ABSTRACT
The benefit of rapid on-site evaluation (ROSE) on the diagnostic accuracy of EUS–guided fine-needle biopsy (EUS-FNB) in patients with pancreatic masses is still matter of debate. Aim of our meta-analysis is to compare the diagnostic outcomes of these two tissue acquisition strategies. Computerized bibliographic search on the main databases was performed through December 2021 and 8 studies were identified (2147 patients). The primary outcome was sample adequacy. Pooled effects were calculated using a random-effects model by means of DerSimonian and Laird test and summary estimates were expressed in terms of odds ratio (OR) or mean difference and 95% confidence Interval (CI). There was no difference in terms of baseline variables between the two groups. Pooled sample adequacy was 95.5% (95% CI 93.2%–97.8%) and 88.9% (83.4%-94.5%) in the EUS-FNB + ROSE and EUS-FNB groups, respectively (OR = 2.05, 0.94–4.49; P = 0.07). Diagnostic accuracy resulted significantly superior in the EUS-FNB + ROSE group (OR = 2.49, 1.08–5.73; P = 0.03), particularly when the analysis was restricted to reverse bevel needle (OR = 3.24, 1.19–8.82, P = 0.02), whereas no statistical difference was observed when newer end-cutting needles were used (OR = 0.71, 0.29–3.61, P = 0.56). Diagnostic sensitivity was not significantly different between the two groups (OR = 1.94, 0.84–4.49; P = 0.12), whereas pooled specificity was 100% with both approaches. The number of needle passes needed to obtain diagnostic samples was not significantly different (mean difference 0.07,-0.22 to 0.37; P = 0.62). Our meta-analysis stands for a non-superiority of EUS-FNB + ROSE over EUS-FNB with newer end-cutting needles, whereas ROSE could have still a role when reverse bevel needles are used.
Keywords: Accuracy, EUS, fine-needle biopsy, pancreas, rapid on-site evaluation, sensitivity
INTRODUCTION
EUS plays a pivotal role in the diagnostic algorithm of solid pancreatic lesions; however, simple morphological evaluation is not sufficient for definitive characterization of pancreatic masses, hence EUS-guided tissue sampling for cytopathological and histological diagnosis by means of fine-needle aspiration (FNA), and more recently, fine-needle biopsy (FNB) is usually needed.[1,2]
Cellular acquisition through EUS-FNA does not necessarily retain the stroma or associated architecture of surrounding tissue, which may be necessary to provide a definitive diagnosis. EUS-FNB, particularly with newer end-cutting design, was shown to preserve the cellular architecture and it has become an increasingly useful tool in establishing a definitive diagnosis of malignancy in a variety of solid lesions.[3,4,5]
In spite of controversial results on previous studies,[6,7] rapid on-site cytological evaluation (ROSE) represents a useful addition to EUS-FNA with convincing advantages of providing timely feedback on sample adequacy and optimizing the number of needle passes performed. Interestingly enough, the efficacy results of EUS-FNA + ROSE were found to be similar to those of EUS-FNB without ROSE, even with newer end-cutting needles.[8]
However, the complexity and the costs of pancreatic cytopathological expertise development and availability of on-site cytologic evaluation have restricted the use of ROSE only to a limited number of high-volume centers, particularly in the USA.[9]
The eventual role of ROSE as an addition to EUS-FNB is still open to debate, with a recent large multicenter randomized-controlled trial (RCT) questioning the utility of ROSE in this setting[10] whereas results from previous studies[11] were controversial.
Theoretically, the availability of both cytological and histological specimens could represent a diagnostic advantage of ROSE over EUS-FNB alone, as suggested by recent large retrospective studies,[11,12] particularly because valuable cytological specimens can also be used for touch-imprint cytology (TIC) technique.[13]
Therefore, we decided to conduct a meta-analysis comparing EUS-FNB + ROSE versus EUS-FNB alone in patients with solid pancreatic lesions. The primary endpoint was sample adequacy. The secondary outcomes were diagnostic accuracy, sensitivity, specificity, and mean number of needle passes. Safety data were also analyzed.
