Abstract
This study investigated the spatial distribution, abundance, and infection rates of human schistosome-transmitting snails and related physicochemical parameters and environmental factors in 11 districts in KwaZulu-Natal (KZN) province, South Africa, from December 2020–February 2021. Snail sampling was carried out in 128 sites by two people for 15 min using scooping and handpicking methods. Geographical information system (GIS) was used to map surveyed sites. In situ measurements of physicochemical parameters were recorded, while remote sensing was used to obtain measurements for climatic factors required to achieve the study's objective. Cercarial shedding and snail-crushing methods were used to detect snail infections. Kruskal-Wallis test was used to test the differences in snail abundance among snail species, districts, and habitat types. A negative binomial generalized linear mixed model was used to identify the physicochemical parameters and environmental factors influencing the abundance of snail species. A total of 734 human schistosome-transmitting snails were collected. Bu. globosus were significantly more abundant (n = 488) and widely distributed (found in 27 sites) compared to B. pfeifferi (n = 246) found in 8 sites. Bu. globosus and B. pfeifferi had infection rates of 3.89% and 2.44%, respectively. Dissolved oxygen and normalized difference vegetation index showed a statistically positive relationship, while normalized difference wetness index showed a statistically negative relationship with the abundance of Bu. globosus. However, there was no statistically significant relationship between B. pfeifferi abundance, physicochemical parameters, and climatic factors. Our study described the current distribution, abundance, and infection status of human schistosome-transmitting snails in KZN province, which will contribute to informing control measure policies for schistosomiasis.
Keywords: Schistosomiasis, Bulinus globosus, Biomphalaria pfeifferi, Cercarial shedding, Physicochemical parameters, KwaZulu-Natal
1. Introduction
Schistosomiasis, also known as bilharzia, is a neglected tropical disease (NTD) mainly prevalent in poor and developing countries with limited health care, poor water, sanitation, and hygiene practices. There is an association between schistosomiasis infection and low socio-economic conditions [1,2]. Domestic and recreational activities such as laundry, fetching water, and swimming in water infested with snails infected with schistosome parasites predisposes people to schistosomiasis. In 2019, more than 105.4 million people were treated for schistosomiasis out of 236.6 million people who required preventive treatment [3]. The transmission of schistosomiasis has been reported in 78 countries and preventive treatment required in 52 endemic countries with moderate-to-high transmission [3,4]. It is estimated that 85–95% of global schistosomiasis infections are from sub-Saharan Africa (SSA), with the highest prevalence and intensity of infection found among school-aged children [5,6]. In South Africa, about 25.7 million people are at risk of contracting schistosomiasis, with 4.5 million infected with S. haematobium [7,8]. The national department of health of South Africa in KwaZulu-Natal (KZN) province reported the prevalence of S. haematobium among school-aged children in KZN province ranging from 1.87 to 25.42%, while the prevalence of S. mansoni was less than 2% [9].
Approximately 5000 species of snails inhabit different habitats worldwide, with about 350 snail species being of medical or veterinary importance [10]. Freshwater snails belonging to the family Planorbidae are intermediate hosts of parasitic blood flukes of the genus Schistosoma which cause schistosomiasis in Africa, Asia, and America. Most intermediate hosts of human Schistosoma parasites belong to the three genera: Biomphalaria spp. serves as the intermediate host snails for S. mansoni in Africa and America, Bulinus spp. serves as the intermediate hosts of S. haematobium in Africa and the Eastern Mediterranean, as well as of S. intercalatum in Africa and Oncomelania spp. serves as the intermediate host of S. japonicum in southeast Asia [11,12]. Schistosomiasis transmission occurs when eggs from infected humans are released into freshwater via faeces or urine. The eggs hatch into miracidia and penetrate specific intermediate host snails (IHS). Cercariae released into the water from infected snails penetrate the skin of humans that have direct contact with the water [11,12]. The schistosomulae (cercariae that lose their fork tail after penetrating the human host skin) get the liver through venous circulation for development and exit after maturation. Sexual reproduction takes place between the adult male and female worms. The female worm deposits eggs, which move to the large intestine and bladder for S. mansoni and S. haematobium, respectively [13].
Praziquantel (PZQ) is the medication used in treating all forms of schistosomiasis. Substantial progress has been made in scaling up the distribution of PZQ from 7 million in 2006 to over 57 million people and more than 90.7 million people in 2015 and 2020, respectively [4,5]. However, studies have shown that preventive chemotherapy with PZQ alone is insufficient to control schistosomiasis. Hence, there is a need for an integrated approach involving water and sanitation hygiene (WASH) provision and snail control [14].
Suitable water bodies and intermediate host snails are factors necessary for the distribution of snail-borne diseases. However, disease transmission cannot be established based solely on these two factors as other underlying environmental, climatic, and physicochemical conditions could interfere with parasite development, reproduction of parasite in the snail, snail growth, and survival rate [15]. Physicochemical water properties like velocity, temperature, turbidity, salinity, electrical conductivity, dissolved oxygen, and pH; biological factors such as competition; and climatic factors such as rainfall, land surface temperature (LST), normalized difference vegetation index (NDVI) and altitude are known to influence the distribution and abundance of intermediate host snails for schistosomiasis [16,17]. Information on the geographical distribution and habitats for Bulinus africanus, Biomphalaria. pfeifferi, Bulinus globosus, and the climatic factors that influence its distribution have been reported [[18], [19], [20], [21], [22], [23]]. Kalinda et al. [23] studied the effect of temperature on the Bu. globosus and concluded that a rise in temperature influences fecundity, growth, survival, and parasite development in snails, thus dictating the time it takes the parasite to complete the life cycle, which has implications for the transmission of schistosomiasis. Manyangadze et al. [22] described the distribution of schistosomiasis intermediate host snails in the Ndumo area of uMkhanyakude district, KZN province, South Africa, on a micro-geographical scale. Bu. globosus snails were widely distributed with higher infection rates compared to B. pfeifferi. In addition, climatic and physicochemical parameters such as rainfall, minimum LST, and dissolved oxygen were found to affect schistosomiasis intermediate host snails.
