Abstract
Background
Although chest tube-omitted video-assisted thoracoscopic surgery (VATS) has been proven to be safe and efficacious, its universal application is precluded by a varying morbidity rate due to a lack of standardization. Since digital chest drainage has already shown improved accuracy and consistency in the management of postoperative air leak, we incorporated it in the strategy of intraoperative chest tube withdrawal, aiming to achieve better results.
Methods
We collected the clinical data of 114 consecutive patients who underwent elective uniportal VATS pulmonary wedge resection at the Shanghai Pulmonary Hospital from May 2021 to February 2022. Their chest tubes were withdrawn intraoperatively after an air-tightness test facilitated by digital drainage: the end flow rate had to be kept ≤30 mL/min for >15 s at the setting of −8 cmH2O suctioning. The recordings and patterns of the air suctioning process were documented and analyzed as potential standards of chest tube withdrawal.
Results
The mean age of the patients was 49.7±11.7 years. The mean size of the nodules was 1.0±0.2 cm. The location of the nodules encompassed all lobes, and 90 (78.9%) patients received preoperative localization. The postoperative morbidity and mortality rates were 7.0% and 0%, respectively. Six patients had clinically overt pneumothorax and two patients had postoperative bleeding that required intervention. All of the patients recovered on conservative treatment except for one case of pneumothorax that required additional tube thoracostomy. The median length of postoperative stay was 2 days; and the median time of suctioning, peak flow rate, and end flow rate were 126 s, 210 mL/min, and 0 mL/min, respectively. The median numeric rating scale for pain was 1 on postoperative day (POD) 1 and 0 on the day of discharge.
Conclusions
Chest tube-free VATS assisted by digital drainage is feasible with minimal morbidity. Its strength of quantitative air leak monitoring produces important measurements for the prediction of postoperative pneumothorax and future standardization of the procedure.
Keywords: Chest tube omission, video-assisted thoracoscopic surgery (VATS), digital pleural drainage, air leak, morbidity
Highlight box.
Key findings
• The morbidity and mortality rates after chest tube-free video-assisted thoracoscopic surgery were 7.0% and 0%.
What is known and what is new?
• It is known that intraoperative chest tube withdrawal performed according to subjective criteria is associated with varying morbidity.
• Digital drainage may play a potential role in the objective indications for intraoperative chest tube withdrawal.
What are the implications, and what should change now?
• Digital drainage should be applied more widely in intraoperative chest tube withdrawal to provide quantitative measurements for future standardization of the technique.
Introduction
Chest tube drainage following pulmonary resection has long been considered the gold standard in thoracic surgery. This technique offers monitoring for various postoperative complications, including air leaks, hemothorax, chylothorax, empyema, etc. On the other hand, it also displays important signs regarding the progress of patient recovery, which further dictates chest tube withdrawal and discharge thereafter. However, the biggest problem with chest tubes lies in the associated pain that often interferes with voluntary coughing and ambulation, which themselves are important components of postoperative rehabilitation.
With the development of minimally invasive surgery and enhanced postoperative recovery, alternative methods to chest tube placement have become an area of growing interest. Gómez-Caro et al. (1) reduced the traditional double chest tube drainage to a single tube and proved their equivalence in a randomized trial. This is consistent with our routine practice after resection up to lobectomy and does not present any increased risk. A more aggressive attempt with tubeless pulmonary resection that completely omits tracheal intubation and chest tube drainage was also reported to be safe and feasible in select patients (2-10). However, a varying incidence of pneumothorax (7.6–40%) was reported despite improving criteria (2,3,7,8,11), casting doubts on the universal application of this technique.
This could be partly due to the lack of standardization in the visual assessment of air leaks, which is overly subjective but used by most teams. Since digital chest drainage was developed to deal with postoperative prolonged air leak, it has already shown improved consistency and accuracy, with the strengths of constant, customizable negative pleural pressure that is more aligned with normal respiratory physiology (12-15). To achieve better standardization, we incorporated a digital drainage system that features quantifiable air leak monitoring and dynamic graphic visualization (12-15) in the chest tube removal process. This case series describes our initial experience with this enhanced strategy by digital drainage. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1749/rc).
