ABSTRACT
Background and Objectives:
The background of this study was to evaluate the outcomes of perihilar cholangiocarcinoma (pCCA) patients treated with EUS-guided hepaticogastrostomy (EUS-HGS).
Methods:
All patients with pCCA who underwent EUS-HGS from 2010 to 2020 were analyzed. The primary outcome was clinical success; the secondary outcomes were technical success, adverse events (AEs), stent patency, and oncological outcomes. Cox proportional-hazards regression and Kaplan–Meier curves were analyzed to identify variables related to survival.
Results:
Thirty-four patients (50% females, 76 years old) were included; 24 (70.6%) presented with distant metastasis. Indications for EUS-HGS were ERCP failure (64.7%), duodenal stricture (23.5%), postsurgical anatomy (5.9%), and dilation limited to the left intrahepatic duct (5.9%). The technical success rate was 97.1%. The clinical success rate was 64.7%. Nine (26.5%) presented AEs, 2 fatal (bleeding and leakage). The overall survival was 91 (31-263) days. On multivariate analysis, EUS-HGS clinical success (Exp[b]: 0.23 [0.09-0.60]; P = 0.003) and chemotherapy (Exp[b]: 0.06 [0.02-0.23]; P < 0.001) were significantly associated with survival. The survival was longer in patients who achieved EUS-HGS clinical success (178[61-393] vs. 15[73-24] days; hazard ratio: 6.3; P < 0.001) and in those starting chemotherapy (324[178-439] vs. 31 [9-48]; hazard ratio: 1.2; P < 0.001).
Conclusions:
EUS-HGS is effective in pCCA patients despite a not negligible AE rate. Clinical success, potentially leading to jaundice resolution and chemotherapy start, significantly improves survival.
Keywords: Cholangitis, guidelines, jaundice, Klatskin, malignant biliary obstruction
INTRODUCTION
Patients with extrahepatic cholangiocarcinoma (CCA) are usually diagnosed with locally advanced or metastatic disease due to the presentation of cholestasis and biliary obstruction. While its indication is still debated in patients with resectable tumors, biliary drainage (BD) is the main therapeutic measure to guarantee access to treatment to patients with unresectable CCA.[1,2,3]
Perihilar localization, or Klatskin tumor, accounts for more than 50% of the whole CCA spectrum.[4,5] The management of patients diagnosed with perihilar CCA (pCCA) is even more challenging, due to the difficulty of achieving correct tumor staging, the complexity of surgical interventions, and strategy of BD.[6,7]
Available guidelines recommend achieving the drainage of more than 50% of liver segments;[8,9] indeed, patients’ survival is directly dependent from the amount of liver volume effectively drained.[10] ERCP represents the first-line treatment approach in this setting; however, endoscopic multisegmental drainage is technically challenging, harboring a significant risk of infection in undrained segments, precluding the access to subsequent treatments. The combination of endoscopic approach to percutaneous transhepatic biliary drainage (PTBD) or EUS-guided BD (EUS-BD) has been suggested, especially in case of complex strictures.[11,12,13,14,15]
In the last decade, palliative treatments for CCA have expanded, and to date, they range from systemic chemotherapy or immunotherapy to locoregional treatments, such as radiofrequency ablation, radioembolization, or external beam radiotherapy.[2,3] These new approaches led to improved outcomes; however, patients may benefit from these only after an efficient BD.
To date, knowledge on EUS-hepaticogastrostomy (EUS-HGS) is inhomogeneous, mainly based on studies including both distal and proximal obstructions, and various malignant or benign conditions.[16,17] Finally, data on oncological follow-up of patients who underwent EUS-HGS are lacking. Therefore, we aimed to assess the clinical outcomes of patients affected by pCCA who underwent EUS-HGS. The primary outcome was clinical success of EUS-HGS, while the secondary outcomes were technical success, adverse events (AEs), stent patency, chemotherapy start, and overall survival.
MATERIALS AND METHODS
Study design
A retrospective analysis of a prospectively collected database was conducted in February 2020, retrieving all patients with pCCA who underwent EUS-HGS between July 2010 and January 2020 at Hôpital Privé Jean Mermoz in Lyon. The study was conducted in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh, 2000); informed consent was obtained from all patients, and the Institutional Review Board (Ethics Committee) of Ramsay Générale de Santé approved the study protocol in June 2020.
Methods used for data extraction, pCCA diagnosis, EUS-HGS, and outcomes definitions are described in Appendix 1.
EUS-guided hepaticogastrostomy
All patients were evaluated by a multidisciplinary team. BD was indicated in case of jaundice (total bilirubin >35 μM), sepsis (fever and positive cultures from blood samples), or cholangitis (jaundice and sepsis). EUS-HGS was indicated based on previous ERCP failure, patient anatomy, underlying disease, or location/extension of biliary stricture.
EUS-HGS procedures were performed by three different operators, under general anesthesia in supine position. Systemic broad-spectrum antibiotics (fluoroquinolone or second-generation cephalosporin) were given to all patients before the intervention.
