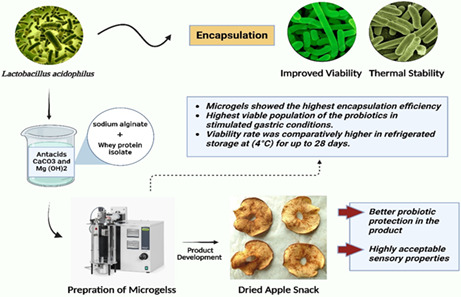
Abstract
In the current study, Lactobacillus acidophilus was encapsulated in sodium alginate and whey protein isolate, with the addition of antacids CaCO3 or Mg(OH)2. The obtained microgels were observed by scanning electron microscopy. Encapsulated and free probiotics were subjected to vitality assay under stressed conditions. Furthermore, dried apple snack was evaluated as a carrier for probiotics for 28 days. A significant (p ≤ .05) effect of antacid with an encapsulating agent was observed under different stressed conditions. During exposure to simulated gastrointestinal conditions, there were observations of 1.24 log CFU and 2.17 log CFU, with corresponding 0.93 log CFU and 2.63 log CFU decrease in the case of SA + CaCO3 and WPI + CaCO3 respectively. Likewise, high viability was observed under thermal and refrigerated conditions for probiotics encapsulated with SA + CaCO3. In conclusion, the results indicated that alginate microgels with CaCO3 are effective in prolonging the viability of probiotics under stressed conditions.
Keywords: antacid, apple snack, encapsulation, gastroprotective microgels, probiotics, viability
The addition of antacids (CaCO3 or Mg (OH)2) has a key role in protecting encapsulated probiotics under stressed conditions. The production of dried apple snacks with encapsulated probiotics is appealing for improving the human health. Microencapsulation with gastro protective is a versatile technique for the production of functional foods.
1. INTRODUCTION
Probiotics can influence and enhance the microbiota of the host (Omar et al., 2013), by boosting immunity and inhibit the pathogens to grow (Tripathi & Giri, 2014). Thus, they provide abundant health‐promoting benefits that includes improving overall immunity, lowering serum cholesterol, and improving lactose intolerance (Agrawal, 2005). To obtain the desired results, probiotic foods must contain at least 106–107 CFU/g live cells at the moment of ingestion (Terpou et al., 2019). Numerous factors, such as oxidative stress, drying process, and storage temperature, can have an impact on probiotic viability throughout food preparation and storage stages (Fiocco et al., 2020). Furthermore, the majority of the probiotics should remain intact during their transit through the gastrointestinal path until they reach the specific sites where they exhibit therapeutic effects (Doherty et al., 2012; Iqbal et al., 2021).
Microencapsulation has received a great deal of study to present (Chen et al., 2017). Probiotic microencapsulation not only protects the probiotic cells from harsh exterior environment but also allows the cells for their controlled release at specific places (Awuchi et al., 2022; Kia et al., 2018). Evidence has suggested that the survival chances of microencapsulated or un‐encapsulated probiotic cells during simulated gastric and intestinal circumstances depend on the strain (Oguntoye et al., 2021). Encapsulation methods employing macro, micro, and nano materias have been proven to be of great significance in improving probiotic utilization (Islam, Noman, et al., 2022; Islam, Saeed, et al., 2022; Morya et al., 2022). In addition, the shielding effect varies with different wall materials, microencapsulation techniques, and artificial intestinal and gastric conditions (Anitha & Sellamuthu, 2021; Rafiq et al., 2022; Xing et al., 2015). More systematic research is needed to offer more information regarding the protective effects of microencapsulation on probiotics.
Bifidobacterium longum entrapped in alginate hydro beads with chitosan layer showed an enhanced survivability after transit through simulated digestive conditions (Yeung et al., 2016). However, limited studies have shown that colloidal delivery methods may reliably sustain the viability of probiotics even after exposure of 2 h under gastric environment (Yeung et al., 2016). The major purpose behind the poor gastric viability of microgel encapsulated probiotics is due to the inactivated bacteria in which hydrogen ions were diffused through the hydrogel network. Zheng et al. (2017) prepared alginate microbeads that contained antacid Mg(OH)2, which could neutralize hydrogen ions and therefore maintain a neutral internal pH even when the microgels were inoculated in gastric conditions. These antacid‐loaded microgels are therefore useful for enhancing the probiotic cell viability through oral route (Yao et al., 2017).
In the current study, probiotic bacterial strain, Lactobacillus acidophilus, was encapsulated in sodium alginate and whey protein isolate microgels containing two different types of antacids, CaCO3, and Mg (OH)2. Afterward, the encapsulated microgels were added to dried apple snacks and observed for probiotic viability. The obtained results from this study provided valuable material for improving the performance of probiotic‐loaded delivery systems for various applications in functional foods and beverages.
2. MATERIALS AND METHODS
2.1. Experimental study
The glassware was purchased from Thermofisher, USA. Encapsulating materials and chemicals were purchased from Merck, USA. The freeze‐dried culture of L. acidophilus (ATTCC 8826) was obtained from the National Institute of Food Science & Technology (NIFSAT), University of Agriculture Faisalabad, Pakistan. Apples (variety Kala Kulu) were purchased from Jhang bazar market of Faisalabad (Pakistan). The laboratory equipments were available in different laboratories of the Department of Food Sciences, Government College University Faisalabad, and were used for research purposes.
2.2. Activation of bacterial culture
Activation of freeze‐dried cell culture was done by following the method of Afzaal, Saeed, Ateeq, et al. (2020); Afzaal, Saeed, Hussain, et al. (2020); Afzaal, Saeed, Saeed, et al. (2020), with slight modifications. Solution of Man, Rogosa, and Sharpe agar (M.R.S agar, LAB093; Lab M Limited) was prepared by adding 70 g of agar in 1 L distilled water. The media was dissolved and autoclaved and plates were prepared for propagation of L. acidophilus (ATTCC 8826). Bacterial culture was incubated in an anaerobic environment at 37°C for 24 h using an incubator (BC‐5501; Memmert). Afterward, the obtained cells were centrifuged (750286 EA; Thermo Fisher Scientific Inc.) at 4000 rpm at 4°C for 10–15 min and the media was decanted. Cells were again suspended in freshly made MRS media and incubation (37°C) was done for an additional 20 h. The cells were harvested, weighed in, and data were recorded. The cell concentration was adjusted at 1010 CFU/ml.
