Abstract
Rationale
Stressful events can have lasting and impactful effects on behavior, especially in terms of appropriate fear regulation and reward seeking. Our prior work in rats has shown baseline sex differences in fear expression and sucrose seeking in a discriminative reward-fear-safety conditioning task.
Objectives
The objectives of the current study were to determine how prior stress may affect alcohol consumption across a reward-fear-safety learning task, and how prior alcohol history may interact with stress to impact learning in this task.
Methods
Male and female Long Evans rats were given home cage intermittent 24 h access to both water and alcohol for 5 weeks. A subset of rats then received exposure to stress (15 unsignaled footshocks), while remaining unstressed rats received context exposure without shock. One week later, all rats were trained on the same reward-fear-safety cue task while having continuous home cage access to both water and alcohol.
Results
All rats increased consumption (g/kg/24 h) across the 5 weeks of intermittent access, with females showing higher consumption levels. Stress exposure did not alter alcohol consumption in the week following stress, but did increase home cage alcohol consumption during later reward-fear-safety cue learning. Stress in both sexes also elevated freezing levels to the reward cue resulting in decreased sucrose seeking and was positively correlated with home cage alcohol consumption.
Conclusions
While stress increased drinking in both males and females, the effects of stress were particularly pronounced in females, indicating our results could be capturing a higher propensity for females to display stress-induced drinking.
Keywords: Stress, Alcohol, Safety, Fear, Reward, Sex differences
Introduction
Post-traumatic stress disorder (PTSD) has become a growing public health concern, with a lifetime prevalence of approximately 6.4% of the US population (Pietrzaka et al., 2011). Following exposure to distressing events, psychological trauma can manifest into debilitating symptoms of reexperiencing, avoidance, numbing, and hyperarousal (American Psychiatric Association, 2000). Increasing evidence suggests that these symptoms are a result of neurobiological alterations in the ability to discriminate stimuli. When a person is unable to differentiate between threatening and non-threatening stimuli, they may have an exaggerated response to neutral stimuli (van der Kolk, 1997). PTSD has a high rate of comorbidity of additional psychiatric disorders. Among these, alcohol abuse or dependence is prevalent in approximately 41.8% of people with PTSD (Kessler et al., 2012; Pietrzaka et al., 2011). Alcohol can suppress previously established traumatic memories and possibly prevent the formation of new memories (Nutt, 2000). Chronic use of alcohol could further impair the ability to discriminate between stimuli (Broadwater and Spear, 2013).
To investigate the discrimination of stimuli signifying reward, fear, and safety, our prior work has used a behavioral Pavlovian-based task in which a reward cue is paired with sucrose, a fear cue is paired with footshock, and a safety cue that is paired with neither foot shock or sucrose (Sangha et al, 2013, 2014a, 2014b; Ng et al, 2018; Müller et al, 2018; Greiner et al, 2019; Woon et al, 2020). While male and female Long Evans rats show similar discrimination among this set of cues, we have found that females are more resistant to subsequent fear extinction and do not demonstrate conditioned inhibition of freezing when the fear and safety cues are presented concurrently (Greiner et al, 2019). We have also shown that prior stress (the same used in the present study) in male rats leaves conditioned inhibition intact, but profoundly impairs subsequent fear extinction (Woon et al, 2020). Since contributors to drug relapse include stress, the environmental context, and cues associated with drug use (Venniro et al, 2020), there could be an interaction between prior stress and alcohol history on cue discrimination.
While many studies in adult rodents have explored the effects of stress on established drinking patterns, very few have used both male and female subjects (reviewed in Mineur et al., 2022). The lack of inclusion of females in these studies is disappointing as stress has been shown to increase alcohol intake in women (Peltier et al 2019). One previous study in male rats, using the same stress procedure as in the present study, showed that exposure to stress during withdrawal does not affect previously established drinking habits, while stress given 10 days prior to the start of drinking increased drinking (Meyer et al, 2013). Overall, the results are mixed in the literature regarding the effects of stress on alcohol consumption and how sex may additionally contribute to these effects (reviewed in Mineur et al., 2022).
The present study examined the interacting effects of alcohol and stress on sucrose seeking and freezing behaviors during a reward-fear-safety cue discrimination task in male and female rats. Since intermittent access models have been shown to increase motivation to seek and take biologically altering substances (Venniro et al, 2020), subjects were first exposed to a 5-week period of intermittent access to a two-bottle choice drinking paradigm consisting of 15% alcohol and water. Stress was then introduced to examine possible stress-induced increases in alcohol consumption. Following stress, and while still having access to home cage alcohol, rats then underwent reward-fear-safety cue discrimination training. Our results indicate that stress increased alcohol consumption in both males and females, but not immediately, and this was associated with elevated freezing levels during the reward and safety cues.
Methods
Subjects
Eighteen male and 18 female Long Evans rats (46–49 days old upon arrival; Envigo, Indianapolis, IN) were single-housed with enrichment upon arrival and allowed to acclimate for 1 week prior to any handling. Rats were then handled daily for 1 week. Estrus was not monitored. All procedures were implemented during the light cycle (7am lights on, 7 pm lights off). All animal procedures were approved by the Purdue University Animal Care and Use Committee.
Five-week baseline intermittent 2-bottle choice
At 60–63 days of age, male rats were given 22–24 g of chow per day, and females 20–22 g, at the time of bottle exchange or immediately after a behavioral session. Intermittent access to both 15% alcohol and water (2-bottle choice) also started, 24 h at a time, 3 times per week (bottles on Monday, Wednesday, Friday; bottles off Tuesday, Thursday, Saturday) for 5 weeks (Fig. 1). A separate, visually distinct, water bottle was available on remaining days. Alcohol and water bottles were weighed at the end of each 24 h 2-bottle choice period. The positions (left/right) of the alcohol and water bottles were alternated to reduce any influence of side preference. All rat cages remained on the same housing rack along with an empty cage with alcohol and water bottles to measure and account for any spillage and/or evaporation. Rats were briefly handled and weighed at the beginning of each 24 h 2-bottle choice period. Cages were changed every Sunday, in between cycles of intermittent access.
Fig. 1.
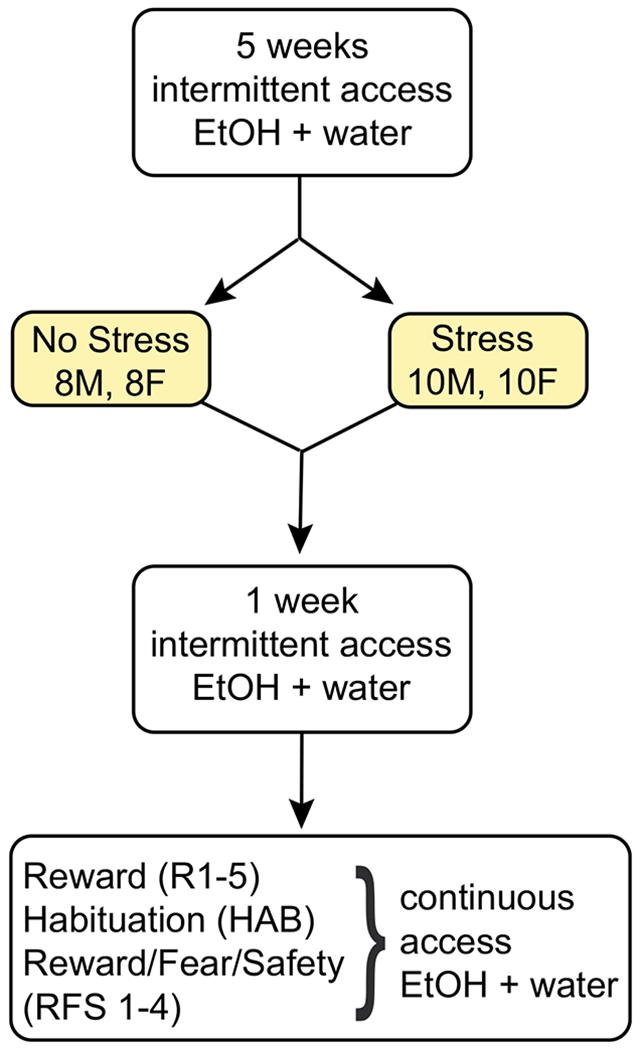
Overview of experimental design. All 18 male and 18 female rats were first exposed to 5 weeks of intermittent access of 15% EtOH. They were then divided into stress (10 M, 10F) or no stress (8 M, 8F) conditions. Stress consisted of 15 unsignaled shocks, while no stress consisted of context exposure without shocks. All rats continued to receive intermittent access of 15% EtOH for 1 week. All rats then underwent 5 reward sessions (R1-5), 1 habituation session (HAB), and 4 reward/fear/safety sessions (RFS1-4), while having continuous access to both 15% EtOH and water. The only procedural difference was exposure to stress or no stress (highlighted)
Stress exposure
After 5 weeks of home cage intermittent 2-bottle choice, rats were exposed to either stress (10 males, 10 females) or no stress (8 males, 8 females) conditions (Fig. 1). Stress consisted of a single 90 min session in Context A in which 15 unsignaled 1 s, 1 mA footshocks were presented (variable ITI, 4–8 min). No stress conditions consisted of a single 90 min session in Context A without any footshocks presented. MedAssociate operant conditioning chambers served as Context A (32 cm length X 25 cm width X 30 cm height Plexiglas boxes encased in sound-attenuating chambers). Background houselights were off and a cotton ball doused with vanilla extract was placed within the sound attenuating chamber but outside of the Plexiglas box to introduce an odor to the context. Footshocks were delivered through a grid floor via a constant current aversive stimulator.
