Abstract
Background
The first reports of West Nile virus (WNV) infection in the United States in 1999 prompted Ontario to establish a surveillance protocol to monitor for the possible spread of the virus into the province. Surveillance components included evaluation of dead birds, sentinel chickens, mosquito pools and human disease. We report the results of human surveillance in 2000.
Methods
Between July 1 and Oct. 31, 2000, an active surveillance program was undertaken in which designated site coordinators in sentinel hospitals identified patients who met the suspect case definition (fever and fluctuating level of consciousness [encephalopathy], with or without muscle weakness). During the same period, following province-wide distribution of educational material, all other patients tested for WNV antibodies were identified through review of provincial laboratory reports (laboratory-based enhanced passive surveillance).
Results
Of the 60 hospitals contacted, 59 agreed to participate in the active surveillance program; 52 provided information on a regular (weekly) basis, and 7 submitted fewer than 8 reports. Thirty-six (61%) of the sentinel sites reported suspect cases. In total, 188 patients were tested (130 identified through active surveillance and 58 through enhanced passive surveillance). Patients identified through active surveillance were more likely than those identified through passive surveillance to meet the suspect case definition (43% [n = 56] v. 7% [n = 4]), to be admitted to hospital (75% [n = 99] v. 16% [n = 9]), to have a longer hospital stay (mean 25 v. 3 days), to have had a second (convalescent) serum sample collected (37% [n = 48] v. 31% [n = 18]), to have had a cerebrospinal fluid (CSF) sample banked (56% [n = 73] v. 14% [n = 8]) and to have had a discharge diagnosis reported (79% [n = 103] v. 28% [n = 16]). Of the 60 patients (32%) who met the suspect case definition, 34 (57% [31 active, 3 passive]) had a discharge diagnosis of encephalitis. Of these, 17 (50% [15 active, 2 passive]) had paired serum samples collected, and 18 (51% [all active]) had a CSF sample banked. The reported causal agents were herpes simplex virus (n = 8), varicella virus (n = 2), Powassan virus (n = 1), echovirus 30 (n = 1) and group B Streptococcus (n = 1); the cause was unknown in 18 cases. One patient died of encephalitis. The remaining 26 patients who met the suspect case definition were ultimately found to have nonencephalitic infections, vascular events or alcohol- or drug-related illness. The 128 (68%) tested for WNV who did not meet the suspect case definition included 9 patients ultimately discharged with a diagnosis of encephalitis. No cases of WNV infection were identified.
Interpretation
Only one-third of the tested patients met the suspect case definition of encephalopathy on admission, and nearly half of them were later found to have another diagnosis; others did not meet the case definition but were later discharged with a diagnosis of encephalitis. This affirms that identification of acute encephalitis on the basis of symptoms at the time of admission is often impossible.
The first known North American outbreak of West Nile virus (WNV) infection occurred in New York City in 1999.1 Sixty-two cases were reported, including 7 deaths involving people over 65 years old.2,3,4,5 The virus may have been introduced through infected birds, legally or illegally entering the United States,6 although other possible routes of transmission through humans or mosquitoes cannot be ruled out. Most cases are asymptomatic, but WNV can cause high fever with encephalitis, profound muscle weakness7 or meningitis in 1%–10% of those infected (mostly older adults)8,9,10 and occasionally other findings.11,12,13
Despite a vector control program in New York State in September 1999, overwintering mosquitoes14 and a red-tailed hawk15 were found to have detectable virus in the late winter of 2000. To ensure the identification of WNV infection in Canada during the mosquito season of 2000, Health Canada organized a surveillance program to be adopted in provinces east of Alberta (a) to educate hospital staff about the virus and clinical findings, (b) to report all suspect cases of meningitis, encephalitis and profound muscle weakness, (c) to ensure that paired serum samples were sent to the provincial public health laboratories for testing and that a sample of cerebrospinal fluid was banked and (d) to initiate a public awareness campaign and take further preventive action to decrease the likelihood of human infection. We report the Ontario experience.
