Abstract
Objective: Sleep disturbance is one of the most prevalent problems in post-menopausal females. The current research intended to evaluate the effects of Dracocephalum on sleep disorder in post-menopausal females.
Method : The current study is a randomized, double-blind controlled trial, in which 110 post-menopausal women were randomly allocated to Dracocephalum or placebo groups. The intervention group took Dracocephalum capsules containing 250 mg Dracocephalum extract twice daily for one month. While, the placebo group took the same capsule containing 250 mg of starch twice daily for one month. Pittsburgh Sleep Quality Index was completed by the participants of both groups before and after the treatment and the data obtained were analyzed with Chi-square, paired and independent t-test in SPSS (version 20).
Results: The mean score of sleep quality before and after the treatment was 12.69 ± 3.98 and 8.58 ± 1.97 in the treatment group, respectively. Also, the score of sleep quality in the placebo group was 13.48 ± 2.60 and 11.21 ± 2.74 at the beginning and end of the research, respectively. The symptoms of sleep disorder in the intervention group significantly improved after the treatment (P < 0.001), while this was not the case with the placebo group (P = 0.155). Besides, there was a significant difference between the two groups in the mean score of sleep quality after the treatment (P = 0.012).
Conclusion: Dracocephalum extracts are effective in reducing symptoms of sleep disorders in post-menopausal women.
Key Words: Dracocephalum, Herbal Medicine, Post-Menopausal Period, Randomized Controlled Trial, Sleep Quality
The frequency of Sleep problems raises in the menopausal transition period (1, 2). Women spend about one-third of their lives in the menopausal stage (3). According to the Iranian National Statistics, 13.8% of the general population are women aged 45 to 60 years (4). Therefore, considering the large population of women, it is important to pay attention to their needs at this age. The frequency of sleep disturbances in post-menopausal women has been reported as high as 65%, and this is one of the main reasons for post-menopausal women to visit health centers (5, 6). Sleep is one of the most basic human needs, which, in addition to maintaining physical and mental health, reduces stress, strengthens reconciliation and increases focus on daily activities (7, 8). During sleep, important functions are performed, such as energy storage, division of skin and bone marrow cells, regulation and secretion of hormones, regulation of the immune system, and also restoration of mental function (9-11). Sleep helps restore mental and physiological abilities and is necessary for taking on new tasks and roles (11, 12). Sleep problems affect health-related quality of life, job productivity and healthcare utilization and may have long-term impacts on well-being and health during the menopausal transition years (13, 14). Usually, the first line of treatment used by clinicians to treat sleep disorders in these people is the administration of hormones. However, many women do not want to use this medicinal method due to various side effects of hormone therapy, including breast sensitivity, nausea, and headache. Also, many women may not be eligible for hormone therapy. On the other hand, there are various complementary medicines and methods to solve this problem. Nowadays, due to the side effects of chemical drugs and the general tendency to use herbal remedies, researchers have become inclined to use herbal remedies (3, 4, 15-17). One of the most common herbs in the treatment of sleep disorders is Dracocephalum, which is a plant from the mint family, and its leaves are used in essential oils, extracts, ointments, and infusions (4). No drug interactions or side effects have been reported so far for Dracocephalum and it has entered pharmaceuticals in various countries such as Germany (4, 18). Cases et al. reported that Dracocephalum improved insomnia by 42% in adults with anxiety and sleep disorders (16). Furthermore, Haybar et al. found a considerable reduction in symptoms of sleep disorders in the Dracocephalum group in comparison to the placebo group in adult patients with chronic stable angina (19). Taavoni et al. investigated the effects of this herbal medicine on sleep disturbances in menopausal women and reported a relative improvement in the sleep status after the intervention (4). However, previous systematic reviews and meta-analyses have shown that there are conflicting results regarding the effects of different medicinal herbs on sleep problems, especially in the post-menopausal period, and more clinical trials are needed to investigate this line of treatment (i.e., herbal medications) for sleep disorders (20, 21).
Although the literature indicates the positive effects of Dracocephalum on the quality of sleep in people with sleep disorders, no study, to the best of our knowledge, has examined the effects of Dracocephalum on post-menopausal-related sleep disorders. Given the limited number of studies investigating the effects of Dracocephalum on sleep disorders and also the lack of studies investigating the effect of Dracocephalum on sleep disturbance in post-menopausal females, this research was administered to evaluate the effect of the extract of Dracocephalum on sleep quality among post-menopausal women.
