ABSTRACT
Background and Objectives:
It remains unclear whether the use of the stylet slow-pull (SP) and wet suction (WS) can improve the yield of endoscopic ultrasound-guided fine-needle biopsy compared to standard suction (SS). The aim of this study was to compare the diagnostic efficacy of the three sampling techniques when using 25G ProCore needles for solid pancreatic lesions.
Materials and Methods:
This multicenter single-blind randomized crossover superiority trial enrolled patients with solid pancreatic lesions (n = 300) from four digestive endoscopic centers in China. All three sampling techniques were performed on each patient using a 25G ProCore needle in a randomized sequence. The diagnostic efficacy, the specimen yield, and quality of each technique, the overall technical success rate and diagnostic yield of the 25G ProCore needle, and rate of adverse events were evaluated.
Results:
A total of 291 patients were analyzed. No significant difference was found in diagnostic efficiency among the three techniques (sensitivity, 82.14% vs. 75.00% vs. 77.86, P = 0.1186; accuracy, 82.82% vs. 75.95% vs. 78.69%, P = 0.1212). The SP had an inferior tissue integrity compared to the SS and WS techniques (71.82% vs. 62.55% vs. 69.76%, P = 0.0096). There was no significant difference in the degree of blood contamination among the three groups (P = 0.2079). After three passes, the overall sensitivity was 93.93%, and the accuracy was 94.16%.
Conclusions:
SS and WS techniques are better choices than SP technique for 25G ProCore needle, for they could provide higher specimen adequacy without increasing the amount of blood contamination. The 25G ProCore needle can provide a satisfactory diagnostic yield for solid pancreatic lesions.
Keywords: EUS-guided fine-needle biopsy, pancreatic cancer, sampling technique
INTRODUCTION
EUS-guided fine-needle biopsy (EUS-FNB) has been widely applied for diagnosing solid pancreatic lesions, as the acquisition of histological specimens by FNB devices allows for the assessment of tissue architecture, immunohistochemical staining, and molecular and genetic analyses, which makes it possible for neoplastic diseases to be further classified and individually treated.[1,2]
In recent years, there has been an intense discussion about how sampling techniques affect the specimen yield of EUS-guided FNB.[3,4,5] Currently, standard suction (SS) is the technique used most commonly and recommended by guidelines for EUS-guided sampling.[6] Nevertheless, some researchers believe that the use of suction tends to increase the damage to the specimens and the amount of blood contamination without improving diagnostic yield; they, therefore, recommend applying stylet slow-pull (SP) or no suction to minimize the negative pressure.[7,8,9,10,11,12] Moreover, there have also been some reports demonstrating that wet suction (WS) can provide a greater suction force while improving the yield and cellularity during tissue acquisition from solid lesions.[13,14,15,16] However, the results of these studies might be affected by individual preferences and varying experience levels of different endoscopists. Furthermore, the selection of the sampling technique might vary with needle design. It was reported by Young Bang et al.[18] that the best outcomes of EUS-FNB can be achieved by tailoring the sampling technique to the needle type. Compared to a 19G or 22G needle, the 25G needle has a smaller diameter and higher flexibility, which might lead to a higher technical success rate and wider applicability, especially in the head/uncinate of the pancreas.[19] However, the actual suction force applied at the 25G needle tip can be weakened as the needle diameter and syringe aspiration volume decreased.[20] Besides, so far, no prospective clinical trial has tested the efficacy of these three techniques for solid pancreatic lesions using a 25G ProCore needle in one trial.
Therefore, this prospective multicenter randomized crossover superiority study with a large sample size was carried out to compare the diagnostic efficacy of the three common sampling techniques in EUS-FNB for solid pancreatic lesions using 25G ProCore needle. Assessing the specimen yield and quality of each technique and the performance of the 25G ProCore needle was also an objective of this study.
MATERIALS AND METHODS
Patients and settings
This study was a multicenter single-blind randomized crossover superiority study. Patients who met the inclusion and exclusion criteria were enrolled from digestive endoscopic centers at four tertiary hospitals in China, including Changhai Hospital, Shenzhen People's Hospital, the Affiliated Hospital of Southwest Medical University, and the Affiliated Hospital of Qingdao University.
The inclusion criteria included age ranging from 18 to 75 years, regardless of sex; diagnosis or suspicion of a solid pancreatic mass based on a previous abdominal imaging examination; lesion diameter larger than 1 cm; and who provided written informed consent. The exclusion criteria included presence of cystic pancreatic lesions; known bleeding disorder that could not be sufficiently corrected with cofactors or fresh frozen plasma; use of anticoagulants/antiplatelet drugs that could not be discontinued; presence of coagulopathy; severe cardiopulmonary dysfunction that limits the use of intravenous anesthesia; and other medical conditions that rendered the patient an unsuitable candidate for EUS-FNB.
Written informed consent was obtained from each participant or from their legally responsible relative. The Ethics Committee (EC) of Changhai Hospital approved the study on June 3, 2019, (CFDA Approval Number: CHEC2019-081). The study was subsequently approved by the EC/IRB of each trial site. We registered the protocol on ClinicalTrials.gov (NCT04100941) before enrolling patients in the study. The study protocol was published on digestive and liver disease before the conclusion of enrollment.[21]
Randomization and blinding
Computer-generated block randomization was used for group assignments before study enrollment. When a patient was enrolled, allocation to one of the six sequences was performed according to the predefined assignment. After randomization, only the endosonographers knew the order of the sampling techniques during the procedure, all of whom had no role in data collecting or analyses. The patients, researchers, and pathologists were blinded throughout the study.
