Abstract
Importance
Solid cancer patients following SARS-CoV-2 vaccination are likely to have a lower seroconversion rate than healthy adults. Seroconversion between those with and without cancer is likely to vary moderately or to be restricted to specific subgroups. Therefore, we sought to conduct a systematic review and meta-analysis to identify risk factors for diminished humoral immune responses in solid cancer patients.
Methods
MEDLINE, Embase, Web of Science, Cochrane Library, and ClinicalTrials.gov were used to search literature through May 1, 2022. Prospective or retrospective studies comparing responders with non-responders against SARS-CoV-2 spike (S) protein receptor-binding domain (RBD) following COVID-19 vaccination were included. Pooled Odds Ratios (pORs) with 95% CIs for binary variables and differences in means (with SDs) for continuous variables were calculated to determine the pooled effect estimates of risk factors for poor antibody response.
Results
Fifteen studies enrolling 3593 patients were included in the analysis. Seroconversion was seen in 84% of the pooled study population. Male gender, age >65 years, and recent chemotherapy were all factors in a poor immune response. Patients under follow-up, those who received immunotherapy or targeted therapy, were more likely to be seropositive. Cancer subtypes, vaccine types, and timing of antibody testing from the 2nd dose of vaccine did not correlate with seroconversion.
Conclusion
Cytotoxic therapy for solid cancer may portend poor immune response following 2 doses of COVID-19 vaccines suggesting a need for booster doses in these patients. Immunotherapy and targeted therapy are likely to be associated with seropositive status, and thus can be considered as an alternative to cytotoxic agents in cases where both therapies are equally efficacious.
Keywords: COVID-19, COVID-19 vaccines, Cancer, Seroconversion
1. Introduction
Individuals with COVID-19 who have active malignancy have a much higher risk of severe COVID-19 disease and associated mortality compared with the general population [1], [2], [3]. Multiple vaccine subtypes have been shown to reduce the viral spread and prevent adverse outcomes in the general population [4], [5], [6]. Vaccination against COVID-19 generally results in an inadequate immune response in patients with cancer, irrespective of vaccination subtype [7], [8], [9], [10].
The seroconversion rate for cancer patients is about 73% (64–81%) following complete COVID-19 immunization [11]. Solid cancer is an umbrella term often used to describe malignancies affecting solid organs [12]. Hematological malignancies are associated with a significantly lower seroconversion than solid cancers (64% vs. 94%) [11], [13]. Approximately 90%–100% of solid cancer patients seroconvert following two vaccine doses [14], [15]. Prior meta-analyses have found a lower seroconversion rate in fully vaccinated solid cancer patients than in those without cancer (risk ratio 0.88 95% CI 0.85, 0.92) [11], [16]. However, a few studies suggest comparable antibody titers for solid cancer patients to those without cancer [17], [18]. There are likely to be specific subgroups of patients with solid cancers with differing rates of seroconversion [19]. Considering that such differences cannot be detected in individual studies, we performed a systematic meta-analysis of studies that sought determinants of humoral immune response to the COVID-19 vaccine in these patients.
2. Methods
2.1. Data sources and searches
An a priori-defined protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022329432) that is in accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guideline was utilized to conduct this meta-analysis [20]. A systematic and comprehensive search was performed independently by two reviewers (D.W. and P.H.) in MEDLINE, Embase, Web of Science, Cochrane Library, and the ClinicalTrials.gov databases between January 1, 2020, and April 1, 2022. We limited our search to the English language and did not include preprint publications. Keywords used were cancer, solid cancer, SARS-CoV-2 vaccine, ChAdOx1 mCoV-19, Oxford-AstraZeneca, mRNA vaccines, BNT162b2, mRNA-1273, BBIBP-CorV, CoronaVac. Discrepancies between reviewers were resolved by consensus or involving a third investigator.
2.2. Study selection and quality assessment
Two reviewers (D.W. and P.H.) conducted an independent assessment of titles, abstracts, and full texts (if required) of all relevant studies. The study selection was defined according to the PICOS criteria: Participants (P): adult patient (>18 years) with solid cancer, Intervention (I): SARS-CoV-2 vaccination, Comparison (C): Seropositive versus seronegative for vaccine-induced antibody against SARS-CoV-2 spike (S) protein receptor-binding domain (RBD), Outcome (O): seroconversion rate, Study (S): Clinical trials, and observational studies including prospective cohort, retrospective cohort, and case-control studies. Studies that did not exclude patients with humoral immunity following COVID-19 infection from their analysis were excluded. Studies investigating neutralizing antibodies, T-cell responses, or antibody assessment following a single vaccination dose were excluded. Two independent reviewers (D.W. and P.H.) conducted a study quality assessment using the Newcastle-Ottawa Scale (NOS) [21]. The assessment comprised three categories: selection quality, comparability, and the outcome of the study population, with a maximum cumulative score of 9 points. Studies with a score of 7 to 9 were considered to have a low risk of bias, 4 to 6 points as moderate, and less than four as a high risk of bias (eTable 1).