Methods
Patients
This retrospective study reviewed all adult patients who underwent elective uniportal video-assisted thoracoscopic surgery (VATS) for pulmonary wedge resection performed by the authors’ team at the Shanghai Pulmonary Hospital from May 2021 to February 2022. The study was approved by the Institutional Review Board (IRB) of Shanghai Pulmonary Hospital (IRB No. K22-319). Individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The inclusion criteria for chest tube withdrawal at the end of surgery were as follows: (I) non-infectious benign or malignant pulmonary nodules; (II) located in the periphery of the lungs; and (III) end flow rate of suctioned air ≤30 mL/min. The exclusion criteria were as follows: (I) comorbidity with diffuse underlying lung disease such as emphysema or interstitial lung disease; (II) history of ipsilateral thoracic surgery; (III) cessation of smoking for <1 month; (IV) coagulopathy with an elevated risk of bleeding; (V) additional concurrent surgery; (VI) conversion to a greater extent of resection or incision; (VII) intraoperative complications; (VIII) extensive adhesion; and (IX) other circumstances that the investigators deemed non-viable.
All patients underwent preoperative thin-section computed tomography (CT) with a thickness of ≤1 mm. Multiple resections were allowed for patients with multiple nodules. However, only the characteristics of the main lesion were recorded and displayed, i.e., size as the greatest dimension of the nodule and depth as the closest distance to the pleura.
Surgical procedure
All patients received general anesthesia with ventilation via a double-lumen endotracheal tube. A urinary catheter was not routinely inserted. Preoperative CT-guided localization was carried out for subpleural nodules with a breast hook-wire Ghiatas (Bard Peripheral Vascular, Tempe, USA), which was determined according to the surgeon’s discretion. A standard lateral decubitus position was chosen and a 3 cm incision was made in the fourth to sixth intercostal space without rib spreading for uniportal VATS (Figure 1A). Intravenous sufentanil-propofol was utilized for analgesia during the procedure.
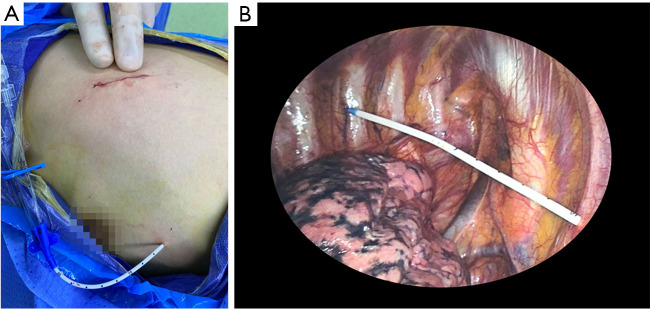
Figure 1.
The incision and the prophylactic catheter. (A) The incision was about 3 cm in length in the 4th–6th intercostal space based on the nodule location. A double-lumen catheter was left for prophylaxis. (B) Internally, the catheter was introduced through the second intercostal space with its tip placed in the apex of the thoracic cavity under direct vision of thoracoscopy.
The wedge resection was performed with an Echelon Flex articulating linear stapler (Ethicon, Cincinnati, USA) along with compatible cartridges. Lymph nodes were not routinely sampled for minimally invasive adenocarcinoma or carcinoma in situ. After removal of the specimen, a double-lumen central venous catheter was introduced to the top of the hemithorax via the second intercostal space for prophylactic air extraction (16,17) (Figure 1B). A 24-Fr chest tube was also placed at the top of the hemithorax through the incision for intraoperative drainage. The muscle layer and connective tissue were closed with interrupted 1-0 and 2-0 sutures, respectively, with the subcutaneous suture around the chest tube left untied.
Digital suctioning and chest tube withdrawal
A Thopaz digital drainage device (Medela Healthcare, Baar, Switzerland) was connected to the chest tube after manual recruitment of the lung at 10 cmH2O for at least 5 s by the anesthesiologist. The suctioning was set at −8 cmH2O and was continued until the flow rate of the air kept static for >15 s. Following suctioning, if the final flow rate was ≤30 mL/min, the tube-free protocol was employed and the chest tube was withdrawn. Otherwise, the protocol was terminated with the chest tube connected to a water-sealed drainage bottle as usual. The previously left stay suture was tied upon chest tube withdrawal. The total duration of suctioning and the peak flow rate was recorded for future analysis. The rest of the incision was closed with subcuticular sutures. In contrast to previous studies (16,17), a prophylactic catheter was connected to a gravity drainage bag rather than being sealed immediately after surgery.
Postoperative management
Patients were extubated in the operating room and transported to the general ward after a transient stay in the post-anesthetic care unit. A patient-controlled analgesia (PCA) pump containing intravenous morphine was routinely administered. Oral loxoprofen or paracetamol was prescribed only as needed. The self-reported pain score was taken based on the numeric rating scale (NRS) (18) on postoperative day (POD) 1 and the day of discharge; a score of 0 denoted no pain and a score of 10 signified the worst pain imaginable.