A curvilinear-array echoendoscope (GF-UCT180, Olympus Corp., Japan) with a dedicated ultrasound processor (Aloka ProSound Alpha-10, Aloka Co. Ltd., Tokyo, Japan, or UE-M2, Olympus Corp., Japan) was used. After identification on B-mode and color-Doppler EUS, a dilated left intrahepatic bile duct was punctured with a 19-gauge fine-needle aspiration needle. Bile was aspirated to confirm the correct positioning of the needle tip and a cholangiogram was obtained through contrast injection. A long 0.025-mm stiff guidewire was advanced and looped into the biliary tree. A 6-Fr cystotome was used over the guidewire to create the fistula and then a biliary self-expandable metal stent (SEMS) was placed with the distal end into the left hepatic duct and the proximal into the gastric lumen was plaed with the distal end into the left hepatic duct and the proximal into the gastric lumen [Figure 1].
Figure 1.
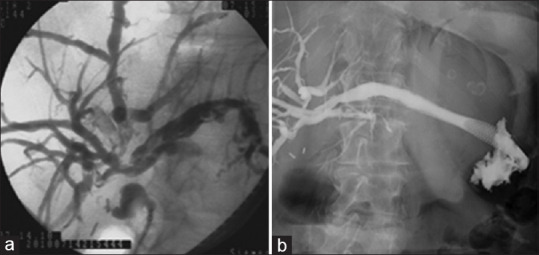
EUS-guided hepaticogastrostomy. Panel A showing cholangiographic image of a perihilar cholangiocarcinoma with Bismuth-Corlette type II hilar stricture. EUS-guided hepaticogastrostomy. Panel B showing a perihilar cholangiocarcinoma with Bismuth-Corlette type II stricture after EUS-HGS placement. EUS-HGS: EUS-guided hepaticogastrostomy
For biliary stenting, two different approaches were used:
Placement of an uncovered SEMS first for anchoring, followed by the co-axial insertion of a fully covered SEMS within the previous one to prevent leakage (WallFlex Biliary, Boston Scientific, US)
Deployment of a single partially covered (hybrid) SEMS, specifically designed for EUS-HGS (Hanarostent® BPD, M. I. Tech, Japan).
Statistical analysis
Categorical variables are expressed as number (n) and proportions (%). Continuous variables are expressed by mean ± standard deviation or median (interquartile range) when appropriate. Univariable and subsequent multivariable logistic regression analyses were performed to explore factors associated with clinical success of EUS-HGS. Odds ratio (OR) and 95% confidence intervals were calculated. Patient survival was evaluated using the nonparametric Kaplan–Meier method, with T0 corresponding to the time of EUS-HGS. Cox proportional-hazards regression model was used to identify variables related to survival after EUS-HGS. Variables with P < 0.2 in this analysis were included in the multivariable logistic regression analysis. Statistical significance was set at P < 0.05. Statistical analysis was performed using MedCalc® Statistical Software version 19.7 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021).
RESULTS
Study population
During the study period, 100 patients underwent EUS-HGS for malignant biliary obstruction; after exclusion of cases with distal obstruction and pathology chart review, 34 patients were finally evaluated [Supplementary Figure 1 (196.7KB, tif) ]. Half of the patients were females; the median age was 76 (67-83) years. Seventeen patients (50.0%) presented with a bilateral hepatic duct involvement (type IV stricture according to Bismuth-Corlette classification) and 24 (70.6%) had distant metastasis at the time of EUS-HGS. A small proportion of cases presented with ascites and signs of portal hypertension (26.5% and 5.9%, respectively). Twenty-four (70.6%) patients had previously undergone ERCP, combined with PTBD in 2 cases (5.9%); the remaining underwent EUS-HGS as a first-line drainage approach. Indications for BD were jaundice (64.7%), sepsis (5.9%), and cholangitis (29.4%). Among the entire population, EUS-HGS was performed because of previous failed ERCP in 22 patients (64.7%), duodenal stricture in 8 (23.5%), altered upper gastrointestinal anatomy in 2 (5.9%), and isolated left hepatic duct dilation in 2 (5.9%). At the time of enrollment, 22 (64.7%) patients were considered fit for chemotherapy [Table 1].
Table 1.
Baseline characteristics of the study population
| Characteristic | Total (n=34) |
|---|---|
| Demographic | |
| Gender (female), n (%) | 17 (50.0) |
| Age (years), median (IQR) | 76 (67-83) |
| Study period (2010-2017), n (%) | 15 (44.1) |
| Study period (2018-2020), n (%) | 19 (55.9) |
| Primary tumor, n (%) | |
| Proven pathology | 28 (82.4) |
| Perihilar cholangiocarcinoma | 25 (73.5) |
| Gallbladder carcinoma | 9 (26.5) |
| Tumor staging, n (%) | |
| Bismuth-Corlette type I | 2 (5.9) |
| Bismuth-Corlette type II | 8 (23.5) |
| Bismuth-Corlette type IIIa | 4 (11.8) |
| Bismuth-Corlette type IIIb | 3 (8.8) |
| Bismuth-Corlette type IV | 17 (50.0) |
| Distant metastasis | 24 (70.6) |
| Ascites | 9 (26.5) |
| Portal hypertension | 2 (5.9) |
| Serum laboratory test, median (IQR) | |
| Total bilirubin (μmol/L) | 226 (121-299) |
| C reactive protein (mg/L) | 71 (18-164) |
| Previous interventions | |
| Previous ERCP, n (%) | 24 (70.6) |
| Number of previous ERCP, mean (range) | 2.4 (1-5) |
| Number of biliary stents, mean (range) | 1.45 (0-3) |
| Percutaneous drainage, n (%) | 2 (5.9) |
| Indication for biliary drainage, n (%) | |
| Jaundice | 22 (64.7) |
| Sepsis without jaundice | 2 (5.9) |
| Cholangitis | 10 (29.4) |
| Indication for EUS-HGS, n (%) | |
| ERCP failure | 22 (64.7) |
| Duodenal stricture | 8 (23.5) |
| Altered anatomy | 2 (5.9) |
| Isolated left hepatic duct dilation | 2 (5.9) |
| Oncological status, n (%) | |
| Fit for chemotherapy | 22 (64.7) |
| Previous chemotherapy | 14 (41.2) |
IQR: Interquartile range, EUS-HGS: EUS-guided hepaticogastrostomy
EUS-hepaticogastrostomy efficacy
EUS-HGS was performed using a hybrid stent in 25 (73.5%) cases. The overall technical success rate was 97.1%. One technical failure was reported: despite correct puncture and cholangiogram, it was not possible to orientate the guidewire in the correct direction. The patient was treated with rescue elective percutaneous BD. Clinical success was achieved in 22 (64.7%) patients after EUS-HGS; three (8.8%) patients presented persistent cholangitis after EUS-HGS and underwent elective percutaneous radiological drainage of the right liver lobe, leading to sepsis resolution and bilirubin drop. Clinical failure was reported in 9 (26.5%) cases [Table 2].