2.3. Outcome measurement and data extraction
The outcome was seroconversion rates following COVID-19 vaccination. We extracted relevant variables such as median age, sex, race, comorbidity, performance status, histological types, anti-cancer therapy, type of vaccine received, and vaccination protocols. Anti-cancer therapy was defined as cytotoxic chemotherapy, immunotherapy, or targeted/biologic therapy within six months from the time of the first dose of the vaccine. In addition, we extracted the number of patients with relevant variables and the total number of responders and non-responders. Responders were defined as per the defined cut-offs of antibody testing in the individual studies. Subgroup analysis was performed for risk factors in which outcomes were reported in ≥ 10 studies. The stratification was also performed by country in which the study was performed: (Israel and others) and sample size employed by each study (study sample size ≤ 150 and study sample size > 150).
2.4. Statistical analysis
Humoral immune response, the host, cancer, or vaccine-related covariates were characterized using descriptive statistics. A meta-analysis was performed with Review Manager software version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) to identify risk factors associated with poor humoral immune response. For each included risk factor, pooled Odds Ratios (pORs) with 95% CIs for binary variables, and differences in means (with SDs) for continuous variables were calculated to determine the pooled effect estimates. The leave-one-out validation method was employed for sensitivity analyses [22].
Publication bias assessment was performed using Funnel Plots and Egger’s regression test for analysis with 10 or more studies [23], [24]. If the p-value of the Egger regression was found to be < 0.1, the publication bias was considered significant [25]. In case of the presence of publication bias, the trim-and-fill method was used to adjust the summary estimate [26]. The I2 statistics were used to assess the heterogeneity of effect size estimates for each study, ranging from 0% to 100%. The I2 of <25% is considered to have low heterogeneity, 25% to 60% as moderate heterogeneity, and >60% as significant heterogeneity [27]. In case of significant heterogeneity, the DerSimonian-Laird random-effects model was utilized; otherwise, the Mantel-Haenszel fixed-effects model was used for moderate-low heterogeneity. Two-tailed p-values of <0.05 were considered to be statistically significant.
2.5. Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to assess the certainty of evidence [28]. Based on the domains of study limitations, inconsistency, indirectness, publication bias, imprecision, effect size, and dose, and plausible effect of residual confounding, the overall certainty of the evidence was rated as high, moderate, low, or very low (eTable 2).
3. Results
3.1. Literature search
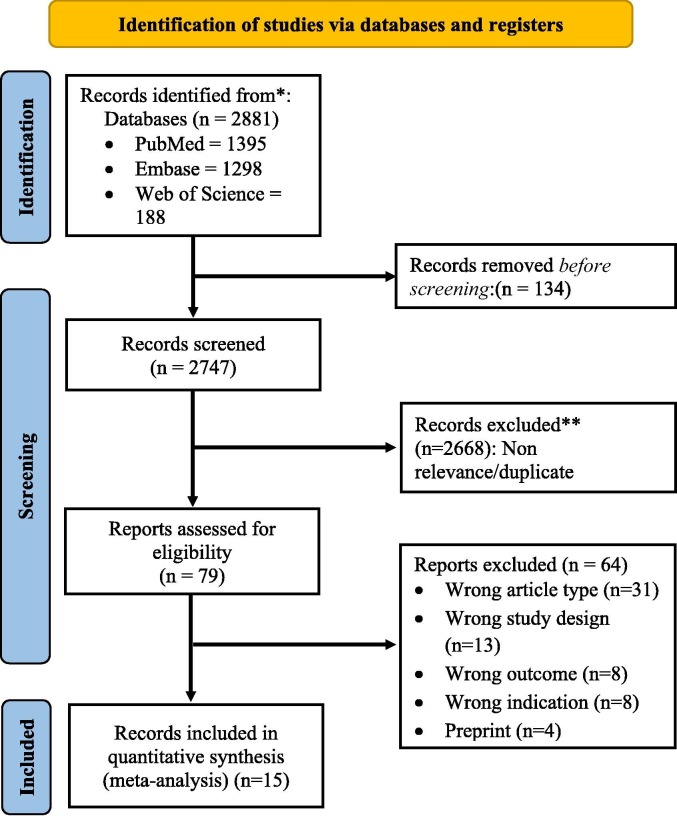
The initial search of databases yielded 1397 studies, 521 studies were removed because of being duplicates, and a further 822 studies were excluded through screening titles and abstracts. The full-text review was performed for 55 studies, of which 40 were excluded because of ineligible study designs, failure to report outcomes of interest, and studies on hematological malignancies. A total of 15 studies were included in the systematic review and meta-analysis [8], [9], [10], [18], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39] (Fig. 1 ). The study characteristics are summarized in Table 1 .
Fig. 1.
PRISMA flow diagram for study selection process.
Table 1.
