Abstract
Since the first uniportal video-assisted thoracoscopic surgery (uVATS) performed in 2010, the uniportal approach has evolved up to a point where even the most complex cases can be done. This is thanks to the experience acquired over the years, the specifically designed instruments and improvements in imaging. However, in these last few years, robotic-assisted thoracoscopic surgery (RATS) has also shown progress and distinct advantages compared to the uniportal VATS approach, thanks to advanced maneuverability of the robotic arms as well as the three-dimensional (3D) view. Excellent surgical outcomes have been reported and so too, the ergonomic benefits to the surgeon. The main limitation we find of the robotic systems is that they are designed for a multiport approach, requiring between three to five incisions to be able to perform surgeries. With the aim to offer the least invasive approach, using the robotic technology we decided to adapt the Da Vinci Xi® in September 2021 to develop the uniportal pure RATS approach (uRATS) performed by a single intercostal incision, without rib spreading and using the robotic staplers. We have now reached a point where we perform all type of procedures, including the more complex sleeve resections. Sleeve lobectomy is now widely accepted as a reliable and safe procedure to allow complete resection of centrally located tumors. Although it is a technically challenging surgical technique, it offers better outcomes when compared to pneumonectomy. The intrinsic characteristics of the robot such as the 3D view and improved maneuverability of instruments make the sleeve resections easier compared to thoracoscopic techniques. As in uVATS vs. multiport VATS, the uRATS approach, due to its geometrical characteristics, requires specific instrumentation, different movements and learning curve compared to multiport RATS. In this article we describe the surgical technique and our initial uniportal pure RATS experience with bronchial, vascular sleeves and carinal resections in 30 patients.
Keywords: Sleeve lobectomy, uniportal robotic-assisted thoracoscopic surgery (uRATS), lung cancer, uRATS sleeve
Introduction
During the past several years, minimally invasive thoracic surgery has evolved from thoracoscopic approaches using three to four ports to a single incision approach (1-3). With the experience acquired with the uniportal video-assisted thoracoscopic surgery (uVATS) approach, we are now able to perform the most complex resections by using a single small incision (4). During this same period of evolution into uVATS surgery, robotic thoracic surgery has gained popularity as an alternative to traditional VATS (5). In 2002, Melfi et al. published the first report on the use of robotic-assisted thoracoscopic surgery (RATS) for the treatment of lung cancer (6). Since then, the adoption of robotic surgery has increased gradually around the globe. In some areas of the world such as the United States, robotic multiport thoracic surgery is now more popular than thoracoscopic techniques or uVATS. The advantages of robotics are the ability to perform surgery more precisely with articulated, wristed instruments, motion scaling, and tremor filtration, as well as improved visualization thanks to three-dimensional (3D) high-definition video. Currently four to five incisions are still necessary to perform anatomic robotic resections through the intercostal spaces using the platforms (7-10). However, after a careful analysis of the Da Vinci Xi platform, we saw that at least three arms could be adapted through a single incision without any problems. Thanks to the experience acquired with uVATS during the last few years by using advanced bimanual instrumentation, we introduced the uniportal RATS (uRATS) approach in September 2021, in Spain, performing the first world cases of single port, fully robotic lobectomies (11-13). Since then, we have improved the technique to encompass all types of surgeries, including sleeve resections (14,15).
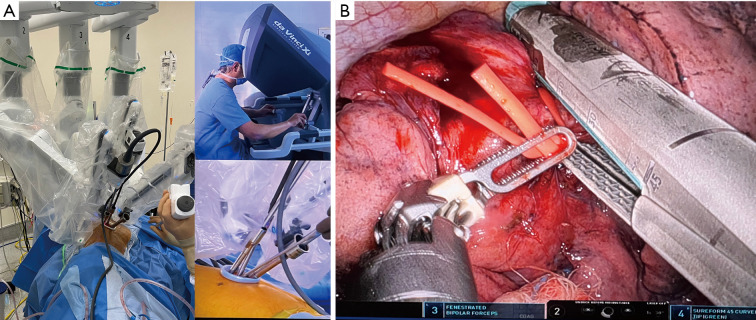
The whole lung exposure and dissection (including stapler insertion) is performed by a single operator comfortably seated in the console (Figure 1). The enhanced 3D view provided by the robotic platform is a remarkable improvement over conventional VATS cameras. The ability of the surgeon to have full control of the camera directly in a stable manner, with better maneuverability and magnification of the field, is also an important advantage. The design of the robotic platform also filters unintended movements caused by tremor. This increases dexterity, restores proper hand-eye coordination and allows for the surgeon to maintain a natural ergonomic position. The uRATS technique can be performed with conventional robotic trocars, but the use of transoral robotic surgery (TORS) trocars, used obviously for transoral surgery, increases the space for the instrumentation and allows more range of movement to avoid arm collision (12).
Figure 1.
Photo showing the arm placement and location of the 12 mm trocar during a middle sleeve lobectomy (A). Combined photo showing the division of posterior fissure and port placement with the 12 mm trocar through the single incision for stapler insertion during a right lower sleeve lobectomy (B).
Location of incision for sleeve resections
The location of the incision is of paramount importance in uVATS, as it can either facilitate or impair the efficiency of the suturing. Placing the incision at the 4th intercostal space (ICS), anterior axillary line, provides direct access to both ends of the bronchial tree and enables suturing in an axis almost perpendicular to that of the anastomosis, just like in open surgery. However, in fully robotic single port surgery, the placement of the incision must be lower in order to have good angle for the articulation of the robotic staplers. In this case, we have to change one of the 8-mm trocars and insert a 12-mm trocar for the stapler, normally at the lower part of the incision (Figure 1). The incision is normally placed at the level of 6th or 7th intercostal space (16). The lower location of the incision is useful for the surgeon at the console but increases the difficulty for the assistant. In case of a hybrid RATS-VATS procedure (using non-robotic thoracoscopic staplers) the incision can be placed at the 4th or 5th ICS for a correct insertion of staplers by the assistant (17).
