The cases
Mr. F, a 65-year-old man, presents to the emergency department with chest and epigastric pain of more than 2 hours' duration. The previous evening he experienced similar pain lasting 30 minutes. He has no history of cardiac disease, known risk factors or other serious illnesses, but he has not seen a physician for many years. His blood pressure is 120/65 mm Hg, the heart rate is 60 beats/min, there are bilateral fine inspiratory crackles in the lung bases, the venous pressure is normal, and there are quiet heart sounds. The electrocardiogram (ECG) shows ST-segment elevation in leads V1 to V4 and reciprocal ST-segment depression in leads II, III and aVF (Fig. 1). A chest x-ray film appears normal. What are the options for reperfusion therapy?
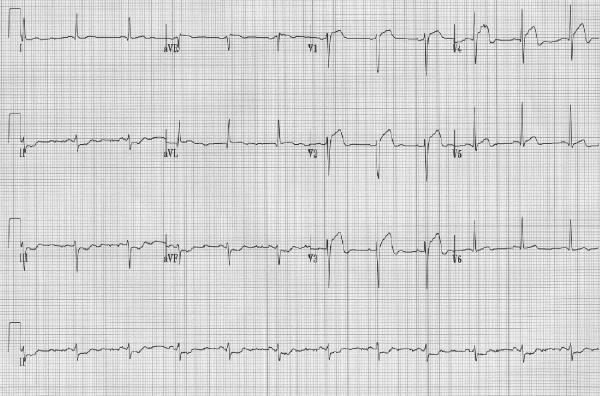
Fig. 1: Case 1. Initial electrocardiogram (ECG), showing ST-segment elevation in leads V1– V4, indicative of evolving anterior myocardial infarction. ST-segment depression is evident in leads II, III and aVF.
Mr. G, a 48-year-old smoker, presents to hospital with unremitting chest pain of 4 hours' duration. He has a 2-week history of episodic central chest pain, for which he did not seek medical attention. His blood pressure is 170/90 mm Hg, the heart rate is 75 beats/min, his chest is clear, the central venous pressure and heart sounds are normal, and there is a bruit heard over the right femoral artery. The initial ECG (Fig. 2) shows inferior T-wave inversion. The baseline levels of creatine kinase (CK), its MB isoenzyme (CK MB) and cardiac troponin I are twice the upper limit of normal. Mr. G is given 325 mg of ASA, 50 mg of metoprolol 3 times daily, nitroglycerin intravenously, unfractionated heparin and tirofiban. His symptoms resolve within 15 minutes after receiving the nitroglycerin. Should Mr. G undergo cardiac catheterization?
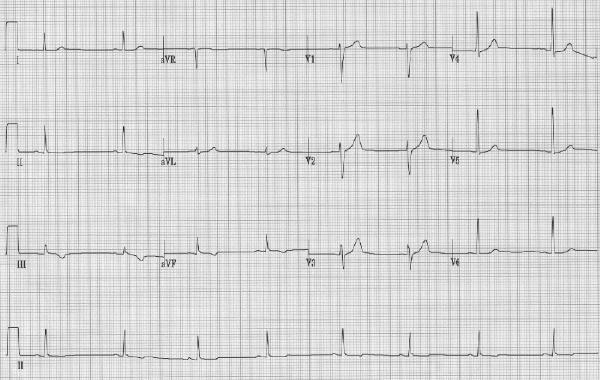
Fig. 2: Case 2. Initial ECG, showing symmetrical T-wave inversion in leads II, III and aVF.
Initially suitable only for highly selected low-risk patients, percutaneous coronary revascularization has evolved into a vital component of the management of acute coronary syndromes (ACS). Remarkable advances in catheters, stents, adjunctive pharmacologic therapy, imaging and operative techniques have expanded the application of percutaneous coronary interventions (PCIs) to patients with clinically and anatomically complex heart disease.
Clinical research conducted in recent years has established sound evidence for the role of coronary angiography and PCIs in patients with ACS. Canadian investigators have made important contributions to this literature. This review is intended to convey the main clinical concepts and lessons from this body of research.
Canadian cardiac catheterization laboratories are limited in number and are highly centralized. Although centralization offers an enviable concentration of expertise and operational efficiencies, it has created special challenges for the majority of clinicians who practise in centres without on-site cardiac catheterization. A challenge to implementing evidence-based care, therefore, is to develop and use a patient transportation infrastructure. We have attempted to highlight these considerations where appropriate.
Modern percutaneous coronary intervention techniques
Two developments in the 1990s dominated the evolution of PCI techniques: stents and improved antithrombotic therapy using glycoprotein IIb/IIIa platelet inhibitors.