Study Characteristics.
| Author, year | Country | Study design | Patients (n) | Vaccines | Doses (n) | Time points of main analysis |
Histology |
Responders |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI | Breast | Thoracic | GU | Gynae | Skin | Others | Patients (n, %) | Median IgG | |||||||
| Addeo, 2021 | Multi-centric (USA, Switz-zerland) | Prospective | 106 | BNT162b2 or mRNA- 1273 | 2 | 1-50d, 2-24d | 16 | 27 | 18 | 20 | 3 | 7 | 15 | I-80/96 (83.3), I-99/101 (98) | I- 44* (4–137), II- 2500 (514–2500) |
| Agbarya, 2021 | Israel | Cross-sectional | 140 | BNT162b2 | 2 | 2->7d | 48 | 30 | 27 | 13 | 8 | – | 13 | 120/140 (85.7) | 2231# (445–8023) |
| Ariamanesh, 2022 | Iran | Prospective | 340 | BBIBP- CorV | 2 | 2-60d | 85 | 160 | – | 20 | 10 | – | 65 | 212/340 (62.35) | – |
| Cavanna, 2021 | Italy | Prospective | 293 | BNT162b2 or mRNA- 1273 | 2 | 2–14-42d | 67 | 70 | 34 | – | 25 | – | 61 | 195/257 (75.8) | – |
| Di Noia, 2021 | Italy | Prospective | 816 | BNT162b2 | 2 | 0, 1-21d, 1-49d | 70 | 250 | 168 | 89 | 46 | – | 73 | 677/816 (83) | 246.09# (16.03–3778.70) |
| Ehmsen, 2021 | Denmark | Prospective | 201 | BNT162b2 or mRNA- 1273 | 2 | 2-36d, 90d | 31 | 69 | 25 | 51& | 51& | 18 | – | 187/201 (93) | – |
| Figueiredo, 2021 | USA | Prospective | 145 | BNT162b2/mRNA- 1273 | 2/3 | 2-42d, 120-180d | 19 | 21 | 39 | – | – | 36 | 30 | 70/87 (80.5) | 8,581 (NA)# |
| Goshen-Lago, 2021 | Israel | Prospective | 232 | BNT162b2 | 2 | 1-10d, 2-14d | 60 | 38 | 43 | 46 | 9 | 4 | 18 | 187/232 (80.6) | – |
| Grinshpun, 2021 | Israel | Retrospective | 172 | BNT162b2 | 2 | 2-77d | 36 | 66 | 38 | 22 | 10 | – | 30 | 150/172 (87.2) | – |
| Karacin, 2021 | Turkey | Prospective | 47 | CoronaVac | 2 | 2-28d | 24 | 7 | 6 | 6 | 3 | – | – | 30/47 (63.8) | – |
| Ligumsky, 2021 | Israel | Retrospective | 326 | BNT162b2 | 2 | 2-60d | 84 | 82 | 45 | 29 | 41 | 13 | 32 | 287/326 (88) | 931 (0–40 000)# |
| Mairhofer, 2021 | Austria | Prospective | 39 | BNT162b2/mRNA- 1273 | 2 | 2-21d | 14 | 11 | – | – | 6 | – | 8 | 34/39 (87.2) | – |
| Masserwah, 2021 | Israel | Prospective | 102 | BNT162b2 | 2 | 2–18-55d | 29 | 18 | 26 | 8 | – | – | 12 | 90/102 (88.2) | 1931 (509–4386)# |
| Oosting, 2021 | Netherlands | Prospective trial | 505 | mRNA-1273 | 2 | 2-28d | 70 | 73 | 211 | 50 | 20 | 64 | 32 | 496/503 (98.6) | – |
| Shmueli, 2021 | Israel | Prospective | 129 | BNT162b2 | 2 | 1–14-28d, 2–14-28d | 55 | 26 | 19 | 10 | – | 14 | 5 | 111/129 (86) | 3.25 (2.7–3.9)^ |
: U/ml, #: AU/ml, ^: geometric mean titer, d: days, GI: Gastrointestinal, GU: Genitourinary, Gynae: Gynecological cancer.
3.2. Study characteristics
A total of 3593 participants (range 39–816) with median age ranging from 62 to 74 years were included in the present study. Breast cancer was the predominant malignancy among the included studies. Four different vaccine subtypes (Pfizer BionNTech (BNT162b2), Moderna (mRNA-1273), Sinovac (CoronaVac), and Sinopharm (BBIBP- CorV) were administered to the study participants. Most participants received the BNT162b2 vaccine, although SARS-CoV-2 vaccines were not mixed among all enrolled participants. All included studies reported immunogenicity rates following the completion of 2 doses, while only one study included participants who were administered a third vaccine dose.
SARS-CoV-2 IgG II Quant assay (Abbott Laboratories) (5 studies) and the SARS-CoV-2 anti-spike (S) S1/S2 IgG assay (Liaison; DiaSorin) (4 studies) were the most frequently utilized methods to assess antibody titers (eTable 3). Seroconversion was seen in 84% (62.3%-98.6%) of the pooled study population. A few studies also reported median IgG levels that ranged between 246 AU/mL and 2231 AU/mL amongst responders.
