ABSTRACT
Background and Objectives:
Although the use of a long metal stent is favored for EUS-guided hepaticogastrostomy (EUS-HGS) for the relief of malignant biliary obstruction (MBO), endoscopic reintervention (E-RI) at the time of recurrent biliary obstruction (RBO) is challenging due to a long intragastric portion. This study evaluated the feasibility and safety of E-RI after a long partially covered metal stent (L-PCMS) placement during EUS-HGS.
Materials and Methods:
We performed a multicenter retrospective study between January 2015 and December 2019 examining patients with MBO who underwent E-RI for RBO through the EUS-HGS route after the L-PCMS placement. Technical and clinical success rates, details of E-RI, adverse events (AEs), stent patency, and survival time were evaluated.
Results:
Thirty-three patients at eight referral centers in Japan who underwent E-RI through the EUS-HGS route were enrolled. The location of MBO was distal in 54.5%. The median intragastric length of the L-PCMS was 5 cm. As the first E-RI attempt, E-RI via the distal end of the existing L-PCMS was successful in 60.6%. The overall technical and clinical success rates of E-RI were 100% and 81.8%, respectively. Liver abscess was noted in one patient. A proximal biliary stricture was associated with the clinical ineffectiveness of E-RI in multivariable analysis (odds ratio, 12.5, P = 0.04). The median survival and stent patency duration after E-RI were 140 and 394 days, respectively.
Conclusions:
Our study findings suggest that E-RI for RBO after EUS-HGS with a L-PCMS is technically feasible and clinically effective, without any severe AEs, especially for patients with distal MBO.
Keywords: EUS, EUS-guided biliary drainage, hepaticogastrostomy, malignant biliary obstruction, reintervention
INTRODUCTION
Since first described two decades ago, EUS-guided biliary drainage (EUS-BD) emerged as a therapeutic alternative to relieve malignant biliary obstruction (MBO) when standard ERCP failed.[1,2,3] Accumulated evidence has demonstrated that EUS-BD has high technical and clinical success rates comparable to ERCP as a palliative means of MBO.[4,5] Over the years, multiple approaches to EUS-BD have been described.[3,6] EUS-BD can be carried out by two major transmural approaches: EUS-guided choledochoduodenostomy and EUS-guided hepaticogastrostomy (EUS-HGS).[2,3] The former involves transmural stenting between the duodenum and the extrahepatic bile duct, whereas the latter involves transmural stenting between the gastric body and the left intrahepatic bile duct. Recent studies, including ours, have demonstrated the equivalent efficacy and safety of both EUS-BD approaches for the palliation of MBO.[7,8] However, from the perspective of reintervention at the time of recurrent biliary obstruction (RBO), EUS-HGS seems disadvantageous for the following reason. When performing EUS-HGS, the use of a long metal stent is favored to prevent stent inward migration into the peritoneal cavity, which is the most serious complication related to EUS-HGS.[9,10] In addition, an intragastric stent length ≥3 cm may be associated with long-term stent patency.[11] Therefore, in EUS-HGS, metal stents with a length of ≥10 cm have been used frequently. However, when RBO occurs, the long intragastric portion owing to the use of a long metal stent makes endoscopic reintervention (E-RI) technically challenging and requires advanced endoscopic skills.
Since survival duration might have been prolonged due to the progress of chemoradiotherapy or immunotherapy for underlying malignancies, the incidence of RBO during the follow-up period increased, which suggests that the clinical needs for E-RI following EUS-HGS are increasing. Nevertheless, literature on the efficacy of reintervention of RBO after EUS-HGS and case reports describing the procedural details of E-RI following EUS-HGS are lacking.[12,13,14] Hence, the present study examined the technical feasibility and clinical efficacy of E-RI for RBO after EUS-HGS using a long metal stent placement with a multicenter experience.
MATERIALS AND METHODS
Patients and study design
This was a multicenter retrospective study conducted at eight referral medical centers that are part of the Therapeutic EUS (TEUS) group in Japan. Consecutive patients who, between January 2015 and December 2019, underwent E-RI for RBO following successful EUS-HGS using a long partially covered metal stent (L-PCMS) were reviewed. RBO was defined as the recurrence of symptoms of MBO, including obstructive jaundice and cholangitis (leukocytosis, fever, and elevated serum liver enzyme and/or bilirubin levels) and biliary dilatation on imaging studies. Electronic medical records and the endoscopic database in each center were used to access data on patients with inoperable MBO who underwent E-RI through the EUS-HGS route for RBO. All included patients had undergone EUS-HGS using L-PCMS to relieve MBO. Exclusion criteria included patients whose jaundice or cholangitis had not been resolved after initial EUS-HGS, patients who underwent EUS-HGS with metal stents different from the L-PCMS, and patients who underwent a reintervention procedure other than the EUS-HGS route. The study protocol was approved by the Institutional Review Board of each affiliated center (Approval Number of the Institutional Review Board of Kindai University Hospital: 30-036), and this study was carried out in accordance with the Helsinki Declaration.