Table 2.
Outcomes of EUS-hepaticogastrostomy
| Total (n=34) | |
|---|---|
| Type of stent, n (%) | |
| Double co-axial biliary SEMS | 8 (23.5) |
| Hybrid SEMS | 25 (73.5) |
| Failed EUS-HGS | 1 (2.9) |
| Technical success, n (%) | |
| Technical success | 33 (97.1) |
| Failure to advance guidewire to hilum | 1 |
| Clinical success, n (%) | |
| Clinical success | 22 (64.7) |
| Complementary elective percutaneous drainage | 3 (8.8) |
| Clinical failure | 9 (26.5) |
| Biochemical response, median (IQR) | |
| Total bilirubin - basal (μmol/L) | 226 (121-299) |
| Total bilirubin - 30-day (μmol/L) | 44 (5-208) |
| Adverse events, n (%) | |
| Any AEs | 9 (26.5) |
| Severe AEs | 3 (8.8) |
| Early AEs | 8 |
| Late AEs | 1 |
| Cholangitis | 5 (14.7) |
| Bleeding | 3 (8.8) |
| Bile leak | 1 (2.9) |
| Stent migration | 0 |
| Periprocedural mortality | 2 (5.9) |
| Stent patency | |
| Stent patency (days), mean (IQR) | 145 (30-222) |
| Stent dysfunction, n (%) | 6 (17.6) |
| Re-intervention, n (%) | 9 (26.5) |
SEMS: Self-expandable metal stent, IQR: Interquartile range, EUS-HGS: EUS-guided hepaticogastrostomy, AE: Adverse event
Clinical success rate did not significantly differ among patients with different stricture extensions, according to Bismuth-Corlette classification. Serum bilirubin levels dropped from 226 (121-299) μmol/L to 44 (5-208) μmol/L (P = 0.01). EUS-HGS had similar technical and clinical success rates, regardless of the presence of ascites (88.9% vs. 100%; P = 0.27 and 55.6% vs. 68.0%; P = 0.22).
Adverse events and stent patency
Nine patients (26.5%) reported an AE: in eight cases, they were early-onset AEs, while one occurred after 2 weeks. The most frequent complications were cholangitis (no. 5) and bleeding (no. 3). One late-onset bleeding was caused by an ulceration on the opposite gastric wall induced by the stent and was treated with argon plasma coagulation with no recurrence. One case of bleeding was related to the underlying presence of severe portal hypertension and coagulopathy. Two patients (5.9%) died because of severe AEs (one bleeding and one bile leakage). No stent migration was observed.
The median stent patency was 145 (30-222) days. Stent patency at 1, 3, and 6 months was 75.8%, 48.5%, and 36.4%, respectively. Nine patients (26.5%) underwent re-intervention. Causes of re-intervention were EUS-HGS dysfunction (no. 6), endoscopic biliary dysfunction (no. 2) treated by ERCP, and disease progression requiring elective complementary drainage (no. 1).
The presence of ascites was not related to a higher incidence of AEs (33.3% vs. 24%; P = 0.67) but was associated with a significantly increased risk of stent dysfunction at 30 days (84.0% vs. 37.5%; P = 0.02) and at 3 months (60.0% vs. 12.5%; P = 0.03).
Oncological outcomes
Among patients who were considered fit for chemotherapy, 15 (68.2%) started antineoplastic treatment after a median of 29 (15-56) days. In the remaining 7 cases, chemotherapy was deemed contraindicated due to general condition deterioration (no. 3), EUS-HGS clinical (no. 2) or technical failure (no. 1), or fatal pulmonary embolism (no. 1). The median overall survival was 91 (31-263) days. Thirty-day and three-month mortality was 23.5% and 47.1%, respectively. The causes of death were disease progression (64.3%), biliary complications (17.9%), EUS-HGS-related AEs (7.1%), pulmonary embolism (3.6%), multiple sclerosis (3.6%), and myocardial infarction (3.6%) [Table 3].
Table 3.