Chest radiography was filmed on POD 1 and POD 21 at the outpatient department. The residual airspace was measured as the maximal distance from the top of the rib cage to the apex of the lung, and a radiological pneumothorax was suspected when the airspace exceeded 3 cm (19). In addition, if the patient complained of shortness of breath or had progressive subcutaneous emphysema, the pneumothorax was considered symptomatic, which would initiate manual air extraction through the prophylactic catheter twice daily (16). If the air extraction failed or the symptoms kept worsening despite aggressive air extraction, closed drainage with chest tube thoracostomy would be considered.
The presence of pleural effusion was defined as the obliteration of the costophrenic angle on chest radiography. It was further stratified as mild or severe based on the need for invasive intervention, for which ultrasound-guided pleural tapping would usually suffice. Other morbidities were defined in a similar manner to what required medical intervention. The chest tube/catheter was withdrawn when adequate lung recruitment, drainage of normal appearance <300 mL/d, and good overall performance and oxygenation for the patient were all achieved. Normally, the patient was discharged on the same day of chest tube/catheter withdrawal.
Statistical analysis
Normally distributed numerical variables were displayed as the mean ± standard deviation, and those that were non-normally distributed were displayed as the median (interquartile range). Categorical variables were displayed as the frequency (percentage). All statistical summaries and graphics are descriptive and were generated by RStudio version 1.4.1717 (RStudio Inc., Boston, USA).
Results
This study identified 114 patients who received pulmonary wedge resection and had their chest tubes withdrawn intraoperatively with the assistance of digital drainage. The patients’ demographic characteristics are shown in Table 1. The mean age of the patients was 49.7±11.7 years, and they were predominantly females (69.3%), most patients presented with a solitary nodule (94.7%), while 5 (4.4%) patients had two nodules and 1 (0.9%) patient had three resected. The location of the nodules encompassed all lobes, and 90 (78.9%) patients received preoperative hook-wire localization. The mean size of the nodules was 1.0±0.2 cm and the mean depth was 0.5±0.5 cm. Postoperative histology was malignant in 100 (87.7%) patients. There were another 26 patients who were intended for chest tube withdrawal but failed to carry out the procedure due to ending air leak >30 mL/min in 13 cases, conversion to anatomic resection in two cases, additional lung sutures in three cases, and at the surgeon’s discretion in the remaining eight cases owing to an increased risk of staple line failure.
Table 1. Demographics of patients undergoing chest tube-free VATS.
| Variables | Chest tube-free VATS (N=114) |
|---|---|
| Age (year) | 49.7±11.7 |
| Gender, n (%) | |
| Male | 35 (30.7) |
| Female | 79 (69.3) |
| Nodules resected, n (%) | |
| 1 | 108 (94.7) |
| 2 | 5 4.4) |
| 3 | 1 (0.9) |
| Location of main lesion, n (%) | |
| Left upper lobe | 30 (26.3) |
| Left lower lobe | 24 (21.1) |
| Right upper lobe | 34 (29.8) |
| Right middle lobe | 9 (7.9) |
| Right lower lobe | 17 (14.9) |
| Size of main lesion (cm) | 1.0±0.2 |
| Depth of main lesion (cm) | 0.5±0.5 |
| Preoperative localization, n (%) | 90 (78.9) |
| Histology, n (%) | |
| Benign | 14 (12.3) |
| Malignant | 100 (87.7) |
Data were displayed as mean ± SD or frequency (percentage) per data type. VATS, video-assisted thoracoscopic surgery; SD, standard deviation.
The postoperative outcomes of the tube-free patients are shown in Table 2. Residual airspace and mild pleural effusion were observed in 13 (11.4%) and 14 (12.3%) patients, respectively, on chest radiography filmed on POD1. Radiological subcutaneous emphysema was detected in 32 (27.2%) patients. However, only 6 (5.3%) patients developed symptomatic pneumothorax and 2 (1.8%) patients had postoperative bleeding that required further intervention. The morbidity rate was 7.0% and the mortality rate was 0%.
