Abstract
The photosynthetic mechanism of crop yields in fluctuating light environments in the field remains controversial. To further elucidate this mechanism, we conducted field and simulation experiments using maize (Zea mays) plants. Increased planting density enhanced the light fluctuation frequency and reduced the duration of daily high light, as well as the light-saturated photosynthetic rate, biomass, and yield per plant. Further analysis confirmed a highly significant positive correlation between biomass and yield per plant and the duration of photosynthesis related to daily high light. The simulation experiment indicated that the light-saturated photosynthetic rate of maize leaves decreased gradually and considerably when shortening the daily duration of high light. Under an identical duration of high light exposure, increasing the fluctuation frequency decreased the light-saturated photosynthetic rate slightly. Proteomic data also demonstrated that photosynthesis was mainly affected by the duration of high light and not by the light fluctuation frequency. Consequently, the current study proposes that an appropriate duration of daily high light under fluctuating light environments is the key factor for greatly improving photosynthesis. This is a promising mechanism by which the photosynthetic productivity and yield of maize can be enhanced under complex light environments in the field.
Introduction
The light environment affects the photosynthesis, growth, and yield of crops under natural conditions. It is generally believed that increased light intensity in the field leads to higher photosynthetic rates, which also causes an enhancement of yield; conversely, the photosynthetic rate under lower light intensity has been found to be low, leading to a marked reduction of yield (Zhu et al., 2010; Raines, 2011; Flannery et al., 2021). It has also been reported that the light-saturated photosynthetic rate of leaves growing under high light is relatively high, as is the ability to dissipate excess excitation, while the opposite photosynthetic properties occur in leaves grown under weak light. The regulation of these photosynthetic functions involves changes in morphological structure, photosynthetic components, enzyme activity, and related gene expression (Brouwer et al., 2012; Li et al., 2014; Wu et al., 2019, 2021). However, these conclusions are mainly based on steady light environments, for which an increasing number of studies have demonstrated that experiments performed under steady light intensity may not correctly reflect the real situation in the field (Matsubara, 2018; Kimura et al., 2020; Yamori et al., 2020).
Natural light environments are highly complex and variable. In particular, light fluctuation occurs in the field. Previous studies have shown that increasing the fluctuation of light intensity can reduce the photosynthetic rate by suppressing the activity of Photosystem I (PSI) and Photosystem II (PSII) (Sejima et al., 2014; Zivcak et al., 2015; Schneider et al., 2019). Under fluctuating light, the delay of nonphotochemical quenching (NPQ) relaxation costs light energy upon a transition from high to low light intensity and may also depress the photosynthetic rate under low light (Kromdijk et al., 2016; Hubbart et al., 2018; Yamori et al., 2020). In addition, under fluctuating light conditions, a slow induction of photosynthesis will also limit the photosynthetic rate, increasing the dissipation of excitation energy and decreasing the efficiency of light utilization after transition from low to high light (Adachi et al., 2019; Acevedo-Siaca et al., 2020; Kimura et al., 2020). Our recent simulation studies with maize demonstrated that boosting the light fluctuation frequency accelerated the initiation of photosynthetic induction and NPQ, thus adapting to the fluctuating light environment and maintaining a certain photosynthetic rate and biomass under growth conditions (Qiao et al., 2021). Therefore, the effects of fluctuating light on photosynthesis, biomass, and yield are very complex. However, some studies have shown that fluctuating light has little effect on the photosystem activity and that increasing the frequency of light intensity rarely affects the photosynthetic rate (Ferroni et al., 2020; Zhang et al., 2020). Clearly, there are still controversies about how and to what extent fluctuating light affects photosynthesis. Thus, the photosynthetic mechanism of crop yield affected by fluctuating light needs to be further studied. The occurrence of weak light under a fluctuating light environment shortens the duration of high light; a slow photosynthetic induction during the transition from low light to high light will increase the time needed for a higher photosynthetic rate to be attained (Qiao et al., 2021). Accordingly, compared with the fluctuation frequency, it can be speculated that the photosynthetic capacity and assimilate accumulation may be affected by the duration of high light to a greater extent under the premise of maintaining the same high light intensity.
High planting density (HD) is an important cultivation measure to improve yield per land area in crop production. However, the biomass and yield per plant decrease rapidly with the increase in density (Liu et al., 2021; Wu et al., 2019, 2022). From the perspective of the light environment, increasing planting density aggravates mutual shading between plants, resulting in a decline of the light intensity that leaves can intercept and enhancing the fluctuation of the light environment. Under these conditions, it remains unclear whether the decrease in light intensity, the increase in fluctuation frequency, or the reduction of the duration of high light results in the declines of yield per plant. Accordingly, in this study, the effects of the frequency of fluctuation and the duration of high light on photosynthesis and yield were carefully investigated by close planting and simulation experiments in maize (Zea mays) plants. These studies are not only of theoretical value for understanding photosynthetic mechanisms, but are also of great importance for crop production.
Results
Effects of planting density on light environments and yield in the field
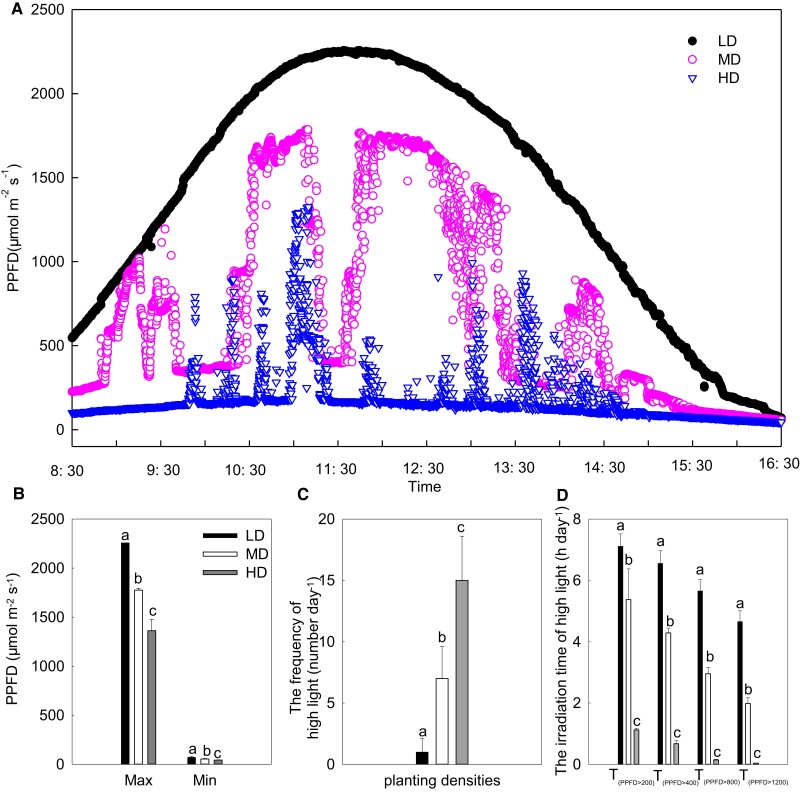
As shown in Figure 1, the effects of diurnal changes in light intensity on the ear leaf on sunny days were determined under all planting densities in maize. The light intensity was low in the morning, increasing gradually to a maximum value at noon, and decreasing thereafter (Figure 1). As planting density increased, the maximum light intensity reduced. In particular, the difference between the maximum and minimum light intensity became more pronounced around noon (Figure 1B). Furthermore, the frequency of light intensity fluctuation increased with the increase of planting density (Figure 1C). Further analysis revealed that the corresponding duration of light intensity exposure decreased with increasing planting density when the light intensities were higher than 200, 400, 800, and 1,200 μmol m−2 s−1 (Figure 1D). Compared with LD, the cumulative duration of light intensity at MD and HD that exceeded 1,200 μmol m−2 s−1 decreased by 58% and by 90% in a diurnal cycle, respectively. Therefore, these data indicated that the increase of planting density shortened the duration of high light.
Figure 1.
Effects of planting density on the diurnal course of light intensity on maize ear leaf. (A) Diurnal course of light intensity; (B) maximum and minimum light intensity; (C) light fluctuation frequency; (D) cumulative illumination time greater than a specific light intensity. Data are means ± standard error (n = 3). Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different densities at the P < 0.05 level. T(PPFD > 200), T(PPFD > 400), T(PPFD > 800), and T(PPFD > 1200) represent the duration of high light when the light intensity was higher than 200, 400, 800, and 1,200 μmol m−2 s−1. LD, low planting density; MD, medium planting density; HD, high planting density; Max, maximum value; Min, minimum value; PPFD, photosynthetic photon flux density.