Sleeve technique
Although technically more demanding, sleeve resections can be performed in a minimally invasive way, thanks to advancements in imaging systems, instrument design and experience in video-assisted thoracic surgery accumulated during the past two decades. Due to certain tumor characteristics, such as size and central location, sleeve resections pose more challenges than conventional lobectomies. Sleeve resections are recommended for the treatment of central tumors involving the origin of the lobar bronchus or extending into the main bronchus and in the case of infiltrated hilar lymph nodes, regardless of the patient’s capacity to tolerate a pneumonectomy. For tumors requiring sleeve resections, most of the surgeons use three to five incisions, either by VATS or RATS. The uniportal technique in expert hands, however, due to its geometric features, could facilitate this type of procedure thus, avoiding additional incisions (18,19). The movements we use for uRATS instrumentation are completely different than multiportal RATS, however, so proper training is mandatory to learn specific tricks (11).
End-to-end anastomosis
The reconstruction of the bronchial tree is facilitated after dissection of lymph node stations 2R, 4R (right side), 4L (left side) and 7 is completed. For the sleeve anastomosis, the most common technique used in minimally invasive surgery is the running suture (19,20). The main reason for this preference is that a continuous suture can reduce the time required for the completion of the anastomosis, as additional sutures are not required, and also tangling is minimized when using a single suture (20).
In uVATS, we normally use a 3-0 double-armed running absorbable suture (single 90 cm thread with two needles) performing a circumferential anastomosis of 360 degrees with both needles (180 degrees with each needle) and a single knot at the most anterior portion of the anastomosis. This technique is more difficult in the case of uniportal robotic sleeve anastomoses. We prefer to use a customized barbed 3/0 suture, 25- or 30-cm lengh with two needles and perform a running suture (Figure 2) (Fibloc, Assut Europe). There is another option with 3-0 V-loc system in two parts. First, we do the posterior wall of the anastomosis and then the anterior wall with another thread. Both ends are tied at the most anterior aspect of the structure. We consider barbed sutures more reliable and safer in uRATS for two reasons: there is no need to take out the threads throughout the incision (that would interfere with robotic arms), and we don’t lose the tension while completing the anastomosis (the maintenance of the tension by the assistant during the procedure would be very difficult).
Figure 2.
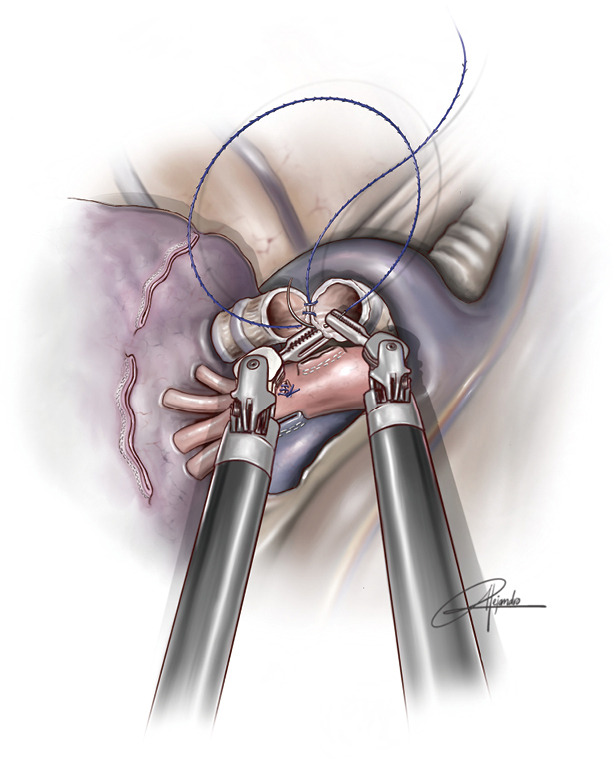
Illustration showing a running barbed suture during an upper and middle bilobectomy sleeve (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article).
The instruments
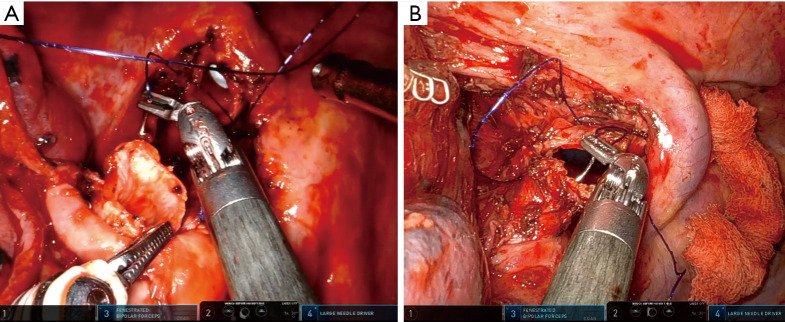
The uRATS technique presents certain differences compared to multiport techniques, attributed largely to the fact that the instruments and the camera are all placed through a single 3–4 cm incision. The role of the assistant at the front side of the patient is very important, more relevant than in uVATS and multiportal RATS. The assistant will need to use instruments to retract the lung, retrieve the lymph nodes and aspirate blood or liquids throughout the procedure. They must also be aware of and coordinate the robotic arm movements to avoid potential collision that could occur during specific moments of the procedure. At the same time, the assistant would also need readapt the position of each arm in case of misconfiguration through the incision. The assistant normally exposes the lung by using two long curved 45.5-cm Dennis Suctions (Scanlan International), that are inserted below the camera port to minimize interference with the other arms. When lymph nodes are dissected, the assistant must remove them by using a 42.4-cm thoracoscopic Node Grasper (Scanlan International). The most difficult maneuver for the assistant is to find a good place to introduce the instruments in coordination with robotic arms, normally in the lower area not occupied by these. The more important robotic instruments in uRATS sleeve resections are the Maryland and bipolar fenestrated for dissection and anatomic resection, and the needle holder and scissors for bronchial sleeving and anastomosis. Due to the robotic scissors offering a 90-degree angulation, the division of the bronchus is facilitated allowing for a more precise cut compared with uVATS (Figure 3). For the bronchial reconstruction, we normally keep a bipolar fenestrated on the left hand for traction (right side arm 3, left side arm 2) and needle holder on the right hand for suturing (right side arm 4, left side arm 1).
Figure 3.