Eleven studies had a low risk of bias; the remaining four had a moderate risk of bias (median NOS score: 8). (eTable1 in the Supplement).
3.3. Determinants of humoral immune responses after COVID-19 vaccines
3.3.1. Host characteristics
The host characteristics of the included studies are shown in Table 1. The results from 12 studies comprising 2636 patients showed that the proportion of males was significantly lower in the seropositive group compared to the seronegative group [OR = 0.76, 95% CI (0.61, 0.96), p = 0.02; I2 = 38%] (eFigure 1).
The median age ranged from 31 to 66 years old in the seropositive group and 47 to 71 years old in the seronegative group. Meta-analysis of studies reporting age as a continuous variable did not show a correlation with seroconversion [MD −1.09 95% CI (-4.03,1.85) p = 0.47; I2 = 83%] (eFigure 2). However, the proportion of patients older than 65 years was lower in the seropositive compared to the seronegative group [OR = 0.60, 95% CI (0.38, 0.97), p = 0.03; I2 = 5%] (eFigure 3).
Patients belonging to non-Hispanic white race [OR = 0.77, 95% CI (0.30, 2.00), p = 0.60; I2 = 0%] (eFigure 4), with comorbidities [OR = 0.83, 95% CI (0.26, 2.63), p = 0.75; I2 = 74%] (eFigure 5) and having poor performance status (ECOG > 1) [OR = 0.58, 95% CI (0.33, 1.01), p = 0.05; I2 = 0%] (eFigure 6) did not show association with seroconversion.
3.3.2. Cancer characteristics
The characteristics related to the diagnosis of cancer subtypes are presented in Table 1. The proportion of patients with gastrointestinal cancer [OR = 0.93, 95% CI (0.69, 1.24), p = 0.61; I2 = 26%] (10 studies) (Table 2 , eFigure 7 in the Supplement), breast cancer [OR = 112, 95% CI (0.83, 1.52), p = 0.45; I2 = 45%] (10 studies) (Table 2 , eFigure 8 in the Supplement), thoracic cancer [OR = 0.95, 95% CI (0.64, 1.42), p = 0.82; I2 = 0%] (9 studies) (Table 2 , eFigure 9 in the Supplement), genitourinary cancer [OR = 0.81, 95% CI (0.51, 1.29), p = 0.38; I2 = 0%] (8 studies) (Table 2 , eFigure 10 in the Supplement), gynecological cancer [OR = 1.65, 95% CI (0.84, 3.24), p = 0.15; I2 = 0%] (6 studies) (Table 2 , eFigure 11 in the Supplement), and cutaneous cancer [OR = 2.58, 95% CI (0.71, 9.44), p = 0.15; I2 = 0%] (4 studies) (Table 2 , eFigure 12 in the Supplement) were similar in both seronegative and seropositive groups. Similarly, the proportion of patients with metastatic disease was similar between the seropositive and seronegative groups [OR = 1.27, 95% CI (0.95, 1.69), p = 0.10; I2 = 58%] (8 studies) (eFigure 13).
Table 2.
Summary of factors associated with immunogenicity after 2 doses of COVID-19 vaccines.
| Risk factor | Humoral immune response, pOR (95% CI) | Pooled difference in humoral immune response, mean (95% CI) | Studies, No. | Evidence certainty (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Host characteristics | Age | NA | −1.09 [-4.03, 1.85] | 6 | Very low | Significant heterogeneity was owed to Figueiredo et al, which upon exclusion, pMD becomes significant (-2.96 [-3.54, −2.38]) |
| > 65 year | 0.60 [0.38, 0.97] | NA | 3 | Very Low | Non-significant pooled estimate after removal of 1 of the following studies : Addeo et al., Cavanna et al. | |
| Male | 0.72 [0.57, 0.91] | NA | 12 | Low | Non-significant pooled estimate after removal of 1 of the following studies : Ariamanesh et al Cavanna et al, | |
| Race: Non-Hispanic white | 0.77 [0.30, 2.00] | NA | 2 | Very low | None | |
| Comorbidity: Present | 0.83 [0.26, 2.63] | NA | 3 | Very low | Significant heterogeneity owed to Shmueli et al. No change in summary estimate after exclusion. | |
| Performance status (ECOG > 1) | 0.41 [0.11, 1.46] | NA | 2 | Very low | None | |
| Cancer characteristics | Cancer types | |||||
| Gastrointestinal | 0.93 [0.69, 1.24] | NA | 10 | Moderate | None | |
| Breast | 1.12 [0.83, 1.52] | NA | 10 | Low | None | |
| Thorax | 0.95 [0.64, 1.42] | NA | 9 | Moderate | None | |
| Genitourinary | 0.81 [0.51, 1.29] | NA | 8 | Moderate | None | |
| Gynecology | 1.59 [0.83, 3.04] | NA | 7 | Low | None | |
| Skin | 2.58 [0.71, 9.44] | NA | 4 | Low | None | |
| Anti-cancer therapy | ||||||
| Follow-up/surveillance | 4.80 [2.22, 10.41] | NA | 5 | Low | None | |