Stenting
Before the advent of stenting, PCI was hampered by 2 problems: occlusive coronary dissection and thrombosis (leading to acute myocardial infarction or emergency bypass surgery), and restenosis (leading to recurrent ischemia). The introduction of coronary stents (Fig. 3) has dramatically altered this landscape. Before stents became available, emergency bypass surgery was required in 2%–3% of patients undergoing PCI; this risk is now below 0.2% despite increasing lesion complexity.1 Stents readily scaffold otherwise occlusive procedure-induced dissections. However, the metallic surfaces of stents can themselves stimulate occlusive thrombosis. Until the stent is entirely covered by endothelial tissue (a process requiring up to several weeks), combined oral antiplatelet therapy with ASA plus clopidogrel or ticlopidine is required.2
Fig. 3: Stents such as this one (the ACS MULTILINK DUET balloon-expandable coronary stent) are commonly used during percutaneous transluminal coronary angioplasty (PTCA) in Canada. The stent is laser-cut from tubular stainless steel in a design providing radial strength and longitudinal flexibility. Stent diameter is determined by the balloon diameter used for deployment. Photo by: Courtesy: Guidant Corporation, Santa Clara, Calif.
Restenosis develops from the additive effects of mechanical vessel recoil plus neointimal proliferation (a healing response). Clinical trials have shown that stents substantially reduce restenosis but do not eliminate it,3,4,5 in large part because stents prevent recoil but do not reduce neointimal proliferation. Data from the British Columbia Cardiac Registries have confirmed the broad effectiveness of stents in most PCI patients and demonstrated that the adoption of routine stenting during the mid-1990s in patients with or without ACS was accompanied by a significant reduction in the incidence of clinical restenosis to below 20% overall, and below 15% among those with stents.1 Currently, over 90% of patients undergoing PCI in British Columbia receive at least 1 stent.
Glycoprotein IIb/IIIa platelet inhibitor therapy
Because PCI itself induces vascular injury, a highly thrombotic coronary environment develops during PCI, even in patients without pre-existing ACS. Not surprisingly, the use of potent glycoprotein IIb/IIIa platelet inhibitors during angioplasty in patients with or without ACS has been shown to result in improved short- and long-term outcomes.6,7 More recently, abciximab and eptifibatide have been tested during stent procedures in placebo-controlled trials that included many patients from Canadian centres (the EPISTENT trial8 and ESPRIT trial9). Both drugs were associated with substantial early reductions in risk of death, myocardial infarction or urgent repeat revascularization.8,9 Early data from a recently completed head-to-head trial comparing tirofiban and abciximab during coronary stent procedures showed abciximab to be slightly superior (30-day mortality, myocardial infarction or emergency revascularization rate 7.6% with tirofiban and 6.0% with abciximab, p = 0.037).10 However, the small absolute difference between agents suggests an important beneficial effect from tirofiban as well. Longer-term data with abciximab and stenting from the EPISTENT study indicates an important sustained reduction in the rates of both myocardial infarction and death through 1 year (Fig. 4) (unpublished data). Whether the benefits of eptifibatide and tirofiban will prove equally durable is yet unknown.
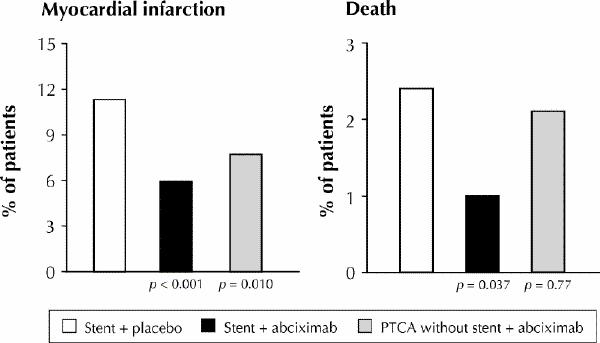
Fig. 4: One-year results from the EPISTENT study, demonstrating the synergy of stenting with intense platelet inhibition using abciximab. Rates of myocardial infarction and death were lower with stenting plus abciximab than with either stenting plus placebo or abciximab plus PTCA without stenting. Photo by: Courtesy: Eric Topol
Although glycoprotein IIb/IIIa inhibitors improve PCI outcomes in patients with a wide variety of indications and clinical characteristics, a consistent preferential benefit exists in those with coexisting ACS.11 Elevated cardiac troponin levels (a marker of intracoronary thrombosis and thromboembolism) further refine the subset of patients most likely to benefit from PCI. Of particular importance to referring physicians in Canada are subgroup analyses from trials testing the administration in the coronary care unit (CCU) of eptifibatide and tirofiban in non-ST-segment elevation ACS: patients subsequently selected for angiography and pre-discharge PCI were found to benefit the most.12,13 Thus, a rational approach to using glycoprotein IIb/IIIa inhibitors in non-ST-segment elevation ACS is to initiate therapy in patients with an elevated level of troponin (or other markers of high risk) when a decision is made to refer for angiography and possible PCI.
