Abstract
Effective vaccination against coronavirus mitigates the risk of hospitalisation and mortality; however, it is unclear whether vaccination status influences long COVID symptoms in patients who require hospitalisation. The available evidence is limited to outpatients with mild disease. Here, we evaluated 412 patients (age: 60 ± 16 years, 65% males) consecutively admitted to two Hospitals in Brazil due to confirmed coronavirus disease 2019 (COVID-19). Compared with patients with complete vaccination (n = 185) before infection or hospitalisation, those with no or incomplete vaccination (n = 227) were younger and had a lower frequency of several comorbidities. Data during hospitalisation revealed that the no or incomplete vaccination group required more admissions to the intensive care unit (ICU), used more corticosteroids, and had higher rates of pulmonary embolism or deep venous thrombosis than the complete vaccination group. Ninety days after hospital discharge, patients with no or incomplete vaccination presented a higher frequency of symptoms (≥ 1) than patients with complete vaccination (40 vs. 27%; p = 0.013). After adjusting for confounders, no or incomplete vaccination (odds ratio [OR] 1.819; 95% confidence interval [CI] 1.175–2.815), female sex (OR 2.435; 95% CI 1.575–3.764) and ICU admission during hospitalisation (OR 1.697; 95% CI 1.062–2.712) were independently associated with ≥ 1 symptom 90 days after hospital discharge. In conclusion, even in patients with severe COVID-19, vaccination mitigates the probability of long COVID symptoms.
Subject terms: Health care, Signs and symptoms, Infectious diseases
Introduction
Coronavirus disease 2019 (COVID-19) promotes a significant burden of symptoms in the subacute and long term post infection1. The commonly reported residual symptoms include fatigue, dyspnoea, and chest pain, among others (collectively described as post-acute COVID-19 syndrome or long COVID)1–4. From 2021, progressive implementation of effective vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has mitigated the risk of complications, including hospitalisations and mortality5,6. Recent reports suggest that vaccination can reduce long COVID in patients who do not need hospitalisation7–10. Therefore, it is unclear whether vaccination status influences the magnitude of long COVID symptoms in patients with more severe disease who frequently require more intensive support and are more susceptible to long-term complications. In the current study, we systematically evaluated several long COVID symptoms 90 days after hospital discharge in consecutive patients, according to vaccination status. We hypothesised that patients with no or incomplete vaccination would present at least one symptom compared to patients with complete vaccination (at least two doses for all vaccines but ≥ 1 dose for the Janssen™ vaccine, as described by others10). Moreover, no or incomplete vaccination would be independently associated with persistence of symptoms after hospital discharge.
Methods
The study was reviewed by an Institutional Review Board (CAAE 45978621.9.0000.5461) and deemed exempt from IRB oversight (Instituto de Ensino e Pesquisa, IEP, Hospital Sírio Libanês). The requirement of informed consent was waived. All methods were performed in accordance with relevant guidelines and regulations. All data were analysed in a secure, anonymised database physically separated from the main production server.
We studied consecutive hospitalised patients at the Hospital Sirio Libanês, Sao Paulo, and Hospital Sirio Libanês, Brasilia, Brazil from May 2021 to February 2022. All patients had a confirmed diagnosis of COVID-19 based on the presence of related symptoms and a positive result on a SARS-CoV-2 polymerase chain reaction assay of both nasal and pharyngeal swab specimens.
All the collected data were reviewed by the study team to ensure accuracy. The registry utilised a web-based case report form in the RedCap™ platform (Nashville, TN, US).
Variables and clinical outcomes collected at hospital
Participant data were collected until hospital discharge. Vaccination status was checked in the medical records (routinely documented through a standard vaccination card). Complete vaccination was considered when patients received two or more doses for all vaccines but ≥ 1 dose for the Janssen™. Otherwise, it was considered an incomplete vaccination10. We compiled data on demographic characteristics, medical history, length of hospital stay, intensive care unit (ICU) admission, mechanical ventilation, pulmonary embolism (PE), deep venous thrombosis (DVT), major bleeding, need for dialysis, and medications used during hospitalisation (including anticoagulants, convalescent plasma, and corticosteroids).