PATIENTS AND METHODS
Inclusion and exclusion criteria
Only studies meeting the following criteria were included: (1) RCTs or retrospective series directly comparing EUS-FNB + ROSE versus EUS-FNB alone or reporting subgroup analysis based on the use of ROSE; (2) studies enrolling patients with solid pancreatic lesions; (3) articles reporting at least one of the following data: diagnostic accuracy (or data useful for its calculation) or sample adequacy (or data useful for its calculation).
We excluded (a) noncomparative single cohort studies, (b) studies not reporting subgroup analysis on pancreatic masses, and (c) studies not reporting any of the aforementioned outcomes.
Search strategy
Bibliographic research was conducted on PubMed, EMBASE, Cochrane Library, and Google Scholar including all studies fulfilling the inclusion criteria published until December 2021. The following search strategy was adopted: (((EUS [MeSH Terms]) AND (fine-needle biopsy[MeSH Terms])) OR (rose[MeSH Terms])) OR (on-site evaluation[MeSH Terms]). Figure 1 reports the search strategy followed in the meta-analysis.
Figure 1.
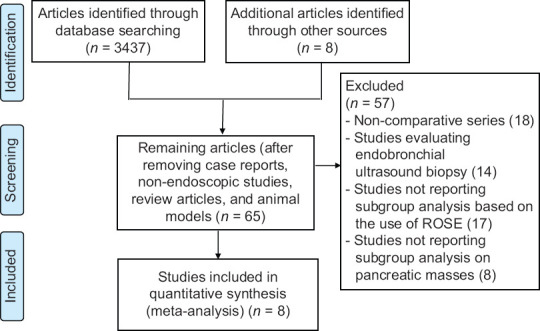
Flow chart of included studies
Relevant reviews and meta-analyses on the use of EUS in pancreatic solid lesions were examined for potential suitable studies. The authors of included studies were contacted to obtain full text or further information when needed. In cases of overlap publications from the same population, only the most recent and complete articles were included.
Data extraction was conducted by two reviewers (AF and DR) using a standardized approach (PRISMA Statement).[14] The quality of the included studies was assessed by two authors independently (AF, SFC) according to the Cochrane Collaboration's tool for assessing the risk of bias for RCTs[15] and the Newcastle-Ottawa scale for nonrandomized studies.[16] Any disagreements were addressed by re-evaluation and following a third opinion (PF).
Outcomes
The primary outcome was sample adequacy, defined as the proportion of samples defined as adequate for diagnosis. The secondary outcomes were diagnostic accuracy, defined as the summary of true positives (TPs) + true negatives (TNs) on the total number of patients, diagnostic sensitivity, computed as the proportion of positives correctly identified with the test (TPs) on the prevalence of disease in the study cohort [(TPs + false negatives (FNs)], diagnostic specificity, calculated as the proportion of negatives correctly identified as such (TNs) among the patients who were not affected by the disease in the study cohort [(TNs + false positives (FPs)], and number of needle passes needed to achieve adequate samples.
The gold standard for the diagnosis was considered surgery or the evolution of the disease assessed for at least 6 months by a combination of clinical course and/or imaging studies.[17]
Statistical analysis
The study outcomes were pooled and compared between the two groups through a random-effects model based on DerSimonian and Laird test, and results were expressed in terms of odds ratio (OR) or mean difference and 95% confidence interval (CI), when appropriate.[18]
The presence of heterogeneity was calculated through I2 tests with I2<30% interpreted as low-level heterogeneity and I2 between 30% and 60% as moderate heterogeneity.[19] Any potential publication bias was verified through the visual assessment of funnel plots.
Sensitivity analyses in the context of the primary outcome were based on study design (RCT versus retrospective), FNB needle used (end-cutting versus reverse bevel), sampling technique (slow-pull versus section), and restricted to full-text articles.