Oncological outcomes
| Total (n=34) | |
|---|---|
| Chemotherapy | |
| Starting chemotherapy, n (%) | 15 (68.2)* |
| Days from EUS-HGS to chemotherapy, median (IQR) | 29 (15-56) |
| Survival, n (%) | |
| Overall survival (days), median (IQR) | 91 (31-263) |
| 30-day mortality | 8 (23.5) |
| 3-month mortality | 16 (47.1) |
| Causes of death, n (%) | |
| Disease progression | 18 (64.3)° |
| Biliary complications | 5 (17.9)° |
| EUS-HGS adverse events | 2 (7.1)° |
| Other causes | 3 (10.7)° |
*Percentage calculated among patients fit for chemotherapy, °Percentages calculated among the number of patients who died during the follow-up. IQR: Interquartile range, EUS-HGS: EUS-guided hepaticogastrostomy
One patient had access to surgery. He was initially considered unresectable, but he was then considered suitable for enlarged left hepatectomy, 41 days after EUS-HGS. He received chemotherapy 77 days after his surgery and died 439 days after EUS-HGS.
Factors related to 30-day and 3-month mortality on univariate and multivariate analyses were reported in Supplementary Tables 1 and 2, respectively. Clinical success was an independent predictor of 30-day survival, while chemotherapy start was independently related to 3-month survival.
Supplementary Table 1.
Risk factors for 30-day mortality after EUS-hepaticogastrostomy for perihilar cholangiocarcinoma
| 30-day mortality after EUS-hepaticogastrostomy | ||||
|---|---|---|---|---|
|
| ||||
| Univariate analysis (OR [95% C.I.) | P | Multivariate analysis (OR [95% C.I.) | P | |
| Gender (male) | NS | NS | - | - |
| Age | 1.16 [1.03-1.31] | 0.02 | 1.23 [1.04-1.45] | 0.02 |
| Study period (2018-2020) | NS | NS | - | - |
| Bismuth-Corlette classification | NS | NS | - | - |
| Distant metastasis | NS | NS | - | - |
| Ascites | 5.25 [0.91-30.2] | 0.06 | NS | NS |
| Unfit for chemotherapy | 12.0 [1.84-78.4] | 0.009 | NS | NS |
| Basal bilirubin levels | NS | NS | - | - |
| Indication for biliary drainage | NS | NS | - | - |
| Clinical success | 0.08 [0.01-0.54] | 0.001 | 0.03 [0.01-0.49] | 0.02 |
| Type of stent | NS | NS | - | - |
| Adverse event | NS | NS | - | - |
| Stent dysfunction | NS | NS | - | - |
| Need for reintervention | NS | NS | - | - |
| Chemotherapy start | 0.15 [0.00-0.41] | <0.001 | NS | NS |
OR: Odd ratio, 95% C.I.: 95% confidence interval, NS: Not statistically significant
Supplementary Table 2.
Risk factors for 3-month mortality after EUS-hepaticogastrostomy for perihilar cholangiocarcinoma
| 3-month mortality after EUS-hepaticogastrostomy | ||||
|---|---|---|---|---|
|
| ||||
| Univariate analysis (OR [95% C.I.) | P | Multivariate analysis (OR [95% C.I.) | P | |
| Gender (male) | NS | NS | - | - |
| Age | NS | NS | - | - |
| Study period (2018-2020) | NS | NS | - | - |
| Bismuth-Corlette classification | NS | NS | - | - |
| Distant metastasis | NS | NS | - | - |
| Ascites | 12.4 [1.31-117.9] | 0.03 | NS | NS |
| Unfit for chemotherapy | 4.67 [1.00-22.8] | 0.05 | NS | NS |
| Basal bilirubin levels | NS | NS | - | - |
| Indication for biliary drainage | NS | NS | - | - |
| Clinical success | 0.10 [0.02-0.61] | 0.01 | NS | NS |
| Type of stent | NS | NS | - | - |
| Adverse event | NS | NS | - | - |
| Stent dysfunction | NS | NS | - | - |
| Need for reintervention | NS | NS | - | - |
| Chemotherapy start | 0.01 [0.00-0.15] | <0.001 | 0.01 [0.00-0.15] | <0.001 |
OR: Odd ratio, 95% C.I.: 95% confidence interval, NS: Not statistically significant
At univariate analysis, the presence of distant metastasis (Exp[b]: 2.53 [1.02-6.30]; P = 0.04), patients unfit for chemotherapy (Exp[b]: 4.11 [1.70-9.93]; P = 0.002), EUS-HGS clinical success (Exp[b]: 0.26 [0.11-0.59]; P = 0.001), stent dysfunction (Exp[b]: 0.39 [0.14-1.05]; P = 0.06), and chemotherapy start (Exp[b]: 0.08 [0.02-0.25]; P < 0.001) were related to long-term mortality. At multivariate analysis, EUS-HGS clinical success (Exp[b]: 0.23 [0.09-0.60]; P = 0.003) and chemotherapy start (Exp[b]: 0.06 [0.02-0.23]; P < 0.001) were independently related to long-term mortality [Table 4]. Patients who achieved clinical success presented a significantly longer overall survival [Figure 2, 178[61-393] vs. 15 [73-24]; hazard ratio: 6.3 [2.2-17.8]; P < 0.001], as well as patients who started chemotherapy [Figure 3, 324[178-439] vs. 31 [9-48]; hazard ratio: 12.2 [4.6-32.3]; P < 0.001].