Variables collected after hospital discharge
The Clinical Outcomes team from Hospital Sirio Libanes prospectively applied a standardised questionnaire 90 days after hospital discharge, contacting all patients over telephone. All members of this team received formal training to identify and characterise symptoms. They had no access to the details of in-hospital admission. We evaluated the presence of symptoms, such as dyspnoea, tiredness or fatigue, cough, chest pain, sore throat, anosmia or ageusia, headache, arthralgia, myalgia, and others (e.g. diarrhoea and vomiting). Long COVID was defined as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection11.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median and interquartile range; categorical variables were described using absolute and relative frequencies. Comparisons of patients according to vaccination status were performed using chi-square tests for categorical variables and Mann–Whitney tests for continuous variables. Multivariate logistic regression analysis for the presence of ≥ 1 symptoms after 90 days of hospital discharge was presented considering variables with biological relevance for the main outcomes. These included vaccination status, age, sex, diabetes, previous cardiovascular disease, chronic kidney disease, ICU admission, PE or DVT, and corticosteroid use (the last three variables during hospitalisation). All analyses were performed using IBM SPSS Statistics version 24 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL).
Results
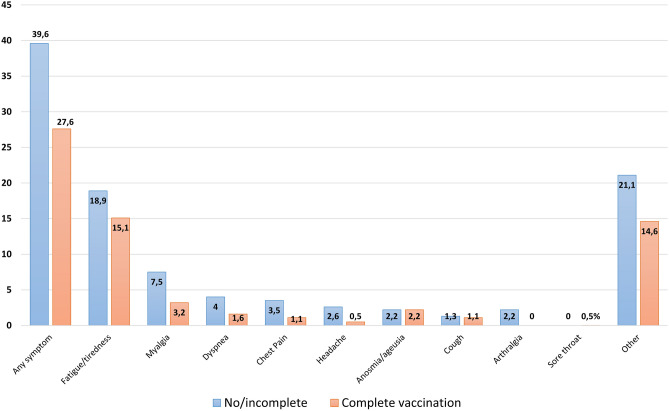
During the study recruitment period, 412 patients were discharged from the hospital and had available data on vaccination status and symptoms after 90 days (227 with no or incomplete vaccination and 185 with complete vaccination). Figure 1 shows the symptoms reported 90 days after discharge. Patients with no or incomplete vaccination had a higher frequency of symptoms (≥ 1) than patients with complete vaccination (p = 0.013). Table 1 shows the main characteristics of the included patients, stratified according to vaccination status. Compared with patients with complete vaccination, those with no or incomplete vaccination were younger and had a lower frequency of hypertension, diabetes, dyslipidaemia, previous cardiovascular disease, and chronic kidney disease. Data during hospitalisation revealed that they required more admissions to the ICU and more corticosteroids and had higher rates of PE or DVT.
Figure 1.
The frequency (A) and number of symptoms reported by patients according to vaccination status.
Table 1.
Characteristics of patients discharged after hospitalisation for COVID-19 stratified according to vaccination status.
| Characteristics | Total | No or incomplete vaccination | Complete vaccination | p-value |
|---|---|---|---|---|
| (n = 412) | (n = 227) | (n = 185) | ||
| Demographic and anthropometric characteristics and comorbidities | ||||
| Age (years), median (interquartile range) | 60 (48–72) | 55 (43–64) | 71 (59–78) | < 0.001 |
| Male, n (%) | 266 (64.6) | 156 (68.7) | 110 (59.5) | 0.051 |
| Self-reported white, n (%)* | 277 (85.5) | 131 (83.4) | 146 (87.4) | 0.475 |
| Body mass index (kg/m2), median (interquartile range) | 28.1 (25.5–31.7) | 28.3 (25.6–32.0) | 28.0 (25.2–31.1) | 0.153 |
| Self-reported previous COVID, n (%) | 5 (1.2) | 1 (0.4) | 4 (2.2) | 0.179 |
| Current smoking, n (%) | 25 (6.1) | 14 (6.2) | 11 (5.9) | 0.255 |
| Hypertension, n (%) | 178 (43.2) | 79 (34.8) | 99 (53.5) | < 0.001 |
| Diabetes, n (%) | 90 (21.8) | 40 (17.6) | 50 (27.0) | 0.022 |
| Dyslipidaemia, n (%) | 93 (22.6) | 40 (17.6) | 53 (28.6) | 0.008 |
| Previous cardiovascular disease, n (%) | 65 (15.8) | 22 (9.7) | 43 (23.2) | < 0.001 |