Procedural technique and specimen analysis
After determining the optimal puncture route, EUS-FNB was performed using a linear-array echoendoscope (GF-UC240PAL5/GF-UCT260, Olympus, Tokyo, Japan, or EG-580UT, Fujifilm, Tokyo, Japan) and a 25G ProCore needle (EchoTip ProCore, Cook Endoscopy, USA) by an expert endosonographer at each site.
Suction was applied as the needle was moved back and forth 20 times within the lesion. The SS technique was applied with the stylet completely withdrawn, and a 10-ml syringe was attached to the end of the needle. For the SP technique, the lesion was punctured with full insertion of the stylet, which was then withdrawn from the needle slowly during its back-and-forth movements. For the WS technique, the stylet was removed from the needle before puncture, and the needle was then flushed with 5 ml of saline to replace the column of air with fluid. After the needle punctured the lesion, a 10-ml syringe was attached to the end of the needle to provide continuous negative pressure suction while the needle moved back and forth.
All three sampling techniques were performed according to the sequence assigned to each patient during randomization. To ensure an acceptable diagnostic yield, extra passes were made if the samples obtained from the three passes were unsatisfactory. The results of the additional passes were not counted in the final statistical analysis.
After each pass, the first drop of the aspirated sample from the needle was placed onto a glass slide by inserting the stylet. Then, the liquid specimen on slide was spread over the surface with another glass slide. The slides were fixed in absolute alcohol for further hematoxylin-eosin staining and were recorded as smear cytology. After removing the stylet, the needle was flushed with saline to collect the remaining aspirated sample and tissue. The liquid sample was preserved in a vial containing BD CytoRich nongyn fixatives (BD SurePath, Franklin Lakes, NJ, USA) for SurePath processing. The slides derived from the liquid-based cytology (LBC) preparation were examined using Papanicolaou staining and recorded as LBC. Tissue specimens were fixed in formalin solution and sent to the pathology laboratory for further staining and examination, which were recorded as histology. The cytological and histological specimens of every pass were marked and handled separately to compare the efficacy of each sampling technique.
Rapid on-site evaluation (ROSE) was not performed during EUS-FNB in this study considering that it might lead to differences in diagnostic outcomes caused by the order of sampling techniques. After EUS-FNB sampling, patients were observed closely for 24 h, and their peripheral blood samples were taken for the detection of procedure-related adverse events (elevated amylase level, acute pancreatitis, bleeding and infection, etc.). A video explaining the standardized protocol had been distributed by the leading center to the other centers before enrollment to ensure that the operation was performed in a consistent fashion across centers.
All specimens were independently examined by two experienced and blinded pathologists in each center, who gave the cytological and histological diagnoses and evaluated specimen quality according to the specifically established criteria [Table 1]. According to the Papanicolaou Society of Cytopathology guidelines for pancreatobiliary cytology, the EUS-FNB diagnoses were divided into nondiagnostic, negative, atypical, neoplastic, suspicious, and positive.[22] Neoplastic, suspicious, and positive results were considered positive EUS-FNB results. If the diagnoses were inconsistent or unclear, the final judgment was made by the pathological quality control expert in the leading center.
Table 1.
Cytological and histological assessment criteria
| Grade | Explanation |
|---|---|
|
| |
| Tissue integrity | |
| Grade A | Presence of a tissue core (defined as an architecturally intact piece of tissue measuring at least 550 µm in the microscope visual field) |
| Grade B | Presence of core fragments (tissue does not meet the criteria for a tissue core, but still can yield a diagnosis based on cell morphology) |
| Grade C | No architecturally intact tissue is present, and it cannot yield a diagnosis |
|
| |
| Cellularity* | |
|
| |
| Grade A | Satisfactory, >4 clusters for adequate cytological interpretation with a minimum of 10 cells |
| Grade B | Adequate, 2-4 clusters for adequate cytological interpretation with a minimum of 10 cells |
| Grade C | Unsatisfactory, <2 clusters for adequate cytological interpretation or probably not representative; or a structure-clear nuclear cell count <50 |
|
| |
| Blood contamination | |
|
| |
| Grade A | Minimal contamination, blood cells present in <25% of the slide |
| Grade B | Moderate contamination, blood cells present in 25%-50% of the slide |
| Grade C | Significant contamination, blood cells present >50% of the slide |
*Cellularity was present for assessment of smear cytology and liquid-based cytology
Definition
The primary outcome measure was the diagnostic efficacy (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy) of EUS-FNB using the three sampling techniques (SS, SP, and WS) in patients with solid pancreatic lesions. The secondary outcomes included the specimens yield and quality (tissue integrity, cellularity, and blood contamination) obtained with the three techniques, the technical success rate and diagnostic yield of the 25G ProCore needle, and the rate of adverse events.
The final diagnosis of each patient was confirmed by three methods: the results of EUS-FNA, surgical pathology, if available, and clinical follow-up for at least 6 months. If the lesion was confirmed to not be neoplastic by surgical pathology or appeared to be stable or spontaneously shrink and the patient showed no signs of deterioration during follow-up, the lesion was defined as benign disease. If the lesion showed pathological evidence of malignancy or neoplasms or showed enlargement or metastasis during follow-up or if the patient presented with aggravated clinical manifestations such as anemia, weight loss, or tumor-related mortality, the lesion was diagnosed as malignant disease.
