Abstract
Background
Anemia affects more than a quarter of non-pregnant women over the globe, with Sub-Saharan Africa bearing a disproportionate share. Although the use of family planning is beneficial in reducing anemia, lack of scientific study on anemia among family planning users of reproductive-age women is notable, particularly in the study setting. The purpose of this study was to determine the extent of anemia and associated factors in women who used family planning.
Methods
A cross-sectional multi-centered study was conducted from March 3 to 29, 2019, among 443 non-pregnant reproductive age (15 to 49 years) women receiving family planning services in Ambo town. Sample size was calculated using Epi-info version 7 software. Participants were selected by systematic random sampling technique. Trained data collectors collected data using a structured pretested questionnaire, as well as venous blood and stool samples. Epi-Data and SPSS were used to enter and analyze data. The effect of independent variables on the outcome variable was determined by binary logistic regression analysis with adjusted odds ratio at 95% confidence interval and 5% margin of error. P-value <0.05 was used to declare statistical significance.
Results
This study revealed 28% (95% CI:23.9%, 32.3%) magnitude of anemia. Age of 25–35 years [AOR:2.84, 95% CI:1.74, 4.64], implantable family planning method [AOR: 0.34, 95% CI: 0.12, 0.96], no previous use of family planning [AOR:2.62, 95% CI: 1.62, 4.24], household food insecurity [AOR: 2.04, 95% CI: 1.06, 3.93], parasite infestations [AOR:2.01, 95% CI: 1.12, 3.63], and regular intake of coffee/tea within 30 minutes post meal [AOR:3.85, 95% CI:1.24, 11.92] were independently associated with anemia.
Conclusion
Anemia is a moderate public health concern among reproductive-age women receiving family planning services in the study area. There are missed opportunities to address the anemia burden during family planning services. This study emphasizes the importance of nutritional screening for early detection and targeted interventions for healthcare workers in reducing missed opportunities to prevent and control anemia in vulnerable populations.
Keywords: anemia, family planning, reproductive age women, Ambo, Ethiopia
Introduction
Anemia is a worldwide public health concern and a major challenging problem in resource-limited countries.1,2 Over one-third of the world population is deficient in single or multiple micronutrients mainly due to inadequate intake.3–5 More than 2.2 billion of the world’s population are affected by anemia at a rate of 33%.6 Pregnant and non-pregnant women of reproductive age (WRA) (15–49 years) and children compose the population group most affected by anemia.7 It affects 273 million (43%) world children, 496 million (30%) of WRA, and 32 million (38%) pregnant women over the globe.6,8,9 This burden is disproportionately concentrated in low socioeconomic groups.9
Anemia is considered unacceptable in any population group or subgroup when the overall prevalence exceeds 5%, which is mostly reflected in LMICs.10 Across LMICs, a great variation in the overall prevalence of anemia in WRA is notable The highest prevalence was reported from countries in the Middle East, South Asia and West African regions in 2018.11 Countries like Yemen (57.4%), India (49.9%), Gambia (50.3%), Senegal (47.3%) and Mali (47.6%) were among the others with high prevalence rates of anemia.11 Ethiopia is one of the countries with the highest rates of anemia in Africa.11 In Ethiopia, the rate of anemia prevalence in WRA was reported to be 23.4% in 2016, nearly half the rate reported in 1990 (48%).12,13
Anemia prevalence among NPWs ranges from 37.7% to 41.5% across the WHO regions of South-East Asia, the Eastern Mediterranean, and Africa.14 Iron deficiency contributes to more than half of the global anemia burden and is caused by insufficient intake, increased requirements, and chronic blood loss.14 Under nutrition in pregnant women can set in motion and perpetuate a cycle of intergenerational malnutrition, infection, and underdevelopment.5 This intern has an impact on the individual and population’s productivity and economic potential.5 Anemia during pregnancy is linked to poor birth outcomes such as maternal and neonatal mortality, as well as impaired and delayed cognitive development in children.15,16 In addition, increased risk of infections, impaired cognitive and physical development as well as poor school performance among offspring are associated with anemia.17
Failing to invest in interventions to reduce anemia among NPWs is estimated to result in 265 million more cases of anemia in women in 2025 than in 2015, as well as nearly 800,000 more child deaths and over 7000–14,000 more maternal deaths.18 In addition to negative health consequences, anemia’s economic impact on human capital results in annual losses of billions of dollars.9 Investing in anemia prevention, on the other hand, could increase the economic productivity of LMIC by $110 billion. Economic returns on investment in this set of interventions are estimated to be $12 for every $1 invested.18
Nutrition, socioeconomic status, health care seeking practice, poor dietary practice, hygiene and sanitary practice, Obstetric and gynecologic factors, health condition, type of family planning used, and women’s BMI are all factors known to contribute to the problem.2,19–26 This problem is exacerbated in Ethiopia by pre-existing food insecurity.27 Unequal household food allocation can expose WRA to anemia because they may lack access to iron-rich foods, particularly in areas where a culture favors older boys, men, and elders, and bias in household food allocation.5,27 The type of women’s diet was also found to influence their risk of developing anemia. In one study, the risk of developing anemia was higher among vegetarians (64.9%) than mixed diet consumers (49.5%) which might be due to low intake of heme iron.28
The use of family planning has been shown to help with anemia. This could be by extending or lengthening the intervals and inhibiting pregnancies. Although hemoglobin levels vary depending on the contraceptive method used, users of any contraceptive method had higher hemoglobin levels than non-users.22,29,30 Studies conducted so far in Ethiopia have shown that non-users of contraceptive methods have a higher prevalence of anemia than contraceptive users WRA.30 A cross-sectional community-based study conducted in four major regions of Ethiopia found that women who used no family-planning methods had a slightly higher prevalence of anemia (37.4%) than their counterparts.25
Using contraceptive methods is associated with a lower risk of anemia. In one study, anemia was 1.38 times more likely among non-users of contraception (21.7%) compared to contraceptive users (11.6%).31 Analytic study of the 2016 EDHS data revealed that the likelihood of developing anemia was 1.5 times (p=0.02) higher among non-pregnant WRA who did not use contraceptive methods than those who used contraception.32 In other studies, not using contraceptive methods was found to be an independent factor associated with anemia in NPW of reproductive age.22,26
Despite this benefit, a significant scarcity of scientific studies and data that estimate the magnitude of anemia and associated risk factors among family planning users NPW of reproductive age is notable Thus, the aim of this study was to determine the extent of anemia and its potential risk factors associated among family planning users NPW of reproductive age in Ambo town, thereby making family planning service a window of opportunity.