Safety data were inconsistently reported; hence, they were analyzed descriptively. All statistical analyses were conducted using RevMan version 5 from the Cochrane collaboration. For all calculations a two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Included studies
From 3445 studies identified using the search strategy, we included 8 studies,[10,11,12,20,21,22,23,24] [Figure 1] recruiting 2147 patients. Out of these 8 studies, 5 were retrospective series,[11,12,20,22,24] 2 RCTs,[10,23] and 1 study was a prospective series.[21] Specifically, 1 large multicenter RCT[10] and 4 retrospective series[12,20,22,24] were studies focused on the direct comparison between EUS-FNB + ROSE versus EUS FNB alone, whereas another retrospective study[11] was a 4-arm retrospective series comparing EUS FNA alone versus EUS-FNA + ROSE versus EUS-FNB alone versus EUS-FNB + ROSE, the prospective series by Gines et al.[21] and the RCT by Nagula et al.[23] reported a subgroup analysis based on the use of ROSE. All the studies were published as full-text manuscripts except the retrospective study by Mahmood et al.[24]
Main baseline characteristics of the included studies are summarized in Table 1. All studies were Western series and the recruitment period ranged from 2012 to 2019. Baseline patient- and lesion-related characteristics were well-balanced between the two study groups, with males representing the majority of participants in the included studies, while mean age was 65 years. Mean lesion size was around 30 mm and most of the sampled masses were located in the head/uncinated process of the pancreas. Particularly, the study by Gines et al.[21] recruited exclusively patients with lesions in the pancreatic head/uncinated process. Slow pull was used predominantly or exclusively in three studies.[10,22,23]
Table 1.
Characteristics of included studies
| Study | Country | Study period/design | Intervention | Age | Gender male | Lesion size (mm) | Location head/uncinated process | Fanning | Suction/slow pull | Needle |
|---|---|---|---|---|---|---|---|---|---|---|
| Crinò, 2021[10] | Multicenter | 2018-2019/RCT | FNB + ROSE: 385 FNB: 386 | 67.5±11.6 67.3±11.4 | 225 (58.4) 210 (54.4) | 31.6±12.6 31±12.8 | 183 (47.5)/42 (10.9) 193 (50)/46 (11.9) | 358 (93) 358 (92.8) | 125 (32.5)/237 (61.6) 120 (31.1)/237 (61.4) | 77 20G side-fenestrated/231 22G Franseen/77 22G Fork-tip 80 20G side-fenestrated/220 22G Franseen, 86 22G Fork-tip |
| De Moura, 2020[11]* | USA | 2016-2019/retrospective | FNB + ROSE: 129 FNB: 249 | Overall: 66.9±12.26 | Overall: 198 (52.1) | Overall: 29.9±13 | Overall: 162 (42.9)/27 (7.1) | 100 | NR/NR | 22G or 25G reverse-bevel, Franseen or Fork-tip |
| Fabbri, 2017[20] | Italy | 2012-2014/retrospective | FNB + ROSE: 140 FNB: 193 | 65.3±12.5 66.8±11.7 | 83 (59.3) 117 (60.6) | 31.8±12.7 32.3±13.5 | 85 (60.7) 136 (70.5) | NR | NRNR | 22G reverse bevel 62.5%/25G reverse bevel 31.8%/19G reverse bevel 5.7% |
| Ginès, 2021[21]* | Spain, Italy | 2018-2019/prospective | FNB + ROSE: 40 FNB: 35 | Overall: 65±13 | Overall: 38 (51) | Overall: 33±12 | Overall: 68 (91)/7 (9) | NR | 100/0 | 19G Menghini-tip |
| Soto-Solis, 2020[22] | Mexico | 2017/retrospective | FNB + ROSE: 13 FNB: 10 | 60 (47-77) 58 (46-72) | 7 (53.8) 4 (40) | <30 (mm): 8 (61.5) <30 (mm): 3 (30) | 8 (61.5)/3 (23.1) 7 (70)/1 (10) | NR | 0/100 | 22G not specified |
| Nagula, 2018[23]* | USA | 2012-2014/RCT | FNB + ROSE: 114 FNB: 25 | Overall: 67.8±12.7 | Overall: 74 (53.2) | Overall: 35.1±23.8 | NR | NR | 0/100 | Reverse bevel |