2.3. Probiotic microencapsulation
2.3.1. Whey protein isolate microgels preparation
Cells obtained by centrifugation (4000 rpm for 10–15 min) and washed thoroughly using sterilize peptone (15 ml) and afterward washed with 22 ml of aseptic distilled water. Probiotic microgels containing antacids were prepared by mixing concentrated L. acidophilus cells (5 ml) with 30 g whey protein isolate (WPI) powder in the presence of an antacid (CaCO3 or Mg (OH)2) (2:2, v/v) (Mehra et al., 2021; Wang et al., 2022). A volume of 125 ml sunflower oil was added to the WPI solution, and both the antacids CaCO3 and Mg (OH)2 (2:2, v/v) were added in the solution separately. Afterward, it was subjected to the preparation of microgels using an encapsulator (Büchi B‐3910 Encapsulator). Microencapsulation of L. acidophilus cells was performed as described by Wang et al. (2022) and Mehra et al. (2021), with minor modifications. An injection nozzle having 180–200 μm diameter was and following operating conditions were followed: vibration frequency = 750 Hz, driving pressure = 500 mbar and electrode potential = 750 V. The samples were collected in a 10% (w:v) calcium chloride solution for microbead formation.
2.3.2. Preparation of alginate microgels
Alginate microgels were prepared by adopting the injection‐gelation method (Zhang, 2020) with some minor amendments. For encapsulation, the cells obtained by centrifugation were washed thoroughly with sterile peptone water (15 ml) and then rewashed twice with 22 ml of distilled water. Microbeads were formed by mixing 5 ml of L. acidophilus cell suspension in 2% w/v sodium alginate solution (200 ml) and both the antacids CaCO3 and Mg (OH)2 at a ratio of 2:2 v/v were added separately. Afterward, buffer solution of phosphate (pH 7) was added dropwise in order to adjust the final pH of the solution and stirred for 60 min at 50°C. Furthermore, the temperature was lowered to 35°C with constant stirring till a uniform solution was obtained. Microgels were obtained by injecting the mixture through a nozzle (180–200 μm diameter) using an encapsulator (Büchi B‐3910 Encapsulator; Flawil). The conditions used to obtain microgels were: 750 Hz, driving pressure = 500 mbar and electrode potential = 750 V. Antacid‐loaded microgels were then held in the calcium solution (0.05 ml) for 15 min at room temperature before being removed to promote hardening of gels. The obtained microgels were filtered and washed twice using sterilized distilled water.
After the preparation of beads from both types of antacids and encapsulating materials, the treatments were given the names, SA + Mg (OH)2 and SA + CaCO3 beads prepared from sodium alginate with both antacids. WPI + Mg (OH)2 and WPI + CaCO3 that were formed from Whey Protein Isolate with both antacids (Mg (OH)2 and CaCO3). Free cells of L. acidophilus were given the name Fc.
2.4. Characterization of encapsulated whey protein isolate and alginate microgels
2.4.1. Particle size determination
The particle size of gastroprotective microbeads was examined immediately following microencapsulation using a compound microscope (Mastersizer S; Malvern Instruments), to make sure that beads were of the correct size and shape.
2.4.2. Scanning electron microscopy (SEM)
A high‐resolution scanning electron microscope (Cube series‐. Emcraft South Korea) available at the physics department‐GCUF was used to collect the micrograph. The prepared microbeads were subjected to structural morphology determination as described by Yao et al. (2017) with slight modification.
2.5. Encapsulation efficiency
The encapsulation efficiency was calculated by adopting the method of Afzaal, Saeed, Ateeq, et al. (2020). The microbeads beads of sodium alginate and whey protein isolate loaded with antacids Mg(OH)2 or CaCO3 were taken randomly, and disintegration of the beads was done using a stomacher with the pour plate technique, the number of cells released was measured. The findings were expressed as units/bead (CFU/bead) forming several colonies. Using the following formula, the importance of encapsulation efficiency was evaluated:
2.6. Survival of un‐encapsulated and encapsulated probiotics in gastrointestinal fluids
Using the approach as defined by Gu et al. (2019), free and encapsulated (sodium alginate and whey protein isolate) microgels were evaluated in simulated gastrointestinal conditions. Particularly, simulated gastric fluid (SGF) was prepared with the addition of sodium chloride (2 g), 6 M hydrochloric acid (7 ml) into 1000 ml distilled water and sterilized to ensure aseptic conditions. Simulated intestinal juice (SIJ) was prepared by dissolving sodium chloride (3.75 M) and calcium chloride (0.25 M) in phosphate buffer (pH 7). Prepared simulated solutions were subjected to autoclave for an aseptic environment before the experiment. Free and encapsulated microgels were consecutively added to SGJ and SIF for 120 min. The survival of unencapsulated and encapsulated probiotics was reported over a time interval of 0, 30, 60, 90, and 120 min. The final readings were noted.
2.7. Analysis of free and encapsulated microgels under heat treatment
The feasibility of L. acidophilus encapsulated microgels and free probiotics was determined by subjecting them to elevated temperature following the procedure of Fang and Bhandari (2012) with some minor amendments. Free cell and encapsulated cells of L. acidophilus (1010 CFU) were inoculated in test tubes having 9 ml saline solution (1% w/v). Additionally, the test tubes were incubated for 10 min in water bath at 63°, 65°, and 72°C. Subsequently after incubation, the test tubes were cooled down to normal room temperature (∼25°C). The viability of the unencapsulated and encapsulated microgels of L. acidophilus was then assessed by spread plate method using MRS agar as a growth medium at 37°C in an incubator (BC‐5501; Memmert) for 24 h.
2.8. Viability of free and encapsulated microgels during refrigeration storage
For the analysis of probiotic resistance against refrigeration temperature, the process of Lemos Junior et al. (2020) and Terpou et al. (2019) were followed with slight modification. The viability of L. acidophilus under refrigeration storage was evaluated by incubating 0.4 ml (approximately 9.5 log CFU/g) of free and encapsulated cells in 1.8 ml of germ‐free sodium chloride solution (0.5%, w/v) and kept in the refrigerator at 4°C for 28 days.
2.9. Product development
Dried apple snacks were prepared by adopting the technique as previously reported by Afzaal, Saeed, Ateeq, et al. (2020); Afzaal, Saeed, Hussain, et al. (2020); Afzaal, Saeed, Saeed, et al. (2020). First, apples were washed using normal tap water ensuring that dirt particles were removed. Peeling was done using a peeler and apples were sliced (diameter 9 mm, width 6 mm). Afterward, apples were blanched using water bath at 75°C for 2–3 min to prevent apples from enzymatic browning.
Lactobacillus acidophilus as a probiotic in both free and encapsulated form, ranging from 9.5–10 log CFU/g were added in sterile peptone water (∼1%), and apple slices were immersed in an aqueous solution (2:4 apple/solution ratio w/v) for 10 min at a room temperature and with constant stirring. Furthermore, apples in peptone water solution were left in a controlled environment for 10–15 min ensuring proper probiotics attachment on apple surface. The apples were then separated from the solution and were dried in a conventional oven (Westpoint Oven‐WF‐4800) at 38–40°C for a maximum of 40–50 min. The dried apple snack obtained was cooled down to ambient room temperature (∼25°C) in a desiccator for 20 min and afterward, stored in airtight food grade packages for further storage. Dried apple snack was stored for 28 days of storage study at 4°C in food graded storage bags and analysis was conducted at an interval of 7 days. The treatments for dried apple snack were named as “AS (SA + Mg (OH)2)” and “AS (SA + CaCO3)” dried apple snack having sodium alginate capsules with antacids. The dried apple snack encapsulated with whey protein isolate were given the names as “AS (WPI + Mg (OH)2)” and “AS (WPI + CaCO3).” However, a controlled sample of dried apple snack was quoted as “AS” and dried apple snack with free cells of L. acidophilus was named as “ASFC.”