Sample size calculation
A recent prospective study reported the rate of adequate core tissue obtained as 52% for SP and as 34% for SS.[12] In another prospective study on EUS-FNA, 85.5% of specimens obtained by WS were considered adequate, and 75.2% of specimens obtained with the SS technique were considered adequate.[14] For a crossover superiority trial with a type I error (α) of 5% and a power (1-β) of 80% (two-sided significance level), 260 patients should be enrolled. Assuming a dropout or withdrawal rate of 15%, we determined a final sample size of 300 patients. Each of the six groups included 50 patients, ensuring that each technique was assessed 300 times. The allocation set was determined after data blinding had been verified. Any file with missing data was excluded from the study.
Statistical analysis
All continuous variables were tested for a normal distribution and expressed as the mean with standard deviation. Categorical variables are expressed as numbers and percentages and were compared with the Chi-square or Fisher's exact test, as indicated; the two-sample t-test, Wilcoxon rank–sum test, or Kruskal–Wallis test were used, as appropriate, to compare continuous data. Differences were considered statistically significant if P < 0.05. The level of diagnostic agreement among the three techniques was evaluated based on Kappa coefficient. Moreover, the multivariate analysis models were set based on baseline characteristics. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC, USA).
RESULTS
Baseline characteristics and technical details
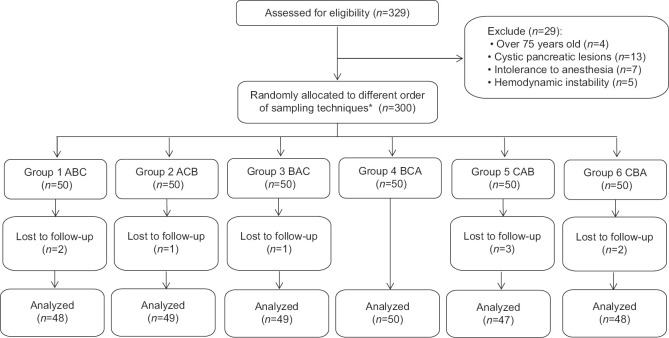
From June 2019 to October 2020, a total of 329 patients suspected to have solid pancreatic lesions were assessed for eligibility, and 300 patients who met the inclusion criteria were enrolled. The participants were randomly allocated and underwent the three sampling techniques for EUS-FNB according to their predefined order. Nine patients were finally excluded, because they were lost to follow-up; therefore, 291 patients were analyzed [Figure 1]. The patient demographics and tumor characteristics are listed in Table 2.
Figure 1.
The CONSORT flow diagram. *Three suction techniques were coded as follows: A, standard suction; B, stylet slow-pull; C, wet suction. Enrolled patients were randomly assigned to one of six sequences according to the predefined assignment: 1. ABC; 2. ACB; 3. BAC; 4. BCA; 5. CAB; and 6. CBA
Table 2.
Patient demographics and tumor characteristics
| Index | Values |
|---|---|
| Patients, n | 291 |
| Age (years), mean±SD | 60.70±8.89 |
| Sex, n (%) | |
| Male | 175 (60.14) |
| Female | 116 (39.86) |
| BMI (kg/m2), mean±SD | 22.20±3.07 |
| Medical history, n (%) | |
| Hypertension | 59 (20.27) |
| Diabetes | 42 (14.32) |
| Cardiovascular event | 5 (1.72) |
| Pancreatitis | 8 (2.75) |
| Tumor | 4 (1.37) |
| Cerebral infarction | 2 (0.69) |
| Lesion site, n (%) | |
| Head/uncinate | 139 (47.76) |
| Neck/body/tail | 152 (52.23) |
| Lesion size (mm), n (%) | |
| <20 | 10 (3.44) |
| 20-40 | 187 (64.26) |
| >40 | 94 (32.30) |
SD: Standard deviation, BMI: Body mass index
EUS-FNB procedures with 25G ProCore needle were technically successful in all patients.
Positive results were obtained by EUS-FNB in 262 patients, and negative results were obtained in 29 patients. After a 6-month follow-up, the final diagnoses were established as malignancy in 280 patients and benign disease in 11 patients [Table 3]. All malignant lesions diagnosed by EUS-FNB were consistent with the final diagnoses, while 18 patients with negative results by EUS-FNB were finally diagnosed by malignancy through surgery or follow-up. There were no procedure-related adverse events in either group.
Table 3.