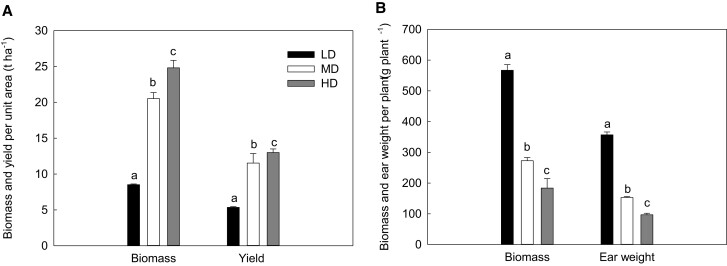
The biomass and yield per unit area increased as the planting density increased (Figure 2A), while the biomass and yield per plant declined (Figure 2B). Compared with LD, the biomass per plant of MD (= 5 × LD) and HD (= 9 × LD) reduced by 52% and 68%, respectively, and the ear weight per plant of MD and HD declined by 57% and 73%, respectively.
Figure 2.
Effects of planting density on biomass and yield of maize. (A) Biomass (n = 5) and yield (n = 20) per unit area; (B) biomass (n = 5) and ear weight (n = 20) per plant. Data are means ± standard error. Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different densities at the P < 0.05 level. LD, low planting density; MD, medium planting density; HD, high planting density.
Effects of light environments on photosynthetic characteristics in the field
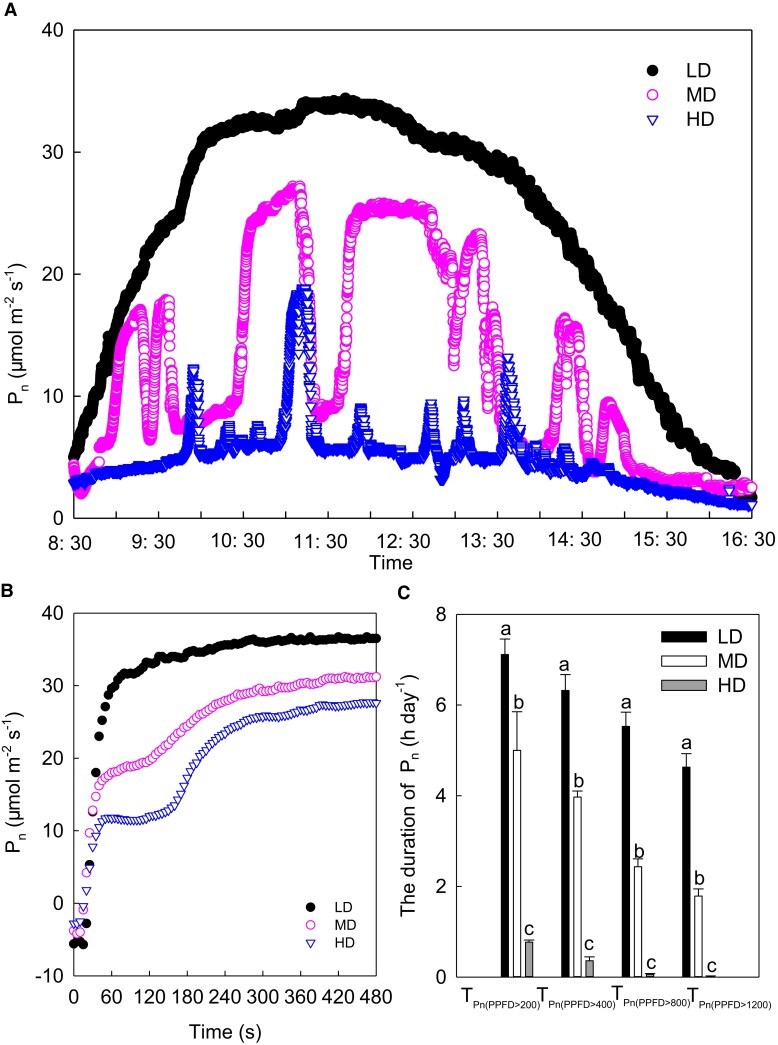
The diurnal time courses of the photosynthetic rates in the ear leaves of maize under different planting densities are shown in Figure 3. The photosynthetic rate was low in the morning, then gradually increased and peaked at noon, and gradually fell henceforth (Figure 3A). The photosynthetic rate fell with increasing planting density, especially at noon. Similar to the diurnal changes of light intensity, the diurnal time course of the photosynthetic rate also showed obvious fluctuations under MD and HD treatments (Figure 3A). When the light intensity was higher than 200, 400, 800, and 1,200 μmol m−2 s−1, the corresponding duration of photosynthesis decreased with increasing plant density (Figure 3C). When light intensity exceeded 1,200 μmol m−2 s−1, compared with LD, the duration of photosynthesis in the MD and HD treatments dropped by more than 60% and 90%, respectively. These findings suggest that the increase of planting density resulted in the reduction of the duration of photosynthesis at a light intensity that is above a certain level. This study also determined the photosynthetic induction of the ear leaves of maize plants grown under various planting densities. In this process, the photosynthetic rate increased rapidly in the primary stage and slowly rose to the maximum value thereafter. Under LD, MD, and HD, the maximum photosynthesis was induced in ∼180, 270, and 330 s, respectively (Figure 3B), suggesting that the increase of planting density reduced the rate of photosynthetic induction.
Figure 3.
Effects of planting density on the diurnal course of gas exchange and photosynthetic induction in maize ear leaves. (A) Diurnal course of photosynthesis (n = 3); (B) photosynthetic induction curve (n = 5); (C) duration of photosynthesis occurring at light levels greater than a specific light intensity (n = 3). Data are means ± standard error. Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different densities at the P < 0.05 level. Pn, net photosynthetic rate; TPn (PPFD > 200), TPn (PPFD > 400), TPn (PPFD > 800), and TPn (PPFD > 1200) represent the duration of photosynthesis when the light intensity was higher than 200, 400, 800, and 1,200 μmol m−2 s−1. LD, low planting density; MD, medium planting density; HD, high planting density.
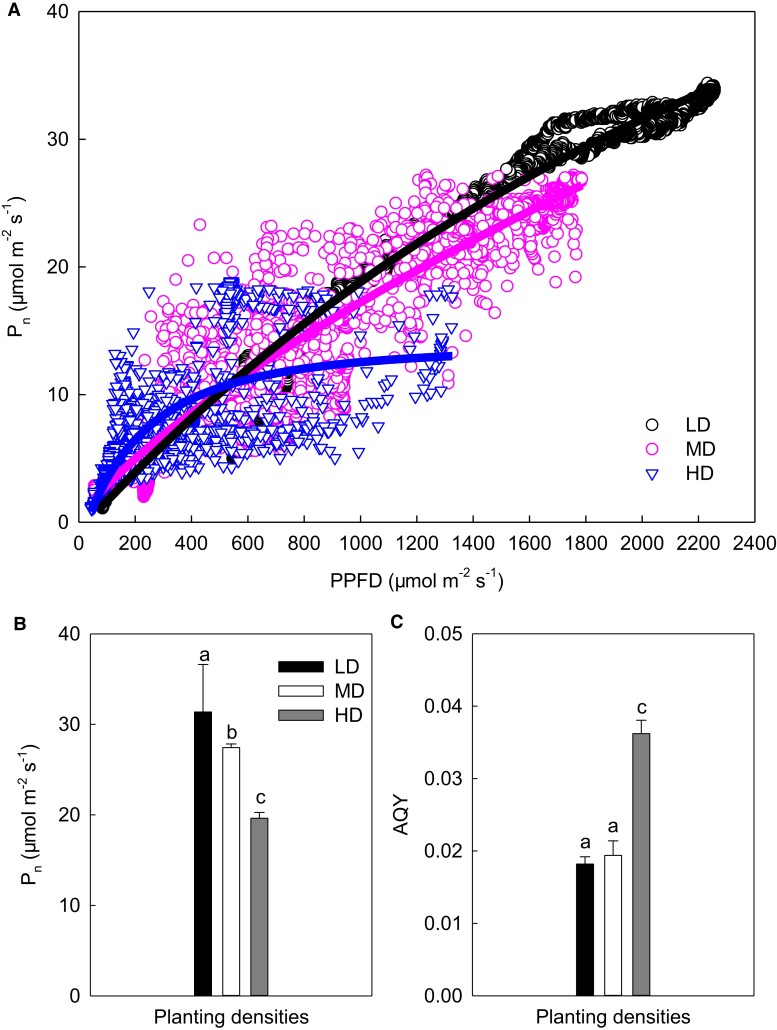
As shown in Figure 4A, the photosynthetic rate rose under different density treatments as the light intensity increased. However, it was noted that the maximum photosynthetic rate was depressed with increasing density (Figure 4B), while the maximum apparent quantum yield (AQY) rose as the density increased (Figure 4C). Relative to LD, the maximum photosynthetic rates of MD and HD declined by 21% and 45%, respectively; while the AQY of MD and HD increased by more than 7% and 90%, respectively, compared with LD. Clearly, although the photosynthetic rate of maize leaves decreased under close planting conditions, the maize leaves maintained a certain photosynthetic rate under weak light intensity by increasing the AQY.
