Abstract
Plants can send long-distance cell-to-cell signals from a single tissue subjected to stress to the entire plant. This ability is termed “systemic signaling” and is essential for plant acclimation to stress and/or defense against pathogens. Several signaling mechanisms are associated with systemic signaling, including the reactive oxygen species (ROS) wave, calcium wave, hydraulic wave, and electric signals. The ROS wave coordinates multiple physiological, molecular, and metabolic responses among different parts of the plant and is essential for systemic acquired acclimation (SAA) to stress. In addition, it is linked with several plant hormones, including jasmonic acid (JA), salicylic acid (SA), and abscisic acid (ABA). However, how these plant hormones modulate the ROS wave and whether they are required for SAA is not clear. Here we report that SA and JA play antagonistic roles in modulating the ROS wave in Arabidopsis (Arabidopsis thaliana). While SA augments the ROS wave, JA suppresses it during responses to local wounding or high light (HL) stress treatments. We further show that ethylene and ABA are essential for regulation of the ROS wave during systemic responses to local wounding treatment. Interestingly, we found that the redox-response protein NONEXPRESSOR OF PATHOGENESIS RELATED PROTEIN 1 is required for systemic ROS accumulation in response to wounding or HL stress, as well as for SAA to HL stress. Taken together, our findings suggest that interplay between JA and SA might regulate systemic signaling and SAA during responses of plants to abiotic stress or wounding.
An antagonistic interaction between salicylic acid and jasmonic acid attenuates reactive oxygen species accumulation in local and systemic tissues during plant responses to light stress or wounding.
Introduction
Plants grow, develop, and reproduce in a dynamic environment that subjects them to rapid changes in conditions such as light intensity, humidity, temperature, and/or the presence of pathogens or pests (Kollist et al., 2019). To successfully thrive in their environment, plants need to rapidly respond and acclimate to these changes (Zandalinas et al., 2019, 2020a). Although occasionally the entire plant is simultaneously exposed to the changing environmental conditions, in many instances only a single tissue of the plant (termed “local” tissue) will sense the different stress conditions before the rest of the plant will. In such instances, the local tissue that first senses the change in environmental conditions will send a rapid systemic signal that spreads to the entire plant within minutes and activates defense and acclimation mechanisms in all other parts of the plant (termed “systemic tissue”), often before the change in environmental conditions will reach them (Kollist et al., 2019; Zandalinas et al., 2020b). This process is known as “rapid systemic signaling” and is essential for the systemic acclimation (termed “systemic acquired acclimation”; SAA) of plants to different abiotic stresses such as excess light or heat stress (HS; Suzuki et al., 2013; Zandalinas et al., 2019, 2020a). In addition, it is essential for the systemic wound response (SWR) to mechanical wounding, that is in many instances associated with insect attack and herbivory (Toyota et al., 2018; Farmer et al., 2020). The rapid activation, or priming, of the entire plant, that occurs within minutes of the sensing of stress by a single tissue (driven by rapid systemic signaling), serves an important role in plant acclimation, triggering many of the slower acclimation responses that include the activation of multiple gene networks and adjustments of metabolism, that may take tens of minutes to hours (Kollist et al., 2019; Mittler et al., 2022).
Over the years, several different rapid systemic signals were identified in plants (Miller et al., 2009; Christmann et al., 2013; Toyota et al., 2018; Farmer et al., 2020; Fichman and Mittler, 2021a, 2021b). These include the reactive oxygen species (ROS) wave, the calcium wave, electric signals, the redox wave, and hydraulic waves. While electric, calcium, and hydraulic signals were found to be dependent on the function of the glutamate receptor-like (GLR) 3.3 and 3.6 channels (Toyota et al., 2018; Farmer et al., 2020; Fichman and Mittler, 2021a), the ROS wave was found to be dependent on the function of the respiratory burst oxidase homolog (RBOH) proteins (RBOHD and RBOHF), and the ROS sensor hydrogen peroxide (H2O2)-induced Ca2+ increases 1 (HPCA1), in Arabidopsis (Arabidopsis thaliana) (Miller et al., 2009; Zandalinas et al., 2020b; Fichman et al., 2022). In addition, many of the different rapid systemic signals were found to be mediated through the plant vascular system and to be dependent on each other (Farmer et al., 2020; Zandalinas et al., 2020b; Zandalinas and Mittler, 2021). However, differences between rapid systemic signaling in response to excess light stress (high light [HL]) and mechanical injury were recently reported (Fichman and Mittler, 2021a), demonstrating that different local stimuli may trigger different combinations of these waves that are regulated by different sets of proteins.
In addition to coordinating systemic transcriptomic and metabolic responses of plants to stress, the ROS wave was recently found to coordinate the systemic stomatal responses of different leaves to a local exposure of HL, HS, or injury (Devireddy et al., 2018, 2020a, 2020b; Zandalinas et al., 2020a). The closure of stomata on treated leaves in response to HL or wounding, or stomatal opening of the local leaf in response to a localized HS, was therefore propagated to the rest of the plant within minutes, in a process that required the function of the ROS wave (Devireddy et al., 2018, 2020a; Zandalinas et al., 2020a). These findings prompted studies of the role of different plant hormones in rapid systemic responses to abiotic stress.
The plant hormones abscisic acid (ABA), salicylic acid (SA), and jasmonic acid (JA) were found to accumulate in local and systemic leaves of plants in response to a local HL or HS treatments (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2020a). ABA accumulation was further found to be required for local and systemic SA and H2O2 accumulation, as well as for systemic stomatal aperture changes, in response to a local HL stress (Devireddy et al., 2018, 2020a). JA was found to be required for local and systemic stomatal aperture changes in response to a local HL stress, and SA was found to be required for systemic, but not local stomatal aperture changes, in response to a local HL stress (Devireddy et al., 2020a; Zandalinas et al., 2020a). Although the studies described above revealed a role for ABA, JA, and SA in rapid systemic signaling, many questions regarding their function remained unanswered. For example, it is unknown what is the role of ABA, JA, and SA in mediating SAA to HL stress. In addition, the role of ABA, JA, and SA in SWRs is unclear. Furthermore, the role of several other stress-related hormones, such as ethylene (ET) and strigolactones (SLs) in rapid systemic responses is unknown. To address these questions, we studied the local and systemic accumulation of ROS in different mutants deficient in ABA, JA, SA, ET, and SL biosynthesis or signaling to HL stress or wounding, as well as the SAA response of these mutants to HL stress.
