Abstract
Background
Selective reporting of antibiotic susceptibility testing (AST) is a recommended antibiotic stewardship strategy, aiming at reducing inappropriate antibiotic prescriptions.
Objectives
Our objectives were to evaluate (i) the feasibility of the implementation of selective reporting of AST for urine cultures for laboratory professionals; and (ii) its acceptability by prescribers and laboratory professionals, to explore facilitators and barriers to its potential implementation on a national scale.
Methods
As part of the ‘ANTIBIO-ciblés’ interventional study (north-eastern France, August 2018–December 2019), we prospectively collected quantitative data on all resources dedicated by the laboratories of the intervention group to implement selective reporting of AST for Escherichia coli-positive urine cultures, and on the numbers and reasons of complete reporting of AST the prescribers requested to the laboratories. We also collected qualitative data using semi-structured interviews and focus groups of GPs and laboratory professionals.
Results
The implementation of selective reporting of AST required around 80 h and cost 23 000 euros. All interviewed professionals were favourable toward the principle of this tool. Most of them found it clear, simple and useful to improve the appropriateness of antibiotic prescriptions and reduce antibiotic resistance. Its major constraint was the necessity for GPs to call the laboratory to obtain the complete reporting of AST, but the number of requests was actually low (1.2% of all selective reporting of AST).
Conclusions
Selective reporting of AST resulted in reasonable human and financial costs, and was well accepted by both GPs and laboratory professionals.
Introduction
The WHO underlines that antimicrobial resistance is one of the 10 most pressing threats to global public health.1 In France, 125 000 persons are infected by MDR bacteria each year, causing 5500 deaths.2 A situation of greatest concern is antibiotic-resistant Escherichia coli (E. coli), the major pathogen for urinary tract infections (UTIs), particularly to third-generation cephalosporins and fluoroquinolones.3 Antibiotic resistance is mainly driven by unnecessary and inappropriate use of antibiotics,4 notably by the use of antibiotics that carry a higher risk of selection of resistance, called ‘critical’ antibiotics in France.5
Antimicrobial stewardship programmes are defined as a coherent set of actions that promote limiting unnecessary and inappropriate antimicrobial use.6 Among the list of potential actions, selective reporting of antibiotic susceptibility testing (AST) results aims at guiding prescriptions to the most appropriate antibiotics, i.e. first-line antibiotics recommended in national guidelines.7 Based on encouraging results from observational retrospective studies, selective reporting of AST has been recommended in several guidelines to limit inappropriate antibiotic prescribing.8–10 However, it has never been evaluated using an experimental design and is not routinely used in France.
In this context, we conducted a large interventional, controlled, before–after study (the ‘ANTIBIO-ciblés’ study) to assess the impact of selective reporting of AST for E. coli-positive urine cultures on the prescription of critical antibiotics in the French outpatient setting.11 As part of the secondary objectives of this study, we present here the evaluation of (i) the feasibility of the implementation of selective reporting of AST for laboratories; and (ii) its acceptability by prescribers and laboratory professionals, as well as facilitators and barriers to inform implementation and scale-up.
Methods
Study design
Details on the ‘ANTIBIO-ciblés’ protocol have been previously published and are available in File S1 (available as Supplementary data at JAC Online).11
To evaluate the feasibility, we prospectively collected (i) quantitative data on all resources dedicated by the laboratories to implement selective reporting of AST; and (ii) the percentage of selective AST reported out of the total number of E. coli-positive urine cultures with AST performed in adult outpatients.
To evaluate acceptability to prescribers, we collected (i) quantitative data on the numbers and percentages of complete reporting of AST requests to the laboratories and their reasons; and (ii) qualitative data from semi-structured individual interviews among GPs. To evaluate acceptability to laboratory professionals, we performed semi-structured focus groups, supplemented by some individual interviews when laboratory professionals could not participate in the focus group. Qualitative investigations comply with the COREQ reporting guidelines (Table S2).12
Study setting and participants
Selective reporting of AST implementation began on 1 September 2018 in the ATOUTBIO group of 21 laboratories located in the Lorraine region (2 306 000 inhabitants according to the 2021 census)13 of north-eastern France.
GPs eligible for the qualitative investigation were those who had received at least one selective reporting of AST from one ATOUTBIO laboratory during the previous year, and were identified from the ATOUTBIO laboratory records. We excluded those with subspecialty practices (e.g. homeopathy, gynaecology), identified through an online directory. GPs were then randomly selected and contacted by phone by a member of the research team (G.L.D.) to explain the present investigation and to ask them for an interview. The randomization was stratified by practice location (rural, suburban, urban). The recruitment continued until data saturation was reached.
