Abstract
Youth worldwide are regularly exposed to pollutants and chemicals (i.e., toxicants) that may interfere with healthy brain development, and a surge in MRI research has begun to characterize the neurobiological consequences of these exposures. Here, a systematic review following PRISMA guidelines was conducted on developmental MRI studies of toxicants with known or suspected neurobiological impact. Associations were reviewed for 9 toxicant classes, including metals, air pollution, and flame retardants. Of 1,264 identified studies, 46 met inclusion criteria. Qualitative synthesis revealed that most studies: (1) investigated air pollutants or metals, (2) assessed exposures prenatally, (3) assessed the brain in late middle childhood, (4) took place in North America or Western Europe, (5) drew samples from existing cohort studies, and (6) have been published since 2017. Given substantial heterogeneity in MRI measures, toxicant measures, and age groups assessed, more research is needed on all toxicants reviewed here. Future studies should also include larger samples, employ personal exposure monitoring, study independent samples in diverse world regions, and assess toxicant mixtures.
Keywords: MRI, brain structure, brain function, toxicant exposure, pollution, development, childhood, adolescence
1. Introduction
Despite increasing concern for the protection of children from chemicals and pollutants with the potential to harm the central nervous system (i.e., toxicants) (Landrigan et al., 2018), toxicant exposure remains widespread in our environment (Hoffman et al., 2018). Toxic chemicals such as heavy metals, pesticides, solvents, plasticizers, and flame retardants can now be found in the air, water, and soil and, increasingly, in food, furniture, clothing, and personal care products. Consequently, national biomonitoring programs routinely detect most major toxicant classes in the blood and urine of American children (Hendryx & Luo, 2018). The presence of these chemicals in children’s bodies is concerning, as occupational exposure studies demonstrate a clear link between toxic chemicals and disrupted neurological function (Flynn & Susi, 2009; Lucchini et al., 2009; Van Maele-Fabry et al., 2012).
Although children typically do not have occupational exposures, they are at even greater risk of toxicant harm than adults due to their physiology and behavior. Children have fewer biological defenses against toxicant exposure than adults (e.g., fewer detoxifying enzymes; (Cole et al., 2003)) and their brains are still-developing (Mills et al., 2016), meaning that they may experience lasting disruptions to brain development, with potential lifelong consequences for personality, cognitive ability, mental health, and social mobility (Reuben et al., 2017, 2019). Children also consume more food, water, and air per unit of body weight than adults (Environmental Protection Agency, 2002), and thus absorb more of a toxic substance than adults when exposed. Children’s behavior also causes them to be at higher exposure: children have greater hand-to-mouth activity (Xue et al., 2007), are closer to the ground where toxicants may settle (Stapleton et al., 2009), and are more likely to eat foods containing higher levels of toxicants (e.g., fruit, juice) (Eskenazi et al., 2007).
While researchers have known for decades that certain toxicants (e.g., lead) have negative behavioral and cognitive effects, research exploring the effects of toxicant exposure on the developing human brain was rare until approximately twenty years ago. At that time, developmental neuroscientists and environmental health researchers began using non-invasive neuroimaging techniques to assess associations between toxicant exposure and developing brain structure and function in vivo. Since then, research in this topic has steadily increased, with most research examining neurobiological effects of individual—rather than cumulative—exposures. This research has begun to answer important questions about which toxic chemicals are of greatest concern for brain development, what brain structures and functions are affected, and how the course of brain development may be impacted.
Thus, the time is right to build on previous work to create the first systematic review following PRISMA guidelines on developmental MRI studies of all toxicants with known or suspected neurobiological impact. This will provide key information about the current state of the MRI literature by indicating what toxicants, age groups, locations, and measures are most often studied and where additional research is needed to fill existing knowledge gaps. Second, by reviewing the effects of more than one toxicant class at once, the current review will provide a point of departure for identifying toxicants with similar effects on the developing brain. Understanding which toxicants may have similar neurobiological effects is critical for identifying candidates for simultaneous study within mixture studies (Maffini & Neltner, 2015), which at present are rare and sorely needed (Sarigiannis & Hansen, 2012). Third, there have been no systematic reviews of the developmental neuroimaging literature on metals, pesticides, flame retardants, phthalates, bisphenols, polychlorinated biphenyls, or solvents–all of which are systematically reviewed here. Given the rapidly growing body of research in this area, this review also acts as an update to the most recent systematic reviews of imaging and air pollution (Balboni et al., 2022; de Prado Bert et al., 2018; Herting et al., 2019).
In sum, the present review has 4 goals: (1) describe the current state of the MRI literature on childhood toxicant exposure, (2) describe trends in findings within toxicant classes, (3) provide evidence regarding which toxicants have similar effects on the developing brain, and (4) provide productive avenues for future research.
2. Methods
This investigation followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). On August 3, 2022, an English-language search was conducted in PubMed and Scopus for the period spanning January 1, 2000 to August 3, 2022. Search terms were prepared by the lead author, reviewed by co-authors, and refined with the help of a Science Librarian at Duke University. Search terms can be found in the Supplement.
To ensure that all relevant studies were included in the present review, our formal search was complemented by examining the reference lists of existing narrative and systematic reviews on toxicant exposure and MRI-assessed brain function and structure in developing populations. We reviewed reference lists from peer-reviewed, published reviews available in English that appeared in our formal search or that were previously known to the authors.
Reference lists reviewed were: Balboni et al. (2022), Herting et al. (2019), and de Prado Bert et al. (2018)—systematic reviews of the imaging literature on air pollution; Rauh & Margolis (2016)—a narrative review tying lead and pesticide exposure to children’s mental health and brain development; and Horton et al. (2014)–a narrative review of studies linking prenatal or childhood exposure to lead, pesticides, and tobacco smoke to brain structure and function. The present review expands on these works by being the first to systematically review the developmental neuroimaging literature on all toxicants under study except air pollution, by providing an update to the extant air pollution reviews’ literature searches, and by integrating findings across the 9 toxicant classes included in the present review. The reference lists of some noteworthy reviews were not reviewed because they focused on associations between brain and exposure in non-human animals (Margolis et al., 2022), focused on non-environmental exposures (Dufford et al., 2021), focused on the aging brain (Power et al., 2016), or did not review imaging studies (Brockmeyer & D’Angiulli, 2016).
Inclusion criteria, established prior to conducting the literature search, were: (1) Study independent variable (exposure measure) must utilize direct or proxy assessments of exposure to suspected environmental or industrial neurotoxicants, including metals, solvents, flame retardants, polychlorinated biphenyls, bisphenols, organophosphate esters, phthalates, pesticides, parabens, per- and polyfluoroalkyl substances, and air pollutants (e.g., polycyclic aromatic hydrocarbons, particulate matter, nitrogen dioxide, ozone). Studies assessing primary or secondary exposure to medications and/or recreational drugs (e.g., tobacco smoking and second-hand smoke) were not considered, nor were studies of radiation exposure or light or noise pollution. (2) Study dependent variable (outcome measure) must utilize MRI, including structural MRI (sMRI, e.g., T1 and T2 imaging), diffusion tensor imaging (DTI), magnetic resonance elastography (MRE), susceptibility weighted imaging (SWI), task functional MRI (fMRI), resting state fMRI (rs-fMRI), arterial spin labeling (ASL), and/or magnetic resonance spectroscopy (MRS). (3) Both exposure and outcome measures must be obtained prior to age 18. Studies with participants over age 18 were included only if the mean sample age at outcome assessment was below 18. Case studies, qualitative studies, animal studies, and studies not utilizing MRI were not considered.
A study protocol was developed and published on the Open Science Framework on May 15, 2021 (https://osf.io/qdwvp/). All studies were reviewed for inclusion criteria by at least two trained independent reviewers using the systematic review management tool Covidence (www.covidence.org). Study titles and abstracts were screened. Full text was read for studies that passed the screening stage.
After review, studies meeting inclusion criteria had the following data extracted by two independent reviewers: lead author, publication year, journal, study design, if part of a broader study (e.g., a cohort), toxicant class, toxicant measure, age at which toxicant measure was obtained, population studied, inclusion and exclusion criteria for MRI sample, number of MRI participants, age range, mean/median age, racial/ethnic composition, sex, MRI scanner type (e.g., 3T, 1.5T), MRI measure (e.g., sMRI, task fMRI, etc.), primary neuroimaging variables used in analyses, main effect of toxicant on the brain, and covariates used in analyses. Following data extraction, the lead author synthesized data by study characteristics and imaging findings. Meta-analysis was not used due to the small number of studies per toxicant and the heterogeneity of the MRI measures.
In addition to study data, study quality was assessed independently by two reviewers using the US National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The Quality Assessment (QA) tool contained 14 items, such as “Was the participation rate of eligible persons at least 50%?” and “Was loss to follow-up after baseline 20% or less?” Questions were answered ‘yes’ (1), ‘no’ (0), or ‘NA’ by each independent reviewer. Studies earning scores of 10 or greater were deemed high quality, 6-9 deemed moderate quality, and 5 or fewer deemed low quality.
3. Results
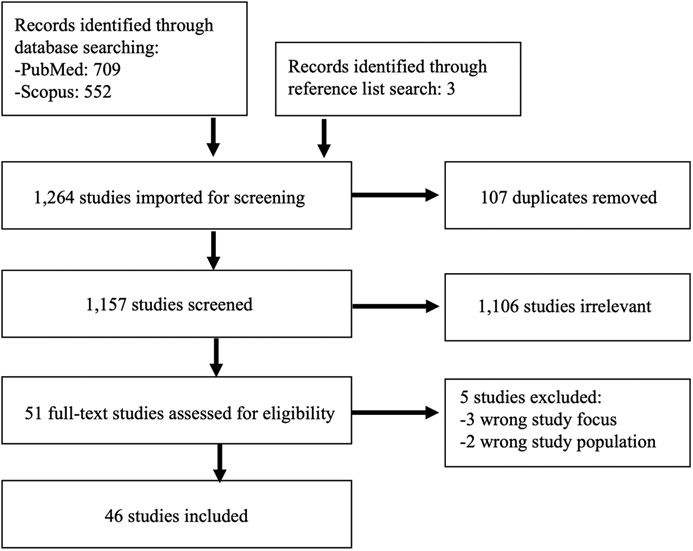
Our search identified 1,158 studies, of which 107 were duplicates. 1,106 were excluded following title and abstract review, and 51 were advanced to full text review. During full text review, 5 further studies were excluded, resulting in a total N= 46 studies in this systematic review. Figure 1 presents a flow diagram of the search stages.
Figure 1.
PRISMA Flow Diagram.
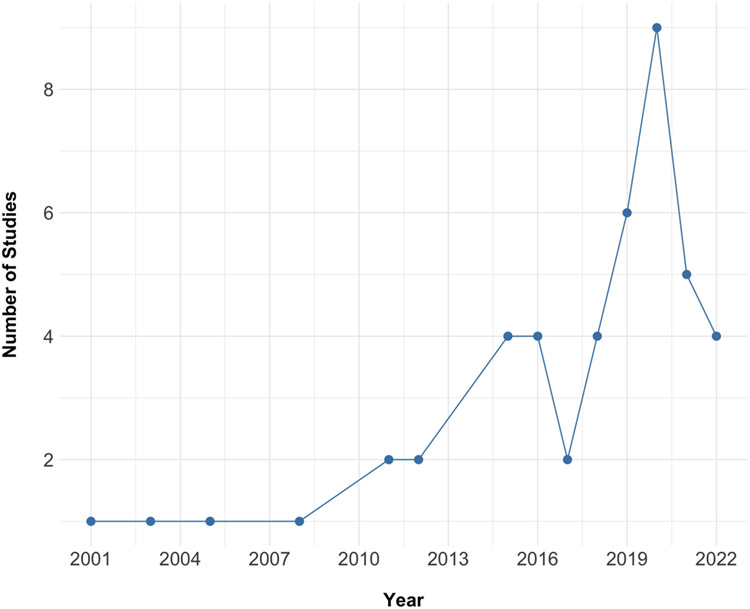
Figure 2 presents the number of studies published by year during the period under study (e.g., 2000 to 2022). Most studies on toxicant exposure and MRI measures of brain structure and function have been published in the last 5 years (i.e., since 2017; 30 studies, 65%).
Figure 2.
Number of studies published on toxicant exposure and MRI-assessed measures of brain structure and function by year.
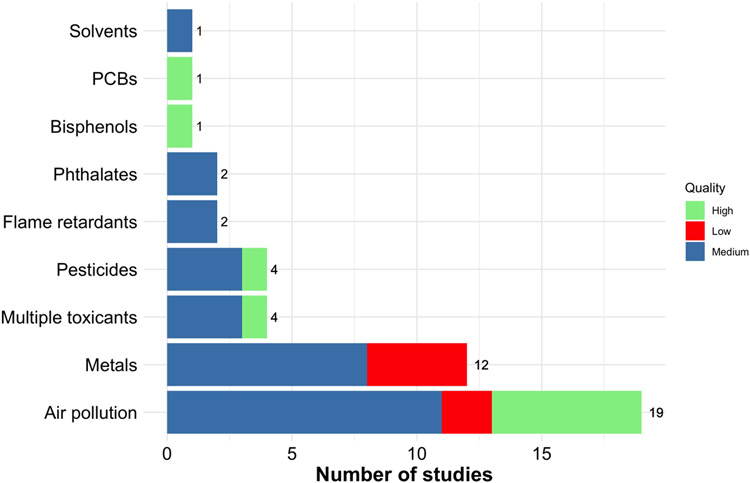
Figure 3 presents the number of studies that investigated each class of toxicants. Air pollution received the greatest attention in the literature (19 studies; 41%), followed by metals (12 studies; 26%), pesticides (4 studies; 9%), and multiple toxicants (4 studies; 9%). All other toxicant classes were investigated by 1 to 2 studies each.
Figure 3.
Number of studies published on toxicant exposure and MRI-assessed brain development by toxicant class (01/01/2000 to 08/03/2022). Quality ratings are displayed on top of frequency counts. Numbers indicate the number of studies per toxicant class.
Following NIH Quality Assessment Tool analysis, 10 (22%) studies were deemed high quality, 30 (65%) moderate quality, and 6 (13%) low quality. The primary reasons studies were rated as moderate or low quality included: recruiting convenience samples, failing to report effect sizes, retaining less than 80 percent of the sample at follow-up, and not making clear whether outcome assessors were blinded to exposure status. Quality assessment ratings by toxicant class are displayed in Figure 3.
3.1. Measures of toxicant exposure
Exposure measurement varied by toxicant class. Among the 19 studies on air pollution, air pollution was measured using land use regression or spatiotemporal modeling (N= 9; i.e., statistical extrapolation of air pollution exposure based on participants’ home address and information about surrounding land use and pollution), high volume sampler (N= 4; i.e., a large, stationary monitoring device), combined use of backpack monitors and urine sampling (N= 1), combined use of backpack monitors and spatiotemporal modeling (N= 1), or comparison of children from a city with substantial air pollution (Mexico City) to those from a city with minimal air pollution (Polotitlan, Mexico) (N= 4).
