Abstract
Introduction
Pneumococcal disease, which presents a substantial health and economic burden, is prevented through pneumococcal vaccination programs. We assessed the impact of switching from a 13-valent-based (PCV13) to lower 10-valent-based (PCV10-GlaxoSmithKline [GSK] or PCV10-Serum Institute of India [SII]) or higher-valent (PCV15 or PCV20) vaccination programs in South Africa.
Methods
A previously published decision-analytic model was adapted to a South African setting. Historical invasive pneumococcal disease (IPD) incidence data were used to project IPD incidence over time for each vaccination program on the basis of serotype coverage. Historical incidence (IPD, pneumonia, otitis media), mortality, costs, and utilities were obtained from the published literature. Cases of disease, direct medical costs (i.e., vaccination, IPD, pneumonia, and otitis media costs) (in 2022 South African rands), life-years, quality-adjusted life-years (QALY), and incremental cost per QALY were estimated over a 5- and 10-year horizon for PCV13 and the PCV10 vaccines. Additionally, a public health impact analysis was conducted comparing PCV13, PCV15, and PCV20.
Results
Continuing use of PCV13 would substantially reduce disease incidence over time compared with switching to either of the PCV10 lower-valent vaccines. Cases of IPD were reduced by 4.22% and 34.70% when PCV13 was compared to PCV10-GSK and PCV10-SII, respectively. PCV13 was also found to be cost saving over 5- and 10-year time horizons compared with PCV10-SII and to be cost-effective over a 5-year time horizon and cost-saving over a 10-year time horizon compared with PCV10-GSK. PCV20 was consistently estimated to prevent more cases than the PCV10 vaccines, PCV13, or PCV15.
Conclusions
Switching from a higher-valent to a lower-valent vaccine may lead to disease incidence re-emergence caused by previously covered serotypes. Maintaining PCV13 was estimated to improve public health further by averting additional pneumococcal disease cases and saving more lives and also to reduce total costs in most scenarios. Higher-valent PCVs can achieve the greatest public health impact in the pediatric vaccination program in South Africa.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00767-4.
Keywords: PCV, PCV13, PCV20, Pneumococcal disease, Vaccine
Key Summary Points
| Why carry out the study? |
| A decision-analytic model was adapted to estimate the impact of switching from a 13-valent-based (PCV13) to lower 10-valent-based (PCV10-GSK or PCV10-SII) or higher-valent (PCV15 or PCV20) vaccination programs in South Africa. |
| Continuing use of PCV13 was estimated to improve public health further and would substantially reduce disease incidence over time compared with switching to either of the PCV10 and would be cost-saving over 5 years or more. |
| What was learned from the study? |
| When higher-valent vaccines (PCV15 or PCV20) are available, switching from PCV13 to PCV20 would have a greater impact on disease burden than switching from PCV13 to PCV15 in South Africa. |
Introduction
Streptococcus pneumoniae (S. pneumoniae) is the leading cause of vaccine-preventable morbidity and mortality for children under 5 years old [1]. Pneumococcal disease can either be invasive (IPD), such as meningitis or bacteremia, or non-invasive, such as non-bacteremic pneumonia and otitis media (OM). Non-invasive disease represents the greatest burden of pneumococcal disease in children in all countries [2].
In South Africa, IPD surveillance began in 1999 and enhanced in 2003 through the Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA), a nationwide, active, laboratory-based surveillance system [3]. Per the GERMS-SA data, between 2005 and 2008 the IPD incidence rate was 9.4 and 1142 cases per 100,000 person-years among all ages and in children aged under 2 years, respectively [4].
Pneumococcal conjugate vaccines (PCVs) have been introduced to the national immunization program (NIP) in South Africa. A 7-valent PCV (PCV7, formulated by Pfizer Inc.) was introduced into South Africa’s national immunization program (NIP) in April 2009 to protect against pneumococcal disease caused by serotypes 4, 6B, 9 V, 14, 18C, 19F, and 23F. The NIP transitioned to 13-valent PCV (PCV13, formulated by Pfizer Inc.) in May 2011 to protect against disease caused by additional serotypes 1, 3, 5, 6A, 7F, and 19A. Since then, a routine pediatric PCV13 NIP has been in place with a recommended dosing schedule at 6 weeks, 14 weeks, and 9 months, with an estimated 90.7% coverage for the final dose in 2019 [5]. In South Africa, PCVs have been highly effective in preventing pneumococcal disease in children. When comparing data aggregated between 2005 and 2008 to data collected in 2012, von Gottberg and colleagues found that IPD incidence rates among children < 2 years old declined by 69% for IPD due to all serotypes and by 89% for PCV7 serotypes [4]. PCV has also provided indirect protection in adults. The relative reduction of IPD due to all serotypes and PCV7 serotypes in adults aged 25 to 44 years was 34% and 57%, respectively.
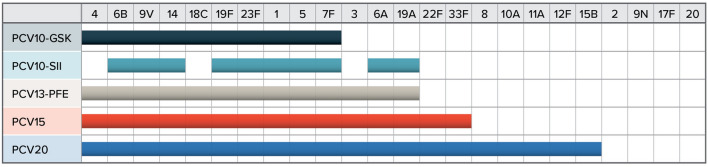
Along with PCV13, two additional 10-valent PCVs are available in South Africa. PCV10-GSK (formulated by GlaxoSmithKline) is indicated to provide protection for three fewer serotypes (3, 6A, and 19A) than PCV13 and has been licensed since 2009. PCV10-SII (formulated by Serum Institute of India) is also indicated to provide protection against three fewer serotypes (3, 4, and 18C) than PCV13. Two higher-valent PCVs (PCV15, formulated by Merck; and PCV20, formulated by Pfizer) may become available in South Africa by 2024 or 2025. These two vaccines share the same serotypes covered by PCV13, while PCV15 covers an additional two serotypes (22F and 33F) and PCV20 covers an additional seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F). Figure 1 illustrates the serotype coverage of each vaccine.
Fig. 1.
Serotype coverage. The serotypes covered by each pneumococcal vaccine. GSK GlaxoSmithKline, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, SII Serum Institute of India
With multiple PCVs available and each with a different clinical profile and cost, cost-effectiveness evaluations of PCVs are increasingly important to identify a vaccine that could maximize health and economic benefits and to provide best value for money, in a country like South Africa, with finite financial resources. Therefore, the primary objective of this study was to assess the cost-effectiveness of maintaining a PCV13 vaccination program compared with switching to a lower-valent PCV10 alternative. Additionally, the study explored the potential public health impact of continuing with PCV13 and switching to PCV15 or PCV20 when they become available.