Treatment of ST-segment elevation acute coronary syndrome
Direct angioplasty
Direct angioplasty refers to the primary use of PCI (instead of thrombolysis) to achieve reperfusion in patients with ST-segment elevation ACS. The principal mechanism of thrombolytic therapy in such patients is myocardial salvage through rapid restoration of antegrade epicardial flow. Widely used thrombolytic regimens currently achieve complete coronary reperfusion by 90 minutes in about 55%–60% of cases and partial reperfusion in an additional 20%–25%.14 Direct PCI generally yields significantly higher rates of complete reperfusion than does thrombolytic therapy. In addition, the underlying coronary stenosis, a substrate for recurrent ischemia, is relieved early in the course of the infarction. The potential of these attributes of direct PCI to improve clinical outcome beyond that achievable with thrombolytic therapy has led to randomized clinical trials directly comparing these 2 approaches. The largest single trial of this type (GUSTO IIB) compared direct angioplasty and accelerated tissue plasminogen activator (tPA) therapy in 1138 patients.15 The primary end point (a composite of death, nonfatal reinfarction or nonfatal disabling stroke at 30 days) occurred in 9.6% of the angioplasty patients and 13.7% of the tPA patients (p = 0.03), for a 33% risk reduction. A secondary analysis at 6 months still favoured angioplasty, although the difference between the 2 strategies was no longer statistically significant (primary end point occurred in 14.1% of angioplatsy patients and 16.1% of tPA patients).15 A meta-analysis of GUSTO IIB plus 9 other randomized trials comparing direct angioplasty and various thrombolytic regimens also supports the superiority of direct PCI when practised in centres with on-site coronary catheterization and experienced operators.16 The widely used end point of 30-day mortality or reinfarction was significantly lower among patients treated with direct PCI than among those given thrombolytic therapy (Fig. 5).
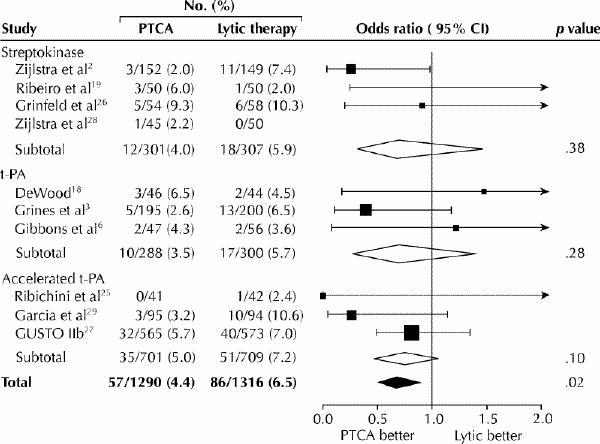
Fig. 5: Mortality at the end of the study period in 10 trials comparing direct PTCA with thrombolytic therapy. The rates for each study are grouped by thrombolytic regimen. Odds ratios with 95% confidence intervals (CIs) are plotted on the right. [Reprinted, with permission, from Weaver WD et al.16 © 1997, American Medical Association.
Because of concerns regarding the safety of stents in the highly thrombotic setting of ST-segment elevation myocardial infarction, direct angioplasty with stents has been tested against angioplasty without stents in randomized trials.17,18 In the Stent-PAMI study17 the prespecified primary 6-month end point (a composite of death, nonfatal myocardial infarction, stroke or target-vessel revascularization) occurred less frequently with stenting than without stenting (12.6% v. 20.1%, p < 0.01). However, a nonsignificant trend toward excess mortality was noted (stenting 5.4% v. no stenting 3.0%). Although likely attributable to chance alone, it has also been suggested that vigorous plaque and thrombus compression associated with direct PCI in general, and stent deployment in particular, may increase athero- and thromboembolic plugging of downstream micro-vessels. Such a phenomenon could paradoxically increase infarct size. This has led to investigations testing the administration of glycoprotein IIb/IIIa inhibitors during direct PCI.
Mechanistic studies have shown that the addition of glycoprotein IIb/IIIa inhibitors during stent-based direct PCI do indeed improve coronary blood flow and reduce infarct size.19,20,21 Preliminary data from the CADILLAC trial, comparing angioplasty with stents, and abciximab with no abciximab, confirmed an overall benefit of abciximab.22 However, the trial was not sufficiently powered to demonstrate an advantage for abciximab in the stent subgroup alone. The primary 6-month end point (a composite of death, target-vessel revascularization, myocardial infarction or disabling stroke) was 19.3% for PTCA plus placebo, 15.2% for PTCA plus abciximab, 10.9% for stent plus placebo and 10.8% for stent plus abciximab). The rates of death were 4.3%, 2.3%, 2.8% and 3.8% respectively. Current practice of direct PCI in Canada and elsewhere now generally involves stenting with concurrent administration of a glycoprotein IIb/IIIa inhibitor.