2.10. Determination of pH of apple snack
The pH of apple snacks was determined by a digital pH meter following AOAC (2009). Apple snack was immersed in peptone water and mixed well before determining the pH. Readings were noted as a mean of three replicates.
2.11. Probiotic enumeration
The viability of probiotics in dried apple snack treatments was determined as described by Nualkaekul et al. (2012). Samples of dried apple snacks were stored at 4°C and were analyzed after an interval of 0, 7, 14, 21, and 28 days. Shortly, all the samples were diluted with deionized water and spread on MRS medium. The Petri plates were incubated at 37°C for a duration of 48 h. The viable cell count was calculated. MRS media and glassware used for viability assessment of probiotics were completely sterilized using the autoclave and hot air oven. After preparation of media, pouring was done in sterilized Petri plates. After complete dilution, the samples were transferred to Petri plates with the help of micro‐dispenser and Petri dishes were incubated for growth.
2.12. Sensory analysis
Sensory analysis was performed by following the procedure of Ranadheera et al. (2012) and Amagwula et al. (2022). The sensory panel included 30 experts (15 female, 15 male) within 20–40 years of age. Participants were asked to evaluate the product (dried apple snack) based on overall liking, color, flavor, mouthfeel, and texture using a 9‐point hedonic scale: 1 = dislike extremely and 9 = like extremely.
2.13. Statistical analysis
Results from all the technological and physicochemical characteristics of the encapsulated microgels and dried apple snacks were taken in triplicate. All the collected data were expressed as mean ± SD. ANOVA was applied to all the collected data using Statistix10.
3. RESULTS AND DISCUSSIONS
3.1. Particle size determination
Two different types of materials CaCO3 and Mg(OH)2 were used along with sodium alginate and whey protein isolate (WPI). Probiotic bacteria were entrapped in these solutions and their particle size was determined as shown in Table 1. The particle size of SA + CaCO3 antacid was observed to be the greatest (621 mm) while the particle size of SA + Mg (OH)2 was 618 mm. However, the particle size of WPI + Mg (OH)2 and WPI+ CaCO3 was 550 mm and 543 mm, respectively. From the data, it is evident that the size of CaCO3 loaded microgels was greater than the Mg (OH)2 microgels. This may be because the solution containing CaCO3 had a greater viscosity than the Mg (OH)2 solution. The same reasons were also suggested by Smidsrød and Skja (1990). Similar studies have also been reported. Awuchi et al. (2019) reported particle size of grains that can be used for microencapsulation.
TABLE 1.
Size of prepared beads (mean ± STD)
| Beads | Size (μm) |
|---|---|
| SA + Mg (OH)2 | 618 ± 0.03 |
| SA + CaCO3 | 621 ± 0.14 |
| WPI + Mg (OH)2 | 550 ± 0.08 |
| WPI + CaCO3 | 543 ± 1.11 |
3.2. Encapsulation efficiency
The mean results obtained for encapsulation efficiency of L. acidophilus is shown in Table 2. From the results, it can be observed that the microgels with SA + CaCO3 showed the highest encapsulation efficiency (95.92%) while WPI + CaCO3 was 89.43% efficient. However, the encapsulation efficiency of SA + Mg (OH)2 was 86.27% while WPI + Mg(OH)2 showed 93.72% efficiency. It was observed that the particle size was influenced by the temperature, viscosity, and concentration of the polymers used along with the encapsulating procedures (Krasaekoopt et al., 2003).
TABLE 2.
Encapsulation efficiency
| Beads | Initial count (before encapsulation) (Log CFU/g) | Final count (after encapsulation) (Log CFU/g) | % Efficiency |
|---|---|---|---|
| SA + Mg (OH)2 | 9.54 ± 0.27 | 8.23 ± 0.01 | 86.27 |
| SA + CaCO3 | 9.56 ± 0.21 | 9.17 ± 0.06 | 95.92 |
| WPI + Mg (OH)2 | 9.55 ± 0.34 | 8.95 ± 0.02 | 93.72 |
| WPI + CaCO3 | 9.55 ± 0.28 | 8.54 ± 0.01 | 89.43 |
3.3. Scanning electron microscopy (SEM)
The detailed microscopy of the microbeads was carried out using SEM. Obtained micrographs are shown in Figure 1. Micrographs showed that antacid‐containing microbeads have an irregular appearance because of antacid particles present in it. The results of the study are in line with the findings of Min Gu et al. (2019) who observed irregular structure in the case of microgels containing antacid. Probiotics were detected in both types of encapsulated microbeads having Mg (OH)2 and CaCO3 antacids.
FIGURE 1.
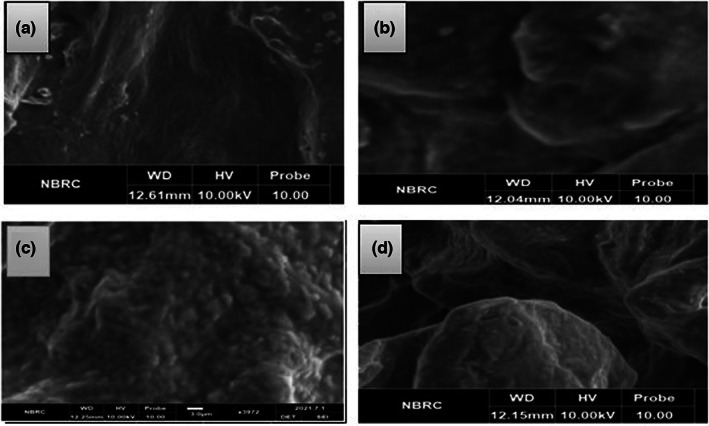
Scanning electron micrographs (a) SA + Mg(OH)2 (Sodium alginate microgels with Mg(OH)2), (b) SA + CaCO3 (Sodium alginate microgels with CaCO3), (c) WPI + Mg(OH)2 (whey protein isolate microgels with Mg(OH)2), (d) WPI + CaCO3 (whey protein isolate microgels with CaCO3)
3.4. Viability of free and encapsulated probiotics under simulated digestion
The probiotic load is reduced to a great extent while passing through the gastro‐intestinal environment and due to this reason their survival in the human gut becomes more difficult (Nazzaro et al., 2012). Therefore, the effect of probiotic microgels with both antacid Mg (OH)2 and CaCO3 was exposed to the simulated gastric and intestinal environment and the mean results were obtained. All the results showed a significant declining trend as shown in Figures 2 and 3. When results for simulated gastric conditions were observed, it showed that the treatment SA + CaCO3 had the highest viable population of the probiotics (8.31 log CFU) while the control sample Fc had the least probiotic survival rate. The WPI antacid microgels, however, showed lower stability than the SA antacid microgels. A log 1.24 CFU/g and log 2.17 CFU/g reduction were noted in the case of Microgels having CaCO3 antacid while a log 1.79 CFU and log 2.42 CFU decrease was observed in the case of Mg (OH)2 antacids. However, a decline of 3.43 log CFU/g was calculated in the control sample.