Methods and Materials
Study Period and Setting
This study was carried out in central Ethiopia in Ambo town public health institutions. Ambo town is the capital of the West Shewa zone, one of the zones in the regional state of Oromia, and is found 114 kilometers from Addis Ababa (the country’s capital city) in the western direction. The town has six kebeles of which three are rural and three are urban kebeles (the last unit of administration in Ethiopia). The town’s total population was 108,000 as of 2018 with 53,400 males and 54,600 females. In Ambo Town, there are thirteen public health institutions of which two are hospitals (1 referral and 1 general), two are health centers and nine are health posts. Twenty-one private clinics are also available in the town.33,34 The study was carried out from March 3 to 29, 2019 in each two public health centers and hospitals.
Populations and Design
A Multicenter institutional-based cross-sectional study design was used among reproductive age (15 to 49 years) women receiving family planning services in Ambo town. All reproductive-age women receiving family planning services in health facilities of the general public in Ambo town were the source population of the current study whereas reproductive-age women receiving family planning services during the study period and fulfilling the inclusion criteria were the study population. However, those women who came for family planning services for the first time, had a history of abortion in the previous three months, gave birth in the previous three months, had a blood transfusion in the previous three months, and were on anemia treatments were excluded.
Sample Size and Sampling Methods
The sample size was calculated using Epi-info version 7 software with a 95% confidence interval, 80% power, and a 1:1 ratio of unexposed to exposed groups based on the type of family planning method used. Available revealed that the proportions of exposed (Barrier methods) and unexposed (Implant) were 41.0% and 27.7%, respectively.35 This results in a sample of 428. Adding a 10% non-response rate yielded a sample of 471. Finally, 471 participants were recruited for this study. However, data were gathered from 443 women who attended family planning clinics.
Measurements and Definitions
Socioeconomic and demographic characteristics (sex, age, educational and literacy status, presence of under-five children in the household, residence, occupation, household income), housing conditions (number of family members in the household, and household sanitation), nutrition and food-related conditions (food security and dietary diversity status), and women’s health conditions (menstruation, pregnancies, history of miscarriages, and body mass index) were independent variables.
Body mass index- is a simple weight-to-height index that is age-independent for adult populations and is the same for both genders. Weight in kilograms divided by height in meters squared (kg/m2) is calculated and classified as follows: <18.5 kg/m2 indicates underweight, 18.5–24.9 kg/m2 indicates normal weight, 25.0–29.9 kg/m2 indicates overweight, and 30.0 kg/m2 indicates obesity.15
Data Collection Methods and Procedures
Socio-demographic, obstetric/gynecologic, food and nutritional, anthropometric measurements, and health-related clinical data were collected by trained clinical nurses using a structured and pretested interviewer-administered questionnaire developed after a review of various pieces of literature,22,37 while four experienced and trained laboratory technicians collected laboratory data. Data were collected in the local language (Afan Oromo) version of data collection tool. Women’s dietary diversity score (WDDS) data were collected using a 24 hrs dietary diversity questionnaire and were analyzed by calculating women’s dietary diversity score from 10 food groups proposed by FANTA to calculate dietary diversity score for women.35–37 Women who scored five or more were classified as having high dietary diversity whereas those scored lower than five were classified as low dietary diversity.37 Household food security data were collected by the HFIAS tool developed by FANTA.35,37–40
Four milliliters of venous blood samples were collected from each study participant. Samples collected from both health centers (Ambo and Awaro) using 4mL of K3 EDTA (ethylenediamine-tetraacetic acid) tube were taken to Ambo general hospital within four hours of collection for analysis.12,14,41,42 Trained laboratory technicians analyzed the samples. Hemoglobin concentration was measured using CELL-DYN 1800® (Abott, USA),12,14,41,42 and it was adjusted only for altitude since there was no smoking reported. Hemoglobin concentration adjustment for altitude was done to subtract the adjustment from the measured Hb concentration at the relevant altitude above sea level to get the sea-level value.10,42–44 Then anemia was diagnosed based on hemoglobin concentration (Hb < 12g/dL) for non-pregnant adult women.8,41,45 Finally, women with a hemoglobin concentration less than 12g/dl were classified as anemic and those with 12g/dl and above were classified as non-anemic.8
Five grams of stool sample was collected from each study participant for parasitological data using clean, wide-mouthed, and leak-proof stool cups. The stool sample was then examined by wet mount preparation within 10–15 minutes of collection. To determine the presence of parasites in the stool, leftover samples were processed for the formol-ether concentration technique.22
Data Quality Management
A structured and pretested interviewer-administered questionnaire which was developed after a review of various pieces of literature,22,37 was used to collect data. The tool was initially prepared in English and then translated into Afan Oromo’s local language, then translated back to English by language experts to check for language consistency, and the Afan Oromo version questionnaires were used for data collection. The data collection tool was pre-tested on 5% of the sample size and necessary amendments were done based on the results before actual data collection. Data collectors were four clinical nurses and four laboratory technicians and they received one-day training on the study’s objectives, data collection methods, and tools. Two supervisors and a principal investigator closely supervised the data collection process. Experienced and trained laboratory technicians collected laboratory-related data and carried out all activities following the manufacturer’s instructions and specific standard operating procedures. The expiry dates of all reagents and quality control materials were checked. Laboratory results were documented using standard reporting formats and unique identification numbers. The collected data were rechecked daily at the end of each day to ensure completeness and consistency.
Data Analysis and Processing
All checked data were coded, entered into Epi-Data version 3.1, cleaned, and exported to SPSS (version 23) for analysis. Data were summarized using descriptive statistics such as frequencies and percentages. Variables with p-values less than 0.20 in the bivariable logistic regression analysis were considered for multivariable logistic regression analysis. The logistic regression model’s fitness was determined using Hosmer-Lemeshow, and it was declared statistically insignificant.