Metals were measured via tap water samples from the child’s home (N= 2), parent-report and tracking via hospital records (N= 1), lifetime residency in an area with versus without metal contamination (N= 1), deciduous teeth (N= 1), geocoded risk for exposure based on census tract (N= 2), and blood sampling (N= 4). Pesticides were measured via urine sampling (N= 2), cord blood at birth (N= 1), and life history calendars/parental status as a farmworker with pesticide exposure (N= 1). Flame retardants were measured via blood sampling (N= 2). Phthalates were measured via urine sampling (N= 2). All other toxicants (e.g., bisphenols, solvents, PCBs, and multi-toxicant exposure) employed the following measures: blood sampling (N= 1), cord blood at birth (N= 1), cord blood and umbilical tissue at birth (N= 1), urine sampling and blood sampling (N= 2), and urine sampling (N= 2).
3.2. Study location and samples
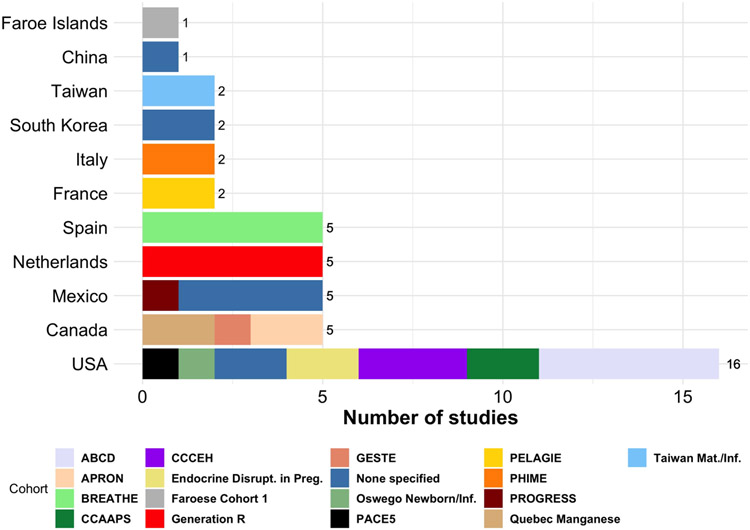
Figure 4 presents the geographic location of each study. All studies were conducted in high-income or middle-income countries. Most studies took place in Western Europe (19 studies, 41%) and the United States (16 studies, 35%). There were no published studies conducted in low-income countries or in South America, Africa, the Middle East, South Asia, or Oceania.
Figure 4.
Studies on toxicant exposure by study location (01/01/2000 to 08/03/2022). Numbers indicate studies per country. Legend displays which cohort samples were employed. Acronyms for cohorts can be found in the Supplement.
Many studies included in the present review used participants from the same samples. For example, 5 studies used the American Adolescent Brain Cognitive Development (ABCD) sample (Garavan et al., 2018), 6 studies used the Spanish Brain Development and Air Pollution Ultra Fine Particles in School Children sample (BREATHE) (Rivas et al., 2014), and 5 studies used the Dutch Generation R sample (Jaddoe et al., 2007). Only 9 studies (19.5%) used an independent sample not from a broader cohort. Figure 4 displays cohorts used in each of the 46 studies.
3.3. Age at exposure measurement and age at MRI
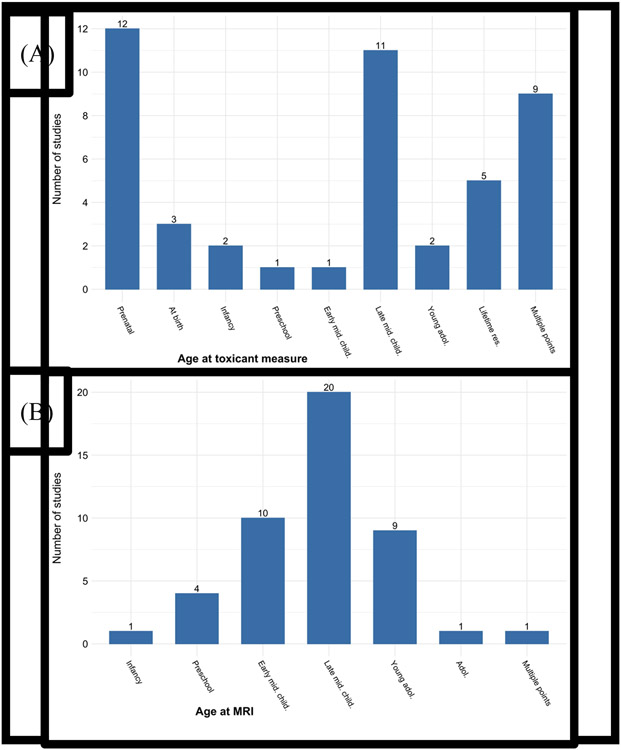
Figure 5a depicts the age group of children at the toxicant measure across studies. Age groups were based on Centers for Disease Control (CDC) guidelines (https://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/index.html). It was most common for toxicant exposure to be measured prenatally, when mothers were pregnant with the child (N= 12). This was followed by at late middle childhood (N= 11; age 9-11), multiple time points (N= 9), lifetime residency in a polluted place (N= 5), at birth (N= 3), infancy (N= 2; age 1 and under), young adolescence (N= 2; age 12-14), preschool age (N= 1; age 3-5), and early middle childhood (N= 1; age 6-8). Figure 5b depicts children’s age group at the MRI measure across studies. MRIs were conducted most often in late middle childhood (N= 20) and early middle childhood (N= 10).
Figure 5.
Age at toxicant (A) and MRI (B) assessment. Lifetime res. refers to studies comparing children who lived in areas with different levels of pollution. Adol. = adolescence. Child. = childhood. Mid. = middle. Res. = residency. Age groups based on CDC guidelines.
3.4. Imaging findings
3.4.1. Air pollution
Air pollution describes the harmful gases and chemicals released into the air as a result of human activity (Mackenzie & Turrentine, 2016). Our search detected 19 studies on air pollution. Across these studies, the primary outcomes measured were differences in cortical white matter, cortical gray matter, subcortical gray matter, and brain function. More than one type of air pollution was measured in these studies, but for simplicity, these are referred to here only as air pollution. The type of air pollution measured as well as more study details can be found in Table 1. Air pollution studies reviewed had a wide range of sample sizes, ranging from fewer than 60 participants (N= 5) to more than 750 (N= 6).
Table 1.
Key findings and study characteristics of studies on air pollution exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| Alemany et al. (2018) | PAH, EC, and NO2 | 163 | 7-11 years | High volume sampler | 7-11 years | Basal ganglia vol. (caudate, putamen, globus pallidus) | Greater exposure to PAH and NO2 associated with smaller caudate vol. only. |
| Beckwith et al. (2020) | ECAT | 135 | First year of life | Land use regression | 11 – 14.71 years | Whole brain CT, Whole brain VBM | High ECAT group had reduced GMV in bilateral cerebellum and left parietal lobe, and thinner parietal and frontal cortex. No other associations. |
| Brunst et al. (2019) | ECAT | 127 | At birth, average from birth to MRI, average past year | Land use regression | At age 12 MRI visit | MRS voxel in bilateral ACC (metabolites : mI, NAA, Cr, Cho, Glu, GLX, GSH). | Elevated past year ECAT associated with elevated mI in bilateral ACC. No associations with other time periods or metabolites detected. |
| Burnor et al. (2021) | PM2.5 | 7602 | 9-10 years | Hybrid spatiotempor al model | 9-10 years | rND, hD, rNO, MD, FA of all white matter fibers in each hemisphere and of ATR, CGC, CGH, CC, CST, FX, UF, IFO, ILF, and SLF in each hemisphere. | Hemisphere-stratified models revealed positive associations between PM2.5 and rNO in L hemisphere tracts (CGH, UF, FX, SLF) and R hemisphere tracts (UF, FX). Negative associations between PM2.5 and MD globally, in L hemisphere tracts (ATR, CGH, FX, SLF, UF, IFO, ILF), and R hemisphere tracts (ILF, UF, CGH, FX). No associations with FA, rND, or hD. |
| Calderon-Garciduenas et al. (2008) | Air pollution (general) | 36 | N/A | Lifetime residency | M=10.71 years | WMH | 7.6% of Polotitlan youth had WMH, vs. 56.5% from Mexico City. |
| Calderon-Garciduenas et al. (2011) | Air pollution (general) | 30 | N/A | Lifetime residency | M= 6.95 years | WMH, cortical GMV and cortical WMV of lobes, CSF, vol. of hippocampus, caudate, putamen, globus pallidus, amygdala | WMH+ children (vs. WMH−) had greater WMV in R parietal lobe. Polotitlan children had greater WMV in bilateral temporal and R parietal, lobes (compared to Mexico City youth). No associations with any other ROI. |
| Calderon-Garciduenas et al. (2012) | Air pollution (general) | 30 | N/A | Lifetime residency | M=7.13 years | WMH, combined GMV and WMV of lobes, vol. of frontal cingulate, parietal cingulate, cerebellum, CC, PFC, insula, hippocampus, caudate, putamen, globus pallidus, amygdala | Between baseline and follow-up, WMH+ children had greater volumetric brain growth (GMV and WMV) in temporal, parietal, and frontal regions, compared to WMH-children. WMH+ and Polotitlan children did not differ. No associations with other ROIs. |
| Calderon-Garciduenas et al. (2015) | Air pollution (general) | 57 | N/A | Lifetime residency | M = 12.45 years | MRS voxels in R and L hippocampus (metabolites : NAA, Cho, Cr, mI) | Children with APOE e4 genotype from Mexico City, compared to children from Polotitlan, had reduced NAA/Cr ratio in R hippocampus. |
| Cserbik et al. (2020) | PM2.5 | 9697 | 9-10 years | Hybrid spatiotemporal model | 9-10 years | 31 SA regions, 27 CT regions, 8 subcortical vols, total SA, total CT, TBV, cortical GMV, subcortical GMV, cortical WMV, CSF, ICV, ventricle vols. | Greater PM2.5 exposure associated with: (1) thinner L frontal, R temporal lobes; (2) thicker R frontal, L temporal lobes, (3) thicker and thinner bilateral cingulate, (4) reduced SA in R frontal pole, L cuneus, (5) greater SA in R lateral OFC, (6) greater R thalamus, R pallidum, L accumbens vol, (7) reduced L putamen, L pallidum vol. No other associations detected. |
| Guxens et al. (2018) | NO2, particulate matter (coarse and fine particles), and absorbance of fine particles | 783 | Prenatal | Land use regression | 6-10 years | Whole brain CT, TBV, cortical GMV, cortical WMV, subcortical GMV, vol. of caudate, putamen, pallidum, accumbens, hippocampu s, amygdala, thalamus, ventricles | Higher fine particle exposure associated with thinner R frontal, R parietal, and L occipital cortex. Higher coarse particle exposure associated with thinner R lateral OFC. Absorbance of fine particles associated with thinner L fusiform. No associations with any other measure. |
| Lubczynska et al. (2021) | NO2, NOx, PM10, PM2.5, PMcoarse, PM2.5 absorbance, PAH, BAP, OC, Cu, Fe, K, Si, Zn, OP, UFP | 3133 | Prenatal, average from birth to MRI | Land use regression | 9-12 years | TBV, cortical GMV, subcortical GMV, cortical WMV, vol. of CC, cerebellum, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, accumbens. Whole brain CT and SA. | Both thinner and thicker cortex associated with exposure, prenatally and across childhood, depending on region. Across childhood exposure associated with less PFC SA, but greater SA in other regions. No associations between pregnancy exposure and SA reported. Both pregnancy and across childhood exposure associated with smaller CC, smaller hippocampus , larger amygdala, larger cerebellum. No associations with other measures. |
| Lubczynska et al. (2020) | NO2, NOx, PM10, PM2.5, PMcoarse, PM2.5 absorbance, PAH, BAP, OC, Cu, Fe, K, Si, Zn, OP, UFP | 2,954 | Prenatal, average from birth to MRI | Land use regression | 9-12 years | Global FA, global MD, follow-up analyses on individual white matter tracts | In single pollutant analyses, prenatal and average childhood exposure predicted lower FA and higher MD for many air pollutants assessed. |
| Mortamais et al. (2017) | Total sum of PAH levels, BAP | 242 | 8-12 years | High volume sampler | 8-12 years | Basal ganglia vol. (caudate, putamen, globus pallidus), brain parenchyma l fraction | Outdoor and indoor BAP predicted smaller caudate vol. Effect of total outdoor PAH on caudate vol. was similar but smaller than the effect of outdoor BAP. No other associations found. |
| Mortamais et al. (2019) | PM2.5 | 186 | Prenatal, average across first 2 years of life, time of MRI | Land use regression | 8-11.7 years | Cortical GMV, cortical WMV, vol. of lateral ventricles and CC sub-regions | Greater 3rd trimester exposure linked to decreased vol. of anterior and body of CC. Associations did not survive correction for multiple comparisons. No associations with other ROIs or time periods. |
| Perez-Crespo et al. (2022) | NOx, NO2, PM2.5, PM2.5 absorbance, PM10 | 2197 | Prenatal, 0-3, 3 – 6, 6 to time of MRI | Land use regression | 9-12 years | Resting state functional connectivity of 6 brain networks | NO2 from 0-3 and NOx from 3-6 both associated with higher visual - task positive network connectivity. PM2.5 absorbance from 0-3 associated with higher connectivity between visual and auditory, task positive, and task negative networks as well as between auditory and task positive and task negative networks, and between task positive and task negative networks. PM2.5 absorbance from 0-3 also associated with greater connectivity within the visual, motor, task positive, and task negative networks. No other associations with other air pollutants or time periods. |
| Peterson et al. (2015) | PAH | 40 | Prenatal, at age 5 | Personal backpack monitor for 48 hours; spot urine sample from child | 7-9 years | Whole brain CT, surface morphology | Prenatal exposure to PAH correlated with reduced vol. in bilateral frontal, superior temporal, and parietal lobes and in L rostro-caudal extent of mesial surface—associations driven by changes in white matter. PAH at age 5 correlated with reduced WMV in bilateral dorsal PFC, especially SFG. No association with CT. |
| Peterson et al. (2022) | PM2.5, PAH | 332 | Prenatal | Personal backpack monitor for 48 hours; spatiotempor al model | 6-14 years | Whole brain CT, FA, ADC, MRS (metabolites: NAA, Cr, Cho, GLX), rCBF, surface morphology | PAH & PM2.5 associated with (1) thicker lateral temporal, posterior inferior, and mesial wall cortices, (2) thinner dorsal parietal cortices, (3) smaller white matter vols in the inferior parietal lobes and cingulate gyrus, (4) larger white matter vols in dorsal convexity, SFG, and posterior inferior brain surface, (5) higher FA in basal ganglia and ACC, (6) lower rCBF in frontal gray matter, (7) higher NAA and Cho in cingulate. ADC alterations different for PM2.5 vs. PAH. |
| Pujol, Fenoll et al. (2016) | Copper | 263 | 8-12 years | High volume sampler | 8-12 years | Whole brain CT,Whole brain VBM, FA, resting state functional connectivity with seeds in PFC and caudate | Greater Cu associated with higher gray matter concentratio n in the caudate, greater FA in white matter in and around the caudate, diffusion changes in the caudate, reduced resting state functional connectivity between the caudate and frontal lobe operculum, and increased CT in L SMA. |
| Pujol, Martinez-Vilavella et al. 2016 | Average of EC, NO2 | 239 | 8-12 years | High volume sampler (EC), passive dosimeter (NO2) | 8-12 years | Whole brain CT, Whole brain VBM, FA, brain activation during sensory task, resting state functional connectivity with seeds in medial frontal, dorsal frontal, PCC, and SMA, MRS voxel in L frontal lobe (metabolite:Cho/Cr ratio) | In the resting state analysis, higher exposure to air pollution was associated with weaker connectivity within DMN and stronger connectivity between the DMN and the lateral boundary of the DMN. In the task analysis, greater exposure to air pollution was associated with lower deactivations in the SMA and somatosenso ry cortex. No association with sMRI, FA, or MRS measures. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
White matter.