Methods
Model Structure
The model structure was based on various previously published decision-analytic forecasting models [6–13] that leverage historical real-world surveillance data to predict future clinical and economic outcomes. Specifically, the model prospectively predicts age-specific incidence trends by individual serotype or grouped serotypes based on observed retrospective incidence and serotype dynamics. We grouped the serotypes according to PCV coverage to allow for estimation of incidence trends with and without serotype coverage. Thus, the serotype groups were as follow: PCV7 minus 4 and 18C; PCV10 minus PCV7; ST3; ST6A; ST19A; ST4; ST18C; ST8; ST12F; PCV15 minus PCV13; PCV20 minus PCV15 minus ST8 and ST12F. This methodology captures both vaccine pressure on covered serotypes on the basis of real-world evidence and replacement of non-vaccine serotypes observed from surveillance data. The projected incidence in pneumococcal disease is used to calculate total cases, deaths, and costs associated with each PCV program.
Vaccination Strategies
Using this model, we formulated five vaccination scenarios: (1) strategy 1, continue with PCV13 (status quo); (2) strategy 2, replace PCV13 with PCV10-GSK; (3) strategy 3, replace PCV13 with PCV10-SII; (4) strategy 4, continue with PCV13 for 3 years then switch to PCV15; and (5) strategy 5, continue with PCV13 for 3 years then switch to PCV20. For the primary objective, the cost-effectiveness of vaccination strategy 1 was compared with strategy 2 and strategy 3. The outcomes of the primary objective included estimated averted disease cases (IPD, pneumonia, and OM), pneumococcal-related deaths, life-years (LYs) saved, quality-adjusted LYs (QALYs) saved, and incremental cost-effectiveness ratio from a payer perspective. For the exploratory objective, the public health impacts of strategy 4 and strategy 5 were compared with those of strategy 2 and strategy 3.
Model Efficacy Assumptions
Historical IPD incidence during the post-PCV13 era in South Africa was used to estimate the future incidences of vaccine-typed IPD assuming scenarios where different vaccination strategies are implemented: PCV10-GSK, PCV10-SII, PCV13, PCV15 or PCV20. On the basis of the licensure indications, we assumed PCV10-GSK does not provide protection against serotypes 3, 6A, and 19A and that PCV10-SII does not provide protection against serotypes 3, 4, and 18C.
For the base-case analyses, we assumed that PCV13 and PCV10-GSK provided protection against all pneumococcal pneumonia for ages under 5 years and protection against only hospitalized pneumonia for ages 5 years and older. For PCV10-SII, we assumed no effect against pneumonia or OM, as no clinical data were available to demonstrate PCV10-SII’s effectiveness against these outcomes. Additionally, there were no real-world data available to support PCV10-SII’s impact on nasopharyngeal carriage, so the model assumes PCV10-SII does not reduce disease among unvaccinated individuals in the base case [14]. Scenario analyses in which PCV10-SII was assumed to offer comparable protection as PCV13 against PCV13-shared serotypes were also conducted.
The model allows for serotype replacement to occur when switching from PCV13 to a lower-valent option. Serotype replacement trends were modeled to behave similarly to dynamics observed in Belgium and were set to re-emerge by a factor of 117–166% for serotypes not covered by PCV13. This may be a conservative assumption considering a previous cost-effectiveness study using the same model and assumption actually underestimated the epidemiologic impact of switching to a lower-valent vaccine in Belgium, where PCV13 was switched to PCV10-GSK in 2015 [15].
Model Inputs
Population and Vaccination Rate
The total population size (58,726,826) was obtained from Statistics South Africa [16] and was stratified into seven age groups (0 to < 2, 2–4, 5–14, 15–24, 25–44, 45–64, and 65 and older). Vaccination coverage for a three-dose vaccination series was assumed to be 90.7% in children less than 2 years of age, regardless of PCV considered in the model [5].
Epidemiology Parameters
Invasive Pneumococcal Disease Incidence
The model defines IPD as a combination of meningitis and bacteremia. Historical age-group-specific IPD incidence and serotype distributions from 2011 to 2019 were sourced from South Africa’s GERMS-SA annual surveillance review [17] (Supplementary Material Tables 1–7). The latest available incidence rates of pneumococcal meningitis and bacteremia from 2019 are included in Table 1.
Table 1.
Model inputs
| Parameter | 0 to < 2 | 2–4 | 5–17 | 18–34 | 35–49 | 50–64 | 65+ |
|---|---|---|---|---|---|---|---|
| Populationa | 2,269,652 | 3,404,478 | 11,195,767 | 9,576,585 | 19,254,757 | 9,513,157 | 3,512,430 |
| Incidence rate (per 100,000) | |||||||
| Bacteremiab | 17.35 | 8.83 | 3.34 | 3.24 | 10.83 | 9.91 | 7.47 |
| Meningitisb,c | 4.22 | 0.45 | 0.20 | 0.22 | 0.54 | 0.50 | 0.18 |
| Non-hospitalized pneumoniad | 3849 | 1018 | 286 | 282 | 836 | 1238 | 2356 |
| Hospitalized pneumoniad | 622 | 238 | 100 | 0 | 74 | 279 | 622 |
| Otitis mediae | 24,290 | 24,290 | 17,294 | ||||
| Otitis media (pre-PCV13 data from South Africa)i | 22,900 | 22,900 | 11,000 | ||||
| Percentage IPD due to meningitisc | 34.80% | 13.10% | 9.50% | 9.00% | 8.80% | 7.10% | 2.40% |
| All-cause mortality per 100,000f | 1000 | 100 | 147 | 147 | 614 | 1508 | 5347 |
| Case fatality ratesc | |||||||
| IPD | 0.036 | 0.038 | 0.069 | 0.145 | 0.171 | 0.222 | 0.342 |
| Hospitalized pneumonia | 0.003 | 0.002 | 0.012 | 0.031 | 0.051 | 0.091 | 0.171 |
| Direct costsg | |||||||
| Bacteremia | R19,827 | R19,827 | R49,411 | R70,246 | R70,246 | R89,326 | R68,102 |
| Meningitis | R26,567 | R26,567 | R66,496 | R94,535 | R94,535 | R120,211 | R91,650 |
| Otitis media (mild) | R349 | R349 | |||||
| Otitis media (moderate/severe) | R699 | R699 | |||||
| Pneumonia (non-hospitalized) | R349 | R349 | |||||
| Pneumonia (hospitalized) | R19,827 | R19,827 | R49,411 | R70,246 | R70,246 | R89,326 | R68,102 |
| Utilityh | 0.94 | 0.94 | 0.94 | 0.94 | 0.89 | 0.82 | 0.76 |
IPD invasive pneumococcal disease, PCV13 13-valent pneumococcal conjugate vaccine
aStatistics South Africa [16]
bNational Institute for Communicable Diseases [17]
cMelegaro and Edmunds [25]
dTempia et al. [18], Moore [19]
eSpanish Ministry of Health [45]
fStatistics South Africa [24]
gPfizer [28], Statistics South Africa (stats sa) Republic of South Africa [29]
hAra and Brazier [30]
iBiagio-de Jager et al. [21]. Incidence calculated from data in the article. Incidence for 2–4 assumed to be the same for < 2
Non-invasive Pneumococcal Disease Incidence
