Abstract
Quantitative Systems Pharmacology (QSP) has emerged as a powerful ensemble of approaches aiming at developing integrated mathematical and computational models elucidating the complex interactions between pharmacology, physiology, and disease. As the field grows and matures its applications expand beyond the boundaries of research and development and slowly enter the decision making and regulatory arenas. However, widespread acceptance and eventual adoption of a new modeling approach requires assessment criteria and quantifiable metrics that establish credibility and increase confidence in model predictions. QSP aims to provide an integrated understanding of pathology in the context of therapeutic interventions. Because of its ambitious nature and the fact that QSP emerged in an uncoordinated manner as a result of activities distributed across organizations and academic institutions, high entropy characterizes the tools, methods, and computational methodologies and approaches used. The eventual acceptance of QSP model predictions as supporting material for an application to a regulatory agency will require that two key aspects are considered: (1) increase confidence in the QSP framework, which drives standardization and assessment; and (2) careful articulation of the expectations. Both rely heavily on our ability to rigorously and consistently assess QSP models. In this manuscript, we wish to discuss the meaning and purpose of such an assessment in the context of QSP model development and elaborate on the differentiating features of QSP that render such an endeavor challenging. We argue that QSP establishes a conceptual, integrative framework rather than a specific and well-defined computational methodology. QSP elicits the use of a wide variety of modeling and computational methodologies optimized with respect to specific applications and available data modalities, which exceed the data structures employed by chemometrics and PK/PD models. While the range of options fosters creativity and promises to substantially advance our ability to design pharmaceutical interventions rationally and optimally, our expectations of QSP models need to be clearly articulated and agreed on, with assessment emphasizing the scope of QSP studies rather than the methods used. Nevertheless, QSP should not be considered an independent approach, rather one of many in the broader continuum of computational models.
Keywords: Quantitative systems pharmacology, PKPD, Model assessment, Regulatory
Introduction
Quantitative Systems Pharmacology (QSP) emerged as the convolution of four distinct areas: (a) systems biology, which focuses on modeling the dynamics of molecular and cellular mechanisms and networks; (b) systems pharmacology, which aims at incorporating links between therapeutic interventions and drug mechanisms; (c) systems physiology, which describes disease mechanisms driving the dynamics of the onset and resolution of a disease and its symptoms in the context of a patient’s physiology, genetics, age, sex, behaviors, and lifestyle, and (d) data science, which enables the integration of relevant, yet diverse, biomarkers of efficacy and safety, disease pathology and phenotype, treatment, and clinical endpoints [1].
These areas were unified under the umbrella of dynamic systems theory [2] for developing integrated, multi-scale models describing the response to treatments and elaborating on patient variability. Arguably, one of the most critical contributions of QSP is the fact that it placed all key players, be it the drug, its molecular target, the disease mechanisms, the patient’s physiology, and treatment options in a unified, underlying context [3–5].
The origins of the field can be traced back to two workshops held at NIH in 2008 and 2009 whose main goals were to (a) characterize the state of the art in systems biology and pharmacology respectively; and (b) to explore whether merging the two into a new discipline, QSP, would significantly advance the discovery, development, and clinical use of therapeutic drugs. The findings were summarized in a white paper which was used as the road map for charting the path forward [6]. So-called “systems” - based approaches, be it either in the context of systems biology, systems pharmacology, or systems physiology, introduced a fundamental shift in terms of how we approach problems moving away from a single target or a specific (sub-)system and instead, consider complex interactions and emergent responses [7–9]. To do so, more holistic methodologies needed to be developed characterizing state evolutions and not just localized events, such as at the site of action of the drug [10]. “Quantitative systems”-based approaches moved things to the next level by introducing complex quantitative (either mathematical or computational) descriptions of the dynamics of the corresponding integrated “systems.”
When using the term QSP, it is always presumed that reference is made to an in silico model, either mathematical (equation-based, mostly ordinary of partial differential equations) or computational (agent-based, rule-based, Boolean, or Bayesian to name a few), describing spatiotemporal dynamic evolution of biomarkers, or outcomes of interest [11]. As such, QSP models materialize in many different ways [12], be it either a data-driven conceptual, qualitative, knowledge-based, or statistical representation; a set of quantifiable phenomenological relations, or a quasi-mechanistic mathematical expressions at a single, or multiple, level of the physiological organization. The way the model is executed can be continuous or discrete (in space and/or time), stochastic or deterministic, synchronous, or asynchronous. In QSP the aim is, broadly, to use (semi-) mechanistic rules that express fundamental phenomena approximating first principles, such as mass action, transport, binding, etc., as a guide [13].
QSP has the potential to impact product development in the pharmaceutical research and development. However, this requires that we establish acceptable levels of confidence and credibility so that QSP models can be reliably used in decision making and regulatory submissions [14]. Thus, the systematic assessment of the quality of a QSP model takes center stage. One could argue that QSP model assessment should have been the same as any other modeling activity since QSP model outcomes constitute attributes which can be formally assessed [15]. Therefore, it is important to address a critical question: does QSP model assessment present any unique challenges requiring different approaches? We will argue that the answer to this question is “yes”, because QSP defines a methodological and conceptual framework rather than an exact and precise computational approach.
QSP and the curse of diversity
A QSP model is at its core a mathematical and computational model (MCM). The deployment of any MCM is divided into two major phases: model development and model execution. Testing, parameter estimation, sensitivity analysis, propagation of uncertainty, robustness, relevance, accuracy, etc., are critical tasks, but all depend on having developed a model and being able to execute it. Often times, these two steps are associated with the processes of validation (how accurately does the model represent reality) and verification (how accurately the model quantifies the conceptual description) [16]. Validation is related to model building and depends on how well the model describes the physical reality, whereas verification is associated with model execution and assesses the quality of the computational solution.