FIGURE 2.
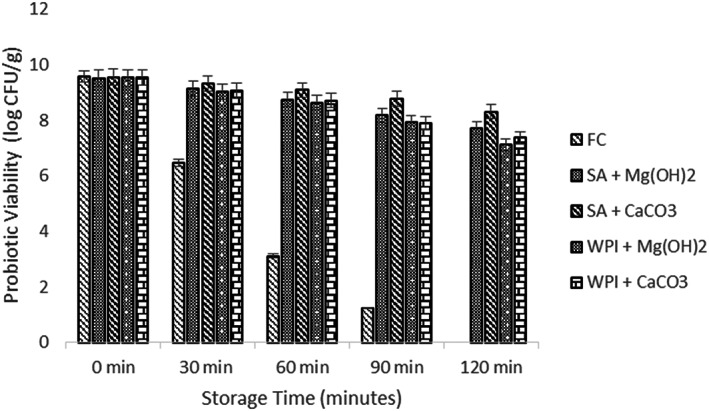
Viability of free and encapsulated (SA and WPI having Mg (OH)2 and CaCO3 antacids) probiotic microgels under simulated gastric conditions during storage intervals (0, 30, 60, 90, and 120 min) compared with control. Each bar represents the mean value for viability of treatments. Fc (un‐encapsulated probiotics), SA + Mg (OH)2 (Sodium alginate microgels with Mg (OH)2), SA + CaCO3 (Sodium alginate microgels with CaCO3), WPI + Mg (OH)2 (whey protein isolate microgels with Mg (OH)2), and WPI + CaCO3 (whey protein isolate microgels with CaCO3).
FIGURE 3.
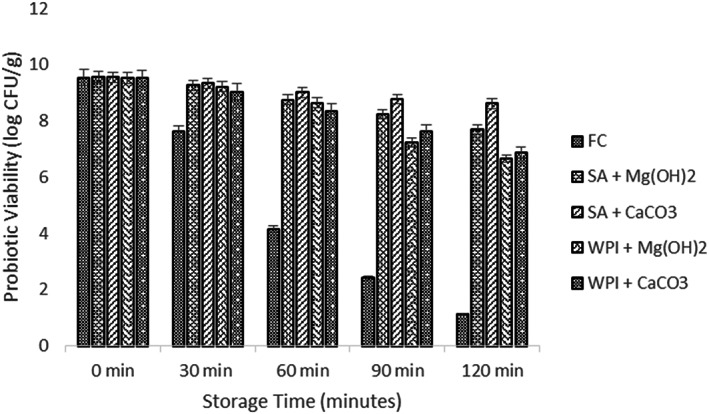
Viability of free and encapsulated (SA and WPI having Mg (OH)2 and CaCO3 antacids) probiotic microgels under simulated intestinal conditions during storage intervals (0, 30, 60, 90, and 120 min) compared with control. Each bar represents the mean value for viability of treatments. Fc (un‐encapsulated probiotics), SA + Mg (OH)2 (Sodium alginate microgels with Mg (OH)2), SA + CaCO3 (Sodium alginate microgels with CaCO3), WPI + Mg (OH)2 (whey protein isolate microgels with Mg (OH)2) and WPI + CaCO3 (whey protein isolate microgels with CaCO3).
The CaCO3 performed better than Mg (OH)2 due to the reason that the pH level of microgels that contained Mg (OH)2 was near to the neutral pH when subjected to gastric incubation. For this reason, we can also assume that there was enough SA + Mg (OH)2 microgel that neutralized the gastric juice and released the probiotics directly in the acidic environment (Zheng et al., 2017). Therefore, microgels that were loaded with CaCO3 gave satisfactory results in the gastric environment than the microgels that were loaded with Mg (OH)2.
After the simulated gastric environment, viability of probiotic microgels was determined under the simulated intestinal environment. After exposure to the simulated intestinal juice, a sharp fall was observed in the control treatment (Fc) and a log 4.11 CFU/g fall was noted after 120 min of study. The maximum peak was obtained by SA + CaCO3 while other treatment samples showed lower survival results. The difference of the mean results (initial and final) showed 0.93 log CFU/g reduction and 2.63 log CFU/g reduction in the case of SA and WPI with CaCO3 antacid. However, 1.87 log CFU/g and 2.88 log CFU/g decline were determined for SA and WPI with Mg (OH)2.
The hidden fact involved in the protective properties of both antacids Mg (OH)2 and CaCO3 is still not clear and will receive further research to reveal the facts. One of the reasons might be that the calcium ions are released at a relatively slower rate and therefore they slowly react with the bile and other digestive salts (Ruiz et al., 2013). Another possible reason could be the size of CaCO3 microgels that was greater than Mg (OH)2, so it dissolved at a slower rate than Mg (OH)2 in aqueous phase (Terpou et al., 2019; Wang et al., 2022).
3.5. Thermal resistance
As L. acidophilus can resist heat shocks at higher temperatures (Saarela et al., 2004), it can therefore be subjected to various elevated temperatures. The mean results were obtained against temperature (63, 65, and 72°C). All the results showed a significant decrease in the bacterial population as shown in Figure 4. However, the results from free probiotics (Fc) showed a sharp decline in viability under elevated temperature.
FIGURE 4.
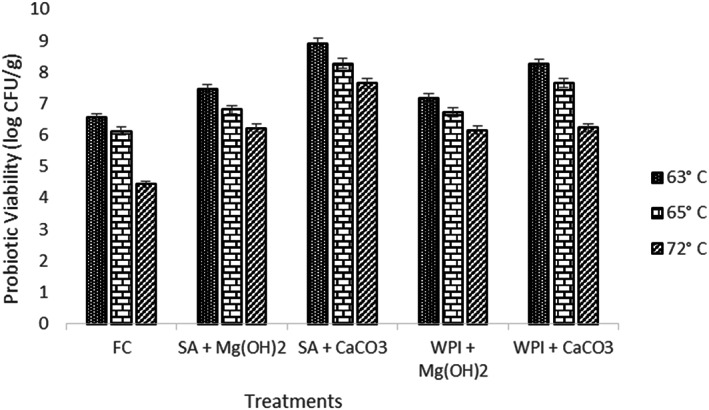
Viability of free and encapsulated (SA and WPI having Mg(OH)2 and CaCO3 antacids) probiotic microgels at elevated temperature (63, 65, and 72°C) for 10 min compared with control. Each bar represents the mean value for viability of treatments. Fc (un‐encapsulated probiotics), SA + Mg (OH)2 (Sodium alginate microgels with Mg (OH)2), SA + CaCO3 (Sodium alginate microgels with CaCO3), WPI + Mg (OH)2 (whey protein isolate microgels with Mg (OH)2), and WPI + CaCO3 (whey protein isolate microgels with CaCO3).