Research on schistosomiasis has generally focused on disease prevalence and intensity of infection among human populations, with few studies trying to identify the intermediate snail hosts within the vicinity of the areas [24]. However, to break the transmission cycle to achieve success in controlling schistosomiasis, both the parasite and IHS must be targeted [24]. To better understand schistosomiasis transmission and inform effective interventions that will lead to control and eventual elimination in endemic areas, it is essential to have sound knowledge of the abundance and distribution of intermediate host snails [25]. However, the distribution and abundance of schistosomiasis IHS have only been studied on a micro-geographical scale in KZN province [21,22]. Thus, the objective of the present study was to determine the distribution, abundance, and infection status of human schistosomiasis-transmitting snails in all the districts in KZN province between December 2020–February 2021. The possible influence of physicochemical parameters: pH, dissolved oxygen, water pressure, and climatic factors: rainfall, oxidation-reduction potential (ORP), normalized difference vegetation index (NDVI), normalized difference wetness index (NDWI), minimum and maximum land surface temperature (LST) on the distribution, abundance and infection status of HSTS will be investigated. Our findings could be used in designing effective control and elimination measures for schistosomiasis.
2. Methods
2.1. Study area
KZN is one of the nine provinces in South Africa (SA). It is coastal and located in the country's southeastern part, sharing borders with Eswatini in the north and Mozambique on the east. It is a gateway to the Indian Ocean and shares borders with the Eastern cape province in the south, Lesotho and Free state provinces on the west, and Mpumalanga province on the northwest. KZN has a tropical to subtropical climate with four seasons: rainy (December–February), post-rainy (March–May), cold/dry (June–August), and hot/dry (September–November). The hot/dry season is suitable for schistosomiasis transmission. There are numerous freshwater sources in the province ranging from big rivers to small streams, used for economic, recreational, and domestic purposes such as laundry and for fetching water for households. KZN province has the second largest population (11.5 million people) in a total area of 94.361 . It has 10 municipality districts and one metropolitan municipality.
2.2. Study design and methods
A cross-sectional snail sampling study was conducted in December 2020 and February 2021 at 128 sites spread across 11 districts. The sites were selected using the stratified random sampling procedure. First, the areas were stratified by districts, followed by simple random sampling. In addition, site selection was based on local knowledge of water contact sites where people go to collect water, do laundry, bath, swim, fish, do agricultural practices, car wash, sand harvesting, and block molding. The study was limited to only one season of the year, the rainy season, precisely because research has shown that rainfall affects snail distribution, abundance, and infection rates. In addition, the study by Manyangadze et al. [22] carried out in uMkhanyakude district, one of the districts in KZN province, showed that more Bu. globosus were found to shed cercariae in the rainy season. Hence, we were interested in having a snapshot of the abundance, distribution, and infection rates of Bu. globosus and B. pfeifferi across the entire province.
Trained field staff collected snails at sampling sites using a combination of long-handled snail scoops described by Appleton [26] and handpicking between 8:00 a.m. and 4:00 p.m. for 15 min per site. At each sampling site, geographical positions were recorded using a global positioning system (GPS). Snails collected from each site were counted, labeled appropriately, and identified to species level using Brown and Kristensen's shell morphological identification key [27]. Bu. globosus and B. pfeifferi snails were shed to detect infections on the same collection day. This was done by placing individual snails in 10-ml plastic vials filled with filtered river water. The vials containing snails were exposed to direct sunlight or electric light to induce cercariae shedding for about 3–4 h. Water from each vial was put in a Petri dish and examined for the presence of cercariae under a dissecting or stereo microscope. Snails that did not shed cercariae on the first exposure were re-exposed on the second day. If they did not shed cercariae after the second day, the crushing method was used to detect developing cercariae or sporocysts under a compound microscope [17,28]. Cercariae were identified morphologically using the keys by Frandsen and Christensen [29], and bifurcate cercariae indicated that the cercariae had a mammalian origin.
2.3. Physico-chemical parameters, environmental and climatic variables
Physico-chemical parameters such as pH, water pressure, total dissolved oxygen, electrical conductivity, total dissolved solids, and salinity influence freshwater snail distribution were measured using a multi-probe meter (Hanna HI 9829 multiparameter). Water temperature was not measured due to technical challenges with the water multiparameter. Hence maximum and minimum LST were used as proxies for water temperature. Three-month average (December 2020–February 2021) climatic data for maximum and minimum LST, Normalized Difference Vegetation Index (NDVI), Normalized Difference Water Index (NDWI), Enhanced Vegetation Index (EVI), and precipitation was obtained through remote sensing. Minimum and maximum LST data was accessed from Climate Engine: Cloud Computing of Climate and Remote Sensing Data (https://clim-engine.appspot.com/climateEngine) while the other data for other climatic variables were accessed through the International Research Institute for Climate and Society (IRI) data library portal (http://iridl.ldeo.columbia.edu/SOURCES/).