Endoscopic procedures
Experienced endoscopists at each center performed EUS-HGS and E-RI. For the initial EUS-HGS, a L-PCMS (Niti-S Biliary Silicone Covered Stent, Taewoong Medical, Gimpo, South Korea, also known as a modified Giobor stent[15]) [Figure 1a] was deployed between the left intrahepatic bile duct and the gastric body under EUS and fluoroscopic guidance [Figure 1b and c]. The L-PCMS was made of braided nitinol wire partially covered by a silicone membrane to prevent bile leakage. The proximal end of the stent had a 1-cm uncovered portion, whereas the distal end of the stent had a flared portion to prevent stent inward migration [Figure 1a]. In the present study, the L-PCMS with a diameter of 8 or 10 mm and a length of 10 or 12 cm was used.
Figure 1.
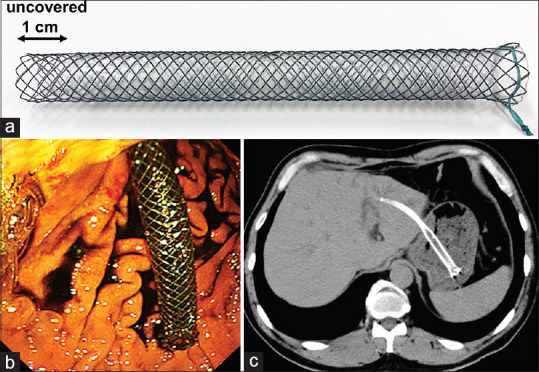
EUS-HGS using a L-PCMS. (a) Illustration of the L-PCMS (Niti-S Biliary Silicone Covered Stent, Taewoong Medical, Gimpo, South Korea) made of braided nitinol wire partially covered by a silicone membrane; the proximal end has a 1-cm uncovered part, and the distal end has a flared portion. (b) Endoscopic view revealing a long intragastric portion of the L-PCMS after EUS-HGS. (c) Computed tomography image showing the L-PCMS deployed between the left intrahepatic bile duct and the gastric body. EUS-HGS: EUS-hepaticogastrostomy; L-PCMS: Long partially covered metal stent
For E-RI, the endoscopic approach through the distal end of the existing EUS-HGS stent was attempted as the first E-RI method [Figure 2a]. Following successful guidewire and catheter insertion, stent cleaning by a retrieval balloon catheter or additional stent deployment via the EUS-HGS stent was performed depending on the findings of cholangiography. If an endoscopic approach through the distal end of the EUS-HGS stent failed, an endoscopic approach through the stent mesh of the EUS-HGS stent [Figure 2b] or through the HGS fistula after removal of the existing EUS-HGS stent [Figure 2c] was attempted. Following guidewire and catheter insertion, additional stent placement was performed through the stent mesh or fistula.
Figure 2.