The decreasing trend in the probiotic population may be due to the reason that high temperatures can cause denaturation and unfolding in the structure of proteins molecules in probiotic cells and can inhibit and denature the enzymatic activity as well which causes the death of live cells of probiotics (Corcoran et al., 2008). Lian et al. (2002) suggested that the wall materials that are used for encapsulation have different physical properties and can act as a barrier against several adverse conditions.
3.6. Refrigeration storage
Probiotic microgel viabilities (free and encapsulated) were compared during refrigerated storage (4°C) for up to 28 days and mean results are shown in Figure 5. Total cell viability of these samples significantly changed during the storage time; however, the survival of the microgels between 0 day and 7 days suggests that an immediate response to the stress conditions was not induced by the process of encapsulation due to which the viability rate was comparatively higher.
FIGURE 5.
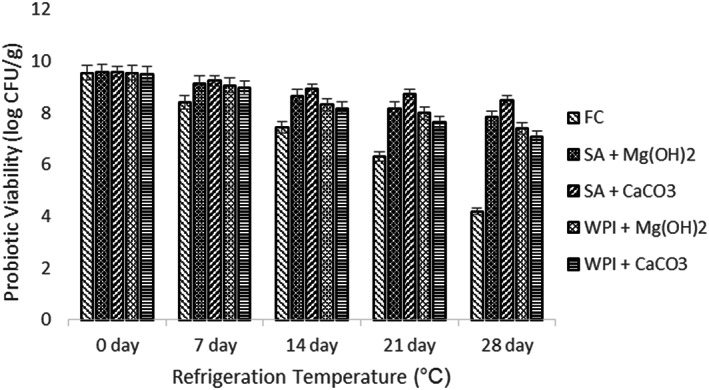
Viability of free and encapsulated (SA and WPI having Mg(OH)2 and CaCO3 antacids) probiotic microgels during refrigeration storage at 4°C during storage intervals (0, 7, 14, 21, and 28 days) compared with control. Each bar represents the mean value for viability of treatments. Fc (un‐encapsulated probiotics), SA + Mg (OH)2 (Sodium alginate microgels with Mg (OH)2), SA + CaCO3 (Sodium alginate microgels with CaCO3), WPI + Mg (OH)2 (whey protein isolate microgels with Mg (OH)2) and WPI + CaCO3 (whey protein isolate microgels with CaCO3).
When all mean results were analyzed, the highest viability was exhibited by the hydrogels that were encapsulated in SA + CaCO3 antacid solution. However, the results for the same antacid in WPI solution showed the least viability among all the antacid solutions. The control treatment Fc showed the viability under acceptable range (106 log CFU).
However, varying degrees of survival of L. plantarum were also reported in earlier studies in free and encapsulated form (Coghetto et al., 2016; Trabelsi et al., 2014). Trabelsi et al. (2014) also reported nearly 8 log CFU/ml decline for L. plantarum in free form during refrigerated storage over 35 days. However, Brinques and Ayub (2011) reported a reduction of L. plantarum BL011 population by half the initial population after about 10 days.
3.7. Analysis of dried apple snack
3.7.1. pH
Dried apple snacks containing microgels were developed and further analyses were carried out to determine the product acceptance as shown in Figure 6. As pH of food is one of the major parameters in determining food quality (Raju et al., 2020), therefore the pH of dried apple snacks impregnated with probiotic microgels was determined. A significant reduction in pH values can be observed. The pH of control treatment (Fc) did not show a sharp decline; instead, it fell gradually. However, pH of apple snacks having free probiotics was reduced at a faster rate. The maximum pH among microgels was analyzed by AS (SA + CaCO3); however, other samples showed lower pH than AS (SA + CaCO3). A study on apple snacks was conducted by Mejía‐Águila et al. (2021) and similar pH conditions were reported.
FIGURE 6.
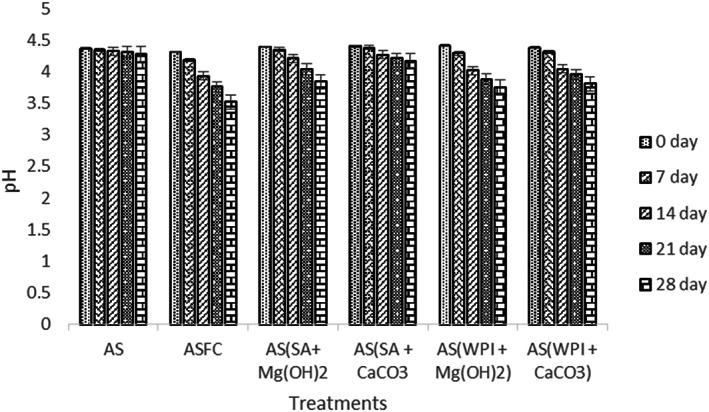
Effect of free (unencapsulated) and encapsulated (SA and WPI having Mg(OH)2 and CaCO3 antacids) L. acidophilus on the pH of dried apple snacks during storage intervals (0, 7, 14, 21, and 28 days) compared with control. Each bar represents the mean value for viability of treatments. AS (control/without probiotics), ASFC (free/unencapsulated cells), AS(SA + Mg(OH)2 (apple snack having sodium alginate microgels with Mg(OH)2), AS(SA + CaCO3) (apple snack having sodium alginate microgels with CaCO3), AS(WPI + Mg(OH)2) (apple snack having whey protein isolate microgels with Mg(OH)2) and AS(WPI + CaCO3) (apple snack having whey protein isolate microgels with CaCO3).
3.8. Probiotic viability
Mean results for probiotic viability are shown in Figure 7. Overall, a gradual significant decreasing trend was observed. The maximum content of probiotics was observed in apple snacks prepared with L. acidophilus encapsulated with alginate and CaCO3 (SA + CaCO3).
FIGURE 7.
