Abstract
The age-related decline in T-cell function among elderly individuals remains unclear. We thus investigated the interrelationship between T-cell subsets and age to identify the changes in T-cell phenotypes and develop an age prediction model for the elderly population. A total of 127 individuals aged >60 years were divided into three groups (youngest-old group, 61–70 years, n = 34; middle-old group, 71–80 years, n = 53; and oldest-old group, ≥ 81 years, n = 40). The percentage of CD8+CD28− cells (P = 0.001) was highest in the oldest-old group and then followed by the middle-old group, while the youngest-old group was the lowest. Both females and males demonstrated significant decreases in the absolute counts of CD4+CD45RA+ cells (P = 0.020; P = 0.002) and CD8+CD28+ cells (P = 0.015; P = 0.005) with age. Multivariate linear regression showed that the percentage of CD8+CD28− cells (P < 0.001) was an independent predictor of aging after adjusting for sex, body mass index, hospitalization duration, smoking, drinking, chronic medical illness, and medications at admission. In conclusion, our results suggest that aging in elderly individuals is accompanied by a decrease in the counts of T-cell subpopulations. CD8+CD28− cells may be potential targets for elderly individuals in antiaging-related immunosenescence.
Keywords: T-cell subsets, immunosenescence, elderly, aging
1. Introduction
Aging is a varied and complex process that occurs with the decline in the immune system [1,2]. T-cell subsets play a key role in the evaluation of immune status and disease course [3]. The reference values of T-cell subpopulations are currently being widely utilized to evaluate the alteration in immune status. Immunosenescence is an inevitable process characterized by the deficiency of the T-cell response as individuals age, which has been a major hallmark of aging.
Aging-related changes in T-cell phenotypic compositions are well-accepted phenomena. Studies have demonstrated that older age is associated with an inversion of the CD4/CD8 ratio, a reduction in naive T cells, and an expansion in memory and effector T cells [2,4]. This process is accompanied by the reduced expression of membrane surface molecules, such as CD45RA, CD27, and CD28 [5,6]. The expression of CD45RA differentiation markers is usually associated with naive T cells [7]. It is recognized that CD45RA re-expressing memory cells display senescence-related proliferative defects but can effectively secrete homeostatic cytokines [8]. CD27 is an immune-checkpoint receptor and plays an important role in T-cell development, activation, and survival [9]. CD28 is involved in T-cell clonal expansion, metabolic activity, and IL-2 production [10]. The loss of CD28 expression is a key indicator of replicative senescence of human T cells [11]. The above molecules have been identified as the main phenotypes involved in T-cell immunosenescence and how they change with age merit further exploration.
Age-related changes in T-cell subsets could be utilized to predict the diagnosis and prognosis of diseases, including autoimmune diseases, cancers, pathogen infections, and primary immunodeficiencies [12,13,14,15]. Immunosenescence is the primary driver of age-related diseases, such as infections, cancers, and cardiovascular diseases [16,17,18]. Modifiable risk factors, such as nutritional status, physical activity, or dietary habits, can affect the senescence of the immune system [19,20]. The T-cell subset composition with aging plays a pivotal role in determining the successful and appropriate immune response of the host. To further explore the implication of senescent T-cell phenotypes in the aging process, we investigated the interrelationship between T-cell subpopulations and age and developed an age prediction model for the elderly population.
2. Materials and methods
2.1. Study design and participants
This retrospective study consecutively recruited 127 elderly inpatients aged >60 years at the Department of Geriatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology from December 2014 to February 2019. The patient population was separated into three age groups: 61–70 years, 71–80 years, and ≥81 years.
The exclusion criteria included the following: (1) serious infections, (2) medication regimens that could influence the immune system, (3) malignant tumor diseases, (4) autoimmune diseases, and (5) HIV infection. Patients who met any of the exclusion criteria were not enrolled in this study. Physical activity in the 7 days before admission was assessed using the physical activity scale for the elderly (PASE) scores [21]. The hospitalization duration refers to the number of days between admission and discharge.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration and has been approved by the Ethics Committee of Tongji Hospital (ID: TJ-C20141112).