The applicability in Canada of the findings from these direct PCI trials is limited. For most patients direct PCI is simply not available without time-consuming transportation. Except in highly organized local referral programs, prompt administration of thrombolytic therapy is almost certainly superior to significantly delayed direct PCI. The observation that direct PCI and thrombolytic therapy result in similar outcomes when applied in community-based, unselected, actual practice verifies the vital role of thrombolysis in most Canadian hospitals.23 Community physicians should be aware, however, that emergency transport for direct PCI may be appropriate for patients with ST-segment elevation ACS and contraindications to thrombolytic therapy. Patients at increased risk of thrombolytic complications (e.g., recent ischemic stroke or surgery, prolonged cardiopulmonary resuscitation and coumadin therapy) may yet be safely treated with heparin and ASA, as PCI requires. On the other hand, patients who are unable to receive therapeutic heparin and ASA owing to active major bleeding are usually not candidates for either thrombolytic therapy or direct PCI.
Rescue angioplasty
Angioplasty following failed thrombolytic therapy is termed “rescue” angioplasty. Thrombolytic failure should be diagnosed clinically when, upon completion of thrombolytic infusion (usually 90 minutes after initiating therapy), ST-segment elevation fails to resolve below half its initial magnitude. This emphasizes the importance of routinely obtaining and reviewing ECGs immediately after thrombolytic therapy, even when the chest pain has disappeared (pain resolution can often be confounded by the administration of narcotics or other analgesics).
Successful rescue angioplasty after failed thrombolytic therapy achieves clinical outcomes that approach those of successful thrombolytic therapy.24 These compelling observations have established rescue angioplasty as standard practice (even when patient transportation is required) yet have hampered its rigorous evaluation in controlled trials. A small randomized trial performed in the early 1990s evaluated rescue angioplasty in the setting of acute anterior myocardial infarction.25 Although not powered to evaluate clinical outcome, the strategy of rescue angioplasty resulted in improved left ventricular ejection fraction on exercise.
The optimum timing for referral for rescue angioplasty when patient transportation is required is unclear. We believe the timing should be individualized according to patient and system factors. For institutions located near coronary catheterization centres, referral should be initiated as soon as thrombolytic failure is suspected 90 minutes after its initiation. Earlier referral is appropriate when hemodynamic compromise is present: patients with acute heart failure face predictably lower rates of thrombolytic success than do stable patients. For institutions located hours from catheterization centres, routine early transportation of high-risk patients (those with heart failure or ECG evidence of extensive myocardial infarction) after initiation of thrombolytic therapy is a possible approach. Stable, low-risk patients with small and uncomplicated infarctions may not warrant transportation despite clinically evident thrombolytic failure.
Cardiogenic shock complicating myocardial infarction
The SHOCK study randomly assigned patients with cardiogenic shock developing up to 36 hours following acute myocardial infarction to either emergency revascularization (with angioplasty or bypass surgery) or initial medical stabilization.26 Follow-up at 1 year showed that the survival rate was substantially higher among patients undergoing early revascularization than among those receiving medical therapy (47% v. 34%, p = 0.025) (Fig. 6). Patients under 75 years of age enjoyed a particular survival advantage from emergency revascularization. Thus, patients with cardiogenic shock up to 36 hours after myocardial infarction should be offered emergency transportation, regardless of transportation time, if on-site facilities for emergency revascularization are unavailable.
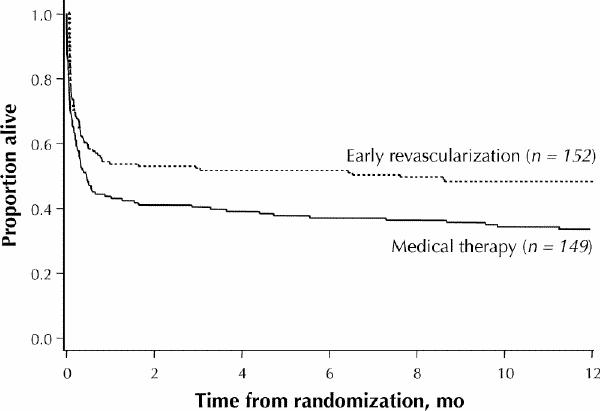
Fig. 6: Kaplan–Meier survival at 1-year follow-up in the SHOCK study. Early revascularization resulted in a 39% relative reduction in mortality. [Reprinted, with permission, from Hochman et al.26 © 2001, American Medical Association.
Angioplasty during convalescence from myocardial infarction
Although it can be argued that routine angiography following the initial treatment of acute myocardial infarction can provide important prognostic information and aid in decision-making, routine angioplasty in patients who are asymptomatic with negative functional test results has been shown to lack benefit. However, when objective ischemia is present following thrombolytic therapy, there is demonstrated benefit to routine revascularization. In the DANAMI study 1008 patients were randomly assigned to either routine angiography and revascularization or medical management alone.27 At 1 year the combined end point of death, reinfarction or unstable angina was reduced from 29.5% to 15.4% with the invasive strategy, a benefit that persisted at least to 3 years. Thus, the routine use of catheterization and appropriate revascularization can be advocated at least for patients in whom there is objective ischemia or spontaneous recurrent angina and for those with heart failure or evidence of a major electrical or mechanical complication.