Laboratory professionals were recruited by laboratory managers in collaboration with the biologist co-investigator (S.F.). Laboratory managers who accepted to participate in the qualitative investigation were asked to recruit at least two of their staff members from various professions (i.e. biologists, technicians and secretaries) to participate in a focus group. If some laboratory professionals could not participate, they were offered to be individually interviewed.
Selective reporting of AST
Selective reporting of AST was performed for all adult outpatients with an E. coli-positive urine culture. Selective reporting of AST was automatically executed by algorithmic software, according to gender and resistance of the isolate.7 Details on the intervention are available in File S3, Table S4 and Table S5.
The ATOUTBIO laboratories informed each prescriber of the change in reporting of AST and that the complete reporting of AST was available upon request to the laboratory (e.g. phone call) in a paragraph of information included in the two first selective AST reports they received.
Data collection
Feasibility
During the selective reporting of AST’s development and adjustment (August 2018–February 2019) periods, ATOUTBIO laboratories’ biologists prospectively collected all material/informatics, financial and human laboratory resources dedicated to the implementation of the selective reporting of AST, using a standardized form provided by the research team. They also collected the number of selective AST reported and the total number of E. coli-positive urine cultures with AST performed from January to December 2019.
Acceptability
From January to December 2019, laboratories’ biologists recorded each prescriber’s request for a complete reporting of AST and their motive.
For the qualitative investigation of acceptability, two interview guides (one for GPs and one for laboratory professionals) were developed by G.L.D. (epistemologist, PhD) and reviewed by N.T. (public health pharmacist, PhD), C.P. (infectious diseases physician, PhD) and J.K. (sociologist, PhD). Questions were defined a priori using the Proctor et al.14 definition of acceptability (i.e. content, complexity, comfort, delivery and credibility) and based on a literature review on barriers frequently faced by GPs in their antibiotic stewardship practices, and their attitude towards new tools to guide their prescriptions. The interview guide for GPs explored five themes: (i) their perceptions about antibiotic resistance; (ii) their opinion on selective reporting of AST; (iii) its perceived impact on their practices; (iv) potential constraints regarding its use; and (v) their expectations (e.g. about information and communication, generalization) (Table S6). The interview guide for laboratory professionals explored the same themes, except for theme (iii) (Table S7). Interviews were conducted in September and October 2019 by G.L.D. and M.S. (PhD student) at the professional’s workplace. After oral consent, they were recorded, anonymized and transcribed by M.S. and G.L.D.
Analyses
Quantitative data were analysed using descriptive statistics (numbers and percentages). Qualitative data (interview transcripts and handwritten notes) were analysed by G.L.D. and M.S. through a thematic analysis using analysis grids. We used themes defined prior to interviews (same themes of the interview guides), and would have allowed new themes to emerge. Each theme and subtheme (detailed in the Results section) were discussed until consensus was found between G.L.D. and M.S. The analyses were conducted using the QSR International N’Vivo 11 and Excel software.
Ethics
This protocol was approved by French national ethics committees [Comité d’expertise pour les recherches, les études et les évaluations dans le domaine de la santé (TPS 29064) and Commission Nationale de l’Informatique et des Libertés (Décision DR-2018–141)].
Registration
ClinicalTrials.gov identifier is NCT03612297.
Results
Feasibility
The development and adjustment of selective reporting of AST resulted in a total cost for the ATOUTBIO laboratory group of 3610 euros for human resources (i.e. 80 working hours of the co-investigator and technician to program, test and adjust the parameters of the software). Besides, 17 760 euros were dedicated to the purchase of the software, and 1335 euros to the annual maintenance, amounting in total to an overall cost of 19 095. In 2019, the ATOUTBIO laboratories reported selective AST for 100% of adult outpatients with an E. coli-positive urine culture.
Prescriber acceptability (quantitative data)
The proportion of selective reporting of AST giving rise to a request for a complete reporting of AST in 2019 was 1.2% (134/11 624; see Table S8 for details per month). The main reasons given by the prescribers for these requests were that (i) their patient had pyelonephritis and they did not want to prescribe one of the antibiotics reported (i.e. amoxicillin or co-trimoxazole), and wanted access to broader-spectrum antibiotics (mostly fluoroquinolones) on the selective reporting of AST; and (ii) they initiated empirical therapy that was not among the reported antibiotics (fluoroquinolones and third-generation cephalosporins mostly mentioned) and did not want to modify the treatment (Table 1).