Methods
Between July 1 and Oct. 31, 2000, active human surveillance for WNV infection was undertaken at selected sentinel hospitals in Ontario, and enhanced passive surveillance was conducted through education and review of laboratory reports of specimens submitted from other hospitals and physicians' offices in the province. Complete surveillance data were disseminated to the public biweekly on the provincial government's Web site (www.gov.on.ca/health).
For the active surveillance, the Ontario WNV Working Group selected 60 sentinel hospitals in key geographic locations, including the metropolitan centres of Windsor, London, Hamilton and Toronto, and regions such as Haliburton–Kawarthas, Niagara, Haldimand and Leeds. Each hospital was contacted, and a local site coordinator was identified and sent a weekly fax summarizing local and North American surveillance data. Designated hospital staff, with their ward-based medical and nursing colleagues and laboratory staff, identified patients who met the suspect case definition: fever and fluctuating level of consciousness, with or without muscle weakness, or infectious encephalopathy, meningitis, meningoencephalitis, transverse myelitis or Guillain–Barré syndrome of unknown causes. These symptoms were purposefully broad to ensure timely identification of a WNV case while excluding more trivial, nonspecific disease. The designated hospital staff ensured WNV testing and provided weekly reports to the site coordinator. The site coordinator then sent weekly faxes to the surveillance coordinator indicating whether or not suspect cases had been identified and, if present, provided demographic and clinical information. Sentinel hospitals failing to provide a weekly fax were contacted by telephone.
For the enhanced passive surveillance, the chief medical officer of health contacted all hospitals and appropriate specialists in the hospital and advised them of the importance of recognizing, testing and reporting cases of encephalitis, meningoencephalitis and adult viral meningitis that may be caused by WNV. Reports were reviewed of WNV testing of specimens submitted to the provincial laboratory from hospitals other than the sentinel hospitals and from physicians' offices.
Patients identified through active or passive surveillance were classified into 5 categories — category 1: the suspect case definition was met, and the discharge diagnosis was encephalitis; category 2: the discharge diagnosis was encephalitis, but the suspect case definition was not met; category 3: the suspect case definition was met, but another cause was found (e.g., vascular, alcohol related); category 4: the discharge diagnosis was meningitis, and the suspect case definition may or may not have been met; and category 5: the suspect case definition was not met, and the patient had a diagnosis other than encephalitis or meningitis.
For testing, paired serum samples were collected: one at presentation (acute sample) and one at 10 to 14 days after the onset of symptoms (convalescent sample). Both serum samples were tested for antibodies to WNV and to eastern equine encephalitis, western equine encephalitis, dengue, Powassan and St. Louis encephalitis viruses with the use of a hemagglutination inhibition assay. Acute arboviral infection was defined as an antibody titre that was at least 4-fold higher or lower in the acute sample than in the convalescent sample. Specimens positive for arboviral infection were forwarded to the National Microbiology Laboratory in Winnipeg for confirmatory testing using the plaque reduction neutralization test in Vero cells,16 enzyme-linked immunosorbent assay or polymerase chain reaction. Where available, a sample of 1–2 mL of cerebrospinal fluid (CSF) was collected, frozen at –70°C and transported to the provincial laboratory for banking until the serologic test results were known.
Surveillance coordinators liaised weekly with the provincial laboratory to determine the status of specimen collection. When necessary, staff at the laboratory requested follow-up specimens from the originating site. Surveillance coordinators contacted the site to obtain information on the patient's length of stay and discharge diagnosis.
Results
Of the 60 sentinel hospitals contacted for active surveillance, 59 agreed to participate in full or in part (i.e., reported only if any patients met the suspect case definition). Weekly faxed reports were provided by the site coordinators at 52 (88%) of the hospitals. The overwhelming majority of cases came from the densely populated Toronto area, reflecting the surveillance base, whereas passively reported cases came from all regions (Fig. 1).