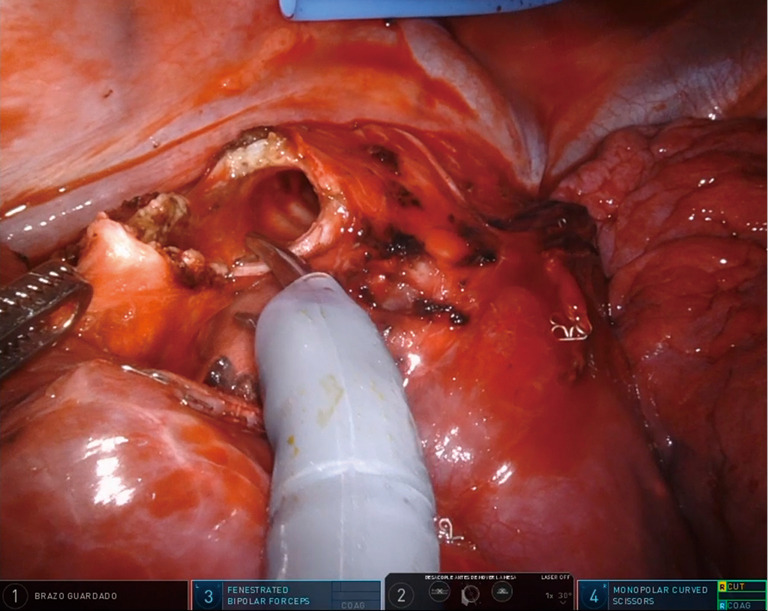
Surgical image showing the use of robotic scissors to divide the intermedius bronchus during a middle sleeve lobectomy.
Arm placement and docking
Since the Xi System has four arms, we must deactivate one arm during surgery. Due to the configuration of the system, we consider that the most appropriate position is the removal of arm 1 in surgeries performed on the right side (using 2-3-4) and arm 4 on the left side (using 1-2-3). The camera must be placed in the upper part of the incision, (most anterior area): on the right side the camera would be arm 2 and on the left side it would be arm 3 (12). Normally, the two arms are placed in the middle and lower part, usually working in parallel. We use three 8-mm trocars and only in case of the use of robotic staplers we will change to a 12-mm trocar, just for stapler insertion (Figure 1).
The 3D vision provided by the Da Vinci Xi is one of the great advantages of the system, especially in the case of sleeve reconstruction, lymphadenectomy and segmentectomies. We recommend the use of a 30-degree camera that will give us a more panoramic and angled view. The angle of camera is normally placed looking down, however, in some specific situations, such as adhesions, it could be necessary to turn the camera 30 degrees up in order to remove these easily.
Types of sleeve resections
Right upper sleeve lobectomy
The incision (4–5 cm long) at the 6th or 7th intercostal space is the most convenient for pure robotic sleeve resections. After division of vessels and opening the fissure, the right main bronchus, upper lobe bronchus and intermediate bronchus must be dissected and sectioned using robotic scissors. The margins should be inspected macroscopically and a frozen section must be performed before starting the anastomosis. We place the first stitch of the continuous barbed 3/0 suture at edge of posterior membranous with cartilaginous portion from inside to outside along the right main bronchus, and then from outside to inside at the same level along the intermedius. We perform a running suture with one needle until we reach the anterior part of the cartilaginous portion. Then we complete the membranous part and the superior cartilaginous part of the anastomosis with the other needle from posterior to anterior to meet the first thread and we tie together at least three times. To compensate for the difference of caliber between both bronchi, the interval between the suture of main bronchus should be slightly larger than the intermedius and should be adjusted during the running suture.
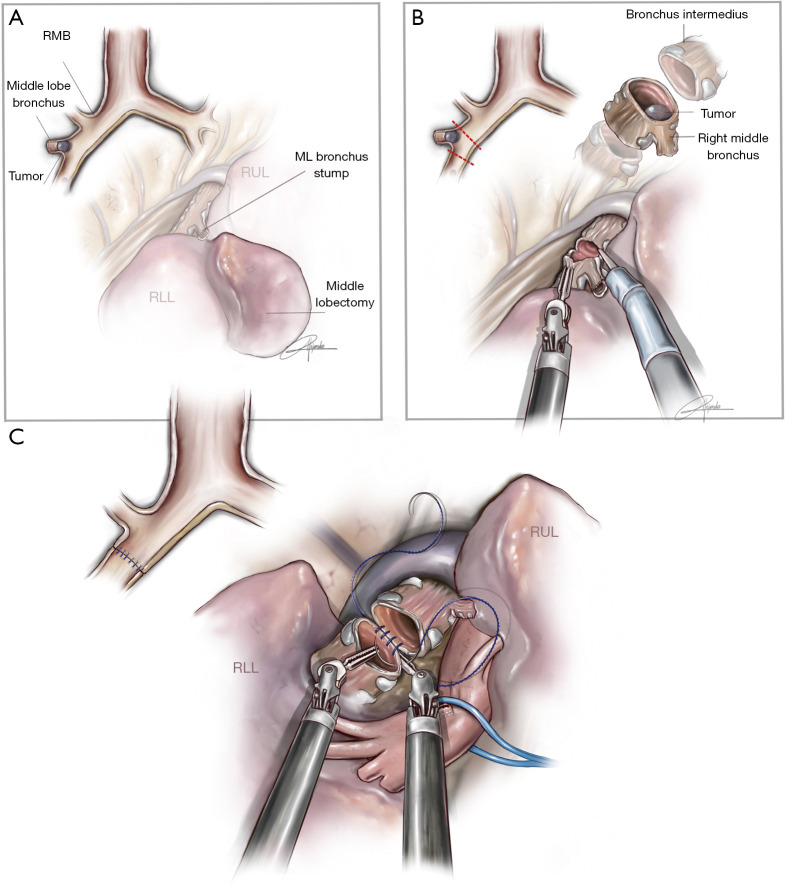
Middle sleeve lobectomy
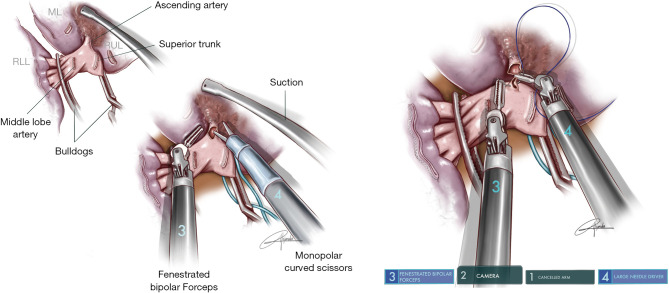
This procedure is performed when the tumor is at the entrance of the middle lobe bronchus (MLB) or at the distal part of the bronchus intermedius (BI), extending into the MLB. The strategy consists of performing a middle lobectomy and performing an anastomosis between the BI and the lower lobe bronchus. Because the MLB and S6B are normally located one in front of each other, the main difficulty is to preserve the S6B when the MLB is divided (Figure 4). We normally recommend encircling the lower lobe artery and retracting it anteriorly in order to facilitate the bronchial anastomosis posteriorly. The subcarinal lymphadenectomy helps the exposure of BI before its division.