2.4. Data analysis
Raw data on snails collected per site were entered in Microsoft Excel, and all statistical analyses were carried out using R Version 4.1.2. The data on the abundance of each snail species were tested for normality using the Shapiro–Wilk normality test, which showed that the data on the abundance of each snail species was not normally distributed. Therefore, Kruskal-Wallis test was used to determine the differences in snail abundance among snail species, districts, and habitat types [30]. The results were presented using box and whisker plots. A negative binomial generalized linear mixed model in the generalized linear mixed models in the ‘glmmTMB’ package was used to identify physicochemical parameters and environmental factors influencing the abundance of the snail species [31]. Variance inflation factor (VIF) was used to determine the relationships and collinearity between variables. A VIF value of more than 5 indicated collinearity [22]. We used Akaike's Information Criterion (AIC) and negative log-likelihood values to compare models and chose the final models with the least AIC [32].
3. Results
3.1. Presence and distribution of human schistosome snail hosts
Bu. globosus and B. pfeifferi snails were the two human schistosome-transmitting snails (HSTS) found in KZN province (Fig. 2). HSTS were found at 31 (24%) of the 128 sampled sites in the 11 districts of KZN province. HSTS was not found in 2 districts (Sisonke and uMgungundlovu) out of the 11 districts in KZN province. Bu. globosus was more distributed in uThukela (found in 6 out of 13 surveyed sites), while Amajuba and eThekwini districts had the least distribution (found in 1 site each). Furthermore, B. pfeifferi was widely and least distributed in uThukela (at 28 sites) and eThekwini (at 8 sites) districts, respectively. Bu. globosus and B. pfeifferi cohabited at 5 of the sampled sites. The number of B. pfeifferi recovered per site ranged from 0 to 145, and those for Bu. globosus ranged between 0 and 86 (Table 1).
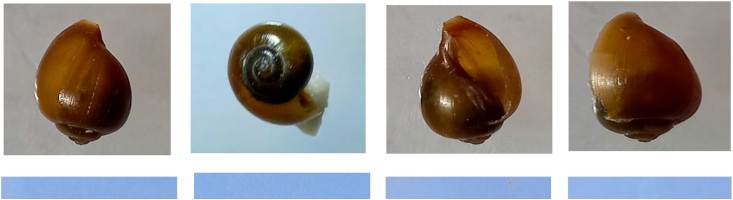
Fig. 2.
Images of the different views of human schistosome transmitting snails: a) Bu. globosus and B. pfeifferi found in KZN province.
Table 1.
Summary of the distribution and abundance of HSTS found in the 11 districts in KZN between December 2020 to February 2021.
| Districts | No. of surveyed sites. | No. of sites with HSTS | No. of sites with B. globosus only (%) | No. of B. globosus found | No. of sites with B. pfeifferi only (%) | No. of B. pfeifferi found | No. of sites with both species | |
|---|---|---|---|---|---|---|---|---|
| 1 | Amajuba | 9 | 1 | 1 (11.1) | 5 | 0 | 0 | 0 |
| 2 | eThekwini | 20 | 5 | 5 (25) | 26 | 1 (5) | 1 | 1 |
| 3 | iLembe | 9 | 1 | 1 (11.1) | 6 | 0 | 0 | 0 |
| 4 | King Cetshwayo | 11 | 5 | 4 (36.4) | 117 | 1 (9.1) | 1 | 0 |
| 5 | Sisonke | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Ugu | 10 | 3 | 3 (30) | 20 | 1 (10) | 145 | 1 |
| 7 | uMkhanyakude | 13 | 4 | 3 (23.1) | 31 | 2 (15.4) | 12 | 1 |