Assessment approaches for model execution have been thoroughly described in the literature. For example benchmarking numerical integration schemes in the context of ODE models [17] or optimization and parameter estimation in systems biology [18] have been assessed extensively. In evaluating the execution method, a bench-mark needs to be established. In tasks such as ODE integration [19], optimization [20], or bioinformatics [21] several libraries of benchmarking problems exist which allow for an objective assessment and benchmarking of the execution of the respective computational tasks.
Historically, MCM approaches that gained widespread acceptance achieve to a great extent a level of universality in their model development phase. These tend to be so-called “physics-based” models where “first-principles” prescribe a recipe for developing the model. Pharmacokinetic/pharmacodynamic (PK/PD) models, one of the closest relatives to QSP, constitute an example of a universally accepted MCM. These rely on a well-established semi-mechanistic underlying background which enables the development of models that can be compared (with each other and/or with experimental data) and assessed, because of the overall agreement on how the model is developed [22]. Alternatives can be evaluated, and the end-user can choose based on preferences which vary from cost to versatility to user-friendliness.
The key features of PK/PD models have been clearly articulated and the model components characterize the links between drug concentration and the response mechanisms are well described [23]. The components express, in various forms, a fundamental sequence of events: disposition kinetics→ biophase distribution→ biosensor process→ biosignal flux→ signal transduction→physiological response. A basic structure, Fig. 1, enhanced with a variety of basic, mostly indirect response, formalisms expressing rate-limiting steps, target binding, etc., define a basic canvas with the help of which different modes of action can be developed in a modular way andbe be extended in a self-similar manner and produce complex PK/PD models. This essential modularity [24] and broad acceptance of model structures [25] has led to the development of standardized approaches to modeling PK/PD. Last, but definitely not least, a comprehensive PK/PD model relies primarily on well-controlled clinical and preclinical trials and in vitro experiments, and are constructed by combining, relatively well-established, simple modules.
Fig. 1.

A PK/PD model is composed of broadly accepted submodules that define broadly accepted mechanisms of a core set of processes (Derendorf and Meibohm [23], Jusko [28], Hosseini, Gajjala et al. [26]). These simpler processes can be combined to produce arb
The ability to combine well accepted simple (r) computational elements using consistent data modalities, has enabled the development of generalized models [26] where these basic elements (sub-models) can be integrated, in a “drag-and-drop” fashion, to create more complex PK/PD structures. These integrated computational models will, almost always, end up being represented by ODEs. The fact that (a) the nature of these elementary structures is ubiquitous and that (b) the data used to parametrize these models are, more or less, standardized pre-clinical PK/PD response data makes such models generalizable. Both the construction of the model as well as its execution (usually an ODE integration) follow well-established and broadly accepted approaches, as evidenced by the availability of several commercially tools for developing and executing complex PK/PD models. Given the broadly accepted nature of PK/PD models, extensions to new areas, for example moving from small molecule drugs to therapeutic proteins, follow the same principles and, as such, it is possible to readily extend our PK/PD understanding, albeit with appropriate modifications [27–29].
A somewhat similar situation describes the state-of-the-art in systems biology, a field also closely relate to QSP [30]. These are models that focus their details at the cellular and molecular level and, primarily, emphasize the modeling of signaling cascades. Advances in the field revealed a certain level of standardization of the modules that come together to establish a complex systems biology model making possible a certain level of uniformity in formalism [31].
To a certain extent PK/PD and systems biology models can be considered “self-similar” in the sense that basic modules exist and those repeat themselves, thus making standardization of model development plausible.
QSP: diversity in approach and purpose
So, if QSP is a manifestation of an MCM, combining PK/PD and systems biology elements and principles, why should its assessment be different? The reason is that QSP models present a unique set of challenges as they capture and describe a far more nuanced landscape: (a) the diversity of information and data used for developing a QSP model originates from a wide variety of, often, unrelated sources which differ for various applications [32]; (b) the complexity of the data structures a QSP model is required to assemble, represent, and integrate, is far superior to what is encountered in a PK/PD model; (c) the plethora of questions a QSP model is often required to address far exceeds that of typical PK/PD model; and (d) the complexity of the physiological and biological realizations that are described by a QSP model, which depend on the available data, require the integration of a variety of modeling approaches and computational tools [33]. In short, in its present form QSP modeling approaches lack ubiquitous underlying first principles!
Therefore, it comes as no surprise that the nature, structure, and type of a QSP model are intimately associated with a specific application, unlike PK/PD models which are more general [25]. QSP deploys the entire arsenal available for mathematical and computational modeling techniques including statistical, and data-driven, logic-based, ODE, PDE, cellular automata, agent-based and machine learning models, essentially spanning the entire range of computational approaches [12]. The model can be expressed in the form of statistical or logic-based expressions; lumped/quasi-mechanistic/mechanistic rate expression at a single or multiple level/scales, Fig. 2. Depending on the nature of the model, its execution can be in the form of a deterministic or stochastic simulation, discrete or continuous in representing time and/or space and include temporal or spatial dimensions.
Fig. 2.