2.2. Flow cytometric analysis
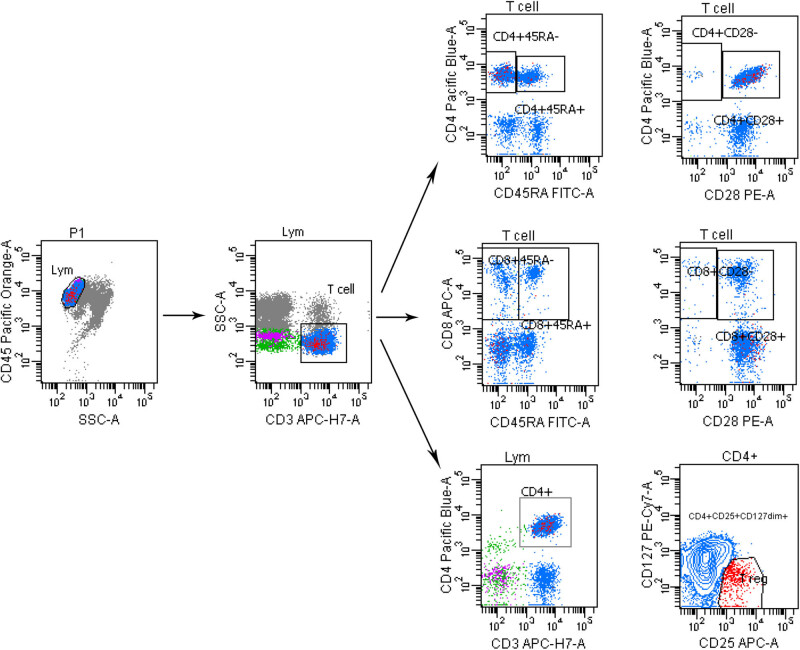
Laboratory tests were completed by the Department of Laboratory Medicine, Tongji Hospital. Peripheral blood specimens were collected into vacutainer tubes containing EDTA as anticoagulant. Peripheral blood mononuclear cells were obtained by density centrifugation using Lymphoprep (StemCell Technologies, Vancouver, BC, Canada). Samples were stained with the antibody cocktail for 30 min at 4°C to analyze antigen expression: CD45 Pacific Orange/CD4 Pacific Blue/CD28 PE/CD45 RA FITC/CD25 PerCP-Cy5.5 (Thermo Fisher Scientific, Waltham, MA, USA), and CD3 APC-H7/CD8 APC (BD Biosciences, San Jose, CA, USA). The proportions of T-cell subsets were acquired from a BD LSRFortessa flow cytometer and analyzed using BD FACS Diva 6.2 software. Absolute T subpopulation numbers were calculated as the cell proportion multiplied by the absolute number of lymphocytes. The absolute counts of lymphocytes were detected by the automatic blood cell analyzer (XN-1000, Sysmex, Kobe, Japan). Treg cells were assessed as CD3+CD4+CD25+CD127dim+ events. Isotype controls and FMO controls were used as appropriate.
2.3. Data analysis
Categorical data were described as the frequency (percentage). Continuous variables are presented as medians with interquartile range (IQR) or means with standard deviation (SD) where appropriate. Comparisons among multiple groups were analyzed by Kruskal–Wallis H test, and between two groups were by Mann–Whitney U test. A chi-square test was used to analyze categorical data. Spearman rank correlation coefficient (r) and associated P values were calculated to determine the correlations between age and T-cell subpopulations. The unstandardized regression coefficient (B) was evaluated using linear regression to indicate the effect size of independent variables on dependent variables. For the decline and rise of the expression of T-cell markers with age, coefficient B represents the pace at which they change over time. Multiple linear regression was performed using a stepwise approach to develop the age prediction models. P values less than 0.05 were considered to be statistically significant. Statistical analyses were conducted by SPSS 24.0 (SPSS, University Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Prism, San Diego, CA, USA).
3. Results
3.1. Profiles of samples
The baseline characteristics of the patients are shown in Table 1. The study sample consisted of 127 subjects with a mean age of 75.25 ± 9.34 years, 101 (79.5%) of whom were males. There were three age groups: youngest old, 61–70 years (n = 34, aged 64.00 ± 2.37); middle old, 71–80 years (n = 53, aged 73.74 ± 2.51); and oldest-old, ≥ 81 years (n = 40, aged 87.83 ± 4.22). Among the three groups, the oldest-old group had the lowest PASE scores (P < 0.001) and mean body mass index (BMI) (P = 0.003). Hospitalization duration was similar in the youngest-old group and the middle-old group but increased in the oldest-old group (P < 0.001). There was no significant difference in other demographic variables between groups.
Table 1.