Materials and Methods
This study is a parallel double-blinded randomized clinical trial that included 110 post-menopausal females aged 50 to 60 years who referred to health centers in the Arak city. The sample size was calculated based on 95% confidence interval, study power 80% and the literature. Finally, considering the attrition rate of 10%, the required sample size for each of the intervention and placebo groups was 55 samples. Convenience sampling was used, and after selecting the eligibility criteria, random allocation was utilized for assigning subjects to intervention or placebo groups through online random number generators. The placebo and treatment groups were masked to both researchers and subjects through coding, and placebo was administered by a third party until the end of the research. The written informed consent was obtained from all eligible subjects and the ethical committee of Arak University of Medical Sciences approved this study. Moreover, this research has been registered in the Iranian Clinical Trial Registry (IRCT code: IRCT2015073110076N5).
The inclusion criteria included the passage of at least one year from the last menstruation after the onset of natural menopause, having a mild to moderate sleep disorder based on the Pittsburg Sleep Quality Index (PSQI) questionnaire, lack of physical and mental illnesses affecting sleep (depression, anxiety, other psychiatric conditions, asthma, thyroid disorders, malignancies and other respiratory chronic diseases), taking no drugs that affect sleep (pain killers, diuretics, sedatives, anti-depressants), no smoking or alcohol dependency, lack of allergy to a drug or particular substance. The exclusion criteria included getting sick physically or mentally during the study, significant changes in sleep conditions due to travel or displacement, taking any medications or sleeping drugs during the study and inability to continue the treatment for more than seven days during the study.
Procedure
In the first stage of the study, the PSQI questionnaire was completed by the subjects through an interview with an experienced researcher in the field of sleep disorders, and women with a sleep quality score of five and above were selected. All eligible subjects were asked for demographic characteristics including the five items of menopause age, number of deliveries, number of children, education level and employment status. In the second stage of this research, participants were randomly divided into two groups. The intervention group took a capsule containing 250 mg of Dracocephalum extract twice daily for one month, while the placebo-controlled group received the same capsule containing 250 mg of starch twice daily for one month. A researcher checked the correct use of the medication and the physical and mental symptoms of the subjects every week by telephone. All participants were followed for one month throughout the intervention. After one month, all participants were re-evaluated through the PSQI questionnaire.
Assessment
Assessment was done before and after the intervention through the PSQI questionnaire. This is a good tool to measure the patterns and quality of sleep in adults. The scale contains 19 items in seven facets of sleep quality (sleep latency, subjective sleep quality, sleep duration, sleep disturbances, habitual sleep efficiency, daytime dysfunction, and consumption of sleeping medications). It has nine main questions (questions 1 to 4 with short and open answers, and questions 5 to 9 with four-point Likert answers). The score of each component is 0 to 3 and thus the total score of the PSQI questionnaire can vary from 0 to 21 (22). A lower score indicates better sleep quality. A total score of five and above indicates poor sleep quality, and the larger this score, the poorer the quality of sleep. The reliability of the Iranian version of the PSQI questionnaire was calculated to be 78% using the test-retest method and 85% using the internal consistency method (Cronbach's alpha). Also, the reliability of the Iranian version of this questionnaire was calculated in another research using the internal consistency method, and its Cronbach's alpha coefficient was 0.76-0.83 (23, 24).
Statistical analysis
After collecting the data, the sleep quality score was calculated based on the standard protocol of the PSQI questionnaire. Marital status and literacy level of the two groups were compared via chi-square test. Moreover, independent t-test was utilized to compare the age of menopause, the number of deliveries and the number of children between the two groups. Kolmogorov-Smirnov test was utilized to check the normality of sleep quality data. A paired t-test was utilized to compare the mean scores before and after the treatment in each group. Moreover, the independent t-test was utilized to compare the mean PSQI scores between the two groups at the beginning and end of the intervention. Analyses were conducted by SPSS (version 20). A P-value less than 0.05 was considered significant.