Pathological types and final diagnoses
| Final diagnosis | n (%) |
|---|---|
| Malignant diseases (n=280) | |
| Pancreatic ductal adenocarcinoma | 249 (85.56) |
| Acinar cell carcinoma | 5 (1.72) |
| Pancreatic neuroendocrine neoplasm | 8 (2.75) |
| Solid pseudopapillary | 1 (0.34) |
| Malignant (unclear type) | 17 (5.84) |
| Benign diseases (n=11) | |
| Chronic pancreatitis | 3 (1.03) |
| Autoimmune pancreatitis | 5 (1.72) |
| Acute pancreatitis | 1 (0.34) |
| Inflammatory pseudotumor | 2 (0.68) |
Specimen adequacy and diagnostic outcomes
Specimen quality with different sampling techniques
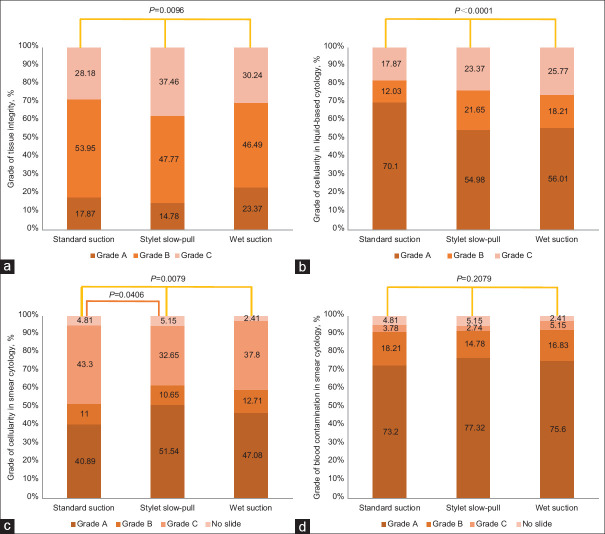
The tissue integrity rate was significantly higher with SS and WS than with SP (P = 0.0096). The cellularity of LBC was the highest with SS compared to SP and WS (P < 0.0001). In smear cytology, statistically significant difference was found between SP and SS (P = 0.0406), but not between WS and SP or WS and SS. The blood contamination rate was higher with SS and WS than with SP, but the difference was insignificant (P = 0.2079) [Figure 2a-d].
Figure 2.
Characteristics of the cytological and histological specimens obtained with 3 different sampling techniques, grade of tissue integrity (a), grade of cellularity in liquid-based cytology (b), grade of cellularity in smear cytology (c), grade of blood contamination in smear cytology (d)
Diagnostic accuracy with different sampling techniques
The diagnostic accuracy of SS and WS for the core tissue was higher than that of SP, but the difference was insignificant (P = 0.1450). The diagnostic accuracy of LBC for the specimens was significantly higher with SS than with WS and SP (P = 0.0029). For smear cytology, the accuracy with SP was slightly higher than that with SS and WS (P = 0.1084). When combining all the cytological and histological diagnoses, still no statistically significant difference was found in diagnostic sensitivity and accuracy among the three sampling techniques (sensitivity, P = 0.1186; accuracy, P = 0.1212) [Table 4].
Table 4.
Diagnostic outcomes of three different sampling techniques
| Indicators | SS | SP | WS | P | P (SS vs. SP) | P (SS vs. WS) | P (SP vs. WS) |
|---|---|---|---|---|---|---|---|
| Histology | |||||||
| Sensitivity, % (95% CI) | 48.93 (40.56-57.30) | 41.43 (32.47-50.39) | 48.57 (40.17-56.97) | 0.1332 | 0.0746 | 0.9326 | 0.0894 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 50.86 (45.12cy, % ( | 43.64 (37.94-49.34) | 50.52 (44.78-56.26) | 0.1450 | 0.0812 | 0.9939 | 0.0967 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 7.14 (3.07% (95% | 6.29 (2.69-9.89) | 7.10 (3.06-11.14) | 0.9405 | 0.7561 | 0.9874 | 0.7682 |
| Liquid-based cytology | |||||||
| Sensitivity, % (95% CI) | 60.71 (53.37-68.05) | 46.07 (37.47-54.67) | 52.86 (44.82-60.90) | 0.0024 | 0.0005 | 0.0606 | 0.1083 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 62.20 (56.63-67.77) | 48.11 (42.37-53.85) | 54.64 (48.92-60.36) | 0.0029 | 0.0006 | 0.0643 | 0.1151 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 9.09 (3.97-14.21) | 6.79 (2.92-10.66) | 7.69 (3.32-12.06) | 0.7734 | 0.4745 | 0.6820 | 0.7612 |
| Smear cytology | |||||||
| Sensitivity, % (95% CI) | 35.58 (25.95-45.21) | 44.53 (35.56-53.50) | 40.66 (31.52-49.80) | 0.1077 | 0.0352 | 0.2245 | 0.3642 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 37.91 (32.20-43.62) | 46.74 (40.85-52.63) | 42.96 (37.20-48.72) | 0.1084 | 0.0355 | 0.2229 | 0.3683 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 5.49 (2.18-8.80) | 6.96 (2.99-10.93) | 6.36 (2.72-10.00) | 0.8530 | 0.5750 | 0.7302 | 0.8257 |
| Overall | |||||||
| Sensitivity, % (95% CI) | 82.14 (77.19-87.09) | 75.00 (69.14-80.86) | 77.86 (72.35-83.37) | 0.1186 | 0.0394 | 0.2049 | 0.4258 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 82.82 (78.49-87.15) | 75.95 (71.04-80.86) | 78.69 (73.99-83.39) | 0.1212 | 0.0404 | 0.2070 | 0.4284 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 18.03 (8.38-27.68) | 13.58 (6.12-21.04) | 15.07 (6.86-23.28) | 0.7643 | 0.4679 | 0.6446 | 0.7921 |
SS: Standard suction, SP: Stylet slow-pull, WS: Wet suction, CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value
Diagnostic yield of the 25G ProCore needle
Grade A and B tissue integrity and cellularity were considered adequate in this study. Finally, 90.72% (264/291) of the tissue specimens were graded as adequate, the immunohistochemical staining of which could specifically diagnose the neoplastic diseases. Moreover, 97.94% (285/291) of the cytological specimens were graded as adequate. The overall specimen adequacy rate was 96.56% for pass 1, which reached 100% when completing pass 2. The cumulative diagnostic accuracy of passes 1 and 2 was 73.20% and 89.00%, and after completing all three passes, the accuracy reached 94.16% [Table 5].