Demographic characteristics of study population
| Variables | Youngest old (61–70 years; n = 34) | Middle old (71–80 years; n = 53) | Oldest old (≥81 years; n = 40) | P-value |
|---|---|---|---|---|
| Age (years) | 64.00 ± 2.37 | 73.74 ± 2.51a | 87.83 ± 4.22a | <0.001 |
| Sex, male (%) | 27 (79.4%) | 42 (79.2%) | 32 (80.0%) | 0.996 |
| PASE scores | 176.08 (84.88–201.25) | 140.20 (64.60–153.90)a | 25.50 (17.07–66.58) a,b | <0.001 |
| BMI (kg/m2) | 23.74 ± 2.63 | 23.03 ± 2.61 | 22.15 ± 2.31 a | 0.003 |
| Hospitalization duration (days) | 10.00 (4.75–14.25) | 11.00 (5.00–17.00) | 18.00 (12.25–24.50)a,b | <0.001 |
| Smoking (%) | 12 (35.3%) | 16 (30.2%) | 11 (27.5%) | 0.765 |
| Drinking (%) | 9 (26.5%) | 11 (20.8%) | 5 (12.5%) | 0.311 |
| Chronic medical illness | ||||
| Hypertension (%) | 13 (38.2%) | 27 (50.9%) | 25 (62.5%)a | 0.115 |
| Diabetes (%) | 7 (20.6%) | 14 (26.4%) | 12 (30.0%) | 0.652 |
| Cardiovascular disease (%) | 16 (47.1%) | 26 (49.1%) | 20 (50.0%) | 0.968 |
| Cerebrovascular disease (%) | 9 (26.5%) | 14 (26.4%) | 11 (27.5%) | 0.992 |
| Chronic obstructive pulmonary disease (%) | 6 (17.6%) | 4 (7.5%) | 9 (22.5%)b | 0.118 |
| Chronic liver disease (%) | 3 (8.8%) | 1 (1.9%) | 2 (5.0%) | 0.329 |
| Chronic kidney disease (%) | 4 (11.8%) | 3 (5.7%) | 7 (17.5%) | 0.187 |
| Severe infection (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | — |
| Medications at admission | ||||
| Antihypertensive drugs | 13 (38.2%) | 24 (45.3%) | 22 (55.5%) | 0.345 |
| Hypoglycemic agents/insulin | 7 (20.6%) | 13 (24.5%) | 11 (27.5%) | 0.788 |
| Statins | 11 (26.8%) | 18 (34.0%) | 17 (42.5%) | 0.601 |
| Anti-thrombotic agents | 13 (38.2%) | 19 (35.8%) | 20 (50.0%) | 0.363 |
| Oral antibiotics | 1 (2.9%) | 1 (1.9%) | 1 (2.5%) | 0.949 |
Data are presented as mean ± standard deviation, median (interquartile range), and frequency (percentage). P-value was calculated among the three groups and statistically significant P-values are marked in bold. BMI, body mass index; PASE, physical activity scale for the elderly.
a P < 0.05 compared with the youngest-old group.
b P < 0.05 compared with the middle-old group.
3.2. Dynamic changes in T-cell subsets in different age groups
Gating strategies for flow cytometry are shown in Figure A1. The absolute counts of lymphocytes (P = 0.021), T cells (P = 0.006), CD4+ cells (P = 0.005), CD4+CD25+CD127dim+ cells (P = 0.026), CD4+CD28+ cells (P = 0.001), CD4+CD45RA+ cells (P < 0.001), CD8+CD28+ cells (P = 0.007), and CD4/CD8 ratio (P = 0.037) were significantly different in the three groups, which were significantly lower in the oldest-old group than in the youngest-old group (P < 0.05) and the middle-old group (P < 0.05). Absolute counts in different age groups are shown in Table 2.
Table 2.
Absolute counts of T-cell subsets in different age groups
| T-cell subsets (/μl) | Youngest old (61–70 years; n = 34) | Middle old (71–80 years; n = 53) | Oldest old (≥81 years; n = 40) | P-value |
|---|---|---|---|---|
| Lymphocytes | 1610.00 (1330.00–2015.00) | 1700.00 (1225.00–2010.00) | 1265.00 (935.00–1697.50) a,b | 0.021 |
| T cells (CD3+CD45+) | 1073.84 (852.36–1399.15) | 1044.12 (744.35–1284.32) | 717.04 (541.98–1145.76)a,b | 0.006 |
| CD4+ cells | 606.45 (402.20–790.92) | 525.41 (330.80–702.60) | 394.33 (225.58–594.15)a,b | 0.005 |
| CD8+ cells | 364.68 (305.35–580.17) | 426.50 (250.70–587.42) | 353.02 (203.69–493.26) | 0.435 |
| CD4+CD25+CD127dim+cells | 68.59 (52.95–92.38) | 65.17 (43.31–92.16) | 46.94 (29.45–76.72)a,b | 0.026 |
| CD4+CD28+ cells | 566.22 (350.45–706.50) | 491.41 (299.49–661.30) | 340.11 (185.80–524.67)a,b | 0.001 |
| CD4+CD28− cells | 27.91 (7.56–52.03) | 23.20 (10.48–55.78) | 36.60 (15.64–56.83) | 0.318 |
| CD4+CD45RA+ cells | 142.99 (80.18–256.00) | 113.43 (49.78–207.20) | 69.54 (36.65–125.28)a,b | <0.001 |
| CD4+CD45RA− cells | 412.05 (315.25–578.85) | 382.55 (266.81–524.69) | 289.56 (201.85–480.58)a | 0.011 |
| CD8+CD28+ cells | 216.72 (148.36–310.98) | 178.54 (144.36–289.30) | 139.36 (90.93–210.65)a,b | 0.007 |
| CD8+CD28− cells | 166.06 (97.00–244.66) | 194.77 (73.15–289.67) | 178.99 (102.83–270.51) | 0.686 |
| CD8+CD45RA+ cells | 157.12 (81.41–213.36) | 132.57 (100.07–197.54) | 123.89 (56.44–188.67) | 0.477 |
| CD8+CD45RA− cells | 214.26 (142.37–363.62) | 256.81 (138.32–350.78) | 199.18 (126.67–325.14) | 0.584 |
| CD4/CD8 ratio | 1.44 (1.06–2.00) | 1.31 (0.80–1.88) | 0.97 (0.70–1.59) | 0.037 |
Data are represented as median (interquartile range). P-value was calculated among the three groups and statistically significant P-values are marked in bold.
a P < 0.05 compared with the youngest-old group.
b P < 0.05 compared with the middle-old group.
