.16 (0.90, 1.50) | 1.27 (0.96, 1.66) | |
| Education level | 0.250 | ||||
| Junior high school or below | 1.00 (ref) | 1.14 (0.98, 1.32) | 1.14 (0.97, 1.33) | 1.13 (0.95, 1.34) | |
| Senior high school or above | 1.00 (ref) | 0.83 (0.60, 1.14) | 0.88 (0.64, 1.20) | 1.13 (0.84, 1.51) | |
| Place of residence | 0.013 | ||||
| Urban areas | 1.00 (ref) | 1.19 (0.89, 1.60) | 1.11 (0.84, 1.47) | 1.41 (1.07, 1.86) * | |
| Rural areas | 1.00 (ref) | 1.05 (0.90, 1.22) | 1.09 (0.92, 1.28) | 1.01 (0.84, 1.21) | |
| Region of residence | 0.365 | ||||
| Northern regions | 1.00 (ref) | 0.95 (0.76, 1.18) | 1.00 (0.80, 1.25) | 1.02 (0.81, 1.28) | |
| Southern regions | 1.00 (ref) | 1.17 (0.98, 1.39) | 1.14 (0.95, 1.36) | 1.29 (1.06, 1.55) ** | |
| Individual annual income | 0.252 | ||||
| Low | 1.00 (ref) | 1.04 (0.84, 1.29) | 1.05 (0.83, 1.32) | 1.22 (0.95, 1.58) | |
| Medium | 1.00 (ref) | 1.17 (0.94, 1.47) | 1.06 (0.83, 1.34) | 1.05 (0.81, 1.36) | |
| High | 1.00 (ref) | 0.98 (0.74, 1.30) | 1.11 (0.85, 1.44) | 1.17 (0.90, 1.51) | |
| Urbanization | 0.008 | ||||
| Low | 1.00 (ref) | 1.13 (0.91, 1.39) | 1.09 (0.87, 1.36) | 0.99 (0.77, 1.29) | |
| Medium | 1.00 (ref) | 1.01 (0.79, 1.29) | 1.02 (0.79, 1.32) | 0.96 (0.74, 1.24) | |
| High | 1.00 (ref) | 1.08 (0.82, 1.41) | 1.15 (0.86, 1.49) | 1.45 (1.11, 1.89) | |
* p < 0.05, ** p < 0.01.
4. Discussion
In this study, the association between UPF consumption and MetS of Chinese adults aged 18 years and above has been evaluated through longitudinal prospective data from the CHNS. In the present study, higher UPF consumption was found to be positively correlated with MetS. The association was stronger in women, adults aged 45–59, and those living in urban areas.
The results of multiple previous studies were consistent with our findings. Lavigne-Robichaud et al. showed that comparing the lowest quintiles diet quality score, adults with the highest contribution of UPF to total daily dietary energy intake can effectively increase the risk of MetS (OR: 1.9, 95% CI: 1.14–3.17) from a 2005–2009 cross-sectional study in Canada [37]. Dana et al. also found that in adults, high consumption of UPF was associated with a higher risk for MetS (OR: 1.88, 95% CI: 1.31–2.71) and its components [38]. Previous systematic reviews and meta-analyses have shown that the highest UPF consumption was associated with a significant increase in the risk of MetS (OR: 1.79, 95% CI: 1.10–2.90) [39,40].
There are several potential plausible mechanisms that may explain the correlation between UPF consumption and MetS. Firstly, UPFs are typically high in added sugars, salt, and saturated and trans fats; excessive intake of UPFs could result in an increase in C-reactive protein (CRP) levels [41]. Moreover, further inflammatory responses may occur, and it increases the risk of MetS [42]. Secondly, higher UPF consumption is inversely associated with a poor nutritional profile and quality and deficiency intake of dietary fiber, fruit, vegetables, and legumes [43]. In addition, ingredients in UPFs such as artificial sweeteners could result in dysbiosis of gut microbiota, glucose intolerance, insulin resistance, and diverse metabolic disturbance, which then leads to the development of MetS [44,45]. Thirdly, the physical properties of food were altered by a series of industrial processes, which could result in a higher glycemic load and reduction of gut–brain satiety signaling [46,47]. The release of incretin hormones and gastric inhibitory polypeptide may increase insulin secretion and promote a greater appetite and overconsumption [48,49].