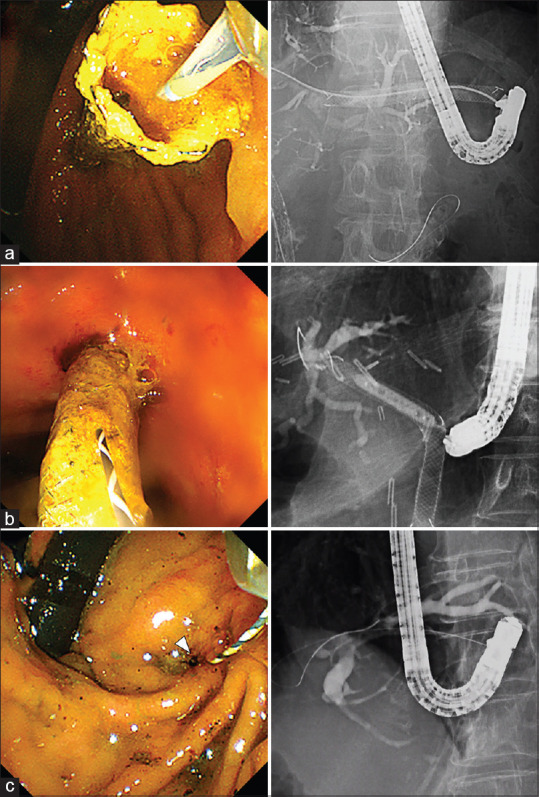
E-RI for recurrent biliary obstruction after EUS-guided hepaticogastrostomy using a L-PCMS. (a) E-RI through the distal end of the existing L-PCMS. (b) E-RI through the stent mesh of the existing L-PCMS. (c) E-RI through the hepaticogastrostomy fistula after removal of the L-PCMS. E-RI: Endoscopic reintervention; L-PCMS: Long partially covered metal stent
Outcome measures and definitions
The primary outcomes of interest in this study were technical and clinical success rates, which were associated with E-RI following EUS-HGS. Technical success was defined as a successful endoscopic approach to RBO via the EUS-HGS route and completion of the intended endoscopic procedure. Clinical success was defined as the improvement of cholangitis or a decrease in the serum bilirubin level, either to a normal level or a reduction rate of more than 50% within 4 weeks following E-RI. The secondary outcome measures included procedural details of E-RI, procedure time, adverse events (AEs), stent patency, and survival durations. The intragastric stent length was measured by computed tomography (CT) image. Distal MBO was defined as a biliary stenosis located ≥2 cm distal to the biliary hilum, and proximal MBO was defined as a biliary stenosis located <2 cm proximal to the biliary hilum based on imaging studies, such as by CT, magnetic resonance imaging, and cholangiography. AEs were defined as any procedure-related complication. Stent patency was defined as the period between the day of E-RI and the day of RBO. If RBO did not present until the time of death or last follow-up, these data were censored. The length of survival was measured from the day of E-RI to the time of death or last follow-up. Factors associated with clinical ineffectiveness were evaluated using a variety of parameters in univariable and multivariable analyses. Patients were followed up and their medical records were reviewed until study termination (December 31, 2020) or death.
Statistical analysis
Data were summarized by numbers with percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. Technical and clinical success rates were presented with a 95% confidence interval (CI). Statistical comparisons were made by performing Fisher's exact test for categorical variables and the Wilcoxon signed-rank test for continuous variables. Stent patency and survival duration were evaluated using Kaplan–Meier plots. The log-rank test was used to compare the stent patency and survival curves while accounting for censored data. To explore factors that affect the clinical ineffectiveness of E-RI, univariable and multivariable logistic regression analyses were performed, for which odds ratios and 95 % CIs were derived. Cutoff values were set at the median for each continuous variable for all patients. Factors with P < 0.1 in the univariable analysis were included in the subsequent multivariable analysis. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
Patient characteristics
During the study period, 211 patients underwent EUS-HGS using the L-PCMS for inoperable MBO at 8 affiliated centers. RBO developed in 16.6% (35/211) of patients. Excluded from these were two cases in which reintervention was performed via the percutaneous approach. Enrolled in this study were the remaining 33 patients who underwent E-RI through the EUS-HGS route. Their baseline characteristics are presented in Table 1. As for the location of MBO, 18 (54.5%) patients had distal MBO and 15 (45.5%) patients had proximal MBO. Duodenal stents were placed in 6 (18.2%) patients. All patients achieved successful relief of MBO after EUS-HGS with a median procedure time of 27 min (IQR, 20–40 min). The types of stents used are shown in Table 1. Notably, stent lengths of 10 and 12 cm were used in 26 (78.8%) cases and 7 (21.2%) cases, respectively. As a result, the median intragastric stent length was 5 cm. The median time to RBO after EUS-HGS was 104 days.
Table 1.