Table 4.
Risk factors for long-term mortality after EUS-hepaticogastrostomy for perihilar cholangiocarcinoma
| Long-term mortality after EUS-hepaticogastrostomy | ||||
|---|---|---|---|---|
|
| ||||
| Univariate, Exp (b) (95% CI) | P | Multivariate Exp (b) (95% CI) | P | |
| Gender (male) | NS | NS | - | - |
| Age | NS | NS | - | - |
| Study period (2018-2020) | NS | NS | - | - |
| Bismuth-Corlette classification | NS | NS | - | - |
| Distant metastasis | 2.53 (1.02-6.30) | 0.04 | NS | NS |
| Ascites | NS | NS | - | - |
| Unfit for chemotherapy | 4.11 (1.70-9.93) | 0.002 | NS | NS |
| Basal bilirubin levels | NS | NS | - | - |
| Indication for biliary drainage | NS | NS | - | - |
| Clinical success | 0.26 (0.11-0.59) | 0.001 | 0.23 (0.09-0.60) | 0.003 |
| Type of stent | NS | NS | - | - |
| Adverse event | NS | NS | - | - |
| Stent dysfunction | 0.39 (0.14-1.05) | 0.06 | NS | NS |
| Need for re-intervention | NS | NS | - | - |
| Chemotherapy start | 0.08 (0.02-0.25) | <0.001 | 0.06 (0.02-0.23) | <0.001 |
95% CI: 95% confidence interval, NS: Not statistically significant
Figure 2.
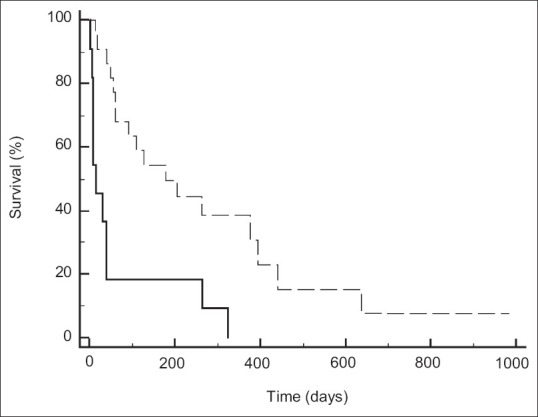
Patients’ survival according to clinical success of EUS-guided hepaticogastrostomy. Patients who achieved clinical success of EUS-HGS (dotted line) compared to patients who do not (continuous line). Overall survival: 178 (61-393) vs. 15 (7-324); hazard ratio: 6.3 (2.2-17.8); P < 0.001. EUS-HGS: EUS-guided hepaticogastrostomy
Figure 3.
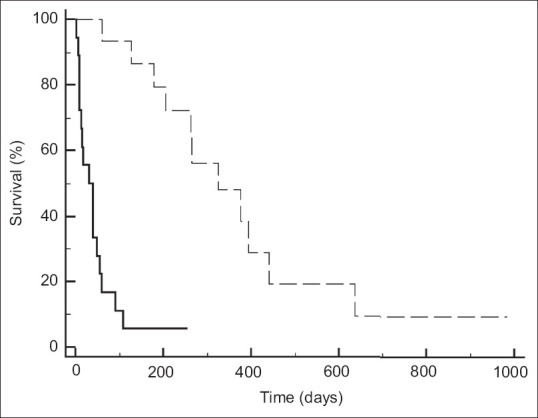
Patients’ survival according to chemotherapy start. Patients who started chemotherapy (dotted line) compared who did not start (continuous line). Overall survival: 324 (178-439) vs. 31 (9-48); hazard ratio: 12.2 (4.6-32.3); P < 0.001
Patients unfit for chemotherapy
In this group, the median overall survival was 15 (7-91) days. Clinical success was the only variable related to mortality [Supplementary Table 3, Exp[b] 0.17 [0.03-0.90]; P = 0.04]. Patients who achieved clinical success presented a significantly longer overall survival [Supplementary Figure 2 (243.1KB, tif) , 91[13-109] vs. 8 [3-40]; hazard ratio: 5.7 [1.3-25.3]; P = 0.02].
Supplementary Table 3.
Risk factors for long-term mortality in patients unfit for chemotherapy who underwent EUS-HGS as best supportive care
| Long-term mortality after EUS-hepaticogastrostomy | ||||
|---|---|---|---|---|
|
| ||||
| Univariate (Exp (b) [95% C.I.]) | P | Multivariate (Exp (b) [95% C.I.]) | P | |
| Gender (male) | NS | NS | - | - |
| Age | NS | NS | - | - |
| Study period (2018-2020) | NS | NS | - | - |
| Bismuth-Corlette classification | NS | NS | - | - |
| Distant metastasis | NS | NS | - | - |
| Ascites | NS | NS | - | - |
| Basal bilirubin levels | NS | NS | - | - |
| Indication for biliary drainage | NS | NS | - | - |
| Clinical success | 0.17 [0.03-0. 90] | 0.04 | 0.17 [0.03-0. 90] | 0.04 |
| Type of stent | NS | NS | - | - |
| Adverse event | NS | NS | - | - |
| Stent dysfunction | NS | NS | - | - |
| Need for reintervention | NS | NS | - | - |
95% C.I.: 95% confidence interval, NS: Not statistically significant
DISCUSSION
The results of our study demonstrate that EUS-HGS is a valuable treatment for patients with advanced pCCA requiring BD. In this field, EUS-HGS could represent an alternative strategy in case of failure of endoscopic or percutaneous approaches, or even a first-line treatment in case of unfeasibility of ERCP or in the presence of isolated left intrahepatic bile duct dilation.