Treatment of non-ST-segment elevation acute coronary syndrome
When and whom to refer to cardiac catheterization and revascularization from among patients with non-ST-segment elevation ACS has long been debated and studied, and a wide range of practice exists. Because non-ST-segment elevation ACS is currently the commonest diagnosis on admission to CCUs, changes in threshold for invasive management of these patients will greatly increase the total demand for cardiac catheterization, angioplasty and bypass surgery. Reciprocally, the per capita resources committed to these procedures within a health care system may influence the threshold for referral through triage processes and wait-listing.
In the past decade, 4 large, well-designed randomized clinical trials have directly addressed the role of routine angiography and revascularization (invasive management) in non-ST-segment elevation ACS.28,29,30,31 In each trial patients were randomly assigned to either routine invasive management or conservative management (in which invasive management was reserved for recurrent or provocable ischemia). Despite their broad similarities, these trials had many key differences in design, including size, enrolment criteria, concurrent medical therapy, interval to angiography and methods for functional testing in conservatively treated patients (Table 1). Furthermore, enrolment in the various trials spanned a decade during which important improvements in medical therapy, PCI procedures and bypass surgery became widely implemented.
Table 1
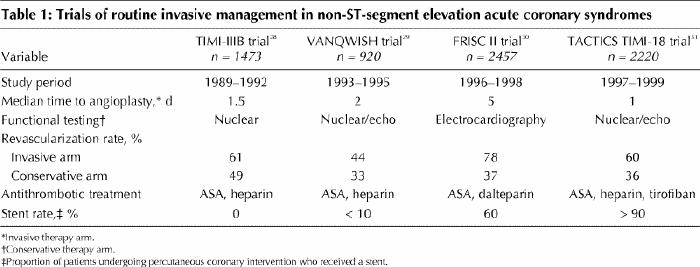
The earlier trials (TIMI-IIIB28 and VANQWISH29) collectively enrolled 2393 patients from 1989 through 1995. These 2 trials showed that routine invasive management reduced symptom burden and hospital length of stay but offered no advantage with respect to subsequent risk of death or myocardial infarction. The 2 more recent trials (FRISC II30 and TACTICS TIMI-1831), which collectively enrolled 4677 patients from 1996 through 1999, confirmed the superiority of routine invasive care with respect to symptom control and demonstrated significant reductions in the composite end point of death, myocardial infarction or urgent revascularization in patients managed invasively compared with those managed conservatively (Figs. 7 and 8). Moreover, at 1-year follow-up, the FRISC II trial showed an independent reduction in mortality. These powerful recent trials better reflect current medical therapy and current PCI and surgical techniques.
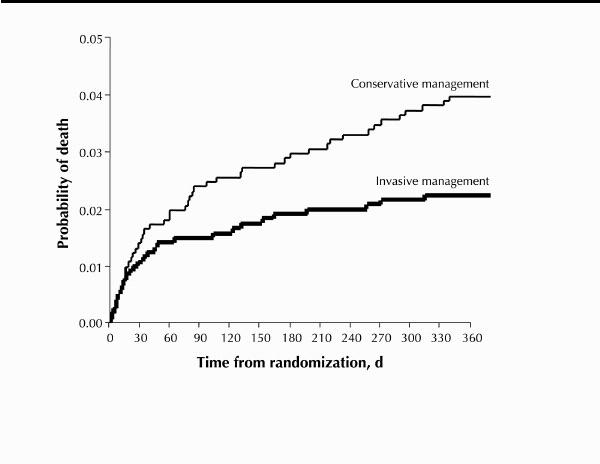
Fig. 7: Kaplan–Meier survival at 1-year follow-up in the FRISC II trial. Routine invasive management (coronary angiography and revascularization) resulted in lower rates of death than did routine conservative management. [Reprinted, with permission, from Wallentin L et al.30 © 2000 The Lancet Ltd.
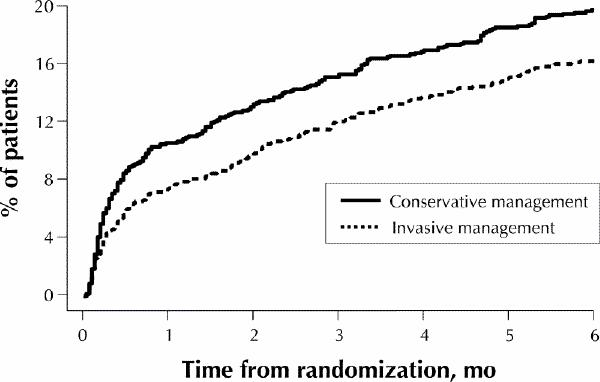
Fig. 8: Incidence of primary end point (composite of death, myocardial infarction or readmission to hospital with acute coronary syndrome) among patients enrolled in the TACTICS TIMI-18 trial. Invasive management reduced the incidence by 22% at 6 months (conservative management 19.4% v. invasive management 15.9%, odds ratio 0.78, 95% confidence interval 0.62–0.97). [Reprinted, with permission, from Cannon et al.31 Copyright © 2001, Massachusetts Medical Society. All rights reserved.