Figure 4.
Illustration showing the procedure of middle sleeve lobectomy (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article). (A) Anatomic location of a tumor requiring middle lobe sleeve; (B) division of bronchus intermedius with robotic scissors and tumor at protruding from middle lobe bronchus; (C) running suture of cartilaginous portion between bronchus intermedius and lower lobe bronchus. The lower lobe artery is retracted with a vessel loop. ML, middle lobe; RLL, right lower lobe; RUL, right upper lobe; RMB, right main bronchus.
Right lower sleeve lobectomy
This kind of sleeve is performed to avoid bilobectomy when the tumor is located at the entrance of the right lower bronchus extending toward, but without involvement, of the MLB. The BI must be divided laterally and the MLB must be sectioned tangentially in order to increase the bronchial surface of the middle lobe and reimplant the MLB to the intermedius with a similar caliber. Due to the small size of the middle bronchus, care must be taken in order to avoid kinking or stenosis of the anastomosis (Figure 5).
Figure 5.
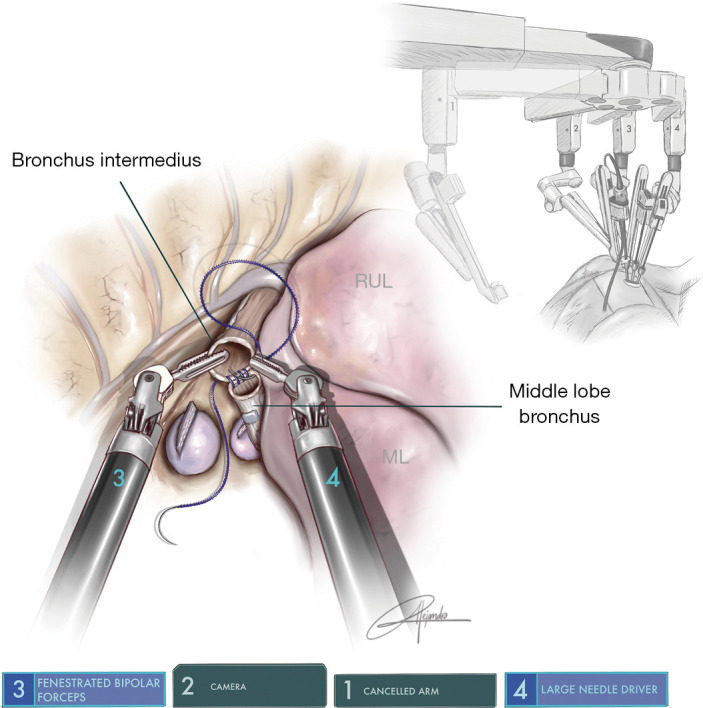
Illustration showing the running suture for a right lower sleeve lobectomy (reimplantation of middle lobe bronchus to intermedius) (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article). ML, middle lobe; RUL, right upper lobe.
Bilobectomy sleeve
On the right side we have two ways of performing a bilobectomy sleeve. 1-Upper and middle bilobectomy sleeve: this situation usually happens when the tumor from the upper lobe extends to the middle lobe on the fissure or more rarely, when there is endobronchial extension from the upper lobe bronchus to intermedius reaching the middle lobe. The anastomosis must be performed from the right main bronchus to intermedius, and the MLB can normally be stapled with the robotic staplers (Figure 6A). 2-Lower and middle bilobectomy sleeve: This surgery is usually performed when the tumor from the lower lobe involves the middle lobe on the fissure and extends endobronchially to the BI, reaching the origin in the bifurcation with the upper bronchus. In these cases, we must divide the right main bronchus and the upper lobe bronchus and remove the lower & middle lobe. Then, an anastomosis of the right main to the right upper must be performed (Figure 6B). The best way is to retract the upper lobe laterally to the left side and do the anastomosis in the same direction as a right upper lobe (RUL) sleeve. It is crucial to localize the membranous portion in the RUL bronchus to perform a correct alignment with the right main bronchus to avoid malrotation of the upper lobe. The anastomosis is performed by using a running 3/0 barbed suture.
Figure 6.
Surgical image of the running anastomosis after a right upper and middle sleeve bilobectomy (intermedius to right main bronchus) (A). Surgical image of the running anastomosis after a right lower and middle sleeve bilobectomy (right upper to right main bronchus) (B).