Post-stress intermittent 2-bottle choice
For the 1 week following stress or no stress exposure, rats were returned to the same intermittent 2-bottle choice schedule and procedure as described above (Fig. 1).
Reward-fear-safety cue discrimination task
One week following stress and intermittent 2-bottle choice, all rats underwent the same discrimination learning task (10 sessions total) in Context B (Fig. 1). Context B consisted of the same MedAssociate boxes described above except the background houselights were turned on and the vanilla odor was removed. All sessions were digitally recorded via a side-mounted video camera for subsequent offline video scoring. Three stimuli were used as cues: a 20 s continuous 3 kHz tone (70 dB) for reward, a 20 s pulsing 11 kHz tone (200 ms on, 200 ms off; 70 dB) for fear, and 20 s continuous lever lights (28 V, 100 mA) for safety. Stimuli were not counterbalanced in this study but our prior work has not indicated differences in learning among these cues across reward, fear, and safety (Sangha et al., 2013; Greiner et al., 2019).
Rats first received 5 sessions of reward training distributed across 5 days (R1-R5). Each session consisted of 25 pairings (ITI, 90-130 s) of the reward cue with a 3 s delivery of 10% liquid sucrose (100 μL pseudorandomly initiated 10–18 s after reward cue onset) into a port. Rats then received one session of habituation training (HAB), which consisted of the same 25 pairings of the reward cue paired with liquid sucrose (3 s delivery pseudorandomly initiated 10–18 s after reward cue onset), along with 5 trials of the future fear cue presented alone, and 5 trials of the future safety cue presented alone (ITI, 90–130 s). This habituation procedure has been used to assess any baseline freezing that may be present to the novel cues with the number of trials presented not being sufficient to produce latent inhibition (Sangha et al, 2013, 2014a, 2014b; Ng et al, 2018; Müller et al, 2018; Greiner et al, 2019; Woon et al, 2020). One day following the habituation session (HAB), rats then received 4 sessions of reward/fear/safety conditioning (RFS1-4) across 4 days; i.e., 1 session per day for 4 days. Each session consisted of 15 trials of the same reward cue paired with liquid sucrose (3 s delivery initiated 18 s after reward cue onset), intermixed with 4 trials of the fear cue paired with footshock (0.5 s, 0.5 mA at cue offset), and 25 trials of the safety cue presented alone without footshock (44 trials total, ITI 100–140 s).
Continuous 2-bottle choice during discrimination learning
At the beginning of discrimination learning, rats were switched from intermittent to continuous 2-bottle choice access to alcohol and water. Bottles and rats were weighed every 24 h, at the time of behavioral training. That is, measurements presented reflect the amount of consumption in the 24 h period following each behavioral training session.
Analyses
Ethanol consumption
Grams of ethanol and water consumed were recorded for each rat over a 24 h period. Grams of ethanol and water that were spilled in the empty cage were subtracted from the ethanol solution consumed (g). Alcohol consumption data were presented as grams of ethanol per kilogram of body weight accounting for the density of ethanol per 24 h session (ETOH g/kg/24 h), and analyzed with a combination of simple linear regressions, two-way repeated measures ANOVAs, and three-way repeated measures ANOVAs, followed by LSD post hoc tests where appropriate.
Behavior
Fear behavior was assessed manually offline from videos by measuring freezing, defined as complete immobility with the exception of respiratory movements, which is an innate defensive behavior (Blanchard & Blanchard, 1969; Fendt & Fanselow, 1999). The total time spent freezing during each 20 s assessment period (cue, post-cue) was quantified and expressed as a percentage of that assessment period (cue, post-cue). Measuring the total time the animal spent inside the reward port and at the entrance of the port with nose positioned at port entrance during assessment period (cue, post-cue) assessed sucrose seeking behavior and was expressed as a percentage of that assessment period (cue, post-cue). Two individuals performed offline manual behavioral scoring and were blind to both condition and sex. Pearson’s correlations of behavioral values between scorers were greater than r = 0.80. Behavioral data were analyzed with three-way repeated measures ANOVAs, followed by LSD post hoc tests where appropriate.
Results
All 18 male and 18 female rats were first exposed to 5 weeks of intermittent access of 15% ethanol (Fig. 1). They were then divided into stress (10 M, 10F) or no stress (8 M, 8F) conditions. Stress consisted of 15 unsignaled shocks, while no stress consisted of context exposure without shocks. All rats continued to receive intermittent access of 15% ethanol for 1 week. All rats then underwent 5 reward sessions (R1-5), 1 habituation session (HAB), and 4 reward/fear/safety sessions (RFS1-4), while having continuous access to both 15% ethanol and water. The only procedural difference was exposure to stress or no stress.
Alcohol consumption during intermittent access before and after stress
Baseline 5-week 2 bottle-choice drinking
Ethanol consumption (g/kg/24 h) was quantified 3 times per week for 5 weeks after 24 h access to both ethanol and water in the home cage (Fig. 2a). A simple linear regression showed that both males (F(1, 258) = 9.45, p = 0.002) and females (F(1, 256) = 8.54, p = 0.004) increased their 24 h consumption over the 5 weeks. A 2-way ANOVA comparing time point and sex was conducted to assess changes in ethanol consumed over the course of the 5 weeks of intermittent access to two-bottle choice. This found a time point by sex interaction (F(14, 168) = 2.41, p = 0.004), and a main effect of sex (F(1, 12) = 11.25, p = 0.006), but no effect of time point (F(14, 168) = 0.60, p = 0.863). Females consumed more ethanol than males.
Fig. 2.
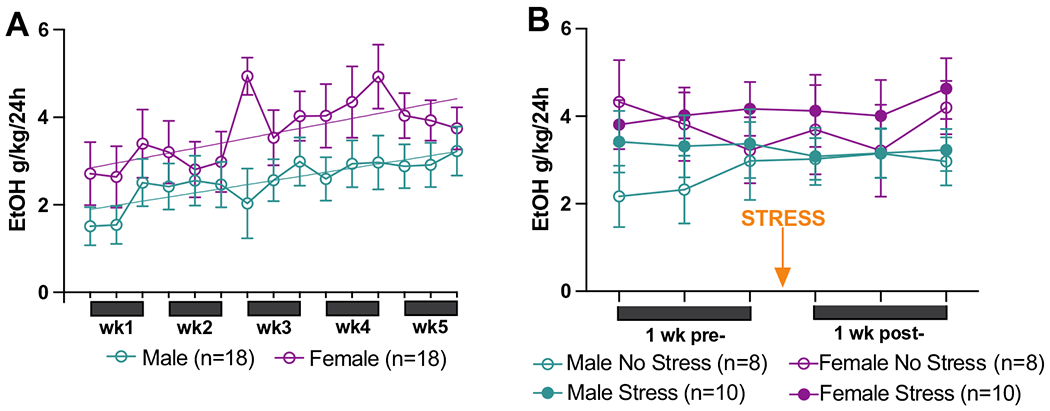
Baseline and post-stress drinking. A Males and females increased ethanol consumption across the 5 weeks of baseline intermittent access to 15% ethanol and water. Females consumed more ethanol than males. B Ethanol consumption did not differ 1 week after stress compared to 1 week prior to stress. Data are presented as mean EtOH g/kg/24 h + / − SEM
One week post-stress drinking
Twenty-four hour of ethanol consumption (g/kg/24 h) was also quantified 3 times for the 1 week following stress/no stress exposure (Fig. 2b) and was compared to ethanol consumption for the 1 week prior to stress/no stress exposure. A 3-way ANOVA comparing time point, sex, and condition found no significant interactions or main effects. Thus, ethanol consumption did not change significantly in the 1 week after stress exposure compared to the 1 week prior to stress.