| Mahmood, 2017[24]+ | USA | 2015-2016/retrospective | FNB + ROSE: 91 FNB: 13 | Overall: 21±20 | Overall: 63 (61) | Overall <20 (mm): 24 (23) | Overall: 62 (60)/7 (7) | NR | NR/NR | Fork-tip |
| Fitzpatrick, 2020[12] | USA | 2014-2019/retrospective | FNB + ROSE: 245 FNB: 79 | Overall: 66±13.5 | Overall: 176 (56) | Overall: 3.1±1.5 | Overall: 176 (54) | NR | NR/NR | Fork-tip |
*Only subgroup data concerning the comparison between FNB + ROSE versus FNB alone were considered, +Conference abstract. Data are reported as absolute n (%) or mean (±SD or with IQR). FNB: Fine needle biopsy; NR: Not reported; ROSE: Rapid on-site cytologic evaluation; SD: Standard deviation; IQR: Interquartile range; RCT: Randomized controlled trial
Four studies[10,12,21,24] used exclusively newer end-cutting FNB needles (22G or 25G Franseen [Acquire®, Boston Scientific, Marlborough, Massachusetts, USA], Fork-tip [SharkCore®, Medtronic, Dublin, Ireland], 20G side-fenestrated forward-facing bevel needle [20G ProCore®, Cook Medical, Bloomington, IN, USA], 19G Menghini-tip needle [EZ Shot®, Olympus; Shinjuku, Tokyo, Japan]); two studies[20,23] used exclusively reverse bevel needles (mainly 22G or 25G ProCore®), other two studies[11,22] used either reverse bevel or end-cutting needles or did not specify the needles used.
Quality assessment of the studies is summarized in Supplementary Table 1. Three studies[10,12,20] were felt to be at low risk of bias, whereas higher risk of outcome reporting bias or selection bias was observed in the other studies.
Supplementary Table 1.
Risk of bias assessment and quality of included studies
| Observational studiesa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Selection | Comparability | Outcome | Overall quality | |||||||||
| de Moura, 2020 | *** | ** | * | Low | ||||||||
| Fabbri, 2017 | *** | ** | ** | High | ||||||||
| Gines, 2021 | ** | ** | * | Low | ||||||||
| Soto Solis, 2020 | ** | ** | * | Low | ||||||||
| Mahmood, 2017 | * | * | * | Low | ||||||||
| Fitzpatrick, 2020 | *** | ** | ** | High | ||||||||
|
| ||||||||||||
| Randomized controlled trialsb | ||||||||||||
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall quality | |||||
|
| ||||||||||||
| Crinò 2021 | Low | Low | High | Low | Low | Low | Low | High | ||||
| Nagula 2017 | Low | Low | High | Low | High | Low | Low | Low | ||||
aStudy quality assessment performed by means of Newcastle/Ottawa Scale (each asterisk represents if the respective criterion within the subsection was satisfied), bCochrane Collaboration’s tool for assessing the risk of bias across 7 domains: 1 (random sequence generation), 2 (allocation concealment), 3 (blinding of participants and personnel), 4 (blinding of outcome assessment), 5 (incomplete outcome data), 6 (selective reporting) and 7 (other bias)
Sample adequacy
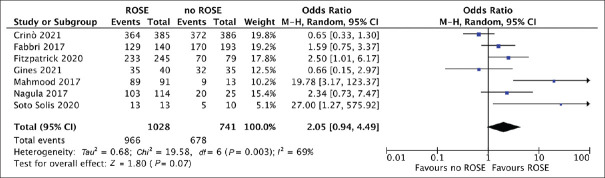
Overall, based on 7 studies[10,12,20,21,22,23,24] (1028 patients in the EUS-FNB + ROSE and 741 in the EUS FNB group), pooled sample adequacy was 95.5% (95% CI 93.2%-97.8%) and 88.9% (83.4% 94.5%) in the EUS-FNB + ROSE and EUS-FNB groups, respectively. As depicted in Figure 2, there was no significant difference between the two approaches (OR = 2.05, 95% CI = 0.94–4.49; P = 0.07). Evidence of high heterogeneity [I2 = 69%, Figure 2] and no publication bias were found [Supplementary Figure 1a (402.4KB, tif) ].