| Cytotoxic | 0.43 [0.34, 0.55] | NA | 14 | Moderate | None | |
| Immunotherapy | 1.76 [1.19, 2.59] | NA | 11 | High | None | |
| Chemo-immunotherapy | 0.98 [0.50, 1.92] | NA | 5 | Low | Masserwah et al contributed to significant heterogeneity | |
| Targeted therapy | 1.66 [1.07, 2.56] | NA | 5 | Low | pOR lost significance after removal 1 of several studies from analysis [Di Noia et al, Goshen-Lago et al.] | |
| Cancer status | ||||||
| Metastatic | 1.27 [0.95, 1.69] | NA | 8 | Very Low | pOR became significant after removal of Cavanna et al., from analysis. | |
| Vaccine characteristics | Vaccine types | |||||
| BNT162b2 | 0.65 [0.28, 1.49] | NA | 3 | Low | None | |
| Time from 2nd COVID-19 vaccine | NA | −1.83 [-5.53, 1.87] | 4 | Very low | Significant heterogeneity owed to 1 of the following: Agbarya et al, Goshen-Lago et al. Significant result after exclusion of Agbarya et al [-3.52 [-6.76, −0.27] | |
pRR, Pooled risk ratios, pMD, Pooled Mean difference, ECOG PS, Eastern cooperative group performance status.
Five studies demonstrated that the proportion of patients under follow-up or clinical surveillance was higher in the seropositive group compared to the seronegative group [OR = 4.80, 95% CI (2.22, 10.41), p < 0.0001; I2 = 0%] (Table 2 , eFigure 14 in the Supplement). Concerning treatment, the results from 14 studies showed that the proportion of patients who received chemotherapy within six months from the first dose of COVID-19 vaccination was significantly lower in the seropositive group in comparison to the seronegative group [OR = 0.43, 95% CI (0.34, 0.55), p < 0.00001; I2 = 48%] (eFigure 15). The proportion of patients who received immunotherapy was statistically higher in the seropositive group compared to the seronegative group [OR = 1.76, 95% CI (1.19, 2.59), p = 0.004; I2 = 0%] (11 studies) (eFigure 16). On the other hand, combination chemo-immunotherapy did not show any association with seroconversion [OR = 0.98, 95% CI (0.50, 1.92), p = 0.96; I2 = 42%] (5 studies) (eFigure 17). Lastly, the proportion of patients who received targeted and/or biological therapy was higher in the seropositive group compared to the seronegative group [OR = 1.66, 95% CI (1.07, 2.56), p = 0.02; I2 = 0%] (5 studies) (eFigure 18).
3.3.3. Vaccine characteristics
The characteristics related to the COVID-19 vaccine are presented in Table 2. The administration of the BNT162b2 vaccine did not affect the seroconversion rate in cancer patients [OR = 0.65, 95% CI (0.28, 1.46), p = 0.31; I2 = 0%] (3 studies) (eFigure 19). Furthermore, no correlation was observed between the timing of antibody testing from the second dose of vaccination and seroconversion status [MD −1.83 95% CI (-5.53,1.87) p = 0.33; I2 = 63%] (4 studies) (eFigure 20).
3.4. Sensitivity analysis and publication bias
The results of the sensitivity analyses are reported in eTable 4. For association analysis of age (continuous) with seroconversion rate, Figueiredo et al. contributed toward significant heterogeneity, subsequent exclusion of which resulted in a statistically significant association [MD −2.96 [95% CI −3.54,-2.38]]. On the other hand, the exclusion of Addeo et al. or Cavanna et al. led to a loss of association of poor response with Age > 65 years. The removal of heterogeneity did not influence the absence of an association of comorbidity with seroconversion rate.
The association of metastatic disease with seroconversion was lost after the exclusion of Cavanna et al. Lastly, the exclusion of Agbarya et al. or Goshen-Lago et al. led to a significant association of timing of antibody testing from 2nd dose of the COVID-19 vaccine with humoral immune response [MD −3.52 (95% CI −6.76,-0.27)].
We found no publication bias for the male sex, gastrointestinal cancer, breast cancer, cytotoxic chemotherapy, and immunotherapy (eFigure 2 in the Supplement).
3.5. Subgroup analysis
Risk factors that satisfied the criteria to be included in subgroup analyses were male sex, gastrointestinal, breast, and thoracic malignancy, cytotoxic chemotherapy, and immunotherapy. The summary of the subgroup analyses is shown in eTable 5.