14 air pollution studies examined associations with white matter (Table 1). 5 of these studies conducted whole brain analyses of white matter structure, and 2/5 found significant associations. Prenatal exposure predicted global reductions in white matter in early middle childhood, and age 5 exposure predicted reduced white matter in the prefrontal cortex (PFC) in the same period (Peterson et al., 2015). However, prenatal exposure predicted both smaller (inferior parietal lobes, cingulate) and larger (PFC, posterior inferior surface) volumes in a larger sub-sample of the same cohort when assessed in late middle childhood (Peterson et al., 2022). In contrast, first year of life exposure (Beckwith et al., 2020) and late middle childhood exposure (Pujol, Fenoll, et al., 2016; Pujol, Martínez-Vilavella, et al., 2016) were not associated with white matter volumes in young adolescence or late middle childhood, respectively.
5 studies assessed fractional anisotropy (FA)—a measure of white matter tract organization throughout the brain, and 3/5 found significant, but conflicting, results. In late middle childhood, greater cross-sectional exposure was associated with higher FA in and around the caudate nucleus (Pujol, Fenoll, et al., 2016), while across childhood and prenatal exposure was associated with lower FA in most white matter tracts in a different study (Lubczyńska et al., 2020). Also in late middle childhood, greater prenatal air pollution exposure was associated with higher FA in the basal ganglia and anterior cingulate (ACC) (Peterson et al., 2022). 2 studies found that cross-sectional exposure in late middle childhood was not associated with FA in any tract (Burnor et al., 2021; Pujol, Martínez-Vilavella, et al., 2016).
2 studies assessed mean diffusivity (MD)—a measure of the permeability of white matter tracts–and both found significant, but opposite, results. In a sub-sample (N= 7,602) of 9 to 10-year-olds from the American ABCD cohort, cross-sectional exposure was associated nonlinearly with reduced MD globally in the left hemisphere and in the left anterior thalamic radiations (ATR), left cingulum adjoining the hippocampus (CGH), left fornix (FX), left superior longitudinal fasciculus (SLF), right inferior longitudinal fasciculus (ILF), and bilateral uncinate fasciculus (UF), and was associated linearly with reduced MD globally in the right hemisphere and in the left inferior frontal occipital (IFO), left ILF, right CGH, and right FX (Burnor et al., 2021). In contrast, prenatal and average childhood exposure were associated with higher global MD in a sub-sample of 9 to 12-year-olds from the Dutch Generation R cohort (N= 2,954) (Lubczyńska et al., 2020).
2 studies used other measures of white matter microstructure, and both found significant results. Restricted isotropic intracellular diffusion (rNO)—a measure of diffusion within glia and cell bodies, restricted directional intracellular diffusion (rND)—a measure of diffusion within an axon, and hindered diffusion (hD)—a measure of extracellular diffusion were measured in Burnor et al. (2021). In this work in the ABCD cohort, cross-sectional exposure was associated with increased rNO in the bilateral UF, bilateral FX, left CGH, and left SLF, but it was not associated with rND or hD. Average diffusion coefficient (ADC)—a measure of the directionless rate of water at each brain voxel—was measured in Peterson et al. (2022), who found that greater prenatal exposure predicted differences in ADC across the cortex depending on the air pollutant.
The other white matter studies were ROI studies examining corpus callosum volume, white matter hyperintensities (e.g., lesions in the white matter), white matter volume of each lobe, and total cortical white matter volume. Of the studies of corpus callosum volume, 2/3 found significant associations. 2/2 found that prenatal air pollution exposure predicted smaller corpus callosum volume in late middle childhood (Lubczyńska et al., 2021; Mortamais et al., 2019), but, in Mortamais et al. (2019), the association did not survive correction for multiple comparisons. A third study comparing children from a polluted city (Mexico City) to those from an unpolluted city (Polotitlan, Mexico) did not find an association with corpus callosum volume (Calderón-Garcidueñas et al., 2012). The study on white matter hyperintensities and on white matter lobe volume also compared children from Mexico City and Polotitlan. Children from Mexico City had more white matter hyperintensities than children from Polotitlan (Calderón-Garcidueñas et al., 2008, 2011). Additionally, children from Polotitlan had greater white matter volume in bilateral temporal and right parietal lobes (Calderón-Garcidueñas et al., 2011) compared to children from Mexico City without white matter hyperintensities. However, Mexico City children with white matter hyperintensities had more white matter volume in the right parietal lobe than Mexico City children without hyperintensities.
Finally, all 4 studies assessing total cortical white matter volume (e.g., the summed volume of the white matter across the cortex) found null results. Cross-sectional (Cserbik et al., 2020), prenatal (Mortamais et al., 2019), and prenatal and across childhood (e.g., birth to MRI; Lubczyńska et al., 2021) exposures were not associated with total cortical white matter volume in late middle childhood. Prenatal (Guxens et al., 2018) exposure was also not associated with total cortical white matter volume in early middle childhood.
Cortical gray matter.
11 studies examined associations between air pollution exposure and cortical gray matter (Table 1). Whole brain analyses of cortical thickness, gray matter volume, and surface area were conducted. Total cortical gray matter volume of the entire cortex and of each lobe were studied as ROIs.
Mixed results were found for studies of air pollution and whole brain cortical thickness. 6/8 studies found significant results, but some studies found associations with both thicker and thinner cortex. Prenatal exposure was associated with thinner right frontal, right parietal, and left occipital lobes in early middle childhood (Guxens et al., 2018) and in late middle childhood (Lubczyńska et al., 2021), although both studies examined overlapping subsamples of the Dutch Generation R cohort at different time points. Mirroring these results, greater first year of life exposure was associated with thinner parietal and frontal cortices in American young adolescents (Beckwith et al., 2020). However, in contrast to these results, Peterson et al. (2022) found that prenatal exposure was associated with both thicker (lateral temporal, posterior inferior, and mesial) and thinner (dorsal parietal) cortices in late middle childhood. Further, in a smaller, younger sub-sample of the same cohort, Peterson et al. (2015) found no association between prenatal or age 5 exposure and cortical thickness in early middle childhood (Peterson et al., 2015).
Studies of late middle childhood exposure also produced mixed results. Exposure in this period was associated cross-sectionally with both thicker and thinner frontal, temporal, and cingulate regions (Cserbik et al., 2020), thinner supplementary motor cortex (Pujol, Fenoll, et al., 2016), and no change (Pujol, Martínez-Vilavella, et al., 2016). Average exposure from birth to late middle childhood was associated with thicker right post central gyrus and thinner left lingual gyrus (Lubczyńska et al., 2021). Finally, total cortical thickness was assessed by 2 studies (Cserbik et al., 2020; Lubczyńska et al., 2021). Neither found significant associations.
Studies of whole brain surface area were more consistent, with 2/2 finding significant associations. Late middle childhood exposure (Cserbik et al., 2020) was cross-sectionally associated with less surface area in frontal and occipital regions, while average exposure from birth to late middle childhood (Lubczyńska et al., 2021) was associated with less prefrontal surface area but greater surface area in several other brain regions. Prenatal exposure (Lubczyńska et al., 2021) was measured in one study of surface area, but the association with surface area was not reported. Total cortical surface area was only assessed by Cserbik et al., (2020) and was not associated with cross-sectional air pollution exposure in late middle childhood.
3 studies measured cortical gray matter volume via whole brain volumetric analysis, and 5 studied total cortical gray matter volume as an ROI. In the whole brain studies, first year of life exposure predicted reduced gray matter volume in the left parietal lobe in young adolescence (Beckwith et al., 2020), but late middle childhood exposure was not cross-sectionally associated with gray matter volume in any region (Pujol, Martínez-Vilavella, et al., 2016). Total cortical gray matter volume was not associated with air pollution in any study (Calderón-Garcidueñas et al., 2011; Cserbik et al., 2020; Guxens et al., 2018; Lubczyńska et al., 2021; Mortamais et al., 2019).
Subcortical gray matter.
10 of 19 studies on air pollution measured subcortical gray matter volume (Table 1). 3 studies employed whole brain volumetric analyses, and 2/3 found significant associations with subcortical structure. First year of life exposure predicted smaller bilateral cerebellum volume in young adolescence (Beckwith et al., 2020). Greater cross-sectional exposure predicted greater gray matter concentration in the caudate nucleus, a sub-region of the basal ganglia, in late middle childhood (Pujol, Fenoll, et al., 2016), but it was not associated with subcortical structure in a different study using the same sample (Pujol, Martínez-Vilavella, et al., 2016).
The remaining studies measured hippocampal, basal ganglia, and amygdala volume as ROIs. Studies of hippocampal and amygdala ROIs largely did not detect associations (Calderón-Garcidueñas et al., 2011, 2012; Cserbik et al., 2020; Guxens et al., 2018). In contrast, 6 of the ROI studies measured basal ganglia volume, and 3 found significant results. Reduced basal ganglia volume at late middle childhood was associated with cross-sectional exposure (Alemany et al., 2018; Cserbik et al., 2020; Mortamais et al., 2017). However, it was not associated with living in a polluted city (Calderón-Garcidueñas et al., 2011, 2012), or with prenatal and average air pollution exposure from birth to late middle childhood (Lubczyńska et al., 2021).
Brain function.
There were 6 studies on air pollution and brain function. (Table 1). 4 of these studies included MRS measures, and 3 found significant results. In American 6 to 14-year-olds, greater exposure to two air pollutants was associated with higher N-acetyl aspartate (NAA) and Choline (Cho) in the cingulate cortex (Peterson et al., 2015). Similarly, in an analysis of NAA, Cho, Cr (Creatinine), and myo-Inositol (mI) in the hippocampus, Mexican young adolescents with the e4 allele of the Apolipoprotein E gene and greater lifetime air pollution exposure had reduced NAA/Creatinine (NAA/Cr) ratio (Calderón-Garcidueñas et al., 2015). In contrast, in young American adolescents, greater past-year exposure to air pollution was associated with elevated mI in the ACC, but it was not associated with NAA, Cr, Cho, Glutamate (Glu), Glutamate and glutamine (GLX), or Glutathione (GSH) (Brunst et al., 2019). Finally, Pujol, Martinez-Vilavella et al. (2016) found no association between Cho/Cr ratio in the left frontal lobe and Spanish 8 to 12-year-olds’ cross-sectional air pollution exposure.
Other brain function studies examined resting state functional connectivity, brain activation, and regional cerebral blood flow. All found significant results. 2 studies assessed cross-sectional exposures. In whole brain analyses of resting state fMRI and an fMRI task, greater cross-sectional exposure was associated with disrupted default mode network connectivity at rest, altered brain activation during a sensory stimulation task, and reduced resting state functional connectivity between the caudate and the frontal lobe in Spanish 8 to 12-year-olds (Pujol, Fenoll, et al., 2016; Pujol, Martínez-Vilavella, et al., 2016). 2 studies assessed prenatal and across childhood exposures. In Perez-Crespo et al. (2022), exposure from birth to age 3 was more likely to be associated with greater resting state functional connectivity between several functional brain networks in Dutch Generation R participants than exposure at other time points. In Peterson et al. (2022), greater prenatal exposure was associated with lower regional cerebral blood flow in several cortical regions in American 6 to 14-year-olds.
3.4.2. Metals
Lead.
A heavy metal, widely known to be a developmental neurotoxicant, lead is often found in dust near roadways (Deocampo et al., 2012) and old homes (Wilson et al., 2022), in old paint and toys (Z. Shen et al., 2018), and in drinking water contaminated by lead pipes (Butler et al., 2016). Our search identified 6 developmental neuroimaging studies on lead. (Table 2).
Table 2.