| 8 | uMgungundlovu | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | uMzinyathi | 7 | 2 | 2 (28.6) | 33 | 0 | 0 | 0 |
| 10 | uThukela | 13 | 7 | 6 (46.2) | 136 | 3 (23.1) | 87 | 2 |
| 11 |
Zululand |
16 |
3 |
3 (18.6) |
114 |
0 |
0 |
0 |
| Total | 128 | 31 | 28 | 488 | 8 | 246 | 5 |
3.2. The abundance of human schistosome transmitting snails
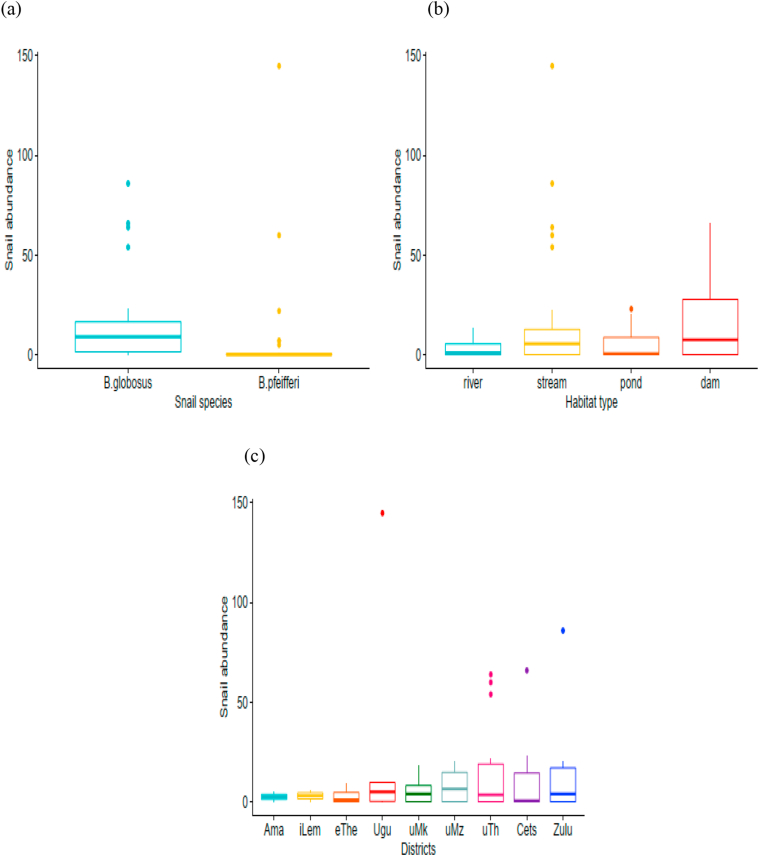
A total of 734 HSTS were collected in KZN province during the study period (December 2020 to February 2021). Bu. globosus, the intermediate host snail for S. haematobium, and B. pfeifferi, the intermediate host snail for S. mansoni, are the HSTS found in the province. The abundance of Bu. globosus (n = 488, 66.5%) was statistically significant compared to B. pfeifferi (n = 246, 33.5%) (Kruskal-Wallis χ2 = 20.269, df = 1, p < 0.0001) (Fig. 3a). Bu. globosus and B. pfeifferi were mainly found in streams, but Bu. globosus was more abundant than B. pfeifferi. However, the abundance of HSTS did not differ significantly amongst districts (Kruskal-Wallis χ2 = 2.404, df = 7, p = 0.93) and habitat type (Kruskal-Wallis χ2 = 0.865, df = 3, p = 0.834) (Fig. 3c and b).).
Fig. 3.
Box and whiskers plots showing (a) the overall abundance of HSTS by species, (b) the overall abundance of HSTS by habitat type, and (d) the overall abundance of HSTS by districts. The box represents the lower quartile (0.25), the median (0.5), and the upper quartile (0.75); the whiskers depict variability outside the lower and upper quartiles. Outliers are shown as individual points. Ama: Amajuba, iLem: iLembe, eThe: eThekwini, uMk: uMkhanyakude, uMz: uMzinyathi, uThu: uThukela, Cets: King Cetshwayo, Zulu: Zululand.
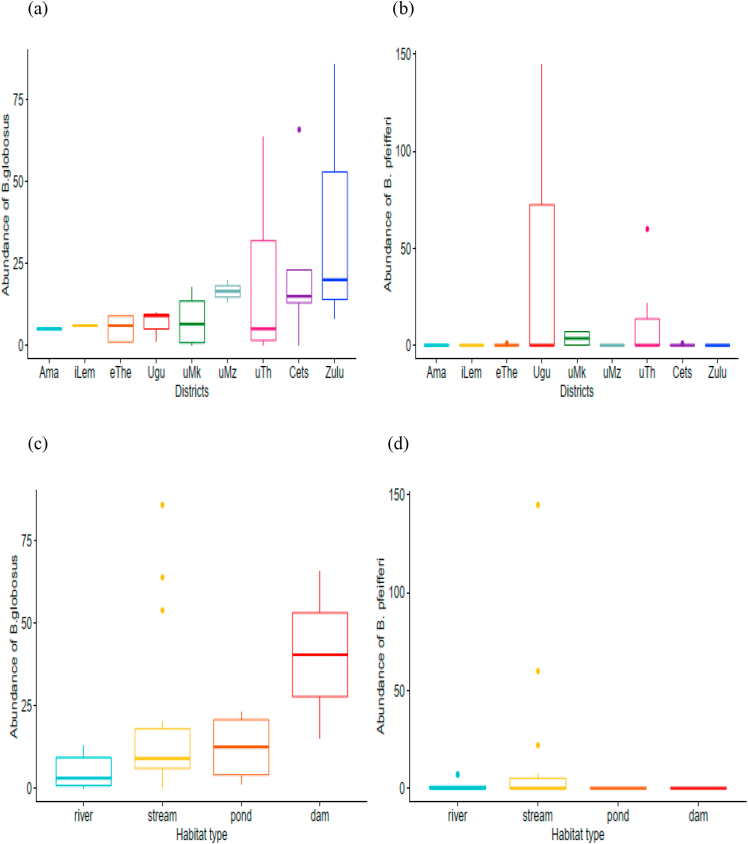
Bu. globosus was the most abundant and widely distributed HSTS species in 28 out of 31 sites inhabited by HSTS. The highest and least abundance of Bu. globosus was found in uThukela (n = 136) and Amajuba (n = 5) districts, respectively (Table 1). However, the difference in abundance of Bu. globosus among different districts was not significant (Kruskal-Wallis χ2 = 7.44, df = 8, p = 0.49). Bu. globosus was found mainly in streams and a few rivers. Furthermore, the difference in the abundance of Bu. globosus amongst different habitat types was not statistically significant (Kruskal-Wallis χ2 = 6.19, df = 3, p = 0.10) (Fig. 4a and (c)).
Fig. 4.
Box and whiskers plots showing (a) the abundance of Bu. globosus collected by districts, (b) the abundance of Bu. pfeifferi collected by districts, (c) the abundance of Bu. globosus collected by habitat type, and (d) the abundance of B. pfeifferi collected by habitat type. The box represents the lower quartile (0.25), the median (0.5), and the upper quartile (0.75); the whiskers depict variability outside the lower and upper quartiles. Outliers are shown as individual points. Ama: Amajuba, iLem: iLembe, eThe: eThekwini, uMk: uMkhanyakude, uMz: uMzinyathi, uThu: uThukela, Cets: King Cetshwayo, Zulu: Zululand.