Table 2. Postoperative outcome of chest tube-free VATS.
| Variables | Chest tube-free VATS (N=114) |
|---|---|
| Residual airspace >3 cm on POD1 | 13 (11.4) |
| Subcutaneous emphysema | 32 (27.2) |
| Pleural effusion on POD1 | |
| None | 100 (87.7) |
| Mild | 14 (12.3) |
| Severe | 0 (0.0) |
| Postoperative stay (days) | 2 [2, 2] |
| Numeric rating scale for pain | |
| POD1 | 1 [0, 2] |
| Day of discharge | 0 [0, 1] |
| Morbidity | 8 (7.0) |
| Pneumothorax | 6 (75.0) |
| Bleeding | 2 (25.0) |
| Mortality | 0 (0.0) |
Data were displayed as median [25th quantile, 75th quantile] or frequency (percentage) per data type. VATS, video-assisted thoracoscopic surgery; POD, postoperative day.
Additionally, the median postoperative hospitalization was 2 days, and the median NRS was 1 on POD1 and 0 on the day of discharge. The median peak flow rate during the air suctioning process was 210 mL/min with an interquartile range (IQR) of 170–270 mL/min (Table 3). All but seven (6.1%) patients had their end flow rate above 0 mL/min, making both the median and quartiles 0 mL/min. The median duration of suctioning was 126 s (IQR, 102.75–151.25 s). The distributions of the peak flow rate and duration of suctioning are shown in Figure 2.
Table 3. Flow rate and duration on digital suctioning.
| Variables | Mean | Median | 25th quantile | 75th quantile |
|---|---|---|---|---|
| Duration of digital suctioning (s) | 139.0 | 126 | 102.75 | 151.25 |
| Peak flow rate (mL/min) | 235.7 | 210 | 170 | 270 |
| End flow rate (mL/min) | 1.1 | 0 | 0 | 0 |
Figure 2.
Distribution of the peak flow rate and duration of digital suctioning. (A) Distribution of peak flow rate; (B) distribution of suction time.
Detailed information about patients with morbidities is shown in Table 4, including six cases of pneumothorax and two cases of postoperative bleeding, as mentioned earlier. Among these, most patients achieved remission after conservative management with manual air extraction for pneumothorax and intravenous hemostatic for bleeding, except for one patient with a pneumothorax who required re-insertion of a chest tube. This protracted the patient’s discharge to POD6 when the air leak ceased and the chest tube was removed. In retrospect, this event might have been predicted by a borderline end flow rate of 30 mL/min with rebound, as well as an excessively long suction time of 335 s.
Table 4. Cases of morbidity.
| Cases | Gender | Age (y) | Nodule size (cm) | Lobe | Peak flow rate (mL/min) |
End flow rate (mL/min) |
Time of suctioning (s) | Size of residual airspace on POD1 (cm) | Morbidity | Management |
|---|---|---|---|---|---|---|---|---|---|---|
| Case A | Male | 63 | 1.0 | Left upper | 210 | 30 | 335 | 0 | Pneumothorax | Chest tube insertion |
| Case B | Female | 58 | 1.2 | Right upper | 150 | 0 | 85 | 0 | Bleeding | IV hemostatic without transfusion |
| Case C | Female | 36 | 0.7 | Left lower | 190 | 0 | 180 | 4.7 | Pneumothorax | Manual air extraction |
| Case D | Male | 44 | 1.0 | Right upper | 150 | 10 | 220 | 4.5 | Pneumothorax | Manual air extraction |
| Case E | Female | 31 | 0.7 | Left lower | 600 | 0 | 155 | 3.1 | Pneumothorax | Manual air extraction |
| Case F | Female | 50 | 0.8 | Left upper | 290 | 0 | 130 | 4.2 | Pneumothorax | Manual air extraction |
| Case G | Female | 35 | 1.0 | Left upper | 210 | 10 | 300 | 4.7 | Pneumothorax | Digital suctioning |
| Case H | Female | 44 | 1.2 | Right upper | 270 | 0 | 120 | 0 | Bleeding | IV hemostatic without transfusion |
POD, postoperative day; IV, intravenous.
Discussion
A drainless strategy that completely omits chest tube placement postoperatively in minor pulmonary surgery has proven feasible and will accelerate patient recovery (2-10). However, varying degrees of morbidity (pneumothorax, 7.6–40%) have been reported (2,3,7,8,11), precluding its wider implementation. In our case series, a digital drainage device with measurable parameters was used in the air suctioning process, and better standardization could thus be achieved.