Table 5.
Pooled diagnostic parameters after each pass
| Pass 1 | Pass 2 | Pass 3 | |
|---|---|---|---|
| Adequate sample, n (%) | 281 (96.56) | 291 (100) | 291 (100) |
| Sensitivity, % (95% CI) | 72.14 (66.61-77.08) | 88.57 (84.82-91.82) | 93.93 (91.04-96.82) |
| Specificity, % (95% CI) | 100 | 100 | 100 |
| Accuracy, % (95% CI) | 73.20 (67.71-78.20) | 89.00 (84.85-92.14) | 94.16 (91.47-96.85) |
| PPV%, (95% CI) | 100 | 100 | 100 |
| NPV%, (95% CI) | 12.36 (6.88-20.96) | 25.58 (14.78-40.38) | 39.29 (21.20-57.38) |
CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value
Predictors of high diagnostic sensitivity for pancreatic mass lesions
Multivariate logistic regression analyses were conducted to identify the factors related to the diagnostic sensitivity of each sampling technique. A pancreatic mass measuring less than 2 cm was associated with a high diagnostic sensitivity, which was statistically significant for the SS group, while insignificant for other groups or in general. Moreover, the diagnostic sensitivity was higher for the mass located in the head/uncinate than that in the body/tail of pancreas, though the difference was not obvious (odds ratio = 1.107, 95% confidence interval 0.61–2.008, P = 0.738) [Table 6].
Table 6.
Multivariate logistic regression analysis to identify factors related to diagnostic sensitivity
| Variables | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Standard suction | |||
| Age (years) | 1.014 | 0.977–1.052 | 0.4637 |
| Sex: Male vs. Female | 1.019 | 0.528–1.966 | 0.9563 |
| BMI (kg/m2) | 1.002 | 0.899–1.117 | 0.9649 |
| Mass location: Head/Uncinate vs. Body/Tail | 0.895 | 0.472–1.697 | 0.7339 |
| Mass size: | |||
| <2 cm vs. 2–4 cm | 3.747 | 0.944–14869 | 0.0603 |
| <2 cm vs. >4 cm | 4.593 | 1.073–19.655 | 0.0398 |
| 2–4 cm vs. >4 cm | 1.226 | 0.597–2.517 | 0.5794 |
| Stylet slow-pull | |||
| Age (years) | 0.988 | 0.958–1.02 | 0.5408 |
| Sex: Male vs. Female | 1.27 | 0.707–2.283 | 0.6393 |
| BMI (kg/m2) | 0.943 | 0.856–1.037 | 1.4634 |
| Mass location: Head/Uncinate vs. Body/Tail | 1.307 | 0.746–2.288 | 0.8765 |
| Mass size: | |||
| <2 cm vs. 2–4 cm | 2.501 | 0.634–9.868 | 0.1905 |
| <2 cm vs. >4 cm | 2.607 | 0.629–10.807 | 1.7442 |
| 2–4 cm vs. >4 cm | 1.042 | 0.564–1.926 | 0.0174 |
| Wet suction | |||
| Age (years) | 1.031 | 0.995–1.069 | 2.9176 |
| Sex: Male vs. Female | 1.172 | 0.466–1.564 | 0.263 |
| BMI (kg/m2) | 0.985 | 0.89–1.09 | 0.0866 |
| Mass location: Head/Uncinate vs. Body/Tail | 1.107 | 0.61–2.008 | 0.1119 |
| Mass size: | |||
| <2 cm vs. 2–4 cm | 2.957 | 0.743–11.762 | 0.1239 |
| <2 cm vs. >4 cm | 1.151 | 0.595–2.227 | 0.1757 |
| 2–4 cm vs. >4 cm | 1.031 | 0.995–1.069 | 2.9176 |
| Overall | |||
| Age (years) | 1.031 | 0.995–1.069 | 0.0876 |
| Sex: Male vs. Female | 0.853 | 0.466–1.564 | 0.6081 |
| BMI (kg/m2) | 0.985 | 0.89–1.09 | 0.7685 |
| Mass location: Head/Uncinate vs. Body/Tail | 1.107 | 0.61–2.008 | 0.738 |
| Mass size: | |||
| <2 cm vs. 2–4 cm | 2.957 | 0.743–11.762 | 0.1239 |
| <2 cm vs. >4 cm | 3.405 | 0.806–14.382 | 0.0956 |
| 2–4 cm vs. >4 cm | 1.151 | 0.595–2.227 | 0.6751 |
CI: Confidence interval
Diagnostic outcomes of different pathological techniques
For the SS technique, the highest diagnostic accuracy was found with LBC, and the lowest was with smear cytology, with significant difference among the three groups (P < 0.0001). Nevertheless, the three pathological techniques showed similar diagnostic efficacy with the SP technique (P = 0.5421). For the WS technique, there was significant difference between LBC and smear cytology (P = 0.0178). The overall diagnostic accuracy and sensitivity were significantly higher with histology and LBC than with smear cytology [Table 7]. The cumulative diagnostic accuracy of the three pathological techniques was significantly higher than adopting any of them only.
Table 7.