A QSP model incorporates a plethora of data modalities as each introduces a different layer of biological, physiological, and pharmacological information. The various data need to be approximated with the appropriate modeling modalities which range from statistical expression to logic-based, to equation driven, machine learning, and hybrid. Finally, the execution of the model also varies depending on the data granularity and the type of question addressed
The diversity of the interpretation, results in QSP applications spanning a wide range of representations. For example, while studying the mechanism of action of statin and anti-PCSK9 therapies and their impact on LDL changes [34] consider a representation using 3 key processes (cholesterol consumption, excretion and utilization; hepatocyte cholesterol synthesis; and therapeutic modulation of cholesterol processing) across 6 major components to evaluate circulatory LDL elimination and the entire model is represented by 9 ODEs, one for each state variable. Therefore, this level of complexity was deemed to have appropriately reduced the complexity of the key processes involved in synthesizing and lowering LDL. The context-specific representation of the physical reality creates a major issue—to be revisited later—as it implies that the path towards a QSP model can, and will, take very different routes, even for the same question if the context changes. To address similar questions [35] developed a QSP model also described by ODEs to account for a multi-cellular model incorporating liver and peripheral cholesterol metabolism as well as statin and anti-PCSK9 function. This model, however, consisted of 74 species (state variables) with their corresponding ODEs. This larger model accounted for processes within the GI tract, plasma, hepatocytes, and peripheral cells while it also described plaque formation. The purpose of the second model was to introduce a more mechanistic description of processes across distinct tissue/organ levels. Despite differences in complexity, both models are eventually described by a set of coupled nonlinear ODEs. Despite increased model complexity, certain aspects cannot be adequately addressed. One such issue concerns spatial proximity and inhomogeneity. One way of approaching this is by invoking modeling tools such as agent-based models (ABM), an approach explore by [36] to approximate computationally plaque progression in a generic artery wall. The model accounted for crucial entities such as B- and T-lymphocytes, macrophages, T-cell receptors, LDP, and LDL. The physical proximity was simulated using a lattice model treating each entity as a separate “agent.” Therefore, this model takes an entirely different approach to describe LDL and plaque formation by adopting a discrete representation. Interestingly, more recent approaches aim at bridging conceptually different methods, such as ODEs and ABM [37] opted for a combination of ODE and agent-based models to resolve the spatial resolution in a tumor growth model. Thus, the dependence of a QSP model on the data and application makes it more difficulty to be transferable [25, 38, 39].
The flexibility, and fuzziness, of QSP models is only expected to increase with the integration of machine learning (ML) components. Without delving into definitions, we will simply state that ML encompasses a compendium of approaches that aim to directly connect observations with outcomes of interest by establishing computational input–output relations [40–47]. One interesting, and challenging, aspect, relates to opportunities emerging from creating so-called hybrid ML-QSP models, Fig. 3 whereby part of the model is “equation-based”, through the integration of systems biology—pharmacology—physiology principles, while part is “data-based”, with ML methods informing the mechanistic model [48] or assisting in developing a structure and/or likely expressions reflecting system dynamics [49]. One of the promises of hybrid QSP-ML models is that they could be used to enable the modeling of components that cannot be readily described with expressions associated with the aforementioned components, thus leading to so-called “hybrid” models, where an ML module expressed part of the response [50]. ML learning is substantially relying on the way data is considered and therefore each expresses a specific bias. Furthermore, data come in a large variety of forms making data representation also a major issue. Therefore, ML algorithms tend to be compared in a specific context rather than in general [51].
Fig. 3.
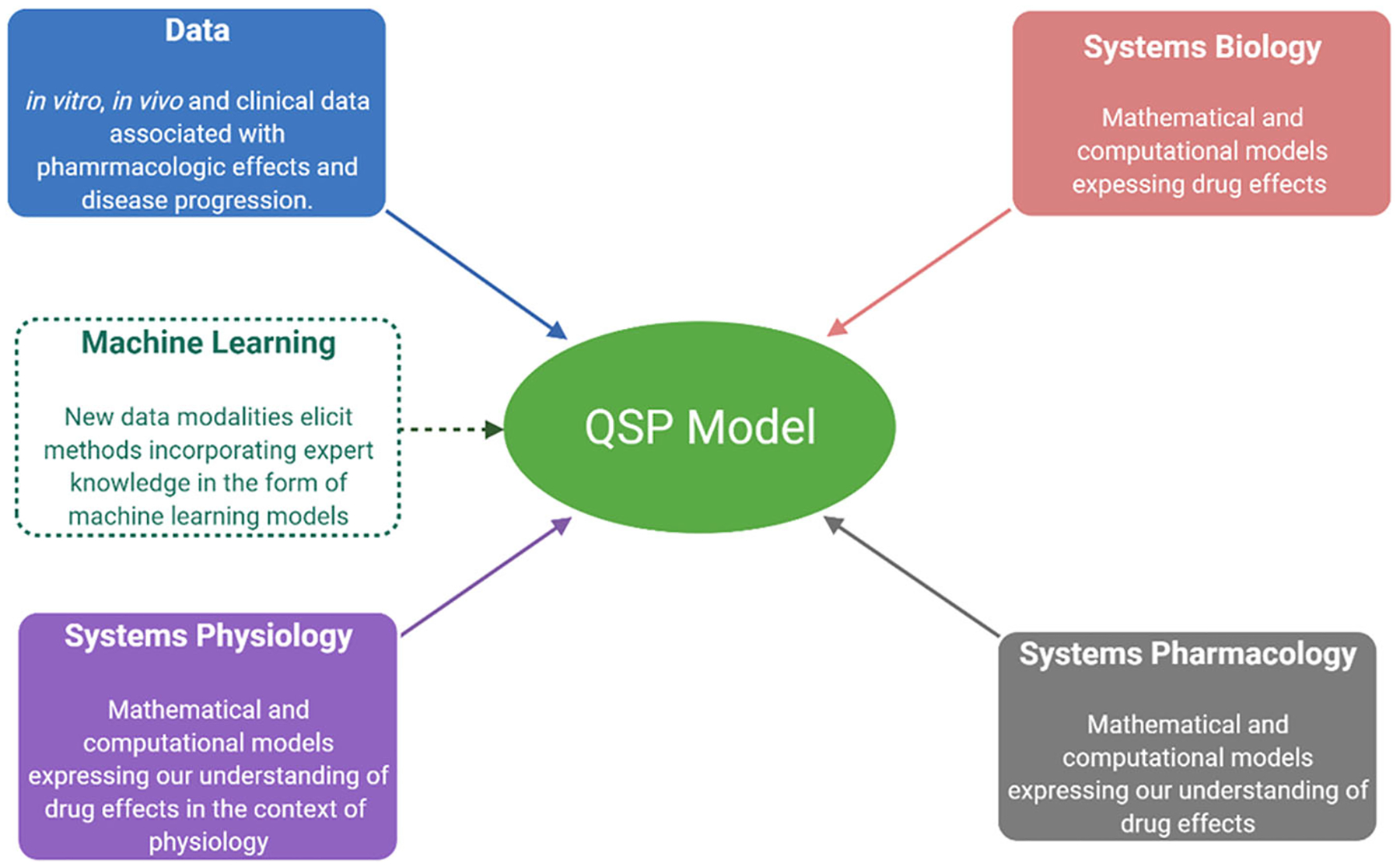
QSP aims at developing computational descriptions of physiology and pharmacology under the umbrella of systems biology. While these models tend to be primarily based on mathematical models (ODE or PDE) with recent advances in machine learning (ML) the scope of the modeling efforts has expanded to include model components expressing either hard to quantify expert knowledge or relations that can be readily expressed in the form of ML computational models
Is standardization in QSP required and if so, what should be standardized?