Results
Socio-Demographic Characteristics
Four hundred seventy-one (471) reproductive-age women who received family planning services were considered for this study and 443 women participated in the survey with a 94.05% response rate. The majority of study participants, 415 (93.7%), were under the age of 35 (Table 1).
Table 1.
Socio-Demographic Characteristics of Women Receiving Family Planning Service in Public Health Facilities in Ambo Town, Central Ethiopia, 2019 (n=443)
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Age of the women | 15–24 | 199 | 44.9 |
| 25–35 | 216 | 48.8 | |
| 36–49 | 28 | 6.3 | |
| Religion | Orthodox | 139 | 31.4 |
| Muslims | 24 | 5.4 | |
| Protestant | 273 | 61.6 | |
| Wakefata | 7 | 1.6 | |
| Marital status | Married | 406 | 91.6 |
| Divorced | 9 | 2.0 | |
| Widowed | 8 | 1.8 | |
| Single | 14 | 3.2 | |
| Separated | 6 | 1.4 | |
| Women’s educational status | No formal education | 123 | 27.8 |
| Primary education | 146 | 33.0 | |
| Secondary and above | 174 | 39.3 | |
| Women’s occupational status | House maker | 279 | 63.0 |
| Government employee | 78 | 17.6 | |
| Merchant | 42 | 9.5 | |
| Daily laborer | 44 | 9.9 | |
| Husband’s educational status | No formal education | 89 | 21.9 |
| Primary education | 104 | 25.6 | |
| Secondary and above | 213 | 52.5 | |
| Husband’s occupational status | Farmer | 72 | 17.7 |
| Government employee | 146 | 36.0 | |
| Merchant | 66 | 16.3 | |
| Daily laborer | 91 | 22.4 | |
| Driver | 31 | 7.6 | |
| Family income(ETB) | <1000 | 94 | 21.2 |
| 1000 and above | 349 | 78.8 | |
| Family size | <5 | 360 | 81.3 |
| >5 | 83 | 18.7 | |
| Under five children in the HH | None | 87 | 19.6 |
| 1 | 308 | 69.5 | |
| 2 and above | 48 | 10.8 | |
| Under two children in the HH | Yes | 270 | 60.1 |
| No | 173 | 39.9 | |
| Source of drinking water | Protected | 402 | 90.7 |
| Unprotected | 41 | 9.3 | |
| ITN use during night | Yes | 224 | 50.6 |
| No | 219 | 49.4 | |
| Toilet facility | Yes | 413 | 93.2 |
| No | 30 | 6.8 |
Abbreviations: HH, Households; ITN, Insect treated nets.
Obstetric, Gynecologic, and Reproductive Characteristics
The majority of women, 249 (56.2%), used injectable contraception, whereas IUCD was used by only 23 (5.2%), and two-thirds, 147 (66.8%), had a birth interval of more than two years (Table 2).
Table 2.
Obstetric and Gynecologic Characteristics of Women Receiving Family Planning Service in Public Health Facilities in Ambo Town, Central Ethiopia, 2019 (n=443)
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Duration of menses in days | ≤5 | 393 | 88.7 |
| >5 | 50 | 11.3 | |
| Number of sanitary pads used daily during menses | ≤2 | 315 | 71.1 |
| >2 | 128 | 28.9 | |
| Parity | None | 56 | 12.6 |
| 1–2 | 249 | 56.2 | |
| 3–5 | 120 | 27.1 | |
| >5 | 18 | 4.1 | |
| Number of live births | ≤2 | 266 | 68.7 |
| 3–5 | 108 | 27.9 | |
| >5 | 13 | 3.4 | |
| Place of delivery | Home | 45 | 11.6 |
| Institutional | 342 | 88.4 | |
| Birth spacing in years | ≤2 | 73 | 33.2 |
| >2 | 147 | 66.8 | |
| Breast feeding | Yes | 228 | 58.9 |
| No | 159 | 41.1 | |
| Duration of breast feeding | 3–11 months | 117 | 51.3 |
| 12–23 months | 107 | 46.9 | |
| 24 months and above | 4 | 1.7 | |
| ANC follow up | Yes | 349 | 90.2 |
| No | 38 | 9.8 | |
| Got IFA supplementation | Yes | 341 | 97.7 |
| No | 8 | 2.3 | |
| Type of FP being used | OCP | 66 | 14.9 |
| Injectable | 249 | 56.2 | |
| Implantable | 105 | 23.7 | |
| IUCD | 23 | 5.2 | |
| Any FP used previously | Yes | 296 | 66.8 |
| No | 147 | 33.2 |
Abbreviations: ANC, Antenatal care; FP, Family planning; IFA, Iron-folic acid; FP, Family planning; OCP, Oral Contraceptive Pill; IUCD, Intra-Uterine Contraceptive Device.
Nutrition and Dietary Characteristics
More than half of the participants, 241 (54.4%), had consumed any form of grain-based meal on a daily basis in the seven days preceding data collection, while vegetables, fruits, and animal sources were consumed less frequently. Almost all of the 417 respondents (94.1%) said they had enough food diversity. The vast majority (389, or 87.8%) stated that they come from food-secure homes (Table 3).
Table 3.