Influence of stress on cued sucrose conditioning
One week following stress/no stress exposure, all rats were subjected to the same appetitive conditioning sessions in which a reward cue was paired with sucrose delivery. Six sessions were given in total, with the last session also including 5 presentations each of the future fear and safety cues, and denoted as “Habituation” (i.e., R1–R5, Hab). In addition to analyzing sucrose seeking and freezing behaviors during each 20 s cue presentation (Fig. 3), we also assessed these behaviors in the 20 s immediately post-cue (Supplemental Fig. 1).
Fig. 3.
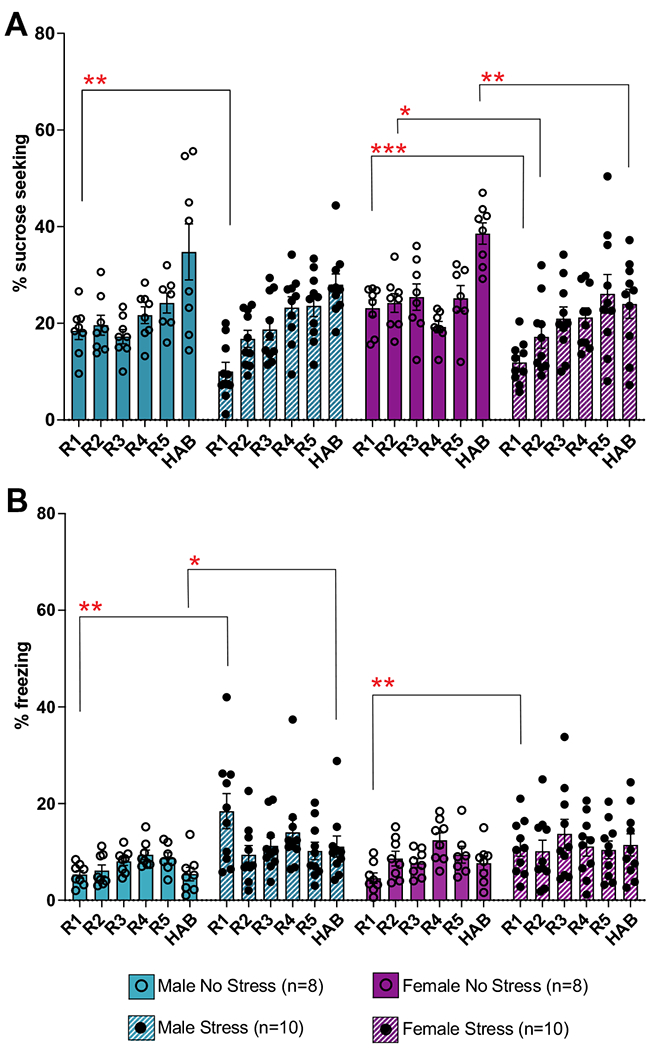
Sucrose seeking and freezing behaviors during reward and habituation sessions. A Sucrose seeking during the 20 s reward cue paired with sucrose. Data are presented as mean % time spent sucrose seeking across reward cues for each session, + / − SEM. Overall, there was decreased sucrose seeking in the stress groups during the cue. B Freezing during the 20 s reward cue paired with sucrose. Data are presented as mean % time spent freezing across reward cues for each session, + / − SEM. Overall, there was increased freezing in the stress groups. *p < 0.05; **p < 0.01; ***p < 0.001; within sex, within session, between condition effects. R1–R5, reward sessions 1–5; HAB, habituation session
Sucrose seeking during reward and habituation sessions
A 3-way ANOVA comparing session, sex, and condition was conducted to assess sucrose seeking during the reward cue during the appetitive conditioning sessions (Fig. 3a). This found a main effect of session (F(4, 112) = 27.20, p < 0.001), a main effect of condition (F(1, 32) = 6.32, p = 0.017), and a session by condition interaction (F(5, 158) = 6.55, p < 0.001). Post hoc analyses yielded significant effects within the first reward session (R1), in which the male stress group spent less time sucrose seeking than the male no stress (p = 0.005) and female no stress (p < 0.0001) groups, and the female stress group also spent less time sucrose seeking than the female no stress (p = 0.0001) and male no stress (p = 0.012) groups. Additionally, during reward session 2 (R2) and habituation, the female stress groups spent less time sucrose seeking than the female no stress group (p = 0.038, p = 0.001, respectively).
A similar 3-way ANOVA comparing session, sex, and condition was conducted to assess sucrose seeking during the 20 s immediately after the reward cue during the appetitive conditioning sessions (Supplemental Fig. 1a). This also found a main effect of session (F(4, 110) = 64.18, p < 0.001), a main effect of condition (F(1, 32) = 23.91, p < 0.001), and a session by condition interaction (F(5, 158) = 31.35, p < 0.001). Post hoc analyses yielded significant effects within every session, except the first reward session (R1) and habituation. The male stress group spent more time sucrose seeking immediately after the reward cue than the male no stress group for reward sessions R2 (p = 0.002), R3 (p = 0.0001), R4 (p < 0.0001), and R5 (p < 0.0001). Similarly, the female stress group also spent more time sucrose seeking immediately after the reward cue than the female no stress group for reward sessions R2 (p = 0.011), R3 (p = 0.0003), R4 (p < 0.0001), and R5 (p = 0.0001). This indicates that the stress groups may be waiting to consume the sucrose until the reward cue is terminated.
Freezing levels during reward and habituation sessions
A 3-way ANOVA comparing session, sex, and condition was conducted to assess freezing during the reward cue during the appetitive conditioning sessions (Fig. 3b). This found a main effect of condition (F(1, 32) = 7.72, p = 0.009) and a session by condition interaction (F(5, 158) = 4.18, p = 0.001). Post hoc analyses yielded significant effects within reward session R1, in which the male stress group froze significantly more than the male no stress (p = 0.006) and female no stress (p = 0.004) groups, and the female stress group also froze more than the female no stress (p = 0.009) and male no stress (p = 0.014) groups. Additionally, during habituation, the male stress (p = 0.045) and female stress (p = 0.040) groups froze significantly more than the male no stress group.
A similar 3-way ANOVA comparing session, sex, and condition was conducted to assess freezing during the 20 s immediately after the reward cue during the appetitive conditioning sessions (Supplemental Fig. 1b). This found a main effect of session (F(3, 88) = 4.03, p = 0.012) and a session by condition interaction (F(5, 158) = 8.22, p < 0.001). Post hoc analyses yielded significant effects within reward session R1, in which the male stress group froze significantly more than the male no stress (p = 0.025) and female no stress (p = 0.018) groups, and the female stress group froze more than the female no stress (p = 0.011) and male no stress (p = 0.020) groups.
Sucrose seeking and freezing during novel cues
During the habituation session, 5 presentations each of the future fear and safety cues were presented to assess behavior during these novel cues before discriminative conditioning began.
A 3-way ANOVA comparing cue period, sex, and condition was conducted to assess sucrose seeking in response to the novel cue presentations (Fig. 4a; Supplemental Fig. 2a). This found a main effect of condition (F(1, 32) = 4.47, p = 0.042) and a cue period by condition interaction (F(1, 32) = 15.29, p = 0.0005). Post hoc analyses showed that during the cue, the male no stress group spent more time sucrose seeking than the male stress (p = 0.012) and female stress (p = 0.009) groups, and the female no stress group also spent more time sucrose seeking than the male stress (p = 0.004) and female stress (p = 0.003) groups. It is important to note that these novel cues did not result in any sucrose delivery. These same differences were not found in the postcue period (Supplemental Fig. 2a).