Figure 2.
Forest plot comparing sample adequacy of EUS-guided fine-needle biopsy with rapid on-site evaluation versus EUS-guided fine-needle biopsy alone. There was no significant difference between the two approaches (odds ratio 2.05, 0.94-4.49; P = 0.07). Evidence of high heterogeneity (I2 = 69%) was observed
As reported in Table 2, sensitivity analyses restricted to study design, needle used, sampling technique and restricted only to full-text articles confirmed the results of the main analysis, mainly with low to moderate heterogeneity. Of note, no heterogeneity was observed in the analysis restricted to end-cutting EUS-FNB needles (OR = 1.85, 0.53–6.51, P = 0.34; I2 = 0%) and a nonsignificant trend toward higher sample adequacy with EUS-FNB + ROSE was registered when reverse bevel needles were used (OR = 2.33, 0.91–5.95, P = 0.08; I2 = 39%).
Table 2.
Sensitivity analysis concerning the primary outcome (sample adequacy)
| Variable | Subgroup | Number of studies | Number of patients | Odds ratio (95% CI) P | Within-group heterogeneity (I2) |
|---|---|---|---|---|---|
| Study design | RCT | 2 | FNB + ROSE: 499 FNB: 392 |
1.13 (0.33-3.92) 0.74 | 49% |
| Retrospective | 5 | FNB + ROSE: 529 FNB: 330 |
2.86 (0.97-7.81) 0.07 | 55% | |
| FNB needle | End-cutting needles | 4 | FNB + ROSE: 761 FNB: 513 |
1.85 (0.53-6.51) 0.34 | 0% |
| Reverse bevel | 3 | FNB + ROSE: 267 FNB: 228 |
2.33 (0.91-5.95) 0.08 | 39% | |
| Sampling technique | Slow-pull | 3 | FNB + ROSE: 512 FNB: 421 |
1.99 (0.43-9.25) 0.38 | 45% |
| Suction | 2 | FNB + ROSE: 425 FNB: 421 |
0.65 (0.35-1.22) 0.18 | 0% | |
| Publication | Full text | 6 | FNB + ROSE: 937 FNB: 728 |
1.34 (0.62-2.90) 0.45 | 37% |
CI: Confidence interval; FNB: Fine-needle biopsy; ROSE: Rapid on-site cytologic evaluation; RCT: Randomized controlled trial
Secondary outcomes
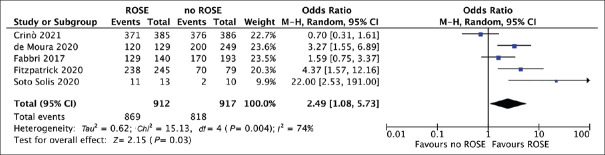
As reported in Table 3 and Figure 3, based on 5 studies,[10,11,12,20,22] diagnostic accuracy resulted significantly superior in the EUS-FNB + ROSE group (OR = 2.49, 1.08–5.73; P = 0.03, I2 = 74%). Again, no evidence of publication bias was observed [Supplementary Figure 1b (402.4KB, tif) ]. Pooled accuracy was 94.2% (91.3%–97.1%) with EUS-FNB + ROSE and 84.2% (76.7%–91.1%) with EUS-FNB alone.
Table 3.
Secondary outcomes
| Outcome | Number of studies | Number of patients | Odds ratio (95% CI), P | Within-group heterogeneity (I2) |
|---|---|---|---|---|
| Diagnostic accuracy | ||||
| Overall | 5 | FNB + ROSE: 912 FNB: 917 |
2.49 (1.08-5.73) 0.03 |
74% |
| End-cutting needles | 2 | FNB + ROSE: 630 FNB: 465 |
0.71 (0.29-3.61) 0.56 |
16% |
| Reverse bevel needles | 3 | FNB + ROSE: 282 FNB: 452 |
3.24 (1.19-8.82) 0.02 |
25% |
| Diagnostic sensitivity | 3 | FNB + ROSE: 899 FNB: 907 |
1.94 (0.84-4.49) 0.12 |
77% |
|
| ||||
| Outcome | Number of studies | Number of patients | Mean difference (95% CI), P | Within-group heterogeneity (I2) |
|
| ||||
| Number of needle passes | 5 | FNB + ROSE: 758 FNB: 851 |
0.07 (−0.22-0.37) 0.62 |
79% |
FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation, CI: Confidence interval
Figure 3.