All-cause and age-specific hospitalized and non-hospitalized pneumonia incidence was estimated using the severe respiratory infection incidence rate reported by Tempia et al.; of the cases discussed by Tempia et al., 41% were assumed to be all-cause pneumonia [18, 19]. The incidence data for hospitalized and non-hospitalized all-cause pneumonia are limited. Therefore, we assumed the historical incidence rates found in our sources were constant from 2013 through 2016 and from 2017 through 2019 for hospitalized pneumonia and that they were constant from 2014 through 2019 for outpatient pneumonia (Table 1). As a result of data limitations, historical OM incidence from Spain for 2014 through 2019, sourced from the Spanish Ministry of Health, was used as a proxy for South Africa [20]. We also conducted a scenario analysis using 2012 OM incidence data from a primary health clinic providing services to the Diepsloot community north of Johannesburg, South Africa [21]. The South African data were used only in a sensitivity analysis because they were collected before the time of routine PCV13 vaccination in a small population and are likely unrepresentative of OM incidence. Non-IPD incidence is presented in Table 1.
Additionally, we assumed that 30% of all-cause pneumonia or OM was caused by S. pneumoniae during the historical period, and we assumed that 18.8% of all-cause pneumonia and 20% of all-cause OM were caused by S. pneumoniae for prospective predicted incidence [22, 23]. Prospective incidence of pneumococcal pneumonia and OM for each age group was estimated assuming a constant proportional relationship to IPD incidence.
Mortality
All-cause mortality per 100,000 was obtained from Statistics South Africa [24]. Case fatality rates for bacteremia, meningitis, and hospitalized pneumonia were obtained from Melegaro and Edmunds [25]. There is no risk of death for OM and non-hospitalized pneumonia (Table 1), a finding that is consistent with epidemiologic data and data from other cost-effectiveness analyses.
Economic Parameters
Vaccine acquisition costs and age-specific, direct disease-related costs were included in the model. Vaccination acquisition costs for PCV13, PCV10-GSK, and PCV10-SII were assumed to be R225.62, R199.95, and R199, respectively [26] (data on file). No vaccination administration costs were assumed.
Age-specific, direct disease-related costs associated with each case of IPD, hospitalized and non-hospitalized pneumonia, and OM were based on the data reported by Melegaro and Edmunds [25] and were inflated to 2022 South African rands (Table 1) [27–29].
Utility Parameters
As a result of lack of data in South Africa, quality of life estimates for patients in other countries were used as a proxy. Age-specific utilities for patients not experiencing pneumococcal-related diseases were estimated using the Health Surveys of England EQ-5D data [30]. Previous cost-effectiveness analyses for PCVs were used to estimate utility decrements for non-hospitalized pneumonia and OM [31]. Bacteremia, meningitis, and hospitalized pneumonia utilities were obtained from a utility assessment conducted in parents of children presenting to the emergency department in the USA [32]. Utility decrements of 0.14, 0.0035, 0.0037, and 0.0079 were estimated for bacteremia/meningitis, OM, non-hospitalized pneumonia, and hospitalized pneumonia, respectively. Age-specific utilities are presented in Table 1.
Analyses
For the base-case analysis, PCV13 was compared with the PCV10 vaccines over a 10-year horizon. Since the model was built on the basis of historical trend line, it is not feasible to conduct one-way or probabilistic sensitivity analyses for the model. However, we conducted various scenario analyses to accommodate uncertainty based on changes in time horizon and vaccine effectiveness assumptions. Scenario analyses with 5-year time horizons were conducted for the primary objective, and 7- and 10-year time horizons were considered for the exploratory objective. A scenario assuming OM incidence equal to pre-PCV13 era data and a scenario regarding the effectiveness of PCV10-SII against non-IPD incidence were also included. Scenario analyses based on a 3% discount rate were also carried out. Costs and outcomes were both discounted at a rate of 5% in the base-case analysis [27] and were presented in 2022 South African rands. The model was based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Public Health Impact and Cost-Effectiveness of Switching to Lower-Valent PCVs
Under a PCV13 program (strategy 1), IPD incidence in children aged under 2 years is predicted to remain relatively stable after 10 years, compared to 15.5% and 73.4% increases in IPD incidence in children aged under 2 years observed under strategy 2 (replace with PCV10-GSK) and strategy 3 (replace with PCV10-SII), respectively (Fig. 2). The increase in incidence in strategy 2 is primarily driven by an increase in uncovered serotypes 6A and 19A, whereas the increase in incidence in strategy 3 is primarily driven by an increase in uncovered serotype 18C, a previously covered PCV7 serotype (Fig. 3).
Fig. 2.
Estimated incidence over time for each vaccination strategy. The historical and projected incidence per 100,000 for PCV13 compared with PCV10-SII (2A) and PCV10-GSK (2B). GSK GlaxoSmithKline, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, SII Serum Institute of India
Fig. 3.
Forecasted serotype distribution of VT serotypes for children aged under 2 years. The current estimated incidence per 100,000 and distribution by serotype and the estimated disease incidence per 100,000 after 10 years for each projected strategy. GSK GlaxoSmithKline, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, SII Serum Institute of India
Table 2 presents the public health impact and cost-effectiveness results from the base-case analyses. Strategies 2 and 3 could result in more pneumococcal disease cases (strategy 2, 217,599; strategy 3, 1,074,862) and deaths (strategy 2, 1733; strategy 3, 16,490) than continuing with strategy 1. As such, cases of IPD were reduced by 4.22% and 34.70% when PCV13 was compared to PCV10-GSK and PCV10-SII, respectively. Translated to LYs and QALYs saved, strategy 1 could save 4484 more LYs and 3191 more QALYs than strategy 2 and could save 43,636 more LYs and 28,900 more QALYs than strategy 3. Although vaccination costs associated with PCV10-SII and PCV10-GSK are lower than those of PCV13, the reduction in disease-related costs with PCV13 could substantially outpace PCV10-GSK or PCV10-SII. Overall, compared with strategy 2 or strategy 3, strategy 1 could be cost-saving over a 10-year time horizon.