A strict interpretation of the FRISC II and TACTICS TIMI-18 trials would conclude that routine angiography and revascularization is indicated in nearly all patients with non-ST-segment elevation ACS. However, patients enrolled in these studies were required to meet entry criteria that bestowed risk: elevated cardiac enzyme levels, ischemic ECG changes or known pre-existing ischemic heart disease.
The results from these recent randomized trials will no doubt continue to increase demand for invasive management of non-ST-segment elevation ACS. However, risk stratification based on history, ECG changes, troponin levels, clinical course and functional test results will remain a sound approach to selecting patients for angiography and revascularization.32
When patient transportation over substantial distances is required, its cost and inconvenience will continue to make thoughtful risk stratification especially important. The many patients with one or more high-risk features who present to community hospitals must be consistently and accurately recognized by their primary care physicians and provided timely access to appropriate tertiary care facilities.
The incidence of non-ST-segment elevation ACS is high, accounting for most admissions to CCUs. Thus, a small increase in the proportion of ACS patients who are selected for angiography levers a large absolute increase in the demand for related services. The implications for Canada regarding education for physicians, transportation infrastructure, tertiary acute care cardiac beds, and catheterization and revascularization procedure volumes are substantial.
The cases revisited
Mr. F has acute ST-segment elevation anterior myocardial infarction. He has presented to a hospital with on-site cardiac catheterization, and direct PTCA is chosen as the preferred acute reperfusion strategy. In the emergency department he receives 325 mg of ASA, a weight-adjusted bolus of unfractionated heparin intravenously, an oral loading dose of 300 mg of clopidogrel, 15 mg of metoprolol intravenously over 15 minutes, oxygen and morphine. Coronary angiography performed 55 minutes after initial presentation shows complete occlusion of the proximal left anterior descending coronary artery (Fig. 9A) and a coincident stenosis of the right coronary artery. Left ventricular angiography shows akinesis of the anterior wall of the left ventricle, with a reduced ejection fraction of 45%. Following administration of abciximab, a stent-based procedure is performed, which relieves the occlusion and restores normal blood flow (Fig. 9B). For safety, treatment of the stenosis of the right coronary artery is deferred until day 5. Mr. F is discharged on day 6 with the following drug regimen: ASA 81 mg/d, clopidogrel 75 mg/d for 4 weeks, coumadin adjusted for an international normalized ratio of 2.5 for 6 months, ramipril, atenolol and simvastatin.
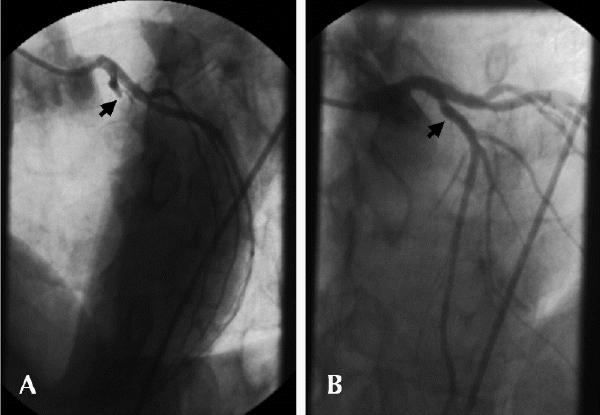
Fig. 9: Case 1. A: Emergent coronary angiography, showing complete occlusion of left anterior descending coronary artery (arrow). B: Normal blood flow is re-established following direct PTCA with stenting.
For Mr. G, who has non-ST-segment elevation ACS, an invasive approach to management is indicated. His high-risk features (ischemic ECG changes and elevated troponin level) justify this decision despite the absence of spontaneous recurrent ischemia and functional testing. Because the patient was admitted to a hospital with on-site cardiac catheterization, coronary angiography is performed 24 hours after admission. Oral and intravenous therapy was continued without interruption before the procedure. A high-grade stenosis is revealed in the middle of the right coronary artery, with associated haziness (suggesting intraluminal thrombus) and impaired blood flow (Fig. 10A). Left ventricular angiography shows mild inferior hypokinesis, with a normal ejection fraction. Stenting performed during the same procedure results in a widely patent vessel with improved distal blood flow (Fig. 10B). Tirofiban therapy is continued for 12 hours after the procedure, and Mr. G is discharged the next morning with the following drug regimen: ASA 325 mg/d, plavix 75 mg/d for 28 days, an angiotensin-converting-enzyme inhibitor and his previously prescribed statin lipid-lowering agent.