Here, we report that JA and SA play antagonistic roles in regulating the ROS wave. While JA suppresses the ROS wave during responses to HL stress or wounding, SA augments the intensity of the ROS wave in response to these treatments. We further show that ET plays a role in the regulation of the ROS wave in response to wounding (but not HL stress), and that SLs do not play a role in responses to these two stresses. Interestingly, we found that the redox-response protein NONEXPRESSOR OF PATHOGENESIS RELATED PROTEIN 1 (NPR1) is required for systemic (but not local) ROS accumulation in response to HL stress or wounding, as well as for local and systemic acclimation to HL stress. This finding suggests that redox changes mediated by the ROS wave (Fichman and Mittler, 2021b) could regulate transcript expression in systemic tissues through SA- or ROS-mediated redox regulation, and that JA antagonizes these responses. Taken together, our findings suggest that SA and JA play antagonistic roles in regulating the ROS wave and that this regulation may be partially mediated by NPR1 in systemic tissues.
Results
ABA biosynthesis is required for systemic ROS responses to wounding and SAA to HL stress
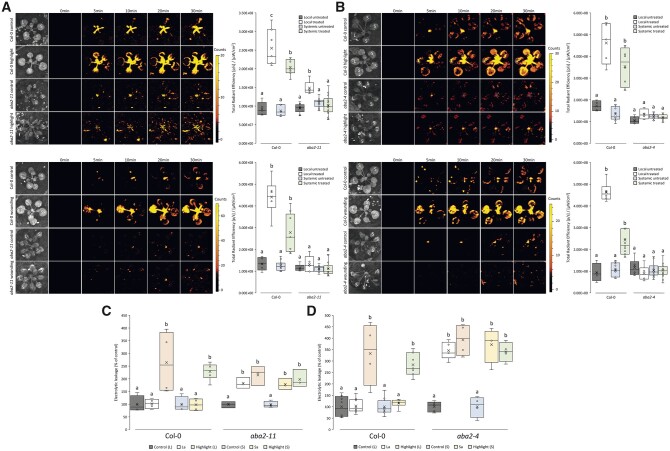
We previously reported that ABA levels transiently accumulate in local and systemic leaves of plants subjected to a local treatment of HL or HS (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2020a). We further revealed that ABA biosynthetic mutants are unable to trigger H2O2 accumulation and stomatal closure in local and systemic leaves in response to a local HL stress treatment (Devireddy et al., 2018, 2020a). However, whether ABA is required for systemic plant acclimation to HL is unknown. In addition, the measurements of H2O2 in local and systemic tissues in response to HL stress were not performed with our highly sensitive whole-plant live ROS imaging method (Fichman et al., 2019; Zandalinas and Mittler, 2021; Fichman et al., 2022), and the role of ABA in local and systemic ROS responses to wounding is unknown. To address these questions, we subjected two different alleles of the ABA biosynthesis mutant aba2 (aba2-11 and aba2-4; mutations in AT1G52340 encoding XANTHOXIN DEHYDROGENASE) to a local treatment of HL stress or wounding and imaged ROS accumulation in live plants grown in soil. aba2 mutants were previously reported to retain 10%–20% of wild-type (WT) ABA levels (González-Guzmán et al., 2002). As shown in Figure 1, A and B, ABA biosynthesis was essential for systemic ROS accumulation of plants in response to a local treatment of HL stress. ABA biosynthesis was also essential for local and systemic ROS accumulation in response to a local wounding treatment. The finding that the aba2-11 mutant accumulated low levels of ROS in local leaves in response to HL stress (Figure 1A) could suggest that residual ABA levels found in some of the aba2 mutants (González-Guzmán et al., 2002) could be sufficient to drive the HL-mediated ABA-dependent ROS accumulation response in local leaves, but that this response is not sufficient to trigger systemic ROS accumulation. As shown in Figure 1, C and D, the two aba2 mutants were unable to acclimate their local and systemic leaves to HL stress, suggesting that ABA is required for plant acclimation to HL stress even if ROS levels are enhanced. The results presented in Figure 1 suggest that ABA plays a key role in local and systemic ROS responses to wounding, in systemic ROS accumulation in response to a local HL stress, and in local and systemic acclimation of plants to HL stress.
Figure 1.
ABA is required for acclimation to HL stress and the initiation of the ROS wave following wounding. A, Arabidopsis plants were subjected to a HL stress or wounding treatment applied to a single leaf (L, Local), and ROS accumulation was imaged, using H2DCFDA, in whole plants. Representative time-lapse images of whole plant ROS accumulation in WT and aba2-11 plants are shown alongside box plots of combined data from all plants used for the analysis at the 0- and 30-min time points (L and systemic [S] leaves). B, Similar to (A), except for the aba2-4 mutant. C, Ion leakage measurements of L and S leaves in Col-0 and aba2-11 plants following HL stress. Local and systemic leaves that were exposed to an extended period of HL stress with no pretreatment (highlight), pretreated with HL for a short period of time and allowed to incubate prior to extended light exposure (local acclimated, La; and systemic acclimated, Sa), and control plants receiving no pretreatment, were measured. D, Similar to (C), except for the aba2-4 mutant. All experiments were repeated at least three times with three plants per repeat. Two-way ANOVA followed by Tukey’s post-hoc test was conducted for statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Whiskers represent 1.5 times the minimum and maximum of the mean (1.5 times of the interquartile range).