As shown in Table 3, the percentages of CD8+ cells and CD8+CD28− cells in the oldest-old group were significantly increased (P < 0.05) compared with the youngest-old group, while the percentages of CD4+CD28+ cells and CD4+CD45RA+ cells, were decreased (P < 0.05). Moreover, the percentage of CD4+CD28− cells in the oldest-old group was significantly higher than those in the youngest-old group (P < 0.05) and the middle-old group (P < 0.05)
Table 3.
Proportions of T-cell subsets in different age groups
| Percentages of T-cell subsets (%) | Youngest old (61–70 years; n = 34) | Middle old (71–80 years; n = 53) | Oldest old (≥81 years; n = 40) | P-value |
|---|---|---|---|---|
| T cells (CD3+CD45+)/lymphocytes | 68.40 (60.00–75.55) | 65.50 (57.50–73.05) | 62.90 (50.10–71.10) | 0.176 |
| CD4+ cells/T cells | 57.75 (49.22–62.87) | 53.80 (40.80–61.10) | 49.55 (39.98–59.50) | 0.111 |
| CD8+ cells/T cells | 35.80 (30.25–41.45) | 41.50 (32.55–51.30) | 48.30 (35.50–54.30)a | 0.013 |
| CD4+CD25+CD127dim+cells/T cells | 6.50 (5.00–7.65) | 7.00 (5.55–8.35) | 6.55 (4.78–8.35) | 0.659 |
| CD4+CD28+ cells/T cells | 53.40 (41.60–61.60) | 48.60 (37.75–59.30) | 44.30 (32.70–56.90)a | 0.045 |
| CD4+CD28− cells/T cells | 2.30 (0.70–4.65) | 2.60 (1.05–5.60) | 5.10 (1.95–11.97)a,b | 0.019 |
| CD4+CD45RA+ cells/T cells | 13.2 (8.00–20.65) | 13.80 (6.55–17.65) | 8.85 (3.15–16.98)a | 0.030 |
| CD4+CD45RA− cells/T cells | 44.10 (32.95–47.75) | 39.70 (31.15–44.3) | 39.90 (32.13–49.45) | 0.359 |
| CD8+CD28+ cells/T cells | 19.30 (14.15–26.45) | 18.30 (14.75–23.70) | 18.60 (13.90–25.18) | 0.723 |
| CD8+CD28− cells/T cells | 13.80 (10.70–20.10) | 19.80 (11.50–30.75)a | 24.90 (19.10–34.45)a | 0.001 |
| CD8+CD45RA+ cells/T cells | 14.3 (8.90–18.90) | 13.1 (10.05–19.35) | 18.20 (9.20–22.65) | 0.291 |
| CD8+CD45RA− cells/T cells | 21.20 (15.60–28.60) | 23.70 (17.1–29.85) | 27.70 (20.55–35.95) | 0.055 |
Data are represented as median (interquartile range). P-value was calculated among the three groups and statistically significant P-values are marked in bold.
a P < 0.05 compared with the youngest-old group.
b P < 0.05 compared with the middle-old group.
3.3. Correlations between age and T-cell subsets
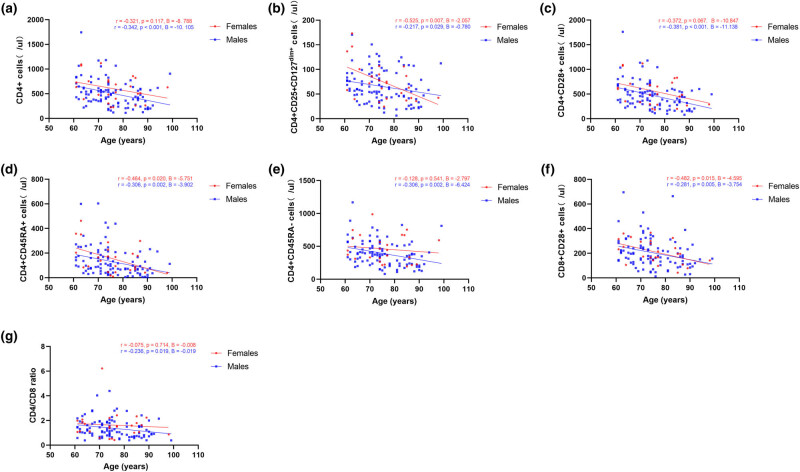
There is skewness in the female/male ratio, so we used different colors to represent different sexes in Spearman's correlation analysis. Both females and males demonstrated significant decreases in the absolute counts of CD4+CD25+CD127dim+ cells (r = −0.525, P = 0.007, B = −2.057; r = −0.217, P = 0.029, B = −0.780), CD4+CD45RA+ cells (r = −0.464, P = 0.020, B = −5.751; r = −0.306, P = 0.002, B = −3.902), and CD8+CD28+ cells (r = −0.482, P = 0.015, B = −4.595; r = −0.281, P = 0.005, B = −3.754) with age. Decreases in the absolute counts of CD4+ cells (r = −0.342, P < 0.001, B = −10.105), CD4+CD28+ cells (r = −0.381, P < 0.001, B = −11.138), CD4+CD45RA− cells (r = −0.306, P = 0.002, B = −6.424), and CD4/CD8 ratio (r = −0.236, P = 0.019, B = −0.019) were observed with increasing age only in the male elderly. Correlation analysis between absolute counts of T-cell subsets and age is shown in Figure 1.