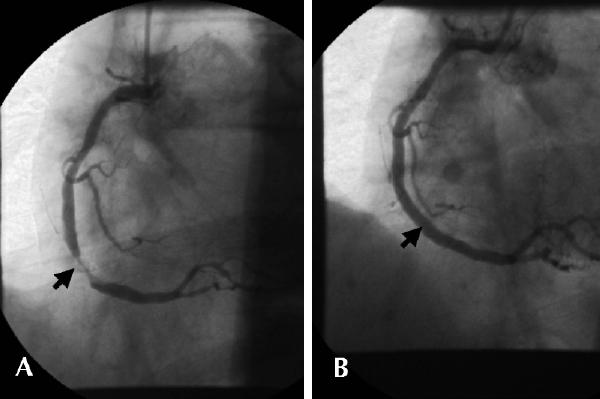
Fig. 10: Case 2. A: Coronary angiography 24 hours after admission, showing high-grade stenosis in middle of right coronary artery with associated haziness consistent with intraluminal thrombus (arrow). B: Vessel is widely patent with improved distal blood flow following PTCA with stenting.
Appendix 1.
Appendix 1. Continued.

Appendix 1. Continued.
Footnotes
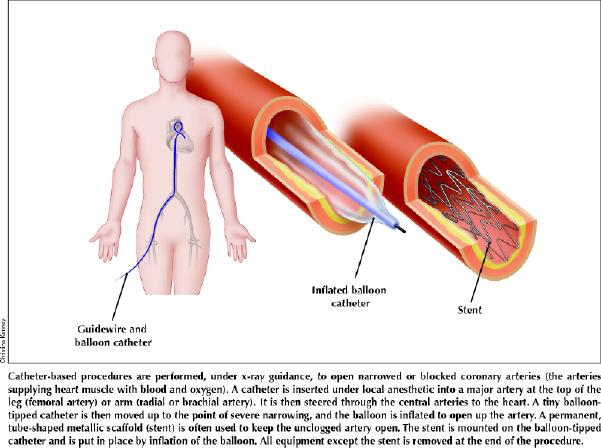
An information sheet for patients appears at the end of the article (see Appendix 1).
Articles to date in this series
Armstrong PW. New advances in the management of acute coronary syndromes [editorial]. CMAJ 2001;164 (9):1303-4.
Fitchett D, Goodman S, Langer A. New advances in the management of acute coronary syndromes: 1. Matching treatment to risk. CMAJ 2001;164(9):1309-16.
Armstrong PW. New advances in the management of acute coronary syndromes: 2. Fibrinolytic therapy for acute ST-segment elevation myocardial infarction. CMAJ 2001;165(6):791-7.
A patient information sheet appears on the next page (Appendix 1).
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Christopher E. Buller, 865–10th Ave. W, Vancouver BC V5Z 1L7; fax 604 875-5403; cehbuller@shaw.ca
References
- 1.Rankin JM, Spinelli JJ, Carere RG, Ricci DR, Penn IM, Hilton JD, et al. Improved clinical outcome after widespread use of coronary-artery stenting in Canada. N Engl J Med 1999;341:1957-65. [DOI] [PubMed]
- 2.Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998;339:1665-71. [DOI] [PubMed]
- 3.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489-95. [DOI] [PubMed]
- 4.Weaver WD, Reisman MA, Griffin JJ, Buller CE, Leimgruber PP, Henry T, et al. Optimum percutaneous transluminal coronary angioplasty compared with routine stent strategy trial (OPUS-1): a randomised trial. Lancet 2000; 355:2199-203. [DOI] [PubMed]
- 5.Buller CE, Dzavik V, Carere RG, Mancini GB, Barbeau G, Lazzam C, et al. Primary stenting versus balloon angioplasty in occluded coronary arteries: the Total Occlusion Study of Canada (TOSCA). Circulation 1999;100:236-42. [DOI] [PubMed]
- 6.Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med 1994;330:956-61. [DOI] [PubMed]
- 7.Lincoff AM, Tcheng JE, Califf RM, Kereiakes DJ, Kelly TA, Timmis GC, et al. Sustained suppression of ischemic complications of coronary intervention by platelet GP IIb/IIIa blockade with abciximab: one-year outcome in the EPILOG trial (Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade). Circulation 1999;99:1951-8. [DOI] [PubMed]
- 8.Lincoff AM, Califf RM, Moliterno DJ, Ellis SG, Ducas J, Kramer JH, et al. Complementary clinical benefits of coronary-artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of Platelet IIb/IIIa Inhibition in Stenting Investigators. N Engl J Med 1999;341:319-27. [DOI] [PubMed]
- 9.Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. The ESPRIT Investigators. Lancet Dec 2000;356:2037-44. [DOI] [PubMed]
- 10.Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. TARGET Investigators. N Engl J Med 2001;344:1888-94 [DOI] [PubMed]
- 11.Lincoff AM, Califf RM, Anderson KM, Weisman HF, Aguirre FV, Kleiman NS, et al. Evidence for prevention of death and myocardial infarction with platelet membrane glycoprotein IIb/IIIa receptor blockade by abciximab (c7E3 Fab) among patients with unstable angina undergoing percutaneous coronary revascularization. EPIC Investigators.Evaluation of 7E3 in Preventing Ischemic Complications. J Am Coll Cardiology 1997;30:149-56. [DOI] [PubMed]
- 12.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. N Engl J Med 1998; 339(7):436-43. [DOI] [PubMed]
- 13.A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med 1998;338(21):1498-505. [DOI] [PubMed]