Table 1.
Reasons given by prescribers for requesting a complete report of AST from January to December 2019 (n = 134 out of the 11 624 selective AST reported in 2019)
| Reasons for the request | Number (%) |
|---|---|
| Pyelonephritis | 45 (33.6) |
| Already initiated antibiotic therapy | 39 (29.1) |
| Reason not specified | 26 (19.4) |
| Particular clinical casea | 12 (9.0) |
| Prostatitis | 4 (3.0) |
| Resistant to all antibiotics | 4 (3.0) |
| Formulation issueb | 4 (3.0) |
Particular clinical cases mentioned were kidney failure, adverse effect, cancer, porphyria, prostatectomy, recurring infection, allergies.
No non-injectable molecule reported, or no injectable molecule reported when patient needed one.
GP acceptability (qualitative data)
Characteristics of the participants
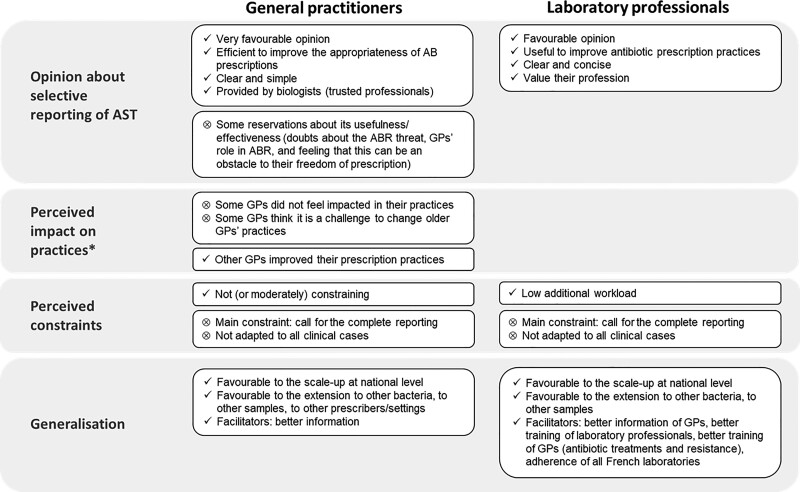
Data saturation was reached after interviewing 21 GPs and we had to contact a total of 74 GPs to recruit these participants (participation rate: 28%); 42 refused to participate (main reason: lack of time) and 11 GPs could not be reached and did not contact us back. Half of the participants practised in a rural area (Table 2). Interviews lasted on average 20 ± 7 min. Figure 1 presents the thematic results of GP acceptability. Table 3 presents a selection of the most illustrative quotes from GP interviews.
Table 2.
Characteristics of the participants in the qualitative interviews
| Number of participants | Profession | Practice settinga | Gender | |
|---|---|---|---|---|
| GPs | ||||
| Individual interviews | 21 | GPs | Rural: 10 Suburban: 6 Urban: 5 |
Female: 9 Male: 12 |
| Laboratory professionals | ||||
| Focus group #1 | 3 | 1 manager biologist 2 biologists |
Urban Suburban Rural |
Female: 2 Male: 1 |
| Focus group #2 | 5 | 1 manager biologist 1 biologist 1 laboratory technician 1 biology student 1 secretary |
Urban | Female: 3 Male: 2 |
| Focus group #3 | 3 | 2 manager biologists 1 secretary |
Urban | Female: 3 |
| Individual interviews | 2 | Manager biologists (including the biologist co-investigator) | Rural Urban Suburban |
Female: 1 Male: 1 |
Rural, <10 000 inhabitants; suburban, 10 000–30 000 inhabitants; urban, >30 000 inhabitants. In France, laboratory biologists can work in multiple laboratories, located in various settings.
Figure 1.
Thematic results of acceptability to GPs and laboratory professionals. * Not explored in the interview guide for laboratory professionals. AB, antibiotic; ABR, antibiotic resistance.
Table 3.