JA is involved in local and systemic ROS responses to HL stress and wounding, as well as in SAA to HL stress
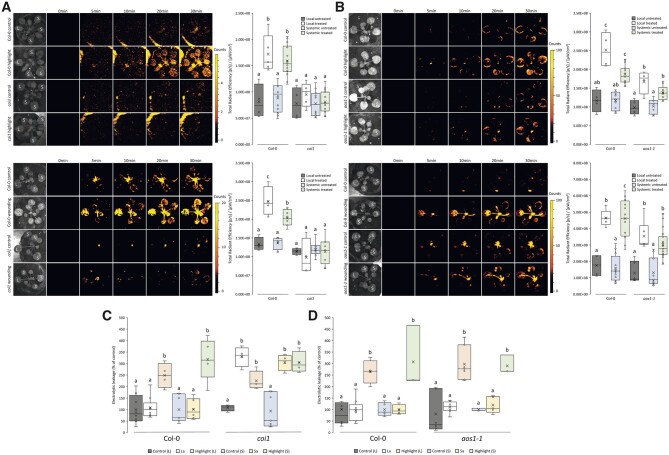
We previously reported that JA accumulates in local and systemic leaves of plants subjected to a local treatment of HL stress, and that JA signaling is required for local and systemic stomatal responses of plants to wounding, HL, and HS (Devireddy et al., 2018, 2020a; Zandalinas et al., 2020a). However, the role of JA in plant acclimation to HL stress or systemic ROS responses to HL or wounding is unknown. To study the role of JA in rapid systemic signaling to HL stress and wounding, we used two different mutants: the JA signaling mutant coi1 that is deficient in JA perception (mutation in AT2G39940 encoding a protein containing Leu-rich repeats and a degenerate F-box motif), and the JA biosynthesis mutant aos1 required for JA biosynthesis (mutation in AT5G42650 encoding ALLENE OXIDE SYNTHASE). coi1 was previously reported to contain high basal levels of JA (Stotz et al., 2011), and aos1 was reported to contain almost no detectable levels of JA (Park et al., 2002). As shown in Figure 2A, the coi1 mutant was deficient in local and systemic ROS accumulation in response to a local treatment of HL stress or wounding. In contrast, the aos1 mutant was not (Figure 2B). As shown in Figure 2C, the coi1 mutant was also deficient in local and systemic acclimation to HL stress. In contrast, the aos1 mutant was not (Figure 2D). The results presented in Figure 2 suggest that JA sensing by COI1 could play a key role in local and systemic ROS responses to wounding and HL stress, as well as in SAA to HL stress. Alternatively, the high basal levels of JA in the coi1 mutant may suppress the ROS wave and SAA via a COI1-independent mechanism (e.g. by antagonizing SA). In contrast to our results obtained with coi1, suppressing JA levels in the aos1 mutant appeared to only decrease ROS signal accumulation in response to HL stress and wounding, but had no effect on SAA to HL.
Figure 2.
JA insensitive mutants fail to acclimate to HL stress or induce a ROS wave response following a local treatment of HL or wounding. A, Arabidopsis plants were subjected to a HL stress or wounding treatment applied to a single leaf (L, Local), and ROS accumulation was imaged, using H2DCFDA, in whole plants. Representative time-lapse images of whole plant ROS accumulation in WT and coi1 plants are shown alongside box plots of combined data from all plants used for the analysis at the 0- and 30-min time points (L and systemic [S] leaves). B, Similar to (A), except for the aos1-1 mutant. C, Ion leakage measurements of L and S leaves in Col-0 and coi1 plants following HL stress. Local and systemic leaves that were exposed to an extended period of HL stress with no pretreatment (highlight), pretreated with HL for a short period of time and allowed to incubate prior to extended light exposure (local acclimated, La; and systemic acclimated, Sa), and control plants receiving no pretreatment, were measured. D, Similar to (C), except for the aos1-1 mutant. All experiments were repeated at least three times with three plants per repeat. Two-way ANOVA followed by Tukey’s post-hoc test was conducted for statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Whiskers represent 1.5 times the minimum and maximum of the mean (1.5 times of the interquartile range).
SA and NPR1 are required for local and systemic acclimation to HL stress, and NPR1 is required for systemic ROS accumulation in response to HL stress or wounding
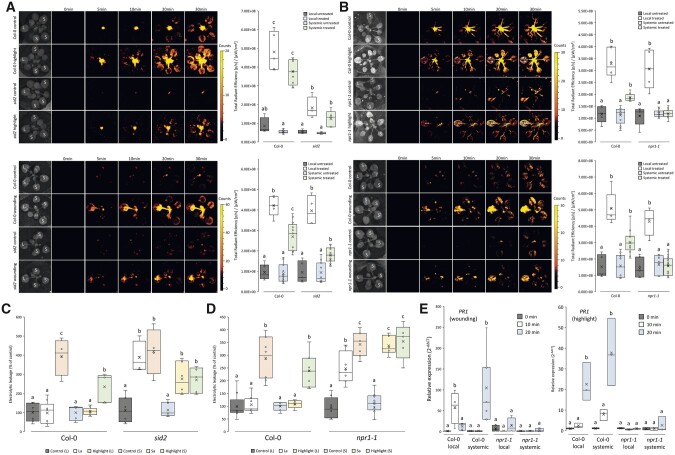
We previously reported that SA accumulates in local and systemic leaves of plants subjected to a local treatment of HL stress, that SA is required for SAA to HS, and that SA is required for systemic stomatal responses to a local treatment of HL stress (Suzuki et al., 2013; Devireddy et al., 2018, 2020a; Zandalinas et al., 2020a). However, the role of SA in plant acclimation to HL stress or systemic ROS responses to HL or wounding is unknown. To study the role of SA in rapid systemic signaling to HL and wounding, we used the sid2 mutant (mutation in AT1G74710 encoding ISOCHORISMATE SYNTHASE 1) that is deficient in SA accumulation (retains 5%–10% of WT SA levels; Nawrath and Métraux, 1999), and the npr1 mutant that is deficient in SA sensing (Spoel et al., 2003; Tada et al., 2008; Caarls et al., 2015; Zhou et al., 2015; Withers and Dong, 2016; Chen et al., 2021). As shown in Figure 3A, local and systemic ROS accumulation was suppressed in the sid2 mutant in response to HL stress. In contrast, only systemic ROS accumulation was suppressed in the sid2 mutant in response to wounding (Figure 3A). As shown in Figure 3B, while local accumulation of ROS was not suppressed in the npr1 mutant in response to a local HL stress or wounding, systemic ROS accumulation was suppressed in the npr1 mutant in response to both treatments. As shown in Figure 3, C and D, local and systemic acclimation to HL stress were abolished in both the sid2 and npr1 mutants. The findings that NPR1 is required for systemic (but not local) ROS accumulation in response to a local wounding or HL stress treatments (Figure 3B), as well as for local and systemic acclimation to HL stress (Figure 3D), suggest that the ROS wave is triggered in local tissues of the npr1 mutant in response to local wounding or HL stress, but that it does not spread to systemic leaves and does not induce SAA to HL stress in local or systemic tissues. To test whether PATHOGENESIS RELATED 1 (PR1) expression is corresponding with this phenotype of the npr1 mutant, we tested the expression of PR1 in local and systemic leaves of WT and npr1 plants subjected to a local HL stress or wounding treatment. As shown in Figure 3E, the expression of PR1, that is associated with responses to pathogens, HL stress and ROS/redox (Spoel et al., 2003; Tada et al., 2008; Zhou et al., 2015), was suppressed in local and systemic leaves of the npr1 mutant in response to a local treatment of HL stress or wounding. This finding demonstrates that NPR1 is required for systemic expression of PR1 in response to wounding or HL stress. Taken together, the findings presented in Figure 3 suggest that SA is essential for local and systemic acclimation to HL stress, and that NPR1 could play a key role in ROS accumulation and signaling in systemic tissues of plants subjected to HL stress or wounding.
