Abstract
To explore the relationship between cognitive function and blood–brain barrier leakage in non-brain metastasis lung cancer and healthy controls. 75 lung cancers without brain metastasis and 29 healthy controls matched with age, sex, and education were evaluated by cognitive assessment, and the Patlak pharmacokinetic model was used to calculate the average leakage in each brain region according to the automated anatomical labeling atlas. After that, the relationships between cognitive and blood–brain barrier leakage were evaluated. Compared with healthy controls, the leakage of bilateral temporal gyrus and whole brain gyrus were higher in patients with lung cancers (P < 0.05), mainly in patients with advanced lung cancer (P < 0.05), but not in patients with early lung cancer (P > 0.05). The cognitive impairment of advanced lung cancers was mainly reflected in the damage of visuospatial/executive, and delayed recall. The left temporal gyrus with increased blood–brain barrier leakage showed negative correlations with delayed recall (r = -0.201, P = 0.042). An increase in blood–brain barrier leakage was found in non-brain metastases advanced lung cancers that corresponded to decreased delayed recall. With progression in lung cancer staging, blood–brain barrier shows higher leakage and may lead to brain metastases and lower cognitive development.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11682-022-00745-3.
Keywords: Lung cancer, Staging, Cognitive impairment, Neuroimaging, Blood–brain barrier
Introduction
Lung cancer (LC) is the most common cancer and the leading cause of cancer death in both men and women(Feng et al., 2019; Sung et al., 2021). Due to untimely diagnosis, most lung cancers are advanced at the time of diagnosis(Alberg et al., 2013). The incidence of brain metastases (BMs) ranges from 22 to 54% and can occur at different stages of tumor development, especially in patients with advanced disease(Alberg et al., 2005). BM is present in 10% of patients at the time of initial diagnosis(Villano et al., 2015). The brain is the only site of tumor recurrence in 50% of patients(Hu et al., 2006). Many chemotherapeutic agents are relatively poor penetrators of the central nervous system (CNS), allowing tumor cells to survive and develop into BM in the CNS. Although recent therapeutic advances including intrathecal chemotherapy, molecular targeted therapies, and immunotherapy have somewhat prolonged the survival time of BM patients, the prognosis of BM remains poor with a median overall survival of 7–13 months(Kondziolka et al., 2005). BM may lead to different focal neurological symptoms and cognitive dysfunction(Chen et al., 2007; Noh & Walbert, 2018).
The blood–brain barrier (BBB) is a selective "gatekeeper" composed of endothelial cells, pericytes, and astrocyte end-foot. BBB separates the brain from systemic circulation and protects it from foreign infectious and/or toxic agents(Arvanitis et al., 2020; Cheng & Perez-Soler, 2018; Fares et al., 2020b). It is estimated that BBB blocks the transport of about 98% of the molecules in systemic circulation(Pardridge, 2005). The integrity of the BBB is essential to block the entry of most tumor cells.
The pathogenesis of BM is complex, and BBB dysfunction is one of its pathogenic mechanisms. Metastasis of lung cancer cells to the brain requires cancer cells to invade the circulatory system from the primary site and colonize in brain parenchyma through the invasion of BBB(Fares et al., 2020a, 2020b). Research has shown that BBB is damaged in this process, resulting in increasing permeability of BBB(Langley & Fidler, 2013). BBB dysfunction (BBBD) is also a feature of other brain diseases, such as stroke(Serlin et al., 2019), subarachnoid hemorrhage (Lublinsky et al., 2019), and epilepsy (Rüber et al., 2018). Recently considered as a potential biomarker for predicting outcome(Bar-Klein et al., 2017; Kamintsky et al., 2020; Lublinsky et al., 2019; Serlin et al., 2019). Although the detailed mechanism of BBBD is unknown, paracellular leakage due to dysfunction or downregulation of tight junction proteins, as well as enhanced endothelial barrier transport across cells, are considered to be pathogenic factors(Andreone et al., 2017; Zhang et al., 2017). Currently, there is no clinically standardized method to objectively assess changes in microvascular permeability in vivo.
Dynamic enhanced magnetic resonance imaging (DCE-MRI) is increasingly being used to assess cerebrovascular permeability in neurological diseases(Sweeney et al., 2019). The widely used DCE-MRI approach is based on extended Tofts or Patlak models(Patlak et al., 1983; Sourbron & Buckley, 2013; Tofts & Kermode, 1991). In lung cancer patients without brain metastasis, there is a lack of quantitative data on BBB leakage and a lack of understanding of the spatial scope of BBB. Therefore, we aim to quantify the BBB leakage in lung cancer patients without brain metastasis and to examine the relationship between BBB leakage and cognitive function.
Materials and methods
Participant
This study was approved by the Ethics Committee of the third affiliated Hospital of Kunming Medical University (NO. SLKYLX202118). all subjects signed informed consent before participating in the research. This work was conducted by the principles of the Declaration of Helsinki and its later amendments.