Figure 4.
Effects of planting density on photosynthesis-light curves in maize ear leaf. (A) The photosynthesis-light curves; (B) maximum photosynthetic rate; (C) maximum apparent quantum yield. The simulation equations in A are as follows: y = 55.66–56.3136/(1 + 9.1824E−010x)1/2.1635E^−006 (R2 = 0.9820); y = −5.229 + 5.7170/(1 + 0.0071x)−1/1.5322 (R2 = 0.9249); and y = 13.8495–15.6104/(1 + 0.0019x)1/0.4249 (R2 = 0.6776). Data are means ± standard error (n = 3). Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different densities at the P < 0.05 level. Pn, net photosynthetic rate; LD, low planting density; MD, medium planting density; HD, high planting density; PPFD, photosynthetic photon flux density; AQY, the maximum apparent quantum yield.
Obviously, planting density has a great influence on light environments within canopy and leaf photosynthesis of maize plants. Apart from light intensity, the fluctuation frequency and the duration of high light are also important characteristics of the light environment. Considering that photosynthesis may respond to the fluctuation frequency and the duration of high light differently, further simulation experiments were performed to reveal the influencing mechanism of yield formation.
Effects of frequent fluctuation of light intensity and duration of high light on photosynthesis
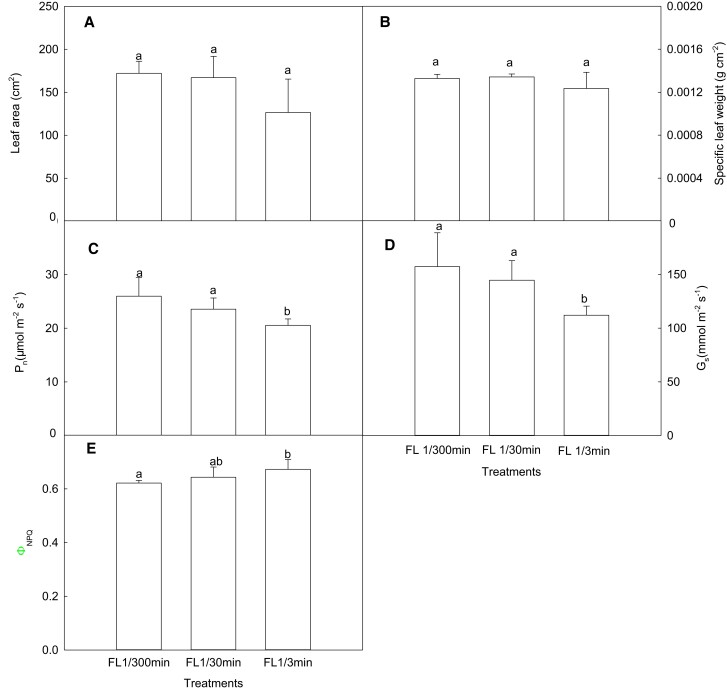
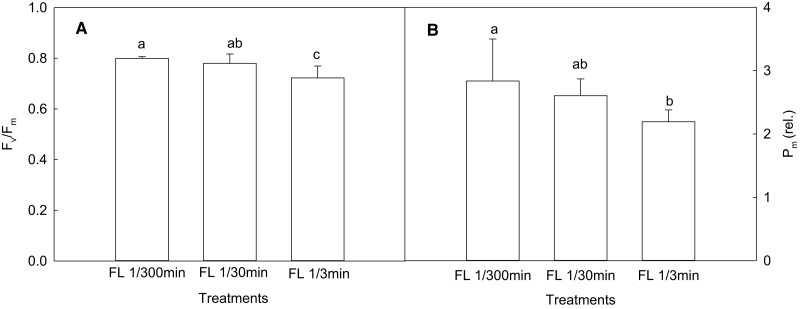
First, we simulated the effect of fluctuation frequency on photosynthesis. The results demonstrated that there were no statistically significant changes in leaf area and specific leaf weight (SLW) as the frequency of light fluctuation increased, while the photosynthetic rate and stomatal conductance gradually decreased (Figure 5, A–D). Compared with FL 1/300 min, the photosynthetic rates decreased by ∼8% and 18% in FL 1/30 min and FL 1/3 min, respectively. As shown in Figure 6A, the values of the maximum quantum yield of PSII (Fv/Fm) in FL 1/30 min and FL 1/3 min were about 2% and 10% lower than those in FL 1/300 min. However, the quantum yield of regulatory energy dissipation(ΦNPQ) of FL 1/30 min and FL 1/3 min increased by 3% and 8%, respectively, compared with FL 1/300 min (Figure 5E). In addition, Pm is P700 maximum oxidation state, reflecting the number of active PSI reaction centers and the degree of PSI photoinhibition (Kim et al., 2005; Sejima et al., 2014). Figure 6B shows that the values of Pm in FL 1/30 min and FL 1/3 min were 8% and 23% lower than those in FL1/300 min, respectively. These data demonstrate that the low frequency of light fluctuation (FL 1/30 min) had only a very slight effect on the leaf photosynthesis of maize, while photosynthetic characteristics were affected to a greater extent by the high frequency of light fluctuation (FL 1/3 min).
Figure 5.
Effects of light intensity fluctuation on leaf morphology, gas exchange, and chlorophyll a fluorescence quenching in maize leaves. (A) Leaf area (n = 5); (B) specific leaf weight (n = 5); (C) photosynthetic rate (n = 6); (D) stomatal conductance (n = 6); (E) the quantum yield of regulatory energy dissipation (n = 6). FL 1/300 min, maize seedlings were exposed alternately to high light (1,600 μmol m−2 s−1) for 300 min and then to weak light (50 μmol m−2 s−1) for 300 min (total 10 h); FL 1/30 min, maize seedlings were exposed alternately to high light and weak light every 30 min (total 10 h); FL 1/3 min, maize seedlings were exposed alternately to high light and weak light every 3 min (total 10 h). Data are means ± standard error. Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different treatments at the P < 0.05 level. Pn, net photosynthetic rate; Gs, stomatal conductance; ΦNPQ, the quantum yield of regulatory energy dissipation.
Figure 6.
Effects of light intensity fluctuation on activities of PSII and PSI in maize leaves. (A) The maximum quantum yield of PSII (n = 20); (B) the maximum photooxidation of P700 (n = 6). FL 1/300 min, maize seedlings were exposed alternately to high light (1,600 μmol m−2 s−1) for 300 min and then to weak light (50 μmol m−2 s−1) for 300 min (total 10 h); FL 1/30 min, maize seedlings were exposed alternately to high light and weak light every 30 min (total 10 h); FL 1/3 min, maize seedlings were exposed alternately to high light and weak light every 3 min (total 10 h). Data are means ± standard error. Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different treatments at the P < 0.05 level. Fv/Fm, the maximum quantum yield of PSII; Pm, the maximum photooxidation of P700.
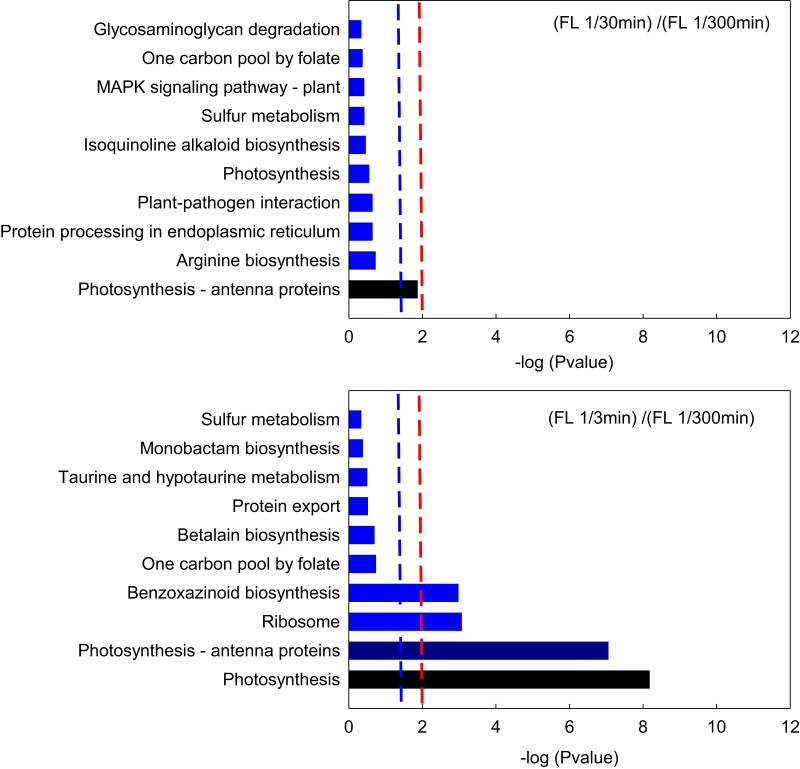
To further test the possible effect of the frequency of light fluctuation on photosynthesis, the enriched KEGG pathways of differentially abundant proteins of leaves under different frequency of light fluctuation were analyzed. The main differential proteins in FL 1/30 min versus FL 1/300 min were the photosynthesis-antenna proteins (P < 0.05). In FL 1/3 min versus FL 1/300 min, the differential proteins were largely focused on photosynthesis, photosynthesis-antenna proteins, and the ribosome (P < 0.01) (Figure 7). These data also reveal that the photosynthesis process was influenced distinctly by the high frequency of light fluctuation (FL 1/3 min), yet not by the low frequency of light fluctuation (FL 1/30 min).