Baseline characteristics of patients who underwent endoscopic reintervention following EUS-guided hepaticogastrostomy
| Patient characteristics | n=33, n (%) |
|---|---|
| Median age, years (IQR) | 72 (67–76) |
| Sex (male/female) | 22 (66.7)/11 (33.3) |
| ECOG performance status (0/1/2) | 15 (45.5)/15 (45.5)/3 (9.1) |
| Underlying malignancy | |
| Gastric cancer | 9 (27.3) |
| Bile duct cancer | 9 (27.3) |
| Pancreatic cancer | 8 (24.2) |
| Hepatocellular carcinoma | 3 (9.1) |
| Others | 4 (12.1) |
| Location of MBO | |
| Distal MBO/proximal MBO | 18 (54.5)/15 (45.5) |
| Duodenal stent placement | 6 (18.2) |
| Indication for EUS-HGS | |
| Failure of duodenal scope insertion | 11 (33.3) |
| Surgically altered gastrointestinal anatomy | 10 (30.3) |
| Failure of biliary cannulation | 12 (36.4) |
| Procedural details of EUS-HGS | |
| Procedure time (min), median (IQR) | 27 (20–40) |
| Type of L-PCMS | |
| Stent diameter (8 mm/10 mm) | 26 (78.8)/7 (21.2) |
| Stent length (10 cm/12 cm) | 26 (78.8)/7 (21.2) |
| Intragastric stent length, median (IQR) | 5 (5.2–5.8) |
| Time to RBO after EUS-HGS (days), median (IQR) | 104 (56–263) |
ECOG: Eastern Cooperative Oncology Group; EUS-HGS: EUS-guided hepaticogastrostomy; IQR: Interquartile range; L-PCMS: Long partially covered metal stent; MBO: Malignant biliary obstruction; RBO: Recurrent biliary obstruction
Outcomes of endoscopic reintervention
Table 2 presents the outcomes of E-RI. RBO occurred due to a reflux of gastroduodenal contents or sludge formation in 19 (57.6%) patients, hyperplasia at the proximal uncovered stent portion in 9 (27.3%) patients, and additional biliary stricture due to tumor invasion in 5 (15.2%) patients, respectively. Examination of the factors of RBO depending on the affected locations of MBO showed that there was no difference in the frequency of RBO caused by food impaction or sludge formation between distal and proximal MBOs (11/18 patients with distal MBO and 8/15 patients with proximal MBO, P = 0.73); however, RBO caused by an additional biliary branch stricture was observed only with proximal MBO cases (0/18 patient with distal MBO and 5/15 patients with proximal MBO, P = 0.01). When the frequencies of RBO occurrence due to food impaction or sludge formation depending on the presence or absence of the duodenal stent were compared, there was no difference between the two groups (5/6 patients with a duodenal stent and 14/27 patients without a duodenal stent, P = 0.21).
Table 2.
Outcomes of endoscopic reintervention following EUS-guided hepaticogastrostomy
| Outcome measure | n=33, n (%) |
|---|---|
| Cause of RBO after EUS-HGS | |
| Food impaction or sludge formation | 19 (57.6) |
| Distal MBO/proximal MBO | 11 (33.3)/8 (24.2) |
| Hyperplasia at uncovered stent part | 9 (27.3) |
| Distal MBO/proximal MBO | 7 (21.2)/2 (13.3) |
| Additional biliary stricture | 5 (15.2) |
| Distal MBO/proximal MBO | 0 (0)/5 (15.2) |
| Technical success | 33 (100) |
| Clinical success | 27 (81.8) |
| Distal MBO/proximal MBO | 17 (94.4)/10 (66.7) |
| Procedure time, median (IQR) | 35 (29–50) |
| Type of endoscope | |
| Gastroscope/duodenoscope/echoendoscope | 5 (15.2)/27 (81.8)/1 (3.0) |
| Location of guidewire and catheter insertion | |
| Distal end of the existing HGS stent | 20 (60.6) |
| Stent mesh of the existing HGS stent | 10 (30.3) |
| HGS fistula after removal of the HGS stent | 3 (9.1) |
| Reintervention method | |
| Stent cleaning | 5 (15.2) |
| Additional HGS stent placement | 20 (60.6) |
| Antegrade stent placement | 5 (15.2) |
| Additional HGS and antegrade stent placement | 3 (9.1) |
| Adverse events | |
| Liver abscess | 1 (3.0) |
HGS: Hepaticogastrostomy; EUS-HGS: EUS-guided HGS; IQR: Interquartile range; MBO: Malignant biliary obstruction; RBO: Recurrent biliary obstruction
As the first E-RI attempt, the guidewire and catheter were successfully inserted through the distal end of the L-PCMS in 60.6% (20/33) of patients. In the remaining 13 patients, E-RI via the distal end of the L-PCMS failed because of the long intragastric L-PCMS. In 30.3% (10/33) of patients, E-RI via the stent mesh of the L-PCMS was successful. In the remaining 9.1% (3/33) of patients, guidewire and catheter insertions were successfully performed via HGS fistula after removal of the EUS-HGS stent. The overall technical and clinical success rates of E-RI for RBO were 100% (33/33 [95% CI, 0.894–1.00]) and 81.8% (27/33 [95% CI, 0.645–0.930]), respectively.