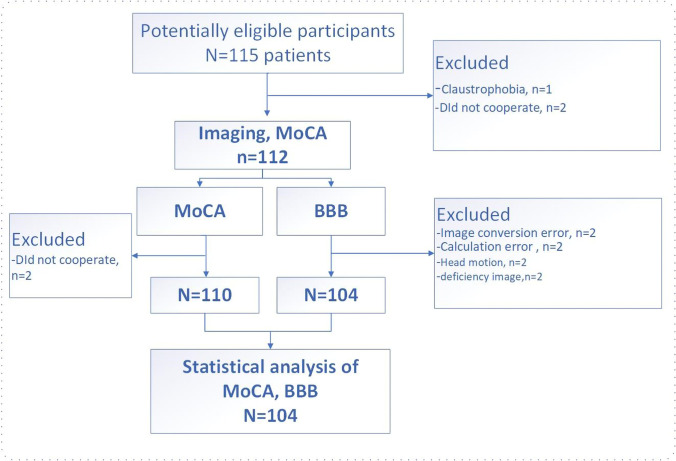
This is a cross-sectional study of lung cancer patients, which plans to scan untreated patients and include age/sex-matched healthy controls (HCs). From August 2021 to February 2022, 75 lung cancer patients (39 patients with early-stage lung cancer and 36 patients with advanced lung cancer) from the Department of Thoracic Surgery, Third Affiliated Hospital of Kunming Medical University, and 29 HCs (enrolled through online advertisement, matched for age and gender) participated in this study. All participants were untreated (i.e., surgery, radiotherapy, or immunotherapy) before pathological confirmation. According to TNM (tumor, node, metastasis) staging (8th edition) (Detterbeck et al., 2017), early-stage patients are those with stage I, advanced-stage patients are those with stage II to IV. Some participants were excluded due to excessive head movement during scanning, contrast allergy, and calculation failure (Fig. 1). Exclusion criteria for all participants were: receipt of prophylactic cranial irradiation; the presence of brain metastases; known history of stroke, cranial trauma, epilepsy, Alzheimer's disease, Parkinson's disease, other acute psychiatric or neurological disorders; history of major medical illness (e.g., anemia, severe heart disease, thyroid dysfunction, or abnormal liver or kidney function); and severe vision or hearing loss.
Fig. 1.
Flow chart of subjects enrollment
MRI acquisition
All data were acquired on a 3.0 T MRI scanner (Discovery MR750, GE Healthcare, Waukesha, Wisconsin, USA) with a 21-channel receiver array head and neck coil, acquired in parallel. Tight but comfortable foam pads are used to minimize head movement; earplugs and headphones are used to reduce scanner noise. For each participant, routine MRI sequences, including T2 and T1 weighted imaging and T2 fluid-attenuated inversion recovery (FLAIR) imaging, were performed to ensure that there were no visible brain lesions or brain metastases.
The sequences for BBB assessment included the following steps: (1) a T1 -weighted 3D axial sequence with variable flip angles (3D-SPGR, TR 5.9 ms, TE 2.0 ms, flip angle 5° and 14°, FOV 24 × 20.4, acquisition matrix 256 × 180, slice thickness 4. 0 mm, interval 0, bandwidth 62.5 kHz); and (3) a T1-weighted 3D axial dynamic scan (LAVA, TR 5.9 ms, TE 2.0 ms, flip angle 14°, FOV 24 × 20.4, acquisition matrix 256 × 180, slice thickness 4.0 mm, interval 0, bandwidth 62.5 kHz) acquired within 650 s after intravenous injection of the magnetic contrast gadolinium (0.05 mmol/kg, flow rate 3.0 mL/s).
BBB data preprocessing
Because the contrast medium leakage caused by the blood–brain barrier leakage increases the T1-weighted signal in the tissue, the contrast medium leakage can be calculated to reflect the BBB leakage. To achieve this, SPM12 (University College London, www.fil.ion.ucl.ac.UK/spm) is first used to preprocess data. The specific steps include: image format conversion; head movement correction; image registration; image segmentation; standardization; smoothing; individual space conversion.
Previous studies have shown that patlak model is the more accurate than other models in diseases with slight BBB damage(Barnes et al., 2016; Heye et al., 2016; Patlak et al., 1983). And using DCE-MRI to measure the subtle leakage of the BBB has moderate to excellent repeatability(Wong et al., 2017). Therefore, the patlak model will be used to calculate the Ktrans value in this study. The Patlak method uses a two-compartment model, in which it is assumed that there is no reflux and infinite flow, so the leakage rate is approximately the product of vascular permeability (P) and the surface area (S) per unit tissue mass. For the Patlak graphic method, the two main determinants of the accuracy of measuring BBB are the estimation of blood concentration curve based on T1 signal intensity and the determination of vascular input function (VIF)(Larsson et al., 2009) (Fig. 2). The common method of T1 mapping is to change the flip angle(Brookes et al., 1999). The determination step of VIF also plays a key role in the estimation of kinetic parameters. The VIF is calculated by selecting the region of interest (ROI) of the superior sagittal sinus(Lavini & Verhoeff, 2010). After that, the Patlak model is used to calculate the volume transfer constant Ktrans (minite−1) based on MATLAB (R2017b). The Ktrans value is used to reflect the leakage of BBB.