In the present study, higher UPF consumption was positively associated with the risk of central obesity (33%), raised TG (26%), reduced HDL-C (25%), and raised BP (16%), while no statistical association was found between the highest quartile group and raised FPG. An increasing body of evidence shows that UPF consumption is linked with overweight/obesity/WC [16,50,51,52]. Li et al. found that higher UPF consumption (≥50 g/d) was associated with an increased risk of overweight/obesity by 45–50% in Chinese adults aged 20 years and above using CHNS (1997–2011) [28]. The poor nutritional profile of diets with saturated fat and free sugar from UPFs contributes to more energy, weight gain, and higher odds of BMI [53]. A prospective Spanish cohort showed that adults with the highest tertile consumption of UPFs had a higher risk of developing hypertension (HR: 1.21, 95% CI: 1.06–1.37) [19]. Similarly, decreased potassium intake and increased sodium intake with UPF consumption may cause sodium/potassium imbalance, thus improving blood pressure levels [54]. In a systematic review and meta-analysis, Pagliai et al. found that no significant correlation was found between the highest UPF consumption and hyperglycemia. However, in two prospective cohort studies of UK and French adults, a diet with a higher proportion of UPFs was associated with an increased risk of type 2 diabetes (T2D) [20,21]. Of note, substances present in food packaging materials such as bisphenol-A (BPA) have been found to have endocrine-disrupting properties and a positive association with increased T2D in previous meta-analyses [55]. Collectively, higher UPF consumption has a certain adverse effect on the components of MetS. The specific mechanism and long-term health outcomes with UPF are warranted to explore.
Our findings suggest that UPF consumption was more associated with higher education levels and higher income. In Western countries, however, the situation is different. People with lower socio-economic profiles or educational levels are more likely to have higher UPF consumption [20,51]. As dietary patterns transition, UPFs are more widely available in China. People can afford to choose more food types due to the improvement in economic level while insufficient nutritional knowledge might lead them to choose durable, palatable, and ready-to-eat UPFs. We also found that adults aged 45–59 years who live in urban areas with higher UPF consumption had higher odds of MetS. With economic growth and development, China has been undergoing a rapid urbanicity and nutrition transition [56,57]. Although urbanization promotes civilization progress, it also brings about some negative consequences on health, such as low physical activity and weight gain [58]. Coincidentally, the high palatability, convenience, and easy availability of UPFs make them more accessible in urban areas and promote overconsumption. There is accumulating evidence implicating UPFs with poor dietary quality [59]. In consequence, residents in urban areas with higher UPF consumption need more attention, thereby reducing the incidence of chronic non-communicable diseases.
To the best of our knowledge, this is the first population-based prospective cohort study to examine the association between UPF consumption and the risk of MetS in Chinese adults. UPFs were classified by an updated NOVA classification system. Robust analysis was performed using three 24 h dietary recalls and data from weighing foods and condiments in household inventories. Nevertheless, there are still some limitations in this study that should be noted. First, there may be a misclassification owing to a lack of food packaging and labeling information for some uncertain food items. Second, dietary information collected by the 24 h retrospective method may lead to recall bias. Third, although potential confounding factors were adjusted, the possibility of residual confusion cannot be completely avoided. Last, the results of 24 h dietary recall may not represent long-term diet habits completely. Future studies are needed to add data from food frequency questionnaires (FFQs) and explore the correlation between UPFs and health outcomes.
5. Conclusions
In conclusion, this study provides prospective evidence that higher UPF consumption is positively correlated with MetS and its single component. Meanwhile, adults aged 45–59 years who live in urban areas with higher UPF consumption had a higher risk of MetS. Further studies such as intervention trials are needed to confirm the mechanism of correlation between UPF consumption and health-related outcomes. From a public health point of view, considering the gradual upward trend of UPF consumption in Chinese residents, nutrition education is warranted to promote a balanced diet and improve the overall dietary quality of residents to reduce the risk of MetS effectively.
Acknowledgments
The authors are grateful to all the staff and participants involved in the CHNS. The authors also thank the research team at the Carolina Population Center, University of North Carolina at Chapel Hill, and the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Author Contributions
Data preparation, original draft preparation and formal analysis, F.P.; investigation, data curation, J.Z., C.S., X.J., W.D., H.J., W.L., L.W. and L.H.; research guidance and administrative support, H.W. and B.Z.; research design, funding support, and review, Z.W., H.W. and G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Committees of the University of North Carolina at Chapel Hill (UNC-CH) and the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention (protocol code: No. 201524, approval date: 20 August 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this study according to the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Conflicts of Interest
The authors declare no potentially relevant conflict of interest.