Key findings and study characteristics of studies on metal exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| Aschner et al. (2015) | Manganese | 58 | Newborn | Survey, medical and pharmacy records, blood sample | Newborn | T1R in globus pallidus, putamen | Greater parenteral nutrition and greater total Mn exposure associated with shorter T1R in globus pallidus and putamen. No association with whole blood Mn. |
| de Water et al. (2018) | Manganese | 15 | Prenatal | Blood sample | 6.3 -7.6 years | Resting state functional connectivity with seeds in bilateral ACC, insula, MFG, superior parietal lobule, R and L globus pallidus | In children with higher prenatal Mn exposure, there was reduced R globus pallidus - dACC functional connectivity and a quadratic association between prenatal Mn and R globus pallidus-IFG connectivity. No other associations survived controlling for all covariates. |
| de Water, Papazahari as et al. (2019) | Manganese | 14 | Prenatal, early postnatal, and childhood | Deciduous teeth | 12 – 18 years | Resting state functional connectivity with seeds in L and R putamen, L and R caudate, L and R pallidum, and bilateral MFG | Adolescents with higher early postnatal Mn exposure showed reduced functional connectivity between R putamen and L pre and post central gyri and increased bilateral MFG - L mPFC functional connectivity. |
| Dion et al. (2016) | Manganese | 23 | 9- 15 years | Water sample from kitchen tap, questionnaire | 9- 15 years | T1R in globus pallidus, standard PI, pericranial PI | High Mn group (vs. low Mn group) had lower signal intensity on the pericranial PI and longer T1R. No difference in standard PI. |
| Iannilli et al. (2016) | Manganese | 14 | N/A | Lifetime residency | 14.65 years | Brain activation during olfactory stimulation task, olfactory bulb vol. | Whole brain task analysis: low Mn group had greater activation in secondary olfactory cortex, cerebellum, and SMA. ROI task analysis: low Mn group had greater activation in bilateral insula, R middle cingulate, and PCC. No association between Mn and olfactory bulb vol. |
| Karcher et al. (2021) | Lead | 10,328 | 9-10 years | Geocoded lead risk | 9-10 years | ICV, cortical GMV, subcortical GMV, total SA, total CT, hippocampa l vol | Lead risk associated with smaller ICV, subcortical GMV, cortical GMV, total SA, and total CT. Hippocampa l vol not associated with lead risk. Air pollution also assessed, but no analyses conducted with MRI measures. |
| Kim et al. (2018) | Lead | 150 | 6 – 17 years | Blood sample | 6 – 17 years | 10-12 frontal lobe CT ROIs | No effect of lead on any ROI. |
| Lao et al. (2017) | Manganese | 23 | 9-15 years | Water sample from kitchen tap | 9-15 years | Basal ganglia vol. (caudate, putamen, globus pallidus) measured volumetrical ly and with mTBM | Using mTBM, high Mn group displayed larger L and bilateral putamen, but no difference when measured volumetrically. |
| Marshall et al. (2020) | Lead | 9,712 | 9 – 10 years | Geocoded lead risk | 9 – 10 years | Total CT, total SA,cortical vol. | No main effect of lead risk on any MRI measure. |
| Marshall et al. (2021) | Lead | 8,524 | 9 – 10 years | Geocoded lead risk | 9 – 10 years | Subcortical GMV, vol. of hippocampus, amygdala, cerebellum, brain stem, CC sub-regions, putamen, caudate, accumbens, pallidum, thalamus, ventricles | Lead risk associated with smaller CC in all sub-regions studied, except anterior CC. No other brain regions associated with lead risk. |
| Meng et al. (2005) | Lead | 12 | 9 – 13 years | Blood sample | 9 – 13 years | MRS voxel in L and R frontal lobe, L and R hippocampus (metabolites : NAA, Cr, Cho) | Peak of NAA, Cr, Cho lower in high lead group in all regions. For NAA, difference between high and low lead groups more pronounced in frontal lobe than hippocampus, and even more pronounced in R frontal lobe than L. |
| Trope et al. (2001) | Lead | 21 | Before age 5 | Blood sample | 8.75 years | MRS voxel in L prefrontal lobe (metabolites : NAA, Cr, Cho, ml). | The NAA/Cr ratio in the L prefrontal lobe was significantly lower in the high lead group. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
Cortical gray matter structure was assessed in 3 lead studies (Kim et al., 2018; Marshall et al., 2020); 2/3 reported null findings. Kim et al. (2018) found null results when assessing the association between concurrent blood lead level and the cortical thickness of frontal lobe ROIs in 150 Korean children aged 6-17, while Marshall et al. (2020) found null results when assessing the cross-sectional association between risk of lead exposure and total cortical thickness, total cortical surface area, and total cortical volume (measured as ROIs) in roughly 9,000 American 9 to 10-year-olds from the ABCD cohort. However, also sampling from the ABCD cohort but with more participants (~10,000), Karcher et al. (2021) found that risk of lead exposure was associated with reduced total cortical thickness, total surface area, cortical gray matter volume, and intracranial volume.
Subcortical gray matter structure was assessed in 2 lead studies (Karcher et al., 2021; Marshall et al., 2021) both conducted in the ABCD cohort. 1/2 found significant results. Despite using the same sample, total subcortical gray matter volume was only associated with risk of lead exposure in Karcher et al. (2021). This is likely because Karcher et al. (2021) had the larger sub-sample from the cohort: N= 10,328 compared to N= 8,524 in Marshall et al. (2021). Additionally, Marshall et al. (2021) also measured the volume of the hippocampus, amygdala, ventricles, cerebellum, brain stem, and sub-regions of the basal ganglia. None of these were associated with risk of lead exposure.
Corpus callosum structure was assessed in 1 lead study (Marshall et al., 2021). In 10,328 9 to 10-year-olds from the ABCD cohort, greater risk of lead exposure was associated with smaller corpus callosum volume, except in the anterior sub-region.
Brain function was assessed in the other 2 lead studies, and both reported significant findings. Trope et al. (2001) reported that American children with elevated blood-lead levels (range: 23–65 μg/dL) had significantly lower NAA/Cr ratios in the left frontal lobe than children with never-elevated blood-lead levels. Similarly, Meng et al. (2005) reported that Chinese children with high concurrent blood-lead levels (mean: 37.7 μg/dL), compared to Chinese children with lower blood-lead levels (mean: 5.4 μg/dL), displayed reduced peak values of NAA, Cr, and Cho in the frontal lobes and hippocampus. Notably, these studies on brain function and lead had relatively small sample sizes: N= 21 (Trope et al., 2001) and N= 12 (Meng et al., 2005).
Manganese.
Manganese (Mn) exposure often occurs in mining or welding occupations (Flynn & Susi, 2009) or through contact with contaminated water (Eaton, 2021). Our search yielded 6 developmental neuroimaging studies on Mn exposure. Sample sizes were small, ranging from N= 12 (Iannilli et al., 2016) to N= 58 (Aschner et al., 2015). See Table 2.
Analyses of brain structure found that, in a sample of young adolescents, higher Mn exposure was associated with greater volume in the bilateral putamen, when cross-sectional ROI analyses of basal ganglia sub-regions were conducted (Lao et al., 2017).
Brain function in the context of Mn exposure was measured by 3 studies—all of which completed whole brain analyses, and all but 1 (Iannilli et al., 2016) analyzed resting state fMRI data. 3/4 studies found associations between metal exposure and basal ganglia function. Specifically, higher prenatal Mn exposure was associated with reduced resting state basal ganglia – PFC functional connectivity in 15 Mexican 6 and 7-year-olds (de Water et al., 2018; Pujol, Fenoll, et al., 2016). However, in another study of 14 Italian young adolescents, only postnatal Mn exposure—but not prenatal or childhood Mn exposure (as indexed by landmarks on deciduous teeth)—was associated with reduced connectivity between the basal ganglia and other brain regions (de Water, Papazaharias, et al., 2019). Finally, in the same sample of Italian adolescents as de Water, Papazaharias et al. (2019), whole brain analyses of task fMRI data revealed that higher lifetime Mn exposure was associated with reduced brain activation in olfactory and motor processing regions during an olfactory stimulation task (Iannilli et al., 2016).
Lastly, 2 of the Mn studies specifically examined T1 relaxation time, a measure of MRI signal intensity, in the basal ganglia, and both found significant associations with Mn exposure. Because Mn is paramagnetic, it appears as hyperintense on MRI images. This hyperintensity is associated with a shorter relaxation time, so a shorter relaxation time can indicate an accumulation of Mn in the brain in those at high exposure (Aschner et al., 2015). Correspondingly, in a study of newborn infants, infants with high recent Mn exposure had a shorter T1 relaxation time in the basal ganglia compared to infants at low Mn exposure (Aschner et al., 2015). In conflict with this result, however, a study of 9 to 15-year-old Canadians found the opposite: youth with higher concurrent Mn exposure had a longer T1 relaxation time in the basal ganglia (Dion et al., 2016).
3.4.3. Pesticides
Pesticides are designed to control pests, weeds, and pathogens and prevent damage to crops and plants (Damalas & Eleftherohorinos, 2011). Our search yielded 4 MRI studies on pesticides. 2 studied brain structure, and 2 studied brain function. Sample size ranged from N= 40 (Rauh et al., 2012) to N= 518 (van den Dries et al., 2020). See Table 3.
Table 3.
Key findings and study characteristics of studies on pesticide exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure | Exposure main effect |
|---|---|---|---|---|---|---|---|
| Rauh et al. (2012) | CPF | 40 | At birth | Cord blood | 5.9 – 11.2 years | Whole brain CT, surface morphology | High CPF group showed enlarged bilateral temporal and parietal lobes and in R frontal and occipital lobes. Enlargement due to white matter. High CPF group had smaller dorsal and mesial SFG and thinner dorsal parietal and frontal cortices. |
| Binter et al. (2020) | OP metabolites | 92 | Prenatal | First morning void urine sample | 10 – 12 years | Whole brain activation analysis of Go / No-go task | Higher metabolite levels associated with reduced PFC activation when successfully inhibiting. |
| van den Dries (2020) | OP metabolites (DM, DE) | 474 | Prenatal | 3 spot urine samples | 9 – 12 years | TBV, WMV, cortical GMV,subcortical GMV, vol. of CC, hippocampus, amygdala, caudate, thalamus, pallidum, putamen, accumbens. Whole brain CT and SA. FA and MD of tracts and globally. | No association with brain vol., CT, or SA. Higher metabolite levels associated with lower global FA and higher global MD. Higher DM associated with lower FA and higher MD in all tracts except L UF, forceps major, and R corticospinal tract. Higher DE associated with lower FA in L superior longitudinal fasciculus, corticospinal tract, and higher MD in L cingulate of cingulum, forceps minor, and L ILF. |
| Bahrami et al. (2022) | Pesticide (general) | 78 | Prenatal, 0-2 years, 3 – 8 years, 8-9 years | Life history calendar, parents work on farms or not | 8-9 years | Resting state functional connectivity: clustering coefficient, connection strength, global efficiency within the DMN and globally. | No differences in clustering coefficient or connection strength across the entire brain, excluding the DMN, between farmworker vs. non-farmworker children. Within the DMN, farmworker children were more likely to have connections between regions with higher clustering coefficients and less likely to have connections between regions with higher global efficiency. These metrics were associated with higher childhood pesticide exposure in the same way. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
In the structural studies, whole brain analyses of cortical thickness (Rauh et al., 2012; van den Dries et al., 2020), white matter (Rauh et al., 2012), and surface area (van den Dries et al., 2020) were conducted, as well as analyses of FA, MD, and volume of cortical and subcortical ROIs. Primary findings were associations with white matter. In (van den Dries et al., 2020), the authors found that prenatal exposure to organophosphate pesticides (OP)—a type of pesticide that disrupts the acetylcholine esterase enzyme—was associated with reduced FA and higher MD—globally and in several white matter tracts in late middle childhood. In Rauh et al. (2012), greater prenatal exposure was associated with greater white matter volume throughout the cortex in early middle childhood. In contrast, no associations with whole brain cortical thickness, surface area, or any ROI was found in van den Dries et al. (2020), while thinner dorsal parietal and frontal cortex was associated with prenatal OP exposure in Rauh et al. (2012).
In the brain function studies, 1 study examined associations between prenatal OP pesticide exposure and whole brain activation during a motor inhibition task in 10 to 12-year-olds (Binter et al., 2020), while the other study assessed differences in resting state network organization between 8 and 9-year-old children of farmworkers and non-farmworkers (Bahrami et al., 2022). The task fMRI study found that prenatal OP exposure correlated with reduced PFC activation during the motor inhibition task in late middle childhood, while the resting state study found that farmworker children had altered default mode network (DMN) organization. Specifically, within the DMN, farmworker children had more connections between regions with higher clustering coefficients—a measure of connection strength—and fewer connections between regions with higher global efficiency—a metric that detects how quickly neural signals are sent between distant regions of the brain.
3.4.4. Flame retardants
Flame retardants are commonly added to furniture, clothing, electronics, infant products, and building materials to slow the rate at which these materials burn (Fromme et al., 2016). Our search yielded 2 developmental MRI studies on brain function and flame retardants (de Water, Curtin, et al., 2019; Margolis et al., 2020), and both found statistically significant associations with prenatal flame retardant exposure. (See Table 4). There are many types of flame retardants, but only the flame retardant class polybrominated diphenyl ethers (PBDE) has been studied in the context of the developing brain. Both studies measured brain function via neural global efficiency. In de Water, Curtin, et al. (2019), functional connectivity was examined via whole brain analyses, while in (Margolis et al., 2020) analyses were conducted for the reading network—a collection of frontal, tempo-parietal, and occipito-temporal regions in the left hemisphere that support reading (Margolis et al., 2020). Both studies were also conducted in the same cohort of 34 children from New York City (Horton et al., 2013). These studies reported that, in 5-year-old children, prenatal exposure to PBDEs was associated with (1) decreased global efficiency of the reading network, and (2) decreased global efficiency throughout the brain in regions associated with visual, auditory, and sensorimotor processing, but increased global efficiency in regions associated with learning and visual attention (de Water, Curtin, et al., 2019). In de Water, Curtin, et al. (2019), local efficiency, a measure of network segregation, was also measured, but no association with prenatal PBDE exposure was detected.
Table 4.
Key findings and study characteristics of studies on flame retardant exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| De Water, Curtin et al. (2019) | PBDE | 34 | Prenatal | Blood sample | 5 years | Whole brain GE, LE | Greater PBDE associated with increased GE in regions associated with learning and visual attention and reduced GE in regions associated with visual, auditory, and sensorimotor processing. |
| Margolis et al. (2020) | PBDE | 33 | Prenatal | Blood sample | 5 years | GE of reading network | Greater PBDE associated with lower GE in the reading network. PBDE-47 and 153 contributed most to this association. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
3.4.5. Plastics: phthalates and bisphenols
Phthalates are a form of plasticizer used in building materials, personal care products, food packaging, and medical tubing (Schettler, 2006). Bisphenols are plasticizers used to produce polycarbonates—hard, clear plastics—or epoxy resins, which can be found in baby bottles, food containers, and some dental products (Geens et al., 2012). Our search yielded 2 studies on phthalate exposure (England-Mason et al., 2020; Park et al., 2015) and 1 on bisphenol exposure (Grohs et al., 2019). 2 of the studies (England-Mason et al., 2020; Grohs et al., 2019) examined white matter, measuring FA and MD of major white matter tracts in preschoolers, while 1 study (Park et al., 2015) measured whole brain cortical thickness in early middle childhood. Sample sizes ranged from N= 76 to N= 115. See Tables 5 and 6.
Table 5.
Key findings and study characteristics of studies on phthalate exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure | Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| England-Mason et al. (2020) | High and low molecular weight phthalates | 76 | Prenatal | Spot urine | 3 – 5 years | FA and MD in major white matter tracts. | HMWP exposure was associated with greater MD of the R IFO, R pyramidal fibers, and bilateral UF. Higher LMWP exposure was associated with reduced FA of the L ILF. |
| Park et al. (2015) | Metabolites of DEHP and DBP | 115 | 6 – 15 years | Spot urine | 6 – 15 years | Whole brain CT | Negative correlation between metabolites of DEHP and CT in the right middle and superior temporal gyri. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
Table 6.
Key findings and study characteristics of studies on bisphenol exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| Grohs et al. (2019) | Bisphenol A | 98 | Prenatal, age 3-4 years | Spot urine from mother and child | 2 – 5 years | FA, MD of white matter tracts | Association between prenatal BPA and greater MD of splenium and R ILF, but this did not survive multiple comparisons. No association with age 3-4 BPA. |
Note. See notes following Table 9 for a comprehensive list of abbreviations.