Fig. 2.
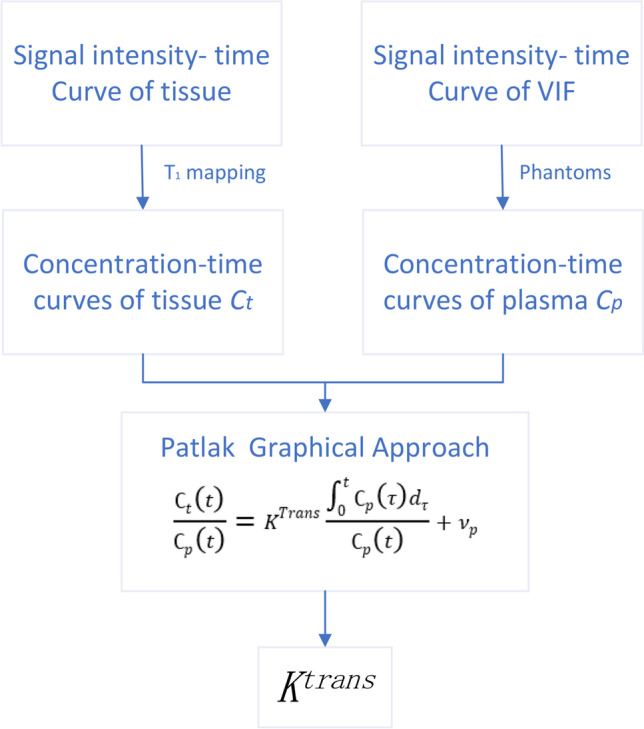
Converts the time-signal strength curve of tissue and vascular input function (VIF) into a time-concentration curve. The leakage rate (Ktrans) was calculated by Patlak graphic method
Ktrans is calculated as voxels. Based on the AAL atlas(Tzourio-Mazoyer et al., 2002).
It is assumed that the increased BBB leakage in the brain regions related to cognitive function, such as frontal gyrus, parietal gyrus, temporal gyrus, hippocampus, and cingulate gyrus(Correa et al., 2017; de Ruiter et al., 2012; Inagaki et al., 2007; Mentzelopoulos et al., 2021; Shiroishi et al., 2017; Simó et al., 2015), leads to the decrease of cognitive function. therefore, we use these brain regions to extract the average Ktrans value of BBB leakage for ROI for analysis.
Statistical analyses
The continuous variables were compared by ANOVA or Kruskal–Wallis test, and the classified variable ratio was compared by the χ 2 test. All statistical analyses are carried out using the software R (version 3.6.3, http://www.r-project.org/). The R language and GraphPad Prism (9.0.0, GraphPad Software, Inc., San Diego, California) were used for visualization. Multiple comparisons are corrected by Bonferroni.
Result
Demographic and clinical features
A summary of all detailed demographic data and histological diagnosis and tumor staging is shown in Table 1. There was no significant difference in sex, age, smoking, and KPS score between LCs and HCs (P > 0.05). The longest diameter of the tumor in the eLC group was smaller than that in the aLC group (P < 0.001). The tumor markers CEA, NSE, CYFRA21-1 and SCC in the aLC group were higher than those in the eLC group.
Table 1.
Demographic and clinical features of patients with Lung cancer
| Controls(n = 29) | Early lung cancer (n = 39) | Advanced lung cancer (n = 36) | χ2/F/μ | pvalue | |
|---|---|---|---|---|---|
| gender | |||||
| male/female | 15/14 | 15/24 | 24/12 | 5.986 | 0.05a |
| Age(mean ± SD), year | 51.07 ± 9.67 | 55.59 ± 8.23 | 56.06 ± 8.99 | 3.059 | 0.051b |
| Smoking(%) | 10/29 | 9/39 | 16/36 | 3.84 | 0.147a |
| Tumor diameter (cm) (mean ± SD) | 1.62 ± 0.94 | 4.93 ± 2.35 | -8.103 | 0.000*c | |
| KPS score(mean ± SD) | 95.00 ± 7.77 | 96.15 ± 7.11 | 94.44 ± 6.95 | 0.568 | 0.568b |