Figure 3.
SA mutants are deficient in acclimation to HL stress and the ROS wave is unable to propagate to the systemic tissues in the npr1-1 mutant. A, Arabidopsis plants were subjected to a HL stress or wounding treatment applied to a single leaf (L, Local), and ROS accumulation was imaged, using H2DCFDA, in whole plants. Representative time-lapse images of whole plant ROS accumulation in WT and sid2 plants are shown alongside box plots of combined data from all plants used for the analysis at the 0- and 30-min time points (L and systemic [S] leaves). B, Similar to (A), except for the npr1-1 mutant. C, Ion leakage measurements of L and S leaves in Col-0 and sid2 plants following HL stress. Local and systemic leaves that were exposed to an extended period of HL stress with no pretreatment (highlight), pretreated with HL for a short period of time and allowed to incubate prior to extended light exposure (local acclimated, La; and systemic acclimated, Sa), and control plants receiving no pretreatment, were measured. D, Similar to (C), except for the npr1-1 mutant. E, RT-qPCR analysis for PR1 steady-state transcript levels in local and systemic leaves of WT (Col-0) and npr1-1 plants following highlight or wounding of a single leaf. Transcript expression is represented as the relative quantity (2−ΔΔCT) compared with an internal control (elongation factor 1α) in unwounded local tissue of WT (time 0). All experiments were repeated at least 3 times with three plants per repeat. Two-way ANOVA followed by Tukey’s post-hoc test was conducted for statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Whiskers represent 1.5 times the minimum and maximum of the mean (1.5 times of the interquartile range).
JA suppresses the ROS wave, while SA augments it in response to a local treatment of HL stress or wounding
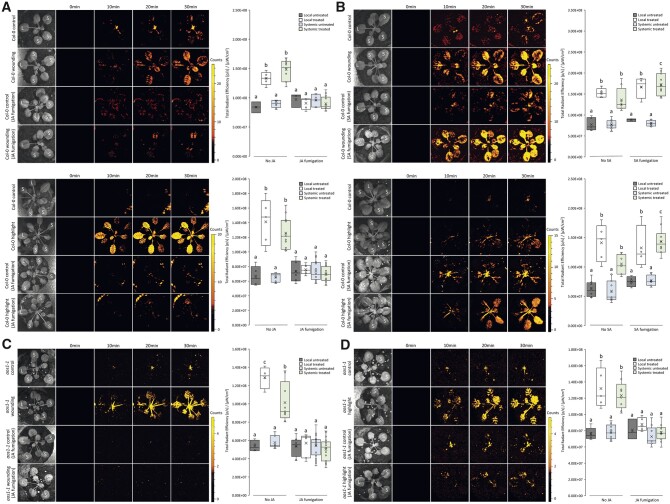
JA and SA were previously found to play antagonistic roles in mediating the response of plants to pathogens and other stresses, and this role was partially linked to the activation and nuclear localization of NPR1 (e.g. Spoel et al., 2003; Tada et al., 2008; Caarls et al., 2015; Zhou et al., 2015; Withers and Dong, 2016; Chen et al., 2021). Because our findings suggest that JA and SA could play antagonistic roles in mediating the ROS wave during responses to HL stress and wounding (Figures 2 and 3), we tested whether application of JA or SA would affect the ROS wave triggered by these stresses. For this purpose, we applied 20 µM JA or 1 mM SA to WT plants via fumigation prior to local stress application and measured local and systemic ROS accumulation in the presence or absence of JA or SA. As shown in Figure 4A, application of JA prior to the local HL stress or wounding treatments suppressed the ROS wave response of plants to these treatments. In contrast, as shown in Figure 4B, application of SA prior to the local HL stress or wounding treatments augmented the systemic ROS wave response of plants to these treatments. The findings presented in Figure 4 support our findings presented in Figures 2 and 3 and suggest that JA and SA play antagonistic roles in regulating the ROS wave response of plants; JA suppresses it, while SA promotes it.
Figure 4.
JA suppresses the ROS wave while SA augments it in response to either HL or wounding of a local tissue. A, Arabidopsis plants were untreated or pretreated with JA, subjected to wounding (Top) or a HL stress (Bottom) applied to a single leaf (L, Local), and ROS accumulation was imaged, using H2DCFDA, in whole plants. Representative time-lapse images of whole plant ROS accumulation in treated and untreated WT plants are shown alongside box plots of combined data from all plants used for the analysis at the 0- and 30-min time points (L and systemic [S] leaves). B, Similar to (A), except that plants were untreated or pretreated with SA before wounding treatments or HL stress were applied. C, Similar to (A) (Top), except for the aos1-1 mutant. D, Similar to (A) (Bottom), except for the aos1-1 mutant. All experiments were repeated at least three times with three plants per repeat. Two-way ANOVA followed by Tukey’s post-hoc test was conducted for statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Whiskers represent 1.5 times the minimum and maximum of the mean (1.5 times of the interquartile range).
Because the basal levels of JA and SA could play a key role in triggering the ROS wave, we measured the levels of JA, SA, and ABA in the local and systemic leaves of untreated WT and the coi1, aos1-1, sid2, and npr1-1 mutants. As shown in Supplemental Figure S1A, and in agreement with Stotz et al. (2011), the levels of JA were higher in untreated coi1 plants, compared with untreated WT plants. In contrast, the levels of JA were lower than that of control in local leaves of untreated aos1-1 and npr1-1 plants. As shown in Supplemental Figure S1B, the levels of SA were suppressed in the local or systemic leaves of the coi1 and sid2 mutants, further supporting the finding that coi1 has higher levels of JA. Interestingly, as shown in Supplemental Figure S1C, the levels of ABA were higher than control in the local leaves of all untreated mutants.
Because JA was able to suppress the ROS wave in WT plants subjected to a local treatment of HL stress or wounding (Figure 4A), and the ROS wave was not suppressed in the aos1-1 mutant (Figure 2B), that has suppressed levels of JA (Supplemental Figure S1A), we tested whether application of JA to the aos1-1 mutant will suppress the ROS wave in this mutant in response to a local treatment of HL or wounding. As shown in Figure 4, C and D, prior treatment of the aos1-1 mutant with JA suppressed the ROS wave triggered in this mutant in response to HL stress or wounding. This finding supported the role of JA in suppressing the ROS wave during responses to HL stress or wounding.