We observe that patients with pCCA undergoing EUS-HGS could achieve clinical success, in terms of jaundice or cholangitis resolution, in a significant amount of cases (up to 70%), with a consequent significant overall survival benefit. Despite the retrospective design and the relatively small population, this study represents one of the first evidence in a homogeneous cohort of patients with advanced pCCA, focusing not only on procedural but also on oncological outcomes. To date, most studies assessing the performance of EUS-HGS have included both malignant and benign conditions, distal and proximal strictures, or even patients treated with different procedures.[16,18] Moreover, to the best of our knowledge, this is the first study assessing not only technical and procedural outcomes but also the subsequent clinical impact on the course of the disease. These results confirm that EUS-HGS could be performed with optimal technical success rate (>95%). The clinical success rate reflects the complexity of selected cases and biliary obstruction; in our study, all patients had proximal obstruction due to pCCA, with complex strictures and previous ERCP failure in most cases, explaining the suboptimal result observed in terms of clinical success rate (64.7%).
The most innovative information arising from the results of the current study is the impact of performing EUS-HGS on subsequent patients’ clinical management and outcomes. Access to chemotherapy after EUS-HGS was evaluated in only two other studies[19,20] reporting rates ranging from 30% to 55%. In our study, the majority (68.2%) of patients considered suitable before BD was able to start chemotherapy with a median delay of 29 days. The main result of this study could be easily expected and understood but still presents some interesting aspects. We observe that both EUS-HGS clinical success and chemotherapy start were independently related to long-term patients’ mortality. It is quite straightforward that patients who achieved jaundice or sepsis resolution will present longer survival; moreover, the impact of systemic chemotherapy in patients with advanced pCCA has already been demonstrated.[8,9] In our opinion, the most insightful result is that these two factors appear to be independent and not consequently related to each other. In detail, EUS-HGS clinical success prolongs survival both in patients fit and unfit for subsequent chemotherapy, and starting chemotherapy guarantees a further survival increase in patients achieving clinical success.
Interestingly, the presence of ascites is not associated with poorer outcomes: clinical success and AE rates are similar in both populations. Moreover, on multivariate analysis, ascites is not identified as an independent variable related to either short- or long-term mortality. The association found between the presence of ascites and stent dysfunction remains unclear. Finally, the results about the presence of ascites do not lead to an absolute contraindication to EUS-HGS. These results are in line with Alvarez-Sanchez et al.'s, demonstrating that EUS-HGS was safe even in patients with malignant biliary obstruction and ascites.[21]
The incidence and proportion of AEs occurring in our study could be considered in line with data available in the literature.[13] We report three cases of EUS-HGS complicated by bleeding; one patient died as a direct consequence of the serious AE. In the second case, EUS-HGS triggered a massive variceal bleeding in a patient with portal hypertension and coagulopathy. This issue suggests that the presence of portal hypertension should be carefully evaluated before EUS-HGS and, when possible, patients should be carefully treated to correct the coagulopathy.
A Non-negligible proportion of enrolled patients (35%) were considered unfit for future chemotherapy and underwent EUS-HGS as the best supportive care for jaundice or cholangitis. Even in this subgroup of particularly complex cases, the clinical success achieved through EUS-HGS led to a significant median overall survival increase (from 8 to 91 days). This finding suggests that, despite suboptimal clinical success rate and safety profile of the procedure, EUS-HGS could not be considered a futile procedure, even in the best supportive care setting. Indeed, in a recent editorial, Dr. Bang and Dr. Varadarajulu suggested that patients with no indication for future treatments and an estimated life expectancy shorter than 6 months could represent the best candidate for EUS-HGS, avoiding the inconvenience and AEs of percutaneous external drainage.[22,23]
Moreover, as for other EUS-BD approaches, EUS-HGS is supposed to have a longer stent patency compared to ERCP because of the distance of the stent from the malignant stricture, reducing the risk of tumor ingrowth.[24] This was the case in the present study (144.5 days), with slightly higher values than those previously reported by Minaga et al. (61 days) and Kanno et al. (112 days).[14,25] Although EUS-HGS is known to be associated with less re-intervention rates than ERCP, we found a re-intervention rate of 26.5% which is a bit higher than rates reported in Paik's randomized trial and Moryoussef's (15.6% and 16.7%). These results may be explained by the fact that the patients in our cohort needed a re-intervention not only due to HGS stent dysfunction, as usually described in other studies, but also in case of dysfunction of stents placed by ERCP.
We acknowledge that this study presents several limitations. The main limit is related to its retrospective nature, theoretically leading to several biases. To date, this study presents the first clinical results of EUS-HGS performed in a homogeneous group of patients with hilar CCA. A prospective multicenter study should be designed in order to assess the reproducibility of these results. The study included patients enrolled over a 10-year period; however, neither the study period nor the technical innovations (namely, the use of a dedicated hybrid SEMS) were correlated to EUS-HGS clinical success or patients’ survival. Finally, due to the technical complexity of the procedure, our results reflect mainly the experience of third-level referral centers rather than a worldwide real-life setting.