Although QSP models promise to offer several advantages and critical insights, broad acceptance is still lagging, especially in the regulatory arena. Several reasons, all linked in some way, have been identified [52], including:
The field emerged primarily as a research activity. As such, models tend to case-specific and many of their details remain hidden.
QSP models tend to be overly complex, which is expected given the magnitude of the problems they wish to address.
QSP models evolve and come in all types of granularities as new or different data are incorporated—this is unlike more traditional PK/PD models which tend to use broadly accepted data types, and
QSP models have encountered unnecessary institutional resistance driven by divisions between pharmacometrics and systems approaches as different stakeholders fail to realize that both are part of the same broader model development endeavor.
All the above have substantially, and possibly unreasonably, raised the bar for accepting QSP. However, it must be recognized that these hurdles are not something unprecedented for a new approach to science. The less than 20 years of QSP activity pale in comparison to the, almost, half-century since the first signs of what eventually became known as bioinformatics made their first appearance. However, even that field still struggles to clarify several standardizations and interoperability issues [53, 54].
QSP modeling and the dual meaning of “fit-for-purpose”
PK/PD models are often considered to be “fit-for-purpose” [55]. However, they are the result of a careful assembly of a well-defined set of submodules [13, 26, 28]. The basic principles on which a PK/PD model is founded are represented by a well-defined set of submodules which are appropriately combined defining a framework for describing a wide variety of applications. In a classical PK/PD model “fit-for-purpose” implies that while the parameter set is uniquely associated with an application, the functional forms are, mostly, well-defined, and often ubiquitous since they are associated with the accepted principles. Comparisons between models are easier given that the building blocks are universally acceptable, rendering also model interchangeability (use and reuse) plausible [56].
The quest to develop holistic QSP representations, results in more “nuanced” approaches [57]. In a QSP model, the sequence is reversed, and “fit-for-purpose” implies that the process begins with a (specific) application and the (specific) data that is available for that application, which drives assumptions regarding the ways the model is conceived. These models are often explored to provide an inductive framework used to generate hypotheses. Thus, QSP models are about creating a scaffold starting with the most comprehensive hypothesis and then attempting to “fill in the blanks” depending on data availability. On the other hand, deductive models, such as PK/PD, quantify standardized representations of defined hypotheses [58] thus providing a scaffold that is consistently populated and quantified by data. The lack of a common scaffold in QSP leads to difficulties in standardizing the approach thus further complicating the assessment of the model.
Because of the focus on conceptualization, most attempts at formalizing the QSP process, focus on the workflow [59] rather than a specific approach. This “reversing” of the model development process poses a major challenge as often the elements of the workflow are unique to a specific application and therefore need to be assessed and evaluated each time, further complicating model interchangeability (use and reuse) of a QSP model in a different context [57]. The critical question therefore becomes: can the conceptualization of a QSP model be standardized?
The technical limits of standardization in QSP
The systems biology community eventually recognized that for the domain to flourish and for genome-scale models to move to the next level, collaborative hurdles and barriers needed to be shattered. That would not be possible without a common, formal and standardized representation of these complex systems [60]. Standardization implied a broad, agreement in the way questions are conceived, represented and mathematical expressed, while focusing less on computational implementation and execution. The latter can be harmonized, although the stakeholders need to agree as to what kind of options will be available.
What is not clear, is whether the conceptualization in the QSP domain could (or should) be standardized. As earlier noted, a QSP model defines a continuum of approaches and a road map. Even when QSP workflows are standardized [32] they define what should be done, and not how, which necessitates the assessment of the model development methodology, rather than the model itself. Recent works [61] have attempted to standardize QSP model development sessions and workflows. Such approaches, however, come with caveats including, among others, that the computational environment needs to be agreed upon as well as the model expression and execution.1 Because of the excessive variability and uncertainty associated with the wide range of data used, the modeling and computational techniques and software used, the development, quantification, and application of QSP models vary enormously. Therefore, the community is actively pursuing establishing “best practices” not so much as far as the model is concerned, but rather providing guidelines assisting the development of consistent workflows [62].
However, standardization in QSP is limited by a number of factors. Unlike disciplines dominated by physics-based models, QSP lack ubiquitous first principles and rely on quantifying hypotheses about components and behaviors with a diversity of purposes exploring a wide range of approaches based on a variety of biological and physiological data modalities. Standardization could be plausible if the domains (i.e., context) is restricted. On the other hand, standardization efforts need to be balanced so as to not stifle creative in the attempt to fully harmonize models and approaches.