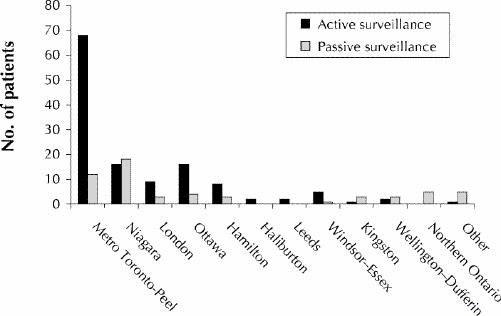
Fig. 1: Geographic distribution of patients identified through active (n = 130) and enhanced passive (n = 58) surveillance for West Nile virus testing in Ontario from July 1 to Oct. 31, 2000.
Overall, 188 patients (100 men) were tested for WNV infection: 130 (69%) were identified through active surveillance and 58 (31%) through enhanced passive surveillance (Table 1). The majority of patients (n = 119) had discharge diagnoses provided (Table 1). Nine patients had a hospital discharge diagnosis of encephalitis but did not meet the suspect case definition (category 2). Nearly half of all reported cases (46% [87/188]) did not meet the case definition and had a diagnosis other than encephalitis or meningitis (category 5); proportionally more were identified through passive surveillance than through active surveillance (55% v. 45%) in this group. Five patients (3%) died; the causes were encephalitis, suspected Creutzfeld–Jakob disease, sudden death of unknown cause, ischemic bowel and respiratory failure.
Table 1
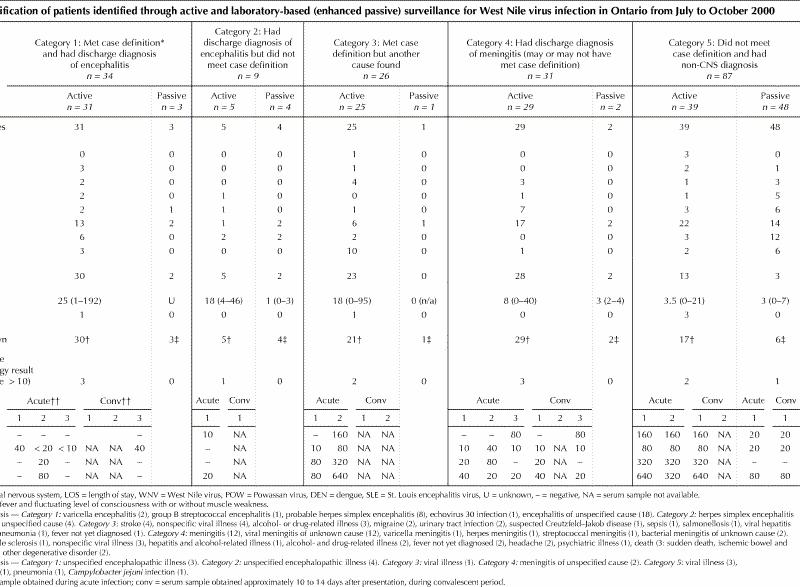
Patients reported through active surveillance were more likely than those identified through passive surveillance to meet the suspect case definition (43% [n = 56] v. 7% [n = 4]), to be admitted to hospital (75% [n = 99] v. 16% [n = 9]), to have a longer mean length of stay in hospital (25 v. 3 days), to have had a second (convalescent) serum sample collected (37% [n = 48] v. 31% [n = 18]), to have had a CSF sample banked (56% [n = 73] v. 14% [n = 8]) and to have had a discharge diagnosis provided (79% [n = 103] v. 28% [n = 16]).
Active human surveillance
Of the 130 cases reported by the sentinel hospitals, 56 (43%) met the suspect case definition (categories 1 and 3). Of the 128 patients for whom age was known, most (67%) were 18–65 years old; 13% were older than 65, and 20% were younger than 18.
In category 1, there were 31 patients with encephalopathy (Table 1), of whom 30 (97%) were admitted to hospital (mean stay 25 days). Serologic testing of acute samples was performed for 28 (90%) of the patients; 3 (11%) were found to have arboviral antibodies. Serological testing of convalescent samples was performed for 15 patients (48%). CSF samples were banked for 18 patients (58%). CSF test results were available for 28 patients, and 16 (57%) were found to have pleocytosis (white blood cell count > 5 х 106/L). One patient, a 25-year-old woman in the Toronto area, died of encephalitis of unknown cause.