Clinical success was achieved at 94.4% (17/18) for distal MBO and 66.7% (10/15) for proximal MBO. In all six patients with clinical failure, jaundice and/or cholangitis did not improve, despite the additional stent placement through the L-PCMS stent. Additional EUS-BD was performed in four patients after failed E-RI through the EUS-HGS route. Two cases of EUS-BD for the right intrahepatic biliary branch via the duodenum achieved clinical success, whereas no clinical improvement was obtained for two cases with EUS-BD for the other left intrahepatic biliary branch via the stomach. In the remaining two patients, no additional drainage was performed due to worsened general condition. Univariable analysis did not determine significant factors affecting clinical ineffectiveness; however, subsequent multivariable analysis found the proximal biliary stricture to be a positive factor associated with clinical ineffectiveness [odds ratio, 12.5, P = 0.04, Table 3].
Table 3.
Univariable and multivariable analyses of factors associated with clinical failure of endoscopic reintervention following EUS-guided hepaticogastrostomy
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age>72 | 7.27 | 0.99-149.7 | 0.09 | 10.97 | 1.25–257.7 | 0.06 |
| Female sex | 2.38 | 0.37-15.5 | 0.35 | |||
| Performance status >1 | 2.50 | 0.10–31.7 | 0.49 | |||
| Pancreatobiliary cancer | 2.16 | 0.36–17.5 | 0.42 | |||
| Proximal biliary stricture | 8.50 | 1.16–175.5 | 0.07 | 12.52 | 1.44–292.3 | 0.04 |
| Presence of ascites | 2.88 | 0.32–21.1 | 0.30 | |||
| Presence of cholangitis | 1.00 | 0.16–8.20 | 0.99 | |||
| Biliary stent diameter of 8 mm | 0.45 | 0.07–3.93 | 0.43 | |||
| Biliary stent length of 12 cm | 0.70 | 0.03–5.59 | 0.76 | |||
| Intragastric stent length >5 cm | 0.80 | 0.13–5.02 | 0.81 | |||
| Duodenal stent placement | 4.00 | 0.43–33.2 | 0.36 | |||
| Time to stent occlusion >104 days | 0.93 | 0.15–5.82 | 0.93 | |||
| Time of procedure >35 min | 1.01 | 0.17–6.76 | 0.93 | |||
| Reintervention via stent mesh | 2.86 | 0.44–18.9 | 0.26 | |||
| Balloon cleaning only | 0.88 | 0.04–7.32 | 0.92 | |||
CI: Confidence interval; OR: Odds ratio
The median procedure time of E-RI was 35 min (IQR, 29–50 min), which was longer than that of the initial EUS-HGS (P = 0.03). In the majority of patients (28/33), additional stent placement was performed, as presented in Table 2. Regarding AEs associated with E-RI, liver abscess was noted in one case, which improved with antibiotic treatment.
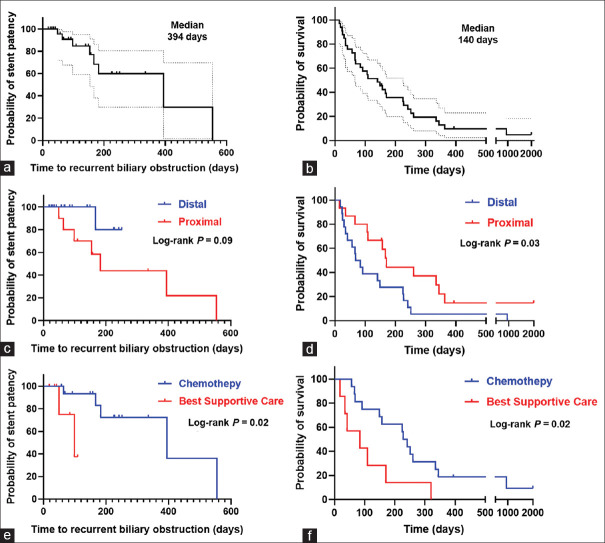
The median follow-up period was 140 days (IQR, 57–251 days). Regarding the treatment for underlying malignancy, among patients who received chemotherapy after EUS-HGS and showed clinical success with E-RI, 69.6% (16/23) could resume chemotherapy after E-RI. The median stent patency and survival periods after E-RI were 394 days (95% CI, 85.7–702.3 days) [Figure 3a] and 140 days (95% CI, 70.8–209.2 days) [Figure 3b], respectively. Then, the stent patency and survival periods were compared between the distal and proximal MBO groups and between groups with and without resumption of chemotherapy after E-RI. Stent patency time did not differ depending on the location of MBO [Figure 3c], whereas the survival time was considerably longer in the proximal MBO group [Figure 3d]. Both the stent patency and survival periods were substantially longer in the chemotherapy resumption group than in the best supportive care group [Figure 3e and f].