Funding Statement
This study was supported by the Study of Diet and Nutrition Assessment and Intervention Technology (No. 2020YFC2006300) from the Active Health and Aging Technologic Solutions Major Project of the National Key R&D Program; the Chinese Center for Disease Control National Institute of Nutrition and Health, the National Institutes of Health (NIH) (R01-HD30880 and R01-HD38700); The Ministry of Finance of the Republic of China (13103110700015005).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu D., Reynolds K., Wu X., Chen J., Duan X., Reynolds R.F., Whelton P.K., He J., Inter A.C.G. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 4.He Y., Li Y., Bai G., Zhang J., Fang Y., Zhao L., Zhao W., Yang X., Ding G. Prevalence of metabolic syndrome and individual metabolic abnormalities in China, 2002–2012. Asia Pac. J. Clin. Nutr. 2019;28:621–633. doi: 10.6133/apjcn.201909_28(3).0023. [DOI] [PubMed] [Google Scholar]
- 5.Yao F., Bo Y., Zhao L., Li Y., Ju L., Fang H., Piao W., Yu D., Lao X. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. Nutrients. 2021;13:4475. doi: 10.3390/nu13124475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godos J., Zappala G., Bernardini S., Giambini I., Bes-Rastrollo M., Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017;68:138–148. doi: 10.1080/09637486.2016.1221900. [DOI] [PubMed] [Google Scholar]
- 7.Mankowski R.T., Aubertin-Leheudre M., Beavers D.P., Botoseneanu A., Buford T.W., Church T., Glynn N.W., King A.C., Liu C., Manini T.M., et al. Sedentary time is associated with the metabolic syndrome in older adults with mobility limitations--The LIFE Study. Exp. Gerontol. 2015;70:32–36. doi: 10.1016/j.exger.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H., Lee K., Rebholz C.M., Kim J. Plant-based diets and incident metabolic syndrome: Results from a South Korean prospective cohort study. PLoS Med. 2020;17:e1003371. doi: 10.1371/journal.pmed.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiani R., Naldini G., Chiavarini M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. Nutrients. 2019;11:2056. doi: 10.3390/nu11092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteiro C.A., Cannon G., Moubarac J.C., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21:5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteiro C.A., Cannon G., Levy R.B., Moubarac J.C., Louzada M.L., Rauber F., Khandpur N., Cediel G., Neri D., Martinez-Steele E., et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019;22:936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srour B., Kordahi M.C., Bonazzi E., Deschasaux-Tanguy M., Touvier M., Chassaing B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022;7:1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 13.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., Chung S.T., Costa E., Courville A., Darcey V., et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019;30:67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauber F., Chang K., Vamos E.P., da Costa Louzada M.L., Monteiro C.A., Millett C., Levy R.B. Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021;60:2169–2180. doi: 10.1007/s00394-020-02367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beslay M., Srour B., Mejean C., Alles B., Fiolet T., Debras C., Chazelas E., Deschasaux M., Wendeu-Foyet M.G., Hercberg S., et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: A prospective analysis of the French NutriNet-Sante cohort. PLoS Med. 2020;17:e1003256. doi: 10.1371/journal.pmed.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canhada S.L., Luft V.C., Giatti L., Duncan B.B., Chor D., Fonseca M., Matos S.M.A., Molina M., Barreto S.M., Levy R.B., et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Public Health Nutr. 2020;23:1076–1086. doi: 10.1017/S1368980019002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaranni P., Cardoso L.O., Chor D., Melo E.C.P., Matos S.M.A., Giatti L., Barreto S.M., da Fonseca M.J.M. Ultra-processed foods, changes in blood pressure and incidence of hypertension: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Public Health Nutr. 2021;24:3352–3360. doi: 10.1017/S136898002100094X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezende-Alves K., Hermsdorff H.H.M., Miranda A., Lopes A.C.S., Bressan J., Pimenta A.M. Food processing and risk of hypertension: Cohort of Universities of Minas Gerais, Brazil (CUME Project) Public Health Nutr. 2021;24:4071–4079. doi: 10.1017/S1368980020002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendonca R.D., Lopes A.C., Pimenta A.M., Gea A., Martinez-Gonzalez M.A., Bes-Rastrollo M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017;30:358–366. doi: 10.1093/ajh/hpw137. [DOI] [PubMed] [Google Scholar]