Nutrition and Dietary Related Characteristics of Women Using Family Planning Methods at Public Health Facilities in Ambo Town, Central Ethiopia, 2019 (n=443)
| Variable | Category | Frequency | Percentage |
|---|---|---|---|
| Intake of food made from cereals/grains in the last seven days | Once | 8 | 1.8 |
| Twice | 31 | 7.0 | |
| Often | 163 | 36.8 | |
| Always | 241 | 54.4 | |
| Intake of food made from pulses in the last seven days | Never | 5 | 1.1 |
| Once | 39 | 8.8 | |
| Twice | 160 | 36.1 | |
| Often | 185 | 41.8 | |
| Always | 54 | 12.2 | |
| Intake of vegetables in the last seven days | Never | 17 | 3.8 |
| Once | 116 | 26.2 | |
| Twice | 168 | 37.9 | |
| Often | 115 | 26.0 | |
| Always | 27 | 6.1 | |
| Intake of fruits in the last seven days | Never | 100 | 22.6 |
| Once | 154 | 34.8 | |
| Twice | 94 | 21.2 | |
| Often | 73 | 16.5 | |
| Always | 22 | 5.0 | |
| Intake of meats in the last seven days | Never | 116 | 26.2 |
| Once | 156 | 35.2 | |
| Twice | 107 | 24.2 | |
| Often | 48 | 10.8 | |
| Always | 16 | 3.6 | |
| Intake of eggs in the last seven days | Never | 83 | 18.7 |
| Once | 123 | 27.8 | |
| Twice | 113 | 25.5 | |
| Often | 99 | 22.3 | |
| Always | 25 | 5.6 | |
| Intake of dairy products in the last seven days | Never | 122 | 27.5 |
| Once | 79 | 17.8 | |
| Twice | 84 | 19.0 | |
| Often | 79 | 17.8 | |
| Always | 79 | 17.8 | |
| Intake of sugar or honey in the last seven days | Never | 58 | 13.1 |
| Once | 75 | 16.9 | |
| Twice | 111 | 25.1 | |
| Often | 67 | 15.1 | |
| Always | 132 | 29.8 | |
| Intake of oil, fats or butter in the last seven days | Never | 33 | 7.4 |
| Once | 42 | 9.5 | |
| Twice | 79 | 17.8 | |
| Often | 78 | 17.6 | |
| Always | 211 | 47.6 | |
| Dietary diversity status | Adequate | 417 | 94.1 |
| Low | 26 | 5.9 | |
| Food security status | Food secure | 389 | 87.8 |
| Food insecure | 54 | 12.2 | |
| BMI(Kg/m2) | <18.5 | 32 | 7.2 |
| 18.5–25 | 411 | 92.8 |
Abbreviation: BMI, Body mass index.
Health and Lifestyle-Related Characteristics
The majority of study participants, 407 (91.9%), were free of sickness for at least two weeks previous to data collection, whereas 66 (14.9%) had one or more intestinal parasites. G.lambia (28.4%), H.worm (23.9%), A.lumbericoids (22.4%), and E.histolytica (16.4%) were the most prevalent parasites detected (Table 4).
Table 4.
Health and Lifestyle-Related Characteristics of Women Receiving Family Planning Service in Public Health Facilities in Ambo Town, Central Ethiopia, 2019 (n=443)
| Variables | Categories | Frequency | Percentage |
|---|---|---|---|
| Intake of coffee/tea after meal within 30 minutes regularly | Yes | 15 | 3.4 |
| No | 428 | 96.6 | |
| Intake of alcohol in the last seven days | Yes | 44 | 9.9 |
| No | 399 | 90.1 | |
| Smoking | Yes | 0 | 0 |
| No | 443 | 100 | |
| Chronic illness | Yes | 36 | 8.1 |
| No | 407 | 91.9 | |
| Acute illness | Yes | 28 | 6.3 |
| No | 415 | 93.7 | |
| Intestinal parasite | Yes | 66 | 14.9 |
| No | 377 | 85.1 | |
| Type of intestinal parasite | H.worm | 16 | 23.9 |
| A.lumbericoids | 15 | 22.4 | |
| E.histolytica | 11 | 16.4 | |
| G.lambia | 19 | 28.4 | |
| E.vermicularis | 2 | 3.0 | |
| T.trichuria | 2 | 3.0 | |
| T.saginata | 2 | 3.0 |
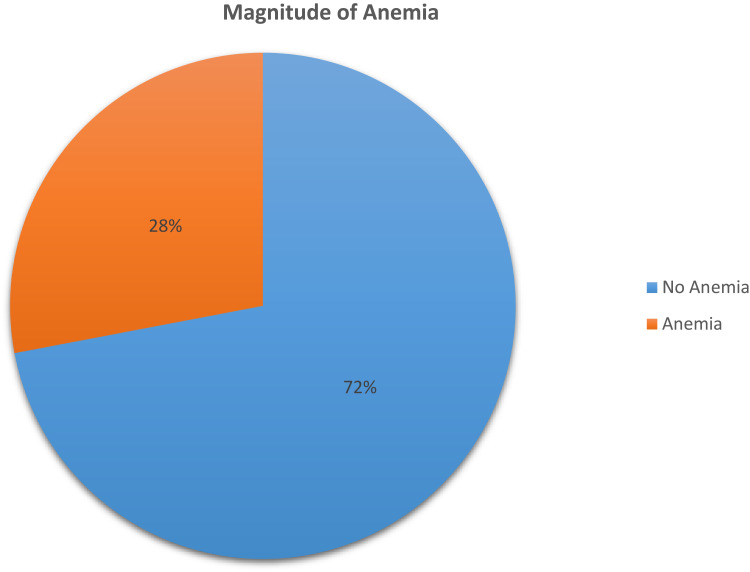
Magnitude of Anemia
In this study, the prevalence of anemia was 28.0% (95% CI: 23.9, 32.3). This prevalence was 2.84 times higher among women aged 25–35 years (AOR: 2.84, 95% CI: 1.74, 4.64) than among women aged 15–24 years (AOR: 2.84, 95% CI: 1.74, 4.64) (Figure 1).
Figure 1.
Magnitude of anemia among women using family planning in Ambo town, Ethiopia, 2019.
Independent Factors Associated with Anemia
In the multivariate analysis, women who used an implantable form of family planning device were 66% less chance of developing anemia [AOR= 0.34, 95% CI: (0.12, 0.96)] than women who used IUCD. Women who had not previously used any type of family planning had 2.62 (AOR=2.62, 95% CI: 1.62, 4.24) higher odds of developing anemia than women who had previously used family planning. Women from food-insecure households had twice [AOR= 2.04, 95% CI: 1.06, 3.93] higher odds of developing anemia than those from food-secure households. Regularly taking coffee or tea within 30 minutes of post-meal was associated with four [AOR= 3.85, 95% CI: 1.24, 11.92] times higher chance of developing anemia. The study also revealed that having an intestinal parasite infection substantially doubles the risk of anemia [AOR= 2.01, 95% CI: 1.12, 3.63] (Table 5).