In category 2, 5 patients were reported by sentinel hospitals with fever or fluctuating level of consciousness, not both, and had a discharge diagnosis of encephalitis. All 5 were admitted to hospital (mean stay 18 days) and had serologic testing of acute samples; serologic testing of convalescent samples was performed in 2 cases, and CSF samples were banked in 3 cases. One patient was found to have arboviral antibodies (Table 1).
There were 25 patients in category 3; all met the suspect case definition, but encephalitis was ruled out in favour of another diagnosis (Table 1). Twenty-three (92%) were admitted to hospital (mean stay 18 days).
Twenty-nine patients were reported to have meningitis (category 4); in 3 the cause was bacterial. Four patients were less than 18 years of age. Most (97% [28/29]) were admitted to hospital (mean stay 8 days). Serologic testing of acute samples was undertaken in 27 (93%) of cases; convalescent samples were tested in 16 cases (55%), and 26 (90%) had a CSF sample banked. Three patients were found to have arboviral antibodies (Table 1).
There were 39 patients who did not meet the suspect case definition and who had a diagnosis other than encephalitis or meningitis (category 5). Only one-third were admitted to hospital (mean stay 3.5 days). The other two-thirds were seen in emergency departments or in doctors' offices. Acute serum samples were tested in 34 cases (87%), convalescent samples were tested in 8 cases (21%), and 14 (36%) had a CSF sample banked. Two patients were found to have arboviral antibodies.
One patient, less than 18 years of age, was reported to have Guillain–Barré syndrome and was admitted to hospital for 19 days. Serologic testing showed no arboviral antibodies.
Enhanced passive surveillance
Of the 58 patients identified through the review of public health laboratory reports of WNV testing, 48 (83%) did not meet the suspect case definition. Of the remaining 10 patients, 3, 4, 1 and 2 were in categories 1 through 4 respectively (Table 1).
Arboviral testing
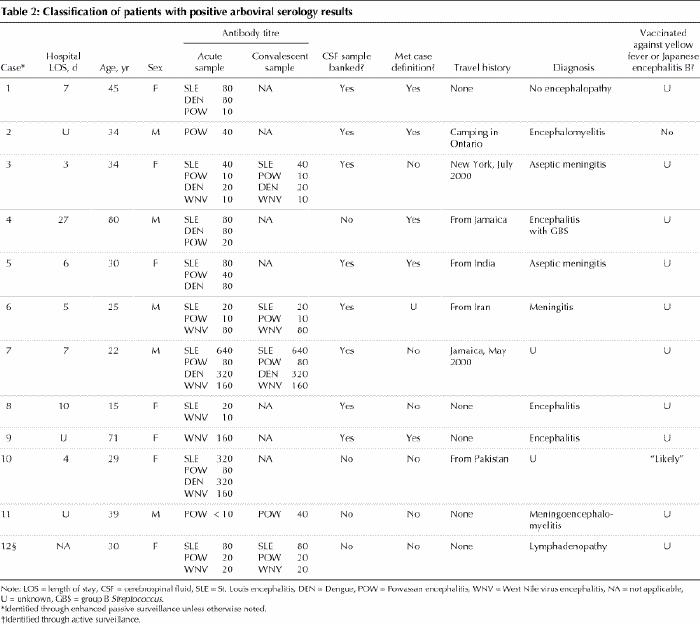
There were no patients for whom neutralizing antibodies to WNV were demonstrated. Twelve patients had evidence of previous exposure to an arbovirus, as determined by the presence of flavivirus antibodies using antigens to dengue, St. Louis encephalitis, Powassan and Japanese encephalitis viruses (Table 2). One patient had evidence of acute Powassan virus infection (Table 1: category 1, patient 3; Table 2: case 11). Of these 12 patients, 11 were identified through active surveillance and 1 through passive surveillance.