Figure 3.
Stent patency time and survival time after E-RI. Kaplan–Meier curve showing time to recurrent biliary obstruction after E-RI (a) and survival time after E-RI (b). Comparison between distal and proximal malignant biliary obstruction groups in relation to stent patency time after E-RI (not reached and 182 days, respectively, log-rank P = 0.09) (c) and survival time (76 and 170 days, respectively, log-rank P = 0.03) (d). Comparison between patients treated with chemotherapy or with best supportive care groups of stent patency time after E-RI (394 and 98 days, respectively, log-rank P = 0.02) (e) and survival time (234 and 84 days, respectively, log-rank P = 0.02) (f). E-RI: Endoscopic reintervention
DISCUSSION
According to some recent meta-analyses, the technical success rate of EUS-BD has been reported to be remarkably high at 90%–95%.[2,16,17] Over the years, applications of EUS-BD have extended beyond a salvage drainage of MBO to primary BD and benign etiologies.[18,19] While EUS-BD has gained popularity as an option for BD, the aforementioned meta-analyses have shown high procedure-associated AE rates of 17%–23%.[2,16,17] Since there is an anatomical gap between the gastric body and the liver, EUS-HGS has the potential risk of stent inward migration, which is one of the most serious AEs associated with mortality.[9,10] Thus far, several preventive devices for EUS-HGS have been proposed to prevent stent migration.[20,21,22] Some metal stents dedicated for EUS-HGS have distal antimigration flaps or hooks.[20,22] Cho et al. reported the clinical outcomes of EUS-HGS using a metal stent with anchoring flaps. The mean length of the stents used was 7.9 cm, and no stent migration occurred with a long-term follow-up period.[20] Unfortunately, as these dedicated metal stents are not commercially available, we used a 10- or 12-cm long L-PCMS without flaps or hooks. As a result, the median intragastric length of L-PCMS after EUS-HGS was 5 cm, which was similar to that of a previous study of EUS-HGS using the same L-PCMS.[23] Long stents are useful for preventing stent migration but make E-RI of RBO difficult. This is supported by the fact that, in the present study, E-RI took longer than the initial EUS-HGS.
One potential advantage of EUS-BD over standard ERCP is that EUS-BD may require fewer reinterventions because EUS-BD does not traverse the biliary stricture.[24,25] However, the risks of encountering RBO after EUS-BD have increased due to the growing clinical experience of EUS-BD and prolonged prognosis of the underlying disease. Previous studies have shown that the incidence of RBO following EUS-BD ranges from 11% to 25%,[26,27] which is equivalent to that of 16.6% in the current study. In EUS-HGS, the reflux of food residue from the gastric lumen into the stent is thought to be a major factor of RBO. In the present study, food impaction or sludge formation causes RBO in more than half of patients. Of note, tissue hyperplasia at the uncovered part of the L-PCMS was the second common factor of RBO. Although the uncovered portion of the L-PCMS is important in preventing blockage of small biliary branches, tissue hyperplasia at an uncovered portion of a metal stent is an inevitable limitation.[23] Recently, a case of RBO with severe hyperplasia after the L-PCMS placement required radiofrequency ablation has been reported.[28] Thus, we should be cautious that the use of L-PCMS may increase the risk of RBO due to hyperplasia formation.
To date, several E-RI techniques after EUS-HGS with a long metal stent have been described in isolated case reports.[12,13,14] E-RI can be divided into the following three approaches: E-RI through the distal end of the existing L-PCMS, E-RI through the stent mesh of the existing L-PCMS, and E-RI through the HGS fistula after removal of the L-PCMS. In the present study, E-RI via the distal end of the existing L-PCMS was attempted at first in all patients, and technical success was achieved in approximately 60% of the patients. E-RI via the stent mesh of the L-PCMS adjacent to the gastric wall was successful in the majority of the remaining cases. The complexity of this method is that the stent mesh must be expanded using a balloon or cautery dilator during the insertion of devices, including additional stents. The frequency of E-RI from the HGS fistula after L-PCMS removal was the lowest in this study. Although this technique is simple, caution is needed, because L-PCMS cannot be removed easily after a long-term indwelling. In the three cases evaluated by this study, E-RI was performed within 2 months after L-PCMS placement. Unfortunately, although the optimal E-RI method was not definitively determined from this study, E-RI through the EUS-HGS route after EUS-HGS with the L-PCMS placement seems technically feasible. Nonetheless, it is desirable to develop a more ideal metal stent exclusively for EUS-HGS that can prevent stent migration and facilitate E-RI.