| Previous cerebrovascular disease, n (%) | 8 (1.9) | 4 (1.8) | 4 (2.2) | > 0.999 |
| Chronic obstructive pulmonary disease/asthma, n (%) | 36 (8.7) | 19 (8.4) | 17 (9.2) | 0.770 |
| Chronic kidney disease, n (%) | 13 (3.2) | 1 (0.4) | 12 (6.5) | < 0.001 |
| Self-reported diagnosis of anxiety, n (%) | 22 (5.3) | 16 (7.0) | 6 (3.2) | 0.087 |
| Self-reported diagnosis of depression, n (%) | 26 (6.3) | 12 (5.3) | 14 (7.6) | 0.344 |
| Data during hospitalisation | ||||
| Days of hospitalisation, median (interquartile range) | 8.0 (4.0–13.0) | 8.0 (4.5–14.0) | 8.0 (4.0–13.0) | 0.435 |
| Intensive care unit admission, n (%) | 107 (26.0) | 71 (31.3) | 36 (19.5) | 0.007 |
| Mechanical ventilation, n (%) | 43 (10.4) | 28 (12.3) | 15 (8.1) | 0.163 |
| Pulmonary embolism, n (%) | 19 (4.6) | 15 (6.6) | 4 (2.2) | 0.032 |
| Deep vein thrombosis, n (%) | 10 (2.4) | 10 (4.4) | 0 (0) | 0.003 |
| Dialysis, n (%) | 7 (1.7) | 6 (2.6) | 1(0.5) | 0.135 |
| Major bleeding, n (%) | 13 (3.2) | 9 (4.0) | 4 (2.2) | 0.298 |
| Critical illness polyneuropathy, n (%) | 9 (2.2) | 7 (3.1) | 2 (1.1) | 0.195 |
| Convalescent plasma, n (%) | 10 (2.4) | 5 (2.2) | 5 (2.7) | 0.759 |
| Prophylactic or therapeutic anticoagulants, n (%)* | 402 (97.6) | 220 (96.9) | 182 (98.4) | 0.522 |
| Corticoids, n (%) | 353 (85.7) | 207 (91.2) | 146 (78.9) | < 0.001 |
*n = 324 (some patients refused to report this variable).
Bold numbers indicates variables with statistical significance.
After adjusting for multiple confounders, no or incomplete vaccination, female sex, and ICU admission during hospitalisation were independently associated with at least one symptom 90 days after hospital discharge (Table 2).
Table 2.
Multivariate analysis evaluating the independent variables associated with symptoms 90 days after hospitalization due to COVID-19.
| Coefficient | Odds ratio | 95% confidence interval | p value | ||
|---|---|---|---|---|---|
| Group (no or incomplete vaccination) | 0.598 | 1.819 | 1.175 | 2.815 | 0.007 |
| Age (years) | 0.003 | 1.003 | 0.987 | 1.018 | 0.737 |
| Sex (female) | 0.890 | 2.435 | 1.575 | 3.764 | < 0.001 |
| Diabetes (yes) | 0.395 | 1.485 | 0.890 | 2.478 | 0.130 |
| Previous cardiovascular disease (yes) | − 0.167 | 0.846 | 0.451 | 1.589 | 0.604 |
| Chronic kidney disease (yes) | − 0.813 | 0.444 | 0.086 | 2.292 | 0.332 |
| Intensive care unit admission during hospitalization (yes) | 0.529 | 1.697 | 1.062 | 2.712 | 0.027 |
| Pulmonary embolism or deep venous thrombosis during hospitalization (yes) | − 0.634 | 0.531 | 0.197 | 1.427 | 0.209 |
| Corticoid use during hospitalization (yes) | 0.508 | 1.662 | 0.840 | 3.287 | 0.145 |
| Constant | − 1.481 | ||||
Bold numbers indicates variables with statistical significance.
Discussion
This study evaluated the potential impact of vaccination on long COVID symptoms after hospital discharge and the following results were obtained: despite patients with complete vaccination being older and having a substantially high frequency of several comorbidities (probably because worldwide vaccination strategies initially focused on vulnerable individuals), they presented a lower frequency of symptoms (≥ 1) 90 days after hospital discharge. This result may be partially explained by the fact that patients with no or incomplete vaccination had more complications during hospitalisation than those who received complete vaccination (Table 1). However, multivariate analysis revealed that complete vaccination was independently associated with a lower rate of symptoms (≥ 1) 90 days after hospital discharge. Taken together, these results emphasise the role of vaccination in promoting a lower chance of long COVID after hospital discharge.