Unlike Bu. globosus, the presence of B. pfeifferi was limited. Ugu district (n = 145) had the highest abundance of B. pfeifferi, while the least abundance of B. pfeifferi was recorded in King Cetshwayo (n = 1) and eThekwini (n = 1) (Table 1). The difference in abundance of B. pfeifferi among different districts (Kruskal-Wallis χ2 = 7.44, df = 8, p = 0.70) and habitat types were not statistically significant (Kruskal-Wallis χ2 = 2.44, df = 3, p = 0.49) (Fig. 4b and (d)). They were found in habitats that had the presence of aquatic plants. The actual count of B. pfeifferi and Bu. globosus snails are presented in S1 Table 1.
3.3. Physicochemical parameters, climatic and environmental factors
The mean, standard deviation, range of physicochemical parameters, and environmental and climatic factors of each surveyed site are presented in S2 Table 2. Physicochemical parameter measurements for sites in uMkhanyakude were not taken due to technical challenges with the multi-parameter. Large variability was observed in the measured physicochemical parameters and could be due to the distinct climatic conditions in each district. The least mean rainfall was recorded in Ugu district, 72.49 ± 4.72 mm (range 67.38–82.20 mm), while the mean maximum rainfall was recorded in uMkhanyakude district, 180.28 ± 65.64 mm (range 101.68–312.88 mm). uThukela had the least ORP value, 86.47 mV, while uMzinyathi had the highest ORP value, 149.95 mV, presented in S2 Table 2.
Table 2.
Output of the negative binomial regression model in the generalized linear model to model the effect of physicochemical parameters and environmental exploratory variables on snail species abundance in the “glmmTMB package in R4.1.2.
| Species | Fixed variables | Estimates | Confidence interval | p-value | Random effects |
|
|---|---|---|---|---|---|---|
| Variance | Standard deviation | |||||
| Bulinus globosus | Intercept | −4.39 | −14.41 to 5.62 | 0.39 | 8.396e-10 4.312e-11 | 2.898e-05 6.567e-06 |
| Rainfall | 0.01 | −0.005 to 0.02 | 0.25 | |||
| Minimum LST | −0.12 | −0.40 to 0.17 | 0.43 | |||
| Maximum LST | 0.06 | −0.29 to 0.42 | 0.74 | |||
| NDVI | 5.36 | 0.35 to 10.37 | 0.04∗ | |||
| Dissolved oxygen | 0.24 | 0.05 to 0.43 | 0.01∗ | |||
| pH | 0.48 | −0.39 to 1.36 | 0.29 | |||
| ORP | −0.01 | −0.02 to −0.001 | 0.01∗ | |||
| NDWI | −5.84 | −10.09 to −1.60 | 0.007∗ | |||
| Pressure | −0.01 | −0.12 to 0.10 | 0.86 | |||
| Biomphalaria pfeifferi | Intercept | 4.81 | −92.74 to 102.37 | 0.92 | 7.71e-09 | 8.781e-05 |
| Rainfall | −0.03 | −0.14 to 0.09 | 0.65 | |||
| Minimum LST | −1.19 | −4.25 to 1.87 | 0.45 | |||
| Maximum LST | 0.72 | −2.99 to 4.43 | 0.71 | |||
| NDVI | 8.72 | −45.96 to 63.39 | 0.76 | |||
| Dissolved oxygen | −0.53 | −3.46 to 2.40 | 0.73 | |||
| pH | −0.04 | −6.07 to 5.98 | 0.99 | |||
| ORP | −0.04 | −0.09 to 0.01 | 0.12 | |||
| NDWI | 3.09 | −42.20 to 48.38 | 0.89 | |||
| Pressure | −0.005 | −0.24 to 0.23 | 0.97 | |||
LST Land surface temperature.
NDVI Normalized difference vegetation index.
ORP oxidation-reduction potential.
NDWI Normalized difference wetness index.
∗Significant at p < 0.05.
The output of the negative binomial regression model in the generalized linear model is shown in Table 2. NDVI and dissolved oxygen showed a positive, statistically significant relationship with the abundance of B. globosus (p-value < 0.05). In contrast, ORP and NDWI showed a statistically significant negative relationship with the abundance of B. globosus (p-value < 0.05) (Table 2). In contrast, none of the physicochemical parameters and climatic factors showed a statistically significant relationship with the abundance of B. pfeifferi (Table 2).