Table 2
Interpretation
The goal of the active surveillance was the early detection of WNV infection in patients admitted to hospital with encephalopathy. The criteria for the suspect case definition were deliberately broad to ensure collection of the first serum sample early in the course of illness. The insidious onset and nonspecific findings of encephalitis17 meant that more serum samples were tested at the acute stage of illness than was ultimately necessary. Many of the patients ultimately had alternative diagnoses (category 3). The frequency of findings suggestive of encephalitis has been reported to be lower at admission than later in the hospital stay: pleocytosis (59% v. 63%), electroencephalogram abnormalities (87% v. 96%) and neuroimaging abnormalities (37% v. 69%).18 This presents a challenge to any surveillance system for encephalitis and underscores the importance of an accurate discharge diagnosis.
Serum samples were often not obtained during the convalescent phase. Serologic testing of such samples is vital for the confirmation of a WNV diagnosis. Evaluation of any patient with encephalitic symptoms should always include the collection of paired serum samples as well as an attempt to detect a microbial organism through culture or molecular techniques. Such systematic investigation may yield the definite or probable microbial cause in 40% of cases and a possible cause in another 26%.18 The strength of purported etiologic associations in our study (category 1) is unknown; that is, a presumptive diagnosis of herpes simplex virus encephalitis may actually be caused by enterovirus 71, Mycoplasma pneumoniae, Epstein–Barr virus or other agents.18
The overwhelming majority of the sentinel hospitals complied with the active surveillance protocol. Many cases that did not meet our suspect case definition and were not admitted to hospital were identified passively through the laboratory from the Niagara region following findings of WNV in birds in adjacent counties of New York State. Our surveillance program complemented other surveillance efforts for WNV infection in Ontario in 2000, including investigation of dead birds, sentinel chickens and mosquitoes19 (C.L.: unpublished data). No WNV activity was demonstrated by any of these surveillance methods.
Although WNV is suspected to have entered Ontario, owing to the discovery of WNV-positive birds found in neighbouring counties of New York State, it was not documented in 2000. WNV should now be considered as established in North America, if not yet endemic.20 WNV infection should be included in the differential diagnosis of infectious encephalopathy originating within North America; the virus may have spread to Florida, eastern Canada, the Caribbean and Mexico with migrating wild bird populations. Should WNV become apparent in Canada, we will have to remain vigilant to reduce the likelihood of outbreaks and minimize human infection. The public has been advised to use judicious mosquito control measures, including limiting outdoor activities between dusk and dawn, wearing long pants and sleeves outdoors when mosquitoes are most active, eliminating sources of standing water on their properties, repairing screens on doors and windows and using insect repellants that contain DEET (N-diethyl-meta-toluamide). Nevertheless, even in regions where mosquitoes carry the virus, less than 1% of mosquitoes are infected and, of humans cases, less than 1% will become severely ill. Among those with severe illness, the case- fatality rate is 3%–15% and involves mostly elderly people.
Provincial initiatives in Ontario for the 2001 season included enhanced surveillance of dead birds (especially crows) and human surveillance coordinated through medical officers of health, emphasizing the vital need for the collection of a second serum at 10 to 14 days after presentation in patients with diagnoses of encephalitis. Of 19 patients admitted to hospital during the epidemiologic investigation of the New York outbreak, 79% were not reported to the department of health. Physician liaison with public health departments is vitally important for the early detection of outbreaks. Just as management of the US epidemic was a tribute to public health organizations and dedicated physicians,21 so, too, is the Canadian initiative.
Footnotes
§A list of the working group members appears at the end of the article.
This article has been peer reviewed.
Acknowledgements: We acknowledge the strong collaboration of all provincial health departments and medical officers of health in this initiative. The secretarial support of Sue Weller is appreciated.
This work was supported through a contract with the Ontario Ministry of Health and Long-term Care.