Selection of the most illustrative quotes from GP interviews
| Theme | Subtheme | Quote (interview number) |
|---|---|---|
| Opinion about selective reporting of AST | Favourable opinion | ‘This is a progress this method. A progress. This is a progress that makes us work better, be more efficient’ (no. 20) ‘I think this is useful, and I think that helps us… Adapt to new guidelines easily…’ (no. 10) |
| Clarity/simplicity | ‘I think it was well thought/designed. It was clear and accurate’ (no. 2) ‘Something reduced is really better, clearly’ (no. 19) |
|
| Reservations | ‘However, when we asked for a urine culture in case of pyelonephritis suspicion, that [the AST selective report] was much less relevant’ (no. 14) ‘The GP who is used to prescribe ‘wrong’ […] Maybe he/she will keep his/her habits, will do it [prescribe ‘wrong’], and will not consider the AST results. Because he/she is used to it working like that’ (no. 15) |
|
| Perceived impacts on practices | No impact | ‘So it does not change my practice’ (no. 7) ‘I barely noticed [the change in AST report], it’s only when I heard about it and I took a step back, and I said ah yes’ (no. 12) |
| Positive impact | ‘So the selective reporting of AST doesn’t impact your prescriptions?—It does, for example, last time, I prescribed Bactrim® [co-trimoxazole] that I had never prescribed before’ (no. 15) | |
| Perceived constraints | Not or moderately constraining | ‘I was not bothered in my practice, in my patients’ care, in the selection of the treatment to choose’ (no. 5) ‘It doesn’t disturb me at all’ (no. 8) |
| Asking for the complete report | ‘I need to know if Augmentin® [amoxicillin/clavulanate] works, if Oroken® [cefixime] works, if the main fluoroquinolones work’ (no. 6) ‘Because when I have an antibiotic that is not on the selective reported list, it means calling the laboratory. Usually, they make me talk to the biologist, after a while, and either I have the patient in front of me, or it is late at night, so I wait…’ (no. 7) |
|
| Expectations regarding information and generalization | Better information | ‘It would have been interesting to have the information, because our reasoning…’ (no. 7) ‘A letter of explanation would have made it clearer’ (no. 14) |
| Extension to other bacteria and/or other samples | ‘I think the best is what you did, target the most common [bacteria, i.e. E. coli]. And do that in other pathologies’ (no. 2) ‘Yes of course! It might be done for all germs’ (no. 14) |
|
| Extension to other prescribers | ‘Why not do it for everyone? That’s better’ (no. 2) | |
| National implementation | ‘Do you think it might be implemented at a national scale?—Sure. Sure. For me, this is a good thing’ (no. 2) |
Opinion about selective reporting of AST
Most GPs (15/21) had already understood the principle and objectives of selective reporting of AST before the interview, most frequently after explanations from the laboratory professionals during the implementation. The others (6/21) understood it through explanations provided at the beginning of the interview.
Once the principle and objectives were understood, all GPs were very favourable toward selective reporting of AST. Most of them described it as an adapted, efficient and time-saving tool to improve the appropriateness of antibiotic prescriptions, and tackle antibiotic resistance, which the majority (18/21) perceived as a major public health threat. Most GPs (15/21) highlighted the clarity and the simplicity of the selective report, which provided the list of appropriate antibiotics according to guidelines in a concise manner. Moreover, some GPs (7/21) highlighted that this tool was provided by biologists, who they trusted and who advised them appropriately.
However, despite this favourable opinion, a few GPs (9/21) had some reservations about the usefulness and effectiveness of selective reporting of AST, mainly due to: the belief that GPs do not have a main role in antibiotic resistance; or the feeling this tool was an obstacle to their prescription freedom.
Perceived impacts on practices
GPs’ perceptions about the impact of selective reporting of AST on practices were mixed.
Many GPs (13/21), especially those who understood the principle of the tool at the beginning of the interview only, did not feel impacted in their practices.
The others (8/21) felt that this tool led them to modify their prescription practices and to improve their compliance with guidelines. It often prompted them to abandon ‘critical’ antibiotics, and sometimes gave them the opportunity to discover antibiotics they never prescribed before.
However, some GPs were convinced that certain colleagues (especially older ones) will never accept change to their prescription practices.
Perceived constraints regarding the use of selective reporting of AST
Most GPs found the use of selective reporting of AST not, or moderately, constraining. The main constraint (8/21 GPs) was the necessity to call the laboratory to obtain the complete reporting, and sometimes to wait several days for it.
GPs requested the complete reporting of AST for several reasons consistent with results from quantitative data (Table 1) and provided the following explanations: (i) for some GPs, selective reporting of AST was not adapted to their patient’s case (pyelonephritis, recurrent UTIs, or allergies); (ii) for pyelonephritis, a few GPs were used to prescribing fluoroquinolones, these being considered as the most appropriate choice, and did not accept changing; (iii) they did not want to change the treatment after receiving the selective reporting to avoid scheduling another appointment with the patient to prescribe another antibiotic, and preferred to continue the treatment initially prescribed if it was effective.