The evidence supporting the impact of vaccination on long COVID is scarce and limited to mild cases7–10,12. In a survey conducted in a community-dwelling population from the United Kingdom10, 23.7% reported long COVID symptoms regardless of severity, at least once during follow-up (at least 12 weeks post infection). The likelihood of long COVID symptoms decreased after vaccination10. However, only 3.2% of this population required hospitalisation during the acute phase. A recent systematic review12 of six studies (including preprints) revealed that vaccination before SARS-CoV-2 infection could reduce the risk of subsequent long COVID. Overall, the studies reported long COVID in patients who were not hospitalised12. Therefore, our study adds to the literature not only by providing consistent results but also by demonstrating that the benefits of vaccination on long COVID are also observed in more severe cases (i.e. patients with SARS-CoV-2 infection who require hospitalisation).
To date, there have been no definitive answer to why there is a lower rate of long COVID after vaccination. Vaccination can increase antibody titres and potentially eliminate viral reservoirs13. It is conceivable that abnormalities in T cells, platelets, vascular endothelium, and clotting factors, related to COVID-1914,15, can be mitigated by vaccination. Consistent with these previous findings, hospitalised patients who completed the vaccination had a lower rate of significant complications, including thrombotic events.
Our study has some strengths. We included consecutive patients hospitalised due to confirmed COVID-19, from two hospitals. Further, we used standard procedures for checking vaccination status and performing a structured interview looking for symptoms 90 days after hospital discharge, with no access to the vaccination status or the severity of hospitalisation. However, we need to acknowledge the following study limitations: (1) the influence of vaccine type on outcomes (long COVID) could not be tested. There are several potential combinations that that limit our ability to detect any statistically significant differences in long COVID symptoms. (2) At the time of data collection, the third and fourth doses were just implemented in the Brazilian Vaccination Program. Therefore, the number of patients hospitalised despite receiving three or four doses was small. In addition, this finding is explained by the higher protection offered to patients who received extra doses during hospital admissions16,17. (3) Another limitation is that the follow-up was short and the 90-day symptoms relied on the patients’ own notification. Multiple evaluations overtime would be interesting to explore the potential impact of complete vaccination on the dynamic changes of symptoms. (4) Finally, we did not explore whether the impact of complete vaccination on long COVID might be influenced by variants of SARS-CoV-2. The lack of genetic confirmation and the relative sample size prevented the utility of this analysis.
In conclusion, our results suggest that effective vaccination may decrease the burden of long COVID in patients who require hospitalisation. Therefore, these findings add to the recent literature suggesting the multiple benefits of vaccination in COVID-19 infection, hospital admissions, severity of hospitalisation, and long COVID.
Acknowledgements
The authors thank Gianni Santos, Stat, for careful statistical analysis.
Author contributions
T.C.D.N. and L.F.D. wrote the initial draft of the manuscript with input from all authors. All authors contributed substantially to the concept and design of the study, and acquisition, analysis, and interpretation of data, or a combination thereof. All authors critically reviewed the manuscript for important intellectual content and approved the final version.
Funding
Hospital Sírio Libanes.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Teresa Cristina D. C. Nascimento, Email: teresa.cdcnascimento@hsl.org.br
Luciano F. Drager, Email: luciano.drager@gmail.com
References
- 1.Huang C, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A, et al. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cares-Marambio K, et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron. Respir. Dis. 2021;18:14799731211002240. doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehme M, COVICARE Team et al. COVID-19 symptoms: Longitudinal evolution and persistence in outpatient settings. Ann. Intern. Med. 2021;174:723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-On YM, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N. Engl. J. Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jara A, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli M, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immunol. 2022;103:154–162. doi: 10.1016/j.bbi.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherlinger M, Pijnenburg L, Chatelus E, et al. Effect of SARS-CoV-2 vaccination on symptoms from post-acute sequelae of COVID-19: Results from the nationwide VAXILONG study. Vaccines. 2021;10:46. doi: 10.3390/vaccines10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayoubkhani, et al. Trajectory of long COVID symptoms after COVID-19 vaccination: Community based cohort study. BMJ. 2022;377:e069676. doi: 10.1136/bmj-2021-069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO definition. https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition.
- 12.Notarte, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massey, et al. Change in symptoms and immune response in people with post-acute sequelae of SARS-CoV-2 infection (PASC) after SARS-CoV-2 vaccination. medRxiv. 2021 doi: 10.1101/2021.07.21.21260391v2. [DOI] [Google Scholar]
- 14.Visco V, et al. Post-COVID-19 syndrome: Involvement and interactions between respiratory, cardiovascular and nervous systems. J. Clin. Med. 2022;11:524. doi: 10.3390/jcm11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kell DB, et al. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barda N, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magen O, et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.