Left upper lobe sleeve resection
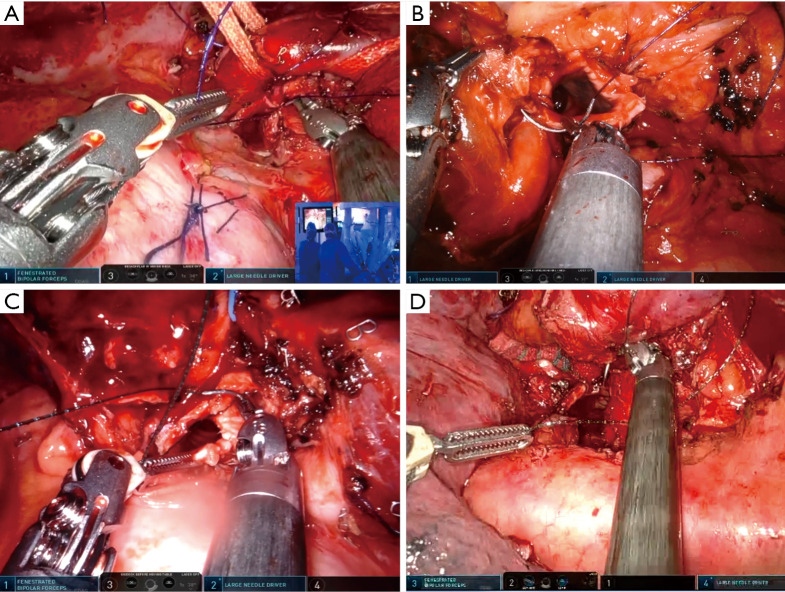
The left upper lobe (LUL) sleeve is one of the most complex bronchoplastic procedures. The interference with the aortic arch and main pulmonary artery (PA) as well as the short length of the upper lobe bronchus, makes the anastomosis more difficult than on the right side. We recommend once again to do the anastomosis from the hilum with anterior view (running double needle 3-0 barbed suture with two needles). Care must be taken during the cut of the left main to avoid too proximal a section. Otherwise, the stump of left main could be retracted too deep behind the esophagus, making reconstruction more difficult. The use of the robotic scissors helps with its angulation to avoid this problem. If the PA covers the bronchus, there are several tricks to improve the exposure of the bronchus such as dissecting the left main PA and retracting with two vessel loops posteriorly attached to the parietal pleura (Figure 7). A stitch to the pericardium could increase the exposure when the left main is too deeply located (Figure 7A).
Figure 7.
Running barbed suture on left-sided sleeve resections and tracheal anastomosis. (A) Surgical photo showing arm placement and the running barbed anastomosis of left main bronchus to left lower bronchus during a left upper sleeve lobectomy. (B) Surgical image showing the running barbed suture of left main bronchus to trisegmental B1-3 bronchus after a left lower + lingulectomy sleeve lobectomy. (C) Surgical image of second and third carinal sleeve anatomic segmentectomy showing the anastomosis of left main bronchus to left lower bronchus as the initial step. (D) Surgical image showing distal tracheal resection and reconstruction with barbed suture.
Left lower sleeve resection
As in other pure robotic lobectomies, the location of the incision for left lower resections must be lower than with uVATS, normally at the 6th ICS. This lower location helps with the anastomosis of lower sleeve lobectomies due to the orientation of the instruments and their position with respect to the left main bronchus. As in all left-sided procedures, arm 4 must be deactivated. The camera should be placed in arm 3, left bipolar fenestrated in arm 1 and needle holder in arm 2. The left lower sleeve lobectomy can be technically more complex because of the presence of the PA, the atrium and upper lobe vein. The orientation to perform the anastomosis is also difficult because the upper lobe bronchus needs to be re-implanted on the main bronchus from an anterior view position, and the stump of left main bronchus is usually deeply located (Figure 8). The robotic approach is very helpful in performing this kind of anastomosis. The placement of several sutures on the surface of the posterior mediastinal pleura attached to the posterior parietal pleural improves the visualization and exposure of left main bronchus stump. The LUL bronchus must be dissected completely before its division. In case of lower lobe tumors extending into the lingula, the procedure can also be performed with en-bloc left lower lobe (LLL)+ lingulectomy, with anastomosis of the left main bronchus to trisegmental B1-3 bronchus to avoid pneumonectomy (Figure 7B).
Figure 8.
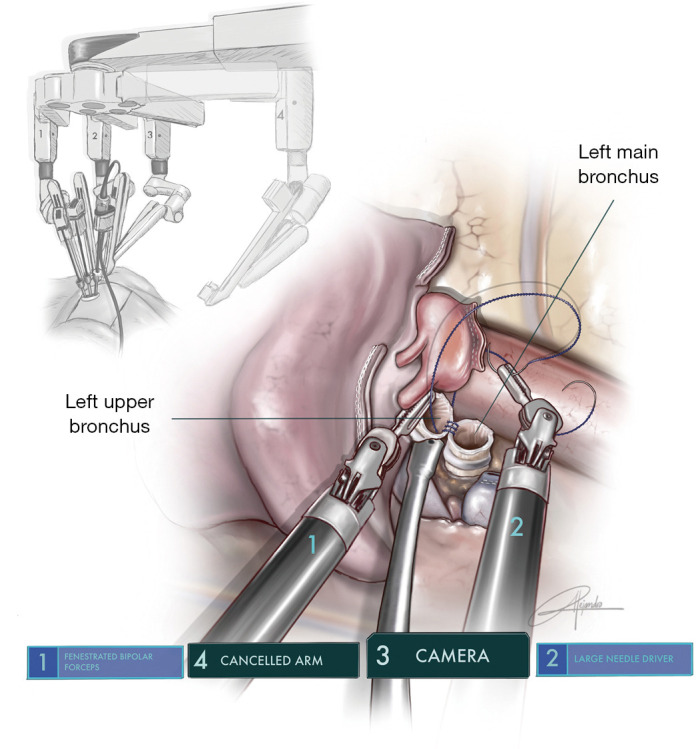
Illustration showing the running barbed suture of left main bronchus to left upper lobe bronchus after a left lower sleeve lobectomy (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article).