Table 5.
Factors Associated with Anemia Among Women Receiving Family Planning Service in Public Health Facilities in Ambo Town, Central Ethiopia, 2019 (n=443)
| Variables | Categories | Anemia | COR 95% CI | AOR 95% CI | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Age of the women | 15–24 | 37 (29.8%) | 162 (50.8%) | 1 | |
| 25–35 | 76 (61.3%) | 140 (43.9%) | 2.38 (1.51, 3.74) | 2.84 (1.74, 4.64)* | |
| 36–49 | 11 (8.9%) | 17 (5.3%) | 2.83 (1.23, 6.55) | 2.48 (0.96, 6.38) | |
| Educational status of the women | No formal education | 46 (37.1%) | 77 (24.1%) | 2.46 (1.46, 4.15) | 1.65 (0.89, 3.06) |
| Primary education | 44 (35.5%) | 102 (32.0%) | 1.78 (1.06, 2.97) | 1.69 (0.97, 2.93) | |
| Secondary and above | 34 (27.4%) | 140 (43.9%) | 1 | ||
| Under two children in the house hold | Yes | 66 (53.2%) | 204 (63.9%) | 1.56 (1.02, 2.37) | 1.34 (0.80, 2.20) |
| No | 58 (46.8%) | 115 (36.1%) | 1 | ||
| Toilet facility | Yes | 111 (89.5%) | 302 (94.7%) | 1 | |
| No | 13 (10.5%) | 17 (5.3%) | 2.08 (0.98, 4.42) | 1.25 (0.40, 3.17) | |
| Sanitary pads used daily during menses | 1–2 | 80 (64.5%) | 235 (73.7%) | 1 | |
| >2 | 44 (35.5%) | 84 (26.3%) | 1.54 (0.99, 2.40) | 1.46 (0.90, 2.36) | |
| Parity | None | 11 (8.9%) | 45 (14.1%) | 1 | |
| 1–2 | 66 (53.2%) | 183 (57.4%) | 1.48 (0.72,3.02) | 2.33 (0.91, 5.95) | |
| 3–5 | 41 (33.1%) | 79 (24.8%) | 2.12 (0.99,4.54) | 2.17 (0.74, 6.42) | |
| >5 | 6 (4.8%) | 12 (3.8%) | 2.05 (0.63,6.67) | 1.17 (0.25, 5.42) | |
| Type of family planning being used | OCP | 17 (13.7%) | 49 (15.4%) | 0.32 (0.12, 085) | 0.72 (0.23, 2.24) |
| Injectable | 75 (60.5%) | 174 (54.5%) | 0.40 (0.17, 0.94) | 0.66 (0.25, 1.75) | |
| Implantable | 20 (16.1%) | 85 (26.6%) | 0.22 (0.08, 0.56) | 0.34 (0.12, 0.96)* | |
| IUCD | 12 (9.7%) | 11 (3.4%) | 1 | ||
| Any other type of FP used previously | Yes | 69 (55.6%) | 227 (71.2%) | 1 | |
| No | 55 (44.4%) | 92 (28.8%) | 1.97 (1.28, 3.02) | 2.62 (1.62, 4.24)* | |
| Food security | Food secure | 103 (83.1% | 286 (89.7%) | 1 | |
| Food insecure | 21 (16.9%) | 33 (10.3%) | 1.77 (0.98, 3.20) | 2.04 (1.06, 3.93)* | |
| Intake of coffee/tea after a meal | Yes | 9 (7.3%) | 6 (1.9%) | 4.08 (1.42, 11.72) | 3.85 (1.24, 11.92)* |
| No | 115 (92.7%) | 313 (98.1%) | 1 | ||
| Chronic illness | Yes | 15 (12.1%) | 21 (6.6%) | 1.95 (0.97, 3.93) | 1.39 (0.65, 2.98) |
| No | 109 (87.9%) | 298 (93.4%) | 1 | ||
| Intestinal parasite | Yes | 28 (22.6%) | 38 (11.9%) | 2.16 (1.26, 3.70) | 2.01 (1.12, 3.63)* |
| No | 96 (77.4%) | 281 (88.1%) | 1 | ||
Note: *Significant at P-value <0.05.
Abbreviations: COR, Crude odds ratio; AOR, Adjusted odds ratio; CI, Confidence interval; FP, Family planning; OCP, Oral Contraceptive Pill; IUCD, Intra Uterine Device.
Discussion
Anemia affects 28% of the people in the study area. According to the study, the age of the women, history of previous family planning use, type of family planning method being used, household food security status, intestinal parasite infection, and coffee or tea consumption within thirty minutes post meal were identified as independent factors associated with anemia. Participants who were found to be anemic and harboring intestinal parasites were treated with the consultation of physicians as needed.
Anemia affects 28% of the people in this study. This finding is comparable to those in Serbia (27.7%),46 Ethiopia in which 30.4% WRA25 and 26.3% NPW30 of reproductive age women were anemic. The prevalence observed in this study is higher than that reported in Brazil41(18.6%) and Jordan (19.3%).47 This might be due to the difference in the socio-economic status of the population and the nutrition intervention programs the countries were undertaking. Other studies in Ethiopia reported a prevalence lower than this study.22,48 The difference might be attributed to the method used to assess the concentration of hemoglobin.49 However this finding is lower than study conducted in Tanzania,50 Sierra Leone51 and Côte d’Ivoire,52 and contraceptive users from Sub-Saharan African countries and Egypt,20,53,54 India,55 Pakistan,2 and Bangladesh.56 The discrepancy might be because the country’s inflammation exposure status in the first two51 while it might be the time gap in the latter studies. Further, the population difference in socio-economic and demographic background and the countries’ higher prevalence rate might be possible reasons for the variations.