The traditional method for air-tightness testing involved expulsion of the residual air by controlled manual inflation of the operated lung while the external end of the chest tube was submerged in sterile saline. Air-tightness was confirmed by the cessation of the underwater bubbles, similar to the evaluation of water-sealed closed drainage. Although different methods have been proposed to secure the test result (2,3), the subjective nature of visual assessment is unchanged, making it vulnerable to interobserver variability (20). Such weakness, however, can be overcome through the application of digital drainage, which has already shown excellent results with prolonged air leaks (12-15). In addition, the digital drainage system provides constant, customizable negative pleural pressure, which is not only more aligned with normal respiratory physiology but also has potential for the future investigation of the optimal setting. Therefore, it would be reasonable to adopt digital drainage to check air-tightness during intraoperative chest tube withdrawal.
Indeed, Liu et al. (21) previously used digital drainage intraoperatively in a series of tubeless VATS. In their series, the standard chest tube was replaced by a 16-Fr pigtail catheter, and the suctioning pressure was set at −15 cmH2O. However, unlike our procedures, air suctioning was left unattended while simultaneously closing the wound; if the flow rate read 0 mL/s when the wound closure was completed, catheter removal was then indicated.
To gain a better understanding of draining mechanics and provide clues for further investigations, we conducted ad hoc documentation of the draining procedure. In our cohort, the median duration of suctioning was 126 s (IQR, 102.75–151.25 s), confirming a shorter duration than that of a normal wound closure procedure. Nevertheless, we prefer to end suctioning sooner rather than later because we employed a standard chest tube instead of a pigtail catheter. Delayed withdrawal may result in an unclosed dead space at the tube site that interferes with wound healing. Moreover, if a persistent air leak is suspected, a standard chest tube may be better than a pigtail catheter, as the former has a lower risk of displacement and occlusion.
To our knowledge, our study is the first to report numeric measurements recorded during intraoperative digital drainage, highlighting its proclaimed advantage of objective evaluation (21). In our series, the median peak and end flow rates were 210 mL/min (IQR, 170–270 mL/min) and 0 mL/min (IQR, 0–0 mL/min), respectively (Table 3). Both the peak flow rate and the duration of suctioning followed a slightly right-skewed distribution, yet the same parameters were scattered dispersedly in cases with radiological pneumothorax (Figure 2). However, for cases with clinical pneumothorax, at least one of the three parameters exceeded the respective IQRs (Table 4). Careful patient selection reduces postoperative morbidity (22). Nevertheless, due to the exploratory nature of this study, the determination of an explicit cutoff to predict postoperative morbidity was not possible, but our findings suggest that using a combination of drainage parameters rather than the flow rate alone may have a better ability to identify patients with elevated risk. Notably, a rebound in the flow rate after it reached the lowest point was observed in Case A (Table 4), which was the only included case that required chest thoracostomy, in addition to another four cases that were already excluded due to an excessive end flow rate, highlighting the importance of dynamic profiling. Taken together, the results of this study indicated that air leak is a complex process, and standardization of the criteria for intraoperative chest tube removal should be further improved with ongoing data collection.
The prophylactic use of an indwelling catheter was inspired by Zhang et al. (16,17). In their trial, remedial air extraction via the catheter could be used as a salvage measure in the case of postoperative pneumothorax, which was correlated with significantly less pain than a routine chest tube (16). Although the prophylactic catheter did save four patients with symptomatic pneumothorax from further chest tube insertion, it cannot be viewed as a completely drainless strategy and would be utilized in patients who failed to omit the chest tube indiscriminately. The postulated benefit of a thoracic catheter may be achieved with careful patient selection and vigilant postoperative monitoring. Therefore, future investigations should focus on improving the risk-stratifying algorithm based on digital recording.
Admittedly, our study is subject to several limitations that should be noted and considered. Firstly, this is a retrospective case series. Although the inclusion and exclusion criteria were set a priori, selection bias is inherent and might have confounded the results. Furthermore, the exploratory nature of the study made the comparison between digital and traditional drainage difficult. Also, the sample size was small and stratified analysis was thus precluded. Given that the feasibility of this procedure is confirmed, we plan to perform further comparative investigations on a larger scale to validate its efficacy. Finally, our current strategy has not fully utilized information generated from digital drainage, such as the air leak volume and the curve pattern. Our future work will examine the mechanics and dynamics of air leaks more comprehensively.