QSP models in the regulatory arena: scope vs. methods
In the pharmaceutical development industry, the ultimate test for a new idea, approach, method, or product to escape the boundaries of a purely research activity is to be accepted by regulatory agencies, thus becoming a commercial product. In the context of QSP, we argue, that this breakpoint will occur when three conditions are met: (a) stakeholders should feel comfortable accepting the validity of the underlying assumptions and mathematical formalisms of QSP models; (b) QSP models should provide insights and understanding above and beyond what is currently achieved using traditional modeling; and finally, (c) regulatory agencies should provide guidance for QSP model results to be accepted evidence in the filling process. A tall order with success strongly depending on a rigorous process for assessing QSP models [63].
The FDA has made the development of computational modeling a key priority in supporting regulatory decision-making [64]. Even though the current emphasis is on imaging for disease screening and diagnosis, the path forward points towards developing modeling, simulation, and statistical techniques which, in conjunction with methodologies for assessing their credibility, will facilitate faster, cheaper, and better pathways to market [60]. In 2011, the FDA’s strategic plan [65] suggested the development of in silico computational methods having identified opportunities to, among others: develop virtual clinical trials connecting individual patient characteristics to outcomes; develop computer models simulating cells and organs to predict safety and efficacy; and integrate PK/PD with safety data to better predict patient-specific clinical risk–benefit. FDA’s support was materialized by the development of the “Model-informed Drug Development” pilot program2 to initiate discussions between regulators and sponsors.
The acceptance of a computational prediction requires that models and approaches are “credible” and “reliable.” Thus, the need for establishing “best practices” to increase the standardization of computational models and set common expectations, which will enable the assessment of model credibility and reliability. The role of “best practices” is to clearly articulate not only “how” (i.e., methods) systems are modeled but, more importantly, “what” (i.e., scope) is modeled. The latter implicitly constraints to some extent the data to be used. This, we believe, is exemplified in the recently proposed (non-binding) FDA recommendations to the pharmaceutical industry regarding the development, evaluation, and use of physiologically based PK (PBPK) analyses for biopharmaceutics applications [66]. The expectation is that new (oral) drug applications will be complemented with PBPK models and simulations that combine physiology, population, drug substance, and product characteristics to describe the PK of the drug more mechanistically, although the recommendations warrant against the use of mathematical models to support major manufacturing changes. However, the recommendations articulated 3 critical steps for model development and evaluation in supporting an application: (1) the objective(s) of the model, i.e., quality issues and questions to be addressed, should be clearly described; (2) the development of the model should consist of clear descriptions of the structure, assumptions, and parameters used; and (3) the model must be validated for its intended purposes.
It is interesting that the FDA recommendations explicitly acknowledge that “[B]ecause the focus of the model is on in vivo dissolution and absorption, it is appropriate to combine a mechanistic absorption model with a simplified disposition model for the prediction of systemic exposure following absorption.” In other words, the expectation is that the scope of the model would be the same, regardless of the application. So, even though the applicants are provided certain degrees of freedom in terms of components of the model, the central aim (dissolution and absorption) is well defined. An interesting, and relevant, analog exists in the domain of medical devices where regulatory agencies more pro-actively consider the use of computational models, albeit primarily as supporting evidence, in marketing applications [67], targeting once again the scope.
The challenge in defining a universal scope in QSP models
The analysis of recent FDA submissions points towards a substantial increase in the use of modeling tools, broadly falling under the “QSP” category, in submissions, starting with the first appearance in an application submitted in 2013, eventually reaching about 60 by 2020. The trend clearly indicates that QSP modeling is becoming increasingly more accepted by the broader community. The FDA recently concluded a scientific exchange and review of clinical drug applications in various therapeutic areas [68]. On the surface it may appear that the QSP challenges should be similar to PBPK and device applications, namely model development, calibration, and validation. However, the analysis revealed a rather complicated landscape and the review recognized: (a) the existence of a wide range of approaches for QSP model development, from de novo models to adaptation of existing ones; (b) the strategies and methods for calibration varied with the approach, endpoints, and type of data used, and (c) the methods and extent of model validation varied based on the scope of the model, and available data. Further, the analysis revealed interesting methodological ambiguities, and produced hard evidence and data justifying the conceptual uniqueness of QSP models that we articulated earlier:
The use of the term “QSP” does not correspond to a unique and well-defined modeling approach, but rather is used to encompass applications described by terms such as: mechanistic model, in silico model, systems biology, systems model, systems pharmacology, quantitative systems pharmacology, system toxicology, quantitative systems toxicology
The level of detail and nature of the identified “QSP” models varied substantially ranging from qualitative descriptions to detailed modeling and simulation results, and from simple models to complex networks.
In short, applications were strongly context-dependent and generally selected in light of the intended predictions to be made with the model and the data that were selected to be used. The approach and scope of QSP models remain highly variable, not to mention that even the exact definition of what constitutes a “QSP” model is not unique. One can argue that precise definitions of approach and scope are in order (i.e., what constitutes a QSP model, how it should be constructed, and what should it predict.) However, we run the risk that, although such exact definitions may lead to transparent, standardized, and assessable models, they may also substantially reduce flexibility and possibly curb innovation in an evolving field.
It is reasonable therefore to argue that a universal scope should not be defined in QSP. Instead of treating QSP as a ubiquitous tool, it may be more advantageous to identify areas of interest and align QSP approaches with those areas. So, maybe we should not be thinking of “the” QSP approach that will be applicable across the board, but rather a collection of QSP approaches tailored to a specific context.
QSP and the computational modeling continuum
Without a doubt, QSP applications are gaining increasing recognition in recent years [69, 70] driven by the desire to answer complex questions and time appears right: scientific, technological, and computational advances generate a wealth of information at a time when the pharmaceutical industry desires to improve its productivity [71]. QSP emerged as a framework to provide an integrated understanding of pathology in the context of therapeutic interventions aiming to integrate data on all conceivable scales. Due to the ambitious nature of the problems and the fact that QSP emerged asynchronously through efforts distributed across multiple organizations, a high degree of entropy characterizes the tools, methods, and approaches. At the same time, models have been primarily academic or developed within companies fit-for-(very specific) purposes. Like any new(er) technology, QSP created expectations and is progressively reaching the point where stakeholders anticipate results.