| Years of education (mean ± SD) | 10.00 ± 3.67 | 8.95 ± 3.50 | 7.92 ± 4.89 | F = 2.102 | 0.127 |
| Pathological type | χ2 | ||||
| Squamous cell carcinoma | 7 | 13 | 7.396 | 0.092a | |
| Adenocarcinoma | 29 | 18 | |||
| Small Cell Lung Cancer | 3 | 5 | |||
| Tumor markers (25%,50%,75%) | Z | ||||
| CEA | 1.53, 2.25, 3.57 | 1.87,4.38, 10.33 | 446.50 | 0.007*d | |
| NSE | 9.60, 11.80, 13.40 | 11.75, 13.70,21.96 | 397.50 | 0.001*d | |
| CYFRA21-1 | 1,40, 2.00,2.60 | 2.625,4.500,7.025 | 223.00 | 0.000*d | |
| SCC | 0.70,0.80,1.00 | 0.72,1.10,1.47 | 493.00 | 0.026*d | |
Data are expressed as ‾x ± s, n (%) or Interquartile Range (P25,P50,P75). aThe P values are obtained by using the χ2 test. bThe P values are obtained by using one-way ANOVA. cThe P values are obtained by using two-sample t-test. dThe P values are obtained by the Kruskal–Wallis test. *P < 0.05 is considered significant. CEA, carcinoembryonic antigen. NSE, neuron-specific enolase. CYFRA21-1, cytokeratins21-1. SCC, squamous cell carcinoma antigen
The BBB leakage in patients with lung cancer at different stages
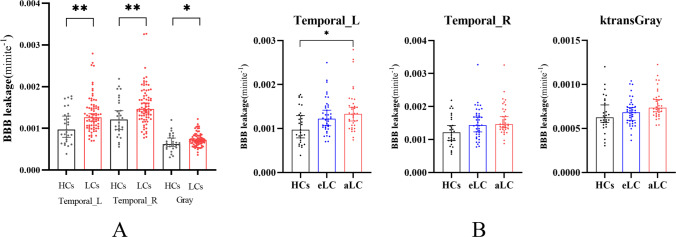
We extracted and analyzed the average Ktrans values of BBB in bilateral frontal gyrus, parietal gyrus, temporal gyrus, hippocampus and cingulate gyrus. Compared with HCs, the Ktrans of bilateral temporal gyrus and whole brain gyrus were increased in LCs. mainly in patients with advanced lung cancer (P < 0.05), but not in patients with early lung cancer (P < 0.05). As shown in Figs. 3 and 4.
Fig. 3.
Comparison of BBB leakage between patients with LC HCs. A Comparison of LCs and HCs. B Comparison of eLCs, aLCs and HCs gyrus
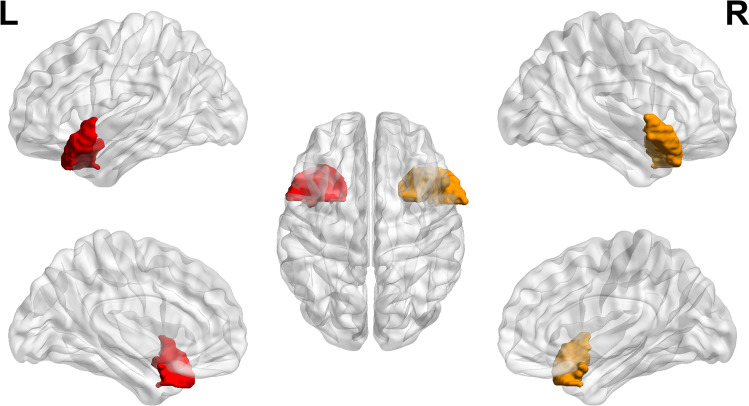
Fig. 4.
Location and distribution of hyperosmotic brain areas in the brain
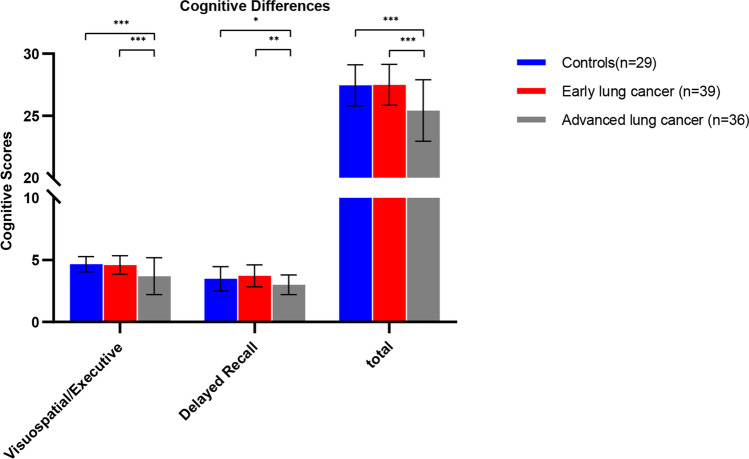
Cognitive impairment in patients with advanced lung cancer
The cognitive impairment of aLCs was mainly reflected in the dysfunction of visuospatial/executive, and delayed recall. There was no significant difference between eLCs and HCs. and it was considered that the cognitive function of eLCs had not been impaired. Moreover, the damage of advanced lung cancer is more obvious than that of early patients. As detailed in Fig. 5.
Fig. 5.