In current study, women between the ages of 25 and 35 were more affected by anemia than women between the ages of 15 and 24. This could be because women between the ages of 25 and 35 might experience repeated pregnancies and deliveries and less likely to use health care services than those aged 15–24 years, which increases the risk of anemia. The Tanzanian study and other Ethiopian investigations support this finding.22,50 In contrast, a Brazilian study found that being under the age of 19 increased the risk of anemia.41 This difference could be attributable to the fact that the more preventive measures among WRA has been done by Brazilian government than our country.41
The study also revealed that no previous use of family planning is connected with higher anemia. Women who had no previous use of family planning were experienced higher proportion of anemia. This is supported by Indian study.28 This could be because women who previously employed family planning methods had extended birth spacing that can help them to have good adequate nutrition by inhibiting pregnancy, delivery, and breastfeeding associated risks.
The type of family planning method used has showed association with anemia in this study. Women who used an implantable type of family planning method were less affected by anemia than those who used an IUCD. This is consistent with studies in Tanzania57 and Sub-Saharan African countries.58 This could be because hormonal contraceptives lessen the risk of anemia by reducing excessive bleeding, which is a non-contraceptive advantage of hormonal contraceptives.53,57 On the other hand, an increase in menstrual flow with the use of IUCD can cause a drop in hemoglobin concentration and contribute to anemia.
Moreover, anemia was connected with household food insecurity among family planning users in this study. Similar finding was evidenced in studies conducted in Pakistan2 and Bangladesh.56 This could be because women from food-insecure households were unable to meet their higher body demands. It could be related to unbalanced and biased household food allocation that favors older boys, males, and seniors, making WRA vulnerable to anemia because of low access or insufficient intake.5
Further, consistent with other studies,22,24,25,28 the current study discovered that study participants infected with intestinal parasites had a higher proportion of anemia. This could be because intestinal parasites compete for nutrients and contribute to increased loss and malabsorption of iron, and red cell destructions.22,28
Finally, the proportion of anemia was connected with drinking coffee or tea within thirty minutes after a meal. Women who reported regular consumption of either coffee or tea within 30 minutes post meal had about four times higher proportion of anemia than those who did not. This might be due to the fact that coffee and tea have inhibitory effects on iron absorption. Anemia is a moderate public health concern for women receiving family planning services based on the WHO cut-off values for NPW of reproductive age.42 Even though family planning services create a window of opportunities that help avoid anemia among NPW, there are missed opportunities to prevent and control anemia among family planning users.
Strengths and Limitations
The study used primary data and large sample size, and discovered a window of opportunity to prevent and control anemia among WRA. Despite these merits, women who used family planning at health posts, on the other hand, were not included in the study, which might have underestimated the prevalence of anemia. Although efforts made to reduce recall bias by probing, there may still be recall bias because study participants were asked about past occurrences. Furthermore, the time of data collection, which occurred during post-harvest in the local area, could have an impact on household food security status.
Conclusions
Anemia is a moderate public health concern among WRA receiving FP services in the study setting. Being in the age of 25–35 years, having no previous use of the FP method, household food insecurity, harboring intestinal parasites, having a regular intake of coffee or tea within 30 minutes after a meal, and using an implantable type of FP method were independent factors significantly connected with the magnitude of anemia. The study nude that opportunities to address the burden of anemia during FP services were missed. As a result, this study urges all stakeholders to prioritize incorporating and integrating proactive strategies such as nutritional screening, nutritional education, and counseling programs as standard practice in all healthcare facilities. A comparative study is also recommended to determine the magnitude of anemia from other direction in the study setting.
Acknowledgments
The authors expressed their heartfelt gratitude and humble appreciation to Haramaya University for giving ethical permission and financially supporting data collection. All study respondents, data collectors, and supervisors who made this study feasible were also grateful. Finally, all cooperative staffs of the Ambo town health bureau and public health facilities, as well as all individuals who contributed directly or indirectly to the accomplishment of the study were thankful.
Funding Statement
This work was financially supported by Haramaya University. The funder had no role in the study selection, data collection, analysis, conclusion, interpretation and manuscript writing.
Abbreviations
AOR, Adjusted Odds Ratio; CI, Confidence Interval; COR, Crude Odds Ratio; EDHS, Ethiopian Demographic Health Survey; FANTA, Food and Nutrition Technical Assistance Project; FAO, Food and Agriculture Organization of the United Nations; FCS, Food Consumption Score; FP, Family planning; HDDS, Household Dietary Diversity Score; HFIAS, Household Food Insecurity Access Scale; IUCD, Intra Uterine Contraceptive Device; LMICs, Low and middle-income countries; MDD, Minimum Dietary Diversity; MDD-W, Minimum Dietary Diversity for Women of Reproductive Age; MOH, Ministry of Health; NPW, Non-pregnant women; OCP, Oral Contraceptive Pills; WDDS, Women Dietary diversity score; WRA, Women of Reproductive Age.
Data Sharing Statement
All datasets pertinent to the work are included within the article. Detailed raw data analyzed for the work reported can be available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
This research was carried out in accordance with the Helsinki Declaration. Haramaya University’s Institutional Health Research and Ethics Review Committee granted ethical approval. All study participants aged 18-49 years provided written, voluntarily signed consent and parental informed consent was obtained for study participants aged 15–18-year-old after being informed about the study’s goal, risks, and benefits. No personal identifiers used throughout the execution of the study. Standard safety measures were implemented strictly during the data collection process.
Author Contributions
All authors made substantial intellectual contributions to the work reported, whether in the conception, study design, execution and acquisition of data, analysis, and interpretation, or in all of these parts; participated in drafting the manuscript, critical review or revision of the article; have read and approved the final version; selected and settled the journal to which the article submitted; and approved accountability in all aspects of the work reported.
Disclosure
The authors declare that there is no financial or other competing interest that can be constructed as a potential conflict of interest in the work reported.
References
- 1.World Health Organization. Monitoring the Building Blocks of Health Systems, a Handbook of Indicators and Their Measurement Strategies. World Health Organization; 2010. [Google Scholar]
- 2.Soofi S, Khan GN, Sadiq K, et al. Prevalence and possible factors associated with anaemia, and vitamin B 12 and folate deficiencies in women of reproductive age in Pakistan, analysis of national-level secondary survey data. BMJ open. 2017;7(12):e018007. doi: 10.1136/bmjopen-2017-018007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF. Micronutrients; 2018. Available from: https://www.unicef.org/nutrition/index_iodine.html. Accessed November 25, 2018.