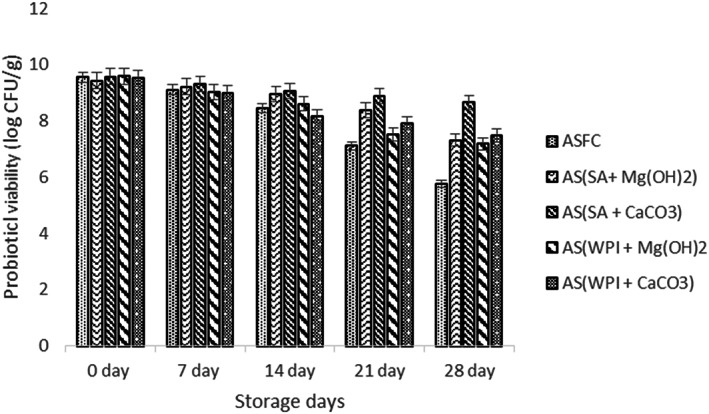
Effect of free (unencapsulated) and encapsulated (SA and WPI having Mg(OH)2 and CaCO3 antacids) L. acidophilus on probiotic viability of dried apple snacks during storage intervals (0, 7, 14, 21, and 28 days) compared with control. Each bar represents the mean value for viability of treatments. AS (control/without probiotics), ASFC (free/unencapsulated cells), AS(SA + Mg(OH)2 (apple snack having sodium alginate microgels with Mg(OH)2), AS(SA + CaCO3) (apple snack having sodium alginate microgels with CaCO3), AS(WPI + Mg(OH)2) (apple snack having whey protein isolate microgels with Mg(OH)2) and AS(WPI + CaCO3) (apple snack having whey protein isolate microgels with CaCO3).
A 0.92 log CFU/g decrease was noted at the end of storage study. However, a log of 2.03 CFU/g was determined in dried apple snacks that contained WPI + CaCO3 antacid. Similarly, probiotic viability of the snacks containing Mg (OH)2 was observed as log 2.12 CFU/g while log 2.40 CFU/g was detected in apple snack having SA + Mg (OH)2 and WPI + Mg (OH)2. However, in the case of dried apple snacks having free probiotics (ASFC), a piercing drop was monitored in the growth of probiotics. From these results, it can be concluded that microgels containing sodium alginate exhibited better probiotic protection and increased their survival rate. Similar results were also obtained by Afzaal, Saeed, Hussain, et al. (2020). In another study, Lactobacillus bulgaricus was encapsulated in wall material and showed enhanced viability. The results of present research are also in accordance with the findings of Li et al. (2018), who observed satisfactory results for L. plantarum in apple snacks.
3.9. Sensory
Sensory results suggested that additions of probiotics affect the sensory profile of any food product. However, the results of the current study revealed that the dried apple snack having microgels can be acceptable by the consumers. The assimilation of probiotic microgels affected significantly (p < .05) the sensory parameters (color, texture, appearance, and general perception) of apple snacks as compared to the snack having free probiotic cells. However, the sample (ASFC) was not appreciated by the panelists. However, dried apple snack with SA + CaCO3 was highly acceptable along with the control sample. A study on dried apple snacks conducted by Afzaal, Saeed, Ateeq, et al. (2020); Afzaal, Saeed, Hussain, et al. (2020); Afzaal, Saeed, Saeed, et al. (2020) also suggested that the wall material incorporated into the apple snack was appreciated by the consumers without changing the sensory profile of the product (Figure 8).
FIGURE 8.
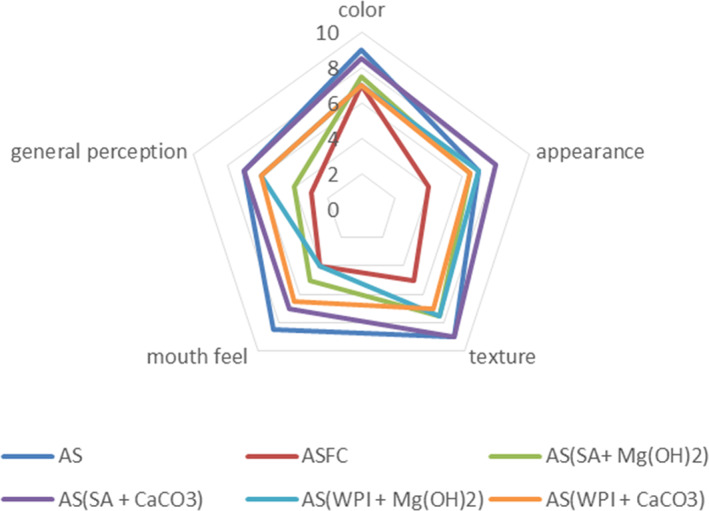
Effect of free (unencapsulated) and encapsulated (SA and WPI having Mg(OH)2 and CaCO3 antacids) L. acidophilus on sensory profile of dried apple snacks during storage intervals (0, 7, 14, 21, and 28 days) compared with control. Each line represents mean value for viability of treatments. AS (control/without probiotics), ASFC (free/unencapsulated cells), AS(SA + Mg(OH)2 (apple snack having sodium alginate microgels with Mg(OH)2), AS(SA + CaCO3) (apple snack having sodium alginate microgels with CaCO3), AS(WPI + Mg(OH)2) (apple snack having whey protein isolate microgels with Mg(OH)2) and AS(WPI + CaCO3) (apple snack having whey protein isolate microgels with CaCO3).
4. CONCLUSION
In the present study, microgels loaded with antacids were evaluated for their effect on probiotics viability under stressed conditions. Results indicated that the use of antacids has a key role in sustaining a neutral pH within microgels and ensures safe passage of probiotics through extremely acidic gastric juices and augment the viability of probiotics. Conclusively, CaCO3 showed as a more effective antacid agent than Mg (OH)2 for protecting the probiotics. Overall, the microgels prepared in this study showed better stability under stress as well as during product storage.
FUNDING INFORMATION
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
CONSENT TO PARTICIPATE
Corresponding and all the co‐authors are willing to participate in this manuscript.
CONSENT FOR PUBLICATION
All authors are willing for publication of this manuscript.
ACKNOWLEDGMENTS
Authors are thankful to Government College University for providing literature collection facilities, which helped in the study.
Afzaal, M. , Saeed, F. , Ateeq, H. , Akhtar, M. N. , Imran, A. , Ahmed, A. , Aamir, M. , Islam, F. , Yasmin, I. , Shah, Y. A. , Hussain, M. , Hameed, A. , Kumar, R. , & Awuchi, C. G. (2023). Probiotics encapsulated gastroprotective cross‐linked microgels: Enhanced viability under stressed conditions with dried apple carrier. Food Science & Nutrition, 11, 817–827. 10.1002/fsn3.3116
Contributor Information
Muhammad Afzaal, Email: muhammadafzaal@gcuf.edu.pk.
Fakhar Islam, Email: fakhar.ft440@gmail.com.
Chinaza Godswill Awuchi, Email: awuchi.chinaza@kiu.ac.ug, Email: awuchichinaza@gmail.com.