Expectations regarding information and generalization
Most GPs (16/21) would have liked to be better informed of the implementation of the selective reporting of AST (e.g. by a dedicated letter from the laboratory).
Overall, GPs were in favour of the extension of the selective reporting of AST to other bacteria, to other samples than urinary ones, and to other settings (e.g. hospitals). They were also all favourable toward its scale-up at national level.
Laboratory professional acceptability (qualitative data)
Characteristics of the participants
Of four laboratory managers contacted, three agreed to participate in the qualitative investigation. Overall, nine biologists, one biology student, one technician and two secretaries were interviewed (see characteristics in Table 2). The focus groups lasted on average 29 ± 2 min. Figure 1 presents the thematic results of acceptability to laboratory professionals. Table 4 presents a selection of the most illustrative quotes.
Table 4.
Selection of the most illustrative quotes from laboratory professional interviews
| Theme | Subtheme | Quote (focus group or interview number) |
|---|---|---|
| Opinion about selective reporting of AST | Favourable opinion | ‘So our laboratory, anyway, we adhered at 100%’ (no. 2) ‘Of course, it’s a great strategy, that’s for sure’ (no. 3) |
| Clarity/simplicity | ‘It will guide them [prescribers], it will target antibiotics that should be used’ (no. 4) ‘I think we’re heading for simplicity for the GP’ (no. 5) |
|
| Useful tool to value their profession | ‘This is not an effort; we are ready to participate because this is interesting and it values our profession’ (no. 1) ‘No this is rewarding! But it is only the beginning’ (no. 3) |
|
| Perceived constraints | Minimal constraint | ‘And we did not really have barriers to the realization of this project’ (no. 2) ‘We only have few requests from GPs who want complete AST results’ (no. 4) |
| Complete reporting requests | ‘At the beginning, some GPs were unsatisfied’ (no. 4) ‘Maybe the first weeks, there were some GPs who were a little aggressive, telling ‘I give this or that antibiotic, I would like the complete report’’ (no. 2) |
|
| Not adapted to all clinical cases | ‘For cases of pyelonephritis or prostatitis, it was trickier’ (no. 2) ‘They [the prescribers] are fed up, for each pyelonephritis, to have to request an appropriate molecule. We totally understand that’ (no. 5) |
|
| Expectations regarding information and generalization | Better information/training | ‘My training on antibiotic therapy is outdated, so we really need to keep abreast, even for biologists younger than me. So we really need support’ (no. 2) |
| Prescribers’ information | ‘Technically, to sum up, it should be an information before starting’ (no.1) ‘I think we have to deliver information before, yes, but not for too long’ (no. 2) |
|
| Extension to other bacteria and/or other samples | ‘We could extend it to other samples. That’s the point also, to try to target more and more things, not only urine cultures’ (no. 5) | |
| National implementation | ‘So we all agree that to extend it to a national scale and for long term is good?—Yes!’ (no. 3) | |
| Competitive sector | ‘Be sure that all biologists adhere. That we are not the only ones to annoy and pressure the medical profession. We must be united with our biologist colleagues. But this is not easy. […] We are a competitive sector so sometimes we disregard the antibiotic stewardship pressure, and we submit to the physician pressure to please him/her’ (no. 3) |
Opinion about selective reporting of AST
All laboratory professionals were in favour of selective reporting. They found it interesting and useful to improve prescription practices and tackle antibiotic resistance, which all perceived as a significant threat. They believed that its clear and concise presentation facilitated prescribers’ choice and guided them to prescribe antibiotics in accordance with guidelines.
Moreover, selective reporting of AST was considered as a necessary tool to value their profession and support their role in the global effort against antibiotic resistance.
Perceived constraints regarding selective reporting implementation
The laboratory professionals perceived the additional workload of the implementation of selective reporting of AST as very low. It had mainly fallen on the biologist co-investigator.
The main constraint was calls from unsatisfied prescribers (mostly at the beginning of the implementation) who asked for the complete reporting. The information about the modification of reporting of AST was not seen by many prescribers who called, perceiving selective reporting as an error or low-cost AST.
However, as the number of phone calls was very low and related mainly to a lack of information, laboratory professionals perceived this constraint as minimal and bearable. Moreover, several laboratory professionals believed that the tool was not completely adapted to some particular clinical cases (e.g. pyelonephritis and prostatitis), and thus were understanding towards GPs.