- 4.World Health Organization, UNICEF. Preventing and Controlling Micronutrient Deficiencies in Populations Affected by an Emergency. Organization WH, UNICEF; 2007:2. [Google Scholar]
- 5.Girard AW, Self JL, McAuliffe C, Olude O. The effects of household food production strategies on the health and nutrition outcomes of women and young children, a systematic review. Paediatr Perinat Epidemiol. 2012;26:205–222. doi: 10.1111/j.1365-3016.2012.01282.x [DOI] [PubMed] [Google Scholar]
- 6.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilbert J. The world health report 2002–reducing risks, promoting healthy life. Educ Health. 2003;16(2):230. doi: 10.1080/1357628031000116808 [DOI] [PubMed] [Google Scholar]
- 8.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011, a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi: 10.1016/S0140-6736(10)62304-5 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. World Health Organization; 2011. [Google Scholar]
- 11.Kinyoki D, Osgood-Zimmerman AE, Bhattacharjee NV, Kassebaum NJ, Hay SI, Hay SI. Anemia prevalence in women of reproductive age in low-and middle-income countries between 2000 and 2018. Nat Med. 2021;27(10):1761–1782. doi: 10.1038/s41591-021-01498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WorldBank. Prevalence of anemia among women of reproductive age (% of women ages 15–49); 2018. Available from: https://data.worldbank.org/indicator/SH.ANM.NPRG.ZS. Accessed November 25, 2018.
- 13.EDHS. Ethiopian demographic and health survey; 2016. Available from: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf. Accessed November 17, 2018.
- 14.World Health Organization. The Global Prevalence of Anaemia in 2011. World Health Organization; 2015. [Google Scholar]
- 15.World Health Organization. Global Nutrition Policy Review, What Does It Take to Scale Up Nutrition Action? World Health Organization; 2013. [Google Scholar]
- 16.World Health Organization. WHO, 2014, Global nutrition targets 2025, anaemia policy brief; 2014. Available from: https://www.who.int/nutrition/topics/globaltargets_anaemia_policybrief.pdf. Accessed November 25, 2018.
- 17.Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;2015:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters D, Kakietek J, Julia DE, Shekar M. An investment framework for meeting the global nutrition target for anemia; 2017.
- 19.Habyarimana F, Zewotir T, Ramroop S. Spatial distribution and analysis of risk factors associated with anemia among women of reproductive age, case of 2014 Rwanda demographic and health survey data. Open Public Health J. 2018;11:1. doi: 10.2174/1874944501811010425 [DOI] [Google Scholar]
- 20.Hassan EO, El-Hussinie M, El-Nahal N. The prevalence of anemia among clients of family planning clinics in Egypt. Contraception. 1999;60(2):93–99. doi: 10.1016/S0010-7824(99)00066-9 [DOI] [PubMed] [Google Scholar]
- 21.Lakew Y, Biadgilign S, Haile D. Anaemia prevalence and associated factors among lactating mothers in Ethiopia, evidence from the 2005 and 2011 demographic and health surveys. BMJ open. 2015;5(4):e006001. doi: 10.1136/bmjopen-2014-006001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asres Y, Yemane T, Gedefaw L. Determinant factors of anemia among nonpregnant women of childbearing age in southwest Ethiopia, a community based study. Int Sch Res Notices. 2014;2014:1–8. doi: 10.1155/2014/391580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejigu BA, Wencheko E, Berhane K. Spatial pattern and determinants of anaemia in Ethiopia. PLoS One. 2018;13(5):e0197171. doi: 10.1371/journal.pone.0197171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebremedhin S, Enquselassie F. Correlates of anemia among women of reproductive age in Ethiopia, Evidence from Ethiopian DHS 2005. Ethiop J Health Dev. 2011;25(1):22–30. doi: 10.4314/ejhd.v25i1.69842 [DOI] [Google Scholar]
- 25.Haidar J. Prevalence of anaemia, deficiencies of iron and folic acid and their determinants in Ethiopian women. J Health Popul Nutr. 2010;28(4):359–368. doi: 10.3329/jhpn.v28i4.6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wondu T, Bijlsma M. “The hidden hunger”, Understanding the burden of anaemia and its determinants among pregnant and non-pregnant women in Ethiopia. African J Food Agric Nutr Dev. 2012;12:7. doi: 10.18697/ajfand.55.11015 [DOI] [Google Scholar]
- 27.Derara Furgasa TD. Household food security situation in central Oromia, Ethiopia, a case study from becho wereda in southwest Shewa zone. Glob J Hum Soc Sci. 2016;16:2. [Google Scholar]
- 28.Ganapathi KC, Kumar KS. A cross-sectional study of anemia among women of reproductive age group (15-49 years) in a rural population of Tamil Nadu. Int J Med Sci Public Health. 2017;6(3):524–530. [Google Scholar]
- 29.Jamil S, Khan RA, Dilshad H, Fatima S. haematologic variations associated with the long term use of contraceptives in young females. Int J Med Res. 2014;2(5):580–586. [Google Scholar]
- 30.Tsehayu A, Tamiru D, Mebratu W. Anaemia and associated factors among non-pregnant women of reproductive age in Ethiopia. Afr J Midwifery Womens Health. 2022;16(1):1–10. doi: 10.12968/ajmw.2020.0045 [DOI] [Google Scholar]
- 31.Debelo O, Shiferaw Y. Correlates of anemia status among women of reproductive age in Ethiopia; 2019.