DATA AVAILABILITY STATEMENT
Even though adequate data have been given in the form of Tables and Figures, however, all authors declare that if more data are required then the data will be provided on request basis.
REFERENCES
- Afzaal, M. , Saeed, F. , Ateeq, H. , Ahmed, A. , Ahmad, A. , Tufail, T. , Ismail, Z. , & Anjum, F. M. (2020). Encapsulation of Bifidobacterium bifidum by internal gelation method to access the viability in cheddar cheese and under simulated gastrointestinal conditions. Food Science & Nutrition, 8(6), 2739–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzaal, M. , Saeed, F. , Hussain, S. , Mohamed, A. A. , Alamri, M. S. , Ahmad, A. , Ateeq, H. , Tufail, T. , & Hussain, M. (2020). Survival and storage stability of encapsulated probiotic under simulated digestion conditions and on dried apple snacks. Food Science & Nutrition, 8(10), 5392–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzaal, M. , Saeed, F. , Saeed, M. , Azam, M. , Hussain, S. , Mohamed, A. A. , Alamri, M. S. , & Anjum, F. M. (2020). Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal and thermal conditions. International Journal of Food Properties, 23(1), 1899–1912. [Google Scholar]
- Agrawal, R. (2005). Probiotics: An emerging food supplement with health benefits. Food Biotechnology, 19(3), 227–246. [Google Scholar]
- Amagwula, I. O. , Osuji, C. M. , Omeire, G. C. , Awuchi, C. G. , & Okpala, C. O. R. (2022). Combined impact of freezing and soaking times on different cowpea varieties' flour functionality and resultant gel strength, sensory and product yield of moi‐moi. AIMS Agriculture and Food, 7(4), 762–776. 10.3934/agrfood.2022047 [DOI] [Google Scholar]
- Anitha, D. P. M. , & Sellamuthu, P. S. (2021). Microencapsulation of probiotics in finger millet milk complex to improve encapsulation efficiency and viability. Food Science and Technology International, 28, 216–232. [DOI] [PubMed] [Google Scholar]
- Awuchi, C. G. , Morya, S. , Dendegh, T. A. , Okpala, O. D. R. , & Korzeniowska, M. (2022). Nanoencapsulation of food bioactive constituents and its associated processes: A revisit. Bioresource Technology Reports, 18, 101088. 10.1016/j.biteb.2022.101088 [DOI] [Google Scholar]
- Awuchi, C. G. , Owuamanam, I. C. , Ogueke, C. C. , & Igwe, C. C. (2019). Evaluation of patulin levels and impacts on the physical characteristics of grains. International Journal of Advanced Academic Research, 5(4), 10–25. [Google Scholar]
- Brinques, G. B. , & Ayub, M. A. Z. (2011). Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. Journal of Food Engineering, 103(2), 123–128. [Google Scholar]
- Chen, H. Y. , Li, X. Y. , Liu, B. J. , & Meng, X. H. (2017). Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. Journal of Functional Foods, 29, 248–255. [Google Scholar]
- Coghetto, C. C. , Brinques, G. B. , Siqueira, N. M. , Pletsch, J. , Soares, R. M. D. , & Ayub, M. A. Z. (2016). Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. Journal of Functional Foods, 24, 316–326. [Google Scholar]
- Corcoran, B. M. , Stanton, C. , Fitzgerald, G. , & Ross, R. P. (2008). Life under stress: The probiotic stress response and how it may be manipulated. Current Pharmaceutical Design, 14(14), 1382–1399. [DOI] [PubMed] [Google Scholar]
- Doherty, S. B. , Auty, M. A. , Stanton, C. , Ross, R. P. , Fitzgerald, G. F. , & Brodkorb, A. (2012). Application of whey protein micro‐bead coatings for enhanced strength and probiotic protection during fruit juice storage and gastric incubation. Journal of Microencapsulation, 29(8), 713–728. [DOI] [PubMed] [Google Scholar]
- Fang, Z. , & Bhandari, B. (2012). Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation technologies and delivery systems for food ingredients and nutraceuticals (pp. 73–109). Woodhead Publishing. [Google Scholar]
- Fiocco, D. , Longo, A. , Arena, M. P. , Russo, P. , Spano, G. , & Capozzi, V. (2020). How probiotics face food stress: They get by with a little help. Critical Reviews in Food Science and Nutrition, 60(9), 1552–1580. [DOI] [PubMed] [Google Scholar]
- Gu, M. , Zhang, Z. , Pan, C. , Goulette, T. R. , Zhang, R. , Hendricks, G. , McClements, D. J. , & Xiao, H. (2019). Encapsulation of Bifidobacterium pseudocatenulatum G7 in gastroprotective microgels: Improvement of the bacterial viability under simulated gastrointestinal conditions. Food Hydrocolloids, 91, 283–289. [Google Scholar]
- Iqbal, R. , Liaqat, A. , Jahangir Chughtai, M. F. , Tanweer, S. , Tehseen, S. , Ahsan, S. , Nadeem, M. , Mehmood, T. , Ur Rehman, S. J. , Saeed, K. , Sameed, N. , Aziz, S. , Tahir, A. B. , & Khaliq, A. (2021). Microencapsulation: A pragmatic approach towards delivery of probiotics in gut. Journal of Microencapsulation, 38, 437–458. [DOI] [PubMed] [Google Scholar]
- Islam, F. , Noman, M. , Afzaal, M. , Saeed, F. , Ahmad, S. , Zubair, M. W. , Zahra, S. M. , Hussain, M. , Ateeq, H. , & Awuchi, C. G. (2022). Synthesis and food applications of resistant starch‐based nanoparticles. Journal of Nanomaterials, 2022, 8729258. 10.1155/2022/8729258 [DOI] [Google Scholar]
- Islam, F. , Saeed, F. , Afzaal, M. , Ahmad, A. , Hussain, M. , Khalid, M. A. , Saewan, S. A. , & Khashroum, A. O. (2022). Applications of green technologies‐based approaches for food safety enhancement: A comprehensive review. Food science & nutrition, 10(9), 2855–2867. 10.1002/fsn3.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia, E. M. , Ghasempour, Z. , Ghanbari, S. , Pirmohammadi, R. , & Ehsani, A. (2018). Development of probiotic yogurt by incorporation of milk protein concentrate (MPC) and microencapsulated Lactobacillus paracasei in gellan‐caseinate mixture. British Food Journal, 120, 1516–1528. [Google Scholar]
- Krasaekoopt, W. , Bhandari, B. , & Deeth, H. (2003). Evaluation of encapsulation techniques of probiotics for yoghurt. International Dairy Journal, 13(1), 3–13. [Google Scholar]
- Lemos Junior, W. , Guerra, A. F. , Tarrah, A. , da Silva Duarte, V. , Giacomini, A. , Luchese, R. H. , & Corich, V. (2020). Safety and stability of two potentially probiotic lactobacillus strains after in vitro gastrointestinal transit. Probiotics and antimicrobial proteins, 12(2), 657–666. 10.1007/s12602-019-09565-2 [DOI] [PubMed] [Google Scholar]