The 2 studies that focused on white matter measures both found that greater prenatal exposure to phthalates and bisphenols were associated with reduced FA and greater MD of white matter tracts at preschool age (England-Mason et al., 2020; Grohs et al., 2019), although the association in 1 study did not survive correction for multiple comparisons (Grohs et al., 2019). The third study (Park et al., 2015) focused on concurrent exposure to phthalates and whole brain cortical thickness in early middle childhood, finding an association between higher exposure and reduced cortical thickness in the temporal lobe.
3.4.6. Polychlorinated biphenyls
Polychlorinated biphenyls (PCBs) are legacy industrial contaminants that were once used as coolant fluids in electrical transformers or as sealants in caulks and paints; although no longer used in the U.S., they persist in the environment because of their long biological half-life (Carpenter, 2006). Our search yielded only 1 study on PCBs (Stewart et al., 2003), which assessed whether high versus low prenatal exposure to PCBs was related to the size of sub-regions of the corpus callosum in early middle childhood (N= 60 children; see Table 7). No significant associations were reported.
Table 7.
Key findings and study characteristics of studies of polychlorinated biphenyl (PCB) exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| Stewart et al. (2003) | PCB | 60 | At birth | Cord blood | ~ 7.8 years | Size of genu, splenium | No association of PCB with splenium size. Association between genu and PCB not reported. |
Note. See notes following Table S9 for table notes and a comprehensive list of abbreviations.
3.4.7. Solvents
Our search yielded 1 study (Table 8) on solvent exposure and brain function (Binter et al., 2019). The authors studied glycol ethers—a type of organic solvent used in paint, varnish, ink, cosmetics, and agriculture. Exposure was assessed prenatally, and whole brain activation patterns during a motor inhibition task were assessed in 71 French 10 to 12-year-olds. When inhibiting responses (whether correctly or not), higher exposure correlated with greater activation in the right precuneus and left cuneus; when successfully inhibiting responses, greater exposure was associated with reduced activation in left temporal and medial prefrontal regions and increased activation in right inferior frontal regions.
Table 8.
Key findings and study characteristics of studies on solvent exposure.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure |
Exposure main effect |
|---|---|---|---|---|---|---|---|
| Binter et al. (2019) | Glycol ether metabolites: | 71 | Prenatal | First morning void urine | 10-12 years | Whole brain activation analysis of go/no-go task | When inhibiting, moderate exposure was associated with greater activation in R precuneus, L cuneus, and R IFG. When successfully inhibiting, high exposure was associated with reduced activation in L temporal and medial frontal regions. |
Note. See notes following Table 9 for table notes and a comprehensive list of abbreviations.
3.4.8. Exposure to more than one toxicant class (multi-toxicant exposure)
Our search yielded 4 studies that measured more than one toxicant class (Table 9) (C.-Y. Shen et al., 2021; Sussman et al., 2022; Weng et al., 2020; White et al., 2011). Weng et al. (2020) conducted whole brain resting state fMRI analyses in a sample of 59 adolescents about whom they also had information about their prenatal exposure to phthalates, cadmium, arsenic, per- and polyfluoroalkyl substances (PFAS), and methylmercury. Associations were found between prenatal exposures to methylmercury and PFAS and altered functional connections between the basal ganglia and other brain regions. For PFAS, these associations held even when controlling for exposure to heavy metals and phthalates. In a sub-sample of 49 adolescents from the same cohort, C.-Y. Shen et al. (2021) assessed associations between prenatal exposure to the same toxicants and white matter micro-structure and gray and white matter volume. Exposures were generally negatively associated with brain volumes, general fractional anisotropy and normalized quantitative anisotropy (measures of white matter fiber tract organization), but positively associated with the isotropic value of the orientation distribution function (a measure of disorganized diffusion).
Table 9.
Key findings and study characteristics of studies on exposure to more than one toxicant class.
| First author (study year) |
Toxicant | N | Age at toxicant measure |
Toxicant measure |
Age at MRI |
MRI measure | Exposure main effect |
|---|---|---|---|---|---|---|---|
| White et al. (2011) | PCB, MeHg | 6 | At birth | Umbilical cord tissue and cord blood | 14 – 15 years | Activation analyses from photic stimulation task and finger tapping task | During the photic stimulation task, the high exposure group had greater activation in bilateral visual association areas and in R temporal and frontal lobes. During the finger tapping task, the high exposure group had greater activation in bilateral premotor and motor cortex, compared to the low exposure group whose activations were largely one-sided in motor regions. |
| Weng et al. (2020) | Phthalates , cadmium, arsenic, lead, MeHg, types of PFAS | 59 | Prenatally | Spot urine sample, blood sample | 13 – 16 years | mFALFF, mReHo | Higher phthalate exposure was associated with reduced mFALFF in R SFG and MFG and increased mReHo in L medial and inferior temporal gyrus; higherPFOS was associated with reduced mFALFF in R putamen and insula; higher PFNA with reduced mReHo in bilateral putamen and L caudate; higher lead with greater mFALFF in bilateral cuneus; higher MeHg with greater mFALFF in L superior temporal gyrus and decreased mReHo in R putamen and caudate. |
| Shen et al. (2021) | Phthalates , cadmium, arsenic, lead, MeHg, types of PFAS | 4 9 | Prenatally | Spot urine, blood sample, cord blood at birth | 13 – 16 years | Whole brain VBM, GFA, NQA ISO | Phthalates, perfluorochemicals , and heavy metals were negatively associated with cingulate, cerebellum, frontal, temporal, hippocampal, and calcarine gyrus vol, CC, corona radiata, SLF, external capsule vol. Perfluorochemicals and heavy metals were negatively associated with GFA and NQA in the CC and internal capsule. Heavy metals were positively associated with ISO in CC external capsule, SFO, and SLF. |
| Sussman et al. (2022) | PCB, PBDE | 46 | Prenatally | Blood sample | 9-11 years | ROI analyses of R IFC, SMA, R AI from Simon spatial incompatibility fMRI task | On incongruent > congruent trials, higher exposure to 2 PCBs and their sum was associated with decreased activation in the right IFC, and higher exposure to PBDE was associated with decreased activity in the rAI. No other associations detected. |
Note. Abbreviations are as follows. Abbreviations articulated earlier in the paper are not repeated. ADHD = attention deficit hyperactivity disorder. BAAN = benz[a]anthracene. BAP = benzo[a]pyrene. BEP – benzo[e]pyrene. BGP = benzo[g, h, ijperylene. CHRYS = chrysene. CPF = chlorpyrifos. CSF = cerebrospinal fluid volume. CST= corticospinal tract. CT = cortical thickness. DAP = dialkylphosphate. DBP = di-n-butyl phthalate. DE = diethylphosphate. DEHP = di(2-ethylhexyl) phthalate. DM = dimethylphosphate. EC = elemental carbon. ECAT = elemental carbon due to traffic. Fe = iron. GE = global efficiency. GFA = generalized fractional anisotropy. GMV = gray matter volume. ICV = intracranial volume. HMWP = High molecular weight phthalates. IFG = inferior frontal gyrus. ISO= isotropic value of the orientation distribution function. K = potassium. L = left. LE = local efficiency. LMWP = low molecular weight phthalates. MeHg = methylmercury. mFALFF = mean fractional amplitude of low frequency fluctuations. MFG = medial frontal gyrus. mPFC = medial prefrontal cortex. mReHo = mean regional homogeneity. mTBM = mean tensor-based morphometry. NICU = neonatal intensive care unit. N02 = nitrogen dioxide. Nox = nitrogen oxides. NQA = normalized quantitative anisotropy. OC = organic carbon. OFC = orbitofrontal cortex. OP = oxidative potential of PM2.5· PAH = polycyclic aromatic hydrocarbons. PCC= posterior cingulate cortex. PFOS = perfluorooctane sulfonate. PI = pallidal index. R = right. rCBF = regional cerebral blood flow. RN = reading network. SA = surface area. SFG = superior frontal gyrus. Si = silicon. SMA = supplementary motor area. TBV = total brain volume. T1R = T1 Relaxation time. UF = uncinate fasciculus. UFP = ultra-fine particles. VBM = voxel-based morphometry. Vol = volume. WMV = white matter volume. WMH = white matter hyperintensities. WMH+ = positive for presence of white matter hyper-intensities. WHM− = negative for presence of white matter hyperintensities. WMV = white matter volume. Zn = zinc.
In White et al. (2011), associations between whole brain activation and prenatal exposure to PCBs and methylmercury in 6 Faroese 15-year-old boys were assessed. Combined exposure to both, compared to low exposure to both, was associated with greater bilateral activation—compared to expected unilateral activation—during a photo stimulation task and a finger tapping task. In Sussman et al. (2022), prenatal exposure to PCBs and PBDEs were assessed in 46 Canadian 9 to 11-year-olds. In an ROI analysis of right inferior frontal cortex (IFC), right anterior insula (AI), and bilateral supplementary motor area activation during an inhibitory control task, prenatal PCB exposure predicted decreased activation in the right IFC, while prenatal PBDE exposure predicted decreased activation in the right AI.
4. Discussion
In the present study, we reviewed all MRI studies published in the last 22 years (01/01/2000 – 08/03/2022) that examined the association between exposure to any known or suspected neurotoxicant and brain structure or function in youths under age 18 years.
One goal of the present review was to describe the current state of the literature. On this score, our review has produced several high-level findings. First, most studies on toxicant exposure and MRI-assessed measures of the developing brain have focused on a few toxicant classes—namely air pollutants and metals. Second, most studies were of moderate quality (65%), with few high quality (22%) and few low quality (13%) studies. Third, most studies were conducted in high-income, North American or Western European locations. Our search identified no studies from low-income countries, and no studies that took place in South America, Africa, the Middle East, South Asia, or Oceania. Fourth, this field is rapidly expanding: most articles (65%) reviewed here have been published since 2017. Fifth, it was most common for reviewed studies to measure toxicant exposure prenatally or at birth (32.6%) and to conduct imaging in late middle childhood (43.5%). Finally, few studies (19.5%) used a truly independent sample. Most research was conducted using participants from a few overlapping cohorts.
In addition to assessing the current state of the literature, the second goal of the present review was to describe trends in findings within toxicant classes. These are discussed in sections 4.1 - 4.7.
4.1. Air pollution
Across the air pollution literature, two trends emerged. First, regardless of age of exposure or age at MRI, summative measures of volume, thickness, and surface area did not seem to be associated with air pollution exposure. For example, 4 studies assessed associations between total cortical white matter volume (e.g., the summed volume of white matter across the cortex), and 0 detected associations between this variable and air pollution exposure regardless of timing of exposure (age 9-10, prenatal, average from birth to approx. age 10) or timing of MRI—although all MRIs were conducted in late middle or early childhood (Cserbik et al., 2020; Guxens et al., 2018; Lubczyńska et al., 2021; Mortamais et al., 2019). Similarly, 5 studies assessed associations between total cortical gray matter volume (e.g., the summed volume of gray matter across the cortex), and 0 found associations between this variable and air pollution—despite measuring exposure at many different time points (at age 9-10, prenatally, as an average across childhood, across the first 2 years of life, as lifetime residency in a polluted area) and measuring cortical gray matter volume in late and early middle childhood (Calderón-Garcidueñas et al., 2011; Cserbik et al., 2020; Guxens et al., 2018; Lubczyńska et al., 2021; Mortamais et al., 2019). Finally, the evidence regarding mean total cortical thickness (e.g., mean thickness across the cortex) and total surface area (e.g., sum of surface areas of cortical brain regions) is weaker as both were only studied by the same 2 studies (Cserbik et al., 2020; Lubczyńska et al., 2021). However, both found null associations with air pollution. This does not mean, of course, that air pollution exposure is not associated with brain structure. Based on other findings from the present review, it likely means that different brain regions are impacted differently, creating a null additive result. Also, given that most studies had a large time lapse between assessing toxicant exposure and conducting brain imaging, it is possible that an effect exists, but measures of exposure and brain need to be closer together in time to detect it.
The second trend in the air pollution literature was that air pollution exposure was that cross-sectional exposure in late middle childhood was associated with reduced basal ganglia volume. 3 ROI studies examined associations between cross-sectional exposure to air pollution and basal ganglia volume in late middle childhood, and all 3 found the same result—smaller volumes in basal ganglia sub-regions (Alemany et al., 2018; Cserbik et al., 2020; Mortamais et al., 2017). In contrast, 3 other ROI studies of the basal ganglia and its sub-regions did not find significant results, but these studies examined exposure to air pollution in the prenatal period, as an average across all of childhood (e.g., from birth to late middle childhood), and by using lifetime residency as a proxy. Additionally, other subcortical ROIs (e.g., hippocampus, amygdala) were typically not associated with air pollution exposure. Only 1/5 studies found significant associations between air pollution exposure and other subcortical ROIs (Lubczyńska et al., 2021). One possible explanation for this pattern of results is that the basal ganglia may be more vulnerable to air pollution exposure than other regions of the subcortex. Parkinson’s disease—which is associated with the systematic degradation of the basal ganglia—has been associated with greater air pollution exposure in case-control studies (Fleury et al., 2021; Ritz et al., 2016). Moreover, the fact that the associations were observed for exposure occurring around age 10-11 could be due to the fact that this is a period of extremely rapid brain development coinciding with puberty (Blakemore et al., 2010), and the basal ganglia continue to develop into adolescence (Wierenga et al., 2014). However, these were ROI studies; whole brain studies are needed to empirically test the effects of exposure between brain regions.
4.2. Metals
The major trend evident in the extant metal literature was an association between exposure to Mn and disrupted basal ganglia structure and function. This association was present regardless of age of exposure or age at imaging and was present in 5/6 studies. It should be noted, however, that 3/6 studies on Mn studied the basal ganglia as an ROI, meaning that brain regions other than the basal ganglia might have been associated with Mn exposure in these studies but were not identified. One explanation for this association could be that Mn deposits preferentially in the basal ganglia. Support for this hypothesis comes from the alterations in signal intensity associated with Mn exposure observed in the present review. Because Mn is paramagnetic, it shows up as hyperintense on MRI images of the brain, an indication of its neuro-accumulation (Aschner et al., 2015). Possibly, accumulation of Mn in the brain changes signal intensity, alters structure, and disrupts function.
4.3. Pesticides
For pesticides, emerging trends were challenging to identify because there were only 4 studies: 2 on brain structure and 2 on brain function. Within the brain function studies completely different metrics (whole brain activation in response to a task, organization of the default mode network) were employed, making results difficult to compare. On the other hand, within the 2 brain structure studies, both found associations between greater prenatal OP exposure and alterations in white matter in early (Rauh et al., 2012) and late middle childhood (van den Dries et al., 2020). However, even here these studies employed different white matter measures, with one assessing whole brain white matter via surface morphology, and the other assessing primarily MD and FA. If indeed white matter is more vulnerable to pesticide exposure, these changes may be explained by neuroinflammation. Rodent studies demonstrate that OP exposure increases inflammatory cytokines (Henderson et al., 2002) and activates microglia (dos Santos et al., 2016), which could trigger oxidative damage—to which white matter is vulnerable.