Expectations regarding information and generalization
Laboratory professionals would have liked to be more informed and trained on the purpose and the conception of the selective reporting of AST, to provide more accurate support to prescribers. They were convinced that prescribers should be better informed about the implementation of selective reporting to improve acceptability. They also believed that better training of prescribers on antibiotics and awareness on antibiotic resistance would increase the impact of selective reporting of AST.
All laboratory professionals were in favour of the extension of selective reporting of AST to other bacteria and other samples (e.g. ear/nose/throat or gynaecological). Some professionals also suggested to add some clinical information to the algorithmic software (e.g. fever, lumbar pain), to help differentiate clinical situations and adapt the antibiotics reported to the indication.
Finally, all laboratory professionals strongly supported the scaling-up of selective reporting of AST at national level. However, some of them highlighted that, in a competitive context, this would require global adherence of all laboratories to implement it.
Discussion
Main results
Selective reporting of AST for E. coli-positive urine cultures, implemented as part of the ‘ANTIBIO-ciblés’ French interventional study, required about 80 working hours from laboratory staff and costed about 23 000 euros. Considering that there are 400 laboratory groups in France, the national implementation of selective reporting of AST would have resulted in 32 000 working hours and 9.2 million euros.15
All GPs and laboratory professionals interviewed were favourable toward the principle of selective reporting of AST. Most of them described it as a clear, simple and useful tool to improve the appropriateness of antibiotic prescriptions and reduce antibiotic resistance. Its major constraint was the necessity for GPs to call the laboratory to obtain the complete reporting of AST but the number of requests was actually low. All professionals supported the extension of this tool to other bacteria, samples and the scaling-up at national level, accompanied by information of prescribers.
Comparison with the literature
The acceptability of selective reporting of AST was poorly studied in the literature. Results from two French case-vignette studies showed that 81% of GPs16 and 71% of trainees in general medicine17 who experimented with selective reporting of AST felt that it made their therapeutic choices easier. In a French qualitative study that explored perceptions regarding antibiotic stewardship interventions by different medical specialists, most participants had a positive opinion on selective reporting of AST.18 However, another study in primary care showed that French GPs were reluctant to accept measures that restrict their freedom of practice, and selective reporting of AST might have been perceived as such.19 To the best of our knowledge, no previous study has evaluated the acceptability of selective reporting of AST by laboratory professionals.
The high acceptability of selective reporting of AST by GPs we recorded reveals their will and readiness to the use of this type of guiding tool to improve their antibiotic prescriptions.18 Moreover, GPs highlighted the important mission of laboratory professionals to advise them on appropriate prescribing. Indeed, it has been shown that clinical microbiologists can have a key role in antibiotic stewardship programmes and a profound impact on prescribing habits.20
Main perceived constraint: requests for complete reporting
Despite infrequent requests for the complete reporting of AST (1.2% of cases), some GPs found the necessity to call the laboratory to obtain the complete reporting as constraining. These requests were motivated by two main reasons, observed in both the quantitative and qualitative results.
First, some GPs believed that selective reporting of AST was not adapted to all clinical cases, such as pyelonephritis. While some molecules recommended as targeted therapy in pyelonephritis (e.g. co-trimoxazole) were always reported on the selective reporting, GPs wanted to prescribe fluoroquinolones, reported only in cases of resistance to first-line agents. The determination of some GPs to prescribe fluoroquinolones might be related to prescription habits they were not ready to change. Some GPs also perceived that selective reporting of AST was not adapted for recurrent UTIs or allergies, whereas those cases had been taken into account in the development of the selective report. Better training of GPs on antibiotic prescribing recommendations might help them better understand the relevance of the selective report and thus improve the appropriateness of their antibiotic prescriptions.
Second, some GPs had initiated empirical therapy and did not want to modify this treatment for an antibiotic reported in the AST. They may be reluctant to make another appointment with their patient, which is constraining for them, in a context of heavy workload, as for their patients. Telemedicine consultations, which have become more frequent in France since the COVID-19 pandemic, may help increase re-evaluation opportunities.21 Moreover, some GPs might wonder whether it is relevant to modify a 7 day antibiotic therapy for uncomplicated pyelonephritis that has already been initiated for few days, considering the potential risk of exposing patients to multiple antibiotic classes.
Perspectives towards the scale-up of selective reporting of AST
Both GPs and laboratory professionals were favourable toward national implementation of selective reporting of AST, and to its extension to other bacteria responsible for UTIs and other samples. In addition, our study showed the feasibility of implementation of selective reporting of AST for laboratories. The additional workload for the development and adjustment of selective reporting mostly concerned the biologist co-investigator and a technician, while other biologists’ workload was not impacted.