Figure 7.
Enriched KEGG pathways of differentially abundant proteins in maize leaves under different light intensity fluctuations. The vertical coordinates and the horizontal coordinates represent the KEGG pathway and −log (P-value), respectively. The right line indicates a P-value of 0.01, and the left line shows a P-value of 0.05. When the right edge of the bar is past the left line or the right line, the biological process it represents is significant, with extremely significant biological processes beyond the right line and significant processes beyond the left line. KEGG, Kyoto encyclopedia of genes and genomes.
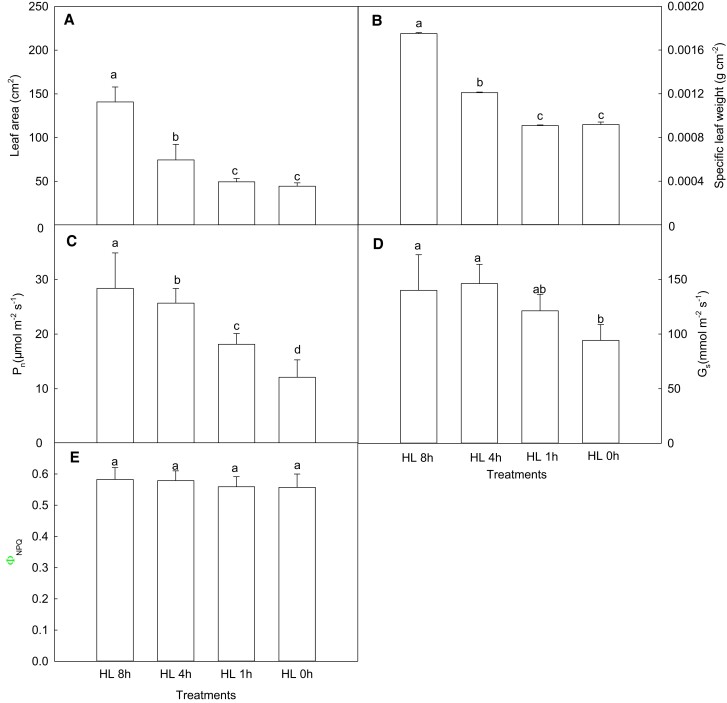
Since fluctuation frequency has a slight effect on photosynthesis, we speculated that the photosynthetic performance of maize leaves may be determined more by the duration of high light. To elucidate this hypothesis, further simulation experiments were conducted in maize plants. Our investigation showed that the leaf area and SLW markedly dropped with the reduction of the duration of high light (Figure 8A, B). Similarly, shortening the duration of high light also induced significant declines in the photosynthetic rate and stomatal conductance (Figure 8C, D). The photosynthetic rates in HL 4 h, HL 1 h, and HL 0 h fell by 10%, 36%, and 57%, respectively, compared with HL 8 h. Thus, reducing the duration of high light (<4 h) resulted in a notable decrease in the photosynthetic rate. Furthermore, the values of ΦNPQ in HL 4 h, HL 1 h, and HL 0 h were only 0.7%, 4%, and 4.3% lower than those in HL 8 h, respectively. Obviously, ΦNPQ decreased only very slightly as the duration of high light reduced (Figure 8E).
Figure 8.
Effects of the duration of high light on leaf morphology, gas exchange, and chlorophyll a fluorescence quenching in maize leaves. (A) Leaf area (n = 5); (B) specific leaf weight (n = 5); (C) photosynthetic rate (n = 6); (D) stomatal conductance (n = 6); (E) the quantum yield of regulatory energy dissipation (n = 6). HL 0 h represents weak light (200 μmol m−2 s−1); HL 1 h, HL 4 h, and HL 8 h represent treatments in which the duration of high light increased by 1, 4, and 8 h on top of weak light (200 μmol m−2 s−1), respectively. Data are means ± standard error. Different lowercase letters indicate statistically significant (1-way ANOVA and LSD test) differences between different treatments at the P < 0.05 level. Pn, net photosynthetic rate; Gs, stomatal conductance; ΦNPQ, the quantum yield of regulatory energy dissipation.
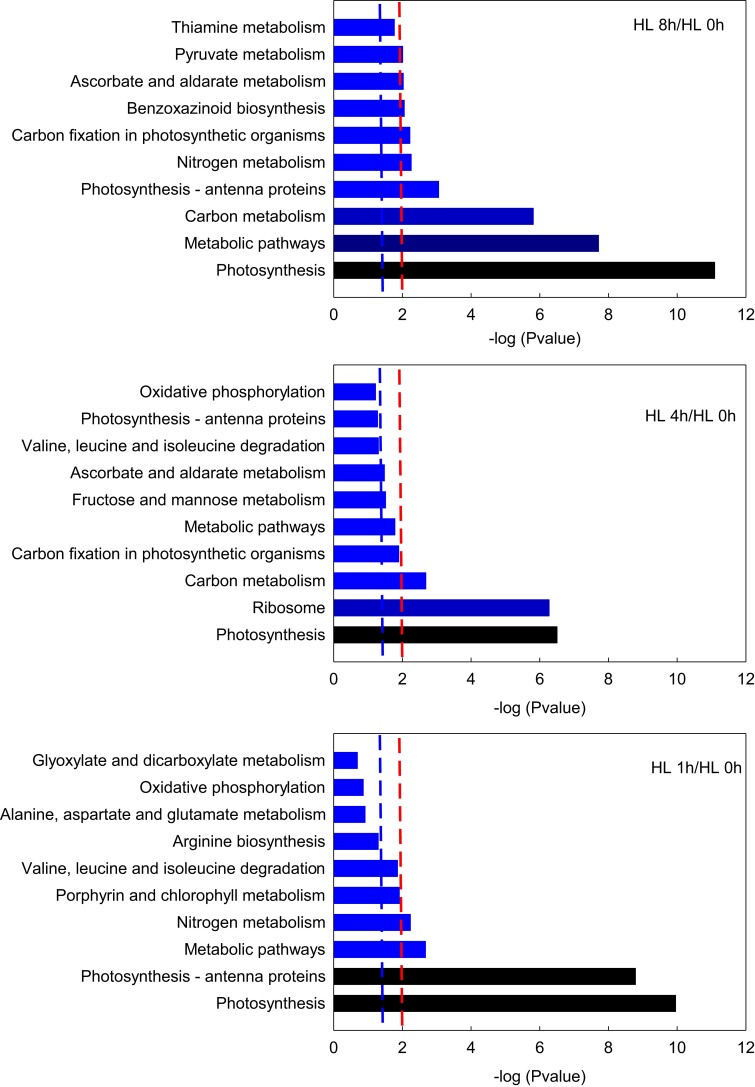
The proteomic data also showed that the differentially abundant proteins in HL 8 h versus HL 0 h were mainly focused on photosynthesis, metabolic pathways, and carbon metabolism. Three pathways were significantly enriched in HL 4 h versus HL 0 h, consisting of photosynthesis, ribosome, and carbon metabolism (P < 0.01). In HL 1 h versus HL 0 h, the differential proteins were largely concentrated in photosynthesis, photosynthesis-antenna proteins, and metabolic pathways (Figure 9). These findings clearly demonstrate that the biological processes related to the duration of high light are mainly centered on photosynthesis.
Figure 9.
Enriched KEGG pathways of differentially abundant proteins in maize leaves under different durations of high light. The vertical coordinates and the horizontal coordinates represent the KEGG pathway and −log (P-value), respectively. The right line indicates a P-value of 0.01, and the left line shows a P-value of 0.05. When the right edge of the bar is past the left line or the right line, the biological process it represents is significant, with extremely significant biological processes beyond the right line and significant processes beyond the left line. KEGG, Kyoto encyclopedia of genes and genomes.