Regarding the location of MBO, EUS-HGS has been applied not only to distal MBO but also to hilar MBO.[29,30] However, as shown in the present study, a newly developed biliary branch stricture due to tumor infiltration is a characteristic RBO factor in proximal MBO cases. Although the risk of bias is high owing to the analysis with a small number of cases, proximal MBO was negatively associated with the clinical success of E-RI after EUS-HGS, which may partially be because of the development of an additional biliary branch stricture caused by disease progression during the follow-up period. This notion is supported by the fact that clinical success was finally achieved after additional EUS-BD for the right intrahepatic bile duct in two patients for whom E-RI through the existing EUS-HGS stent failed to achieve clinical improvement. In cases where clinical success was achieved after E-RI, stent patency duration did not differ, regardless of the location of MBO; survival time was longer in the hilar MBO group. This may have been because all eight cases of pancreatic cancer with a dismal prognosis were included in the distal MBO group.
This study had several limitations. First, this was a retrospective study without a control group involving a relatively small number of patients despite having recruited patients from eight specialized centers. Selection bias is inevitable in this setting. Second, there was moderate heterogeneity in the underlying malignancy that led to the development of MBO. It is well known that biological behaviors of gastric cancers are quite different from those of hepatobiliary–pancreatic cancers. Third, procedural details of E-RI including the drainage method and stent types used for E-RI were heterogeneous between the affiliated centers, although our TEUS group regularly assembled every 3 months to share and refine our knowledge of EUS-BD and its E-RI procedure. Fourth, we only used a single type of L-PCMS for the initial EUS-HGS, although this stent has been commonly used in Japan. Future studies of E-RI that compare other EUS-HGS stents, including laser-cut metal stents or plastic stents, are required. According to a recent study on a large case series using a dedicated plastic stent for EUS-HGS, the stent patency rate after 2 months of stent placement was >90%.[31] Considering the excellent patency as well as the economic impact and the ease of stent exchange at the time of RBO, plastic stents may be suitable as the initial EUS-HGS for MBO; however, this concept can be confirmed if future studies compare the efficacy and safety of the L-PCMS and plastic stents in a large cohort of patients with inoperable MBO. Despite these limitations, the main strength of this study is that this is the first multicenter study that specifically addresses the feasibility and efficacy of E-RI after EUS-HGS.
CONCLUSION
Our multicenter experience suggests that E-RI after EUS-HGS with L-PCMS is a feasible and safe technique with a high clinical success rate in the hands of experienced endoscopists. Further large-scale prospective studies implementing a standardization of the reintervention procedure are required to establish an optimal E-RI technique following EUS-HGS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 2.Moole H, Bechtold ML, Forcione D, et al. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore) 2017;96:e5154. doi: 10.1097/MD.0000000000005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc. 2018;30:38–47. doi: 10.1111/den.12910. [DOI] [PubMed] [Google Scholar]
- 4.Hathorn KE, Bazarbashi AN, Sack JS, et al. EUS-guided biliary drainage is equivalent to ERCP for primary treatment of malignant distal biliary obstruction: A systematic review and meta-analysis. Endosc Int Open. 2019;7:E1432–41. doi: 10.1055/a-0990-9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Z, Wei Y, Lin H, et al. Endoscopic ultrasound-guided versus endoscopic retrograde cholangiopancreatography-guided biliary drainage for primary treatment of distal malignant biliary obstruction: A systematic review and meta-analysis. Dig Endosc. 2020;32:16–26. doi: 10.1111/den.13456. [DOI] [PubMed] [Google Scholar]
- 6.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: A review. Clin J Gastroenterol. 2014;7:94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minaga K, Ogura T, Shiomi H, et al. Comparison of the efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: Multicenter, randomized, clinical trial. Dig Endosc. 2019;31:575–82. doi: 10.1111/den.13406. [DOI] [PubMed] [Google Scholar]