Table 2.
Base-case cost-effectiveness results for PCV13 vs. PCV10 vaccines (10-year time horizon, 5% discount on costs and outcomes)
| Outcomes | Strategy 1 (continue with PCV13) | Strategy 2 (replace with PCV10-GSK) | Strategy 3 (replace with PCV10-SII) | Strategy 1 vs. strategy 2 (PCV13 vs. PCV10-GSK) | Strategy 1 vs. strategy 3 (PCV13 vs. PCV10-SII) |
|---|---|---|---|---|---|
| Disease cases | |||||
| Bacteremia | 19,094 | 19,900 | 25,720 | − 806 | − 6626 |
| Meningitis | 10,212 | 10,643 | 14,755 | − 431 | − 3543 |
| Otitis media | 7,338,651 | 7,526,296 | 8,413,513 | − 187,645 | − 1,074,862 |
| OP pneumonia | 1,436,160 | 1,451,981 | 1,533,229 | − 15,821 | − 97,069 |
| IP pneumonia | 208,944 | 221,839 | 335,981 | − 12,895 | − 127,037 |
| Total cases | 9,012,012 | 9,229,611 | 10,321,149 | − 217,599 | − 1,309,137 |
| Deaths | |||||
| IPD | 5402 | 5665 | 7524 | − 263 | − 2122 |
| Pneumonia | 19,189 | 20,659 | 33,557 | − 1470 | − 14,368 |
| Costs | |||||
| Vaccination | R5,164,513,407 | R4,576,822,980 | R4,555,166,135 | R587,690,427 | R609,347,272 |
| IPD | R1,547,786,005 | R1,604,710,463 | R2,100,711,820 | − R56,924,458 | − R552,925,815 |
| Pneumonia | R10,106,056,299 | R10,667,029,408 | R15,976,423,582 | − R560,973,109 | − R5,870,367,283 |
| Otitis media | R708,405,647 | R757,024,469 | R986,708,283 | − R48,618,822 | − R278,302,636 |
| Total | R17,526,761,358 | R17,605,587,321 | R23,619,009,821 | − R78,825,963 | − R6,092,248,462 |
| Outcomes | |||||
| Life-years | 472,915,088 | 472,915,088 | 472,871,453 | 4484 | 43,636 |
| QALY | 333,062,901 | 333,059.710 | 333,034,002 | 3191 | 28,900 |
| ICER | PCV13 cost-saving | PCV13 cost-saving | |||
GSK GlaxoSmithKline, ICER incremental cost-effectiveness ratio, IP inpatient, IPD invasive pneumococcal disease, OP outpatient, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, QALY quality-adjusted life-year, SII Serum Institute of India
The scenario analysis based on a 5-year time horizon and 5% discount rate on costs and outcomes resulted in lower incremental costs and lower QALYs than did the results from the base-case analysis, but strategy 1 remained cost-effective and cost-saving compared with strategy 2 and strategy 3, respectively (Table 3). Additionally, we conducted scenario analyses in which a 3% discount rate was assumed and found that strategy 1 remains cost-effective and cost-saving against strategy 2 and strategy 3, respectively, in both a 5- and 10-year time horizon. The results from the scenario analysis in which pre-PCV13 OM incidence data from South Africa were used as an input in the model mimicked the base-case results (Table 4). Assuming PCV10-SII has comparable protection as PCV13, strategy 1 remains cost-saving over a 10-year and near cost-effective over a 5-year horizon when compared with strategy 3 (Table 4).
Table 3.
Scenario analysis of cost-effectiveness results for PCV13 vs. PCV10 vaccines (5-year time horizon, 5% discount on costs and outcomes)
| Outcome | Strategy 1 (continue with PCV13) | Strategy 2 (replace with PCV10-GSK) | Strategy 3 (replace with PCV10-SII) | Strategy 1 vs. strategy 2 (PCV13 vs. PCV10-GSK) | Strategy 1 vs. strategy 3 (PCV13 vs. PCV10-SII) |
|---|---|---|---|---|---|
| Disease cases | |||||
| Bacteremia | 9413 | 9710 | 11,750 | − 296 | − 2337 |
| Meningitis | 5034 | 5193 | 6284 | − 158 | − 1250 |
| Otitis media | 3,497,734 | 3,569,173 | 3,902,467 | − 71,439 | − 404,733 |
| OP pneumonia | 650,389 | 656,100 | 693,600 | − 5710 | − 43,211 |
| IP pneumonia | 97,490 | 101,666 | 137,351 | − 4177 | − 39,862 |
| Total cases | 4,260,060 | 4,341,842 | 4,751,453 | − 81,781 | − 491,392 |
| Deaths | |||||
| IPD | 2578 | 2666 | 3362 | − 88 | − 784 |
| Pneumonia | 8295 | 8711 | 12,552 | − 416 | − 4257 |
| Costs | |||||
| Vaccination | R2,959,421,521 | R2,622,658,943 | R2,610,250,335 | R336,762,578 | R349,171,186 |
| IPD | R855,080,797 | R879,537,248 | R1,082,816,654 | − R24,456,452 | − R227,735,857 |
| Pneumonia | R5,280,860,245 | R5,489,901,243 | R7,409,877,679 | − R209,040,998 | − R2,129,017,434 |
| Otitis media | R400,151,202 | R421,379,547 | R520,185,773 | − R21,228,045 | − R120,034,271 |
| Total | R9,495,514,064 | R9,413,476,981 | R11,623,130,441 | R82,037,083 | − R2,127,616,376 |
| Outcomes | |||||
| Life-years | 261,119,119 | 261,118,147 | 261,109,270 | 973 | 9849 |
| QALY | 203,062,832 | 203,061,886 | 203,054,388 | 945 | 8444 |
| ICER | PCV13 cost-effective | PCV13 cost-saving | |||
GSK GlaxoSmithKline, ICER incremental cost-effectiveness ratio, IP inpatient, IPD invasive pneumococcal disease, OP outpatient, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, QALY quality-adjusted life-year, SII Serum Institute of India
Table 4.