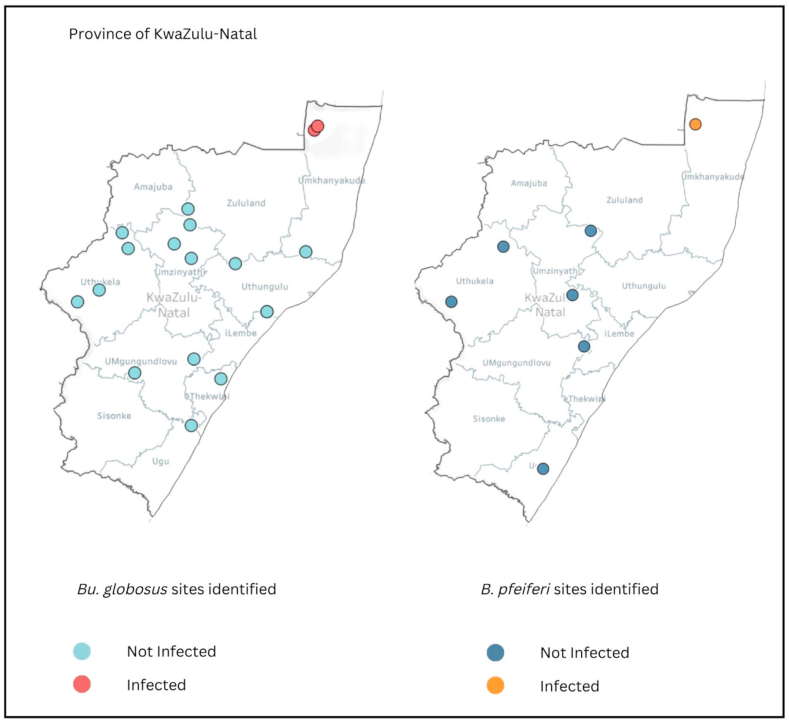
Out of the 31 sites where HSTS were found, infected snails were found in 2 sites. One of the 2 sites had Bu. globosus infected with mammalian cercariae, while the other site had infected Bu. globosus and B. pfeifferi. Both sites were in uMkhanyakude district (Fig. 1). As an entire province, the number of snails shedding Schistosoma spp. cercariae were very low for both Bu. globosus and B. pfeifferi, with snails shedding mammalian cercariae found in only one district. The overall infection rate of Bu. globosus was 3.89% and that of B. pfeifferi is 2.44% (Table 3).
Fig. 1.
Map of KwaZulu Natal province showing sites with Bu. globosus, B. pfeifferi snails, and positive infections.
Table 3.
Infection rate of human schistosomiasis transmitting snails (HSTS) in KZN.
| Bu. globosus | B. pfeifferi | |
|---|---|---|
| No. of snails screened | 488 | 246 |
| No. infected with human mammalian cercariae | 19 | 6 |
| Total (%) | 3.89 | 2.44 |
4. Discussion
Our results show that Bu. globosus is about twice as much as the number of B. pfeifferi at the sampled sites. Furthermore Bu. globosus was more widely distributed compared to B. pfeifferi. Both snail species were more abundant in streams (54.8% of the sites where we found HSTS were streams), followed by rivers at 25.8%, suggesting that streams may be more conducive for Bu. globosus and B. pfeifferi. Few snails were also found in ponds and dams, as was reported by Ofulla et al. [33] in western Kenya.
NDWI is an important environmental factor that influences the abundance and survival of intermediate host snails. The effect of NDWI on snail abundance and distribution has been demonstrated in other studies [21,22]. In our study, NDWI was negatively correlated with the abundance of B. globosus; this could be because the snail sampling was carried out during the rainy season when most water bodies were full and might have been washed away due to floods. Although water velocity was not measured in this study, Bu. globosus snails are not found in water exceeding 0.3 m/s as they cannot attach themselves to the rocks [34]. Annual rainfall higher than 1192.2 mm leads to higher humidity, negatively impacting snail populations. In addition, water is one of the necessary conditions for the growth and reproduction of snails, but the humidity requirements for snail growth vary across the different stages of development.
NDVI is another environmental factor that affects snail abundance [35]. It indicates the quantity of vegetation present at each location, with higher values representing more intensive vegetation cover. In our study, NDVI has a positive relationship with the abundance of Bu. globosus, indicating that an increase in the value of NDVI leads to an increase in snail abundance. This could be because the freshwater habitats had the right amount of vegetation cover that provided a conducive breeding site for freshwater snails, which increased snail abundance and growth. In addition, a suitable snail habitat has appropriate green vegetation cover, especially aquatic weeds, which the snails feed on.
Pulmonate freshwater snails breathe by absorbing dissolved oxygen from the water through their lungs for their metabolic activities. Hence, high levels of dissolved oxygen are needed to survive and reproduce. The mean values of dissolved oxygen in the different districts in KZN province ranged from 4.33 ppm to 9.40 ppm, which is within the desired concentration (0.4 ppm–16.0 ppm) of dissolved oxygen for intermediate host snails [36]. The positive relationship between snail abundance and dissolved oxygen can be attributed to flowing water that washes pollutants away. The cleaner the water becomes, the more suitable it is for snail survival. Also, B. globosus may be sensitive to water pollution. Low dissolved oxygen levels indicate poor water quality from organic and anthropogenic pollution. Furthermore, low oxygen content in water affects the development of intermediate host snails hence reduction in snail abundance, egg size, and variations in shapes [37]. This low oxygen in water also increases the active organic decomposition of the sediments, leading to the suffocation and death of freshwater snails. Boelee and Laamrani [38] and Salawu and Odaibo [36] reported similar findings on the positive relationship between snail density and dissolved oxygen.
The infection rate of HSTS indicates active transmission of schistosomiasis in KZN province. Bu. globosus snails had higher infection rates than B. pfeifferi, suggesting that S. haematobium is more prevalent than S. mansoni. This result is similar to that of Manyangadze et al. [22]; except that their study was carried out in one district, i.e., uMkhanyakude district in KZN province. Furthermore, our results complement the parasitology survey carried out by the KZN department of health in 2017, where schistosomiasis among humans was 1.87%–25.42% for S. haematobium and less than 2% for S. mansoni. Although uMkhanyakude recorded the highest HSTS infection rate, other districts had HSTS found in them, indicating potential transmission [26].