Diagnostic abilities of the three pathology techniques
| Indicators | Core tissue (A) | Liquid-based cytology (B) | Smear cytology (C) | P | P (A vs. B) | P (A vs. C) | P (B vs. C) |
|---|---|---|---|---|---|---|---|
| SS | |||||||
| Sensitivity, % (95% CI) | 48.93 (40.56-57.30) | 60.71 (53.37-68.05) | 35.58 (25.95-45.21) | <0.0001 | 0.0051 | 0.0016 | <0.0001 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 50.86 (45.12-56.60) | 62.20 (56.63-67.77) | 37.91 (32.20-43.62) | <0.0001 | 0.0058 | 0.0019 | <0.0001 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 7.14 (3.07-11.21) | 9.09 (3.97-14.21) | 5.49 (2.18-8.80) | 0.4842 | 0.5545 | 0.534 | 0.2274 |
| SP | |||||||
| Sensitivity, % (95% CI) | 41.43 (32.47-50.39) | 46.07 (37.47-54.67) | 44.53 (35.56-53.50) | 0.5302 | 0.2681 | 0.465 | 0.7176 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 43.64 (37.94-49.34) | 48.11 (42.37-53.85) | 46.74 (40.85-52.63) | 0.5421 | 0.2795 | 0.459 | 0.7439 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 6.29 (2.69-9.89) | 6.79 (2.92-10.66) | 6.96 (2.99-10.93) | 0.9671 | 0.8514 | 0.8041 | 0.9515 |
| WS | |||||||
| Sensitivity, % (95% CI) | 48.57 (40.17-56.97) | 52.86 (44.82-60.90) | 40.66 (31.52-49.80) | 0.0145 | 0.3104 | 0.0613 | 0.0041 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 50.52 (44.78-56.26) | 54.64 (48.92-60.36) | 42.96 (37.20-48.72) | 0.0178 | 0.3192 | 0.0694 | 0.0051 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 7.10 (3.06-11.14) | 7.69 (3.32-12.06) | 6.36 (2.72-10.00) | 0.8973 | 0.8443 | 0.7895 | 0.6428 |
| Overall | |||||||
| Sensitivity, % (95% CI) | 73.57 (67.55-79.59) | 76.79 (71.15-82.43) | 65.36 (58.47-72.25) | 0.0081 | 0.3786 | 0.0348 | 0.0029 |
| Specificity, % (95% CI) | 100 | 100 | 100 | ||||
| Accuracy, % (95% CI) | 74.57 (69.57-79.57) | 77.66 (72.87-82.45) | 66.67 (61.25-72.09) | 0.0087 | 0.3816 | 0.0363 | 0.0031 |
| PPV, % (95% CI) | 100 | 100 | 100 | ||||
| NPV, % (95% CI) | 12.94 (5.80-20.08) | 14.47 (6.56-22.38) | 10.19 (4.48-15.90) | 0.6654 | 0.7775 | 0.5498 | 0.3773 |
CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, SS: Standard suction, SP: Stylet slow-pull, WS: Wet suction
DISCUSSION
To the best of our knowledge, this prospective multicenter randomized crossover study is the first to simultaneously compare three common sampling techniques (SS, SP, and WS) for the EUS-FNB of solid pancreatic lesions and the largest prospective study to determine the diagnostic performance of 25G ProCore needles.
First, no significant difference was found in overall diagnostic sensitivity and accuracy among the three sampling techniques when using 25G ProCore needles. However, an inferior tissue integrity was observed with the SP technique compared to the SS and WS techniques. The SP technique could reduce blood contamination, though indistinctively. Second, for cytological specimens, the diagnostic outcomes of the SP technique were excellent for smear cytology, but poor for LBC; interestingly, the opposite was true for the SS and WS techniques. Moreover, the LBC diagnostic efficiency with SS was significantly higher than with WS and SP. Finally, a sensitivity of 93.93% and diagnostic accuracy of 94.16% were obtained after three passes with 25G ProCore needles. According to the multivariate analysis, a mass measuring smaller than 2 cm was significantly associated with a higher diagnostic sensitivity in the SS group.
In prior studies, the SS technique was used most frequently for EUS-FNA/FNB;[6] however, a range of studies put forward that SP or WS may improve specimens yield and reduce damage to the specimen.[7,8,9,10,11,12,13,14,15,16] Bang et al.[10] found that using suction could increase the number of passes needed and specimen bloodiness when using 22G and 25G FNA needles. Later, they designed another prospective study comparing four 22G FNB needles using 20-ml negative-pressure suction, SP, or no suction, and found that a decrease in suction force significantly lowered the diagnostic performance of ProCore and Menghini-tip needles, but not Franseen- and fork-tip needles.[18] However, another study performed by Lee et al.[12] showed that SP could provide the best cellularity when using 22G ProCore needles. Whereas a high-quality multicenter randomized trial by Saxena et al.[17] demonstrated that SS and SP offered high and comparable diagnostic sensitivities for the diagnosis of solid pancreatic lesions when using 22G FNA needles. Furthermore, WS was considered a technique that could improve the specimen yield and quality in previous studies. Recently, Wang et al.[13] found in a prospective multicenter study that WS could significantly increase the yield of adequate specimens without increasing the amount of blood contamination compared to SS when using 22G ProCore needles for sampling pancreatic and other gastrointestinal lesions.