Figure 1.
Correlation analysis between absolute counts of T-cell subsets and age. (a–g) Correlation between age and absolute counts of CD4+ cells, CD4+CD25+CD127dim+ cells, CD4+CD28+ cells, CD4+CD45RA+ cells, CD4+CD45RA− cells, CD8+CD28+ cells, and CD4/CD8 ratio. Red indicates females, and blue indicates males.
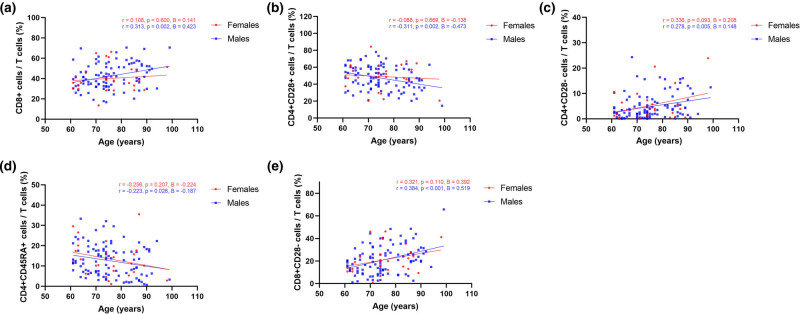
For T-cell proportions, no independent correlation between age and T-cell subsets was shown in females. The percentages of CD8+ cells (r = 0.313, P = 0.002, B = 0.423), CD4+CD28− cells (r = 0.278, P = 0.005, B = 0.148), and CD8+CD28− cells (r = 0.384, P < 0.001, B = 0.519) were all positively correlated with aging in males. In contrast, a decreasing trend in the percentages of CD4+CD28+ cells (r = −0.311, P = 0.002, B = −0.473) and CD4+45RA+ cells (r = −0.223, P = 0.026, B = −0.187) was found to be associated with aging in males. Correlation analysis between proportions of T-cell subsets and age is shown in Figure 2.
Figure 2.
Correlation analysis between proportions of T-cell subsets and age. (a–e) Correlation between age and proportions of CD8+ cells, CD4+CD28+ cells, CD4+CD28− cells, CD4+CD45RA+ cells, and CD8+CD28− cells. Red indicates females, and blue indicates males.
3.4. Multiple linear regression analysis
Table 4 features the multivariate linear regression showing independent predictor variables for aging, including PASE scores (B = −0.06, P < 0.001), history of hypertension (B = 3.37, P = 0.012), the percentages of total T cells (B = −0.12, P = 0.018), and CD8+CD28− cells (B = 0.24, P < 0.001). Further adjusted for sex, BMI, hospitalization duration, smoking, drinking, chronic medical illness, and medications at admission, PASE scores (B = −0.07, P < 0.001), history of hypertension (B = 6.51, P = 0.024), and the percentage of CD8+CD28− cells (B = 0.20, P < 0.001) were still independent predictors.
Table 4.
Association between T-cell subsets, clinical data, and age using multivariable linear regression analysis
| Biomarker | Model 1 | Model 2 | ||
|---|---|---|---|---|
| B (95% CI) | P-value | B (95% CI) | P-value | |
| PASE scores | −0.06 (−0.08 to –0.03) | <0.001 | −0.07 (−0.09 to –0.04) | <0.001 |
| Hypertension | 3.37 (0.77 to 5.97) | 0.012 | 6.51 (0.86 to 12.16) | 0.024 |
| T cells (CD3+CD45+)/lymphocytes (%) | −0.12 (−0.22 to–0.02) | 0.018 | −0.11 (−0.21 to 0.01) | 0.052 |
| CD8+CD28− cells/T cells (%) | 0.24 (0.13 to 0.36) | <0.001 | 0.20 (0.08 to 0.32) | <0.001 |
Model 1: results of stepwise multiple linear regression; model 2: further adjusted for sex, BMI, hospitalization duration, smoking, drinking, chronic medical illness, and medications at admission. Statistically significant P-values are marked in bold. BMI, body mass index. PASE, physical activity scale for the elderly.