4.4. Flame retardants
In the flame retardant literature, the emerging trend was that greater prenatal exposure to PBDE flame retardants was associated with alterations in neural global efficiency—a measure of how quickly neural signals are transmitted between distant regions of the brain (de Water, Curtin, et al., 2019; Margolis et al., 2020). Although there were only 2 flame retardant studies yielded by the search, evidence for the specificity of this trend comes from the fact that 1 study (de Water, Curtin, et al., 2019) also measured local efficiency but did not find a significant association. Because neural global efficiency is supported by white matter integrity (McDonough & Siegel, 2018), these results may implicate the developing white matter. PBDEs’ neurotoxicity may result from disrupted thyroid hormone function, damage from oxidative stress, or interference with calcium signaling and neurotransmission (Costa et al., 2014)—all of which could potentially damage white matter, and thus, alter global efficiency.
4.5. Plastics: bisphenols and phthalates
The literature on bisphenols and phthalates produced 2 primary findings: (1) greater prenatal exposure was associated with greater permeability (MD) and reduced organization (FA) of white matter tracts, and (2) greater childhood exposure was associated with differences in temporal lobe structure. Disruptions in thyroid hormone could explain these findings. Both phthalates and bisphenols have been observed to affect thyroid hormone (Ghisari & Bonefeld-Jorgensen, 2009; Moriyama et al., 2002). A well-recognized target of the thyroid hormone is the oligodendrocyte, the glial cell that makes and maintains the myelin sheath of the white matter (Anderson, 2001) and is particularly active during fetal and neonatal life (Schug et al., 2015). This means that phthalate or bisphenol exposure could alter thyroid hormones, disrupting oligodendrocytes, and, in turn, early white matter development. However, both bisphenols and phthalates have also been observed to increase oxidative stress (Quagliariello et al., 2019; Waits et al., 2020). This process might better explain the decrease in cortical thickness of the temporal lobe found in Park et al. (2015), as decreases in cortical thickness have been previously attributed to neuronal death (Spear, 2013).
4.6. Polychlorinated biphenyls and solvents
For solvents and polychlorinated biphenyls, our search produced few trends. Only 2 studies, 1 on each toxicant, were yielded by the search: Binter et al. (2019) and Stewart et al. (2003). Binter et al. (2019) reported that prenatal glycol ether exposure correlated with altered activation throughout the brain during a motor inhibition task at school age. Mechanistically, one possibility is that glycol ethers may have a similar effect on the brain to another well-known solvent—ethyl alcohol—which alters brain development through acute toxicity and by increasing oxidative stress (Ehrhart et al., 2019). Indeed, Binter et al.’s (2019) findings mirror results reported in children with prenatal alcohol exposure (Ware et al., 2015). In contrast, regarding polychlorinated biphenyls (PCB), Stewart et al. (2003) reported no association between prenatal PCB exposure and splenium or genu size in school age children. PCB has been theorized to disrupt brain development by producing thyroid hormone dysfunction (Ghassabian & Trasande, 2018), but no alterations consistent with thyroid hormone dysfunction were observed.
4.7. Exposure to more than one toxicant class
Our search yielded 4 studies that measured more than one toxicant class: C.-Y. Shen et al. (2021) and Weng et al. (2020), both of whom measured prenatal lead, phthalates, PFAS, methylmercury, cadmium, and arsenic, Sussman et al. (2022) measured prenatal PCB and PBDE, and White et al. (2011) measured prenatal PCB and methylmercury. Possibly due to the different MRI measures assessed (e.g., 2 task fMRI studies employing different tasks, 1 resting state fMRI study, 1 DTI study), there were no clear trends evident in the results of these studies. Despite the different toxicants and neural outcomes studied, both PCB and PFAS are highly biologically persistent, and both are thought to impact brain development by disrupting thyroid function (Ghassabian & Trasande, 2018)—similar to flame retardants, phthalates, and bisphenols.
4.8. Evidence for similar effects of different toxicants
The third goal of the present review was to provide evidence regarding which toxicants have similar effects on the developing brain. This evidence could be used to inform which different toxicant classes might be good candidates for study together in cumulative effect or mixture studies, which are currently rare. Because youth are regularly exposed to more than one toxicant at once (Gaylord et al., 2020), understanding how exposures interact in their effects is critical (Sexton & Hattis, 2007). In the present review, toxicants with overlapping effects largely fell into two categories – those impacting the basal ganglia and those impacting white matter. First, manganese and air pollution were both associated with effects on the basal ganglia. In the case of manganese, these effects were associated with both brain structure and function, regardless of age at toxicant exposure (prenatal, infancy, late middle childhood, adolescence all assessed) or age at MRI (infancy, early middle childhood, adolescence all assessed). In the case of air pollution, basal ganglia effects were mostly confined to effects on structure in late middle childhood. These associations may suggest a benefit of studying combined manganese and air pollution exposure in late middle childhood and measuring basal ganglia volume. Whole brain studies would be particularly beneficial, as these would help demonstrate if the associations hold when multiple brain regions are assessed. Two other groups of toxicants that might be good candidates for study together in cumulative effects studies are pesticides and flame retardants, as both displayed preliminary evidence of acting on white matter structure in the present review. Nevertheless, because few studies assessed the same toxicant and MRI measures at the same ages, comparisons of neurobiological effects across toxicants should be viewed with caution and will require future research to definitively address them.
4.9. On sensitive periods and exposures
Brain development occurs at a particularly rapid pace prenatally, in early childhood, and in adolescence (Johnson, 2001; Spear, 2008), and the brain may be more vulnerable to experience during these periods (Dunn et al., 2018; Gee, 2016). However, whether the brain is more vulnerable to toxicant exposure during periods of rapid maturation remains unknown. To determine this empirically would not only require multiple studies to measure exposures and brain outcomes at multiple time points, but also would require these studies to use the same brain outcome measure. Of 46 reviewed studies, 9 measured exposures at multiple time points, and only 1 measured the brain at multiple time points. Moreover, there was great heterogeneity among brain measures employed. For example, among studies measuring exposure prenatally (N= 12), the maximum number of studies that employed the same brain outcome measure at the same time point (e.g., fMRI task of inhibitory control in late middle childhood) was 3—and only 2 of these studies used the same task. Moreover, none of these studies measured the same toxicant exposure. Future longitudinal studies that begin in the prenatal period and measure both exposure and outcome across multiple time points are now needed to determine if neural sensitivity to toxicants differs based on developmental timing of exposure. Another possibility is that exposure may be harmful at certain critical windows of exposure that are unrelated to sensitive periods of brain development. Again, longitudinal studies with multiple measures of exposure and brain would help clarify.
4.10. Limitations
This review has limitations. First, we could not conduct a meta-analysis given the heterogeneity of study conditions (age groups, exposure measures, outcomes) and the small number of studies for some classes of toxicants. Second, we only reviewed English-language publications. Our search may not have detected studies that took place in countries where English is not the primary language for scientific writing and research.
In addition to these methodological limitations, the existing literature is also limited, and these limitations—in turn—constrain the reliability and generalizability of the conclusions of this review. Small sample sizes (e.g., N ≤ 60), use of indirect exposure measures, and measurement of toxicants with short half-lives were all common in the studies included in the present review. Small sample sizes reduce study power and precision of effect size estimates (Dick et al., 2021); use of indirect exposure measures may misclassify exposure; and, because toxicants with short half-lives (e.g., pesticides, phthalates, bisphenols, solvents) are metabolized and cleared from the body rapidly (Casas et al., 2018), exposure values for these toxicants are more likely to change. Because these practices may cause unreliability in the original studies, they make the inferences of this review less reliable. In addition, the field has yet to conduct any studies in low-income countries or in South America, Africa, the Middle East, South Asia, or Oceania. Moreover, most studies included in the present review drew from overlapping samples of existing cohort studies. 19 studies reviewed here came from just 4 cohorts: the American ABCD cohort, the Dutch Generation R cohort, the Spanish BREATHE cohort, and the American Columbia Center for Children’s Environmental Health (CCCEH) cohort. In fact, only 9 studies reviewed did not specify sampling from an existing cohort. Given that many findings reported come from a handful of well-studied individuals in Western Europe and North America, this raises questions about the generalizability of the present findings. Moreover, it is possible that some of the cohorts themselves may have problems with generalizability. For example, the Dutch Generation R cohort deliberately oversampled children for prenatal exposure to drugs, alcohol, and psychiatric medication and with behavior problems. Even the ABCD study, which is impressive for its attempts at national representativeness, acknowledges that rural American youth may be under-represented (Garavan et al., 2018). Finally, most studies (65%) in the present review were of moderate quality, which may further constrain reliability and generalizability of the review.
4.11. Future directions
The fourth and final goal of this review was to determine avenues for future research. First, more studies are needed on toxicant exposure and developing brain structure and function, regardless of toxicant class, age at exposure, or age at MRI. Even looking within air pollution—the most studied toxicant in this review—there were few studies that had comparable study designs. For example, among 5 studies on air pollution and FA, only 3 studies measured cross-sectional associations between air pollution exposure and FA in late middle childhood. Yet, even within these studies there were key differences: one study had a sample size nearly 7 times the other two; one study measured air pollution exposure via hybrid spatiotemporal modeling, while the other two assessed exposure via a high-volume sampler; not all 3 studies even measured the same air pollutant (see Table 1). More studies with comparable designs, employing the same or similar MRI and toxicant measures and measuring both at the same or similar ages, are needed regardless of toxicant class. Until more studies are conducted, the findings in this systematic review remain preliminary and subject to change as more evidence comes available.
Further, some studies have received more attention in the literature than others. Bisphenols, flame retardants, phthalates, organophosphate esters, and parabens are widely present in children’s homes and bodies (Frederiksen et al., 2013; Hoffman et al., 2018; Stapleton et al., 2009), but these toxicants have, to date, been investigated by no studies or by 1 to 2 studies each. More studies specifically on these toxicants are needed to elucidate how these toxicants interact with human brain development. Studies with comparable study designs (e.g., assessing toxicant and brain at similar ages via similar measures) would be most helpful.
Across all toxicant classes, larger sample sizes, personal exposure monitoring, more studies with specific and independent populations are also all needed. One promising and low-cost personal exposure measure is silicone wristbands, which non-invasively and reliably (K. A. Anderson et al., 2017; O’Connell et al., 2014) measure personal exposure to many toxicants, such as flame retardants, pesticides, and some air pollutants (Dixon et al., 2018; Donald et al., 2016; Hammel et al., 2016). While more research on all age groups is necessary, the fewest studies to date have taken place in infants and preschoolers—even though these are periods of rapid brain development—and suggests the need for more studies specifically in these age groups. Additionally, given that only 9 studies used samples not drawn from existing cohorts and that some existing cohort studies may have problems with representativeness, more studies of independently recruited and representative samples are needed for all toxicants reviewed.
Further, even though a majority (26/46) of the studies yielded by the search were longitudinal, only 1 longitudinal study reported developing brain results at more than one time point (Calderón-Garcidueñas et al., 2012). Critically, longitudinal studies must also recruit representative samples, maintain excellent participant retention (e.g., 80 percent or more) between study waves, and report effect sizes—as problems on these points were some of the main reasons why studies were rated moderate quality and not high quality. More studies conducting MRI at multiple time points are needed to demonstrate the persistence of identified associations. Moreover, many studies conducted ROI analyses only, which may limit our understanding of the associations of exposures across the whole brain. More studies of whole brain associations are needed. Further, only 6/46 studies assessed developing brain structure and function with more than one imaging modality. Future studies might consider using multi-modal imaging analysis to provide a more comprehensive understanding of MRI phenotypes.
Finally, additional research should investigate toxicants and brain development in youth from low-income, non-Western nations. Because these countries have unique exposure patterns and sociocultural and economic contexts (Young et al., 2021), the field cannot assume that the associations observed in the present review will generalize to these nations, nor that toxicants deemed safe in one context at one dosage will be safe in all contexts. Our findings also suggest the importance of characterizing how diverse exposures interact in their effects. Only 4 studies yielded by our search assessed more than one toxicant class. Moreover, despite measuring multiple toxicants in a study, only 1 of the multi-toxicant exposure studies controlled for exposure to the other measured toxicants in their analyses to demonstrate the unique effect of an individual toxicant, and only 1 toxicant study expressly examined the effects of combined exposure. Multiple exposures may interact additively or synergistically, as has been found in the study of toxicants and the aging brain (Cabezudo et al., 2020). Researchers should test this hypothesis by collecting brain data and exposure data on diverse toxicants. Mixture modeling could assess which combinations of toxicants are most harmful. Such information would, in turn, be critical for proper regulation of individual toxicants and toxicant mixtures.
5. Conclusion
In this systematic review, we followed PRISMA guidelines to review the last 22 years of MRI studies on toxicant exposure and the developing brain. Associations were reviewed for 9 different toxicant classes. This is the first paper to systematically review the developmental neuroimaging literature on metals, pesticides, flame retardants, phthalates, bisphenols, polychlorinated biphenyls, solvents, and multi-toxicant exposure, as well as the first to integrate this literature with the literature on air pollution. Results revealed that most studies: (1) investigated air pollutants or metals, (2) assessed exposures prenatally and the brain in late middle childhood, (3) took place in North America or Western Europe, (4) sampled predominantly from a few cohort studies, and (5) have been published since 2017.
Supplementary Material
Highlights.
We conducted a systematic review following PRISMA guidelines of MRI studies investigating associations of toxicant exposure with youth brain structure and function published in the last 22 years (01/01/2000 – 08/03/2022).
9 toxicant classes were reviewed: air pollution, metals, pesticides, flame retardants, phthalates, bisphenols, polychlorinated biphenyls, solvents, and multi-toxicant exposure.
Most reviewed studies (67%) studied air pollution or metals, assessed exposures prenatally (26%), and conducted neuroimaging in late middle childhood (43%).
Most reviewed studies also took place in Western Europe or North America (78%), drew samples from existing cohort studies (80%), and have been published since 2017 (65%).
More research on all toxicant classes reviewed here is needed. Future studies should also include larger samples, employ personal exposure monitoring, study independent samples in diverse world regions, and assess toxicant mixtures.
Acknowledgements
We would like to thank Heather M. Stapleton for providing invaluable feedback on this article, Eve Puffer for providing support during the research process, and Jodi Psoter for assistance with developing search terms and implementing search strategy. This research was supported by a Duke Institute for Brain Sciences Germinator Award and a Duke Psychology & Neuroscience Department Lafitte Award. A. Reuben was supported by the National Institute of Environmental Health Sciences, grant # F32ES34238-01.
Funding Source:
This work was supported by a Duke Institute for Brain Sciences Germinator Award and a Duke Psychology & Neuroscience Department Lafitte Award. A. Reuben was supported by the National Institute of Environmental Health Sciences, grant # F32ES34238-01. The funding sources did not have a role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none.