The results of the subgroup analyses suggested that ethnicity or geographical location significantly modifies the effect of immune response in males (p = 0.003) and breast cancer patients (p = 0.0004). For males, the proportion of patients in studies conducted in countries other than Israel was significantly lower in the seropositive group compared to the seronegative group [OR = 0.55, 95% CI (0.40, 0.75), p = 0.0002; I2 = 20%]. No such association was observed in studies conducted in Israel [OR = 1.04, 95% CI (0.72, 1.50), p = 0.84; I2 = 0%]. However, the proportion of breast cancer patients enrolled in Israeli studies was significantly lower in the seropositive group compared to the seronegative group [OR = 0.63, 95% CI (0.40, 0.98), p = 0.04; I2 = 0%]; whereas the proportion of these patients enrolled in other countries was significantly higher in the seropositive group compared to the seronegative group [OR = 1.83, 95% CI (1.19, 2.82), p = 0.006; I2 = 0%].
The results of the subgroup analyses suggest that sample size significantly modifies the effect of immune response on chemotherapy outcome. The proportion of chemotherapy patients in studies with>150 sample sizes was significantly lower in the seropositive group as compared to the seronegative group [OR = 0.36, 95% CI (0.27, 0.46), p < 0.00001; I2 = 24%]. No such association was observed in studies with<150 participants [OR = 0.88, 95% CI (0.51, 1.50), p = 0.63; I2 = 44%].
4. Discussion
This systematic review and meta-analysis of 15 studies on solid cancer patients revealed that males over 65 who received cytotoxic chemotherapy within six months from the first dose of the COVID-19 vaccine might have a higher risk of inadequate humoral immune response. Patients who were under follow-up or clinical surveillance, or had received immunotherapy or targeted therapy, were more likely to have seropositive status. The majority of the studies included exhibited a low-to-moderate risk of bias. Geographical region and sample size may influence the effect of these risk factors on the COVID-19 vaccine immunological response.
Sex-specific differences in the antibody response and vaccine efficacy against viruses have been reported [40]. Women exhibit expanded humoral and cell-mediated immune responses to antigenic stimulation and vaccination than men, likely due to the critical role in immune response regulation played by several genes on the X chromosome, including FoxP3, CD40L, and TLR7 [41], [42]. A prior meta-analysis of clinical trials on COVID-19 vaccines showed a 33% reduced risk of COVID-19 infection in males compared to females [43]. Contrarily, a population-based study has demonstrated higher seropositivity in women than in men following the second COVID-19 vaccine dose (either BNT162b2 or CHDAdOx1)[44]. Our data on gender variations in antibody response largely resembles the differences seen in the general population. Untangling these sex disparities should focus on vaccination research and may aid in understanding gender-based variations in COVID-19 results.
Following two doses of mRNA vaccination, evidence shows that older individuals mount a comparable humoral immune response to the adult group [45]. However, a lower neutralizing antibody response, particularly against Variant Of Concerns (VOC), and decreased production of interferon-γ and interleukin-2 induced by SARS-CoV-2 spike-specific T cells in the elderly age group, along with declining antibody titers, have been used to justify booster doses in these populations [45], [46], [47]. Patients with solid cancer over 65 years old are likely to develop inadequate humoral immune responses and may benefit from booster doses. The optimal vaccine types and the role of heterologous vaccines in these patients require further studies.
Vaccine-induced immune responses are likely to be impaired by cancer therapies. We observed that patients who underwent recent chemotherapy are almost twice as likely to be seronegative, which corresponds with the impact of chemotherapy on vaccine efficacy against viruses in the current literature [48], [49]. This effect, however, was only seen in studies with a large sample size (>150 participants). Furthermore, any form of cancer therapy, chemotherapy, or steroid treatment has shown impaired T-cell responsiveness to COVID-19 vaccination [15], [50]. Evidence indicates that the timing of vaccination to ongoing chemotherapy may not contribute to suboptimal vaccine efficacy, necessitating no schedule adjustements [51], [52]. Due to the scarcity of data, we could not comment on the timing of vaccination to chemotherapy, although we did learn that the timing of antibody testing from the 2nd dose of the vaccine showed no relationship with seroconversion.
Cancer immunotherapy and COVID-19 vaccination interact in a complicated and multifaceted manner. VOICE, a prospective, multicenter, non-inferiority trial comprising 505 solid cancer patients, concluded an optimal antibody response to mRNA-1273 COVID-19 vaccine following chemotherapy, immunotherapy, or both [18]. Certain studies have raised concerns about the safety of combining ICIs with the COVID-19 vaccine, citing an increase in vaccine-associated adverse effects and the onset of cytokine release syndrome (CRS) in patients receiving long-term anti-PD-1 therapy [53], [54]. However, evidence from the VOICE trial suggests that COVID-19 vaccines are safe for these patients [18]. Despite a complex yet evolving paradigm, our findings imply that cancer immunotherapy may augment seroconversion in solid cancer patients. Based on this finding, immunotherapy or targeted therapy may be considered a promising alternative in cases where chemotherapy and these agents have equivalent efficacy to reduce the patient’s COVID-19 mortality risk. Evidence from influenza vaccination indicates that cancer patients treated with immune checkpoint inhibitors have an improved survival [55], [56]. Building on this fact, further investigation into the reciprocal benefits of COVID-19 vaccines on the oncological outcomes of ICIs is warranted.