Difference in cognitive function between patients with lung cancer in different stages and HCs. Total = total score of cognitive function
Correlation analysis between increased brain leakage and cognitive function in patients with lung cancer at different stages
To examine the correlation between increased brain areas of BBB leakage and cognitive function, we analyzed the correlation between Ktrans value and cognitive function of participants, and controlled age and gender factors. It was found that the Ktrans of the left temporal gyrus was negatively correlated with delayed recall (r = -0.201, P = 0.042). We further correlated the maximum tumor diameter, serum markers (CEA, NSE, CYFRA21-1, SCC) and brain areas with increased Ktrans in LCs and controlled age and gender factors, and we did not find any correlation between them. We also examined the relationship between leakage increased brain areas and tumor size and serum tumor markers and controlled age and gender factors, and we also found no correlation between them (Table 2, Fig. 6).
Table 2.
Partial correlation between BBB leakage and cognitive function in patients with LC
| BBB leakage | cognitive function | R | P |
|---|---|---|---|
| Temporal_L | Delayed recall | -0.201 | 0.042 |
Fig. 6.

Correlation of left temporal gyrus with impaired delayed recall in patients with lung cancer and HCs
Discussion
This study was the first to quantitatively image BBB leakage in lung cancer patients who had not yet developed brain metastases and found that aLC exhibited higher levels of BBB leakage in some brain regions and correlated with cognitive impairment in patients. These findings provide a critical step in determining the role of BBBD in the pathogenesis of brain metastases from lung cancer and highlight the BBB as a potential diagnostic and predictive target.
BMs cause severe, uncontrollable symptoms and reduce quality of life, such as paralysis, increased intracranial pressure, or seizures, and the incidence of BM has shown an upward trend in the last decade, but there has been little progress in treatment options and poor outcomes(Soffietti et al., 2017). Therefore, it is important to be able to detect early BM in patients with lung cancer who are more prone to develop BM and to provide enhanced adjuvant therapy for them. In this study, BBB leakage examination was performed in patients who had not yet developed visible brain metastases, and increased leakage was found in patients with progressive disease. This suggests an increased risk of brain metastasis.
The development of BM involves a series of interrelated steps, starting with the invasion of cancer cells into the intravascular and/or lymphatic system. Due to the lack of a lymphatic system in the central nervous system (CNS), the only possible way for cancer cells to reach the brain is through blood circulation. The circulating tumor cells (CTCs) may circulate into the brain's microcirculation and adapt to the brain's microenvironment, resulting in the formation of micro-metastases, which eventually form visible tumors through "metastatic colonization"(Hanahan & Weinberg, 2011; Paduch, 2016). However, metastatic cells that invade the CNS parenchyma must pass through the BBB. During this process, changes occur in the cerebral vascular endothelium(Bart et al., 2000), including impaired tight junction structures and increased perivascular gaps(Liebner et al., 2000). In addition, windows corresponding to the surrounding vascular system can be found in these vessels, and the number and activity of cytosolic vesicles are increased(Shibata, 1989). Therefore, these vessels may reflect those of the tumor tissue rather than those of the CNS endothelium. As a result of these structural alterations leading to increased BBB leakiness, plasma leaks into the extracellular space outside the vasculature(Nduom et al., 2013). And our findings coincide with this process.
DCE-MRI allows the leakage of the extracellular gap within each voxel to be assessed by pharmacokinetic parameters (Ktrans), detecting leakage of the BBB in response to the destruction of the BBB. Ktrans is defined as the volume transfer constant between the plasma and the extracellular space outside the blood vessel, and it is often used as a synonym for permeability(Tofts & Kermode, 1991). DCE-MRI has been widely used in neuro-oncology imaging(Bar-Klein et al., 2017; Kamintsky et al., 2020; Lublinsky et al., 2019; Rüber et al., 2018; Serlin et al., 2019). However, measuring leakage from a relatively intact BBB is not common. And the leakage of BBB we need to measure may be microleakage, so the patlak model is used to calculate Ktrans because it is more accurate in measuring microleakage(Barnes et al., 2016; Heye et al., 2016; Patlak et al., 1983).
Our findings confirm that, compared to the HCs, LCs have higher levels of BBB leakage in bilateral temporal gyrus and whole brain gyrus, especially more pronounced in aLCs. It suggests that as LC progress, higher leakage of the BBB may occur in brain areas and the brain BBB integrity suffers disruption. And these changes indicate changes in the cerebral vascular endothelium in these brain regions, damage to tight junction structures and increased perivascular gaps(Soffietti et al., 2017). This signals the possibility of micro-metastasis, or a shift from BBB to BTB, as BTB is often considered “leakier” than BBB. Therefore, without further clinical intervention, visible BM may develop. Early detection of increased BBB leakage and enhanced clinical intervention are important to prevent the development of BMs.