Fig. 4.
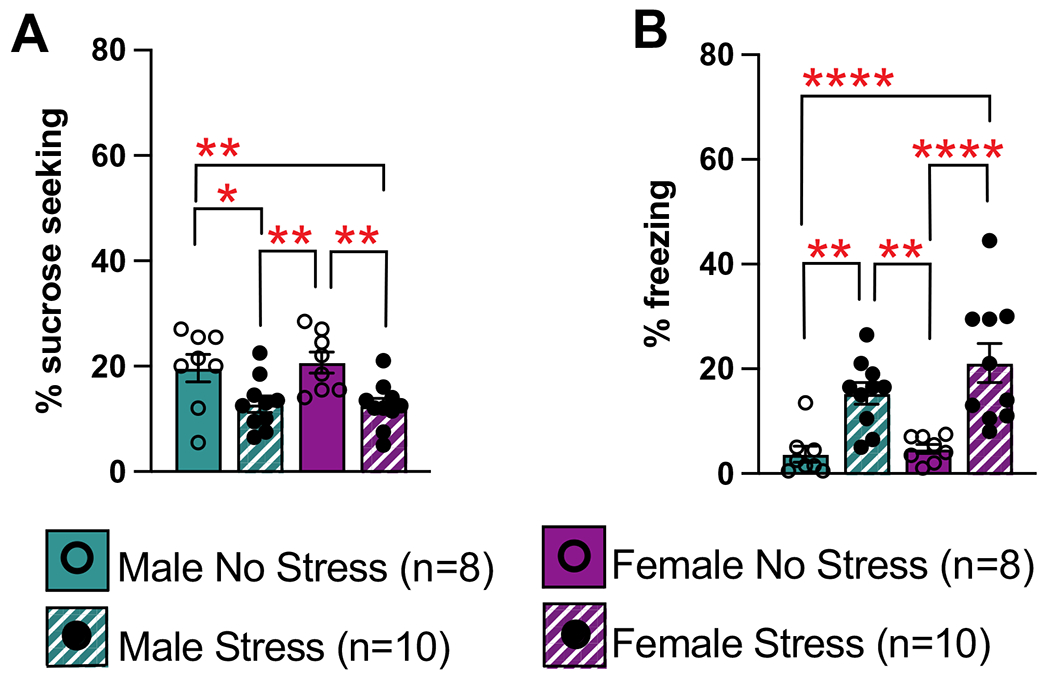
Sucrose seeking and freezing behaviors during novel cue presentations of habituation session. Novel cues were not presented with sucrose or footshocks. A Sucrose seeking during the novel cue shown as mean % time spent sucrose seeking + / − SEM. During the novel cue, both no stress groups showed higher sucrose seeking than stress groups. *p < 0.05; **p < 0.01. B Stress increased freezing levels during the novel cue shown as mean % time spent freezing + / − SEM. **p < 0.01; ****p < 0.0001
A similar 3-way ANOVA comparing cue period, sex, and condition was conducted to assess freezing in response to the novel cue presentations (Fig. 4b; Supplemental Fig.2b). This found a main effect of condition (F(1, 32) = 20.50, p < 0.001), a period by sex interaction (F(1, 32) = 8.62, p = 0.006), and a period by condition interaction (F(1, 32) = 11.17, p = 0.002). Post hoc analyses showed that during the cue, the male no stress group froze less than the male stress (p = 0.003) and female stress (p < 0.0001) groups, and the female no stress group also froze less than the male stress (p = 0.006) and female stress (p < 0.0001) groups. During the post-cue period (Supplemental Fig. 2b), the female no stress group froze less than the female stress (p = 0.006) and male stress (p = 0.009) groups. It is important to note that these novel cues did not result in any footshocks.
Influence of stress on reward/fear/safety conditioning
One day following the habituation session, all rats began 4 sessions (1 per day) of reward/fear/safety conditioning (RFS1-4) in which the same reward cue was paired with sucrose, intermixed with trials of a fear cue paired with footshock (0.5 mA, 0.5 s) and a safety cue presented alone without sucrose or footshock. As above, in addition to analyzing sucrose seeking and freezing behaviors during each 20 s cue presentation (Figs. 5 and 6), we also assessed these behaviors in the 20 s immediately post-cue (Supplemental Figs. 3 and 4).
Fig. 5.
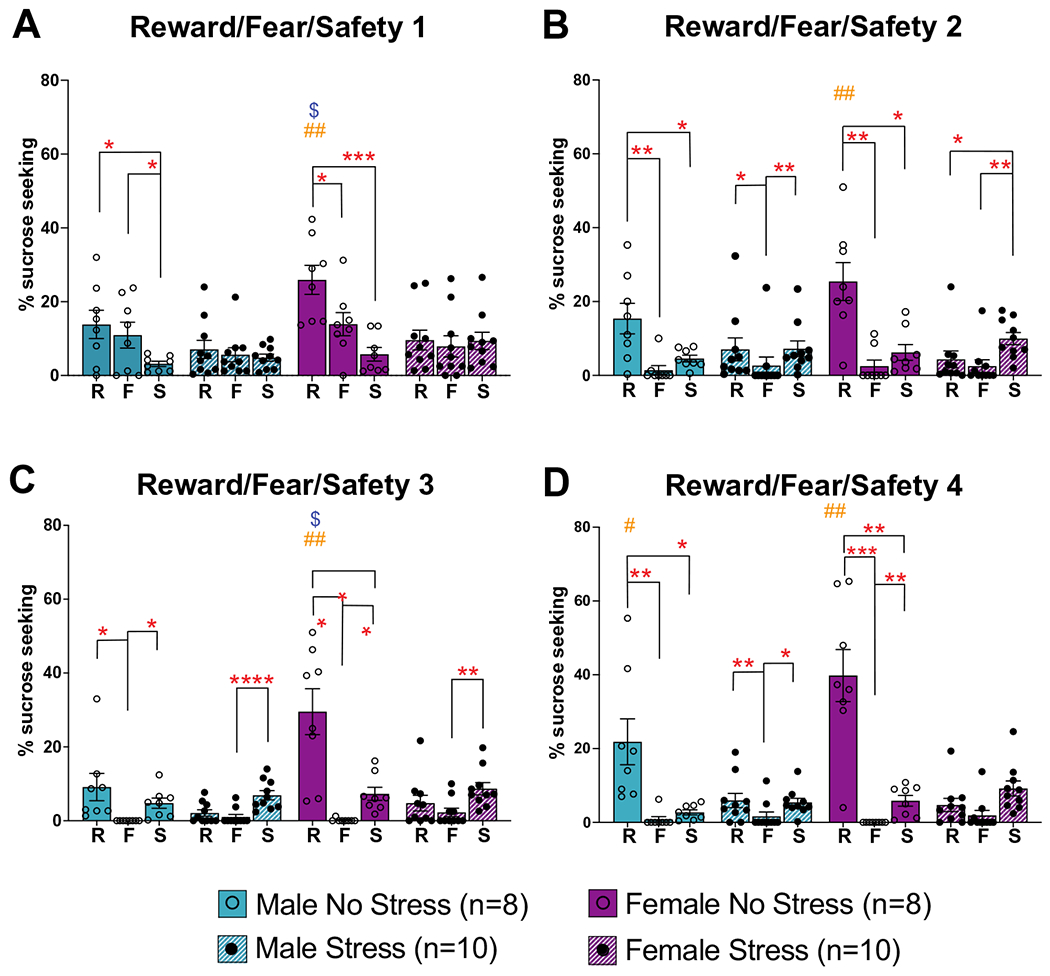
Sucrose seeking during reward/fear/safety sessions. Sucrose seeking during the 20 s cue period across the 4 reward/ fear/safety sessions in response to the reward (R; paired with sucrose), fear (F; paired with footshock), and safety (S; no sucrose or footshock) cues. In most cases, sucrose seeking was the highest during the reward (R) cue (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 indicate within sex, within condition, between cue effects). In general, male and female stress groups showed decreased reward seeking compared to their control counterparts (#p < 0.05; ##p < 0.01 indicate within sex, within cue, between condition effects). In sessions 1 and 3 (A and C), female no stress rats showed increased reward seeking compared to male no stress rats ($p < 0.05 indicates within condition, within cue, between sex effects). Data are shown as mean % time spent sucrose seeking + / − SEM
Fig. 6.