Results
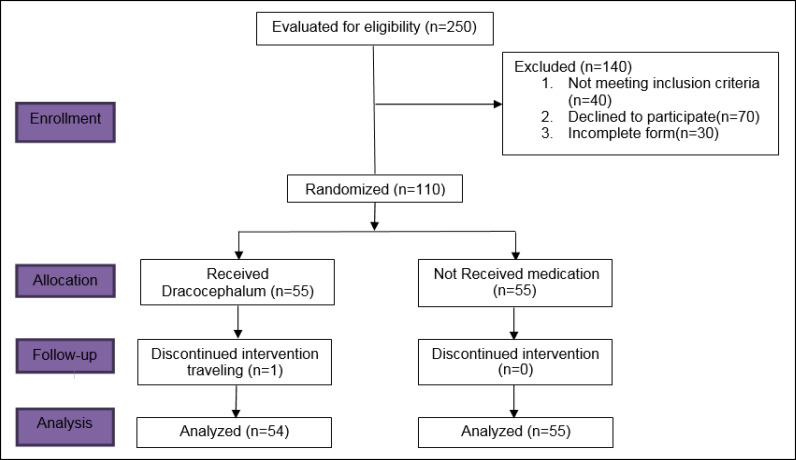
First, it should be noted that one woman was excluded from the intervention group because of travelling. Figure 1 indicates the CONSORT diagram of the processes involved in the different stages of this parallel randomized trial for both intervention and placebo-controlled groups. According to Table 1, there was no significant difference between the two groups in terms of demographic characteristics, including marital status (P = 0.120) and literacy rate (P = 0.146). Furthermore, as indicated in Table 2, the two groups were similar in terms of menopause age, number of deliveries and number of children (P > 0.05).
Figure 1.
CONSORT Diagram of the Processes Involved in the Different Stages of the Parallel Randomized Trial for Both Intervention and Placebo-Controlled Groups
Table 1.
Distribution of the Participants based on Demographic Variables in Both Intervention and Placebo Groups
| Variables |
Intervention group
N (%) |
Placebo group
N (%) |
P-value | |
|---|---|---|---|---|
| Marital status | Single/widow/divorced | 8 (15.4) | 1 (1.9) | 0.12 |
| Married | 44 (84.6) | 54 (98.1) | ||
| Literacy rate | Illiterate | 20 (38.5) | 15 (27.2) | 0.146 |
| High school diploma | 21 (40.4) | 30 (54.5) | ||
| Diploma and higher | 11 (21.2) | 55 (18.1) | ||
Table 2.
Baseline Characteristics of Both Intervention and Placebo Groups
| Variables |
Intervention group
(Mean ± SD) |
Placebo group
(Mean ± SD) |
P-value |
|---|---|---|---|
| Menopause Age (years) | 53.17 ± 4.88 | 52.4 ± 9.17 | 0.085 |
| Number of children | 3.1 ± 1.29 | 3.21 ± 1.45 | 0.627 |
| Number of deliveries | 4.20 ± 2.38 | 4.2 ± 2.35 | 0.857 |
Table 3 shows the sleep quality scores for the two groups and its changes before and after the treatment. As shown, the mean score of sleep quality, before and after the intervention, was 12.69 ± 3.98 and 8.58 ± 1.97 in the Dracocephalum group, respectively. Furthermore, the mean sleep quality score in the placebo group was 13.48 ± 2.60 and 11.21 ± 2.74 at the beginning and end of the research. The paired t-test revealed that the symptoms of sleep disorder in the intervention group significantly improved after treatment (P < 0.001), while this was not the case for the placebo group (P = 0.155). Also, the independent t-test showed that there was a significant difference in the mean score of sleep quality between the two groups after the intervention (P = 0.012). It should be noted that based on the results of the independent t-test, there was no significant difference between the intervention group and the placebo-controlled group in the sleep quality score before the treatment (P > 0.05). As shown in Table 3, the mean changes in PSQI scores were -4.11 and -2.27 (post minus pre) for the intervention and placebo groups, respectively. There was also a significant difference between the two groups in the mean change of the PSQI score (P < 0.001).
Table 3.