We thank Harvey Artsob, Mike Drebot and Kimberly Holloway, National Microbiology Laboratory, Winnipeg, for providing confirmatory testing for West Nile virus and advice on arboviral infection. We also thank the following people who contributed directly to the success of the surveillance protocol at their sites: Roxanne Strong, Campbellford Hospital; Lynne Johnston and Donna Stey, Haliburton Site, and Denise Northey and Shirley Perreault, Minden Site, Haliburton Highlands Health Services; Myonne Allan, Northumberland Health Care Corporation; Leanne Harding, Ross Memorial Hospital; Marnie Green, Myrna Cooper and Lyne Hyatt, Haldimand War Memorial Hospital; Barbara Pond, Norfolk General Hospital; Dale Murray, West Haldimand Hospital; Jane Burden, Jim Gauthier and Shirley McDonald, Kingston General Hospital; M. Peggy Perkins, Tom Reiss and T.C. Stavro Sholoff, Ajax–Pickering Health Centre; Nazira Gallani and Ian Kitai, Centenary Health Centre; William Geddie, Angela Jackson-Lee, Betty Ann Jolley, Charles Price, Neil Rau and Marilyn Sarina, Credit Valley Hospital; Jane Fader, Etobicoke Campus, and Donna McCallum, Brampton Campus, William Osler Health Care Corporation; Shirley Lanza, Faye Matthews, Ruby Norris and Della Redwood, Oakville Trafalgar Memorial Hospital; Doris Tam, Guelph General Hospital; Mary Jane Adams, St. Joseph's Hospital and Home; Anne Bialachowski and Brian Egier, Hamilton Site, Jan Olde, Henderson Site, and Diane Thornley and Deborah Yamamura, McMaster and Chedoke Sites, Hamilton Health Sciences Corporation; Sandra Callery and May Griffiths-Turner, St. Joseph's Hospital, Hamilton; Grant Large, Scarborough General Hospital; Linda Davis, Margaret McMahon, David Rose and Felix J. Tyndel, Scarborough Hospital – Grace Division; Linda Sharpen and microbiology staff, Toronto East General; Helen Heurter, The Hospital for Sick Children; A.E. Simor, Sunnybrook Health Science Campus, and Elizabeth Van Horne, Sunnybrook & Women's College Health Sciences Centre; Marta Garcia, General Division, and Zahir Hirji and Cathy Kennedy, Western Division, University Health Network; Melody Radu, Welland County General Hospital; Nicole Lamirande and Sylvie Denise Nault, Montfort Hospital; Charlie Dickey and Inez Landry, Queensway Carelton Hospital; Christine Moore and Cindi Wigston, Mt. Sinai Hospital; Annette Blanchard, Hotel-Dieu Grace Hospital, Windsor; Donna Segeon, Sandra Whittle and emergency department staff, Leamington District Memorial Hospital; George Barclay, Lois Hollingshead, Sangeeta Joshi and D. Munkley, Greater Niagara General Hospital; Jo Ann Kieller, Sigmund Krajden and Sandy Noble, St. Joseph's Health Centre, Toronto; Mary Lou Card, Alice Newman and Leesa Round, St. Joseph's Health Centre, London; Lorraine Ashby, Susan Cooper and Bill Fayle, Brockville General Hospital; Michael John and Kathryn McGhie, North Campus, and Marilyn Austin, South Campus, London Health Sciences University; Delena Bragg, Finch Site, and Terri Anisko, Church Site, Humber River Regional Hospital; Debra Bruckswaiger, Children's Hospital of Western Ontario; Anne Augustin, North York Branson Hospital; Diane White, North York General Hospital; Janice Hartley and Diane Weinwurm, Trillium Health Centre, Mississauga Site; Donna Grey, Almonte General Hospital; Sandra Dunnett, St. Catharines General Hospital; Peggy Dennis and Viral Laboratory staff, Children's Hospital of Eastern Ontario; Barbara Licata, Windsor Regional Hospital, Metro Campus; Sherrill Ireton, Kemptville District Hospital; Elaine Cheung, Wilson Chiang, Gladys Chen See and Patrice D. Simmons, Central Public Health Laboratory, Toronto.
Competing interests: None declared.