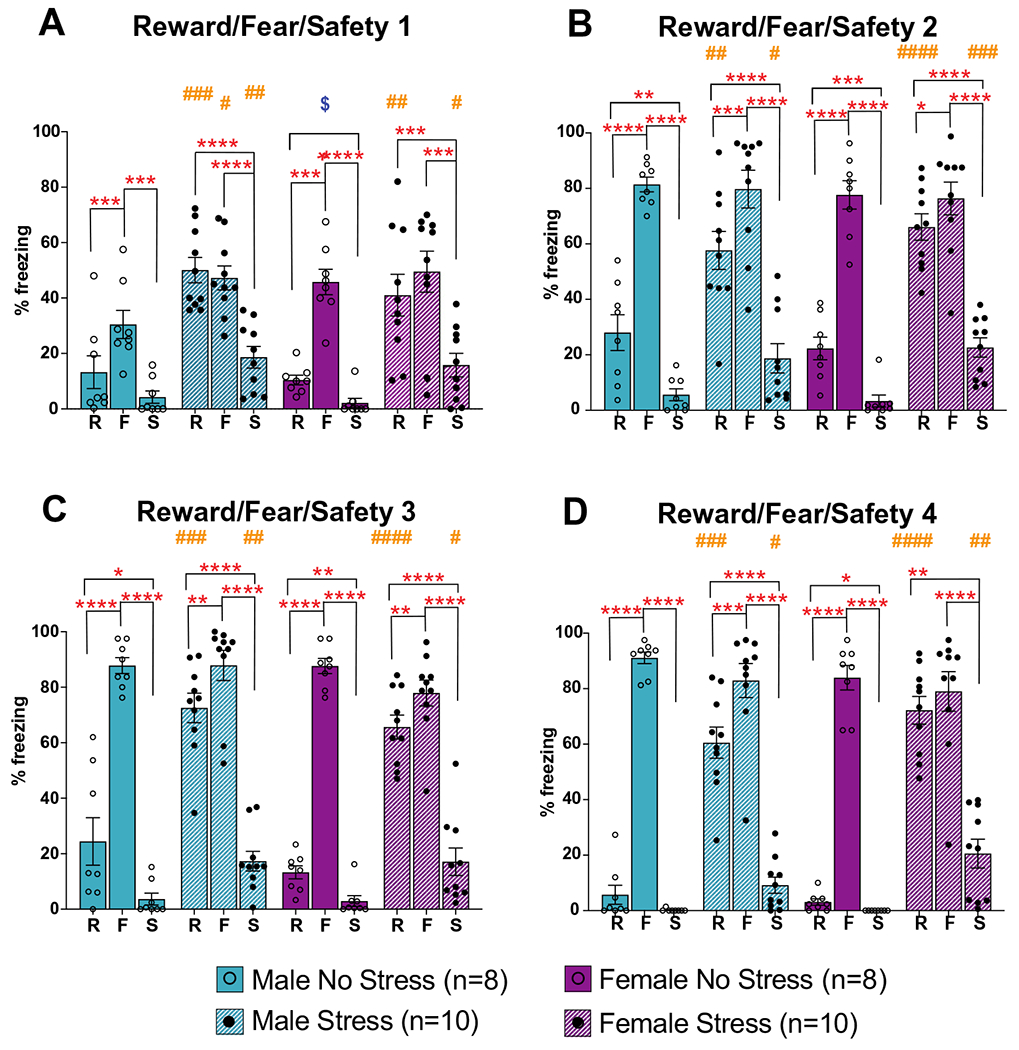
Freezing during reward/fear/safety sessions. A Freezing during the 20 s cue period across the 4 reward/fear/safety sessions in response to the reward (R; paired with sucrose), fear (F; paired with footshock), and safety (S; no sucrose or footshock) cues. For all groups freezing was elevated during the fear (F) cue (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 indicate within sex, within condition, between cue effects). In general, stress increased freezing to the reward and safety cues regardless of sex (#p < 0.05; ##, p < 0.01; ###, p < 0.001; ####, p < 0.0001 indicate within cue, within sex, between condition effects). In the first session (A), female no stress rats froze significantly more than male no stress rats ($p < 0.05 indicates within cue, within condition, between sex effects). Data are shown as mean % time spent freezing + / − SEM
Sucrose seeking during reward/fear/safety sessions
A separate 3-way ANOVA for each reward/fear/safety conditioning session, RFS1-4, was conducted to compare cue, condition, and sex to assess sucrose seeking during each cue (Fig. 5a–d). For reward/fear/safety session RFS1, we found main effects of cue (F(2, 62) = 19.40, p < 0.0001), condition (F(1,32) = 5.08, p = 0.031), and sex (F(1,32) = 4.36, p = 0.045), as well as a cue by condition interaction (F(2,64) = 14.22, p < 0.0001). For reward/fear/safety session RFS2, we found a main effect of cue (F(2, 46) = 28.35, p < 0.0001) and significant interactions of cue by condition (F(2, 64) = 22.75, p < 0.0001) and for cue by sex by condition (F(2,64) = 3.34, p = 0.042). For reward/fear/safety session RFS3, main effects were found for cue (F(2, 40) = 23.57, p < 0.0001), condition (F(1,32) = 9.78, p = 0.004), and sex (F(1,32) = 12.81, p = 0.001). Significant interactions were also found for cue by sex (F(2,64) = 7.30, p = 0.001), cue by condition (F(2,64) = 21.69, p < 0.0001), sex by condition (F(1,32) = 4.68, p = 0.038), and cue by sex by condition (F(2,64) = 5.69, p = 0.005). Finally, for reward/fear/safety session RFS4, we found main effects of cue (F(2,37) = 50.34, p < 0.0001), sex (F(1,32) = 4.15, p = 0.05), and condition (F(1,32) = 13.86, p = 0.0008). Significant interactions were also found for cue by condition (F(2,64) = 41.87, p < 0.0001) and cue by sex by condition (F(2,64) = 5.46, p = 0.006).
Post hoc analyses indicated that across sessions, the male and female no stress groups showed more sucrose seeking during the reward cue compared to the fear or safety cues (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 in Fig. 5a–d). This was not apparent for the stress groups for either males or females, indicating an effect of prior stress on appropriate sucrose seeking during a sucrose-paired cue. Furthermore, the female no stress group showed significantly more sucrose seeking during the reward cue compared to the female stress group for all 4 reward/fear/safety session sessions, while the male no stress group only showed this effect compared to male stress rats in reward/fear/safety session RFS4 (#p < 0.05, ##p < 0.01 in Fig. 5a–d). Finally, sex differences were found within the no stress groups, with females showing more sucrose seeking than males for reward/fear/safety sessions RFS1 and RFS3 ($, p < 0.05 in Fig. 5a and c, respectively).
Similar to the above, separate 3-way ANOVAs for each discriminative conditioning session, RFS1-4, was conducted to compare condition, cue, and sex to assess sucrose seeking during the 20 s immediately after each cue (Supplemental Fig. 3). For both reward/fear/safety sessions RFS1 and RFS2, we found main effects of cue (F(2, 50) = 119.5, p < 0.0001; F(2, 40) = 156.3, p < 0.0001), sex (F(1,32) = 7.71, p = 0.009; F(1,32) = 4.26, p = 0.047) and condition (F(1,32) = 18.57, p = 0.0001; F(1,32) = 12.93, p = 0.001). Significant interactions were also found for cue by sex (F(2,64) = 7.72, p = 0.001; F(2,64) = 5.63, p = 0.006) and cue by condition (F(2,64) = 18.25, p < 0.0001; F(2,64) = 34.16, p < 0.0001). For reward/fear/safety session RFS3, main effects of cue (F(2, 39) = 182.1, p < 0.0001) and condition (F(1,32) = 21.97, p < 0.0001) were found, as well as a significant interaction of cue by condition (F(2,64) = 55.51, p < 0.0001). During reward/fear/safety session RFS4, we found main effects of cue (F(2, 54) = 144.6, p < 0.0001) and condition (F(1, 32) = 17.68, p = 0.0002). Significant interactions were also found for cue by sex (F(2, 64) = 3.35, p = 0.042), and cue by condition (F(2, 64) = 23.93, p < 0.0001).