- Li, C. U. I. , Niu, L. Y. , Li, D. J. , Liu, C. Q. , Liu, Y. P. , Liu, C. J. , & Song, J. F. (2018). Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks. Journal of Integrative Agriculture, 17(1), 247–255. [Google Scholar]
- Lian, W. C. , Hsiao, H. C. , & Chou, C. C. (2002). Survival of bifidobacteria after spray‐drying. International Journal of Food Microbiology, 74, 79–86. [DOI] [PubMed] [Google Scholar]
- Mehra, R. , Kumar, H. , Naveen, K. , Suvartan, R. , Atanu, J. , Harpal, S. B. , Istvan, G. T. , Awuchi, C. G. , Okpala, C. O. R. , Korzeniowskg, M. , & Guiné, R. F. P. (2021). Whey proteins processing and emergent derivatives: An insight perspective from constituents, bioactivities, functionalities to therapeutic applications. Journal of Functional Foods., 87, 104760. 10.1016/j.jff.2021.104760 [DOI] [Google Scholar]
- Mejía‐Águila, R. A. , Aguilar‐Galvez, A. , Chirinos, R. , Pedreschi, R. , & Campos, D. (2021). Vacuum impregnation of apple slices with Yacon (Smallanthus sonchifolius Poepp. & Endl) fructooligosaccharides to enhance the functional properties of the fruit snack. International Journal of Food Science & Technology, 56(1), 392–401. [Google Scholar]
- Morya, S. , Awuchi, C. G. , & Menaa, F. (2022). Advanced functional approaches of nanotechnology in food and nutrition. In Chowdhary P., Kumar V., Kumar V., & Hare V. (Eds.), Environmental Management Technologies: Challenges and Opportunities (pp. 257–272). CRC Press, Taylor & Francis. 10.1201/9781003239956-16 [DOI] [Google Scholar]
- Nazzaro, F. , Orlando, P. , Fratianni, F. , & Coppola, R. (2012). Microencapsulation in food science and biotechnology. Current Opinion in Biotechnology, 23(2), 182–186. [DOI] [PubMed] [Google Scholar]
- Nualkaekul, S. , Lenton, D. , Cook, M. T. , Khutoryanskiy, V. V. , & Charalampopoulos, D. (2012). Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydrate Polymers, 90(3), 1281–1287. [DOI] [PubMed] [Google Scholar]
- Oguntoye, M. A. , Ezekiel, O. O. , & Oridupa, O. A. (2021). Viability of Lactobacillus rhamnosus GG in provitamin A cassava hydrolysate during fermentation, storage, in vitro and in vivo gastrointestinal conditions. Food Bioscience, 40, 100845. [Google Scholar]
- Omar, J. M. , Chan, Y. M. , Jones, M. L. , Prakash, S. , & Jones, P. J. (2013). Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. Journal of Functional Foods, 5(1), 116–123. [Google Scholar]
- Rafiq, S. , Sofi, S. A. , Kumar, H. , Kaul, R. K. , Mehra, R. , Awuchi, C. G. , Okpala, C. O. R. , & Korzeniowska, M. (2022). Physicochemical, antioxidant, and polyphenolic attributes of microencapsulated freeze‐dried kinnow peel extract powder using maltodextrin as wall material. Journal of Food Processing and Preservation, 46(1), e16177. 10.1111/jfpp.16177 [DOI] [Google Scholar]
- Raju, R. , Bridges, G. E. , & Bhadra, S. (2020). Wireless passive sensors for food quality monitoring: Improving the safety of food products. IEEE Antennas and Propagation Magazine, 62(5), 76–89. [Google Scholar]
- Ranadheera, C. S. , Evans, C. A. , Adams, M. C. , & Baines, S. K. (2012). Probiotic viability and physico‐chemical and sensory properties of plain and stirred fruit yogurts made from goat's milk. Food Chemistry, 135(3), 1411–1418. [DOI] [PubMed] [Google Scholar]
- Ruiz, L. , Margolles, A. , & Sánchez, B. (2013). Bile resistance mechanisms in Lactobacillus and Bifidobacterium . Frontiers in Microbiology, 4, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela, M. , Rantala, M. , Hallamaa, K. , Nohynek, L. , Virkajärvi, I. , & Mättö, J. (2004). Stationary‐phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria . Journal of Applied Microbiology, 96(6), 1205–1214. [DOI] [PubMed] [Google Scholar]
- Smidsrød, O. , & Skja, G. J. T. (1990). Alginate as immobilization matrix for cells. Trends in Biotechnology, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- Terpou, A. , Papadaki, A. , Lappa, I. K. , Kachrimanidou, V. , Bosnea, L. A. , & Kopsahelis, N. (2019). Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients, 11(7), 1591. 10.3390/nu1107159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi, I. , Ayadi, D. , Bejar, W. , Bejar, S. , Chouayekh, H. , & Salah, R. B. (2014). Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. International Journal of Biological Macromolecules, 64, 84–89. [DOI] [PubMed] [Google Scholar]
- Tripathi, M. K. , & Giri, S. K. (2014). Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods, 9, 225–241. [Google Scholar]
- Wang, G. , Chen, Y. , Xia, Y. , Song, X. , & Ai, L. (2022). Characteristics of probiotic preparations and their applications. Foods (Basel, Switzerland), 11(16), 2472. 10.3390/foods11162472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Y. , Xu, Q. , Jiang, L. , Cao, D. , Lin, H. , Che, Z. , Ma, Y. , Li, X. , & Cai, Y. (2015). Effect of different coating materials on the biological characteristics and stability of microencapsulated Lactobacillus acidophilus . RSC Advances, 5(29), 22825–22837. [Google Scholar]
- Yao, M. , Wu, J. , Li, B. , Xiao, H. , McClements, D. J. , & Li, L. (2017). Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocolloids, 72, 228–236. [Google Scholar]
- Yeung, T. W. , Üçok, E. F. , Tiani, K. A. , McClements, D. J. , & Sela, D. A. (2016). Microencapsulation in alginate and chitosan microgels to enhance viability of Bifidobacterium longum for oral delivery. Frontiers in Microbiology, 7, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. D. H. (2020). Recent advances in probiotics encapsulation by electrospinning. ES Food & Agroforestry, 2, 3–12. [Google Scholar]
- Zheng, H. , Gao, M. , Ren, Y. , Lou, R. , Xie, H. , Yu, W. , Liu, X. , & Ma, X. (2017). An improved pH‐responsive carrier based on EDTA‐Ca‐alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydrate Polymers, 155, 329–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Even though adequate data have been given in the form of Tables and Figures, however, all authors declare that if more data are required then the data will be provided on request basis.