Several facilitators have been identified to optimize the implementation of selective reporting on a large scale. First, better information for GPs might further improve acceptability, as well as the addition of educational interventions (e.g. training on antibiotic prescribing guidelines, awareness of antibiotic resistance). Second, laboratory professionals expressed their need to be better trained to answer prescribers’ requests and enhance their role in the fight against antibiotic resistance. Finally, in the case of a national scale-up of selective reporting of AST, all laboratories should be incentivized to participate, as community biology laboratories work in a competitive sector.
Limitations
To the best of our knowledge, this is the first study assessing the feasibility of selective reporting of AST, and its acceptability to both GPs and laboratory professionals. However, this study presents some limitations. First, it might present a selection bias (participation rate: 28%) and GPs who participated might be more interested than others in issues related to antibiotic resistance and more inclined to change their practices. However, the low proportion of requests for complete reporting of AST seems to confirm the high acceptability found in the interviews. Moreover, participants were selected from one French north-eastern region. Therefore, our sample might not be representative of the opinions of French GPs and laboratory professionals. For both these reasons, generalization should be considered carefully. Second, we focused on the acceptability evaluation as defined by Proctor et al.,14 and did not use a broader model or framework for implementation research. Future studies might use such a model (e.g. consolidated framework for implementation research, promoting action on research implementation in health services)22,23 to strengthen our results. Finally, we estimated the global resources that might be dedicated to the implementation of selective reporting of AST at a national level. But these results might be biased, considering uncertainties and lack of knowledge about the number of French laboratory groups that have already purchased algorithmic software allowing the edition of AST results reports, and the current expertise of professionals of all laboratories regarding antibiotic stewardship and the software.
Conclusions
In summary, implementation of selective reporting of AST for E. coli-positive urine cultures resulted in reasonable human and financial costs and was well accepted by both prescribers and laboratory professionals.
Supplementary Material
Acknowledgements
We would like to thank the ANTIBIO-ciblés Scientific Committee: Alexandre CHARMILLON, Virginie CHOPARD, Patrice DE MONCHY, Marion DELPUECH, Anne FAGOT-CAMPAGNA, Alain LOZNIEWSKI, Amandine LUC, Violaine MAUFFREY, Christian RABAUD, Emmanuelle VARON, and all GPs and laboratory professionals who accepted to participate in the study.
Contributor Information
Gaëlle Le Dref, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France.
Maïa Simon, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France; Département Méthodologie, Promotion, Investigation, Université de Lorraine, CHRU-Nancy, Nancy F-54000, France.
Aurélie Bocquier, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France.
Sébastien Fougnot, Laboratoire ATOUTBIO, unité de microbiologie, Nancy F-54000, France.
Joëlle Kivits, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France.
Alain Duda, Laboratoire ATOUTBIO, unité de microbiologie, Nancy F-54000, France.
Céline Pulcini, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France; Service des maladies infectieuses et tropicales, Université de Lorraine, CHRU-Nancy, Nancy F-54000, France.
Nathalie Thilly, EA 4360 APEMAC, Université de Lorraine, Nancy F-54000, France; Département Méthodologie, Promotion, Investigation, Université de Lorraine, CHRU-Nancy, Nancy F-54000, France.
ANTIBIO-ciblés Scientific Committee‡:
Alexandre CHARMILLON, Virginie CHOPARD, Patrice DE MONCHY, Marion DELPUECH, Anne FAGOT-CAMPAGNA, Alain LOZNIEWSKI, Amandine LUC, Violaine MAUFFREY, Christian RABAUD, and Emmanuelle VARON
Funding
This study was supported by a grant from the French Ministry of Health (Programme de Recherche sur la Performance du Système de Soins, 2017, numéro d’enregistrement: PREPS-17-0026).
Transparency declarations
All authors have no conflict to declare.
Author contributions
Conceptualization: C.P., N.T., S.F.; methodology: C.P., N.T., S.F., G.L.D., J.K.; software: S.F., A.D.; formal analysis: G.L.D., M.S.; writing—original draft: M.S.; writing—review and editing: G.L.D., A.B., N.T., C.P., S.F., A.D.
Supplementary data
Files S1 and S3, and Tables S2 and S4–S8 are available as Supplementary data at JAC-AMR Online.
References
- 1. WHO . 10 global health issues to track in 2021. 2020.https://www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021.
- 2. Santé publique France . Résistance aux antibiotiques. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques.