Forest plot comparing diagnostic accuracy of EUS-guided fine-needle biopsy with rapid on-site evaluation versus EUS-guided fine-needle biopsy alone. Diagnostic accuracy resulted significantly superior in the EUS-FNB + ROSE group (odds ratio 2.49, 1.08-5.73; P = 0.03, I2 = 74%). ROSE: Rapid on-site evaluation
As reported in Table 3, these findings were confirmed in the subgroup analysis restricted to reverse bevel needle (OR = 3.24, 1.19–8.82, P = 0.02), whereas no statistical difference was observed when newer end-cutting needles were used (OR = 0.71, 0.29–3.61, P = 0.56). Of note, heterogeneity was low (I2 = 25% and 16%, respectively) in the subgroup analyses thus confirming that the design of the needle used was the main source of heterogeneity.
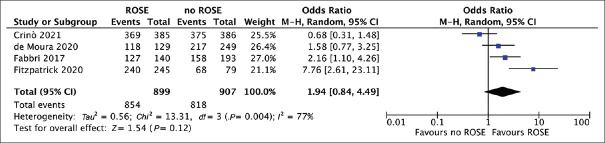
Based on 4 studies,[10,11,12,20] diagnostic sensitivity was not significantly different between the two groups (OR = 1.94, 0.84–4.49; P = 0.12), with high heterogeneity [I2 = 77%; Figure 4]. Specifically, pooled sensitivity was 94.3% (86.9%-95.8%) with EUS-FNB + ROSE and 91.5% (85.9%-94.1%) with EUS-FNB alone, whereas pooled specificity was 100% with both approaches.
Figure 4.
Forest plot comparing diagnostic sensitivity of EUS-guided fine-needle biopsy with rapid on-site evaluation versus EUS-guided fine-needle biopsy alone. Diagnostic sensitivity was not significantly different between the two groups (odds ratio 1.94, 0.84-4.49; P = 0.12), with high heterogeneity (I2 = 77%)
As reported in Table 3 and Supplementary Figure 2 (203.7KB, tif) , based on 5 studies,[10,11,20,22,24] the number of needle passes needed to obtain diagnostic samples was not significantly different (mean difference 0.07,-0.22 to 0.37; P = 0.62, I2 = 79%).
Only the study by Crinò et al.[10] reported adverse events, specifically six episodes of acute pancreatitis managed conservatively, 1 pancreatic leak with pseudo cyst formation treated endoscopically, and 1 episode of stroke in the EUS-FNB + ROSE group while in the EUS-FNB alone arm 1 patient had acute pancreatitis that was managed conservatively, there was 1 case of bleeding with a >2 g/dL drop in hemoglobin not requiring transfusion, 1 case of syncope, and 1 death after a single combined session of EUS sampling followed by ERCP from unexplained causes.
DISCUSSION
EUS-guided tissue sampling plays a pivotal role in the diagnostic algorithm of solid pancreatic lesions but it is still unclear whether newly introduced EUS-FNB needles have recently achieved a significantly better diagnostic accuracy to obviate to the need of ROSE.
With a meta-analysis of 8 studies, of which 2 RCTs, we made several key observations. First, there was no significant difference between EUS-FNB + ROSE versus EUS-FNB alone in terms of sample adequacy, although a non-significant trend toward higher adequacy rates was observed in the overall analysis (OR = 2.05, 0.94–4.49; P = 0.07). Of note, high heterogeneity (I2 = 69%) was found. Sensitivity analyses were able to explore the sources of this heterogeneity, in particular the needle design was identified as a major responsible of the heterogeneity observed. In fact, the analysis restricted to end-cutting EUS-FNB needles showed a decreased difference between the two approaches (OR = 1.85, 0.53–6.51, P = 0.34) with no evidence of heterogeneity (I2 = 0%); on the other hand, again a nonsignificant trend toward higher sample adequacy with EUS-FNB + ROSE was registered when the older reverse bevel needle was used (OR = 2.33, 0.91–5.95, P = 0.08). As confirmed in recent meta-analyses,[8,25,26] newer EUS-FNB needles determine better diagnostic performances as compared to reverse bevel FNB; hence, the higher adequacy rates might make the addition of ROSE superfluous in this setting.