Lung-sparing sleeve resections and second carinal reconstruction
Bronchial sleeve resections with preservation of parenchyma should be attempted whenever possible when treating endoluminal tumors located on distal trachea, carina or endobronchial tumors rather than performing a lobectomy. Generally, the main indications for this surgical technique without lung resection include bronchial low malignant tumors (such as typical carcinoid, mucoepidermoid carcinoma and adenoid cystic carcinoma) and some benign diseases. We do not recommend pure bronchial resection in the treatment of patients with invasive non-small cell lung cancer. The anastomosis in these cases is more difficult as the overlying undivided lobar structures limit the exposure for mobilization and suturing during lung-sparing sleeve procedures. For endobronchial carcinoid tumors located on the right main bronchus with extension to the entrance of the right upper bronchus, the main and the RUL bronchus should be dissected and exposed after the subcarinal, hilar and peribronchial lymph nodes are removed. The BI must be cut proximally just below the origin of the RUL bronchus. The anastomosis is commenced by joining the lateral wall of the upper and intermedius bronchus to create a second neocarina. Then the neocarina is anastomosed to the origin of the right main bronchus or even at the level of the main carina, depending on the resection. The whole procedure is performed from the posterior aspect, keeping lung traction anteriorly. Compared to the right bronchial sleeve resection, the thoracoscopic reconstruction of the second carina on the left side is usually more complex because the anastomosis of the upper and lower lobe bronchus and the left main bronchus is often hidden in the rear of the left PA. These types of procedures should be approached from the anterior hilum and we recommend retraction of the upper lobe vein anteriorly (with a vessel loop attached to pericardium) and the main PA posteriorly (attached to the posterior pleura). In rare cases of carcinoid tumors extending into segmental bronchus such as B1-2-3 or lingular bronchus, an anatomic segmentectomy can be performed in order to avoid lobectomy (second and third lung segment sparing carinal sleeve segmentectomy) (Figure 7C).
Tracheal and carinal resection-reconstruction
Tumors invading the distal trachea or carina represent a challenge due to the complexity of airway reconstruction and management. The classical open surgery approach for distal trachea or carinal resections is to gain access through the right side or median sternotomy. However, these procedures can be performed by uVATS or uRATS in expert hands. Tumors in this location require total coordination with the anesthesiologist during airway resection and a significant experience in airway management and reconstruction, as well as a preoperative plan in case of emergency. For this reason, when this procedure is performed through uRATS, we recommend two options in order to maintain lung ventilation: the use of high frequency jet ventilation or with the help of extracorporeal membrane oxygenation (ECMO). The jet tube can be introduced through the endotracheal tube and thanks to the small diameter of the catheter for ventilation, it doesn’t interfere with the anastomosis of the membranous portion (21). This way we do not need intra-field intubation (Figure 7D).
For RUL tumors involving the carina, the most difficult part of the operation is to make an optimal reconstruction because of the caliber mismatch between the distal trachea, left main bronchus and BI. To avoid mismatch, an initial end-to-end anastomosis of the main stem bronchus to the distal trachea with re-implantation of the BI to the created neocarina at the distal trachea, can be performed. This is useful in maintaining oxygenation and facilitates the anastomosis of the left side wall of the main stem bronchus and distal trachea. Another option is the resection of the carina, followed by a total end-to-end anastomosis between the left main bronchus and the trachea with an anastomosis of the right main bronchus to the lateral, cartilaginous wall of the trachea (at least 2 cm above the first anastomosis).
The fully uniportal robotic approach should be performed at the 6th intercostal space. The division of the azygos vein enables a better exposure to perform the RUL and carinal resection. A high-frequency ventilation through a 2-mm endotracheal catheter is a good choice to ventilate the left lung. A running suture (V-loc 3/0) facilitates the reconstruction: first formed by the left side wall between the trachea and left main bronchus, then suturing the membranous trachea and the left main bronchus. Thereafter, the neo-carina of the left main bronchus and the intermediate bronchus. Finally, the front wall of the trachea, left main bronchus and intermediate bronchus must be anastomosed. To cover the anastomosis, we use a thymus flap, and we always recommend the use of hemostatic agents at the end of the procedure, especially for oozing areas after lymph node dissection (4SEAL® Hemostatic Powder, GRENA LTD.).
Double sleeve and vascular reconstructions
For tumors involving the PA we have to options to control the main PA: tourniquets or RelianceTM Bullodog clamps (Scanlan international). Clamping the main PA and the distal end of the vessel with a Reliance TM Bulldog clamp is considered to be the most appropriate choice when the procedure is performed fully robotically (Figure 9). The other option is to use tourniquets to clamp both, main artery and distal artery (22). This step should be done by removing the robot and using the uVATS approach (Figure 10).
Figure 9.
Illustration of vascular reconstruction using the bulldog clamps (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article). ML, middle lobe; RUL, right upper lobe; RLL, right lower lobe.
Figure 10.
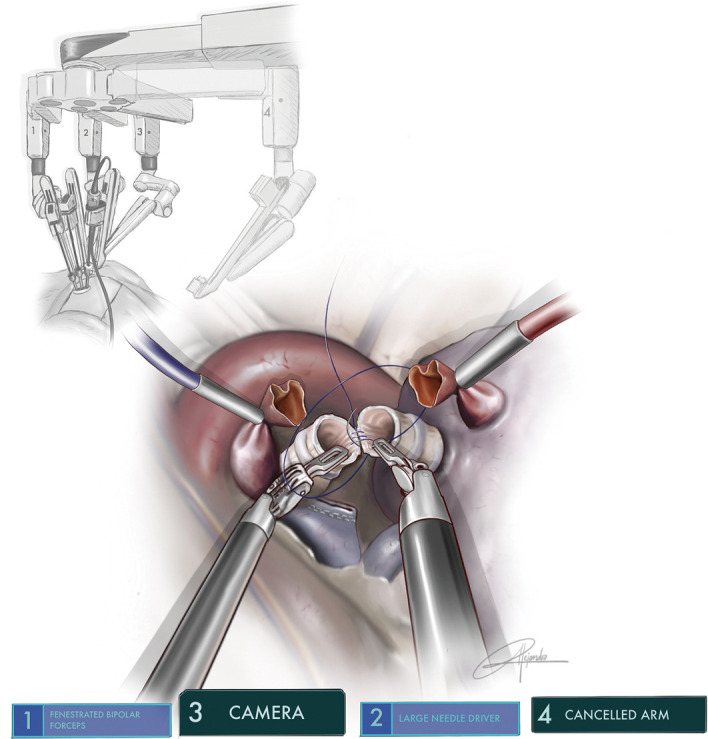
Illustration showing the running barbed suture of left main bronchus to lower lobe bronchus after a double sleeve lobectomy (permission provided from medical illustrator Alejandro Garcia, who is also a co-author of this article).