Scenario analyses for PCV13 vs. PCV10-GSK and PCV10-SII
| Discount rate | Time horizon | Incremental cost (R) | Incremental effect (QALYs) | ICER | |
|---|---|---|---|---|---|
| Strategy 1a vs. strategy 2b | 3% | 5-year | 84,156,250 | 1066 | PCV13 cost-effective |
| 10-year | − 105,094,058 | 4063 | PCV13 cost-saving | ||
| South Africa (pre-PCV13) OM incidence data | 3% | 5-year | 85,450,438 | 1053 | PCV13 cost-effective |
| 10-year | − 101,970,209 | 4031 | PCV13 cost-saving | ||
| 5% | 5-year | 83,251,902 | 933 | PCV13 cost-effective | |
| 10-year | − 76,043,487 | 3163 | PCV13 cost-saving | ||
| Strategy 1a vs. strategy 3c | |||||
| South Africa (pre-PCV13) OM incidence data | 3% | 5-year | − R2,260,722,788 | 9429 | PCV13 cost-saving |
| 10-year | − 6,844,024,888 | 36,396 | PCV13 cost-saving | ||
| 5% | 5-year | − 2,113,137,936 | 8299 | PCV13 cost-saving | |
| 10-year | − 6,031,257,887 | 28,289 | PCV13 cost-saving | ||
| No effect on non-IPD for PCV10-SII | 3% | 5-year | − 2,276,367,192 | 9586 | PCV13 cost-saving |
| 10-year | − 6,913,918,958 | 37,096 | PCV13 cost-saving | ||
| Comparable effect as PCV13 for PCV10-SII | 3% | 5-year | 129,970,934 | 845 | R153,772/QALY |
| 10-year | − 423,322,076 | 4466 | PCV13 cost-saving | ||
| 5% | 5-year | 126,311,356 | 755 | R167,219/QALY | |
| 10-year | − 342,099,591 | 3516 | PCV13 cost-saving | ||
GSK GlaxoSmithKline, ICER incremental cost-effectiveness ratio, IPD invasive pneumococcal disease, OM otitis media, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, QALY quality-adjusted life-year, SII Serum Institute of India
aContinue with PCV13 (status quo)
bReplace PCV13 with PCV10-GSK
cReplace PCV13 with PCV10-SII
Public Health Impact of Switching to Higher-Valent PCVs
Based on estimated prospective disease incidence that potentially can be prevented by continuing use of PCV13 for 3 years and switching to PCV15 or PCV20 when they are available at year 4, the model assessed the public health impact as compared with strategy 2 or strategy 3 (Table 5). The result showed that strategy 5 (switch to PCV20) and strategy 4 (switch to PCV15) could, when compared with strategy 2, prevent 3420 and 921 more IPD cases, respectively, in 10 years, as well as 1238 and 427 more IPD cases, respectively, in 7 years. When compared with strategy 3, the result showed that strategy 5 and strategy 4 could prevent 12,352 and 9853 more IPD cases, respectively, in 10 years, as well as 6370 and 5559 more IPD cases, respectively, in 7 years. In general, compared with strategy 2, strategy 5 could prevent about 3.7 to 2.9 (3420 vs. 921 or 1238 vs. 427) times more IPD cases than strategy 4, but about 1.3 to 1.1 (12,352 vs. 9853 or 6370 vs. 5559) times more IPD cases when compared with strategy 3 over 10 years or 7 years. For non-IPD, strategy 5 was consistently estimated to prevent more cases than strategy 4, strategy 2, or strategy 3.
Table 5.
Public health impact of switching from PCV13 to either PCV15 or PCV20 vs. PCV10-GSK or PCV10-SII (5% discount rate)
| Scenario | Time horizon | IPD cases averted | IP pneumonia cases averted | OP pneumonia cases averted | OM cases averted | Life-years saved | QALYs saved |
|---|---|---|---|---|---|---|---|
| Strategy 5a vs. PCV10-GSK | 7 yearsc | 1238 | 11,822 | 14,379 | 164,123 | 2568 | 2183 |
| Strategy 4b vs. PCV10-GSK | 427 | 6104 | 7709 | 81,541 | 1801 | 1390 | |
| Strategy 5a vs. PCV10-SII | 6370 | 73,629 | 69,890 | 708,604 | 20,797 | 15,941 | |
| Strategy 4b vs. PCV10-SII | 5559 | 67,910 | 63,220 | 626,022 | 20,029 | 15,148 | |
| Strategy 5a vs. PCV10-GSK | 10 yearsd | 3420 | 30,901 | 34,431 | 402,538 | 8437 | 6280 |
| Strategy 4b vs. PCV10-GSK | 921 | 12,602 | 13,501 | 142,295 | 4433 | 2914 | |
| Strategy 5a vs. PCV10-SII | 12,352 | 145,042 | 115,679 | 1,289,755 | 47,588 | 31,988 | |
| Strategy 4b vs. PCV10-SII | 9853 | 126,744 | 94,748 | 1,029,512 | 43,584 | 28,622 |
GSK GlaxoSmithKline, IP inpatient, IPD invasive pneumococcal disease, OM otitis media, OP outpatient, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, PCV15 15-valent pneumococcal conjugate vaccine, PCV20 20-valent pneumococcal conjugate vaccine, QALY quality-adjusted life-year, SII Serum Institute of India
aContinue with PCV13 then switch to PCV20
bContinue with PCV13 then switch to PCV15
cPCV13 for 4 years and PCV15 or PCV20 for 3 years
dPCV13 for 4 years and PCV15 or PCV20 for 6 years
Discussion
We developed a decision-analytic model to forecast and compare the economic and public health impact of various PCV vaccination strategies. Specifically, we estimated the economic and health impact of sustained use of PCV13 (strategy 1), and compared this with a change in PCV recommendation to a lower-valent vaccine (i.e., PCV10-GSK [strategy 2], PCV10-SII [strategy 3]) in the pediatric vaccination program in South Africa. The analysis estimated that PCV13 would substantially reduce disease incidence over time when compared with switching to either of the PCV10 lower-valent vaccines. PCV13 was also found to be cost-saving over 5- and 10-year time horizons as compared with PCV10-SII and to be cost-effective over a 5-year time horizon and cost-saving over a 10-year time horizon when compared with PCV10-GSK.