- 32.Diress G, Endalifer ML. Effect of alcohol consumption on haemoglobin level among non-pregnant reproductive age women in Ethiopia, a cross-sectional secondary data analysis of the 2016 Ethiopian demographic health survey. BMJ open. 2022;12(2):e046458. doi: 10.1136/bmjopen-2020-046458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demissie DB, Bulto GA, Terfassa TG. Involvement of male in antenatal care, birth preparedness and complication readinessand associated factors in Ambo town, Ethiopia. J Health Med Nurs. 2016;27(5):14–23. [Google Scholar]
- 34.Bulto GA, Fekene DB, Moti BE, Demissie GA, Daka KB. Knowledge of neonatal danger signs, care seeking practice and associated factors among postpartum mothers at public health facilities in Ambo town, Central Ethiopia. BMC Res Notes. 2019;12(1):549. doi: 10.1186/s13104-019-4583-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FAO aF. Minimum Dietary Diversity for Women, a Guide for Measurement. Rome: University of California; 2016. [Google Scholar]
- 36.Mohammadi F, Omidvar N, Houshiar-Rad A, Khoshfetrat MR, Abdollahi M, Mehrabi Y. Validity of an adapted household food insecurity access scale in urban households in Iran. Public Health Nutr. 2012;15(1):149–157. doi: 10.1017/S1368980011001376 [DOI] [PubMed] [Google Scholar]
- 37.FAO F. Minimum Dietary Diversity for Women, a Guide for Measurement. Vol. 82. Rome: FAO; 2016. [Google Scholar]
- 38.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool, process, current status, and outstanding issues. J Nutr. 2006;136(5):1449s–1452s. doi: 10.1093/jn/136.5.1449S [DOI] [PubMed] [Google Scholar]
- 39.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access, indicator guide, version 3; 2007.
- 40.INDDEX Project. Data4Diets, Building Blocks for Diet-Related Food Security Analysis. Boston, MA: Tufts University; 2018. [Google Scholar]
- 41.Bezerra A, Leal V, Lira P, et al. Anemia and associated factors in women at reproductive age in a Brazilian Northeastern municipality. Rev Bras Epidemiol. 2018;21:e180001–e180001. doi: 10.1590/1980-549720180001 [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity, 2011 and for adjustment; 2011. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed November 25, 2018.
- 43.Gonete KA, Tariku A, Wami SD, Derso T. Prevalence and associated factors of anemia among adolescent girls attending high schools in Dembia District, Northwest Ethiopia, 2017. Arch Public Health. 2018;76(1):79. doi: 10.1186/s13690-018-0324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Document Reference WHO. NMH/NHD/MNM/11.1. Geneva S; 2011. Available from: http://www.who.int/entity/vmnis/indicators/haemoglobin. Accessed February 2, 2023. [Google Scholar]
- 45.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 46.Rakic L, Djokic D, Drakulovic M, Pejic A, Radojicic Z, Marinkovic M. Risk factors associated with anemia among Serbian non-pregnant women 20 to 49 years old. A cross-sectional study. Hippokratia. 2013;17(1):47. [PMC free article] [PubMed] [Google Scholar]
- 47.Abdo N, Douglas S, Batieha A, et al. The prevalence and determinants of anaemia in Jordan. East Mediterr Health J. 2018;25(5):341–349. doi: 10.26719/emhj.18.047 [DOI] [PubMed] [Google Scholar]
- 48.Kibret KT, Chojenta C, D’Arcy E, Loxton D. Spatial distribution and determinant factors of anaemia among women of reproductive age in Ethiopia, a multilevel and spatial analysis. BMJ open. 2019;9(4):e027276. doi: 10.1136/bmjopen-2018-027276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adam I, Ahmed S, Mahmoud MH, Yassin MI. Comparison of HemoCue® hemoglobin-meter and automated hematology analyzer in measurement of hemoglobin levels in pregnant women at Khartoum hospital, Sudan. Diagn Pathol. 2012;7(1):30. doi: 10.1186/1746-1596-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Msemo OA, Bygbjerg IC, Møller SL, et al. Prevalence and risk factors of preconception anemia, A community based cross sectional study of rural women of reproductive age in northeastern Tanzania. PLoS One. 2018;13(12):e0208413. doi: 10.1371/journal.pone.0208413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirth JP, Rohner F, Woodruff BA, et al. Anemia, micronutrient deficiencies, and malaria in children and women in Sierra Leone prior to the Ebola outbreak-findings of a cross-sectional study. PLoS One. 2016;11(5):e0155031. doi: 10.1371/journal.pone.0155031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohner F, Northrop-Clewes C, Tschannen AB, et al. Prevalence and public health relevance of micronutrient deficiencies and undernutrition in pre-school children and women of reproductive age in Cote d’Ivoire, West Africa. Public Health Nutr. 2014;17(9):2016–2028. doi: 10.1017/S136898001300222X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gebremedhin S, Asefa A. Association between type of contraceptive use and haemoglobin status among women of reproductive age in 24 sub-Saharan Africa countries. BMJ Sex Reprod Health. 2018;bmjsrh-2018–200178. doi: 10.1136/bmjsrh-2018-200178 [DOI] [PubMed] [Google Scholar]
- 54.Haile ZT, Kingori C, Teweldeberhan AK, Chavan B. The relationship between history of hormonal contraceptive use and iron status among women in Tanzania, A population-based study. Sex Reprod Healthc. 2017;13:97–102. doi: 10.1016/j.srhc.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 55.Naaz S, Khan AA, Begum W, Mukhtar F, Ainuddin N, Tarannum S. Prevalence of anemia in reproductive age females in Aligarh, a retrospective study. Group. 2005;18:42. [Google Scholar]
- 56.Kamruzzaman M, Rabbani M, Saw A, Sayem M, Hossain M. Differentials in the prevalence of anemia among non-pregnant, ever-married women in Bangladesh, multilevel logistic regression analysis of data from the 2011 Bangladesh Demographic and Health Survey. BMC Women’s Health. 2015;15(1):1–8. doi: 10.1186/s12905-015-0211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilunda C, Massawe S, Jackson C. Determinants of moderate‐to‐severe anaemia among women of reproductive age in T anzania, analysis of data from the 2010 T anzania demographic and health survey. Trop Med Int Health. 2013;18(12):1488–1497. doi: 10.1111/tmi.12199 [DOI] [PubMed] [Google Scholar]
- 58.Gebremedhin S, Asefa A. Association between type of contraceptive use and haemoglobin status among women of reproductive age in 24 sub-Saharan Africa countries. BMJ Sex Reprod Health. 2019;45(1):54–60. [DOI] [PubMed] [Google Scholar]