For a double sleeve procedure on the left side, or in a case needing bronchovascular reconstruction, initially the main PA should be dissected with removal of lymph node stations 5–6. When involvement of the tumor is too proximal, the division of the ductus arteriosus could enlarge approximately 1 cm for the PA on the left side. After dividing the superior pulmonary vein, the left main bronchus, upper lobe bronchus and lower lobe bronchus can be dissected easily. Then the fissure should be divided for the evaluation of tumor invasion in the lower lobe artery. At this point is when it can be confirmed that the double sleeve procedure is feasible or not. Before clamping the left PA, 5,000 units of heparin must be injected intravenously. The bronchial anastomosis is performed using an absorbable 25- or 30-cm barbed double needle 3/0. A continuous suture technique is used, facilitating a secure wound closure without knot-tying. The membranous portion sutures first, then the cartilaginous portion in a 365 degrees manner. The bronchial anastomosis is normally easier as we don’t have the interference with the artery (Figure 10).
The vascular anastomosis is more challenging by the uRATS approach due to the difficulties in keeping the tension on the thread. We cannot use barbed sutures and we cannot take out the thread through the incision during the suturing as we normally do during uVATS sleeve, in order to maintain the tension. Sometimes the anastomosis can be performed in two steps, starting with the posterior wall initially with a running suture and doing a second running suture at the superior part of the artery, tying together both threads. Recently, we have developed a customized 25-cm double needle 5/0 prolene suture to make the procedure faster. At the end of the anastomosis, we recommend injecting heparin to fill the artery and prevent thrombus. The distal clamp should be released first to check distal flow and the possibility of bleeding.
Results
Since we started the uRATS program in September 2021 until December 2022, we have performed 30 uRATS sleeve resections (73% male, 27% female). The mean age was 59.9±1.3 years and 73% were smokers. The most frequent histology was squamous cell (27%), adenocarcinoma (23%) and carcinoid (23%), with a mean tumoral size of 3.6±0.2 cm. The left upper sleeve lobectomy was the most frequent procedure (20%) and there were 4 cases of sparing lung surgery (1 tracheal, 1 main carinal and 2 second carinal reconstruction). The mean surgical time was 178±5.5 minutes and the mean hospital stay was 6.6±0.5 days. One patient died during the first 30 postoperative days due to an acute respiratory distress syndrome (ARDS). All results are showed in the Tables 1,2.
Table 1. Clinical characteristics.
| Characteristics | Values |
|---|---|
| Demographic | |
| Gender | |
| Male | 22 [73] |
| Female | 8 [27] |
| Age (years) | 59.9±1.3 |
| Smoking | 22 [73] |
| FEV1 (%) | 78.9±1.4 |
| DLCO (%) | 74.0±1.4 |
| CVRF | 23 [77] |
| Surgical | |
| Adhesions | 15 [50] |
| Resection | |
| RUL | 3 [10] |
| ML | 2 [7] |
| RLL | 5 [17] |
| Bilobectomy | 4 [13] |
| LUL | 6 [20] |
| LLL | 4 [13] |
| LLL+ lingula | 2 [7] |
| Tracheal/carinal | 2 [7] |
| Second carina | 2 [7] |
| Sleeve type | |
| Bronchial | 26 [87] |
| Arterial | 1 [3] |
| Double | 3 [10] |
| Anatomopathological | |
| Tumor (cm) | 3.6±0.2 |
| Histology | |
| Adeno | 7 [23] |
| Squamous | 8 [27] |
| Adenosquamous | 2 [7] |
| Giant cell | 1 [3] |
| Small cell | 1 [3] |
| Carcinoid | 7 [23] |
| Sarcomatoid | 2 [7] |
| Benign tumor | 2 [7] |
| TNM | |
| IA | 7 [27] |
| IB | 6 [23] |
| IIA | 4 [15] |
| IIB | 3 [12] |
| IIIA | 6 [23] |
Discrete data are expressed as number with percentages: n [%]; continuous data are expressed as mean ± standard deviation. FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity for carbon monoxide; CVRF, cardiovascular risk factors; RUL, right upper lobe; ML, middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; TNM, tumor node metastasis.
Table 2. Perioperative outcomes.
| Outcomes | Values |
|---|---|
| Operative | |
| Time | 178±5.5 |
| Complication | 0 [0] |
| Conversion | 0 [0] |
| Lymphadenectomy | |
| LN (number) | 16.7±0.9 |
| Stations (number) | 4.3±0.1 |
| Mortality | 0 [0] |
| Postoperative (30 days) | |
| Complication | 5 [17] |
| Opioid usage | 6 [20] |
| Air leak (>5 days) | 4 [13] |
| Chest drain (days) | 4.8±0.5 |
| ICU (days) | 1.5±0.3 |
| Hospitalisation (days) | 6.6±0.5 |
| Reintervention | 2 [7] |
| Mortality | 1 [3] |
Discrete data are expressed as number with percentages: n [%]; continuous data are expressed as mean ± standard deviation. LN, lymph nodes; ICU, intensive care unit.
Discussion
Multiport RATS sleeve lobectomy has been successfully performed for many years across thoracic surgical centers throughout the world. Aside from the surgical and survival outcomes, thoracic surgeons are sometimes concerned about the learning curve associated with robotic sleeve lobectomy (23). Even with conventional thoracotomy, this technique is still challenging due to the lack of volume in the different thoracic departments. Following the research of the early pioneers, the indications for RATS are becoming increasingly similar to VATS. For many years, researchers have been investigating the feasibility and safety of bronchoplasty in RATS (24,25). For locally advanced lung tumors, RATS sleeve lobectomy or bronchoplasty may be preferable over VATS due to the ergonomic characteristics of the robot (26). In 2019, O’Sullivan and colleagues conducted a systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. They discovered that transfusions, complications, length of stay, and 30-day mortality were all lower with RATS than with VATS or open surgery (27). Previously, RATS lobectomies with two, three, and four ports were performed for the removal of lung tumors (28,29).
Liu et al. performed a single utility port approach in robotic-assisted sleeve segmentectomy for bronchial carcinoid tumor, but they added another 4-cm incision for an endoscopic arm (28). Qu et al., performed two-port robotic bronchial sleeve lobectomies on 15 consecutive patients showing good outcomes (29). Due to the important experience gained by our team in uVATS complex sleeve resections during the the last few years (4,30,31), the learning curve for uniportal robotic sleeve was easier (13,14). To our knowledge, this is the first report of fully robotic sleeve cases performed by a single incision. To date, we have performed 30 uRATS sleeve procedures with excellent outcomes having only one postoperative death due to an ARDS in a high-risk patient. The limitation of our analysis is that our follow-up time was short, and we evaluated only postoperative outcomes. Considering that in uRATS both working arms are parallel through a single incision the technique of suturing via uRATS is different compared with multiport RATS and several tricks must be learned to master the technique.