References
- Alemany S, Vilor-Tejedor N, García-Esteban R, Bustamante M, Dadvand P, Esnaola M, Mortamais M, Forns J, van Drooge BL, Àlvarez-Pedrerol M, Grimalt JO, Rivas I, Querol X, Pujol J, & Sunyer J (2018). Traffic-related air pollution, APOE ∈4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, spain). Environmental Health Perspectives, 126(8), 1–11. 10.1289/EHP2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GW (2001). Thyroid Hormones and the Brain. Frontiers in Neuroendocrinology, 22, 1–17. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Points GL, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG , Hoffman PD, Herbstman JB, & O’Connell SG (2017). Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. Journal of Exposure Science and Environmental Epidemiology, 27(6), 551–559. 10.1038/jes.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Anderson A, Slaughter JC, Aschner M, Steele S, Beller A, Mouvery A, Furlong HM, & Maitre NL (2015). Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. American Journal of Clinical Nutrition, 102(6), 1482–1489. 10.3945/ajcn.115.116285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami M, Simpson SL, Burdette JH, Lyday RG, Quandt SA, Chen H, Arcury TA, & Laurienti PJ (2022). Altered default mode network associated with pesticide exposure in Latinx children from rural farmworker families. NeuroImage, 256, 119179. 10.1016/j.neuroimage.2022.119179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni E, Filippini T, Crous-Bou M, Guxens M, Erickson LD, & Vinceti M (2022). The association between air pollutants and hippocampal volume from magnetic resonance imaging: A systematic review and meta-analysis. Environmental Research, 204, 111976. 10.1016/j.envres.2021.111976 [DOI] [PubMed] [Google Scholar]
- Beckwith T, Cecil K, Altaye M, Severs R, Wolfe C, Percy Z, Maloney T, Yolton K, LeMasters G, Brunst K, & Ryan P (2020). Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS ONE, 15(1), 1–19. 10.1371/journal.pone.0228092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binter AC, Bannier E, Saint-Amour D, Simon G, Barillot C, Monfort C, Cordier S, Pelé F, & Chevrier C (2020). Exposure of pregnant women to organophosphate insecticides and child motor inhibition at the age of 10-12 years evaluated by fMRI. Environmental Research, 188, 109859. 10.1016/j.envres.2020.109859 [DOI] [PubMed] [Google Scholar]
- Binter A-C, Bannier E, Simon G, Saint-Amour D, Ferré J-C, Barillot C, Monfort C, Cordier S, Chevrier C, & Pelé F (2019). Prenatal exposure to glycol ethers and motor inhibition function evaluated by functional MRI at the age of 10 to 12 years in the PELAGIE mother-child cohort. Environment International, 133(Pt A), 105163. 10.1016/j.envint.2019.105163 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–933. 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer S, & D’Angiulli A (2016). How air pollution alters brain development: The role of neuroinflammation. Translational Neuroscience, 7(1), 24–30. 10.1515/tnsci-2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Ryan PH, Altaye M, Yolton K, Maloney T, Beckwith T, LeMasters G, & Cecil KM (2019). Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12 years. Environmental Research, 175(February), 71–78. 10.1016/j.envres.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnor E, Cserbik D, Cotter D, Palmer C, Hedyeh A, Eckel S, Berhane K, McConnell R, Chen JC, Schwartz J, Jackson R, & Herting MM (2021). Association of Outdoor Ambient Fine Particulate Matter With Intracellular White Matter Microstructural Properties Among Children ∣ Child Development ∣ JAMA Network Open ∣ JAMA Network. JAMA Network Open, 4(12), e2138300–e2128300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LJ, Scammell MK, & Benson EB (2016). The Flint, Michigan, Water Crisis: A Case Study in Regulatory Failure and Environmental Injustice. Environmental Justice, 9(4), 93–97. 10.1089/env.2016.0014 [DOI] [Google Scholar]
- Cabezudo D, Baekelandt V, & Lobbestael E (2020). Multiple-Hit Hypothesis in Parkinson’s Disease: LRRK2 and Inflammation. Frontiers in Neuroscience, 14(April). 10.3389/fnins.2020.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H , Jewells V, Torres-Jardón R, Romero L, Monroy-Acosta ME, Bryant C, González-González LO, Medina-Cortina H, & D’Angiulli A (2011). Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain and Cognition, 77(3), 345–355. 10.1016/j.bandc.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Melo-Sánchez G, Rodríguez-Díaz J, Torres-Jardón R, Styner M, Mukherjee PS, Lin W, & Jewells V (2015). A critical proton MR spectroscopy marker of Alzheimer’s disease early neurodegenerative change: Low hippocampal NAA/Cr ratio impacts APOE ε 4 Mexico City children and their parents. Journal of Alzheimer’s Disease, 48(4), 1065–1075. 10.3233/JAD-150415 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henríquez-Roldán C, Pérez-Guillé B, Torres-Jardón R, Herrit L, Brooks D, Osnaya-Brizuela N, Monroy ME, González-Maciel A, Reynoso-Robles R, … Engle RW (2008). Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain and Cognition, 68(2), 117–127. 10.1016/j.bandc.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H, Torres-Jardón R, Carlos E, Solorio-López E, Medina-Cortina H, Kavanaugh M, & D’Angiulli A (2012). White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. Journal of Alzheimer’s Disease : JAD, 31(1), 183–191. 10.3233/JAD-2012-120610 [DOI] [PubMed] [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, Manzano-Salgado CB, Brochot C, Zeman F, de Bont J, Andrusaityte S, Chatzi L, Donaire-Gonzalez D, Giorgis-Allemand L, Gonzalez JR, Gracia-Lavedan E, Grazuleviciene R, Kampouri M, Lyon-Caen S, … Vrijheid M (2018). Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environment International, 121(May), 561–573. 10.1016/j.envint.2018.09.046 [DOI] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, & Shih DM, … & Furlong CE (2003). Expression of human paraoxonase (PON1) during development. Pharmacogenetics and Genomics, 13(6), 357–364. [DOI] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, & Pellacani C (2014). A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicology Letters, 230(2), 282–294. 10.1016/j.toxlet.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserbik D, Chen JC, McConnell R, Berhane K, Sowell ER, Schwartz J, Hackman DA, Kan E, Fan CC, & Herting MM (2020). Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environment International, 143, 105933. 10.1016/j.envint.2020.105933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas CA, & Eleftherohorinos IG (2011). Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. International Journal of Environmental Research and Public Health, 8(5), 1402–1419. 10.3390/ijerph8051402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO (2006). Polychlorinated Biphenyls (PCBs): Routes of Exposure and Effects on Human Health. Reviews on Environmental Health, 21(1), 1–24. 10.1515/REVEH.2006.21.1.1 [DOI] [PubMed] [Google Scholar]
- de Prado Bert P, Mercader EMH, Pujol J, Sunyer J, & Mortamais M (2018). The Effects of Air Pollution on the Brain: A Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Current Environmental Health Reports, 5(3), 351–364. 10.1007/s40572-018-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Curtin P, Zilverstand A, Sjödin A, Bonilla A, Herbstman JB, Ramirez J, Margolis AE, Bansal R, Whyatt RM, Peterson BS, Factor-Litvak P, & Horton MK (2019). A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 60(9), 1010–1020. 10.1111/jcpp.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Papazaharias DM, Ambrosi C, Mascaro L, Iannilli E, Gasparotti R, Lucchini RG, Austin C, Arora M, Tang CY, Smith DR, Wright RO, & Horton MK (2019). Early-life dentine manganese concentrations and intrinsic functional brain connectivity in adolescents: A pilot study. PLoS ONE, 14(8), 1–13. 10.1371/journal.pone.0220790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Proal E, Wang V, Martinez Medina S, Schnaas L, Tellez-Rojo MM, Wright RO, Tang CY, & Horton MK (2018). Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. NeuroToxicology, 64, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deocampo DM, Reed PJ, & Kalenuik AP (2012). Road Dust Lead (Pb) in Two Neighborhoods of Urban Atlanta, (GA, USA). International Journal of Environmental Research and Public Health, 9(6), 2020–2030. 10.3390/ijerph9062020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Lopez DA, Watts AL, Heeringa S, Reuter C, Bartsch H, Fan CC, Kennedy DN, Palmer C, Marshall A, Haist F, Hawes S, Nichols TE, Barch DM, Jernigan TL, Garavan H, Grant S, Pariyadath V, Hoffman E, … Thompson WK (2021). Meaningful associations in the adolescent brain cognitive development study. NeuroImage, 239, 118262. 10.1016/j.neuroimage.2021.118262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion L-A, Bouchard MF, Sauvé S, Barbeau B, Tucholka A, Major P, Gilbert G, Mergler D, & Saint-Amour D (2016). MRI pallidal signal in children exposed to manganese in drinking water. Neurotoxicology, 53, 124–131. 10.1016/j.neuro.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, & Anderson KA (2018). Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Analytical and Bioanalytical Chemistry, 410(13), 3059–3071. 10.1007/s00216-018-0992-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, & Anderson KA (2016). Silicone wristbands detect individuals’ pesticide exposures in West Africa. Royal Society Open Science, 3(8). 10.1098/rsos.160433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos AA, Naime AA, de Oliveira J, Colle D, dos Santos DB, Hort MA, Moreira ELG, Suñol C, de Bem AF, & Farina M (2016). Long-term and low-dose malathion exposure causes cognitive impairment in adult mice: Evidence of hippocampal mitochondrial dysfunction, astrogliosis and apoptotic events. Archives of Toxicology, 90(3), 647–660. 10.1007/s00204-015-1466-0 [DOI] [PubMed] [Google Scholar]
- Dufford AJ, Spann M, & Scheinost D (2021). How prenatal exposures shape the infant brain: Insights from infant neuroimaging studies. Neuroscience & Biobehavioral Reviews, 131, 47–58. 10.1016/j.neubiorev.2021.09.017 [DOI] [PubMed] [Google Scholar]
- Dunn EC, Nishimi K, Gomez SH, Powers A, & Bradley B (2018). Developmental timing of trauma exposure and emotion dysregulation in adulthood: Are there sensitive periods when trauma is most harmful? Journal of Affective Disorders, 227(August 2017), 869–877. 10.1016/j.jad.2017.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton A (2021). Assessment of Manganese Occurrence in Drinking Water in the United States. ACS ES&T Water, 1(11), 2450–2458. 10.1021/acsestwater.1c00293 [DOI] [Google Scholar]
- Ehrhart F, Roozen S, Verbeek J, Koek G, Kok G, van Kranen H, Evelo CT, & Curfs LMG (2019). Review and gap analysis: Molecular pathways leading to fetal alcohol spectrum disorders. Molecular Psychiatry, 24(1), 10–17. 10.1038/s41380-018-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England-Mason G, Grohs MN, Reynolds JE, MacDonald A, Kinniburgh D, Liu J, Martin JW, Lebel C, & Dewey D (2020). White matter microstructure mediates the association between prenatal exposure to phthalates and behavior problems in preschool children. Environmental Research, 182(December 2019), 109093. 10.1016/j.envres.2019.109093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency. (2002). Child-Specific Exposure Factors Handbook. [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, & Jewell NP (2007). Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environmental Health Perspectives, 115(5), 792–798. 10.1289/ehp.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury V, Himsl R, Joost S, Nicastro N, Bereau M, Guessous I, & Burkhard PR (2021). Geospatial analysis of individual-based Parkinson’s disease data supports a link with air pollution: A case-control study. Parkinsonism and Related Disorders, 83, 41–48. 10.1016/j.parkreldis.2020.12.013 [DOI] [PubMed] [Google Scholar]
- Flynn MR, & Susi P (2009). Neurological risks associated with manganese exposure from welding operations—A literature review. International Journal of Hygiene and Environmental Health, 212(5), 459–469. 10.1016/j.ijheh.2008.12.003 [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Nielsen JKS, Mørck TA, Hansen PW, Jensen JF, Nielsen O, Andersson AM, & Knudsen LE (2013). Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. International Journal of Hygiene and Environmental Health, 216(6), 772–783. 10.1016/j.ijheh.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Fromme H, Becher G, Hilger B, & Völkel W (2016). Brominated flame retardants – Exposure and risk assessment for the general population. International Journal of Hygiene and Environmental Health, 219(1), 1–23. 10.1016/j.ijheh.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, & Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord A, Osborne G, Ghassabian A, Malits J, Attina T, & Trasande L (2020). Trends in neurodevelopmental disability burden due to early life chemical exposure in the USA from 2001 to 2016: A population-based disease burden and cost analysis. Molecular and Cellular Endocrinology, 502(March 2019), 110666. 10.1016/j.mce.2019.110666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG (2016). Sensitive Periods of Emotion Regulation: Influence of Parental Care on Frontoamygdala Circuitry and Plasticity. In Maternal brain plasticity: Preclinical and human research and implications for intervention. (Vol. 153, pp. 87–110). 10.1002/cad [DOI] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon J-P, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet A-M, Pussemier L, Scippo M-L, Van Loco J, & Covaci A (2012). A review of dietary and non-dietary exposure to bisphenol-A. Food and Chemical Toxicology, 50(10), 3725–3740. 10.1016/j.fct.2012.07.059 [DOI] [PubMed] [Google Scholar]
- Ghassabian A, & Trasande L (2018). Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Frontiers in Endocrinology, 9, 204. 10.3389/fendo.2018.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisari M, & Bonefeld-Jorgensen EC (2009). Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicology Letters, 189(1), 67–77. 10.1016/j.toxlet.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Grohs MN, Reynolds JE, Liu J, Martin JW, Pollock T, Lebel C, & Dewey D (2019). Prenatal maternal and childhood bisphenol a exposure and brain structure and behavior of young children. Environmental Health : A Global Access Science Source, 18(1), 85. 10.1186/s12940-019-0528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Lubczyńska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G, van der Lugt A, Verhulst FC, White T, Brunekreef B, Tiemeier H, & El Marroun H (2018). Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biological Psychiatry, 84(4), 295–303. 10.1016/j.biopsych.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster T, Anderson KA, & Stapleton HM (2016). Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environmental Science and Technology, 50(8), 4483–4491. 10.1021/acs.est.6b00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, March TH, Sopori ML, Tesfaigzi Y, & Ménache MG (2002). Response of Rats to Low Levels of Sarin. Toxicology and Applied Pharmacology, 184(2), 67–76. 10.1006/taap.2002.9495 [DOI] [PubMed] [Google Scholar]
- Hendryx M, & Luo J (2018). Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environmental Science and Pollution Research, 25(6), 5336–5343. 10.1007/s11356-017-0874-5 [DOI] [PubMed] [Google Scholar]