The incremental clinical impact of PCV13 compared to PCV10-GSK was primarily driven by the additional protection offered by PCV13 against serotypes 3, 6A, and 19A, which has been supported not only by immunogenicity data but also by real-world evidence. For example, in Sweden, the childhood immunization program with PCV7 was replaced by either PCV13 or PCV10-GSK starting in October 2009 in different regions, and it showed no incidence of IPD caused by serotype 19A in children younger than 5 years in PCV13 regions from 2013 to 2016, whereas the incidence was 1.1 per 100,000 in PCV10 regions [33]. The incremental clinical impact of PCV13 compared to PCV10-GSK was also driven by the prediction of significant re-emergence of disease caused by serotypes not covered by lower-valent vaccines. Although it is uncommon to switch from a higher-valent to a lower-valent PCV, the evidence from Belgium demonstrated the risks associated with the change. In 2015, in consideration of vaccine cost, Belgium switched from PCV13 to PCV10-GSK. Almost immediately, there was a rapid increase in IPD incidence from 38.6 cases per 100,000 in 2013–2014 to 58.4 cases per 100,000 in 2017–2018, primarily driven by an increase in serotype 19A incidence [34]. Switching from a higher-valent vaccine (PCV13) to a lower-valent vaccine (PCV10-GSK or PCV10-SII) in South Africa could result in a similar phenomenon. Our model may underestimate the true impact of switching from PCV13 to a PCV10 in South Africa, considering that a previous cost-effectiveness study from Belgium using the same model found that switching from PCV13 to PCV10-GSK led to more observed pneumococcal disease cases and deaths than the model estimated [15]. Therefore, it is important to retain a broad serotype coverage, as this remains relevant to epidemiological considerations.
The incremental clinical impact of PCV13 compared to PCV10-SII was primarily driven by the additional protection offered by PCV13 against serotypes 3, 4, and 18C and by the fact that PCV10-SII has not demonstrated protection against pneumococcal pneumonia and OM in children aged under 5 years or protection against hospitalized pneumonia in children aged 5 years and older. By removing PCV13 pressure on serotype 18C, the model predicts that 18C will re-emerge as a dominant serotype and cause the majority of disease, potentially even returning to pre-PCV incidence levels. In addition, PCV10-SII has not shown any impact on nasopharyngeal carriage to date, and its herd effects are still unknown. PCV10-SII was compared to PCV10-GSK in the phase 3 pivotal non-inferiority study; this was inconsistent with World Health Organization guidance, which recommends that comparators for PCV non-inferiority trials be driven by the number of common serotypes [35, 36]. Since PCV13 was not used as the comparator, there are no matched immunogenicity responses for 6A and 19A; this could be problematic especially with respect to 19A, because this serotype requires a particularly strong immune response due to a high correlated protection threshold [37, 38]. Nevertheless, PCV13 is predicted to have greater public health impact in all scenarios and to be cost-saving or cost-effective in most scenarios.
The human immunodeficiency virus (HIV) epidemic in South Africa may contribute to an increase in pneumococcal disease incidence if PCV13 is replaced with PCV10-GSK or PCV10-SII. Prior to the introduction of PCV7 in South Africa, the incidence of IPD was greatest among HIV-infected individuals [39]. Infection with HIV greatly increases the risk of IPD in both children and adults, and UNAIDS estimates that up to 370,000 children under the age of 14 years in South Africa are currently living with HIV [40]. Although there has been a reduction in IPD in HIV-infected children over time, HIV-infected children under 2 years old are at a 21.1 times greater risk of IPD compared to HIV-uninfected children [41, 42]. Because of this, it is possible that newly resurgent serotypes following a switch from PCV13 to either PCV10-GSK or PCV10-SII may cause more IPD cases than originally estimated. Although we did not conduct a specific analysis in children infected with HIV, we would expect that PCV13 might provide more protection than lower-valent vaccines in the HIV-infected and HIV-exposed pediatric populations, as a study showed that the targeted immunization of South African infants has been temporally associated with a decline in vaccine-serotype colonization among HIV-infected individuals, including HIV-infected women [43].
Despite the success of PCV13 in South Africa, a significant proportion of pneumococcal disease burden is attributed to non-vaccine serotypes, particularly serotypes 10A, 11A, and 15B. To address the additional burden of pneumococcal disease caused by non-vaccine serotypes, two higher-valent PCVs (PCV15 and PCV20) are currently in development and could be available in South Africa by 2024 or 2025. Considering the potential availabilities of higher-valent vaccines soon, we estimated the public health impact of maintaining PCV13 then switching to a higher-valent vaccine (PCV15 [strategy 4] and PCV20 [strategy 5]) in 2024 versus both PCV10-SII and PCV10-GSK. In this analysis, we found that, when compared to both PCV10s, switching to higher-valent vaccines could potentially prevent many more pneumococcal cases; we also found that switching from PCV13 to PCV20 in 2024 would have a greater impact on disease burden over both 7- and 10-year periods than switching from PCV13 to PCV15 in 2024.
This is the first study to model the cost-effectiveness of PCV13 in South African children. It demonstrated that maintaining PCV13 in the NIP could have substantial public health impact when compared to lower-valent PCV options. However, the burden of disease in South African adults also remains high. In 2017, the South African guidelines for management of community-acquired pneumonia recommended PCV13 use in all adults aged 50 years and older starting in 2017. A recent study compared the cost-effectiveness of PCV13 and pneumococcal polysaccharide vaccine (PPSV23) in South African adults and estimated that PCV13 would have substantial public health impact by averting more cases of IPD, non-bacteremia pneumonia, and deaths than PPSV23 [44]. Along with vaccinating infants, direct protection via increased PCV13 vaccination in adults, particularly the elderly and HIV-infected individuals, may be an important strategy for reducing the burden of pneumococcal disease in South Africa.