Comparing the Mean Score of Sleep Quality between Two Intervention and Placebo Groups Before and After the Intervention
| Groups | PSQI score | Mean change | P-Value ‡ | |
|---|---|---|---|---|
|
Before intervention
Mean ± SD |
After intervention
Mean ± SD |
|||
| Intervention | 12.69 ± 3.98 | 8.58 ± 1.97 | 4.11 | < 0.001 |
| Placebo | 13.48 ± 2.60 | 11.21 ± 2.74 | 2.27 | 0.155 |
| P-Value† | 0.876 | 0.012 | < 0.001 | |
PSQI: Pittsburg Sleep Quality Index. SD: standard deviation
Based on independent t-test;
Based on paired t-test
Discussion
Menopause is one of the most crucial periods of life for some women (1, 25). Of the most prevalent problems of females in this period are sleep disorders and poor quality of sleep. Hence, the current work was designed with the aim of evaluating the effect of Dracocephalum extract on improving the sleep quality of post-menopausal women. Based on our findings, we can conclude that Dracocephalum extract could be effective in reducing sleep disorders. This conclusion is consistent with previous researches that investigated the effect of this herbal medicine on sleep disorders (26, 27). For instance, Haybar et al. investigated the effect of Dracocephalum as a supplement in patients with chronic stable angina and reported that an eight-week treatment with this supplement can reduce the symptoms of anxiety, depression, stress and sleep disorders in patients (19). Heydari et al. confirmed the effect of this herbal medicine on reducing symptoms of sleep disorder and anxiety by investigating the effect of Dracocephalum on teenage girls with premenstrual syndrome (28). Experimental studies have also shown the sedative effects of Dracocephalum. In the study by Gorgi et al., Dracocephalum significantly increased the duration of sleep in laboratory rats in comparison to the control group (29). Despite the positive effects reported for Dracocephalum, so far few studies have investigated its mechanisms of effect on the sleep process. Dracocephalum contains a phytochemical agent that inhibits the catabolism of gamma-aminobutyric acid (GABA), and as a result, leads to an increase in the concentration of this neurotransmitter, which can alleviate the symptoms of sleep disorders (27, 30). It should be noted that previous studies have reported decreased levels of GABA in people with sleep disorders (31, 32). There are also reports of the effect of Dracocephalum on adenosine and 5HT-5α receptors, which are important receptors in sleep regulation and sleep disorders (33, 34).
In addition to Dracocephalum, other medicinal herbs have also been investigated to improve sleep problems in pre- or in post-menopausal women. For example, Lipoval et al. showed, in a cross-over study, that taking 80 mg of red clover daily could reduce sleep problems in post-menopausal women, as compared to the placebo group (35). In a parallel study, Shamshad Begum et al. showed that fenugreek bark extract can improve insomnia in menopausal women, as compared to the placebo group (36). Kamalifard et al. reported that either 500 mg of bitter orange or lavender flower significantly improved sleep quality in pre- and post-menopausal women, as compared to the placebo group (37). Yang et al. showed that French maritime pine bark extract could significantly improve sleep problems in pre-menopausal women, as compared to the placebo group (38). In line with these studies, our findings support the use of medicinal herbs, with minimal side effects, for managing postmenopausal sleep problems. However, some clinical trials have reported no positive effects for these herbs on sleep disorders. Liu et al. found no significant effect of soy or daidzein on insomnia in post-menopausal women, compared to the placebo group (39). Moreover, Park and Kim found no significant effect of 784 mg of Scisandra chinensis extract on sleep quality in postmenopausal women (40). In general, it should be noted that most studies have reported no or mild to moderate clinical effects for herbs, including Dracocephalum. Therefore, future research should focus more on these medicinal herbs as a complementary treatment alongside common standard care.
Limitation
One of the limitations of this study was the probability of the influence of confounding variables on the sleep quality score. To solve this limitation, random sampling was employed to select the research samples. More importantly, the only way to evaluate the sleep quality of the subjects in this study was through the PSQI questionnaire, which is a subjective self-report method. Therefore, lack of objective and biological evaluations of subjects for a more accurate assessment of sleep quality is one of the serious limitations of the current research.
Conclusion
Dracocephalum extract is an effective treatment for enhancing the quality of sleep in post-menopausal females as well as for decreasing symptoms of sleep disturbances. This study showed that sleep quality increased after the consumption of 250 mg of Dracocephalum extract for one month. Therefore, the use of complementary herbal medicines can reduce sleep disorders in post-menopausal women. Future research should focus more on the use of Dracocephalum in different populations as an adjunctive treatment alongside common standard care.
Acknowledgment
We express our deep gratitude toward all dear colleagues and subjects taking part in this study for their sincere cooperation. We are also thankful to the Deputy of Research and Technology of Arak University of Medical Sciences for providing facilities and opportunities to conduct this study. This article is the result of a research project approved by Arak University of Medical Sciences No. 2222, which has been registered with the code IRCT2015073110076N5.