Correspondence to: Dr. E. Lee Ford-Jones, Division of Infectious Diseases, Hospital for Sick Children, 555 University Ave., Toronto ON M5G 1X8
References
- 1.Quagliarello V. Emergence of West Nile virus encephalitis. Curr Infect Dis Rep 2000;2:325-6. [DOI] [PubMed]
- 2.Outbreak of West Nile virus encephalitis. MMWR Morb Mortal Wkly Rep 1999;48:845-9. [PubMed]
- 3.WestNile-like viral encephalitis. MMWR Morb Mortal Wkly Rep 1999;48: 890-2. [PubMed]
- 4.West Nile-like viral encephalitis. MMWR Morb Mortal Wkly Rep 1999;48: 944-6. [PubMed]
- 5.Fine A, Layton M. Lessons from the West Nile viral encephalitis outbreak in New York City, 1999: implications for bioterrorism preparedness. Clin Infect Dis 2001;32:277-82. [DOI] [PubMed]
- 6.Rappole J, Rappole JH, Derrickson SR, Hubalek Z, Migratory birds and spread of West Nile virus in the western hemisphere. Emerg Infect Dis 2000;6: 319-28. [DOI] [PMC free article] [PubMed]
- 7.Ahmed S, Libman R, Wesson K, Ahmed F, Einberg K, Guillain–Barré syndrome: an unusual presentation of West Nile virus infection.Neurology 2000; 55: 144-6. [DOI] [PubMed]
- 8.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania.Lancet 1998;352:767-71. [DOI] [PubMed]
- 9.Weir E. West Nile fever heads north. CMAJ 2000:163(7):878. Available: www.cma.ca/cmaj/vol-163/issue-7/0878.htm [PMC free article] [PubMed]
- 10.Hubalek Z, Halouzka J. West Nile fever: a re-emerging mosquito-borne viral disease in Europe.Emerg Infect Dis 1999;5:643-50. [DOI] [PMC free article] [PubMed]
- 11.Han LL, Popovici F, Alexander JP Jr, Laurentia V, Tengelsen LA, Cernescu C, et al. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996.J Infect Dis 1999;179:230-3. [DOI] [PubMed]
- 12.Asnis DS, Conetta R, Teixeira AA, Waldman G, Sampson BA. The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience.Clin Infect Dis 2000;30:413-8. [DOI] [PubMed]
- 13.Cunha BA, Minnaganti V, Johnson DH, Klein NC. Profound and prolonged lymphocytopenia with West Nile encephalitis. Clin Infect Dis 2000;31:1116-7. [DOI] [PubMed]
- 14.Update: West Nile virus activity — northeastern United States, January–August 7, 2000. MMWR Morb Mortal Wkly Rep 2000; 49:714-7. [PubMed]
- 15.Garmendia AE, Van Kruiningen HJ, French RA, Anderson JF, Andreadis TG, Kumar A, et al. Recovery and identification of West Nile virus from a hawk in winter. J Clin Microbiol 2000;38:3110-1. [DOI] [PMC free article] [PubMed]
- 16.Artsob H, Spence L, Th'ng C, West R. Serological survey for human arbovirus infections in the province of Quebec Can J Public Health 1980;71:341-6. [PubMed]
- 17.Marra C. Encephalitis in the 21st century. Semin Neurol 2000;20:323-7. [PubMed]
- 18.Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, Jamieson F, et al. Etiology of acute childhood encephalitis at the Hospital for Sick Children, Toronto, 1994–1995. Clin Infect Dis 1998;26:398-409. [DOI] [PubMed]
- 19.Fearon M, LeBer C, Ford-Jones L, Alves D, Barker I, Rossatte R, et al. West Nile virus surveillance in Ontario. American Society for Microbiology; Florida; May 2001.
- 20.Jordan I, Briese T, Fischer N, Lau JY, Lipkin WI. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J Infect Dis 2000; 182: 1214-7. [DOI] [PubMed]
- 21.Tyler KL. West Nile virus encephalitis in America. N Engl J Med 2001; 344: 1858-9. [DOI] [PubMed]