Cognitive impairment in cancer patients has been of concern and increases the risk of developing dementia(Heck et al., 2008; Lovelace et al., 2019). Functional impairment of visuospatial/executive and delayed recall was found in patients with advanced lung cancer, and the decrease was more obvious with the progression of the disease. It is suggested that the development of lung cancer will impair cognitive function, giving priority to visuospatial/executive and delayed recall, but the specific mechanism is unclear. Some neuroimaging studies have shown that the structural and functional changes of frontal gyrus, temporal gyrus, parietal gyrus and hippocampus are related to cognitive impairment(Correa et al., 2017; de Ruiter et al., 2012; Inagaki et al., 2007; Mentzelopoulos et al., 2021; Shiroishi et al., 2017; Simó et al., 2015). Our study found that the BBB leakage in left temporal gyrus was increased and negatively correlated with delayed recall. It is well known that temporal lobe is associated with verbal and episodic memory(Buckner & Wheeler, 2001; McDermott et al., 2000; Pievani et al., 2011). Therefore, we speculate that BBB damage may be caused by the secretion of cytokines from lung cancer. Because it contains a complex network of tissue stroma, infiltrating immune cells and tumor cells, all of these cells can produce cytokines(Seruga et al., 2008). Some clinical studies have shown that peripheral cytokines are associated with cognitive decline(Andreotti et al., 2015; Ma et al., 2016; Wu et al., 2016).So, in the treatment of lung cancer, it is necessary to consider the cognitive impairment of patients, avoid inappropriate treatment, aggravate the cognitive impairment of patients, and reduce the quality of life.
The advantages of this study include no recruitment bias, and the time between clinical evaluation and neuroimaging regimens is close, usually, after clinical evaluation, an MRI examination is completed within 1 day. However, the sample size of our cohort is small, and our imaging scheme requires a gadolinium-based contrast agent, which may cause potential damage to the kidney. BBB imaging without contrast agents may have more extensive applicability.
Conclusion
This study confirmed for the first time that there is an increase in BBB permeability in patients with non-brain metastasis before treatment, which is related to cognitive impairment, indicating that the occurrence and development of lung cancer itself can cause cognitive impairment, which is not related to treatment. In the treatment of lung cancer, the cognitive dysfunction should be fully considered to avoid aggravating the damage and reducing the quality of life. Therefore, this study provides new preliminary evidence for individualized treatment of lung cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study is a joint effort of many investigators and staff members, and their contribution is gratefully acknowledged. We especially thank all patients who participated in this study.
Abbreviations
- LC
Lung cancer
- HC
Healthy control
- BBB
Blood-brain barrier
- BBBD
BBB dysfunction
- BMs
Brain metastases
- eLC
Early lung cancers
- aLC
Advanced lung cancers
- CNS
Central nervous system
- DCE-MRI
Dynamic enhanced magnetic resonance imaging
- ROI
Region of interest
- VIF
Vascular input function
- AAL
Anatomical labeling
- CEA
Carcinoembryonic antigen
- NSE
Neuron-specific enolase
- CYFRA21-1
Cytokeratins21-1
- SCC
Squamous cell carcinoma antigen
- Temporal_L
Left temporal gyrus
- Temporal_R
Right temporal gyrus
- Gyrus
The whole brain gyrus matter
Author contributions
Da-Fu Zhang: Data curation, Methodology, Software, Writing-Original draft preparation.
Yu-Qi Chen: Conceptualization, Writing- Reviewing and Editing.
Zhen-hui Li: Visualization, Investigation.
Xiu-Feng Xu: Supervision.
Ying-Ying Ding: Writing- Reviewing and Editing.
Bin-li Shang: Software.
Zhi-Ping Zhang, Yin-Fu He: Data curation.
All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82060259, 81760296, 82001986); Yunnan Province High-Level Health Technical Talents (leading talents) (L-2019004, L-2019011); Yunnan Province Special Project for Famous Medical Talents of the “Ten Thousand Talents Program” (YNWRMY- 2018–040, YNWR-MY-2018–041); Yunnan digitalization, development and application of biotic resource (202002AA100007); The Outstanding Youth Science Foundation of Yunnan Basic Research Project (202101AW070001). Innovation Team of Kunming Medical University (CXTD202110). The Applied Basic Research Projects of Yunnan Province(2019FE001-083).
Data Availability
The data from this article cannot be shared publicly due to the privacy of the individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Declarations
Ethical Approval and Consent to Participate
This study was approved by the Ethics Committee of the third affiliated Hospital of Kunming Medical University (NO. SLKYLX202118). all participants signed informed consent before participating in the research. This work was conducted by the principles of the Declaration of Helsinki and its later amendments.
Consent to Publish
The Author confirms:
That the work described has not been published before (except in the form of an abstract or as part of a published lecture, review, or thesis);
That it is not under consideration for publication elsewhere;
That its publication has been approved by all co-authors;
That its publication has been approved by the responsible authorities at the institution where the work is carried out.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Da-Fu Zhang and Zhen-Hui Li contributed equally.
Contributor Information
Xiu-Feng Xu, Email: xfxu2004@sina.com.
Ying-Ying Ding, Email: dingyingying0428@163.com.
Yu-Qi Cheng, Email: yuqicheng@126.com.