Nevertheless, the SP and WS techniques were not found to be superior to the SS technique in this study. Instead, SP had a significantly lower tissue integrity rate than SS and WS. Given that it was a multicenter randomized crossover study, biases in experience and the individual preferences of the endosonographers were substantially eliminated. It is worth discussing the role of needle size in this finding. Katanuma et al.[20] reported that the suction force generated by the SP technique was evidently weaker than that generated by the normal sampling technique and was 4.8% of that generated by a 20-mL syringe for a 19 G needle, 3% of that with a 22 G needle, and 1.4% of that with a 25 G needle. The suction force generated by a 25G needle is weaker than that generated by a 22G or 19G needle, and applying the SP technique would further reduce the suction force, which could affect the diagnostic yield.
For cytological specimens, the diagnostic outcomes with the SP technique were excellent for smear cytology, but poor for LBC, and interestingly, the opposite was true for the SS and WS techniques. This could be due to the uneven distribution of cytological specimens. During the EUS-FNB procedure, the aspirated sample was collected on smears first; then, the remaining liquid specimen was collected for liquid-based preparation, which might cause sample deficiency for one of the cytology techniques. According to our previous study, specimens with less blood contamination are more suitable for smear cytology, while the more contaminated specimens should be processed by LBC, the process of which can greatly reduce the level of contamination.[23] Therefore, although the difference in blood contamination among the three groups was nonsignificant, it might lead to a discrepancy in the distribution of cytological specimens. Thus, the use of smear cytology is more recommended when using the SP technique rather than the SS or WS technique.
Compared to 19G or 22G needles, 25G needles have the characteristics of a smaller diameter and higher flexibility, which makes them easier to handle, especially in the duodenum, where it is difficult to keep the echoendoscope straight.[3] In addition, using a 25G needle reduces the possibilities of needle bending and tissue damage at sites that are difficult to access, such as the pancreatic head, uncinate, and distal segment of the bile duct.[24] However, there have been doubts about whether 25G needles are inferior to 22G or 20G needles for obtaining core tissue.[3,25] However, no previous prospective studies with large sample sizes have explored this issue. Therefore, the acquisition ability of a 25G ProCore needle was further determined through this study. After completing three passes, the overall specimen adequacy rate was 100%, the diagnostic sensitivity was 93.93%, and the accuracy was 94.16%. This diagnostic efficacy was higher than that of 22G ProCore needles in another prospective study that we performed in the same period.[26] Moreover, 90.72% of the tissue specimens acquired with the 25G ProCore needle were adequate for immunohistochemical staining, so that neoplastic diseases could be specifically diagnosed. Furthermore, in previous studies involving 22G needles, a lower diagnostic accuracy was often observed for lesions located in the head and uncinate or those with diameters <2 cm,[10,12,19,27] but this was not the case in the present study. According to the multivariate analysis, a mass measuring smaller than 2 cm was even significantly associated with a higher diagnostic sensitivity in the SS group. There was no significant distinction in diagnostic sensitivity between masses located in the head/uncinate and those in the body/tail. This finding can be attributed to the high degree of flexibility of the 25G needle. Recently, novel needles with specific designs such as Franseen or fork-tip needles have reached the forefront of academic research. Several studies have reported that these novel needles could obtain samples with the highest cellularity.[18,28] However, the use of these novel needles is not as widespread as ProCore needles, and no 25G novel needle is available in China at the present.
The major strength of this study is that the biases in individual preference and experience of the endosonographers and in basic characteristics of the surgical patients were eliminated through the multicenter randomized crossover design. Nevertheless, there are some limitations in this study. First, the study involved only solid pancreatic masses, so the findings might not be applicable to other gastrointestinal lesions or cystic pancreatic lesions. Second, only one type of FNB needle was used. To further determine the optimal sampling technique for different needles and verify the superiority of the 25G ProCore needle for head/uncinate or small lesions, more needle types, especially the novel FNB needle, should be evaluated. Finally, ROSE was not performed in this study, because it might lead to differences in diagnostic outcomes caused by the sequence of sampling techniques, thus affecting the diagnostic efficacy. However, according to a recent study, ROSE has a little effect when the diagnostic accuracy of EUS-FNB is sufficiently high.[28] The criteria for assessing specimen quality were set based on the experience of a single institution and need further validation.
CONCLUSIONS
This study demonstrates that when using a 25G ProCore needle in EUS-FNB to acquire tissues from solid pancreatic lesions, the SS and WS techniques can provide a higher specimen adequacy than SP technique without increasing the amount of blood contamination. Thus, the SS and WS techniques are better choices when using a 25G ProCore needle to acquire specimens from solid pancreatic lesions through EUS-FNB. This study also reveals that 25G ProCore needles can provide a satisfactory diagnostic yield for solid pancreatic lesions, especially lesions located in the head and uncinate of the pancreas or with diameters less than 2 cm.
Novelty and impact statement
To the best of our knowledge, this prospective multicenter randomized crossover study is the first to simultaneously compare three common sampling techniques (standard suction, stylet slow-pull, and wet suction) for the EUS-FNB of solid pancreatic lesions and the largest prospective study to determine the diagnostic performance of 25G ProCore needles.
Writing assistance
This manuscript has been edited and proofread by American Journal Experts.
Financial support and sponsorship
Funded by the Medical Discipline Construction Project of Pudong New Area Commission of Health and Family Planning (Grant No. PWYgf2018-01).
Conflicts of interest
Zhao-Shen Li is an Honoary Editor-in-Chief of the journal, and Zhen-Dong Jin is an Associate Editor. This article was subject to the journal's standard procedures, with peer review handled independently of the editors and their research group.