ET is essential for systemic ROS accumulation in response to local wounding, while SLs appear to not play a role in systemic responses to HL or wounding stresses
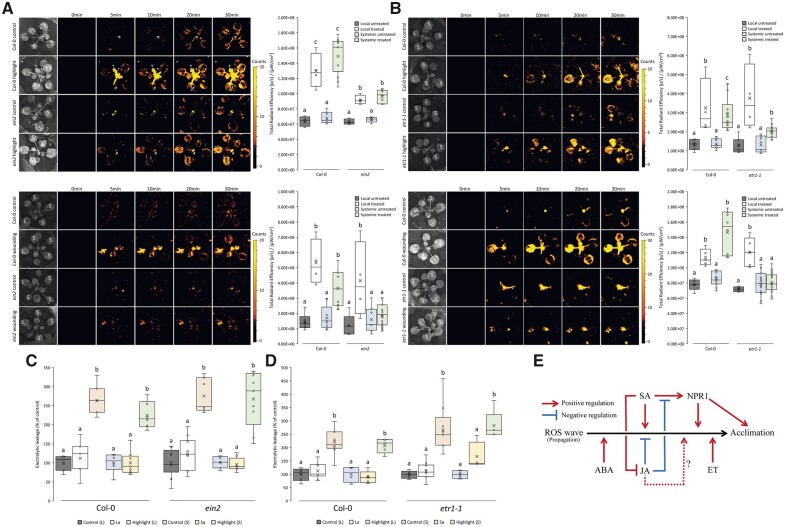
ET is involved in many stress responses of plants, as well as in different developmental processes (Gamble et al., 1998; Alonso et al., 1999). However, little is known about the role of ET in systemic ROS responses of plants to stress. To study the potential roles of ET in local and systemic responses to HL stress and wounding, we subjected two ET sensing mutants, ein2 (mutation in AT5G03280) and etr1 (mutation in AT1G66340), both transmembrane proteins required for triggering ET responses in Arabidopsis (Gamble et al., 1998; Alonso et al., 1999), to a local treatment of HL stress or wounding and measured local and systemic ROS accumulation. As shown in Figure 5, A and B, ET sensing by EIN2 and ETR1 was not required for local or systemic ROS accumulation in response to HL stress. In contrast, ET sensing by these two proteins was required for systemic, but not local, ROS accumulation in response to wounding (Figure 5, A and B). As shown in Figure 5, C and D, both etr1 and ein2 could acclimate to HL stress. The findings presented in Figure 5, A–D reveal that ET could be playing a role in systemic ROS accumulation in response to wounding.
Figure 5.
ET insensitive mutants are unable to mount a systemic ROS wave response following a local wounding treatment, and a model. A, Arabidopsis plants were subjected to a HL stress or wounding treatments applied to a single leaf (L, Local), and ROS accumulation was imaged, using H2DCFDA, in whole plants. Representative time-lapse images of whole plant ROS accumulation in WT and ein2 plants are shown alongside box plots of combined data from all plants used for the analysis at the 0- and 30-min time points (L and systemic [S] leaves). B, Similar to (A), except for the etr1-1 mutant. C, Ion leakage measurements of L and S leaves in Col-0 and ein2 plants following HL stress. Local and systemic leaves that were exposed to an extended period of HL stress with no pretreatment (highlight), pretreated with HL for a short period of time and allowed to incubate prior to extended light exposure (local acclimated, La; and systemic acclimated, Sa), and control plants receiving no pretreatment, were measured. D, Similar to (C), except for the etr1-1 mutant. E, A model depicting the interactions between different plant hormones, the ROS wave and plant acclimation. All experiments were repeated at least three times with three plants per repeat. Two-way ANOVA followed by Tukey’s post-hoc test was conducted for the statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Whiskers represent 1.5 times the minimum and maximum of the mean (1.5 times of the interquartile range).
SLs play important roles in plant–pathogen interactions and developmental responses to abiotic stress (Saeed et al., 2017). To test whether SLs play an important role in local and systemic responses to HL stress and wounding, we used the SL mutants max2 involved in SL sensing (mutation in AT2G42620 encoding an F-box leucine-rich repeat protein), and max3 involved in SL biosynthesis (mutation in AT2G44990 encoding CAROTENOID CLEAVAGE DIOXYGENASE 7). As shown in Supplemental Figure S2, max2 and max3 were not deficient in their local or systemic ROS accumulation in response to wounding or HL stress, or in SAA to HL. These results suggest that SLs might not be involved in rapid responses to wounding or HL stress.
Discussion
We previously reported that ABA, JA, and SA play important roles in regulating local and systemic stomatal responses to a local application of HL stress (Devireddy et al., 2018, 2020a; Zandalinas et al., 2020a). ABA, SA, and JA rapidly accumulate in local and systemic leaves of plants in response to a local HL or HS treatments (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2020a), while JA rapidly accumulates in local and systemic leaves in response to wounding (Glauser et al., 2009). ABA is required for local and systemic SA and H2O2 accumulation, as well as for systemic stomatal aperture changes, in response to a local HL stress (Devireddy et al., 2018, 2020a), while JA is required for local and systemic stomatal aperture changes in response to a local HL stress, and SA is required for systemic, but not local stomatal aperture changes, in response to a local HL stress (Devireddy et al., 2020a; Zandalinas et al., 2020a). The rapid accumulation of these hormones in local and systemic tissues in response to stress is thought to result from rapid synthesis (JA; Glauser et al., 2009), or release from conjugated/stored forms (SA/ABA; Suzuki et al., 2013; Kollist et al., 2019). Nevertheless, the role some of these hormones (e.g. ABA) play in SAA to HL stress, or SWR is not clear. Our current study reveals an important function for ABA and SA in plant acclimation to HL stress, as well as in regulating the ROS wave in response to HL stress and wounding (Figures 1 and 3). In addition, we reveal a role for ABA and ET in systemic ROS responses to wounding (Figures 1 and 5). Interestingly, while ABA was required for local and systemic ROS responses to HL and wounding (that may involve ET responses; Cheng et al., 2009; please see below), ET signaling was only required for systemic ROS responses to wounding (Figures 1 and 5). This finding is in agreement with our previous findings that the systemic ROS response of plants to wounding is different than that to HL stress (i.e. depended on different regulators and could occur through different plants tissues; e.g. systemic ROS responses to HL are mediated through the vascular system and may not require glutamate-like receptors 3.3 and 3.6, while systemic ROS responses to wounding are mediated through the vascular system or mesophyll cells and are dependent on glutamate-like receptors 3.3 and 3.6; Zandalinas et al., 2020b; Fichman and Mittler, 2021a; Zandalinas and Mittler, 2021). It is also possible that HL stress or wounding trigger different sources of ROS production (Fichman et al., 2021; Xiong et al., 2021), and that although both require ABA, local ROS responses to wounding do not require ET. Previous studies have shown that ABA and ET have antagonistic interactions and that in the aba2 mutant some ET responses are suppressed (Cheng et al., 2009). This finding could explain why in the aba2 mutant systemic ROS wave responses to wounding, that require ET signaling (Figure 5), are suppressed (Figure 1). Further studies are required to address the sources of local ROS produced during HL stress or wounding and their interactions with different plant hormones and other regulators (e.g. phytochrome B; Fichman et al., 2021; Xiong et al., 2021). In addition, the role of the chloroplast, which is the initial site of ABA, SA, and other plant hormone biosynthesis during these responses, as well as the different plant tissues involved, need to be defined in future studies in different local and systemic tissues during responses to different stresses.