Israel had an efficient COVID-19 vaccination rollout which could be attributed to the limited geographical and population size, prioritization of efficient delivery systems, and adoption of a comprehensive web-based immunization registry for the COVID-19 vaccine [57], [58]. Such a rollout has not only provided direct benefits in preventing COVID-19 mortality but also reduced COVID-19 cases in unvaccinated individuals indicating a pseudo-herd immunity effect [59], [60]. Nevertheless, implementing this strategy globally has been challenging. Vaccine equity, affordability, access, and logistics issues restrict rapid population coverage in other countries [61]. These findings may explain the gender and cancer subtype-specific subgroup disparities in our results.
This review addressed a few pertinent research questions while some areas remained unexplored. Elderly, male solid cancer patients who had recently received chemotherapy are most likely to have suboptimal humoral immune responses. Checkpoint inhibitors and targeted therapy may boost vaccination responses in these patients. However, the clinical benefit of COVID-19 vaccines in terms of breakthrough infections and subsequently severe COVID disease and mortality in solid cancer patients remains undetermined. Assessment of neutralizing antibody responses and cell-mediated immunity might shed light on this issue. Lack of data precludes stratification of results by VOCs. Further investigations into the safety profile and clinical efficacy of mRNA vaccines for cancer patients are required compared to heat-killed viruses or protein-based vaccines. The optimal timing of chemotherapy and ICIs, particularly in the case of therapy-induced cytopenia or T-cell immunosuppression, requires further research. This study has evidence of the influence of geographical region and sample size, indicating plausible residual confounding effects. Moreover, sensitivity analyses and overall outcomes of certain variables have revealed considerable inconsistencies. Apart from gastrointestinal cancer and immunotherapy, the GRADE system determined that the overall quality of evidence was low.
5. Conclusions
Understanding the determinants of the humoral immune response following the COVID-19 vaccine among solid cancer patients has the potential to alter current vaccination protocols, acknowledging that most of these schedules were designed for the non-cancer population. Of the factors examined, elderly, male patients with recent chemotherapy were at higher risk of lower seroconversion, which may require additional vaccine doses. However, immunotherapy and targeted therapy may boost the vaccine-induced immune response in these patients and may be considered an alternative to cytotoxic agents in scenarios where both therapies have equivalent efficacy. Further examination of the clinical efficacy of the COVID-19 vaccine in these patients is required.
Notes:
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The datasets generated during and/or analysed during the current study are available in the Open Science Framework repository, DOI https://doi.org/10.17605/OSF.IO/9B7PN without third-party permission.
References:
- 1.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesulu B.P., Chandrasekar V.T., Girdhar P., et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Can Spectr. 2021;5 doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagano L., Salmanton-García J., Marchesi F., et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):1–15. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med Published online 2020. [DOI] [PMC free article] [PubMed]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med Published online 2020. [DOI] [PMC free article] [PubMed]
- 6.Sadoff J., Gray G., Vandebosch A., et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linardou H., Spanakis N., Koliou G.-A., et al. Responses to SARS-CoV-2 vaccination in patients with cancer (ReCOVer Study): a prospective cohort study of the hellenic cooperative oncology group. Cancers (Basel). 2021;13(18):4621. doi: 10.3390/cancers13184621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shmueli E.S., Itay A., Margalit O., et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy–a single centre prospective study. Eur J Can. 2021;157:124–131. doi: 10.1016/j.ejca.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ligumsky H., Safadi E., Etan T., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. JNCI J Natl Can Inst. 2022;114(2):203–209. doi: 10.1093/jnci/djab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agbarya A., Sarel I., Ziv-Baran T., et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers (Basel) 2021;13(16):4191. doi: 10.3390/cancers13164191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., et al. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Can. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazebnik Y. What are the hallmarks of cancer? Nat Rev Can. 2010;10(4):232–233. doi: 10.1038/nrc2827. [DOI] [PubMed] [Google Scholar]
- 13.Sakuraba A., Luna A., Micic D. Serologic response following SARS-COV2 vaccination in patients with cancer: a systematic review and meta-analysis. J Hematol Oncol. 2022;15(1):1–22. doi: 10.1186/s13045-022-01233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrabi Nejad M.-M., Moosaie F., Dehghanbanadaki H., et al. Immunogenicity of COVID-19 mRNA vaccines in immunocompromised patients: a systematic review and meta-analysis. Eur J Med Res. 2022;27(1):1–13. doi: 10.1186/s40001-022-00648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fendler A., Shepherd S.T.C., Au L., et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Can. 2021;2(12):1305–1320. doi: 10.1038/s43018-021-00274-w. [DOI] [PubMed] [Google Scholar]