References
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: Looking to the future. Journal of Clinical Oncology. 2005;23(14):3175–3185. doi: 10.1200/jco.2005.10.462. [DOI] [PubMed] [Google Scholar]
- Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron. 2017;94(3):581–594.e585. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, King AA, Macy E, Compas BE, DeBaun MR. The Association of Cytokine Levels With Cognitive Function in Children With Sickle Cell Disease and Normal MRI Studies of the Brain. Journal of Child Neurology. 2015;30(10):1349–1353. doi: 10.1177/0883073814563140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nature Reviews Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H, Senatorov VV, Jr, Swissa E, Rosenbach D, Elazary N, Milikovsky DZ, Milk N, Kassirer M, Rosman Y, Serlin Y, Eisenkraft A, Chassidim Y, Parmet Y, Kaufer D, Friedman A. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140(6):1692–1705. doi: 10.1093/brain/awx073. [DOI] [PubMed] [Google Scholar]
- Barnes SR, Ng TS, Montagne A, Law M, Zlokovic BV, Jacobs RE. Optimal acquisition and modeling parameters for accurate assessment of low Ktrans blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magnetic Resonance in Medicine. 2016;75(5):1967–1977. doi: 10.1002/mrm.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart J, Groen HJ, Hendrikse NH, van der Graaf WT, Vaalburg W, de Vries EG. The blood-brain barrier and oncology: New insights into function and modulation. Cancer Treatment Reviews. 2000;26(6):449–462. doi: 10.1053/ctrv.2000.0194. [DOI] [PubMed] [Google Scholar]
- Brookes JA, Redpath TW, Gilbert FJ, Murray AD, Staff RT. Accuracy of T1 measurement in dynamic contrast-enhanced breast MRI using two- and three-dimensional variable flip angle fast low-angle shot. Journal of Magnetic Resonance Imaging. 1999;9(2):163–171. doi: 10.1002/(sici)1522-2586(199902)9:2<163::aid-jmri3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nature Reviews Neuroscience. 2001;2(9):624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: Clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668–1675. doi: 10.1002/cncr.22565. [DOI] [PubMed] [Google Scholar]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. The Lancet Oncology. 2018;19(1):e43–e55. doi: 10.1016/s1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- Correa DD, Root JC, Kryza-Lacombe M, Mehta M, Karimi S, Hensley ML, Relkin N. Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: A pilot study. Brain Imaging and Behavior. 2017;11(6):1652–1663. doi: 10.1007/s11682-016-9608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, Lavini C, Linn SC, Boven E, van Dam FS, Schagen SB. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Human Brain Mapping. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduction and Targeted Therapy. 2020;5(1):28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J, Kanojia D, Rashidi A, Ulasov I, Lesniak MS. Landscape of combination therapy trials in breast cancer brain metastasis. International Journal of Cancer. 2020;147(7):1939–1952. doi: 10.1002/ijc.32937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: Good or bad news from the 2018 Global Cancer Statistics? Cancer Communications (Lond) 2019;39(1):22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heck JE, Albert SM, Franco R, Gorin SS. Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy. Journal of the American Geriatrics Society. 2008;56(9):1687–1692. doi: 10.1111/j.1532-5415.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- Heye AK, Thrippleton MJ, Armitage PA, Valdés Hernández MDC, Makin SD, Glatz A, Sakka E, Wardlaw JM. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. NeuroImage. 2016;125:446–455. doi: 10.1016/j.neuroimage.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, Komaki R, Liao Z. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106(9):1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Kamintsky L, Cairns KA, Veksler R, Bowen C, Beyea SD, Friedman A, Calkin C. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2020;26:102049. doi: 10.1016/j.nicl.2019.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Martin JJ, Flickinger JC, Friedland DM, Brufsky AM, Baar J, Agarwala S, Kirkwood JM, Lunsford LD. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005;104(12):2784–2791. doi: 10.1002/cncr.21545. [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. The biology of brain metastasis. Clinical Chemistry. 2013;59(1):180–189. doi: 10.1373/clinchem.2012.193342. [DOI] [PubMed] [Google Scholar]
- Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magnetic Resonance in Medicine. 2009;62(5):1270–1281. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- Lavini C, Verhoeff JJ. Reproducibility of the gadolinium concentration measurements and of the fitting parameters of the vascular input function in the superior sagittal sinus in a patient population. Magnetic Resonance Imaging. 2010;28(10):1420–1430. doi: 10.1016/j.mri.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathologica. 2000;100(3):323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Lovelace DL, McDaniel LR, Golden D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. Journal of Midwifery & Women's Health. 2019;64(6):713–724. doi: 10.1111/jmwh.13012. [DOI] [PubMed] [Google Scholar]