In contrast to SA and ABA, the involvement of JA in regulating the rapid systemic response of plants to HL stress or wounding appears to be more complicated (Figures 2 and 4). JA was initially shown to be required for local and systemic stomatal responses to HL stress (Devireddy et al., 2018, 2020a), and as shown in Figure 2, JA sensing by COI1 is also required for local and systemic ROS production and plant acclimation to a local treatment of HL stress. However, because the aos1 mutant that does not accumulate JA (Park et al., 2002; Supplemental Figure S2) can still accumulate local and systemic ROS and acclimate to a local HL stress treatment (Figure 2), it is possible that the role of COI1 in these responses is independent of JA signaling. In this respect, it should be noted that COI1 was found to have JA-independent functions (e.g. Stotz et al., 2011). An alternative possibility, that appears more plausible, is that in the coi1 mutant the basal levels of JA are high (due to a positive feedback loop on JA synthesis; similar to what happens in the abi1 mutant with ABA; Devireddy et al., 2018), and that these high levels of JA antagonize SA function and cause the coi1 mutant to not acclimate or accumulate ROS. In this respect it should be noted that the coi1 mutant was found to have high basal levels of JA, supporting this possibility (Stotz et al., 2011; Supplemental Figure S2). Moreover, treatment of the aos1-1 mutant with JA, suppressed the ROS wave in this mutant in response to HL or wounding (Figure 4, C and D), further suggesting that JA plays a suppressing role in ROS wave propagation.
Antagonistic interactions between SA and JA were previously reported in many studies (e.g. Spoel et al., 2003; Tada et al., 2008; Caarls et al., 2015; Zhou et al., 2015; Withers and Dong, 2016; Chen et al., 2021). We previously observed that when a combination of HS and HL was applied to the same local Arabidopsis leaf, the ROS wave response originating from this leaf was suppressed (Zandalinas et al., 2020a). Both HS and HL treatments resulted in the accumulation of SA and JA in the local leaf, suggesting that this suppression could result from antagonistic interactions between JA and SA (Zandalinas et al., 2020a). Indeed, we found that in the aos1 mutant the suppression of the ROS wave at the local leaf during the stress combination was removed, supporting the hypothesis that SA and JA antagonize the function of each other, and that JA might suppress the initiation of the ROS wave (Zandalinas et al., 2020a). In the current work, we clearly show that application of JA suppresses, and application of SA promotes, the ROS wave in response to HL stress or wounding (Figure 4). Taken together, our results suggest that in the coi1 mutant the high basal levels of JA (Stotz et al., 2011; Supplemental Figure S2) antagonize the function of SA, and that SA and JA have antagonistic functions in regulating the ROS wave (Figures 4 and 5E). Of course, JA sensing could still play an important role in plant acclimation to HL stress and further studies are needed to address this question.
One protein, previously proposed to be at the core of SA–JA antagonistic interactions, is NPR1 (e.g. Spoel et al., 2003; Tada et al., 2008; Caarls et al., 2015; Zhou et al., 2015; Withers and Dong, 2016; Chen et al., 2021). SA was shown to promote the monomerization of NPR1 via TRX-h3/5 that results in its nuclear localization and activation of transcriptional responses, while JA was shown to promote S-nitrosylation of NPR1 by S-nitrosoglutathione (GSNO) that keeps it as a multimer in the cytosol and prevents the activation of transcript expression (Caarls et al., 2015; Withers and Dong, 2016; Chen et al., 2021). NPR1 was further shown to be posttranslationally regulated by ubiquitinoylation, SUMOylation, and other posttranslational modifications (Chen et al., 2021). Activation of transcriptional responses by SA was further shown to antagonize JA function and reverse its S-nitrosylation via GSNO reductase (Caarls et al., 2015; Withers and Dong, 2016; Chen et al., 2021). Interestingly, in our hands, NPR1 was required for the systemic accumulation of ROS in response to a local treatment of HL stress or wounding, and for local and systemic acclimation to a local treatment of HL stress (Figure 3). We further show that in the absence of NPR1 (npr1) the expression of PR1 is suppressed in local and systemic tissues of plants subjected to a local treatment of HL stress or wounding (Figure 3). Taken together, these findings suggest that NPR1 is required for SA to promote the ROS wave and trigger some of the transcripts required for plant acclimation to HL stress (Figure 5E). In this respect, it should be noted that NPR1 was reported to play a key role as a master regulator of redox-driven responses in the nuclei and to connect environmental cues with the circadian clock of plants (Zhou et al., 2015). Because the ROS wave is accompanied by a redox wave (Fichman and Mittler, 2021b), it could trigger different transcriptomic responses through NPR1 (and other transcriptional regulators such as MYB30; Fichman et al., 2020), that are modulated by an interplay between JA and SA (Figure 5E). ROS and redox signaling could therefore intersect with SA and JA signaling through NPR1 and control systemic accumulation of ROS and systemic plant acclimation to abiotic stresses or wounding (Figure 5E).
Materials and methods
Plant material, growth conditions, and stress treatments
WT Arabidopsis (A. thaliana; Col-0) and homozygous knockout mutants (Col-0 background) of coi1 (SALK_095916C), aos1-1 (SALK_017756C), sid2 (SALK_093400C), npr1 (SALK_204100C), ein2 (CS3071), etr1 (CS237), aba2 (CS3835 and aba2-11), max2 (SALK_028336C), and max3 (SALK_023975C) were grown on peat pellets (Jiffy 7; Jiffy International, Kristiansand, Norway) for 4 weeks under controlled short-day light conditions of 10-h light/14-h dark, 50 µmol m−2s−1, and 21°C room temperature. Homozygosity of each SALK line was determined via PCR (primers used are described in Supplemental Table S1). HL stress was applied using a ColdVision fiber optic LED light source (Schott, Southbridge, Massachusetts, USA) as previously described (Fichman et al., 2019, 2022). Wounding stress was applied by puncturing a single leaf with 18 dressmaker pins simultaneously as described in Fichman et al. (2019).