Second, diagnostic accuracy resulted significantly superior in the EUS-FNB + ROSE group (P = 0.03), with pooled accuracy rates of 94.2% with EUS-FNB + ROSE and 84.2% with EUS-FNB alone. Interestingly, while this finding was confirmed in the reverse bevel group, no statistical difference was observed when newer end-cutting needles were used (OR = 0.71, 0.29–3.61, P = 0.56).
Our results are in line with the recent RCT by Crinò et al.[10] and can be explained again in light of the higher performance of newer end-cutting needles.
Similarly, no difference in terms of diagnostic sensitivity (P = 0.12) and specificity (P = 0.75) was observed between the two strategies. We did not see difference also concerning the number of needle passes needed to obtain adequate samples and this finding represents another aspect of similarity between the two sampling strategies.
Therefore, on the basis of these results, centers with an established ROSE service can re-evaluate the need for it, whereas institutions lacking a ROSE service can reach an outstanding diagnostic accuracy using EUS-FNB alone.
Only the study by Crinò et al.[10] reported adverse events, mainly cases of mild acute pancreatitis managed conservatively, although it should be noted that all of the included studies were underpowered to detect differences in adverse event rate between the two groups.
Unfortunately, a specific analysis concerning procedural time was not feasible during to lack of data; however, previous studies showed that the sampling procedure was significantly shorter in the absence of ROSE[10] and a RCT demonstrated a shorter pathology viewing time for histologic compared with cytologic samples,[27] suggesting that EUS-FNB with off-site histologic evaluation can be time-saving and cost-effective, especially in high-volume centers.
There are some limitations to our study. First of all, the low number of included studies and the high heterogeneity observed in most of the analyses require particular caution in interpreting our findings. However, several sensitivity analyses were performed to explain the sources of heterogeneity and the FNB needle design was identified as a major responsible of the heterogeneity observed.
Second, specific analyses based on the needle size were not feasible due to the lack of subgroup data; given the recent evidence of a trend toward higher adequacy and accuracy rates with 22G as compared to 25G end-cutting FNB needles,[8] it could be argued that ROSE could be still useful when using smaller caliper needles. Further studies are needed to answer this question.
Third, the use of TIC in some studies[10] or differences in tissue sample handling could represent further sources of heterogeneity in our analysis. Finally, a cost-effectiveness analysis was beyond the scope of this study.
In conclusion, despite these weaknesses, our meta-analysis stands for a nonsuperiority of EUS FNB + ROSE over EUS-FNB alone with newer end-cutting needles in the tissue acquisition of solid pancreatic lesions, whereas ROSE could still play a role when older reverse bevel FNB needles are used. Further RCTs are needed to confirm these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
Pietro Fusaroli is a Senior Associate Editor of the journal. This article was subject to the journal's standard procedures, with peer review handled independently of the editor and his research group.