In our study, there were no significant differences in the abundance of HSTS among districts and habitat types, and there was no statistically significant relationship between the abundance of B. pfeifferi, physicochemical parameters, and climatic factors. This might be because the study is cross-sectional, and more information might be obtained if a longitudinal study is carried out.
The study's limitations include that the cercarial shedding and crushing techniques used for detecting infection rates have been found to have lower detection rates due to their inability to detect prepatent infections compared to molecular techniques. In addition, cercariae were identified morphologically under a microscope, but since some schistosomiasis cercariae are indistinguishable under the microscope, we suggest the use of molecular xeno-monitoring for cercariae differentiation in future studies. Therefore, the reported infection rate might be underreported of the actual prevalence in the area. Finally, although the study provides the current distribution, abundance, and infection rates of HSTS, snail sampling was carried out only in the rainy season, which could have masked the results obtained.
5. Conclusion
To the best of our knowledge, this study is the first to determine the distribution, abundance, and infection rates of HSTS across all eleven districts in KZN. Bu. globosus was widely distributed and more abundant compared to B. pfeifferi. The results showed that NDWI and ORP were significantly inversely correlated to the abundance of Bu. Globosus, while NDVI and dissolved oxygen had a significant direct correlation with the abundance of Bu. globosus. In contrast, none of the physico-chemical properties and climatic factors were significantly correlated with the abundance of B. pfeifferi. Furthermore, the infection rate of Bu. globosus snails were higher than that of B. pfeifferi snails. The active transmission sites identified need to be included in strategies to control schistosomiasis in the province.
Contributions
OEN conceived and designed the experiment; performed the experiments; analyzed and interpreted the data; wrote the paper. T.M conceived and designed the experiment; analyzed and interpreted the data. M.J.C conceived and designed the experiment; contributed reagents and materials.
Ethical approval
The University of KwaZulu Natal biomedical research ethics committee (BREC) issued the ethical approval (Ref No: BREC/00001305/2020). This review is part of the approved thesis protocol.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
This research was conducted as part of the Ph.D. work of the first author. This research was commissioned by the National Institute for Health Research (NIHR) Global Health Research program (16/136/33), UK, and the University of KwaZulu-Natal through a Ph.D. studentship bursary awarded to O.E.N by the College of Health Sciences. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The authors thank the research team members and community research assistants for the assistance rendered during fieldwork. We also thank the administrative staff, Ms. Nokwanda Majola and Ms. Sambulo Ntombela, for their support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2022.e12463.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
S1 Table: Snail abundance, habitat type, districts, and GPS coordinates of sampling sites.
S2 Table: Descriptive statistics of physicochemical and environmental parameters used to assess HSTS distribution, abundance, and infection status in KZN province.
References
- 1.Adenowo A.F., Oyinloye B.E., Ogunyinka B.I., Kappo A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015;19:196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhumuza S., Kitimbo G., Oryema–Lalobo M., Nuwaha F. Association between socio economic status and schistosomiasis infection in Jinja District, Uganda. Trop. Med. Int. Health. 2009;14:612–619. doi: 10.1111/j.1365-3156.2009.02273.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO . 2022. Schistosomiasis. [Google Scholar]
- 4.WHO Schistosomiasis and soil-transmitted helminthiases: progress report, 2020–Schistosomiase et géohelminthiases: rapport de situation. Wkly. Epidemiol. Rec.= Relevé Épidémiol. Hebd. 2021;2020(96):585–595. [Google Scholar]
- 5.Mazigo H.D. Participatory integrated control strategies and elimination of schistosomiasis in sub-Saharan Africa. Lancet Global Health. 2019;7:e998–e999. doi: 10.1016/S2214-109X(19)30271-2. [DOI] [PubMed] [Google Scholar]
- 6.Randjelovic A., Frønæs S., Munsami M., Kvalsvig J., Zulu S., Gagai S., Maphumulo A., Sandvik L., Gundersen S., Kjetland E.F. A study of hurdles in mass treatment of schistosomiasis in KwaZulu-Natal, South Africa. S. Afr. Fam. Pract. 2015;57:57–61. [Google Scholar]
- 7.Lothe A., Zulu N., Øyhus A.O., Kjetland E.F., Taylor M. Treating schistosomiasis among South African high school pupils in an endemic area, a qualitative study. BMC Infect. Dis. 2018;18:1–10. doi: 10.1186/s12879-018-3102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magaisa K., Taylor M., Kjetland E.F., Naidoo P.J. A review of the control of schistosomiasis in South Africa. South Afr. J. Sci. 2015;111:1–6. [Google Scholar]
- 9.Precious M.M. 2017. SCHISTOSOMIASIS AND SOIL-TRANSMITTED HELMINTHIASIS MAPPING IN KWAZULU-NATAL AND LIMPOPO PROVINCES. [Google Scholar]
- 10.Oloyede O.O., Otarigho B., Morenikeji O. Diversity, distribution and abundance of freshwater snails in Eleyele dam, Ibadan, south-west Nigeria. Zool. Ecol. 2017;27:35–43. [Google Scholar]