Discussion
Photosynthetic capacity depends on the duration of high light under fluctuating light
The results of the present study show that the maximum light intensity decreased by ∼40% (from 2,200 to 1,300 μmol m−2 s−1) during the diurnal changes of light intensity as planting density increased from LD to HD. The average light intensity under the 3 planting density treatments decreased from 1,300 to 700 and 150 μmol m−2 s−1, respectively. Evidence from previous studies indicates that reducing the light intensity in plants leads to a decline of photosynthetic capacity (Brouwer et al., 2012; Wu et al., 2019, 2021). More importantly, previous experiments focused mainly on investigating the effect of changes in light intensity on photosynthesis under the premise of maintaining the same illumination time (Vialet-Chabrand et al., 2017; Pao et al., 2019; Schneider et al., 2019; Wei et al., 2021). However, the effect of light intensity on photosynthesis is influenced by both light intensity itself and the duration of high light. In the simulation study of the duration of high light, when the high light illumination time reduced from 8 to 4, 1, and 0 h, the photosynthetic rate fell by ∼10%, 36%, and 57%, respectively (Figure 8C). The results of the present study showed that high-density planting not only resulted in a decline in overall light intensity but also caused a marked reduction in the duration of high light in the field (Figure 1). Under the 3 density treatments of LD, MD, and HD, the durations during which high light exceeded 1,200 μmol m−2 s−1 in a day were 4.66, 1.98, and 0.4 h, respectively (Figure 1D). Apparently, the duration of high light (>1,200 μmol m−2 s−1) in the HD treatment diminished by at least 90%. Although the fluctuation frequency of the light environment in the HD treatment increased by 15 times (Figure 1C), the simulation experiment showed that the photosynthetic rate of leaves dropped by 8% at the fluctuation frequency of 10 times per day (FL 1/30 min) and by 18% at the change frequency of 100 times per day (FL 1/3 min) (Figure 5C). The maximum photosynthetic rate under HD reduced by more than 45% in the field (Figure 4B). Thus, it is asserted in the present study that the reduced duration of high light reduces the photosynthetic capacity to a greater extent than the frequency of fluctuating light (Figure 5 and 6). Proteome data also demonstrated that the abundance of proteins related to photosynthesis was affected by the duration of high light and that photosynthesis was the primary biological process regulated by the duration of high light (Figure 9). By contrast, the frequency of fluctuating light had only minor effects on the abundance of photosynthesis-related proteins (Figure 7). In addition, we noticed that leaf area and SLW were largely affected by the duration of high light and not by the fluctuation frequency of the light (Figures 5and 8). Hence, the duration of high light is a key factor affecting the photosynthetic capacity of maize plants under complex light environments in the field.
Previous studies on fluctuating light mainly focused on the effects of different light intensities on photosynthesis under identical durations of high light (Vialet-Chabrand et al., 2017; Wei et al., 2021). It has previously been shown that higher photosynthetic rates follow increased average light intensity under fluctuating light (Vialet-Chabrand et al., 2017). However, the enhancement of the average light intensity in these studies was accompanied by the extension of the duration of high light. For example, a recent report by Vialet-Chabrand et al. (2017) demonstrated that although the total illumination time remained the same under fluctuating light conditions, the durations during which light intensity exceeded 230 μmol m−2 s−1 under 2 fluctuating light treatments (fluctuating low light and fluctuating high light) were ∼5.3 and 6.8 h, respectively. The durations during which the light intensity was higher than 460 μmol m−2 s−1 were ∼3.7 and 5.3 h for low and high light, respectively, and the durations in which the light intensity surpassed 690 μmol m−2 s−1 were ∼1 and 3.7 h for low and high light, respectively. Obviously, in the study reported by Vialet-Chabrand et al. (2017), higher photosynthetic rates were related to longer durations of high light. Therefore, the enhancement of the photosynthetic rate caused by the increase in average light intensity under fluctuating light conditions can be realized by prolonging the duration of high light.
The results of the simulation experiment in this study showed a relatively small decrease (∼10%) in the photosynthetic capacity when the duration of high light reduced from 8 to 4 h, whereas there were considerable decreases of ∼36% and 57% when the duration of high light continuously diminished from 4 to 1 h and from 1 to 0 h, respectively (Figure 8C), demonstrating that the photosynthetic capacity under a specific light intensity requires a certain continuous duration of high light during growth. Proteome data also showed that the abundance of proteins related to carbon metabolism maintained at a relatively high level under 8 and 4 h of illumination time, which was consistent with the higher photosynthetic rate (Figure 9). Specifically, the excitation energy captured by plants grown in high light cannot be fully utilized by photosynthesis, and excessive excitation energy may induce photooxidation of photosynthesis (Takahashi and Murata, 2008; Dietz, 2015). To avoid photooxidative damage, plants have evolved various photoprotective mechanisms, of which the most common and important is energy dissipation depending on the xanthophyll cycle (Niyogi, 1999). ΦNPQ reflects the quantum yield of regulatory light-induced NPQ (Hendrickson et al., 2005). In the present study, it was observed that the ΦNPQ under 8 h of high light maintained at a relatively high level, demonstrating that plants require a strong regulatory energy dissipation capacity to avoid serious photooxidative damage under continuous high light for a long period. With the decrease in the duration of high light, maize plants gradually and slightly decreased their photoprotection ability, while greatly reducing the photosynthetic rate (Figure 8C, E). Hence, an appropriate duration of high light may be conducive to greatly improving photosynthesis when the capacity of regulatory energy dissipation changes a little in maize plants.
Photosynthetic mechanism of maize yield under fluctuating light conditions
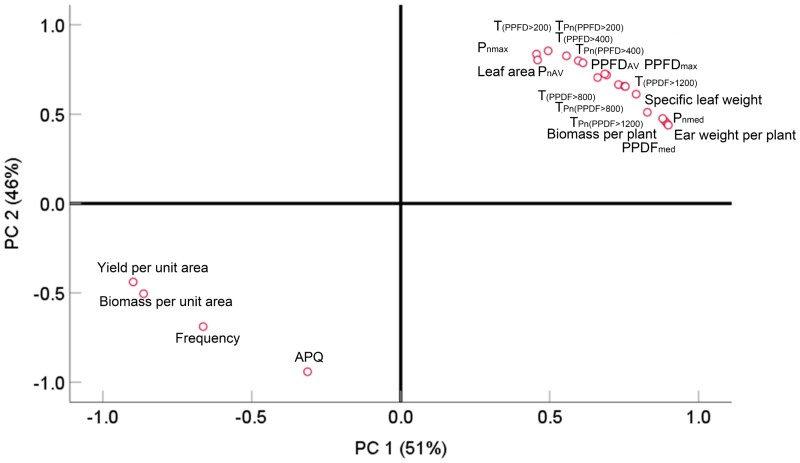
In the field, the AQY of HD was the highest among the 3 plant density treatments (Figure 4C), indicating that maize plants could acclimate to the light environment by regulating photosynthesis. The increase of AQY under HD treatment may be associated with the alteration of the distribution of photosynthetic components among various cell layers (Yabiku et al., 2020; Wu et al., 2021). Hence, maize plants under high-density planting conditions may maintain a certain photosynthetic rate by increasing the AQY so that the biomass and yield per plant will not fall excessively. A distinct reduction in the biomass and yield per plant produced by high-density planting was also accompanied by an increased frequency of light fluctuation (Figure 2). More frequent light fluctuation will lead to repeated decay and induction of photosynthetic dark reactions. Previous studies have also shown that the slow induction of photosynthesis may cost daily carbon assimilation when leaves are suddenly returned to high light from low light or dark conditions (Taylor and Long, 2017; Acevedo-Siaca et al., 2020; Kimura et al., 2020; Qiao et al., 2021). By contrast, the faster induction response in high-yield rice increased the daily CO2 assimilation compared with low-yield rice (Oryza sativa) (Adachi et al., 2019). More importantly, increasing the light fluctuation frequency could considerably reduce the duration of high light (Figure 1D). The results of the present study show that, in the MD treatment, it took at least 4.5 min for leaf photosynthesis to be fully induced after the transition from low light to high light. Considering that 7 fluctuations occurred in the MD treatment, the duration of a steady or higher photosynthetic rate reduced by no less than 10.5 min [(4.5 −3) min × 7 = 10.5 min] compared with LD. For the HD treatment, the full induction of photosynthesis was observed when leaves were re-exposed to high light for 5.5 min after weak light. Accordingly, in a comparison with LD, the duration of a steady or higher photosynthetic rate was delayed by at least 35 min [(5.5 −3) min × 15 = 37.5 min] during the whole period of high light exposure (> 1,200 μmol m−2 s−1) (Figure 3). Based on this calculation, when the light intensity was higher than 1,200 μmol m−2 s−1, the frequent light fluctuations resulted in a 30% decrease in the duration of photosynthesis under the MD treatment, while there was not enough time for full photosynthetic induction in the HD treatment. Principal component analysis revealed a greater correlation between the duration of high light and the corresponding photosynthetic rate in the ear leaf and the ear weight and biomass per plant during the diurnal time course. Moreover, the duration of the photosynthetic rate had the best linear relationship with the ear weight and biomass per plant when light intensity exceeded 1,200 μmol m−2 s−1 (Figure 10). Consequently, these findings show that the main cause of the decrease in biomass and yield per plant was the shortened duration of the high photosynthetic rate associated with higher growth light intensity that occurred under HD.