REFERENCES
- 1.Kitano M, Yoshida T, Itonaga M, et al. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19–32. doi: 10.1007/s00535-018-1519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AY, Yachimski PS. Endoscopic management of pancreatobiliary Neoplasms. Gastroenterology. 2018;154:1947–63. doi: 10.1053/j.gastro.2017.11.295. [DOI] [PubMed] [Google Scholar]
- 3.Artifon EL, Guedes HG, Cheng S. Maximizing the diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration biopsy. Gastroenterology. 2017;153:881–5. doi: 10.1053/j.gastro.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 4.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Wani S. Basic techniques in endoscopic ultrasound-guided fine-needle aspiration: Role of a stylet and suction. Endosc Ultrasound. 2014;3:17–21. doi: 10.4103/2303-9027.123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 7.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen JY, Ding QY, Lv Y, et al. Slow-pull and different conventional suction techniques in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid lesions using 22-gauge needles. World J Gastroenterol. 2016;22:8790–7. doi: 10.3748/wjg.v22.i39.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JM, Lee HS, Hyun JJ, et al. Slow-pull using a fanning technique is more useful than the standard suction technique in EUS-guided fine needle aspiration in pancreatic masses. Gut Liver. 2018;12:360–6. doi: 10.5009/gnl17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang JY, Navaneethan U, Hasan MK, et al. Endoscopic ultrasound-guided specimen collection and evaluation techniques affect diagnostic accuracy. Clin Gastroenterol Hepatol. 2018;16:1820–8.e4. doi: 10.1016/j.cgh.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Bor R, Vasas B, Fábián A, et al. Prospective comparison of slow-pull and standard suction techniques of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of solid pancreatic cancer. BMC Gastroenterol. 2019;19:6. doi: 10.1186/s12876-018-0921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KY, Cho HD, Hwangbo Y, et al. Efficacy of 3 fine-needle biopsy techniques for suspected pancreatic malignancies in the absence of an on-site cytopathologist. Gastrointest Endosc. 2019;89:825–31.e1. doi: 10.1016/j.gie.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wang RH, Ding Z, et al. Wet- versus dry-suction techniques for endoscopic ultrasound-guided fine-needle aspiration of solid lesions: A multicenter randomized controlled trial. Endoscopy. 2020;52:995–1003. doi: 10.1055/a-1167-2214. [DOI] [PubMed] [Google Scholar]
- 14.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Villa NA, Berzosa M, Wallace MB, et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramai D, Singh J, Kani T, et al. Wet – Versus dry-suction techniques for EUS-FNA of solid lesions: A systematic review and meta-analysis. Endosc Ultrasound. 2021;10:319–24. doi: 10.4103/EUS-D-20-00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena P, El Zein M, Stevens T, et al. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: A multicenter randomized trial. Endoscopy. 2018;50:497–504. doi: 10.1055/s-0043-122381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young Bang J, Krall K, Jhala N, et al. Comparing needles and methods of endoscopic ultrasound-guided fine-needle biopsy to optimize specimen quality and diagnostic accuracy for patients with pancreatic masses in a randomized trial. Clin Gastroenterol Hepatol. 2021;19:825–35.e7. doi: 10.1016/j.cgh.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Bhullar F, Alaber O, et al. Comparative diagnostic accuracy of EUS needles in solid pancreatic masses: A network meta-analysis. Endosc Int Open. 2021;9:E853–62. doi: 10.1055/a-1381-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katanuma A, Itoi T, Baron TH, et al. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J Hepatobiliary Pancreat Sci. 2015;22:379–85. doi: 10.1002/jhbp.201. [DOI] [PubMed] [Google Scholar]
- 21.Li SY, Zhou W, Shi L, et al. Diagnostic efficacy of three suction techniques for endoscopic ultrasound-guided fine-needle biopsy of solid pancreatic lesions: Protocol for a multicenter randomized cross-over clinical trial. Dig Liver Dis. 2020;52:734–9. doi: 10.1016/j.dld.2020.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: The papanicolaou society of cytopathology guidelines. Cytojournal. 2014;11:3. doi: 10.4103/1742-6413.133343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W, Gao L, Wang SM, et al. Comparison of smear cytology and liquid-based cytology in EUS-guided FNA of pancreatic lesions: Experience from a large tertiary center. Gastrointest Endosc. 2020;91:932–42. doi: 10.1016/j.gie.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909–15. doi: 10.1016/j.gie.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 25.van Riet PA, Cahen DL, Biermann K, et al. Agreement on endoscopic ultrasonography-guided tissue specimens: Comparing a 20-G fine-needle biopsy to a 25-G fine-needle aspiration needle among academic and non-academic pathologists. Dig Endosc. 2019;31:690–7. doi: 10.1111/den.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Li SY, Jiang H, et al. Optimal number of needle passes during EUS-guided fine-needle biopsy of solid pancreatic lesions with 22G ProCore needles and different suction techniques: A randomized controlled trial. Endosc Ultrasound. 2021;10:62–70. doi: 10.4103/EUS-D-20-00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crinò SF, Di Mitri R, Nguyen NQ, et al. Endoscopic ultrasound-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: A randomized controlled non-inferiority trial. Gastroenterology. 2021;161:899–909.e5. doi: 10.1053/j.gastro.2021.06.005. [DOI] [PubMed] [Google Scholar]