Conflict of Interest
None.
References
- 1.Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and Sleep Disorders in the Menopausal Transition. Sleep Med Clin. 2018;13(3):443–56. doi: 10.1016/j.jsmc.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaleghi A, Mohammadi MR, Zandifar A, Ahmadi N, Alavi SS, Ahmadi A, et al. Epidemiology of psychiatric disorders in children and adolescents; in Tehran, 2017. Asian J Psychiatr. 2018;37:146–53. doi: 10.1016/j.ajp.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Asltoghiri M, Ghodsi Z. The effects of Reflexology on sleep disorder in menopausal women. Procedia Soc Behav Sci. 2012;31:242–6. [Google Scholar]
- 4.TAAVONI S, Nazem Ekbatani N, HAGHANI H. The Effect of lemon Balm on sleep disorder in menopausal women 60-50 years old. Complementary Medicine Journal. 2013;2(4):344–54. [Google Scholar]
- 5.Taavoni S, Ekbatani N, Kashaniyan M, Haghani H. Effect of valerian on sleep quality in postmenopausal women: a randomized placebo-controlled clinical trial. Menopause. 2011;18(9):951–5. doi: 10.1097/gme.0b013e31820e9acf. [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18(2):221–8. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fund N, Green A, Chodick G, Orin M, Koren G, Shalev V, et al. The epidemiology of sleep disorders in Israel: results from a population-wide study. Sleep Med. 2020;67:120–7. doi: 10.1016/j.sleep.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Tao MF, Sun DM, Shao HF, Li CB, Teng YC. Poor sleep in middle-aged women is not associated with menopause per se. Braz J Med Biol Res. 2016;49(1):e4718. doi: 10.1590/1414-431X20154718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han KS, Kim L, Shim I. Stress and sleep disorder. Exp Neurobiol. 2012;21(4):141. doi: 10.5607/en.2012.21.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venter RE. Role of sleep in performance and recovery of athletes: a review article. S Afr J Res Sport Phys Educ Recreat. 2012;34(1):167–84. [Google Scholar]
- 11.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 12.Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68(3):224–32. doi: 10.1016/j.maturitas.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014;21(12):1301–18. doi: 10.1097/GME.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 14.Ornat L, Martínez-Dearth R, Chedraui P, Pérez-López FR. Assessment of subjective sleep disturbance and related factors during female mid-life with the Jenkins Sleep Scale. Maturitas. 2014;77(4):344–50. doi: 10.1016/j.maturitas.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. 2011;15(2):99–106. doi: 10.1016/j.smrv.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Cases J, Ibarra A, Feuillère N, Roller M, Sukkar SG. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med J Nutrition Metab. 2011;4(3):211–8. doi: 10.1007/s12349-010-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 18.Gooneratne NS. Complementary and alternative medicine for sleep disturbances in older adults. Clin Geriatr Med. 2008;24(1):121–38. doi: 10.1016/j.cger.2007.08.002. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haybar H, Javid AZ, Haghighizadeh MH, Valizadeh E, Mohaghegh SM, Mohammadzadeh A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin Nutr ESPEN. 2018;26:47–52. doi: 10.1016/j.clnesp.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Leach MJ, Page AT. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med Rev. 2015;24:1–12. doi: 10.1016/j.smrv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Khadivzadeh T, Abdolahian S, Ghazanfarpour M, Kargarfard L, Dizavandi FR, Khorsand I. A Systematic Review and Meta-analysis on the Effect of Herbal Medicine to Manage Sleep Dysfunction in Peri- and Postmenopause. J Menopausal Med. 2018;24(2):92–9. doi: 10.6118/jmm.2018.24.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrahi J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Psychometric properties of the Persian version of the Pittsburgh Sleep Quality Index addendum for PTSD (PSQI-A) Sleep Breath. 2009;13(3):259–62. doi: 10.1007/s11325-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadbeigi A, Absari R, Valizadeh F, Saadati M, Sharifimoghadam S, Ahmadi A, et al. Sleep Quality in Medical Students; the Impact of Over-Use of Mobile Cell-Phone and Social Networks. J Res Health Sci. 2016;16(1):46–50. [PMC free article] [PubMed] [Google Scholar]