4. Discussion
By 2050, the world population aged 60 years and older will account for approximately 22%, more than double the 10% of the elderly population in 2010 [22]. As the aging population rapidly grows, the complex physiological process of aging leads to a serious increase in the social medical burden [22,23]. In this study, we measured T-cell subpopulations to identify aging-associated factors in elderly individuals and found that the percentage of CD8+CD28− cells was an independent predictor.
In accordance with previous studies, our results demonstrated that indicated alterations in CD4+ T-cell subsets with aging characterized by a decline in the expression of CD28 and CD45RA [24]. No significant differences in the absolute counts of T-cell subsets were observed between the youngest-old group and the middle-old group, which demonstrated a significant T-cell exhaustion in the elderly patients over 80 years old. However, the differences between the three age groups cannot determine when changes in T-cell markers start and further studies are necessary. Although our results showed no statistically significant correlation between the majority of T-cell subsets and age in the female elderly, similar trends were apparent in the male elderly. Our multivariate regression analysis was performed to adjust for the skewness in the data.
There was no significant difference in the expression of CD45RA on CD8+ T cells between the three age groups in this study. These findings indicated that the proliferation ability of CD4+ cells might be impaired more significantly with age than that of CD8+ cells in the elderly population [25]. It has been proposed that T cells can be divided into naive T cells (CCR7+CD45RA+), central memory T cells (CCR7+CD45RA−), effector memory T cells (CCR7−CD45RA−), and terminally differentiated T cells (CCR7−CD45RA+). The expression of CCR7 was not measured in our samples, which requires investigation in the future.
With increasing age, healthy individuals usually experience a loss of CD28 expression [26]. Similar to previous results [26,27], the percentages of CD8+CD28− cells and CD4+CD28− cells were highest in the oldest-old group and then followed by the middle-old group, while the youngest-old group was lowest. Lack of CD28 expression could inhibit T-cell survival and cytokine production in elderly individuals [11,28]. In addition, the results of multiple linear regression showed that CD8+CD28− was an independent index for predicting age. Regarding the correlations between age and proportions of T-cell subsets, the highest coefficient B for the percentages of CD8+CD28− cells indicates that this subpopulation has increased with aging at the fastest pace (Figure 2e). To date, CD8+CD28− T-cell alterations have been reported to be a strong contributor to age-related diseases such as diabetes [29], cardiovascular diseases [30], and chronic infections [31].
This present study indicated that the absolute count of CD4+CD25+CD127dim+ cells in the oldest-old group was significantly lower than that in the youngest-old group and the middle-old group, which was similar to previous reports [32]. Autoimmune diseases and inflammatory disorders such as rheumatoid arthritis [33], systemic lupus erythematosus [34], and coronavirus disease 2019 (COVID-19) infection [35] can also decrease CD4+CD25+CD127dim+ cells. The gradually decreased count of CD4+CD25+CD127dim+ cells might exacerbate dysregulation of immune homeostasis and impact the progression of diseases [36,37]. Further investigation into the role of CD4+CD25+CD127dim+ cells in elderly individuals is warranted.
Alterations in T-cell subsets with aging may lead to the development of chronic diseases and frailty status [38,39], and several lifestyle modifications can prevent or slow down the age-related decline in health [40]. Previous studies demonstrated that exercise induced increased expression of CD28 and CD45RA on the T-cell surface [41,42]. Vitamin supplementation could affect the levels of CD28+ T-cell subsets [43,44]. Caloric restriction has been shown to attenuate the age-related shift from naive to memory T cells in aged animals [45]. Regular physical exercise and appropriate nutritional interventions could help older populations avoid immune-related diseases, which merits further investigation.
The present study focused on the alteration of T-cell subsets with age in older people. There are some limitations to this study. First, the ratio of women to men in the included individuals stood at 1:4. Second, our analysis was based on a single-center retrospective study. Third, due to the retrospective design, CCR7 and cytokines cannot be complemented for detection and need further exploration. Finally, the major limitation of this study is the inability to provide isotype controls and FMO controls.
5. Conclusions
The present study suggests that aging in the elderly population is accompanied by a decrease in the count of T-cell subsets, characterized by a decline in the expression of CD28 and CD45RA. Furthermore, CD8+CD28− cells could be a useful factor in the age prediction model. This study reports the correlation between T-cell subpopulations and age among elderly individuals, which could be used to guide immunological evaluation for anti-aging strategies.
Acknowledgements
We are extremely grateful to all participants who contributed to this study.
Appendix
Figure A1.
Gating strategies for flow cytometry.
Footnotes
Funding information: This work is supported by the Guangdong Basic and Applied Basic Research Foundation (2021A1515220123) and National Key R&D Program of China (2020YFC2008000).
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Qing Zhang, Email: qingzhang196@163.com.
Cun-Tai Zhang, Email: ctzhang0425@163.com.