QSP faces multiple challenges: within the pharmaceutical industry QSP needs to demonstrate that it can reliably and consistently add value above and beyond what traditional pharmacometrics delivers, while regulatory agencies must identify consistent requirements for accepting complex mathematical and computational QSP models. The eventual adoption of QSP as information supporting decision making and regulatory applications, will require that two conditions are met: (1) increase confidence in the QSP framework; and (2) careful articulation of expectations. In this paper, we have argued that standardization will empower rigorous assessment of QSP models, thus increasing confidence, whereas clear articulation of context will focus the application domain, including the questions asked and the data used.
However, the key message we wish to convey is that QSP should not be treated as an approach orthogonal, or independent, of other modeling and computational activities. Doing so, requires that we redefine and initiate a de novo assessment. Instead, QSP should be considered part of the computational modeling continuum and invoked judiciously when considered to add value above and beyond what could possibly be accomplished with “simpler” models. Doing so will justify the uniqueness of a QSP model and its strong context-dependence and will enable the development of acceptable, case-dependent, assessment criteria.
Acknowledgements
IPA acknowledges support from NIH GM131800.
Footnotes
In (Hosseini, Feigelman et al. [61]) the environment was MATLAB whereas the models were assumed to be expressed in the form of standard ODEs.
References
- 1.Azer K, Kaddi CD, Barrett JS, Bai JPF, McQuade ST, Merrill NJ, Piccoli B, Neves-Zaph S, Marchetti L, Lombardo R, Parolo S, Immanuel SRC, Baliga NS (2021) History and future perspectives on the discipline of quantitative systems pharmacology modeling and its applications. Front Physiol 12:637999. 10.3389/fphys.2021.637999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae D (2020) Introduction to dynamical systems analysis in quantitative systems pharmacology: basic concepts and applications. Transl Clin Pharmacol 28(3):109–125. 10.12793/tcp.2020.28.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Androulakis IP (2016) Quantitative systems pharmacology: a framework for context. Curr Pharmacol Rep. 10.1007/s40495-016-0058-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao RT, Scherholz ML, Hartmanshenn C, Bae SA, Androulakis IP (2016) On the analysis of complex biological supply chains: from process systems engineering to quantitative systems pharmacology. Comput Chem Eng. 10.1016/j.compchemeng.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Androulakis IP (2015) Systems engineering meets quantitative systems pharmacology: from low-level targets to engaging the host defenses. Wiley Interdiscip Rev Syst Biol Med 7(3):101–112. 10.1002/wsbm.1294 [DOI] [PubMed] [Google Scholar]
- 6.Allerheiligen S, Abernethy D, Altman RB, Brouwer K, Califano A, David Z, D’argenio, Iyengar R, Jusko W, Lalonde R, Lauffenburger D, Shoichet B, Stevens J, Sorger P, Subramaniam S, Graaf PD, Vicini P, Ward RJ (2011) Quantitative and systems pharmacology in the post-genomic era : new approaches to discovering drugs and understanding therapeutic. In: An NIH White Paper by the QSP Workshop Group. [Google Scholar]
- 7.Vodovotz Y, An G, Androulakis IP (2013) A systems engineering perspective on homeostasis and disease. Front Bioeng Biotechnol 1:6. 10.3389/fbioe.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danhof M (2016) Systems pharmacology—towards the modeling of network interactions. Eur J Pharm Sci 94:4–14. 10.1016/j.ejps.2016.04.027 [DOI] [PubMed] [Google Scholar]
- 9.Kitano H (2010) Grand challenges in systems physiology. Front Physiol 1:3. 10.3389/fphys.2010.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Greef J, McBurney RN (2005) Rescuing drug discovery: in vivo systems pathology and systems pharmacology. Nat Rev Drug Discov 4(12):961–967. 10.1038/nrd1904 [DOI] [PubMed] [Google Scholar]
- 11.Knight-Schrijver VR, Chelliah V, Cucurull-Sanchez L, Le Novere N (2016) The promises of quantitative systems pharmacology modelling for drug development. Comput Struct Biotechnol J 14:363–370. 10.1016/j.csbj.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheff JD, Kamisoglu K, Androulakis IP (2016) Mechanistic modeling of inflammation. In: Mager DE, Kimko HHC (eds) Systems pharmacology and pharmacodynamics. Springer International Publishing, Cham, pp 325–352. 10.1007/978-3-319-44534-2_15 [DOI] [Google Scholar]
- 13.Ayyar VS, Jusko W (2020) Transitioning from basic towards systems pharmacodynamic models: lessons from corticosteroids. Pharmacol Rev 72(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison TM, Hariharan P, Funkhouser CM, Afshari P, Goodin M, Horner M (2019) Assessing computational model credibility using a risk-based framework: application to hemolysis in centrifugal blood pumps. ASAIO J 65(4):349–360. 10.1097/MAT.0000000000000996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanujan S, Chan JR, Friedrich CM, Thalhauser CJ (2019) A flexible approach for context-dependent assessment of quantitative systems pharmacology models. CPT Pharmacomet Syst Pharmacol 8(6):340–343. 10.1002/psp4.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross F, MacLeod M (2017) Prospects and problems for standardizing model validation in systems biology. Prog Biophys Mol Biol 129:3–12. 10.1016/j.pbiomolbio.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 17.Stadter P, Schalte Y, Schmiester L, Hasenauer J, Stapor PL (2021) Benchmarking of numerical integration methods for ODE models of biological systems. Sci Rep 11(1):2696. 10.1038/s41598-021-82196-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degasperi A, Fey D, Kholodenko BN (2017) Performance of objective functions and optimisation procedures for parameter estimation in system biology models. NPJ Syst Biol Appl 3:20. 10.1038/s41540-017-0023-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzia F, Cash JR, Soetaert K (2012) A test set for stiff initial value problem solvers in the open source software R: package deTestSet. J Comput Appl Math 236(16):4119–4131. 10.1016/j.cam.2012.03.014 [DOI] [Google Scholar]