Conclusions
In conclusion, chest tube-free VATS assisted by a digital drainage device is a feasible strategy with minimal morbidity and mortality. Its strength of quantitative air leak monitoring produces important measurements for the prediction of postoperative pneumothorax and would be a useful tool to facilitate standardization of the procedure in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital (IRB No. K22-319). Individual consent for this retrospective analysis was waived.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1749/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1749/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1749/coif). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Gómez-Caro A, Roca MJ, Torres J, et al. Successful use of a single chest drain postlobectomy instead of two classical drains: a randomized study. Eur J Cardiothorac Surg 2006;29:562-6. 10.1016/j.ejcts.2006.01.019 [DOI] [PubMed] [Google Scholar]
- 2.Yang SM, Wang ML, Hung MH, et al. Tubeless Uniportal Thoracoscopic Wedge Resection for Peripheral Lung Nodules. Ann Thorac Surg 2017;103:462-8. 10.1016/j.athoracsur.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Yang R, Sun Y. Tubeless uniportal thoracoscopic wedge resection with modified air leak test and chest tube drainage. BMC Surg 2020;20:301. 10.1186/s12893-020-00910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe A, Watanabe T, Ohsawa H, et al. Avoiding chest tube placement after video-assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg 2004;25:872-6. 10.1016/j.ejcts.2004.01.041 [DOI] [PubMed] [Google Scholar]
- 5.Cui F, Liu J, Li S, et al. Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively. J Thorac Dis 2016;8:2226-32. 10.21037/jtd.2016.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S, Jiang L, Ang KL, et al. New tubeless video-assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017;51:689-93. 10.1093/ejcts/ezw364 [DOI] [PubMed] [Google Scholar]
- 7.Nakashima S, Watanabe A, Mishina T, et al. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today 2011;41:774-9. 10.1007/s00595-010-4346-5 [DOI] [PubMed] [Google Scholar]
- 8.Liao HC, Yang SM, Hung MH, et al. Thoracoscopic Surgery Without Drainage Tube Placement for Peripheral Lung Nodules. Ann Thorac Surg 2020;109:887-93. 10.1016/j.athoracsur.2019.10.048 [DOI] [PubMed] [Google Scholar]
- 9.Liu CY, Hsu PK, Leong KI, et al. Is tubeless uniportal video-assisted thoracic surgery for pulmonary wedge resection a safe procedure? Eur J Cardiothorac Surg 2020;58:i70-6. 10.1093/ejcts/ezaa061 [DOI] [PubMed] [Google Scholar]
- 10.Laven IEWG, Franssen AJPM, van Dijk DPJ, et al. A No-Chest-Drain Policy After Video-assisted Thoracoscopic Surgery Wedge Resection in Selected Patients: Our 12-Year Experience. Ann Thorac Surg 2022. [Epub ahead of print]. doi: . 10.1016/j.athoracsur.2022.04.030 [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Kehlet H, Holbek BL, et al. Efficacy and safety of omitting chest drains after video-assisted thoracoscopic surgery: a systematic review and meta-analysis. J Thorac Dis 2021;13:1130-42. 10.21037/jtd-20-3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerfolio RJ, Varela G, Brunelli A. Digital and smart chest drainage systems to monitor air leaks: the birth of a new era? Thorac Surg Clin 2010;20:413-20. 10.1016/j.thorsurg.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 13.Brunelli A, Salati M, Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;37:56-60. 10.1016/j.ejcts.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. 10.1016/j.athoracsur.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 15.Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardiothorac Surg 2011;39:584-8. 10.1016/j.ejcts.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 16.Zhang JT, Dong S, Chu XP, et al. Randomized Trial of an Improved Drainage Strategy Versus Routine Chest Tube After Lung Wedge Resection. Ann Thorac Surg 2020;109:1040-6. 10.1016/j.athoracsur.2019.11.029 [DOI] [PubMed] [Google Scholar]
- 17.Zhang JT, Tang YC, Lin JT, et al. Prophylactic air-extraction strategy after thoracoscopic wedge resection. Thorac Cancer 2018;9:1406-12. 10.1111/1759-7714.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240-52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 19.Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. 10.1378/chest.119.2.590 [DOI] [PubMed] [Google Scholar]
- 20.Varela G, Jiménez MF, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28-31. 10.1016/j.ejcts.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Liu CY, Hsu PK, Chien HC, et al. Tubeless single-port thoracoscopic sublobar resection: indication and safety. J Thorac Dis 2018;10:3729-37. 10.21037/jtd.2018.05.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CK, Leong KI, How CH, et al. Drainless thoracoscopic surgery should be avoided in primary spontaneous pneumothorax with pleural adhesion. Interact Cardiovasc Thorac Surg 2022;35:ivac237. 10.1093/icvts/ivac237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as