References
- [1].Fan ZY, Yang Y, Zhang CH, Yin RY, Tang L, Zhang F, et al. Prevalence and patterns of comorbidity among middle-aged and elderly People in China: A cross-sectional study based on CHARLS data. Int J Gen Med. 2021;14:1449–55. [DOI] [PMC free article] [PubMed]
- [2].Ramasubramanian R, Meier HCS, Vivek S, Klopack E, Crimmins EM, Faul J, et al. Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the health and retirement study. Immun Ageing. 2022;19(1):33. [DOI] [PMC free article] [PubMed]
- [3].Xu K, Miao L, Chen W, Wu H, Gong Y, Tu X, et al. Establishment of the reference intervals of lymphocyte subsets for healthy Chinese Han adults and its influencing factors. Ann Transl Med. 2021;9(19):1495. [DOI] [PMC free article] [PubMed]
- [4].Aiello AE, Feinstein L, Dowd JB, Pawelec G, Derhovanessian E, Galea S, et al. Income and markers of immunological cellular aging. Psychosom Med. 2016;78(6):657–66. [DOI] [PMC free article] [PubMed]
- [5].Li M, Yao D, Zeng X, Kasakovski D, Zhang Y, Chen S, et al. Age related human T cell subset evolution and senescence. Immun Ageing. 2019;16:24. [DOI] [PMC free article] [PubMed]
- [6].Sun X, Nguyen T, Achour A, Ko A, Cifello J, Ling C, et al. Longitudinal analysis reveals age-related changes in the T cell receptor repertoire of human T cell subsets. J Clin Invest. 2022;132(17):e158122. [DOI] [PMC free article] [PubMed]
- [7].Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. 2017;8(1):1473. [DOI] [PMC free article] [PubMed]
- [8].Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012;24(4):476–81. [DOI] [PubMed]
- [9].Grant EJ, Nussing S, Sant S, Clemens EB, Kedzierska K. The role of CD27 in anti-viral T-cell immunity. Curr Opin Virol. 2017;22:77–88. [DOI] [PubMed]
- [10].Molon B, Liboni C, Viola A. CD28 and chemokine receptors: Signalling amplifiers at the immunological synapse. Front Immunol. 2022;13:938004. [DOI] [PMC free article] [PubMed]
- [11].Vallejo AN. CD28 extinction in human T cells: Altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–69. [DOI] [PubMed]
- [12].Perry DJ, Titov AA, Sobel ES, Brusko TM, Morel L. Immunophenotyping reveals distinct subgroups of lupus patients based on their activated T cell subsets. Clin Immunol. 2020;221:108602. [DOI] [PMC free article] [PubMed]
- [13].Huang M, Wang Y, Ye J, Da H, Fang S, Chen L. Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19). Ann Transl Med. 2020;8(18):1145. [DOI] [PMC free article] [PubMed]
- [14].Jablonka A, Etemadi H, Adriawan IR, Ernst D, Jacobs R, Buyny S, et al. Peripheral Blood Lymphocyte Phenotype Differentiates Secondary Antibody Deficiency in Rheumatic Disease from Primary Antibody Deficiency. J Clin Med. 2020;9(4):1049. [DOI] [PMC free article] [PubMed]
- [15].Yu Z, Li G, Yu H, Asakawa T. Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study. Open Life Sci. 2021;16(1):1002–9. [DOI] [PMC free article] [PubMed]
- [16].Xu W, Larbi A. Immunity and Inflammation: From Jekyll to Hyde. Exp Gerontol. 2018;107:98–101. [DOI] [PubMed]
- [17].Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: A key player in cancer development. J Hematol Oncol. 2020;13(1):151. [DOI] [PMC free article] [PubMed]
- [18].Shirakawa K, Sano M. T Cell Immunosenescence in Aging, Obesity, and Cardiovascular Disease. Cells. 2021;10(9):2435. [DOI] [PMC free article] [PubMed]
- [19].Tylutka A, Morawin B, Gramacki A, Zembron-Lacny A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 2021;21(1):200. [DOI] [PMC free article] [PubMed]
- [20].Muller L, Pawelec G. Aging and immunity - impact of behavioral intervention. Brain Behav Immun. 2014;39:8–22. [DOI] [PubMed]
- [21].Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. [DOI] [PubMed]
- [22].Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205(10):1589–92. [DOI] [PubMed]
- [23].Williams A. An aging population--burden or blessing? Value Health. 2005;8(4):447–50. [DOI] [PubMed]
- [24].Le Page A, Dupuis G, Larbi A, Witkowski JM, Fulop T. Signal transduction changes in CD4(+) and CD8(+) T cell subpopulations with aging. Exp Gerontol 2018; 105:128–139. [DOI] [PubMed]
- [25].Nguyen THO, Sant S, Bird NL, Grant EJ, Clemens EB, Koutsakos M, et al. Perturbed CD8(+) T cell immunity across universal influenza epitopes in the elderly. J Leukoc Biol. 2018;103(2):321–39. [DOI] [PubMed]