Conclusions
The uRATS technique for sleeve resections is a feasible and reliable technique when performed by experienced surgeons. The 3D view and wider range of movements for suturing provided by the robot are some of the advantages of the uRATS technique compared with thoracoscopic approaches. The use of a double needle barbed sutures allows for a faster, more precise and safer anastomosis. The learning curve of the robotic procedure includes three main aspects, such as the learning of port placement, the optimal coordination with the assistant during surgery and the correct anastomotic technique for alignment of both bronchial and vascular edges. Further studies with a large number of patients are needed to evaluate oncological outcomes of this innovative technique.
Video.

Uniportal RATS fully robotic sleeve resections - initial experience of 30 cases.
Acknowledgments
Funding: None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. 10.1510/icvts.2010.256222 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. 10.1016/j.athoracsur.2012.10.070 [DOI] [PubMed] [Google Scholar]
- 3.Sihoe AD. Uniportal video-assisted thoracic (VATS) lobectomy. Ann Cardiothorac Surg 2016;5:133-44. 10.21037/acs.2016.03.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i6-i16. 10.1093/ejcts/ezv410 [DOI] [PubMed] [Google Scholar]
- 5.Oh DS, Cho I, Karamian B, et al. Early adoption of robotic pulmonary lobectomy: feasibility and initial outcomes. Am Surg 2013;79:1075-80. [PubMed] [Google Scholar]
- 6.Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. 10.1016/s1010-7940(02)00102-1 [DOI] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. 10.1016/j.jtcvs.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BE, Shapiro M, Rutledge JR, et al. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann Thorac Surg 2015;100:229-33; discussion 233-4. 10.1016/j.athoracsur.2015.03.109 [DOI] [PubMed] [Google Scholar]
- 9.Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. 10.1097/SLA.0000000000002346 [DOI] [PubMed] [Google Scholar]
- 10.Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. 10.1016/j.lungcan.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Rivas DG, Bosinceanu M, Dunning J. Uniportal Robotic Surgery: A Step-by-Step Guide to Setup by Diego Gonzalez Rivas. 2022. doi: 10.25373/ctsnet.21092140.v1. [DOI]
- 12.Gonzalez-Rivas D, Bosinceanu M, Motas N, et al. Uniportal robotic-assisted thoracic surgery for lung resections. Eur J Cardiothorac Surg 2022;62:ezac410. 10.1093/ejcts/ezac410 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rivas D, Manolache V, Bosinceanu ML, et al. Uniportal pure robotic-assisted thoracic surgery—technical aspects, tips and tricks. Ann Transl Med 2022. doi: . 10.21037/atm-22-1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Rivas D, Prado RF, GarciaPerez A, et al. Bilateral uniportal robotic-assisted thoracic surgery sleeve lobectomy for a bilateral endobronchial lung cancer. Ann Cardiothorac Surg 2023;12:64-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Rivas D, Essa RA, Motas N, et al. Uniportal roboticassisted thoracic surgery lung-sparing carinal sleeve resection and reconstruction. Ann Cardiothorac Surg 2022. doi: . 10.21037/acs-2022-urats-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunning J, Waterhouse B, Rivas DG. The UK's First Uniportal Robotic Surgery with Diego Gonzales Rivas. 2022. doi: 10.25373/ctsnet.21632624.v1. [DOI]
- 17.E H , Yang C, Wu J, et al. Hybrid uniportal robotic-assisted thoracoscopic surgery (RATS) using videoassisted thoracoscopic surgery (VATS) staplers: technical aspects and results. Ann Cardiothorac Surg 2023;12:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [DOI] [PubMed] [Google Scholar]
- 19.Soultanis KM, Gonzalez-Rivas D. Uniportal video-assisted sleeve resections: how to deal with specific challenges. J Thorac Dis 2019;11:S1670-7. 10.21037/jtd.2019.06.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic sleeve resections: Where are the limits? JTCVS Tech 2020;2:160-4. 10.1016/j.xjtc.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu Y, Yu F, Yao F, et al. The efficacy of high-frequency jet ventilation on intraoperative oxygen saturation compared to cross-field ventilation in patients undergoing carinal resection and reconstruction. J Thorac Dis 2022;14:3197-204. 10.21037/jtd-22-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Rivas D, Garcia A, Chen C, et al. Technical aspects of uniportal video-assisted thoracoscopic double sleeve bronchovascular resections. Eur J Cardiothorac Surg 2020;58:i14-22. 10.1093/ejcts/ezaa037 [DOI] [PubMed] [Google Scholar]
- 23.Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Luo Q, Tan Q, et al. Initial experience of robot-assisted thoracoscopic surgery in China. Int J Med Robot 2014;10:404-9. 10.1002/rcs.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. 10.3978/j.issn.2072-1439.2016.01.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan X, Chen Y, Shi J, et al. Robotic Assisted Extended Sleeve Lobectomy After Neoadjuvant Chemotherapy. Ann Thorac Surg 2015;100:e129-31. 10.1016/j.athoracsur.2015.08.084 [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. 10.1093/icvts/ivy315 [DOI] [PubMed] [Google Scholar]
- 28.Liu A, Zhao Y, Qiu T, et al. Single utility port approach in robot-assisted sleeve segmentectomy for bronchial carcinoid tumor. Thorac Cancer 2022;13:1537-40. 10.1111/1759-7714.14409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu JC, Zhang WT, Jiang L. Two-port robotic sleeve lobectomy using Stratafix sutures for central lung tumors. Thorac Cancer 2022;13:1457-62. 10.1111/1759-7714.14412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Rivas D, Soultanis KM, Garcia A, et al. Uniportal video-assisted thoracoscopic lung sparing tracheo-bronchial and carinal sleeve resections. J Thorac Dis 2020;12:6198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]