In the last decade, several manuscripts focusing on EUS-guided interventions have been published yearly, contributing to the continuously growing literature evidence in this field;[26] among EUS-guided interventions, EUS-HGS represents one of the most complex and technically challenging procedures for interventional endoscopists, requiring technological and technical innovations;[27,28,29] in this particular setting, the development of metal stents dedicated for EUS procedures, such as hybrid stents for EUS-HGS, allows an improvement in technical outcomes and a reduction of AEs.[30] We think that prospective, randomized trials to assess the impact of EUS-HGS in patients with pCCA with high level of evidence should be designed, despite the difficulty in organizing multicenter studies in homogeneous settings.
In conclusion, the results of this study suggest that EUS-HGS could significantly impact the clinical outcomes of patients with malignant biliary obstruction due to pCCA. EUS-HGS is a valid alternative strategy for BD, after the failure or in case of no feasibility of endoscopic approaches, or even as an upfront approach in selected cases. EUS-HGS could allow patients to start chemotherapy early after jaundice or cholangitis resolution in a significant proportion of cases. Finally, EUS-HGS has a dramatic positive impact, not only in patients fit for chemotherapy but also in the setting of best supportive care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Study flowchart
Patients’ survival according to clinical success of EUS-guided hepaticogastrostomy in patients considered unfit for chemotherapy. Patients unfit for chemotherapy who achieved clinical success of EUS-HGS (dotted line) compared to patients who do not (continuous line). 91 (13-109) vs. 8 (3-40); hazard ratio: 5.7 (1.3-25. 3); P = 0.02
SUPPORTING INFORMATION LEGEND
Appendix 1. Description of Material and methods: data extraction, perihilar CCA diagnosis, EUS-HGS, and outcome definitions. EUS-HGS: EUS-guided hepaticogastrostomy, CCA: cholangiocarcinoma
MATERIALS AND METHODS
Data extraction
Relevant data were retrieved from patient's medical records or from a direct contact with referring physicians. Follow-up started at the time of EUS-HGS until June 2020 or until patient death. For each patient, the following data were recorded: age and gender, pathological diagnosis, extent of ductal infiltration according to Bismuth-Corlette classification, presence of distant metastasis, presence of ascites, presence of portal hypertension, previous ERCP, number of stents placed and indication for biliary drainage and for EUS-HGS, basal bilirubin levels, type and size of stent used, stent patency and need for reintervention, adverse events, access to chemotherapy, cause of death and survival.
Perihilar cholangiocarcinoma diagnosis
Diagnosis of perihilar CCA was made by proven histology or by a range of arguments (computed tomography scan, magnetic resonance imaging, ERCP or EUS, tumoral markers, follow-up). Patients were subclassified into three different groups, according to stricture extension and the consequent portion of liver potentially drained by EUS-HGS: in Bismuth-Corlette I, the whole liver should be drained; in Bismuth-Corlette II and IIIA the entire left liver lobe; in Bismuth IIIb and IV only a portion of the left liver lobe.
Definitions of outcomes
Technical success was defined as the correct SEMS placement with immediate bile flow on endoscopy. Clinical success was defined as a 50% decrease of serum bilirubin in case of jaundice, fever resolution and antibiotic withdrawal in case of sepsis and both bilirubin drop and sepsis resolution in case of cholangitis.
All early (within 15 days from EUS-HGS) and late adverse events were recorded. Bleeding was defined as haemoglobin loss >2g/dL or need of blood transfusion. Bile leakage was defined as acute abdominal pain with free abdominal fluid, confirmed by bile aspiration or fistula opacification. Cholangitis was defined as post procedure fever with positive blood culture and/or need to reintroduce antibiotics.
Stent patency was defined as the time to recurrence of jaundice and/or sepsis implying a stent dysfunction (diagnosed on CT or during an endoscopic control) or the time to patient death. Recurrence of jaundice was defined as the re-elevation of serum bilirubin compared to the lowest level achieved. At the time of multidisciplinary team evaluation, patients were considered potentially fit for chemotherapy or undergoing EUS-HGS for best supportive care. The access to systemic chemotherapy after EUS-HGS was recorded. All patients were followed up until the end of June 2020 or until death. Causes of death were recorded and classified as related to biliary complications, related to EUS-HGS adverse events or to disease progression.