Post hoc analyses indicated that across all sessions, all groups showed more sucrose seeking during the 20 s postreward cue period compared to the post-cue period for the fear and safety cues (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 in Supplemental Fig. 3a and b). Also, across all sessions, the no stress groups showed more sucrose seeking post-reward cue compared to their stress counterparts within each sex (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 in Supplemental Fig. 3a and d). Lastly, sex differences were found in the post-reward cue period during reward/fear/safety sessions RFS1 and RFS2, where the female no stress group showed more sucrose seeking than the male no stress group ($$, p < 0.01, $, p < 0.05 in Supplemental Fig. 3a and b respectively).
Freezing levels during reward/fear/safety sessions
A separate 3-way ANOVA for each reward/fear/safety conditioning session, RFS1-4, was conducted to compare cue, condition, and sex to assess freezing during each cue (Fig. 6). In reward/fear/safety session RFS1, we found main effects of cue (F(2, 60) = 107.2, p < 0.0001) and condition (F(1,32) = 19.93, p < 0.0001), as well as significant interactions of cue by sex (F(2, 64) = 5.78, p = 0.005) and cue by condition (F(2, 64) = 15.52, p < 0.0001). For reward/fear/safety sessions RFS2, RFS3, and RFS4, we found main effects of cue (F(2,53) = 314.3, p < 0.0001; F(2,61) = 441.9, p < 0.0001; F(2, 50) = 466.6, p < 0.0001) and condition (F(1, 32) = 16.85, p = 0.0003; F(1, 32) = 31.38, p < 0.0001; F(1,32) = 42.78, p < 0.0001), as well as a significant cue by condition interaction (F(2,64) = 26.11, p < 0.0001; F(2,64) = 61.27, p < 0.0001; F(2,64) = 95.00, p < 0.001).
Post hoc analyses indicated that for the no stress groups, males and females showed more freezing to the fear cue compared to the reward and safety cues for all reward/fear/safety sessions, RFS1-4 (Fig. 6a–d). In contrast, the stress groups only showed this effect for reward/fear/safety sessions RFS2-4 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 in Fig. 6a–d). Despite the appropriate elevation in displayed fear to the fear cue across all groups, the stress groups were notable in their elevated fear to the reward and safety cues compared to no stress groups across all reward/fear/safety sessions, RFS1-4 (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 in Fig. 6a–d). The only sex difference noted was higher freezing during the fear cue for the female no stress group compared to the male no stress group during reward/fear/safety session RFS1 ($p < 0.05 in Fig. 6a).
Similar to the above, separate 3-way ANOVAs for each reward/fear/safety conditioning session, RFS1-4, was conducted to compare cue, condition, and sex to assess freezing during 20 s after each cue (Supplemental Fig. 4). For reward/fear/safety session RFS1, a main effect of condition (F(1,32) = 29.97; p < 0.0001) was found. For both reward/fear/safety sessions RFS2 and RFS3, we found main effects of cue (F(2,64) = 6.34, p = 0.003; F(2,64) = 6.75, p = 0.003) and condition (F(1,32) = 45.31, p < 0.0001; F(1,32) = 71.12, p < 0.0001), as well as significant interactions of cue by condition (F(2,64) = 9.65, p = 0.0002; F(2,64) = 17.55, p < 0.0001) and cue by sex by condition (F(2,64) = 8.08, p = 0.0007; F(2,64) = 5.10, p = 0.009). During reward/fear/safety session RFS4, we found main effects of cue (F(2, 60) = 17.15, p < 0.0001) and condition (F(1, 32) = 85.11, p < 0.0001). Significant interactions were also found for cue by sex (F(2, 64) = 4.64, p = 0.013), cue by condition (F(2, 64) = 22.26, p < 0.0001), and cue by sex by condition (F(2, 64) = 4.41, p = 0.016).
Post hoc analyses for freezing during the 20 s post-cue periods consistently showed that freezing post-reward cue was significantly higher than post-safety cue for both male and female stress groups across most sessions (*p < 0.05, **p < 0.01, ***p < 0.001 in Supplemental Fig. 4a–d). Also consistent in the male and female stress groups were elevated freezing post-reward and post-safety cues compared to their no stress counterparts (#p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 in Supplemental Fig. 4a–d). The only sex differences noted were higher freezing post-fear cue in male stress versus female stress in reward/fear/safety session RFS2, and higher freezing post-safety cue in female stress versus male stress in reward/fear/safety session RFS4 ($p < 0.05 in Supplemental Fig. 4a and d, respectively).
Influence of stress on alcohol consumption
Across behavioral conditioning, all rats had continuous home cage access to both ethanol and water. Ethanol and water consumed were quantified at the time of each behavioral session and data are presented to show how much ethanol was consumed (g/kg/24 h) in the 24 h period after each session.
A 3-way ANOVA was conducted to compare session, condition, and sex to assess alcohol consumed (g/kg/24 h) in the 24 h after each session (Fig. 7a). Main effects of session (F(3, 90) = 9.19, p < 0.0001), condition (F1,32) = 9.45, p = 0.004), and sex (F(1,32) = 7.27, p = 0.011) were found. Post hoc analyses showed that female stress rats consistently consumed more ethanol than male no stress rats after every session except reward session R1 (*p < 0.05, **p < 0.01, ***p < 0.001 in Fig. 7a–d). The female stress group also showed more consumed ethanol compared to male stress rats after reward session R4 (p = 0.021), habituation (p = 0.031), and reward/fear/safety session RFS4 (p = 0.014) ($p < 0.05 in Fig. 7a; *p < 0.05 in Fig. 7b–d). Additionally, the female stress group showed higher ethanol consumed compared to female no stress rats after reward session R4 (p = 0.041), habituation (p = 0.012), and reward/fear/safety session RFS4 (p = 0.035) (%p < 0.05 in Fig. 7a; *p < 0.05 in Fig. 7b–d). Finally, the male stress group showed higher ethanol consumed compared to male no stress rats after reward session R4 (p = 0.009), habituation (p = 0.042), and reward/fear/safety sessions RFS1 (p = 0.034), RFS2 (p = 0.022), and RFS4 (p = 0.004) (#p < 0.05, ##p < 0.01 in Fig. 7a; *p < 0.05, **p < 0.01 in Fig. 7b–d).
Fig. 7.