Similar to other economic analyses, our study has some limitations. First, IPD incidence from 2013–2019 was used to estimate future incidence over the model time horizon (i.e., 2022–2028). Thus, the increases or decreases in serotype-specific incidence may not be precisely estimated. Secondly, there were no available OM incidence data from South Africa nor from any other African country that could be used as a proxy. We chose to use OM incidence data from Spain in the model because, like South Africa, Spain has a mature 2 + 1 PCV13 program with high vaccine uptake. However, we also included a scenario analysis that used local data from the pre-PCV13 period in South Africa and observed comparable cost-effectiveness results. Third, there is no available real-world evidence regarding PCV10-SII’s effectiveness. Since PCV10-SII has been licensed on the basis of immunogenicity and safety data, there are no available efficacy or effectiveness data regarding the vaccine’s effect on invasive or non-invasive disease, herd effects, or nasopharyngeal carriage. Although we conducted scenario analyses in which PCV10-SII was assumed to have the same effect on OM and pneumonia as PCV13, there is no evidence to assess the validity of these assumptions. Future cost-effectiveness analyses using PCV10-SII as a comparator would benefit from using published data on the vaccine’s effects when they become available. Fourth, the distribution of serotypes among non-IPD cases was assumed to vary proportionately to future IPD cases, which might not be true, as data comparing the proportion of all-cause vaccine-preventable non-IPD cases with serotype distribution of IPD are limited. Furthermore, the model structure and design do not allow us to conduct typical probabilistic or sensitivity analyses based on vaccine efficacy or to consider a mixed dosing strategy, an approach that has been introduced in some countries. Finally, this study did not incorporate any differences between single and multidose vial wastage rates, schedule administration nuances, or schedule adherence and completion. Completion of vaccination series and adherence to vaccination schedules are important to prevent breakthrough infections, and the inclusion of a booster in a schedule can increase the duration of direct protection and produce stronger indirect effects.
Conclusion
With the COVID-19 pandemic, governments worldwide are looking for ways to lower healthcare spending, and lower-valent PCVs may seem like an attractive option due to their perceived cost-savings. However, switching from a higher-valent to a lower-valent vaccine may lead to disease incidence re-emergence caused by previously covered serotypes. In South Africa, PCV13 has provided significant clinical and economic benefit since its inclusion in the NIP. Maintaining PCV13 was estimated to improve public health further by averting additional pneumococcal disease cases and saving more lives and was also estimated to reduce total costs in most scenarios. With higher-valent PCVs arriving to protect against disease burden not covered by PCV13, we can soon achieve the greatest public health impact. In summary, with wholistic planning in mind, and in consideration of current and future higher-valent vaccines—with their short-, middle- and long-term disease and economic impacts—this economic evaluation can help inform decision makers on which PCVs may provide the best value for money in South Africa.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was conducted by RTI Health Solutions, Research Triangle Park, NC, under the direction of Pfizer Inc. and was funded by Pfizer Inc., New York, NY, which owns Prevnar 13. Pfizer provided funding for the development of the model and manuscript as well as for the Rapid Service Fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author Contributions
LH, JEP, MRW, SN, and RS contributed to concept of the work. All authors contributed to statistical analysis, drafting, reviewing and revising the manuscript.
Disclosures
This work was sponsored by Pfizer Inc. Michele Wilson and Cheryl McDade are employees of RTI Health Solutions, who were paid consultants to Pfizer in connection with the development of this manuscript. Johnna Perdrizet, Liping Huang, Sophie Warren, and Renilla Sewdas are employees of Pfizer Inc. and may have owned stock or stock option at the time of this study. Susan Nzenze was a paid consultant to Pfizer in connection with the development of this manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Pneumococcal disease: the leading vaccine-preventable cause of death in children under five. 16 November 2016. https://www.gavi.org/pneumococcal-disease-leading-vaccine-preventable-cause-death-children-under-five. Accessed 24 August 2021.
- 2.Obaro S. Seven-valent pneumococcal conjugate vaccines for developing countries. Expert Rev Vacc. 2009;8(8):1051–1061. doi: 10.1586/erv.09.66. [DOI] [PubMed] [Google Scholar]
- 3.von Gottberg A, Cohen C, de Gouveia L, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: South Africa, 2003–2008. Vaccine. 2013;31(38):4200–4208. doi: 10.1016/j.vaccine.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 4.von Gottberg A, De Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 5.Yun J, Kleynhans J, Moyes J, et al. Epidemiology of respiratory pathogens from the influenza-like illness and pneumonia surveillance programmes, South Africa. 2019. https://www.nicd.ac.za/wp-content/uploads/2020/10/EPIDEMIOLOGY-OF-RESPIRATORY-PATHOGENS-FROM-THE-INFLUENZA-LIKE-ILLNESS-AND-PNEUMONIA-SURVEILLANCE-PROGRAMMES-SOUTH-AFRICA-2019.pdf. Accessed 17 August 2022.
- 6.Wasserman M, Palacios MG, Grajales AG, et al. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccin Immunother. 2019;15(3):560–569. doi: 10.1080/21645515.2018.1516491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserman M, Wilson M, Breton M, Peloquin F, McDade C, Farkouh R. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to a lower-valent (PCV10) vaccine in Canada. Presented at the 35th Annual Meeting of the European Society for Paediatric Infectious Diseases; 23–27 May 2017. Madrid, Spain.
- 8.Wasserman M, Wilson M, McDade C, et al. Estimating the clinical and economic impact of maintaining use of 13-valent pneumococcal conjugate vaccine (PCV13) in Mexico. Presented at the IDWeek 2017; 4–8 October 2017. San Diego, CA, USA.
- 9.Perdrizet J, Santana CFS, Senna T, et al. Cost-effectiveness analysis of replacing the 10-valent pneumococcal conjugate vaccine (PCV10) with the 13-valent pneumococcal conjugate vaccine (PCV13) in Brazil infants. Hum Vaccin Immunother. 2021;17(4):1162–1172. doi: 10.1080/21645515.2020.1809266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh S, Wasserman M, Moffatt M, et al. Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and the Netherlands: a cost-effectiveness analysis. Infect Dis Ther. 2020;9(2):305–324. doi: 10.1007/s40121-020-00287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserman MD, Sings HL, Wilson MR, et al. Re-analysis of modeling a switch from a 13-valent to 10-valent pneumococcal conjugate vaccine in Canada: leveraging real-world experience from Belgium. Infect Dis Ther. 2019;8(1):1–3. doi: 10.1007/s40121-018-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson M, Wasserman M, Breton M, et al. Potential clinical and economic impact of switching from the 13-valent to 10-valent pneumococcal conjugate vaccine in Quebec. Presented at the Canadian Immunization Conference; 6–8 December 2016. Ottawa, Ontario, Canada.