- Herring MM, Younan D, Campbell CE, & Chen J-C (2019). Outdoor Air Pollution and Brain Structure and Function From Across Childhood to Young Adulthood: A Methodological Review of Brain MRI Studies. Frontiers in Public Health, 7. https://www.frontiersin.org/articles/10.3389/fpubh.2019.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Hammel SC, Phillips AL, Lorenzo AM, Chen A, Calafat AM, Ye X, Webster TF, & Stapleton HM (2018). Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environment International, 119(February), 26–36. 10.1016/j.envint.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R, Wapner R, & Factor-Litvak P (2013). Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: A cross-sectional study. Environmental Health: A Global Access Science Source, 12(1), 1–13. 10.1186/1476-069X-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannilli E, Gasparotti R, Hummel T, Zoni S, Benedetti C, Fedrighi C, Tang CY, Van Thriel C, & Lucchini RG (2016). Effects of Manganese Exposure on Olfactory Functions in Teenagers: A Pilot Study. PloS One, 11(1), e0144783. 10.1371/journal.pone.0144783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VWV, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, Moll HA, Steegers EAP, Tiemeier H, Uitterlinden AG, Verhulst FC, & Hofman A (2007). The Generation R Study Biobank: A resource for epidemiological studies in children and their parents. European Journal of Epidemiology, 22(12), 917–923. 10.1007/s10654-007-9209-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH (2001). Function brain development in humans. Nature Reviews Neuroscience, 2(July 2001), 475–483. [DOI] [PubMed] [Google Scholar]
- Karcher NR, Schiffman J, & Barch DM (2021). Environmental Risk Factors and Psychotic-like Experiences in Children Aged 9–10. Journal of the American Academy of Child & Adolescent Psychiatry, 60(4), 490–500. 10.1016/j.jaac.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Kim J-W, Lee J-M, Yun HJ, Sohn C-H, Shin M-S, Kim B, Chae J, Roh J, & Kim B-N (2018). Interaction between DRD2 and lead exposure on the cortical thickness of the frontal lobe in youth with attention-deficit/hyperactivity disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 82, 169–176. 10.1016/j.pnpbp.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N. (Nil), Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, … Zhong M (2018). The Lancet Commission on pollution and health. The Lancet, 391(10119), 462–512. 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- Lao Y, Dion L-A, Gilbert G, Bouchard MF, Rocha G, Wang Y, Leporé N, & Saint-Amour D (2017). Mapping the basal ganglia alterations in children chronically exposed to manganese. Scientific Reports, 7, 41804. 10.1038/srep41804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubczyńska MJ, Muetzel RL, El Marroun HE, Basagaña X, Strak M, Denault W, Jaddoe VWV, Hillegers M, Vernooij MW, Hoek G, White T, Brunekreef B, Tiemeier H, & Guxens M (2020). Exposure to air pollution during pregnancy and childhood, and white matter microstructure in preadolescents. Environmental Health Perspectives, 128(2). 10.1289/EHP4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubczyńska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, Hillegers M, Vernooij MW, White T, Tiemeier H, & Guxens M (2021). Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environmental Research. 10.1016/j.envres.2020.110446 [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Martin CJ, & Doney BC (2009). From manganism to manganese-induced parkinsonism: A conceptual model based on the evolution of exposure. NeuroMolecular Medicine, 11(4), 311–321. 10.1007/s12017-009-8108-8 [DOI] [PubMed] [Google Scholar]
- Mackenzie J, & Turrentine J (2016). Air pollution: Everything you need to know. NRDC: Our Stories. https://www.nrdc.org/stories/air-pollution-everything-you-need-know [Google Scholar]
- Maffini MV, & Neltner TG (2015). Brain drain: The cost of neglected responsibilities in evaluating cumulative effects of environmental chemicals. Journal of Epidemiology and Community Health, 69(5), 496–499. 10.1136/jech-2014-203980 [DOI] [PubMed] [Google Scholar]
- Margolis AE, Banker S, Pagliaccio D, De Water E, Curtin P, Bonilla A, Herbstman JB, Whyatt R, Bansal R, Sjödin A, Milham MP, Peterson BS, Factor-Litvak P, & Horton MK (2020). Functional connectivity of the reading network is associated with prenatal polybrominated diphenyl ether concentrations in a community sample of 5 year-old children: A preliminary study. Environment International, 134(March 2019), 105212. 10.1016/j.envint.2019.105212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Liu R, Conceição VA, Ramphal B, Pagliaccio D, DeSerisy ML, Koe E, Selmanovic E, Raudales A, Emanet N, Quinn AE, Beebe B, Pearson BL, Herbstman JB, Rauh VA, Fifer WP, Fox NA, & Champagne FA (2022). Convergent neural correlates of prenatal exposure to air pollution and behavioral phenotypes of risk for internalizing and externalizing problems: Potential biological and cognitive pathways. Neuroscience & Biobehavioral Reviews, 137, 104645. 10.1016/j.neubiorev.2022.104645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A, Betts SA, Kan EC, McConnel R, Lanphear BP, & Sowell ER (2020). Association of lead-exposure risk and family income with childhood brain outcomes. Nature Medicine, 26(1), 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, McConnell R, Lanphear BP, Thompson WK, Herring MM, & Sowell ER (2021). Risk of lead exposure, subcortical brain structure, and cognition in a large cohort of 9- to 10-year-old children. PLOS ONE, 16(10), e0258469. 10.1371/journal.pone.0258469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, & Siegel JT (2018). The relation between white matter microstructure and network complexity: Implications for processing efficiency. Frontiers in Integrative Neuroscience, 12(September), 1–15. 10.3389/fnint.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X-M, Zhu D-M, Ruan D-Y, She J-Q, & Luo L (2005). Effects of chronic lead exposure on 1H MRS of hippocampus and frontal lobes in children. Neurology, 64(9), 1644–1647. 10.1212/01.WNL.0000160391.58004.D4 [DOI] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, & Tamnes CK (2016). Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage, 141, 273–281. 10.1016/j.neuroimage.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, & Nakao K (2002). Thyroid hormone action is disrupted by bisphenol A as an antagonist. Journal of Clinical Endocrinology and Metabolism, 87(11), 5185–5190. 10.1210/jc.2002-020209 [DOI] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, Martínez-Vilavella G, Fenoll R, Reynes C, Sabatier R, Rivas I, Forns J, Vilor-Tejedor N, Alemany S, Cirach M, Alvarez-Pedrerol M, Nieuwenhuijsen M, & Sunyer J (2019). Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environmental Research, 178, 108734. 10.1016/j.envres.2019.108734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, Sabatier R, Rivas I, Grimalt J, Forns J, Alvarez-Pedrerol M, Querol X, & Sunyer J (2017). Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environment International, 105, 12–19. 10.1016/j.envint.2017.04.011 [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, & Anderson KA (2014). Silicone wristbands as person passive samplers. Environmental Science & Technology, 48(6), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, … Moher D (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ, 372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee JM, Kim JW, Cheong JH, Yun HJ, Hong YC, Kim Y, Han DH, Yoo HJ, Shin MS, Cho SC, & Kim BN (2015). Association between phthalates and externalizing behaviors and cortical thickness in children with attention deficit hyperactivity disorder. Psychological Medicine, 45(8), 1601–1612. 10.1017/S0033291714002694 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Bansal R, Sawardekar S, Nati C, Elgabalawy ER, Hoepner LA, Garcia W, Hao X, Margolis A, Perera F, & Rauh V (2022). Prenatal exposure to air pollution is associated with altered brain structure, function, and metabolism in childhood. Journal of Child Psychology and Psychiatry, n/a(n/a). 10.1111/jcpp.13578 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, & Perera F (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry, 72(6), 531–540. 10.1001/jamapsychiatry.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, & Weuve J (2016). Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. NeuroToxicology, 56, 235–253. 10.1016/j.neuro.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Fenoll R, Macià D, Martínez-Vilavella G, Alvarez-Pedrerol M, Rivas I, Forns J, Deus J, Blanco-Hinojo L, Querol X, & Sunyer J (2016). Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain and Behavior, 6(6), e00467. 10.1002/brb3.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Martínez-Vilavella G, Macià D, Fenoll R, Alvarez-Pedrerol M, Rivas I, Forns J, Blanco-Hinojo L, Capellades J, Querol X, Deus J, & Sunyer J (2016). Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage, 129, 175–184. 10.1016/j.neuroimage.2016.01.036 [DOI] [PubMed] [Google Scholar]
- Quagliariello V, Coppola C, Mita DG, Piscopo G, Iaffaioli RV, Botti G, & Maurea N (2019). Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environmental Toxicology and Pharmacology, 69(February), 1–8. 10.1016/j.etap.2019.03.006 [DOI] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, & Peterson BS (2012). Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America, 109(20), 7871–7876. 10.1073/pnas.1203396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Caspi A, Belsky DW, Broadbent J, Harrington H, Sugden K, Houts RM, Ramrakha S, Poulton R, & Moffitt TE (2017). Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA—Journal of the American Medical Association, 317(12), 1244–1251. 10.1001/jama.2017.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Schaefer JD, Moffitt TE, Broadbent J, Harrington H, Houts RM, Ramrakha S, Poulton R, & Caspi A (2019). Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry, 76(4), 418–425. 10.1001/jamapsychiatry.2018.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Lee PC, Hansen J, Lassen CF, Ketzel M, Sørensen M, & Raaschou-Nielsen O (2016). Traffic-related air pollution and parkinson’s disease in Denmark: A case–control study. Environmental Health Perspectives, 124(3), 351–356. 10.1289/ehp.1409313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas I, Viana M, Moreno T, Pandolfi M, Amato F, Reche C, Bouso L, Àlvarez-Pedrerol M, Alastuey A, Sunyer J, & Querol X (2014). Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environment International, 69, 200–212. 10.1016/j.envint.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Sarigiannis DA, & Hansen U (2012). Considering the cumulative risk of mixtures of chemicals – A challenge for policy makers. Environmental Health, 11(1), S18. 10.1186/1476-069X-11-S1-S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T (2006). Human exposure to phthalates via consumer products. International Journal of Andrology, 29(1), 134–139. 10.1111/j.1365-2605.2005.00567.x [DOI] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, & Lawler CP (2015). Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology, 156(6), 1941–1951. 10.1210/en.2014-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, & Hattis D (2007). Assessing cumulative health risks from exposure to environmental mixtures—Three fundamental questions. Environmental Health Perspectives, 115(5), 825–832. 10.1289/ehp.9333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C-Y, Weng J-C, Tsai J-D, Su P-H, Chou M-C, & Wang S-L (2021). Prenatal Exposure to Endocrine-Disrupting Chemicals and Subsequent Brain Structure Changes Revealed by Voxel-Based Morphometry and Generalized Q-Sampling MRI. International Journal of Environmental Research and Public Health, 18(9), 4798. 10.3390/ijerph18094798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Hou D, Zhang P, Wang Y, Zhang Y, Shi P, & O’Connor D (2018). Lead-based paint in children’s toys sold on China’s major online shopping platforms. Environmental Pollution, 241(2018), 311–318. 10.1016/j.envpol.2018.05.078 [DOI] [PubMed] [Google Scholar]
- Spear LP (2008). The psychobiology of adolescence. Authoritative Communities (pp. 263–280). Springer, New York, NY. [Google Scholar]
- Spear LP (2013). Adolescent Neurodevelopment. Journal of Adolescent Health, 52(2, Supplement 2), S7–S13. 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, & Webster TF (2009). Detection of Organophosphate Flame Retardants in Furniture Foam and US House Dust. Environmental Science and Technology, 43(19), 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, & Hauser P (2003). Prenatal PCB exposure, the corpus callosum, and response inhibition. Environmental Health Perspectives, 111(13), 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman TJ, Baker BH, Wakhloo AJ, Gillet V, Abdelouahab N, Whittingstall K, Lepage J-F, St-Cyr L, Boivin A, Gagnon A, Baccarelli AA, Takser L, & Posner J (2022). The relationship between persistent organic pollutants and Attention Deficit Hyperactivity Disorder phenotypes: Evidence from task-based neural activity in an observational study of a community sample of Canadian mother-child dyads. Environmental Research, 206, 112593. 10.1016/j.envres.2021.112593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope I, Lopez-Villegas D, Cecil KM, & Lenkinski RE (2001). Exposure to lead appers to selectively alter metabolism of cortical gray matter. Pediatrics, 107, 1437–1442. [DOI] [PubMed] [Google Scholar]
- van den Dries MA, Lamballais S, El Marroun H, Pronk A, Spaan S, Ferguson KK, Longnecker MP, Tiemeier H, & Guxens M (2020). Prenatal exposure to organophosphate pesticides and brain morphology and white matter microstructure in preadolescents. Environmental Research, 191(May). 10.1016/j.envres.2020.110047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele-Fabry G, Hoet P, Vilain F, & Lison D (2012). Occupational exposure to pesticides and Parkinson’s disease: A systematic review and meta-analysis of cohort studies. Environment International, 46, 30–43. 10.1016/j.envint.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Waits A, Chen HC, Kuo PL, Wang CW, Huang H. Bin, Chang WH, Shih SF, & Huang PC (2020). Urinary phthalate metabolites are associated with biomarkers of DNA damage and lipid peroxidation in pregnant women – Tainan Birth Cohort Study (TBCS). Environmental Research, 188(April). 10.1016/j.envres.2020.109863 [DOI] [PubMed] [Google Scholar]
- Ware AL, Infante MA, O’Brien JW, Tapert SF, Jones KL, Riley EP, & Mattson SN (2015). An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behavioural Brain Research, 278, 137–146. 10.1016/j.bbr.2014.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J-C, Hong CI, Tasi J-D, Shen C-Y, Su P-H, & Wang S-L (2020). The association between prenatal endocrine-disrupting chemical exposure and altered resting-state brain fMRI in teenagers. Brain Structure and Function, 225(5), 1669–1684. 10.1007/s00429-020-02089-4 [DOI] [PubMed] [Google Scholar]
- White RF, Palumbo CL, Yurgelun-Todd DA, Heaton KJ, Weihe P, Debes F, & Grandjean P (2011). Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology, 32(6), 975–980. 10.1016/j.neuro.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, & Durston S (2014). Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage, 96, 67–72. 10.1016/j.neuroimage.2014.03.072 [DOI] [PubMed] [Google Scholar]
- Wilson J, Dixon SL, Wisinski C, Speidel C, Breysse J, Jacobson M, Crisci S, & Jacobs DE (2022). Pathways and sources of lead exposure: Michigan Children’s Lead Determination (the MI CHILD study). Environmental Research, 215, 114204. 10.1016/j.envres.2022.114204 [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Moya J, Freeman N, Beamer P, Black K, Tulve N, & Shalat S (2007). A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Analysis, 27(2), 411–420. 10.1111/j.1539-6924.2007.00893.x [DOI] [PubMed] [Google Scholar]
- Young AS, Herkert N, Stapleton HM, Cedeño Laurent JG, Jones ER, MacNaughton P, Coull BA, James-Todd T, Hauser R, Luna ML, Chung YS, & Allen JG (2021). Chemical contaminant exposures assessed using silicone wristbands among occupants in office buildings in the USA, UK, China, and India. Environment International, 156. 10.1016/j.envint.2021.106727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.