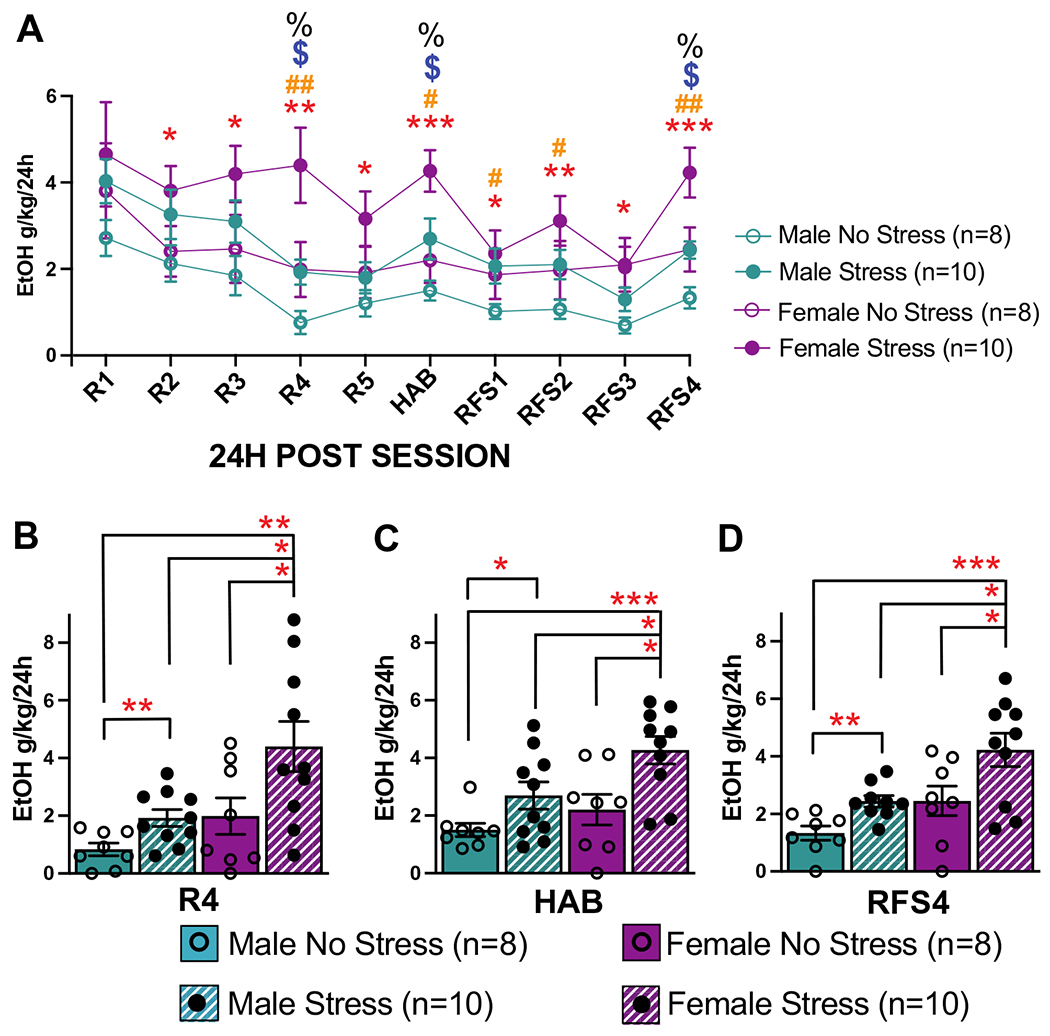
Home cage ethanol consumption during the 24 h after each behavioral session. Both ethanol and water were available to all rats in their home cages between sessions. R1–R5, reward sessions 1–5; HAB, habituation session; RFS1-4, reward/fear/safety sessions 1–4. A Ethanol consumption as g/kg for the 24 h period following each behavioral session averaged for each group (+ / − SEM). Female stress rats consistently consumed more ethanol than male no stress rats after every session except R1 (*p < 0.05; **p < 0.01; ***p < 0.001). The female stress group also showed more consumed ethanol compared to male stress rats ($p < 0.05) and female no stress rats (%p < 0.05) after sessions R4, HAB, and RFS4. The male stress group showed higher ethanol consumed compared to male no stress rats after sessions R4, HAB, RFS1, RFS2, and RFS4 (#p < 0.05; ##p < 0.01). B–D Data for sessions in which multiple significant effects of condition and sex were found are shown in more detail with individual data points (*p < 0.05; **p < 0.01; ***p < 0.001). Overall, the female stress group showed the highest levels of ethanol consumed across behavioral conditioning, with the male stress group also showing higher ethanol consumed compared to male no stress rats across most behavioral conditioning
Data for sessions in which multiple significant effects of condition and sex were found are shown in more detail in Fig. 7b–d. Overall, the female stress group showed the highest levels of ethanol consumed across behavioral conditioning, with the male stress group also showing higher ethanol consumed compared to male no stress rats across most behavioral conditioning.
Correlations between alcohol consumption and behavior
Thus far, it appears that stress reduced sucrose seeking during a reward cue while increasing alcohol consumption in the home cage afterwards, particularly in females. This could also be related to elevated freezing during the reward and safety cues within the stress groups of both sexes. To assess how these all may be linked together, we calculated Pearson’s correlations in males (Fig. 8a) and females (Fig. 8b) in the following measures collapsed across all 4 reward/fear/safety sessions: ethanol consumed 24 h prior to session, ethanol consumed 24 h after session, % time freezing to the reward cue, % time freezing to the safety cue, and % time sucrose seeking to the reward cue. Data are shown as a correlation matrix for each sex.
Fig. 8.
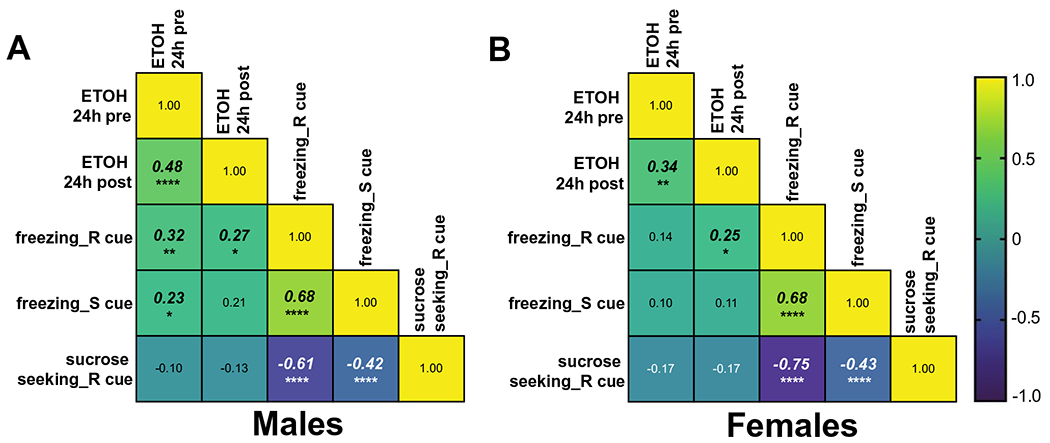
Correlations between behavior and ethanol consumption for males (A) and females (B). Pearson’s correlations are shown for displayed measures collapsed across all 4 reward/fear/safety
In both males and females, ethanol consumed in the 24 h prior to a session was positively correlated with ethanol consumed in the 24 h after the session (M: p < 0.0001; F: p = 0.003). In males, ethanol consumed in the 24 h prior to a session was also positively correlated with the amount of freezing to both the reward (p = 0.007) and safety (p = 0.05) cues. For both males and females, freezing during the reward cue was positively correlated with the subsequent ethanol consumed in the 24 h after the session (M: p = 0.023; F: p = 0.038). Additionally, both sexes showed positive correlations between freezing during the reward cue and freezing during the safety cue (M: p < 0.0001; F: p < 0.0001), and negative correlations between freezing during the reward and safety cues with sucrose seeking during the reward cue (p < 0.0001 for each correlation). Overall, males and females showed similar correlations across these measures, with males demonstrating additional significant correlations between prior ethanol consumed and freezing behavior during the reward and safety cues. The most notable alcohol by behavior correlation was the positive correlation in both males and females between freezing during the reward cue and subsequent alcohol consumption.
Discussion
Our goal in this study was to investigate the potential interaction and effects of prior stress and alcohol history on reward-fear-safety cue discrimination and continued drinking in male versus female rats. Our results showed that females consumed more alcohol than males, and that unstressed females also displayed elevated cued sucrose seeking compared to unstressed males. This seems to indicate generally higher reward seeking in females. Stress exposure reduced sucrose seeking during a reward cue and increased freezing to the reward and safety cues in both males and females. Despite these similar effects of stress on conditioned behaviors, it was notable that stress had a bigger effect in females in home cage alcohol consumption compared to stressed and unstressed males, as well as unstressed females. Directly correlating ethanol consumption with behavior revealed that the amount of freezing during a reward cue was positively correlated with subsequent alcohol consumption in both males and females. This positive correlation may indicate that failure to regulate fear during a reward cue is associated with elevated alcohol consumption.
Also notable was the amount of freezing to the safety cue across sessions in the stress groups compared to their control counterparts. We assessed if higher freezing to the safety cue was also correlated with alcohol consumption. A positive correlation may indicate failure to regulate fear during a safety cue is similarly associated with alcohol consumption. Our results only indicated a positive correlation in the ethanol consumed prior to a session to safety cue freezing in males. It is interesting that males showed a similar positive correlation between prior ethanol consumed and amount of freezing to the reward cue, while females showed neither. For males, it is thus possible that freezing behavior during the reward/fear/safety sessions could have been influenced by having alcohol on board during the training sessions, resulting in higher freezing levels across all cues. While we did not directly test this possibility, given the females showed similarly high freezing levels across the same cues as the males and only showed correlations of freezing with subsequent ethanol consumption, versus prior ethanol consumption, it seems unlikely this would be the case. Nonetheless, this would be a valuable assessment to tease apart for our future studies. We also tested if there was a negative correlation between sucrose seeking during a reward cue and subsequent alcohol consumption which could indicate dysregulated reward seeking after stress. We, however, did not find a significant correlation between sucrose seeking and subsequent alcohol consumption. Taken together, our results point towards a failure to regulate fear during a reward cue or safety cue being associated with increased alcohol consumption.