- 14.The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. The GUSTO Angiographic Investigators. N Engl J Med 1993;329: 1615-22. [DOI] [PubMed]
- 15.A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. N Engl J Med 1997;336:1621-8. [DOI] [PubMed]
- 16.Weaver WD, Simes RJ, Betriu A, Grines CL, Zijlstra F, Garcia E, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA 1997; 278: 2093-8. [PubMed]
- 17.Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1999;341:1949-56. [DOI] [PubMed]
- 18.Suryapranata H, van't Hof AW, Hoorntje JC, de Boer MJ, Zijlstra F. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation 1998;97:2502-5. [DOI] [PubMed]
- 19.Neumann FJ, Blasini R, Schmitt C, Alt E, Dirschinger J, Gawaz M, et al. Effect of glycoprotein IIb/IIIa receptor blockade on recovery of coronary flow and left ventricular function after the placement of coronary-artery stents in acute myocardial infarction. Circulation 1998;98:2695-701. [DOI] [PubMed]
- 20.Schomig A, Kastrati A, Dirschinger J, Mehilli J, Schricke U, Pache J, et al. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction Study Investigators. N Engl J Med 2000;343:385-91. [DOI] [PubMed]
- 21.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 2001;344:1895-903. [DOI] [PubMed]
- 22.Stone GW. CADILLAC trial results: controlled abciximab and device investigation to lower late angioplasty complications [abstract]. Late-Breaking Clinical Trials, American Heart Association Scientific Session; New Orleans; Nov 2000.
- 23.Danchin N, Vaur L, Genes N, Etienne S, Angioi M, Ferrieres J, et al. Treatment of acute myocardial infarction by primary coronary angioplasty or intravenous thrombolysis in the “real world”: one-year results from a nationwide French survey. Circulation 1999;99:2639-44. [DOI] [PubMed]
- 24.Ross AM, Lundergan CF, Rohrbeck SC, Boyle DH, van den Brand M, Buller CH, et al. Rescue angioplasty after failed thrombolysis: technical and clinical outcomes in a large thrombolysis trial. GUSTO-1 Angiographic Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol 1998;31:1511-7. [DOI] [PubMed]
- 25.Ellis SG, da Silva ER, Heyndrickx G, Talley JD, Cernigliaro C, Steg G, et al. Randomized comparison of rescue angioplasty with conservative management of patients with early failure of thrombolysis for acute anterior myocardial infarction. Circulation 1994;90:2280-4. [DOI] [PubMed]
- 26.Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, et al. One-year survival following early revascularization for cardiogenic shock. JAMA 2001;285:190-2. [DOI] [PubMed]
- 27.Madsen JK, Grande P, Saunamaki K, Thayssen P, Kassis E, Eriksen U, et al. Danish multicenter randomized study of invasive versus conservative treatment in patients with inducible ischemia after thrombolysis in acute myocardial infarction (DANAMI). DANish trial in Acute Myocardial Infarction. Circulation 1997;96:748-55. [DOI] [PubMed]
- 28.Anderson HV, Cannon CP, Stone PH, Williams DO, McCabe CH, Knatterud GL, et al. One-year results of the Thrombolysis in Myocardial Infarction (TIMI) IIIB clinical trial. A randomized comparison of tissue-type plasminogen activator versus placebo and early invasive versus early conservative strategies in unstable angina and non-Q wave myocardial infarction. J Am Coll Cardiol 1995;26:1643-50. [DOI] [PubMed]
- 29.Boden WE, O'Rourke RA, Crawford MH, Blaustein AS, Deedwania PC, Zoble RG, et al. Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. N Engl J Med 1998;338:1785-92. [DOI] [PubMed]
- 30.Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet 2000;356:9-16. [DOI] [PubMed]
- 31.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879-87. [DOI] [PubMed]
- 32.Fitchett D, Goodman S, Langer A. New advances in the management of acute coronary syndromes: 1. Matching treatment to risk. CMAJ 2001;164(9): 1309-16. [PMC free article] [PubMed]