- 24.Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P) Sleep Breath. 2012;16(1):79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi MR, Ahmadi N, Khaleghi A, Mostafavi SA, Kamali K, Rahgozar M, et al. Prevalence and Correlates of Psychiatric Disorders in a National Survey of Iranian Children and Adolescents. Iran J Psychiatry. 2019;14(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Taavoni S, Ekbatani N, Haghani H. Effect of Valerian and Lemon Balm Combined Capsules, On Postmenopausal Sleep Disorder, a Triple Blind Randomized Placebo Control Clinical Trial. Eur Psychiatry. 2015;30:1784. [Google Scholar]
- 27.Taavoni S, Nazem Ekbatani N, Haghani H. Valerian/lemon balm use for sleep disorders during menopause. Complement Ther Clin Pract. 2013;19(4):193–6. doi: 10.1016/j.ctcp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Heydari N, Dehghani M, Emamghoreishi M, Akbarzadeh M. Effect of Melissa officinalis capsule on the mental health of female adolescents with premenstrual syndrome: a clinical trial study. Int J Adolesc Med Health. 2018;31(3) doi: 10.1515/ijamh-2017-0015. [DOI] [PubMed] [Google Scholar]
- 29.Miladi-Gorji H, Vafaei AA, Bageri A. To investigate the effect of Portulaca oleracea L. and Melissa officinalis L. extract on sleeping time in mice. Journal of Medicinal Plants. 2011;10(38):95–101. [Google Scholar]
- 30.Hepsomali P, Groeger JA, Nishihira J, Scholey A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front Neurosci. 2020;14:923. doi: 10.3389/fnins.2020.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jie F, Yin G, Yang W, Yang M, Gao S, Lv J, et al. Stress in Regulation of GABA Amygdala System and Relevance to Neuropsychiatric Diseases. Front Neurosci. 2018;12:562. doi: 10.3389/fnins.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–9. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamatsu A, Yamashita Y, Pandharipande T, Maru I, Kim M. Effect of oral γ-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci Biotechnol. 2016;25(2):547–51. doi: 10.1007/s10068-016-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luppi PH, Peyron C, Fort P. Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med Rev. 2017;32:85–94. doi: 10.1016/j.smrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Lipovac M, Chedraui P, Gruenhut C, Gocan A, Kurz C, Neuber B, et al. Effect of Red Clover Isoflavones over Skin, Appendages, and Mucosal Status in Postmenopausal Women. Obstet Gynecol Int. 2011;2011:949302. doi: 10.1155/2011/949302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamshad Begum S, Jayalakshmi HK, Vidyavathi HG, Gopakumar G, Abin I, Balu M, et al. A Novel Extract of Fenugreek Husk (FenuSMART™) Alleviates Postmenopausal Symptoms and Helps to Establish the Hormonal Balance: A Randomized, Double-Blind, Placebo-Controlled Study. Phytother Res. 2016;30(11):1775–84. doi: 10.1002/ptr.5680. [DOI] [PubMed] [Google Scholar]
- 37.Kamalifard M, Farshbaf-Khalili A, Namadian M, Ranjbar Y, Herizchi S. Comparison of the effect of lavender and bitter orange on sleep quality in postmenopausal women: A triple-blind, randomized, controlled clinical trial. Women Health. 2018;58(8):851–65. doi: 10.1080/03630242.2017.1353575. [DOI] [PubMed] [Google Scholar]
- 38.Yang HM, Liao MF, Zhu SY, Liao MN, Rohdewald P. A randomised, double-blind, placebo-controlled trial on the effect of Pycnogenol on the climacteric syndrome in peri-menopausal women. Acta Obstet Gynecol Scand. 2007;86(8):978–85. doi: 10.1080/00016340701446108. [DOI] [PubMed] [Google Scholar]
- 39.Liu ZM, Ho SC, Woo J, Chen YM, Wong C. Randomized controlled trial of whole soy and isoflavone daidzein on menopausal symptoms in equol-producing Chinese postmenopausal women. Menopause. 2014;21(6):653–60. doi: 10.1097/GME.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Kim KH. A randomized, double-blind, placebo-controlled trial of Schisandra chinensis for menopausal symptoms. Climacteric. 2016;19(6):574–80. doi: 10.1080/13697137.2016.1238453. [DOI] [PubMed] [Google Scholar]