- 18.Oosting S.F., van der Veldt A.A.M., GeurtsvanKessel C.H., et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fendler A., de Vries E.G.E., GeurtsvanKessel C.H., et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022:1–17. doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. doi:10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 21.Wells G, Shea B, O’Connell D, Peterson J, Welch V LM. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Published 2013. Accessed September 2, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 22.Patsopoulos N.A., Evangelou E., Ioannidis J.P.A. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 24.Terrin N., Schmid C.H., Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58(9):894–901. doi: 10.1016/j.jclinepi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G.H., Oxman A.D., Sultan S., et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Grinshpun A., Rottenberg Y., Ben-Dov I.Z., Djian E., Wolf D.G., Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO open. 2021;6(6) doi: 10.1016/j.esmoop.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karacin C., Eren T., Zeynelgil E., et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Futur Oncol. 2021;17(33):4447–4456. doi: 10.2217/fon-2021-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mairhofer M., Kausche L., Kaltenbrunner S., et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171–1172. doi: 10.1016/j.ccell.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanna L., Citterio C., Biasini C., et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. Eur J Can. 2021;157:441–449. doi: 10.1016/j.ejca.2021.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Can Cell. 2021;39(8):1091–1098. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ariamanesh M., Porouhan P., PeyroShabany B., et al. Immunogenicity and safety of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV) in patients with malignancy. Can Invest. 2022;40(1):26–34. doi: 10.1080/07357907.2021.1992420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Noia V., Pimpinelli F., Renna D., et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Can Res. 2021;27(24):6815–6823. doi: 10.1158/1078-0432.CCR-21-2439. [DOI] [PubMed] [Google Scholar]
- 37.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Can Cell. 2021;39(8):1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueiredo J.C., Merin N.M., Hamid O., et al. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Can Res. 2021;81(24):6273–6280. doi: 10.1158/0008-5472.CAN-21-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink A.L., Klein S.L. Sex and gender impact immune responses to vaccines among the elderly. Physiology (Bethesda) 2015;30(6):408–416. doi: 10.1152/physiol.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lotter H., Altfeld M. Sex differences in immunity. Semin Immunopathol. 2019;41(2):133–135. doi: 10.1007/s00281-018-00728-x. [DOI] [PubMed] [Google Scholar]
- 43.Bignucolo A., Scarabel L., Mezzalira S., Polesel J., Cecchin E., Toffoli G. Sex Disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines. 2021;9(8):825. doi: 10.3390/vaccines9080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward H., Whitaker M., Flower B., et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1):1–6. doi: 10.1038/s41467-022-28527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jergović M., Uhrlaub J.L., Watanabe M., et al. Competent immune responses to SARS-CoV-2 variants in older adults following two doses of mRNA vaccination. Nat Commun. 2022;13(1):1–8. doi: 10.1038/s41467-022-30617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrotri M., Navaratnam A.M.D., Nguyen V., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollaard A., Schreuder I., Slok-Raijmakers L., Opstelten W., Rimmelzwaan G., Gelderblom H. Influenza vaccination in adult patients with solid tumours treated with chemotherapy. Eur J Can. 2017;76:134–143. doi: 10.1016/j.ejca.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Goossen G.M., Kremer L.C.M., van de Wetering M.D. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. 2013:(8). doi: 10.1002/14651858.CD006484.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKenzie D.R., Muñoz-Ruiz M., Monin L., et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Can Cell. 2021;39(11):1445–1447. doi: 10.1016/j.ccell.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peeters M., Verbruggen L., Teuwen L., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J.-T.-Y., La J., Branch-Elliman W., et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide Veterans Affairs study. JAMA Oncol. 2022;8(2):281–286. doi: 10.1001/jamaoncol.2021.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Au L., Fendler A., Shepherd S.T.C., et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27(8):1362–1366. doi: 10.1038/s41591-021-01387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang C.K., Kim H.-R., Song K.-H., et al. Cell-mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis. 2020;222(11):1902–1909. doi: 10.1093/infdis/jiaa291. [DOI] [PubMed] [Google Scholar]
- 56.Valachis A., Rosén C., Koliadi A., et al. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology. 2021;10(1):1886725. doi: 10.1080/2162402X.2021.1886725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen B., Waitzberg R., Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res. 2021;10(1):6. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leshem E., Wilder-Smith A. COVID-19 vaccine impact in Israel and a way out of the pandemic. Lancet. 2021;397(10287):1783–1785. doi: 10.1016/S0140-6736(21)01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muhsen K., Cohen D. COVID-19 vaccination in Israel. Clin Microbiol Infect. 2021;27(11):1570–1574. doi: 10.1016/j.cmi.2021.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwok KO, Lai F, Wei WI, Wong SYS, Tang JWT. Herd immunity–estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020;80(6):e32-e33. [DOI] [PMC free article] [PubMed]
- 61.Van De Pas R., Widdowson M.-A., Ravinetto R., et al. COVID-19 vaccine equity: a health systems and policy perspective. Expert Rev Vac. 2022;21(1):25–36. doi: 10.1080/14760584.2022.2004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the Open Science Framework repository, DOI https://doi.org/10.17605/OSF.IO/9B7PN without third-party permission.