Imaging of the ROS wave and hormone fumigation
ROS accumulation after administration of stress treatments was imaged and analyzed as previously described (Fichman et al., 2019). Plants were fumigated for 30 min in a glass aquarium using a nebulizer (Punasi Direct, Hong Kong) with solution containing 50 µM H2DCFDA (Sigma-Aldrich, St. Louis, Missouri, USA) in 0.05 M phosphate buffer, pH 7.4, and 0.01% (v/v) Silwet L-77 (LEHLE seeds, Round Rock, Texas, USA). A single local leaf of the fumigated plant was treated with either HL or wounding stress as described above, and images of ROS accumulation were captured over the following 30 min using the IVIS Lumina S5 system (PerkinElmer, Waltham, Massachusetts, USA). Time course images of ROS accumulation were analyzed using the Living Image 4.7.2 software (PerkinElmer). Measurement of total radiant efficiency in regions of interest (the local and systemic leaves) was used for data analysis as described in Fichman et al. (2019). Dye penetration controls were performed by fumigation and imaging with 0.3% (v/v) H2O2 for 10 min following 50 µM H2DCFDA fumigation for 30 min (Fichman et al., 2019; Supplemental Figure S3). The whole-plant live ROS imaging method used in this study was validated in previous studies by measuring H2O2 in local and systemic tissues using the Amplex-Red method as described below (Fichman et al., 2021, 2022; also shown in Supplemental Figure S4). H2O2 in local and systemic leaves was quantified with Amplex-Red (10-Acetyl-3,7-dihydroxyphenoxazine; Thermo Fisher Scientific, Waltham, Massachusetts, USA). Leaves were flash frozen in liquid nitrogen, ground to powder, and resuspended in 50 µL 0.1 M trichloroacetic acid (Thermo Fisher Scientific). Following centrifugation for 15 min at 12,000 g, 4°C, the supernatant was buffered with 1 M phosphate buffer pH 7.4, and the pellet dried and used for dry weight calculation. H2O2 quantification at the supernatant was performed according to the MyQubit-Amplex-Red Peroxide Assay manual (Thermo Fisher Scientific), using an H2O2 calibration curve (Thermo Fisher Scientific). Concentration values were normalized to dry weight of each sample (Fichman et al., 2022). Imaging of the ROS wave following administration of individual hormones was performed as described above with the addition of 20 µM JA (Sigma-Aldrich) or 1 mM SA (Sigma-Aldrich) to the fumigation solution prior to stress treatment and imaging.
SAA following HL stress
Damage caused by HL stress was measured as previously described (Zandalinas et al., 2019; Fichman et al., 2020). HL stress (2,000 µmol photons m−2 s−1) was applied to either a local or a systemic leaf of a plant for 45 min to serve as the HL damage control. Following HL treatment, the exposed leaf was immediately sampled and placed in a tube containing 10 mL of ddH2O and moved to a gentle shaker for 1 h. After 1 h, the electrolytic leakage was measured for each sample (treated, untreated, local, or systemic) using a conductivity meter Oakton CON 700 (Thermo Fisher Scientific, Vernon Hills, Illinois, USA). The samples were then boiled for 20 min. The boiled samples were moved to a shaker for 1 h and the electrolytic leakage was measured for a second time. This process was also performed for plants receiving no HL treatment (untreated controls) and for plants that received 10 min of HL stress followed by a 50-min incubation period under controlled conditions prior to the 45 min of HL that allows for acclimation to occur. The percentage of electrolytic leakage in each sample was determined by dividing the preboiling measurement of electrolytic leakage by the postboiling electrolytic leakage in each sample.
Transcript expression analysis
The transcriptional responses to each stress (HL or wounding) were analyzed in local and systemic leaves at 0-, 10-, and 20-min timepoints after application of the stress treatment as described in Fichman et al. (2020). Local and systemic leaves, located at 137.5° angle from the locally treated leaf in the plant rosette, were sampled for analysis. Following stress application, plants were sampled at the different time points and RNA was isolated. RNA extraction and purification were performed using RNeasy kit (Qiagen, Hilden, Germany) as described by the manufacturer’s instructions and complementary DNA was synthesized for reverse-transcription quantitative polymerase chain reaction (RT-qPCR; Primescript RT Reagent Kit, Takara Bio, Kusatsu, Japan). RT-qPCR analysis was performed for the gene PR1 (AT2G14610) with iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, California, USA) and the CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories) as described in Fichman et al. (2020). The forward and reverse primer sequences used for the analysis of the PR1 transcriptional response were CGAACACGTGCAATGGAGTT and CACTTTGGCACATCCGAGTCT, respectively. Relative gene expression (2−ΔΔCT) was quantified using ELONGATION FACTOR 1A as the internal control (GAGCCCAAGTTTTTGAAGA and TAAACTGTTCTTCCAAGCTCCA). The relative increase in gene expression following stress treatment in each sample is shown as the increase compared with the untreated local sample that was collected alongside the treated samples.
Hormone measurements
Hormone extraction and quantification were performed as previously described (Balfagón et al., 2019; Sinha et al., 2022). Chromatographic separation was conducted on a reverse-phase C18 column (Gravity, 50 × 2.1 mm, 1.8 µm particle size; Macherey-Nagel GmbH, Düren, Germany) using a MeOH: H2O (both supplemented with 0.1% [v/v] acetic acid) gradient at a flow rate of 300 µL min−1. Hormones were quantified with a TQS triple quadrupole mass spectrometer (Micromass, Manchester, UK). All data were acquired and processed using Mass Lynx version 4.1 software.
Statistical analysis
Two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was conducted for statistical analysis. Letters represent a statistically significant difference of at least P < 0.05. Results for each experiment are displayed as box-and-whisker plots, with the borders corresponding to the 25th and 75th percentiles of the data. Each data value is included as a point within each box plot, with the horizontal line representing the median and “X” corresponding to the mean. Data points for ROS imaging are depicted as the determined total radiant efficiency ([p/s]/[µW/cm2]) calculated within a chosen region of interest. Data for SAA experiments are displayed as the relative amount of electrolytic leakage (shown as percent of control, with untreated local or systemic tissue acting as the control).