- 8.Uemura RS, Khan MA, Otoch JP, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: A systematic review and meta-analysis. J Clin Gastroenterol. 2018;52:123–30. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 9.Minaga K, Kitano M, Yamashita Y, et al. Stent migration into the abdominal cavity after EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2017;85:263–4. doi: 10.1016/j.gie.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy. 2010;42(Suppl 2):E126–7. doi: 10.1055/s-0029-1243911. [DOI] [PubMed] [Google Scholar]
- 11.Ogura T, Yamamoto K, Sano T, et al. Stent length is impact factor associated with stent patency in endoscopic ultrasound-guided hepaticogastrostomy. J Gastroenterol Hepatol. 2015;30:1748–52. doi: 10.1111/jgh.13021. [DOI] [PubMed] [Google Scholar]
- 12.Kawakubo K, Isayama H, Kogure H, et al. Exchange of self-expandable metal stent in endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2012;44(Suppl 2 UCTN):E311–2. doi: 10.1055/s-0032-1309779. [DOI] [PubMed] [Google Scholar]
- 13.Yane K, Katanuma A, Maguchi H, et al. Successful re-intervention with metal stent trimming using argon plasma coagulation after endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2014;46(Suppl 1 UCTN):E391–2. doi: 10.1055/s-0034-1377388. [DOI] [PubMed] [Google Scholar]
- 14.Maehara K, Hijioka S, Wu SY, et al. Re-intervention for recurrent biliary obstruction after endoscopic ultrasound hepaticogastrostomy with partially covered self-expandable metal stent. Endoscopy. 2019;51:E297–8. doi: 10.1055/a-0915-1718. [DOI] [PubMed] [Google Scholar]
- 15.Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy. 2016;48:1125–8. doi: 10.1055/s-0042-116595. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and Meta-analysis. Dig Dis Sci. 2016;61:684–703. doi: 10.1007/s10620-015-3933-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 19.Ogura T, Takenaka M, Shiomi H, et al. Long-term outcomes of EUS-guided transluminal stent deployment for benign biliary disease: Multicenter clinical experience (with videos) Endosc Ultrasound. 2019;8:398–403. doi: 10.4103/eus.eus_45_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest Endosc. 2017;85:1067–75. doi: 10.1016/j.gie.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos) Gastrointest Endosc. 2015;82:390–6.e2. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita Y, Itonaga M, Gon C, et al. Usefulness of a newly designed laser-cut metal stent with an anchoring hook and thin delivery system for EUS-guided hepaticogastrostomy in experimental settings (with video) Gastrointest Endosc. 2021;94:999–1008.e1. doi: 10.1016/j.gie.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video) Gastrointest Endosc. 2020;92:623–31.e1. doi: 10.1016/j.gie.2020.03.3856. [DOI] [PubMed] [Google Scholar]
- 24.Lyu Y, Li T, Cheng Y, et al. Endoscopic ultrasound-guided versus ERCP-guided biliary drainage for malignant biliary obstruction: A up-to-date meta-analysis and systematic review. Dig Liver Dis. 2021;53:1247–53. doi: 10.1016/j.dld.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Téllez-Ávila FI, Figueredo-Zacarías MA, Muñoz-Anaya E, et al. EUS-guided biliary drainage in patients with distal malignant biliary obstruction requires fewer interventions and has a lower cost compared to ERCP biliary drainage. Surg Endosc. 2021;35:2531–6. doi: 10.1007/s00464-020-07667-5. [DOI] [PubMed] [Google Scholar]
- 26.Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–34. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 27.Hedjoudje A, Sportes A, Grabar S, et al. Outcomes of endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. United European Gastroenterol J. 2019;7:60–8. doi: 10.1177/2050640618808147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubara S, Nakagawa K, Suda K, et al. Radiofrequency ablation of hyperplasia at an uncovered portion of a partially covered metal stent in endoscopic ultrasound-guided hepaticogastrostomy (with video) J Hepatobiliary Pancreat Sci. 2021;28:e32–3. doi: 10.1002/jhbp.1004. [DOI] [PubMed] [Google Scholar]
- 29.Minaga K, Takenaka M, Kitano M, et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc. 2017;31:4764–72. doi: 10.1007/s00464-017-5553-6. [DOI] [PubMed] [Google Scholar]
- 30.Sundaram S, Dhir V. EUS-guided biliary drainage for malignant hilar biliary obstruction: A concise review. Endosc Ultrasound. 2021;10:154–60. doi: 10.4103/EUS-D-21-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunami Y, Itoi T, Sofuni A, et al. EUS-guided hepaticoenterostomy with using a dedicated plastic stent for the benign pancreaticobiliary diseases: A single-center study of a large case series. Endosc Ultrasound. 2021;10:294–304. doi: 10.4103/EUS-D-20-00232. [DOI] [PMC free article] [PubMed] [Google Scholar]