Funnel plots for assessing the risk of publication bias concerning a) sample adequacy; b) diagnostic accuracy
Forest plot concerning the comparison between ROSE and FNB alone in terms of number of needle passes
REFERENCES
- 1.Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling – Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1174–88. doi: 10.1055/a-1611-5091. [DOI] [PubMed] [Google Scholar]
- 2.ASGE Standards of Practice Committee. Eloubeidi MA, Decker GA, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83:17–28. doi: 10.1016/j.gie.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Facciorusso A, Crinò SF, Muscatiello N, et al. Endoscopic ultrasound fine-needle biopsy versus fine-needle aspiration for tissue sampling of abdominal lymph nodes: A propensity score matched multicenter comparative study. Cancers (Basel) 2021;13:4298. doi: 10.3390/cancers13174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crinò SF, Ammendola S, Meneghetti A, et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology. 2021;21:443–50. doi: 10.1016/j.pan.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest Endosc. 2020;91:14–22.e2. doi: 10.1016/j.gie.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, et al. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451–7. doi: 10.3748/wjg.v20.i28.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 8.Gkolfakis P, Crinò SF, Tziatzios G, et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest Endosc. 2022;95:1067–77.e15. doi: 10.1016/j.gie.2022.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Sahai AV, Teoh A, et al. An international, multi-institution survey on performing EUS-FNA and fine needle biopsy. Endosc Ultrasound. 2020;9:319–28. doi: 10.4103/eus.eus_56_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crinò SF, Di Mitri R, Nguyen NQ, et al. Endoscopic ultrasound-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: A randomized controlled non-inferiority trial. Gastroenterology. 2021;161:899–909.e5. doi: 10.1053/j.gastro.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 11.de Moura DT, McCarty TR, Jirapinyo P, et al. Evaluation of endoscopic ultrasound fine-needle aspiration versus fine-needle biopsy and impact of rapid on-site evaluation for pancreatic masses. Endosc Int Open. 2020;8:E738–47. doi: 10.1055/a-1122-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick MJ, Hernandez-Barco YG, Krishnan K, et al. Evaluating triage protocols for endoscopic ultrasound-guided fine needle biopsies of the pancreas. J Am Soc Cytopathol. 2020;9:396–404. doi: 10.1016/j.jasc.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Crinò SF, Larghi A, Bernardoni L, et al. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology. 2019;30:179–86. doi: 10.1111/cyt.12662. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle – Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [Last accessed on 2021 Dec 30]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm .
- 17.Wani S, Muthusamy VR, McGrath CM, et al. AGA white paper: Optimizing endoscopic ultrasound-guided tissue acquisition and future directions. Clin Gastroenterol Hepatol. 2018;16:318–27. doi: 10.1016/j.cgh.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence – Inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Fuccio L, Fornelli A, et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc. 2017;31:225–30. doi: 10.1007/s00464-016-4960-4. [DOI] [PubMed] [Google Scholar]
- 21.Ginès A, Fusaroli P, Sendino O, et al. Performance of a new flexible 19 G EUS needle in pancreatic solid lesions located in the head and uncinate process: A prospective multicenter study. Endosc Int Open. 2021;9:E1269–75. doi: 10.1055/a-1480-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto-Solis R, Paz MP, Torres-Ruiz MI, et al. Rapid on-site cytologic evaluation during endoscopic ultrasound-guided biopsies of pancreatic solid lesions. Cir Cir. 2020;88:435–40. doi: 10.24875/CIRU.20001572. [DOI] [PubMed] [Google Scholar]
- 23.Nagula S, Pourmand K, Aslanian H, et al. Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018;16:1307–13.e1. doi: 10.1016/j.cgh.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood SK, Abdelfattah A, Joyce AM, et al. Sharkcore pancreatic biopsies with rose (Rapid On-Site Evaluation) have a higher diagnostic yield than sharkcore biopsies without rose. Gastrointest Endosc. 2017;85:AB348–9. [Google Scholar]
- 25.Facciorusso A, Del Prete V, Buccino VR, et al. Diagnostic yield of Franseen and fork-tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: A meta-analysis. Endosc Int Open. 2019;7:E1221–30. doi: 10.1055/a-0982-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan BP, Shakhatreh M, Garg R, et al. Comparison of Franseen and fork-tip needles for EUS-guided fine-needle biopsy of solid mass lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2019;8:382–91. doi: 10.4103/eus.eus_27_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppong KW, Bekkali NL, Leeds JS, et al. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized crossover study. Endoscopy. 2020;52:454–61. doi: 10.1055/a-1114-5903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots for assessing the risk of publication bias concerning a) sample adequacy; b) diagnostic accuracy
Forest plot concerning the comparison between ROSE and FNB alone in terms of number of needle passes