Similar to others, stress did not result in immediate changes in established drinking patterns as ethanol consumption was not affected in the week following stress (e.g., Meyer et al, 2013; Kirson et al, 2021). Our observations of elevated ethanol consumption in the stress groups emerged later during behavioral conditioning. One limitation of our study is that we did not have a parallel group that had 5 weeks of prior alcohol access along with stress, but without behavioral conditioning. It is possible that the increase in alcohol consumption in the stress groups was simply a product of elapsed time. However, the positive correlation between the amount of freezing during the reward cue and amount of alcohol consumed in the subsequent 24 h period indicate that it was related to behavioral conditioning and the arousal caused by it for the stress groups.
In a previous study with male rats, we showed the same stress procedure, but without alcohol exposure, reduced sucrose seeking during a reward cue and increased freezing during the reward and safety cues (Woon et al, 2020). Our data here are consistent with and replicate this effect. In this prior work, we also showed no significant baseline differences between stress and control rats in either the amount of sucrose seeking or freezing prior to cue presentations. Instead, effects seemed to be limited to the cue presentations themselves. This is not surprising given that the intertrial interval in both the prior and current study was 100–140 s across the 44 trials, an ample amount of time for freezing and/or sucrose seeking from the previous trial to dissipate by the next trial. We, however, did not assess pre-cue responding in the current study, and it is possible differences in generalized responding, particularly in freezing, throughout the intertrial intervals could have emerged as a result of the interaction of alcohol and stress. We did assess responding in the 20 s immediately after each cue and freezing returns to almost 0 by the last training session in no stress rats, and dampens to ~ 40% post-cue from > 80% during the cue in the stress rats. Compared to our previous study with stress exposure, the magnitude of elevated freezing to the reward cue was much greater in the current study that also included alcohol exposure, compared to our previous study, without alcohol exposure. More specifically, in our previous study, stress resulted in an increase to ~ 20–30% freezing during the reward cue in males, while in the present study, stress combined with alcohol exposure resulted in ~ 50–70% freezing to the reward cue. While this could be simply due to a difference in different cohorts, it is interesting to speculate that it was the interaction of alcohol history with stress that caused a synergistic effect on increasing fear during the reward cue.
Our prior work has already identified several behavioral sex differences in a similar safety learning task under stress- and alcohol-free conditions (Greiner et al, 2019). Female rats in this prior study showed higher sucrose seeking during earlier training sessions, but after the first session with footshocks, this reduced to the same levels as the male rats. Most striking in that study was the lack of conditioned suppression of freezing in the female rats when the fear and safety cues were presented together as a compound cue, i.e., conditioned inhibition. That is, the females froze equally high to the fear cue and fear + safety cue. As part of that study where we first explored sex differences in our safety task, we assessed a naïve group of male and female rats in their responsiveness to a range of shock intensities since age-matched females are smaller and could have perceived the footshock as more aversive than males. We found no sex differences in freezing or jumping in response to shock intensities across a 0.3–1.0 mA range, tested in 0.05 mA intervals. Despite this lack of sex difference in unstressed, alcohol-free rats, it is possible that prior history of alcohol may influence the perception of shock at time of the stress exposure, especially considering consumption was higher in females and it was the females that showed greater stress-induced drinking. This is a very interesting possibility that would indicate long-term changes in sensory perception caused by chronic alcohol consumption, and we will explore this in future studies by similarly assessing responsiveness in rats with stress and/or alcohol to compare to our prior findings.
Prior work has shown interesting sex differences in the effect of context on responding to an alcohol-predictive cue, where males show context-dependent responding to a discrete alcohol-associated cue but females show equivalent responding to the same cue regardless of context (Segal et al, 2022). The authors proposed that males may have relied more on context whereas females relied more on cues to guide their alcohol seeking behavior. These results could be related to our data showing increased sucrose seeking in unstressed females compared to unstressed males, both during the cue period as well as the post-cue period. While we did not explicitly investigate the influence of context, one may consider the home cage as the “alcohol” context and the conditioning boxes as the “stress” context. Stressed males and stressed females increased home cage alcohol consumption following several training sessions that were associated with cued foot shock and reminiscent of prior stress. This was particularly evident in the stressed females and may be capturing a higher propensity for females to display stress-induced drinking. Whether this was governed by the behavioral conditioning context or responses elicited by conditioned cues, it remains to be further explored. However, we propose, based on the data here and our prior work, that females may be more cue responsive under both fear and reward conditions.
The data presented here show promise in recapitulating some of the reported increases in sensitivity to stress-induced drinking in women, laying the groundwork for a more mechanistic approach at a behavioral, circuit, cellular, and molecular level to better understand sex-specific responses to stress in the context of addiction.
Supplementary Material
Acknowledgements
This work was supported by NIMHR01MH110425 to SS. We thank Yolanda Jonker and Signe Hobaugh for excellent animal care, and to Dr. Christopher Lapish for discussions regarding alcohol paradigms.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00213-022-06206-5.
Conflict of interest The authors declare no competing interests.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders-text revision (DSM-IV-TR). American Psychiatric Press, Washington, DC [Google Scholar]
- Blanchard RJ, Blanchard DC (1969) Crouching as an index of fear. J Comp Physiol Psychol 67:370–375 [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP (2013) Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res 256(1):10–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23:743–760 [DOI] [PubMed] [Google Scholar]
- Greiner EM, Müller I, Norris MR, Ng KH, Sangha S (2019) Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behav Brain Res 368:111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H (2012) Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21(3):169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Steinman MQ, Wolfe SA, Spierling Bagsic SR, Bajo M, Sureshchandra S, Oleata CS, Messaoudi I, Zorrilla EP, Roberto M (2021) Sex and context differences in the effects of trauma on comorbid alcohol use and post-traumatic stress phenotypes in actively drinking rats. J Neurosci Res 99(12):3354–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I (2013) Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res 37:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I, Brinkman AL, Sowinski EM, Sangha S (2018) Adolescent conditioning affects rate of adult fear, safety and reward learning during discriminative conditioning. Sci Rep 8:17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Pollock MW, Urbanczyk PJ, Sangha S (2018) Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue. Neurobiol Learn Mem 147:26. [DOI] [PubMed] [Google Scholar]
- Nutt DJ (2000) The psychobiology of posttraumatic stress disorder. J Clin Psychiatry 61(Suppl 5):24–32 [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA (2019) Sex differences in stress-related alcohol use. Neurobiology of Stress 10:100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzaka RH, Goldstein RB, Southwick SM, Grant BF (2011) Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord 25:456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH (2013) Safety encoding in the basal amygdala. J Neurosci 33:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Greba Q, Robinson PD, Ballendine SA, Howland JG (2014) Heightened fear in response to a safety cue and extinguished fear cue in a rat model of maternal immune activation. Front Behav Neurosci 8:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Davies DA, Greba Q, Howland JG (2014) Alterations in reward, fear and safety cue discrimination after inactivation of the prelimbic and infralimbic cortices. Neuropsychopharm 39:2405–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D, Valyear MB, Chaudhri N (2022) The role of context on responding to an alcohol-predictive cue in female and male rats. Alcohol 99:70–81 [DOI] [PubMed] [Google Scholar]
- van der Kolk BA (1997) The psychobiology of posttraumatic stress disorder. J Clin Psychiatry 58(Suppl 9):16–24 [PubMed] [Google Scholar]
- Venniro M, Banks ML, Epstein HMDH, & Shaham Y, (2020) Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21(11):625–643 [DOI] [PubMed] [Google Scholar]
- Woon E, Seibert T, Urbanczyk P, Ng KH, Sangha S (2020) Differential effects of prior stress on conditioned inhibition of fear and fear extinction. Behav Brain Res 381:112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Garcia-Rivas V, Thomas MA, Soares AR, McKee SA, Picciotto MR. Sex differences in stress-induced alcohol intake: a review of preclinical studies focused on amygdala and inflammatory pathways. Psychopharmacology (Berl). 2022. Mar 31. doi: 10.1007/s00213-022-06120-w. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