- [26].Lazuardi L, Herndler-Brandstetter D, Brunner S, Laschober GT, Lepperdinger G, Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+CD28- T cells from young and elderly persons, but not of CD8+CD28+ T cells. Biogerontology. 2009;10(2):191–202. [DOI] [PubMed]
- [27].Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. 2020;134:110887. [DOI] [PubMed]
- [28].Ramaswamy M, Kim T, Jones DC, Ghadially H, Mahmoud TI, Garcia A, et al. Immunomodulation of T- and NK-cell responses by a bispecific antibody targeting CD28 homolog and PD-L1. Cancer Immunol Res. 2022;10(2):200–14. [DOI] [PubMed]
- [29].Lee YH, Kim SR, Han DH, Yu HT, Han YD, Kim JH, et al. Senescent T Cells predict the development of hyperglycemia in humans. Diabetes. 2019;68(1):156–62. [DOI] [PubMed]
- [30].Kazem N, Sulzgruber P, Thaler B, Baumgartner J, Koller L, Laufer G, et al. CD8+CD28null T lymphocytes are associated with the development of atrial fibrillation after elective cardiac surgery. Thromb Haemost. 2020;120(8):1182–7. [DOI] [PubMed]
- [31].Yu X, Lin Y, Chen H, Wu MJ, Huang LN, Song YY, et al. Distribution and clinical significance of circulating CD8(+)CD28(-) regulatory T cells in the peripheral blood of patients with pulmonary tuberculosis. BMC Pulm Med. 2022;22(1):291. [DOI] [PMC free article] [PubMed]
- [32].Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48(12):1379–86. [DOI] [PubMed]
- [33].Go E, Yoo SJ, Choi S, Sun P, Jung MK, Kwon S, et al. Peripheral Blood from Rheumatoid Arthritis Patients Shows Decreased Treg CD25 Expression and Reduced Frequency of Effector Treg Subpopulation. Cells. 2021;10(4):801. [DOI] [PMC free article] [PubMed]
- [34].Li M, Luo L, Wu Y, Song Z, Ni B, Hao F, et al. Elevated apoptosis and abnormal apoptosis signaling of regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2022;31(12):1441–55. [DOI] [PubMed]
- [35].Liu K, Yang T, Peng XF, Lv SM, Ye XL, Zhao TS, et al. A systematic meta-analysis of immune signatures in patients with COVID-19. Rev Med Virol. 2021;31(4):e2195. [DOI] [PMC free article] [PubMed]
- [36].Zhao Y, Tang Y, Qin H, Feng K, Hu C. The efficient circulating immunoscore predicts prognosis of patients with advanced gastrointestinal cancer. World J Surg Oncol. 2022;20(1):233. [DOI] [PMC free article] [PubMed]
- [37].Pan Y, Wang H, An F, Wu F, Tao Q, Li Y, et al. CD4(+)CD25(+)CD127(low) regulatory T cells associated with the effect of CD19 CAR-T therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Int Immunopharmacol. 2021;96:107742. [DOI] [PubMed]
- [38].Jiang CH, Zhu F, Qin TT. Relationships between chronic diseases and depression among middle-aged and elderly People in China: A prospective study from CHARLS. Curr Med Sci. 2020;40(5):858–70. [DOI] [PubMed]
- [39].Zhang H, Weyand CM, Goronzy JJ, Gustafson CE. Understanding T cell aging to improve anti-viral immunity. Curr Opin Virol. 2021;51:127–33. [DOI] [PMC free article] [PubMed]
- [40].Pataky MW, Young WF, Nair KS. Hormonal and metabolic changes of aging and the influence of lifestyle modifications. Mayo Clin Proc. 2021;96(3):788–814. [DOI] [PMC free article] [PubMed]
- [41].Cao Dinh H, Beyer I, Mets T, Onyema OO, Njemini R, Renmans W, et al. Effects of physical exercise on markers of cellular immunosenescence: A systematic review. Calcif Tissue Int. 2017;100(2):193–215. [DOI] [PubMed]
- [42].Vasconcelos ABS, Aragao-Santos JC, de Resende-Neto AG, Rodrigues LS, Correa CB, Schimieguel DM, et al. Effects of functional and combined training on subsets of memory T cells and functional fitness of postmenopausal women: A randomized controlled trial. Exp Gerontol. 2022;167:111898. [DOI] [PubMed]
- [43].Jankowska M, Marszall M, Debska-Slizien A, Carrero JJ, Lindholm B, Czarnowski W, et al. Vitamin B6 and the immunity in kidney transplant recipients. J Ren Nutr. 2013;23(1):57–64. [DOI] [PubMed]
- [44].Zhou Q, Li L, Chen Y, Zhang J, Zhong L, Peng Z, et al. Vitamin D supplementation could reduce the risk of acute cellular rejection and infection in vitamin D deficient liver allograft recipients. Int Immunopharmacol. 2019;75:105811. [DOI] [PubMed]
- [45].Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: Caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40(11):884–93. [DOI] [PubMed]