- Lublinsky S, Major S, Kola V, Horst V, Santos E, Platz J, Sakowitz O, Scheel M, Dohmen C, Graf R, Vatter H, Wolf S, Vajkoczy P, Shelef I, Woitzik J, Martus P, Dreier JP, Friedman A. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. eBioMedicine. 2019;43:460–472. doi: 10.1016/j.ebiom.2019.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Wu T, Zhao J, Song A, Liu H, Xu W, Huang G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Science and Reports. 2016;6:37486. doi: 10.1038/srep37486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL., 3rd Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event-related fMRI study. Journal of Cognitive Neuroscience. 2000;12(6):965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Mentzelopoulos A, Gkiatis K, Karanasiou I, Karavasilis E, Papathanasiou M, Efstathopoulos E, Kelekis N, Kouloulias V, Matsopoulos GK. Chemotherapy-Induced Brain Effects in Small-Cell Lung Cancer Patients: A Multimodal MRI Study. Brain Topography. 2021;34(2):167–181. doi: 10.1007/s10548-020-00811-3. [DOI] [PubMed] [Google Scholar]
- Nduom EK, Yang C, Merrill MJ, Zhuang Z, Lonser RR. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. Journal of Neurosurgery. 2013;119(2):427–433. doi: 10.3171/2013.3.Jns122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh T, Walbert T. Brain metastasis: Clinical manifestations, symptom management, and palliative care. Handbook of Clinical Neurology. 2018;149:75–88. doi: 10.1016/b978-0-12-811161-1.00006-2. [DOI] [PubMed] [Google Scholar]
- Paduch R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cellular Oncology (Dordrecht) 2016;39(5):397–410. doi: 10.1007/s13402-016-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow and Metabolism. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurology. 2011;10(9):829–843. doi: 10.1016/s1474-4422(11)70158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüber T, David B, Lüchters G, Nass RD, Friedman A, Surges R, Stöcker T, Weber B, Deichmann R, Schlaug G, Hattingen E, Elger CE. Evidence for peri-ictal blood-brain barrier dysfunction in patients with epilepsy. Brain. 2018;141(10):2952–2965. doi: 10.1093/brain/awy242. [DOI] [PubMed] [Google Scholar]
- Serlin Y, Ofer J, Ben-Arie G, Veksler R, Ifergane G, Shelef I, Minuk J, Horev A, Friedman A. Blood-Brain Barrier Leakage: A New Biomarker in Transient Ischemic Attacks. Stroke. 2019;50(5):1266–1269. doi: 10.1161/strokeaha.119.025247. [DOI] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathologica. 1989;78(6):561–571. doi: 10.1007/bf00691283. [DOI] [PubMed] [Google Scholar]
- Shiroishi, M. S., Gupta, V., Bigjahan, B., Cen, S. Y., Rashid, F., Hwang, D. H., Lerner, A., Boyko, O. B., Liu, C. J., Law, M., Thompson, P. M., & Jahanshad, N. (2017). Brain cortical structural differences between non-central nervous system cancer patients treated with and without chemotherapy compared to non-cancer controls: a cross-sectional pilot MRI study using clinically-indicated scans. Proceedings of SPIE the International Society for Optical Engineering, 10572. 10.1117/12.2285971 [DOI] [PMC free article] [PubMed]
- Simó M, Root JC, Vaquero L, Ripollés P, Jové J, Ahles T, Navarro A, Cardenal F, Bruna J, Rodríguez-Fornells A. Cognitive and brain structural changes in a lung cancer population. Journal of Thoracic Oncology. 2015;10(1):38–45. doi: 10.1097/jto.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Villa Freixa SS, Brada M, Carapella CM, Preusser M, Le Rhun E, Rudà R, Tonn JC, Weber DC, Weller M. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of Neuro-Oncology (EANO) Neuro-Oncology. 2017;19(2):162–174. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbron SP, Buckley DL. Classic models for dynamic contrast-enhanced MRI. NMR in Biomedicine. 2013;26(8):1004–1027. doi: 10.1002/nbm.2940. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiological Reviews. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magnetic Resonance in Medicine. 1991;17(2):357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro-Oncology. 2015;17(1):122–128. doi: 10.1093/neuonc/nou099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SM, Jansen JFA, Zhang CE, Staals J, Hofman PAM, van Oostenbrugge RJ, Jeukens C, Backes WH. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: Test-retest reproducibility and its influencing factors. Journal of Magnetic Resonance Imaging. 2017;46(1):159–166. doi: 10.1002/jmri.25540. [DOI] [PubMed] [Google Scholar]
- Wu H, Li N, Jin R, Meng Q, Chen P, Zhao G, Wang R, Li L, Li W. Cytokine levels contribute to the pathogenesis of minimal hepatic encephalopathy in patients with hepatocellular carcinoma via STAT3 activation. Science and Reports. 2016;6:18528. doi: 10.1038/srep18528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Du X, Guo Y, Chen X, Wang S, Fang J, Cao P, Zhang B, Liu Z, Zhang W. Increasing of Blood-Brain Tumor Barrier Permeability through Transcellular and Paracellular Pathways by Microbubble-Enhanced Diagnostic Ultrasound in a C6 Glioma Model. Frontiers in Neuroscience. 2017;11:86. doi: 10.3389/fnins.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this article cannot be shared publicly due to the privacy of the individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.