- 20.Srour B., Fezeu L.K., Kesse-Guyot E., Alles B., Debras C., Druesne-Pecollo N., Chazelas E., Deschasaux M., Hercberg S., Galan P., et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Sante Prospective Cohort. JAMA Intern. Med. 2020;180:283–291. doi: 10.1001/jamainternmed.2019.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy R.B., Rauber F., Chang K., Louzada M., Monteiro C.A., Millett C., Vamos E.P. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin. Nutr. 2021;40:3608–3614. doi: 10.1016/j.clnu.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Duan M.J., Vinke P.C., Navis G., Corpeleijn E., Dekker L.H. Ultra-processed food and incident type 2 diabetes: Studying the underlying consumption patterns to unravel the health effects of this heterogeneous food category in the prospective Lifelines cohort. BMC Med. 2022;20:7. doi: 10.1186/s12916-021-02200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llavero-Valero M., Escalada-San Martín J., Martínez-González M.A., Basterra-Gortari F.J., de la Fuente-Arrillaga C., Bes-Rastrollo M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clin. Nutr. 2021;40:2817–2824. doi: 10.1016/j.clnu.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Martinez Steele E., Juul F., Neri D., Rauber F., Monteiro C.A. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev. Med. 2019;125:40–48. doi: 10.1016/j.ypmed.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro C.A., Moubarac J.C., Cannon G., Ng S.W., Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013;14((Suppl. 2)):21–28. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro C.A., Lawrence M., Millett C., Nestle M., Popkin B.M., Scrinis G., Swinburn B. The need to reshape global food processing: A call to the United Nations Food Systems Summit. BMJ Glob. Health. 2021;6:e006885. doi: 10.1136/bmjgh-2021-006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Wang Z., Du W., Huang F., Jiang H., Bai J., Zhang X., Zhang B., Wang H. Twenty-Five-Year Trends in Dietary Patterns among Chinese Adults from 1991 to 2015. Nutrients. 2021;13:1327. doi: 10.3390/nu13041327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Shi Z. Ultra-Processed Food Consumption Associated with Overweight/Obesity among Chinese Adults-Results from China Health and Nutrition Survey 1997–2011. Nutrients. 2021;13:2796. doi: 10.3390/nu13082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., Shi Z. Association between Ultra-Processed Food Consumption and Diabetes in Chinese Adults-Results from the China Health and Nutrition Survey. Nutrients. 2022;14:4241. doi: 10.3390/nu14204241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Shi Z. Ultra-Processed Food Consumption Associated with Incident Hypertension among Chinese Adults-Results from China Health and Nutrition Survey 1997–2015. Nutrients. 2022;14:4783. doi: 10.3390/nu14224783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Zhai F.Y., Du S.F., Popkin B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014;15((Suppl. S1)):2–7. doi: 10.1111/obr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popkin B.M., Du S., Zhai F., Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010;39:1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y. China Food Composition 2004. Peking University Medical Press; Beijing, China: 2005. [Google Scholar]
- 34.Yang Y., Wang Y., Pan X. China Food Composition 2009. Peking University Medical Press; Beijing, China: 2010. [Google Scholar]
- 35.Heng D., Ma S., Lee J.J., Tai B.C., Mak K.H., Hughes K., Chew S.K., Chia K.S., Tan C.E., Tai E.S. Modification of the NCEP ATP III definitions of the metabolic syndrome for use in Asians identifies individuals at risk of ischemic heart disease. Atherosclerosis. 2006;186:367–373. doi: 10.1016/j.atherosclerosis.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth B.E., Haskell W.L., Whitt M.C., Irwin M.L., Swartz A.M., Strath S.J., O’Brien W.L., Bassett D.R., Jr., Schmitz K.H., Emplaincourt P.O., et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 37.Lavigne-Robichaud M., Moubarac J.C., Lantagne-Lopez S., Johnson-Down L., Batal M., Laouan Sidi E.A., Lucas M. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Quebec, Canada. Public Health Nutr. 2018;21:172–180. doi: 10.1017/S136898001700115X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivancovsky-Wajcman D., Fliss-Isakov N., Webb M., Bentov I., Shibolet O., Kariv R., Zelber-Sagi S. Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int. 2021;41:2635–2645. doi: 10.1111/liv.14996. [DOI] [PubMed] [Google Scholar]