- 13.Wilson M, Wasserman M, Jadavi T, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–371. doi: 10.1007/s40121-018-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdrizet J, Horn EK, Nua W, et al. Cost-effectiveness of the 13-valent pneumococcal conjugate vaccine (PCV13) versus lower-valent alternatives in filipino infants. Infect Dis Ther. 2021;10(4):2625–2642. doi: 10.1007/s40121-021-00538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson MR, McDade CL, Perdrizet JE, Mignon A, Farkouh RA, Wasserman MD. Validation of a novel forecasting method for estimating the impact of switching pneumococcal conjugate programs: evidence from Belgium. Infect Dis Ther. 2021;10(3):1765–1778. doi: 10.1007/s40121-021-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics South Africa. Mid-year population estimates 2018. 23 July 2018. Report No. P0302. https://www.statssa.gov.za/publications/P0302/P03022018.pdf. Accessed 10 August 2022.
- 17.National Institute for Communicable Diseases. National Health Laboratory Service. GERMS-SA Annual Report 2019. http://www.nicd.ac.za/index.php/publications/germs-annual-reports/. Accessed 10 August 2022.
- 18.Tempia S, Moyes J, Cohen AL, et al. The national burden of influenza-like illness and severe respiratory illness overall and associated with nine respiratory viruses in South Africa, 2013–2015. Influenza Other Respir Viruses. 2022;16(3):438–451. doi: 10.1111/irv.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DP. University of the Witwatersrand, Johannesburg thesis 'Epidemiology of childhood pneumonia in the era of antiretroviral therapy and bacterial conjugate vaccines'. 2018.
- 20.Ministerio de Sanidad. Base de Datos Clínicos de Atención Primaria (BDCAP). Consulta Interativa del SNS. 2020. http://www.mscbs.es/. Accessed 14 October 2022.
- 21.Biagio-de Jager L, Swanepoel DW, Laurent C, Lundberg T. Paediatric otitis media at a primary healthcare clinic in South Africa. S Afr Med J. 2014;104(6):431–435. doi: 10.7196/SAMJ.7534. [DOI] [PubMed] [Google Scholar]
- 22.Walaza S, Buys A, Cohen C, Treurnicht F, Hellferscee O, Mcanerney J. Epidemiology of respiratory pathogens from influenza-like illness and pneumonia surveillance programmes. Natl Inst Commun Dis Bull. 2017;16:36–60. [Google Scholar]
- 23.Madhi SA, Govender N, Dayal K, et al. Bacterial and respiratory viral interactions in the etiology of acute otitis media in HIV-infected and HIV-uninfected South African children. Pediatr Infect Dis J. 2015;34(7):753. doi: 10.1097/INF.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistics South Africa. Mortality and causes of death in South Africa: findings from death notification 2018. 15 June 2021. Report No. P0309.3. https://www.who.int/data/gho/data/countries/country-details/GHO/south-africa?countryProfileId=e5bf5e3c-86a3-421f-89cc-18d787c36968. Accessed 22 August 2022.
- 25.Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–4214. doi: 10.1016/j.vaccine.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Pan American Health Organization. PAHO revolving fund vaccine prices for 2022. 2022. https://www.paho.org/en/documents/paho-revolving-fund-vaccine-prices-2022. Accessed 7 September 2022.
- 27.Department of Health. Medicines and Related Substances Act (101/1965) Regulations relating to a transparent pricing system for medicines and scheduled substances: Publication of guidelines for pharmacoeconomic submissions. Pretoria: Government Gazette. 2013.
- 28.Pfizer. Direct medical costs in South Africa. Key opinion leader input. Data on file. 2013.
- 29.Statistics South Africa (stats sa) Republic of South Africa. Consumer price index. 2022. https://www.statssa.gov.za/. Accessed 7 September 2022.
- 30.Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–545. doi: 10.1016/j.jval.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 31.van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–7213. doi: 10.1016/j.vaccine.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Bennett JE, Sumner W, 2nd, Downs SM, Jaffe DM. Parents' utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–48. [PubMed] [Google Scholar]
- 33.Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65(11):1780–1790. doi: 10.1093/cid/cix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desmet S, Lagrou K, Wyndham-Thomas C, et al. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2021;21(1):127–136. doi: 10.1016/s1473-3099(20)30173-0. [DOI] [PubMed] [Google Scholar]
- 35.Clarke E, Bashorun A, Adigweme I, et al. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in the Gambia: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021;21(6):834–846. doi: 10.1016/s1473-3099(20)30735-0. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. WHO expert committee on biological standardization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines, Annex 3, TRS No 977. Replacement of WHO Technical Report Series, No. 927, Annex 2. 2013. https://www.who.int/publications/m/item/pneumococcal-conjugate-vaccines-annex3-trs-977. Accessed 14 October 2022.
- 37.Madhi SA, Knoll MD. An affordable pneumococcal conjugate vaccine after 20 years. Lancet Infect Dis. 2021;21(6):751–753. doi: 10.1016/s1473-3099(21)00002-5. [DOI] [PubMed] [Google Scholar]
- 38.Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–846. doi: 10.1016/s1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
- 39.Meiring S, Cohen C, Quan V, et al. HIV infection and the epidemiology of invasive pneumococcal disease (IPD) in South African adults and older children prior to the introduction of a pneumococcal conjugate vaccine (PCV) PLoS ONE. 2016;11(2):e0149104. doi: 10.1371/journal.pone.0149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UNAIDS. South Africa HIV and AIDS Estimates in 2021. 2022. https://www.unaids.org/en/regionscountries/countries/southafrica. Accessed 7 September 2022.
- 41.Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J. 2000;19(12):1141–1147. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Nunes MC, von Gottberg A, de Gouveia L, et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS ONE. 2011;6(11):e27929. doi: 10.1371/journal.pone.0027929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nzenze SA, von Gottberg A, Shiri T, et al. Temporal changes in pneumococcal colonization in HIV-infected and HIV-uninfected mother-child pairs following transitioning from 7-valent to 13-valent pneumococcal conjugate vaccine, Soweto. South Africa J Infect Dis. 2015;212(7):1082–1092. doi: 10.1093/infdis/jiv167. [DOI] [PubMed] [Google Scholar]
- 44.Feldman C, Dlamini SK, Madhi SA, et al. The cost-effectiveness of using pneumococcal conjugate vaccine (PCV13) versus pneumococcal polysaccharide vaccine (PPSV23), in South African adults. PLoS ONE. 2020;15(1):e0227945. doi: 10.1371/journal.pone.0227945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spanish Ministry of Health. Consulta Interativa del SNS (mscbs.es). Base de Datos Clínicos de Atención Primaria (BDCAP) 2022. https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S/base-de-datos-de-clinicos-de-atencion-primaria-bdcap. Accessed 24 October 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.