Figure 10.
Principal component analysis of morphological and physiological indexes under different planting densities. AQY, the maximum apparent quantum yield; PPDFmax, PPDFmed, and PPDFAV represent the maximum, median, and average value of light intensity, respectively; Pnmax and Pnmed represent the maximum and median net photosynthetic rate, respectively; PC, principal component; T(PPDF > 200), T(PPDF > 400), T(PPDF > 800), and T(PPDF > 1200) represent the duration of high light when the light intensity was higher than 200, 400, 800, and 1,200 μmol m−2 s−1, respectively. TPn (PPDF > 200), TPn (PPDF > 400), TPn (PPDF > 800), and TPn (PPDF > 1200) represent the duration of photosynthesis when the light intensity was higher than 200, 400, 800, and 1,200 μmol m−2 s−1, respectively.
It should be noted that the corresponding photosynthetic rates under the LD and MD treatments were ∼20 μmol m−2 s−1 when the light intensity was 1,200 μmol m−2 s−1 (Figure 4A). When the light intensity was –1,200 μmol m−2 s−1 and the corresponding photosynthetic rate was higher than 20 μmol m−2 s−1, the duration under the LD treatment was ∼5 h, the corresponding time under the MD treatment was only ∼2 h, and the time under HD treatment was close to 0 h (Figure 3C). The biomass and ear weight per plant under the MD treatment declined by more than 50% compared with that under the LD treatment (Figure 2). Therefore, longer durations of the light intensity exceeding 1,200 μmol m−2 s−1 or the photosynthetic rate exceeding 20 μmol m−2 s−1 favored the accumulation of biomass in the whole plant and an increase in ear weight. The light intensity in the canopy decays sharply from the top to the bottom. Previous studies by this research group demonstrated that when the light intensity of ear leaves was ∼1200 m−2 s−1 under LD conditions, the light intensity of the fourth leaf below the ear could reach 600 μmol m−2 s−1 in most cases, which was far greater than the light compensation point of maize leaves (Wu et al., 2019, 2022). In such conditions, the photosynthesis of ear leaves and the adjacent upper and lower leaves mainly supported the growth and development of ears. Under MD and HD conditions, by contrast, the duration of light intensity >1,200 μmol m−2 s−1 in ear leaves decreased rapidly, and the time during which the photosynthetic rate was higher than 20 μmol m−2 s−1 substantially shortened (Figure 4); moreover, most of the time, the light intensity in the fourth leaf below the ear leaf dropped below the photosynthetic light compensation point, and there was little or no net photosynthesis (Wu et al., 2022). In the present study, while the photosynthetic rate and daily total photosynthetic productivity of ear leaves decreased, they were less able to support the metabolism of other organs in the plant. This may have been the primary cause of the remarkable declines in biomass and ear weight per plant under close planting.
Photoinhibition often occurs when the amount of absorbed light energy exceeds its capacity of utilization and dissipation by the photosynthetic processes (Takahashi and Murata, 2008). When severe photoinhibition occurs, the recovery of NPQ may take several minutes to several hours (Zhu et al., 2004; Kromdijk, 2016; Hubbart et al., 2018; Yamori et al., 2020). Zhu et al. (2004) reported that the daily canopy photosynthesis of field crops could be limited by the slow recovery of NPQ relaxation, while the accelerated rate of NPQ relaxation led to a faster recovery of CO2 assimilation efficiency after leaves were transferred from high light to low light, and finally caused a slight enhancement in biomass under fluctuating light. Therefore, researchers have concluded that slow relaxation of NPQ may be energetically costly, which, in turn, decreases the photosynthetic rate (Kromdijk, 2016; Hubbart et al., 2018; Yamori et al., 2020). In our simulation experiment, high fluctuating frequency (FL1/3 min) caused a slight photoinhibition of PSI and PSII compared with low frequency treatment (FL 1/300 min), while the photoinhibition of both photosystems was hardly affected by medium frequency treatment (FL1/30 min) (Figure 6). Actually, in the field, no more than 15 fluctuations per day (about 1 fluctuation/40–60 min) occurred under HD on sunny days, which was obviously less than that of medium frequency treatment (1 fluctuation/30 min). Obviously, there would have been little photoinhibition of PSI and PSII under HD in the field (Li et al., 2015; Wu et al., 2019). Thus, the rapid component accounts for the vast majority of NPQ and should be able to recover within seconds. Consequently, the NPQ of C4 plants such as maize should have a very slight effect on the photosynthetic rate and ear weight per plant under weak light following high light.
Rational close planting is an important cultivation measure to achieve high yields. This study demonstrates that the high yield produced by a reasonable planting density is related to the duration of high light exposure and photosynthesis. However, various studies analyzing long-term records of surface radiation measurements suggest a widespread decrease in the amount of solar radiation reaching the Earth's surface in recent decades, and the intensity of photosynthetically active radiation has also weakened (Stanhill and Gohen, 2001; Wild, 2009; Wang et al., 2015). Furthermore, extreme cloudy and rainy weather results in the attenuation of light intensity. Considering these factors, maintaining the current planting density in China's main production areas or continuing to increase the planting density is likely to lead to a decline of maize yield in the future. This pattern may also occur in the cultivation of other crops. From the perspective of the light environment, it is likely that the gradual reduction of planting density under global climate change conditions in the future will contribute to the stability of crop yield per unit area. Varieties with high yield per plant or varieties that are more suitable for growing in weak light will become increasingly important.
Conclusion
The results of this study show that although the light fluctuation frequency and the duration of high light both affect the photosynthetic characteristics in maize leaves, the appropriate duration of daily high light under fluctuating light environments can substantially improve the photosynthetic performance, while the regulatory energy dissipation changes only slightly in maize plans. This is a key mechanism by which the photosynthetic productivity and yield of maize can be further enhanced under fluctuating light environments in the field.
Materials and methods
Planting materials and experimental design
Experiment 1: The experiment was conducted from 2016 to 2021 at the Institute of Botany, Chinese Academy of Sciences, Beijing (39°28′–41°25′ N, 115°25′–117°30′ E). The maize (Zea mays) hybrid Zhengdan 958, which is the most widely cultivated hybrid in China, was used in the experiment. Maize was sown on April 30, with 3 plant densities: low planting density (LD) (15,000 plants ha−1), medium planting density (MD) (75,000 plants ha−1), and high planting density (HD) (135,000 plants ha−1), respectively. Normal irrigation and fertilizer management practices were conducted throughout the experiment. The light intensity, gas exchange, yield and biomass per unit area, ear weight, and biomass per plant were measured at the grain-filling stage.
Experiment 2: Experiments were carried out in 2017–2018 at the Institute of Botany of the Chinese Academy of Sciences in Beijing (39°28′–41°25′ N, 115°25′–117°30′ E). Maize seedlings (cv. Zhengdan 958) were used as the experimental material. Before the experiment, maize seeds were removed from cold storage and imbibed on wet culture dishes for 48 h in the dark at 25°C. Germinated seeds were then planted in containers (17 cm in diameter and 20 cm in height) that had seepage pores and were filled with a 1:4 mixture of loess and peat. Normal water and fertilizer management practices were performed to avoid potential nutrient and drought stresses. Potted seedlings were first cultured outside for 1 week. Then, the 1-week-old seedlings were transferred to a greenhouse.
In the greenhouse, the light intensity, which was controlled by LED (Philips, Amsterdam, The Netherlands) using a programmable control (DELIXI, Zhengjiang, China), fluctuated between 2 irradiances, of which the high light and weak light were 1,600 μmol m−2 s−1 and 50 μmol photons m−2 s−1, respectively. Maize seedlings were randomly divided into 3 treatments: (1) FL 1/300 min, in which seedlings were exposed alternately to high light (1,600 μmol m−2 s−1) for 300 min and then to weak light (50 μmol m−2 s−1) for 300 min; (2) FL 1/30 min, in which seedlings were subjected alternately to high light and weak light every 30 min (total 10 fluctuations); (3) FL 1/3 min, in which seedlings were exposed alternately to high light and weak light every 3 min (for a total of 100 fluctuations). In all of the treatments, the cumulative duration of high light and low light was 5 h and the photoperiod was 10 h. The temperature was controlled at 32 ± 2°C during the day and 22 ± 2°C during the night in the experiment. Four weeks later, newly fully expanded leaves (7 or 8 leaves) were used for all measurements (leaf area, specific leaf weight (SLW), gas exchange, and chlorophyll a fluorescence quenching) in maize seedlings.