- 39.Pagliai G., Dinu M., Madarena M.P., Bonaccio M., Iacoviello L., Sofi F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021;125:308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X., Zhang Z., Yang H., Qiu P., Wang H., Wang F., Zhao Q., Fang J., Nie J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020;19:86. doi: 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez A.S., Guerrero D.B., Soto M.B., Diaz S.P., Martinez-Olmos M., Vidal O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006;60:802–809. doi: 10.1038/sj.ejcn.1602384. [DOI] [PubMed] [Google Scholar]
- 42.Christ A., Lauterbach M., Latz E. Western Diet and the Immune System: An Inflammatory Connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Batal M., Johnson-Down L., Moubarac J.C., Ing A., Fediuk K., Sadik T., Tikhonov C., Chan L., Willows N. Quantifying associations of the dietary share of ultra-processed foods with overall diet quality in First Nations peoples in the Canadian provinces of British Columbia, Alberta, Manitoba and Ontario. Public Health Nutr. 2018;21:103–113. doi: 10.1017/S1368980017001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 45.Azad M.B., Abou-Setta A.M., Chauhan B.F., Rabbani R., Lys J., Copstein L., Mann A., Jeyaraman M.M., Reid A.E., Fiander M., et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can. Med. Assoc. J. 2017;189:E929–E939. doi: 10.1503/cmaj.161390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7:2338–2346. doi: 10.1039/C6FO00107F. [DOI] [PubMed] [Google Scholar]
- 47.Fardet A., Mejean C., Laboure H., Andreeva V.A., Feron G. The degree of processing of foods which are most widely consumed by the French elderly population is associated with satiety and glycemic potentials and nutrient profiles. Food Funct. 2017;8:651–658. doi: 10.1039/C6FO01495J. [DOI] [PubMed] [Google Scholar]
- 48.Ayton A., Ibrahim A. The Western diet: A blind spot of eating disorder research?—A narrative review and recommendations for treatment and research. Nutr. Rev. 2020;78:579–596. doi: 10.1093/nutrit/nuz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford H.E., Peters V., Martin N.M., Sleeth M.L., Ghatei M.A., Frost G.S., Bloom S.R. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr. 2011;65:508–513. doi: 10.1038/ejcn.2010.291. [DOI] [PubMed] [Google Scholar]
- 50.Mendonca R.D., Pimenta A.M., Gea A., de la Fuente-Arrillaga C., Martinez-Gonzalez M.A., Lopes A.C., Bes-Rastrollo M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016;104:1433–1440. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 51.Julia C., Martinez L., Alles B., Touvier M., Hercberg S., Mejean C., Kesse-Guyot E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Sante study. Public Health Nutr. 2018;21:27–37. doi: 10.1017/S1368980017001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva F.M., Giatti L., de Figueiredo R.C., Molina M., de Oliveira Cardoso L., Duncan B.B., Barreto S.M. Consumption of ultra-processed food and obesity: Cross sectional results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort (2008–2010) Public Health Nutr. 2018;21:2271–2279. doi: 10.1017/S1368980018000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dicken S.J., Batterham R.L. The Role of Diet Quality in Mediating the Association between Ultra-Processed Food Intake, Obesity and Health-Related Outcomes: A Review of Prospective Cohort Studies. Nutrients. 2021;14:23. doi: 10.3390/nu14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juul F., Vaidean G., Parekh N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021;12:1673–1680. doi: 10.1093/advances/nmab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J., Lin Y., Liu Y., Chen K. Antibiotic exposure and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021;28:65052–65061. doi: 10.1007/s11356-021-16781-3. [DOI] [PubMed] [Google Scholar]
- 56.Li X., Song J., Lin T., Dixon J., Zhang G., Ye H. Urbanization and health in China, thinking at the national, local and individual levels. Environ. Health. 2016;15((Suppl. S1)):32. doi: 10.1186/s12940-016-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhai F., Wang H., Du S., He Y., Wang Z., Ge K., Popkin B.M. Prospective study on nutrition transition in China. Nutr. Rev. 2009;67((Suppl. S1)):S56–S61. doi: 10.1111/j.1753-4887.2009.00160.x. [DOI] [PubMed] [Google Scholar]
- 58.Attard S.M., Howard A.G., Herring A.H., Zhang B., Du S., Aiello A.E., Popkin B.M., Gordon-Larsen P. Differential associations of urbanicity and income with physical activity in adults in urbanizing China: Findings from the population-based China Health and Nutrition Survey 1991–2009. Int. J. Behav. Nutr. Phys. Act. 2015;12:152. doi: 10.1186/s12966-015-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elizabeth L., Machado P., Zinocker M., Baker P., Lawrence M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients. 2020;12:1955. doi: 10.3390/nu12071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this study according to the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.