- 20.Floudas CA, Pardalos PM, Adjiman CS, Esposito WR, Gumus ZH, Harding ST, Klepeis JL, Meyer CA, Schweiger CA (1999) Handbook of test problems in local and global optimization. Springer, Berlin. 10.1023/A:1008328212973 [DOI] [Google Scholar]
- 21.Geistlinger L, Csaba G, Santarelli M, Ramos M, Schiffer L, Turaga N, Law C, Davis S, Carey V, Morgan M, Zimmer R, Waldron L (2021) Toward a gold standard for benchmarking gene set enrichment analysis. Brief Bioinform 22(1):545–556. 10.1093/bib/bbz158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouzom F, Ball K, Perdaems N, Walther B (2012) Physiologically based pharmacokinetic (PBPK) modelling tools: how to fit with our needs? Biopharm Drug Dispos 33(2):55–71. 10.1002/bdd.1767 [DOI] [PubMed] [Google Scholar]
- 23.Derendorf H, Meibohm B (1999) Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm Res 16(2):176–185. 10.1023/A:1011907920641 [DOI] [PubMed] [Google Scholar]
- 24.Meibohm B, Derendorf H (1997) Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther 35(10):401–413 [PubMed] [Google Scholar]
- 25.Agoram B (2014) Evaluating systems pharmacology models is different from evaluating standard pharmacokinetic-pharmacodynamic models. CPT Pharmacomet Syst Pharmacol 3:e101. 10.1038/psp.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini I, Gajjala A, Bumbaca Yadav D, Sukumaran S, Ramanujan S, Paxson R, Gadkar K (2018) gPKPDSim: a SimBiology((R))-based GUI application for PKPD modeling in drug development. J Pharmacokinet Pharmacodyn 45(2):259–275. 10.1007/s10928-017-9562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diao L, Meibohm B (2015) Tools for predicting the PK/PD of therapeutic proteins. Expert Opin Drug Metab Toxicol 11(7):1115–1125. 10.1517/17425255.2015.1041917 [DOI] [PubMed] [Google Scholar]
- 28.Jusko WJ (2013) Moving from basic toward systems pharmacodynamic models. J Pharm Sci 102(9):2930–2940. 10.1002/jps.23590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mager DE, Wyska E, Jusko WJ (2003) Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 31(5):510–518. 10.1124/dmd.31.5.510 [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Matsuoka Y, Asai Y, Hsin KY, Kitano H (2011) Software for systems biology: from tools to integrated platforms. Nat Rev Genet 12(12):821–832. 10.1038/nrg3096 [DOI] [PubMed] [Google Scholar]
- 31.Machado D, Costa RS, Rocha M, Ferreira EC, Tidor B, Rocha I (2011) Modeling formalisms in systems biology. AMB Express 1(1):45. 10.1186/2191-0855-1-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadkar K, Kirouac DC, Mager DE, van der Graaf PH, Ramanujan S (2016) A six-stage workflow for robust application of systems pharmacology. CPT Pharmacomet Syst Pharmacol 5(5):235–249. 10.1002/psp4.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ermakov S, Schmidt BJ, Musante CJ, Thalhauser CJ (2019) A survey of software tool utilization and capabilities for quantitative systems pharmacology: what we have and what we need. CPT Pharmacomet Syst Pharmacol 8(2):62–76. 10.1002/psp4.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadkar K, Budha N, Baruch A, Davis JD, Fielder P, Ramanujan S (2014) A mechanistic systems pharmacology model for prediction of LDL cholesterol lowering by pcsk9 antagonism in human dyslipidemic populations. CPT Pharmacomet Syst Pharmacol 3:e149. 10.1038/psp.2014.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ming JE, Abrams RE, Bartlett DW, Tao M, Nguyen T, Surks H, Kudrycki K, Kadambi A, Friedrich CM, Djebli N, Goebel B, Koszycki A, Varshnaya M, Elassal J, Banerjee P, Sasiela WJ, Reed MJ, Barrett JS, Azer K (2017) A quantitative systems pharmacology platform to investigate the impact of alirocumab and cholesterol-lowering therapies on lipid profiles and plaque characteristics. Gene Regul Syst Biol 11:1177625017710941. 10.1177/1177625017710941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappalardo F, Musumeci S, Motta S (2008) Modeling immune system control of atherogenesis. Bioinformatics 24(15):1715–1721. 10.1093/bioinformatics/btn306 [DOI] [PubMed] [Google Scholar]
- 37.Gong C, Ruiz-Martinez A, Kimko H, Popel AS (2021) A spatial quantitative systems pharmacology platform spQSP-IO for simulations of tumor-immune interactions and effects of checkpoint inhibitor immunotherapy. Cancers (Basel). 10.3390/cancers13153751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrich CM (2016) A model qualification method for mechanistic physiological QSP models to support model-informed drug development. CPT: Pharmacomet Syst Pharmacol 5(2):43–53. 10.1002/psp4.12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirouac DC (2018) How do we “Validate” a QSP model? CPT Pharmacomet Syst Pharmacol 7(9):547–548. 10.1002/psp4.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee G, Park C, Ahn J (2019) Novel deep learning model for more accurate prediction of drug-drug interaction effects. BMC Bioinformatics 20(1):415. 