- 3. Santé publique France . Surveillance de la résistance bactérienne aux antibiotiques en soins de ville et en établissements pour personnes âgées dépendantes. Mission PRIMO. Année 2021. https://www.santepubliquefrance.fr/import/surveillance-de-la-resistance-bacterienne-aux-antibiotiques-en-soins-de-ville-et-en-etablissements-pour-personnes-agees-dependantes.-mission-primo.
- 4. WHO . Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- 5. Agence nationale de sécurité du médicament et des produits de santé (ANSM) . Liste des antibiotiques critiques. https://archiveansm.integra.fr/S-informer/Points-d-information-Points-d-information/Les-antibiotiques-consideres-comme-critiques-premieres-reflexions-sur-leur-caracterisation-Point-d-information.
- 6. Dyar OJ, Huttner B, Schouten Jet al. . What is antimicrobial stewardship? Clin Microbiol Infect 2017; 23: 793–8. 10.1016/j.cmi.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 7. Antibioclic: Antibiothéraphie rationnelle en soins primaires . https://antibioclic.com/.
- 8. Ministère des Solidarités et de la Santé . Lutte et prévention en France. https://solidarites-sante.gouv.fr/prevention-en-sante/les-antibiotiques-des-medicaments-essentiels-a-preserver/des-politiques-publiques-pour-preserver-l-efficacite-des-antibiotiques/article/lutte-et-prevention-en-france.
- 9. European Commission . EU Action on Antimicrobial Resistance. https://ec.europa.eu/health/antimicrobial-resistance/eu-action-antimicrobial-resistance_en.
- 10. Barlam TF, Cosgrove SE, Abbo LMet al. . Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binda F, Fougnot S, De Monchy Pet al. . Impact of selective reporting of antibiotic susceptibility test results in urinary tract infections in the outpatient setting: a protocol for a pragmatic, prospective quasi-experimental trial. BMJ Open 2019; 8: e025810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The EQUATOR Network . Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. https://www.equator-network.org/reporting-guidelines/coreq/. [DOI] [PubMed]
- 13. Institut national de la statistique et des études économiques (Insee) . Statistiques. https://www.insee.fr/fr/statistiques.
- 14. Proctor E, Silmere H, Raghavan Ret al. . Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38: 65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vie de bio . Actualités financières des groupes de laboratores de biologie médicale. https://viedebio.com/2022/06/27/sars-cov-2-le-cygne-noir-ou-la-puissance-de-limprevisible/.
- 16. Bourdellon L, Thilly N, Fougnot Set al. . Impact of selective reporting of antibiotic susceptibility test results on the appropriateness of antibiotics chosen by French general practitioners in urinary tract infections: a randomised controlled case-vignette study. Int J Antimicrob Agents 2017; 50: 258–62. 10.1016/j.ijantimicag.2017.01.040 [DOI] [PubMed] [Google Scholar]
- 17. Coupat C, Pradier C, Degand Net al. . Selective reporting of antibiotic susceptibility data improves the appropriateness of intended antibiotic prescriptions in urinary tract infections: a case-vignette randomised study. Eur J Clin Microbiol Infect Dis 2013; 32: 627–36. 10.1007/s10096-012-1786-4 [DOI] [PubMed] [Google Scholar]
- 18. Mauffrey V, Kivits J, Pulcini Cet al. . Perception of acceptable antibiotic stewardship strategies in outpatient settings. Med Mal Infect 2016; 46: 285–93. 10.1016/j.medmal.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 19. Giry M, Pulcini C, Rabaud Cet al. . Acceptability of antibiotic stewardship measures in primary care. Med Mal Infect 2016; 46: 276–84. 10.1016/j.medmal.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 20. Morency-Potvin P, Schwartz DN, Weinstein RA. Antimicrobial stewardship: how the microbiology laboratory can right the ship. Clin Microbiol Rev 2017; 30: 381–407. 10.1128/CMR.00066-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collège de la Médecine Générale (CMG) . Recommandations de bonnes pratiques concernant la prescription d’antibiotiques à l’occasion des infections aigües en soins primaires. 2021. https://lecmg.fr/recommandations-de-bonnes-pratiques-concernant-la-prescription-dantibiotiques-a-loccasion-des-infections-aigu%CC%88es-en-soins-primaires/.
- 22. Damschroder LJ, Reardon CM, Widerquist MAOet al. . The updated consolidated framework for implementation research based on user feedback. Implement Sci 2022; 17: 75. 10.1186/s13012-022-01245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care 1998; 7: 149–58. 10.1136/qshc.7.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.