Experiment 3: Experiments were carried out in 2016–2017 at the Institute of Botany of the Chinese Academy of Sciences in Beijing (39°28′–41°25′ N, 115°25′–117°30′ E). The culture environment before treatment in Experiment 3 were consistent with those in Experiment 2. In the greenhouse, the light intensity was controlled by LED (Philips) using a programmable control (DELIXI, Zhengjiang, China). Maize seedlings were randomly separated into 4 treatments. The durations of high light exposure (1,600 μmol m−2 s−1) on top of weak light (200 μmol m−2 s−1) were 8, 4, 1, and 0 h; these treatments were expressed as HL 8 h, HL 4 h, HL 1 h, and HL 0 h, respectively. Four weeks later, leaf area, SLW, gas exchange, chlorophyll a fluorescence quenching, and the proteome in newly fully expanded leaves (7 or 8 leaves) were determined.
Determination of morphology
Whole plants in the field were carefully sampled, with 5 plants sampled for each treatment. The dry weights (biomass) were measured after drying in an oven at 105°C for 30 min and 85°C for 72 h.
Leaf area was determined using an AM 100 leaf area meter (ADC, Bioscientific Ltd, Hoddesdon, UK). Five replicates were measured for each treatment.
Twenty leaf disks (1 cm in diameter) were drilled from fresh leaves using a perforator. The leaf disks were placed in an oven at 105°C for 30 min, following which they were dried to a constant weight at 85°C. Subsequently, the total dry weight of the leaf disks was measured by using an electronic precision balance, and the SLW (calculated as the leaf dry weight/leaf area) was calculated. Five replicates were performed for each treatment.
Determination of gas exchange
Diurnal variation of light intensity and photosynthesis: The diurnal time course of light intensity and photosynthetic rate were measured every 5 s between 8:30 Am and 4:30 Pm on the ear leaf using an open gas exchange system (Ciras-2, PP Systems, USA). During the procedure, the leaf cuvette maintained a CO2 concentration of 380 ± 20 μmol mol−1, a leaf chamber temperature of 28–33°C, and a humidity of 70%–80%. The leaf chamber was exposed to natural light to measure the daily light intensity and photosynthetic rate. Parameters such as the maximum light intensity, minimum light intensity, changing frequency of high light, duration of high light, average photosynthetic rate, and light-saturated photosynthetic rate were quantified and calculated using Microsoft Excel 2019.
The photosynthetic induction curve: The photosynthetic induction curves of 3 planting densities under a controlled light environment were measured with an open gas exchange system (Ciras-2, PP Systems, USA) between 08:00 and 12:00 on a sunny day. During this process, CO2 and humidity were maintained at 380 ± 20 μmol mol−1 and 75% ± 5%, respectively, with the ambient temperature maintained in the leaf chamber. The light intensity was controlled at 1,600 µmol m−2 s−1. Data were recorded every 5 s. Five repetitions were completed for each planting density.
Light-saturated photosynthetic rate: Gas exchange was measured under an irradiance of 1,600 μmol m−2 s−1 using a portable photosynthesis system (Ciras-2, PP Systems, USA). For all of the measurements, a leaf chamber temperature of 28–33°C, a humidity of 70%–80%, and a CO2 concentration of 380 ± 20 μmol mol−1 were maintained. The net photosynthetic rate (Pn) and stomatal conductance (Gs) in Experiments 2 and 3 were determined between 8:00 and 12:00 on a sunny day. Six replicates were measured for each treatment.
Determination of chlorophyll a fluorescence induction kinetics
Using the method described by Strasser et al. (2004), chlorophyll a fluorescence induction kinetics of maize leaves under fluctuation frequency treatments were determined with a Handy Plant Efficiency Analyzer (Hansatech, UK). Fully dark-adapted leaves (>30 min) were used to determine the chlorophyll a fluorescence transient at 22:00. Chlorophyll a fluorescence transient was recorded during a 1-s pulse of red radiation (3,000 μmol m−2 s−1) provided by an array of 6 light-emitting diodes. The following original data were retained: the maximum fluorescence intensity (Fm) and the fluorescence intensity at 50 μs were considered as minimum intensity (Fo). The maximum quantum yield of PSII (Fv/Fm = (Fm − Fo)/Fm) was calculated according to the method provided by Strasser et al. (2004). Twenty replicates were performed for each treatment.
Determination of chlorophyll a fluorescence quenching
Chl a fluorescence quenching measurement was conducted on intact leaves at room temperature using a pulse-modulated fluorimeter (FMS2, Hansatech, UK). The plants were dark-adapted for 30 min prior to the measurements. The minimal fluorescence (Fo) was determined with the measuring light, while the maximum fluorescence (Fm) was examined after an application of a saturating pulse (SP, 8,000 μmol m−2 s−1). The fluorescence measurement protocol was as follows: dark-adapted leaves were continuously illuminated with actinic light from the FMS-2 light source. The actinic light intensity was 1,600 μmol m−2 s−1. Once a steady state was established, the steady-state fluorescence levels (Fs) and the maximum chlorophyll yield in the light-adapted state (Fm′) were recorded after exposure to actinic light for 20 min and then switching off the actinic light to determine the Fo′ with far-red light. The actual photochemical efficiency of PSII (ФPSII, defined as (Fm′–Fs)/Fm′), the coefficient of photochemical quenching of chlorophyll fluorescence (qP, defined as (Fm′–Fs)/(Fm′–Fo′)), the quantum yield of regulatory energy dissipation (ΦNPQ, defined as Fs/Fm′–Fs/Fm), and the quantum yield of nonregulatory energy dissipation (ΦNO, defined as 1–ФPSII–ΦNPQ) were calculated according to the procedure given by Oxborough and Baker (1997) and Lazar (2015). Six replicates were selected for each treatment.
Determination of the redox state of P700
The redox state of P700 under fluctuation frequency treatments was determined in dark-adapted (30 min) maize leaves using a PAM-101 modulated fluorometer with a dual wavelength emitter-detector ED-P700DW unit and PAM 102 units (Klughammer and Schreiber 1994) as described in detail by Qiao et al (2020) and Kim et al (2005). Far-red light was provided by an FL-101 light source. The P700 maximum oxidation state was evaluated as the absorbance change around 820 nm in a custom-designed cuvette. Six replicates were selected for each treatment.
Proteomic analysis
The samples in Experiments 2 and 3 were used for proteomic analysis. (1) Protein extraction and digestion were performed using the filter-aided sample preparation (FASP) method, as described by Wisniewski et al. (2009). Briefly, 200 μg protein extract was mixed with reducing buffer (10 mM Dl-dithiothreitol, 8 M urea, 100 mM borane-triethylamine complex [TEAB], pH 8.0), and the solution was incubated at 60°C for 1 h. Iodoacetamide was added to reach a final concentration of 50 mM in the dark at room temperature (18°C–22°C) for 10 min. The filter units were centrifuged at 12,000 × g for 20 min, and the flow-through was discarded from the collection tube. Then, 100 μl 100 mM TEAB was added, and the samples were centrifuged at 12,000 × g for 20 min. This step was repeated 3 times. The filter units were transferred to new collection tubes, and 100 μl 100 mM TEAB and 2 μl sequencing-grade trypsin (1 μg/μl) were added to the samples, which were incubated at 37°C for 12 h. The samples were centrifuged at 12,000 g for 20 min, and the peptide was collected. Then, 50 μl 100 mM TEAB was added, and the tube was centrifuged again. The collected solution was mixed again, and the solution was lyophilized. (2) For liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis, the tryptic peptides were fractionated by high pH reverse-phase high-performance liquid chromatography using an Agilent Zorbax Extend-C18 column (2.1 × 150 mm, C18, 5 μm, 120 Å, ChromXP Eksigent, Santa Clara, CA, USA). Briefly, the peptides were eluted at a flow velocity of 300 μl/min. The gradient elution conditions were as follows: 0–8 min, 98% A; 8–8.01 min, 98%–95% A; 8.01–38 min, 95%–75% A; 38–50 min, 75%–60% A; 50–50.01 min, 60%–10% A; 50.01–60 min, 10% A; 60–60.01 min, 10%–98% A; and 60.01–65 min, 98% A. The samples were collected for 8–50 min, and the eluate was collected into the centrifuge tube every other minute. After collecting and freeze-drying, the samples were frozen and stored at −80°C for mass spectrometry analysis. (3) For bioinformatics analysis, the identified proteins were annotated with a common functional database (http://geneontology.org/). The differentially expressed proteins were further analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/pathway.html).