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: ABA2—NM_104113.5; AOS1—NM_123629.4; COI1—NM_129552.4; EIN2—NM_120406.5; ETR1—NM_105305.4; GLR 3.3—NM_103438.3; GLR 3.6—NM_115007.4; HPCA1—NM_124354.3; RBOHD—NM_124165.3; RBOHF—NM_105079.3; MAX2—NM_129823.3; MAX3—NM_001337112.1; NPR1—NM_105102.3; PR1—NM_127025.3; SID2—NM_127025.3.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Basal levels of JA, SA, and ABA in WT and the coi1, aos1-1, sid2, and npr1-1 mutants.
Supplemental Figure S2. SLs are not required for the triggering of the ROS wave in local and systemic tissues, or for plant acclimation to HL stress.
Supplemental Figure S3. Mutants deficient in hormone production or signaling responses show no deficiency in absorption of fluorescent dye via fumigation.
Supplemental Figure S4. H2O2 quantification in local and systemic leaves of WT (Col-0), npr1-1, coi1, aba2-4, and aos1-1, untreated or subjected to a local treatment of HL stress or wounding.
Supplemental Table S1. Primers for genotyping via PCR.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center (Ohio State University) for the seeds of the mutant lines that were used in this study. We apologize to all authors of papers not mentioned in this article due to space limitations.
Funding
This work was supported by funding from the National Science Foundation (IOS-2110017; IOS-1353886, MCB-1936590, and IOS-1932639), the Interdisciplinary Plant Group, and the University of Missouri.
Contributor Information
Ronald J Myers, Jr, The Division of Plant Sciences and Technology, Interdisciplinary Plant Group, College of Agriculture, Food and Natural Resources, Christopher S. Bond Life Sciences Center, University of Missouri, Columbia, Missouri 65201, USA.
Yosef Fichman, The Division of Plant Sciences and Technology, Interdisciplinary Plant Group, College of Agriculture, Food and Natural Resources, Christopher S. Bond Life Sciences Center, University of Missouri, Columbia, Missouri 65201, USA.
Sara I Zandalinas, Department of Biology, Biochemistry and Environmental Sciences, Jaume I University, Castelló de la Plana 12071, Spain.
Ron Mittler, The Division of Plant Sciences and Technology, Interdisciplinary Plant Group, College of Agriculture, Food and Natural Resources, Christopher S. Bond Life Sciences Center, University of Missouri, Columbia, Missouri 65201, USA.
R.J.M., S.I.Z., and Y.F. performed the research. R.M. supervised the research and provided resources, laboratory infrastructure, and funding. R.J.M., Y.F., S.I.Z., and R.M. wrote the manuscript and prepared figures. All authors read and approved the final version of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Ron Mittler (mittlerr@missouri.edu).
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad RK, Mittler R, Zandalinas SI (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181: 1668–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caarls L, Pieterse CM, Van Wees SC (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 6: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang J, Kong M, Freeman A, Chen H, Liu F (2021) More stories to tell: NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1, a salicylic acid receptor. Plant Cell Environ 44: 1716–1727 [DOI] [PubMed] [Google Scholar]
- Cheng WH, Chiang MH, Hwang SG, Lin PC (2009) Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol Biol 71: 61–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Arbogast J, Mittler R (2020a) Coordinated and rapid whole-plant systemic stomatal responses. New Phytol 225: 21–25 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Liscum E, Mittler R (2020b) Phytochrome B is required for systemic stomatal responses and reactive oxygen species signaling during light stress. Plant Physiol 184: 1563–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Gao YQ, Lenzoni G, Wolfender JL, Wu Q (2020) Wound- and mechanostimulated electrical signals control hormone responses. New Phytol 227: 1037–1050 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R (2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2021a) Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J 107: 7–20 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2021b) A systemic whole-plant change in redox levels accompanies the rapid systemic response to wounding. Plant Physiol 186: 4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, Xiong H, Sengupta S, Azad RK, Hibberd JM, Liscum E, Mittler R (2021) Phytochrome B regulates reactive oxygen signaling during abiotic and biotic stress in plants. BioRxiv 2021.11.29.470478 [DOI] [PubMed]
- Fichman Y, Zandalinas SI, Peck SC, Luan S, Mittler R (2022) HPCA1 is required for systemic ROS and calcium cell-to-cell signaling and plant acclimation to stress. Plant Cell 34: 4453–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, Zandalinas SI, Sengupta S, Burks D, Myers RJ, Azad RK, Mittler R (2020) MYB30 orchestrates systemic reactive oxygen signaling and plant acclimation. Plant Physiol 184: 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Apostolova N, Bellés JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodríguez PL (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14: 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2019) Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trend Plant Sci 24: 25–37 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45–ra45 [DOI] [PubMed] [Google Scholar]
- Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F (2022) Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol 23: 663–679. [DOI] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Saeed W, Naseem S, Ali Z (2017) Strigolactones biosynthesis and their role in abiotic stress resilience in plants: a critical review. Front Plant Sci 8: 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Zandalinas SI, Fichman Y, Sen S, Zeng S, Gómez-Cadenas A, Joshi T, Fritschi FB, Mittler R (2022) Differential regulation of flower transpiration during abiotic stress in annual plants. New Phytol 235: 611–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Jikumaru Y, Shimada Y, Sasaki E, Stingl N, Mueller MJ, Kamiya Y (2011) Jasmonate-dependent and COI1-independent defense responses against Sclerotinia sclerotiorum in Arabidopsis thaliana: auxin is part of COI1-independent defense signaling. Plant Cell Physiol 52: 1941–1956 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Withers J, Dong X (2016) Posttranslational modifications of NPR1: a single protein playing multiple roles in plant immunity and physiology. PLoS Pathog 12: e1005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Hua L, Reyna-Llorens I, Shi Y, Chen K-M, Smirnoff N, Kromdijk J, Hibberd JM (2021) Photosynthesis-independent production of reactive oxygen species in the rice bundle sheath during high light is mediated by NADPH oxidase. Proc Natl Acad Sci USA 118: e2022702118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Devireddy AR, Sengupta S, Azad RK, Mittler R (2020a) Systemic signaling during abiotic stress combination in plants. Proc Natl Acad Sci USA 117: 13810–13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Mittler R (2020b) Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell 32: 3425–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R (2021) Vascular and nonvascular transmission of systemic reactive oxygen signals during wounding and heat stress. Plant Physiol 186: 1721–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Sengupta S, Burks D, Azad RK, Mittler R (2019) Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J 98: 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang W, Karapetyan S, Mwimba M, Marqués J, Buchler NE, Dong X (2015) Redox rhythm reinforces the circadian clock to gate immune response. Nature 523: 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.