- 11.Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 13.CDC . Global Health, Division of Parasitic Diseases and Malaria; 2019. Schistosomiasis: Biology. [Google Scholar]
- 14.Fenwick A., Jourdan P. Schistosomiasis elimination by 2020 or 2030? Int. J. Parasitol. 2016;46:385–388. doi: 10.1016/j.ijpara.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Stensgaard A.-S., Jørgensen A., Kabatereine N.B., Rahbek C., Kristensen T.K. Modeling freshwater snail habitat suitability and areas of potential snail-borne disease transmission in Uganda. Geospat. Health. 2006:93–104. doi: 10.4081/gh.2006.284. [DOI] [PubMed] [Google Scholar]
- 16.Ofoezie I.E. Distribution of freshwater snails in the man-made Oyan Reservoir, Ogun State, Nigeria. Hydrobiologia. 1999;416:181–191. [Google Scholar]
- 17.Opisa S., Odiere M.R., Jura W.G., Karanja D.M., Mwinzi P.N. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasites Vectors. 2011;4:1–9. doi: 10.1186/1756-3305-4-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleton C., Madsen H. Human schistosomiasis in wetlands in southern Africa. Wetl. Ecol. Manag. 2012;20:253–269. [Google Scholar]
- 19.De Kock K., Wolmarans C. Distribution and habitats of the Bulinus africanus species group, snail intermediate hosts of Schistosoma haematobium and S. mattheei in South Africa. WaterSA. 2005;31:117–125. [Google Scholar]
- 20.De Kock K., Wolmarans C., Bornman M. Distribution and habitats of Biomphalaria pfeifferi, snail intermediate host of Schistosoma mansoni, in South Africa. WaterSA. 2004;30:29–36. [Google Scholar]
- 21.Manyangadze T., Chimbari M.J., Gebreslasie M., Ceccato P., Mukaratirwa S. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using Maxent in Ndumo area, KwaZulu-Natal Province, South Africa. Parasites Vectors. 2016;9:1–10. doi: 10.1186/s13071-016-1834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manyangadze T., Chimbari M.J., Rubaba O., Soko W., Mukaratirwa S. Spatial and seasonal distribution of Bulinus globosus and Biomphalaria pfeifferi in Ingwavuma, uMkhanyakude district, KwaZulu-Natal, South Africa: implications for schistosomiasis transmission at micro-geographical scale. Parasites Vectors. 2021;14:1–9. doi: 10.1186/s13071-021-04720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalinda C., Chimbari M.J., Mukaratirwa S. Effect of temperature on the Bulinus globosus—Schistosoma haematobium system. Infect. Dis. Poverty. 2017;6:1–7. doi: 10.1186/s40249-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejehu Z., Ekwunife C., Anumba J., Onyido A., Umeanaeto P. Snail fauna and investigations into the incidence of schistosoma infection in Lake of Oguta 1 Region, Imo State, Nigeria. Niger. J. Parasitol. 2017;38:173–178. [Google Scholar]
- 25.Rabone M., Wiethase J.H., Allan F., Gouvras A.N., Pennance T., Hamidou A.A., Webster B.L., Labbo R., Emery A.M., Garba A.D. Freshwater snails of biomedical importance in the Niger River Valley: evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp. Parasites Vectors. 2019;12:1–20. doi: 10.1186/s13071-019-3745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appleton C., Miranda N. Locating bilharzia transmission sites in South Africa: guidelines for public health personnel. South. Afr. J. Infect. Dis. 2015;30:95–102. [Google Scholar]
- 27.Brown D., Kristensen T. Danish Bilharziasis Laboratory publication number; 1989. A Field Guide to African Freshwater Snails, Southern African Species. [Google Scholar]
- 28.Chimbari M., Kalinda C., Siziba N. Changing patterns of Schistosoma host snail population densities in Maun, Botswana. Afr. J. Aquat. Sci. 2020;45:493–499. [Google Scholar]
- 29.Frandsen F., Christensen N. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41:181–202. [PubMed] [Google Scholar]
- 30.Joof E., Sanneh B., Sambou S.M., Wade C.M. Species diversity and distribution of schistosome intermediate snail hosts in the Gambia. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks M.E., Kristensen K., Van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Machler M., Bolker B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400. [Google Scholar]
- 32.Zuur A.F., Ieno E.N., Walker N.J., Saveliev A.A., Smith G.M. Springer; 2009. Mixed Effects Models and Extensions in Ecology with R. [Google Scholar]
- 33.Ofulla A.V., Adoka S.O., Anyona D.N., Abuom P.O., Karanja D., Vulule J.M., Okurut T., Matano A.S., Dida G.O., Jembe T. Spatial distribution and habitat characterization of schistosomiasis host snails in lake and land habitats of western K enya. Lakes Reservoirs Res. Manag. 2013;18:197–215. [Google Scholar]
- 34.Nwoko O.E., Kalinda C., Manyangadze T., Chimbari M.J.J.W. Species diversity, distribution, and abundance of freshwater Snails in KwaZulu-Natal. S. Afr. 2022;14:2267. [Google Scholar]
- 35.Kristensen T., Malone J., McCarroll J. Use of satellite remote sensing and geographic information systems to model the distribution and abundance of snail intermediate hosts in Africa: a preliminary model for Biomphalaria pfeifferi in Ethiopia. Acta Trop. 2001;79:73–78. doi: 10.1016/s0001-706x(01)00104-8. [DOI] [PubMed] [Google Scholar]
- 36.Salawu O.T., Odaibo A.B. Preliminary study on ecology of Bulinus jousseaumei in Schistosoma haematobium endemic rural community of Nigeria. Afr. J. Ecol. 2013;51:441–446. [Google Scholar]
- 37.Oso O.G., Odaibo A.B. Land use/land cover change, physico-chemical parameters and freshwater snails in Yewa North, Southwestern Nigeria. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boelee E., Laamrani H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop. Med. Int. Health. 2004;9:997–1004. doi: 10.1111/j.1365-3156.2004.01301.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.