REFERENCES
- 1.Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:28–37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 2.Fostea RM, Fontana E, Torga G, et al. Recent progress in the systemic treatment of advanced/metastatic cholangiocarcinoma. Cancers (Basel) 2020;12:2599. doi: 10.3390/cancers12092599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eso Y, Seno H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therap Adv Gastroenterol. 2020;13:1756284820948773. doi: 10.1177/1756284820948773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–73. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelen RJ, Ruys AT, Wiggers JK, et al. Development of a risk score to predict detection of metastasized or locally advanced perihilar cholangiocarcinoma at staging laparoscopy. Ann Surg Oncol. 2016;23:904–10. doi: 10.1245/s10434-016-5531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito F, Cho CS, Rikkers LF, et al. Hilar cholangiocarcinoma: Current management. Ann Surg. 2009;250:210–8. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 7.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–8. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593–607. doi: 10.1111/jgh.12128. [DOI] [PubMed] [Google Scholar]
- 9.Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline – Updated October 2017. Endoscopy. 2018;50:910–30. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 10.Caillol F, Bories E, Zemmour C, et al. Palliative endoscopic drainage of malignant stenosis of biliary confluence: Efficiency of multiple drainage approach to drain a maximum of liver segments. United European Gastroenterol J. 2019;7:52–9. doi: 10.1177/2050640618803812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao WC, Angsuwatcharakon P, Isayama H, et al. International consensus recommendations for difficult biliary access. Gastrointest Endosc. 2017;85:295–304. doi: 10.1016/j.gie.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Wiggers JK, Groot Koerkamp B, Coelen RJ, et al. Preoperative biliary drainage in perihilar cholangiocarcinoma: Identifying patients who require percutaneous drainage after failed endoscopic drainage. Endoscopy. 2015;47:1124–31. doi: 10.1055/s-0034-1392559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moryoussef F, Sportes A, Leblanc S, et al. Is EUS-guided drainage a suitable alternative technique in case of proximal biliary obstruction? Therap Adv Gastroenterol. 2017;10:537–44. doi: 10.1177/1756283X17702614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minaga K, Takenaka M, Kitano M, et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg Endosc. 2017;31:4764–72. doi: 10.1007/s00464-017-5553-6. [DOI] [PubMed] [Google Scholar]
- 15.Kongkam P, Orprayoon T, Boonmee C, et al. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: A multicenter observational open-label study. Endoscopy. 2021;53:55–62. doi: 10.1055/a-1195-8197. [DOI] [PubMed] [Google Scholar]
- 16.Khoo S, Do ND, Kongkam P. Efficacy and safety of EUS biliary drainage in malignant distal and hilar biliary obstruction: A comprehensive review of literature and algorithm. Endosc Ultrasound. 2020;9:369–79. doi: 10.4103/eus.eus_59_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahaleh M, Artifon EL, Perez-Miranda M, et al. EUS-guided drainage: Summary of therapeutic EUS consortium meeting. Endosc Ultrasound. 2019;8:151–60. doi: 10.4103/eus.eus_26_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannini M. EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2019;8:S35–9. doi: 10.4103/eus.eus_47_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai H, Takenaka M, Omoto S, et al. Utility of endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Oncology. 2017;93(Suppl 1):69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 20.Ogura T, Nishioka N, Ueno S, et al. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2021;53:369–75. doi: 10.1055/a-1199-5418. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Sánchez MV, Luna OB, Oria I, et al. Feasibility and safety of endoscopic ultrasound-guided biliary drainage (EUS-BD) for malignant biliary obstruction associated with ascites: Results of a pilot study. J Gastrointest Surg. 2018;22:1213–20. doi: 10.1007/s11605-018-3731-z. [DOI] [PubMed] [Google Scholar]
- 22.Bang JY, Varadarajulu S. The promise of EUS-guided hepaticogastrostomy: Miles to go before we sleep, and miles to go before we sleep. Gastrointest Endosc. 2020;92:632–3. doi: 10.1016/j.gie.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Brighi N, Biasco G. Palliative care for cholangiocarcinoma. In: Brandi G, Ercolani G, editors. Cholangiocarcinoma. New York: Nova Science Publisher, Inc; 2015. pp. 497–507. [Google Scholar]
- 24.Park DH, Song TJ, Eum J, et al. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos) Gastrointest Endosc. 2010;71:413–9. doi: 10.1016/j.gie.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Kanno Y, Ito K, Koshita S, et al. EUS-guided biliary drainage for malignant perihilar biliary strictures after further transpapillary intervention has been judged to be impossible or ineffective. Intern Med. 2017;56:3145–51. doi: 10.2169/internalmedicine.9001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabbri C, Luigiano C, Lisotti A, et al. Endoscopic ultrasound-guided treatments: Are we getting evidence based – A systematic review. World J Gastroenterol. 2014;20:8424–48. doi: 10.3748/wjg.v20.i26.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishiwatari H, Satoh T, Sato J, et al. Bile aspiration during EUS-guided hepaticogastrostomy is associated with lower risk of postprocedural adverse events: A retrospective single-center study. Surg Endosc. 2021;35:6836–45. doi: 10.1007/s00464-020-08189-w. [DOI] [PubMed] [Google Scholar]
- 28.Maehara K, Hijioka S, Nagashio Y, et al. Endoscopic ultrasound-guided hepaticogastrostomy or hepaticojejunostomy without dilation using a stent with a thinner delivery system. Endosc Int Open. 2020;8:E1034–8. doi: 10.1055/a-1169-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video) Gastrointest Endosc. 2020;92:623–31.e1. doi: 10.1016/j.gie.2020.03.3856. [DOI] [PubMed] [Google Scholar]
- 30.Leung Ki EL, Napoleon B. EUS-specific stents: Available designs and probable lacunae. Endosc Ultrasound. 2019;8:S17–27. doi: 10.4103/eus.eus_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study flowchart
Patients’ survival according to clinical success of EUS-guided hepaticogastrostomy in patients considered unfit for chemotherapy. Patients unfit for chemotherapy who achieved clinical success of EUS-HGS (dotted line) compared to patients who do not (continuous line). 91 (13-109) vs. 8 (3-40); hazard ratio: 5.7 (1.3-25. 3); P = 0.02