Ear weight and yield
At the physiological maturity stage, 20 ears were selected and removed from the middle 2 rows of each density by manual harvest. These ears were dried and subsequently used to determine the ear weight and grain yield.
Statistical analysis
Data were analyzed using 1-way analysis of variance (ANOVA) and compared with the least significant difference (LSD) multiple comparison test using SPSS (version 25). Differences were significant at P ≤ 0.05. The graphics software SigmaPlot (version 12.5) was used to create the figures.
Contributor Information
Han-Yu Wu, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; Key Laboratory of Oasis Eco-Agriculture, Xinjiang Production and Construction Corps/College of Agronomy, Shihezi University, Shihezi 832003, China.
Mei-Yu Qiao, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.
Ya-Jun Zhang, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.
Wei-Jian Kang, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; State Key Laboratory of Crop Biology, College of Life Sciences, Shandong Agricultural University, Taian, Shandong 271018, China.
Qing-Hu Ma, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.
Hui-Yuan Gao, State Key Laboratory of Crop Biology, College of Life Sciences, Shandong Agricultural University, Taian, Shandong 271018, China.
Wang-Feng Zhang, Key Laboratory of Oasis Eco-Agriculture, Xinjiang Production and Construction Corps/College of Agronomy, Shihezi University, Shihezi 832003, China.
Chuang-Dao Jiang, Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.
Funding
This study was supported by the National Natural Science Foundation of China (31970350, 31571576, and U1803234) and the Strategic Priority Research Program of Chinese Academy of Sciences (XDA26040103). The funders had no role in the study design, data analysis and interpretation, and manuscript writing but just provided the financial support.
References
- Acevedo-Siaca LG, Coe R, Wang Y, Kromdijk J, Quick WP, Long SP (2020) Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol 227(4): 1097–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Tanaka Y, Miyagi A, Kashima M, Tezuka A, Toya Y, Kobayashi S, Ohkubo S, Shimizu H, Kawai-Yamada M, et al. (2019) High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light. J Exp Bot 70(19): 5287–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer B, Ziolkowska A, Bagard M, Keech O, Gardeström P (2012) The impact of light intensity on shade-induced leaf senescence. Plant Cell Environ 35(6): 1084–1098 [DOI] [PubMed] [Google Scholar]
- Dietz KJ (2015) Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J Exp Bot 66(9): 2401–2414 [DOI] [PubMed] [Google Scholar]
- Ferroni L, Živčak M, Sytar O, Kovár M, Watanabe N, Pancaldi S, Baldisserotto C, Brestič M (2020) Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ Exp Bot 178: 104156 [Google Scholar]
- Flannery SE, Hepworth C, Wood WHJ, Pastorelli F, Hunter CN, Dickman MJ, Jackson PJ, Johnson MP (2021) Developmental acclimation of the thylakoid proteome to light intensity in Arabidopsis. Plant J 105(1): 223–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson L, Forster B, Pogson BJ, Chow WS (2005) A simple chlorophyll fluorescence parameter that correlates with the rate coefficient of photoinactivation of photosystem II. Photosynth Res 84(1–3): 43–49 [DOI] [PubMed] [Google Scholar]
- Hubbart S, Smillie I, Heatley M, Swarup R, Foo C, Zhao L, Murchie E (2018) Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun Biol 1(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Chow WS, Lee CH (2005) Photosystem I acceptor side limitation is a prerequisite for the reversible decrease in the maximum extent of P700 oxidation after short-term chilling in the light in four plant species with different chilling sensitivities. Physiol Plant 123(1): 100–107 [Google Scholar]
- Kimura H, Hashimoto-Sugimoto M, Iba K, Terachima I, Yamori W (2020) Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J Exp Bot 71(7): 2339–2350 [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700 + absorbance changes at 830 nm. Planta 192(2): 261–268 [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354(6314): 857–861 [DOI] [PubMed] [Google Scholar]
- Lazár D (2015) Parameters of photosynthetic energy partitioning. J Plant Physiol 175: 131–147 [DOI] [PubMed] [Google Scholar]
- Li T, Liu LN, Jiang CD, Liu YJ, Shi L (2014) Effects of mutual shading on the regulation of photosynthesis in field-grown sorghum. J Photochem Photobiol B 137: 31–38 [DOI] [PubMed] [Google Scholar]
- Li T, Liu YJ, Shi L, Jiang CD (2015) Systemic regulation of photosynthetic function in field-grown sorghum. Plant Physiol Biochem 94: 86–94 [DOI] [PubMed] [Google Scholar]
- Liu GZ, Liu WM, Hou P, Ming B, Yang YS, Guo XX, Xie RZ, Wang KR, Li SK (2021) Reducing maize yield gap by matching plant density and solar radiation. J Integr Agric 20(2): 363–370 [Google Scholar]
- Matsubara S (2018) Growing plants in fluctuating environments: why bother? J Exp Bot 69(20): 4651–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK (1999) PHOTOPROTECTION REVISITED: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50(1): 333–359 [DOI] [PubMed] [Google Scholar]
- Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth Res 54(2): 135–142 [Google Scholar]
- Pao YC, Stützel H, Chen TW (2019) A mechanistic view of the reduction in photosynthetic protein abundance under diurnal light fluctuation. J Exp Bot 70(15): 3705–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao MY, Zhang YJ, Liu LA, Shi L, Ma QH, Chow WS, Jiang CD (2021) Do rapid photosynthetic responses protect maize leaves against photoinhibition under fluctuating light? Photosynth Res 149(1–2): 57–68 [DOI] [PubMed] [Google Scholar]
- Raines CA (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155(1): 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Bolger A, Zeier J, Preiskowski S, Benes V, Trenkamp S, Usadel B, Farré EM, Matsubara S (2019) Fluctuating light interacts with time of day and leaf development stage to reprogram gene expression. Plant Physiol 179(4): 1632–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55(6): 1184–1193 [DOI] [PubMed] [Google Scholar]
- Stanhill G, Cohen S (2001) Global dimming: a review of the evidence for a widespread and significant reduction in global radiation with discussion of its probable causes and possible agricultural consequences. Agric For Meteorol 107(4): 255–278 [Google Scholar]
- Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In Papageorgiou GC, Govindjee eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, pp 321–362 [Google Scholar]
- Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13(4): 178–182 [DOI] [PubMed] [Google Scholar]
- Taylor SH, Long SP (2017) Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philos T R Soc B 372(1730): 20160543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Matthews JSA, Simkin AJ, Raines CA, Lawson T (2017) Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol 173(4): 2163–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Gong W, Hu B, Lin AW, Li H, Zou L (2015) Modeling and analysis of the spatiotemporal variations of photosynthetically active radiation in China during 1961–2012. Renew Sust Energy Rev 49: 1019–1032 [Google Scholar]
- Wei Z, Duan FY, Sun XZ, Song XL, Zhou WB (2021) Leaf photosynthetic and anatomical insights into mechanisms of acclimation in rice in response to long-term fluctuating light. Plant Cell Environ 44(3): 747–761 [DOI] [PubMed] [Google Scholar]
- Wild M (2009) Global dimming and brightening: a review. J Geophys Res 114: D00D16 [Google Scholar]
- Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5): 359–362 [DOI] [PubMed] [Google Scholar]
- Wu HY, Liu LA, Shi L, Zhang WF, Jiang CD (2021) Photosynthetic acclimation during low-light-induced leaf senescence in post-anthesis maize plants. Photosynth Res 150(1–3): 313–326 [DOI] [PubMed] [Google Scholar]
- Wu HY, Qiao MY, Zhang WF, Wang KR, Li SK, Jiang CD (2022) Systemic regulation of photosynthetic function in maize plants at graining stage under vertically heterogeneous light environment. J Integr Agric 21(3): 666–676 [Google Scholar]
- Wu HY, Zhang YJ, Zhang WF, Wang KR, Li SK, Jiang CD (2019) Photosynthetic characteristics of senescent leaf induced by high planting density of maize at heading stage in the field. Acta Agron Sin 45(2): 248–255 [Google Scholar]
- Yabiku T, Akamatsu S, Ueno S (2020) Light reacclimatization of lower leaves in C4 maize canopies grown at two planting densities. Photosynthetica 58(3): 732–739 [Google Scholar]
- Yamori W, Kusumi K, Iba K, Terashima I (2020) Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ 43(5): 1230–1240 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Kaiser E, Marcelis LFM, Yang Q, Li T (2020) Salt stress and fluctuating light have separate effects on photosynthetic acclimation, but interactively affect biomass. Plant Cell Environ 43(9): 2192–2206 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61(1): 235–261 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55(400): 1167–1175 [DOI] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kunderlikova K, Sytar O, Allakhverdiev SI (2015) Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res 126(2–3): 449–463 [DOI] [PubMed] [Google Scholar]