10.1186/s12859-019-3013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guthrie NL, Carpenter J, Edwards KL, Appelbaum KJ, Dey S, Eisenberg DM, Katz DL, Berman MA (2019) Emergence of digital biomarkers to predict and modify treatment efficacy: machine learning study. BMJ Open 9(7):e030710. 10.1136/bmjopen-2019-030710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Sun L, Li W, Liu G, Tang Y (2018) In silico prediction of chemical toxicity for drug design using machine learning methods and structural alerts. Front Chem 6:30. 10.3389/fchem.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Wang G (2018) Machine learning based toxicity prediction: from chemical structural description to transcriptome analysis. Int J Mol Sci. 10.3390/ijms19082358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Engkvist O, Wang Y, Olivecrona M, Blaschke T (2018) The rise of deep learning in drug discovery. Drug Discov Today 23(6):1241–1250. 10.1016/j.drudis.2018.01.039 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Wong YS, Deng J, Anton C, Gabos S, Zhang W, Huang DY, Jin C (2016) Machine learning algorithms for mode-of-action classification in toxicity assessment. BioData Min 9:19. 10.1186/s13040-016-0098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McComb M, Bies R, Ramanathan M (2021) Machine learning in pharmacometrics: opportunities and challenges. Br J Clin Pharmacol. 10.1111/bcp.14801 [DOI] [PubMed] [Google Scholar]
- 47.Zhang T, Androulakis IP, Bonate P, Cheng L, Helikar T, Parikh J, Rackauckas C, Subramanian K, Cho CR, Working G (2022) Two heads are better than one: current landscape of integrating QSP and machine learning: an ISoP QSP SIG white paper by the working group on the integration of quantitative systems pharmacology and machine learning. J Pharmacokinet Pharmacodyn 49(1):5–18. 10.1007/s10928-022-09805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarou G, Chelliah V, Small BG, Walker M, van der Graaf PH, Kierzek AM (2020) Integration of omics data sources to inform mechanistic modeling of immune-oncology therapies: a tutorial for clinical pharmacologists. Clin Pharmacol Ther 107(4):858–870. 10.1002/cpt.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Putnins M, Campagne O, Mager DE, Androulakis IP (2022) From data to QSP models: a pipeline for using Boolean networks for hypothesis inference and dynamic model building. J Pharmacokinet Pharmacodyn. 10.1007/s10928-021-09797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian Z, Zame W, Fleuren L, Elbers P, van der Schaar M (2021) Integrating expert ODEs into neural ODEs: pharmacology and disease progression. Adv Neural Inf Process Syst 34:11364–83 [Google Scholar]
- 51.Uddin S, Khan A, Hossain ME, Moni MA (2019) Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform Decis Mak 19(1):281. 10.1186/s12911-019-1004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topp B, Trujillo ME, Sinha V (2019) Industrialization of quantitative systems pharmacology. CPT: Pharmacomet Syst Pharmacol 8(6):356–358. 10.1002/psp4.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gauthier J, Vincent AT, Charette SJ, Derome N (2019) A brief history of bioinformatics. Brief Bioinform 20(6):1981–1996. 10.1093/bib/bby063 [DOI] [PubMed] [Google Scholar]
- 54.Katayama T, Arakawa K, Nakao M, Ono K, Aoki-Kinoshita KF, Yamamoto Y, Yamaguchi A, Kawashima S, Chun HW, Aerts J, Aranda B, Barboza LH, Bonnal RJ, Bruskiewich R, Bryne JC, Fernandez JM, Funahashi A, Gordon PM, Goto N, Groscurth A, Gutteridge A, Holland R, Kano Y, Kawas EA, Kerhornou A, Kibukawa E, Kinjo AR, Kuhn M, Lapp H, Lehvaslaiho H, Nakamura H, Nakamura Y, Nishizawa T, Nobata C, Noguchi T, Oinn TM, Okamoto S, Owen S, Pafilis E, Pocock M, Prins P, Ranzinger R, Reisinger F, Salwinski L, Schreiber M, Senger M, Shigemoto Y, Standley DM, Sugawara H, Tashiro T, Trelles O, Vos RA, Wilkinson MD, York W, Zmasek CM, Asai K, Takagi T (2010) The DBCLS BioHackathon: standardization and interoperability for bioinformatics web services and workflows. The DBCLS BioHackathon Consortium*. J Biomed Semantics 1 (1):8. 10.1186/2041-1480-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Huang SM (2019) Commentary on fit-for-purpose models for regulatory applications. J Pharm Sci 108(1):18–20. 10.1016/j.xphs.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12(1):1–10. 10.1208/s12248-009-9155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cucurull-Sanchez L, Chappell MJ, Chelliah V, Amy Cheung SY, Derks G, Penney M, Phipps A, Malik-Sheriff RS, Timmis J, Tindall MJ, van der Graaf PH, Vicini P, Yates JWT (2019) Best practices to maximize the use and reuse of quantitative and systems pharmacology models: recommendations from the United Kingdom quantitative and systems pharmacology network. CPT Pharmacomet Syst Pharmacol 8(5):259–272. 10.1002/psp4.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duffull SB (2016) A philosophical framework for integrating systems pharmacology models into pharmacometrics. CPT: Pharmacomet Syst Pharmacol 5(12):649–655. 10.1002/psp4.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng Y, Thalhauser CJ, Smithline S, Pagidala J, Miladinov M, Vezina HE, Gupta M, Leil TA, Schmidt BJ (2017) QSP toolbox: computational implementation of integrated workflow components for deploying multi-scale mechanistic models. AAPS J 19(4):1002–1016. 10.1208/s12248-017-0100-x [DOI] [PubMed] [Google Scholar]
- 60.Drager A, Palsson BO (2014) Improving collaboration by standardization efforts in systems biology. Front Bioeng Biotechnol 2:61. 10.3389/fbioe.2014.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosseini I, Feigelman J, Gajjala A, Susilo M, Ramakrishnan V, Ramanujan S, Gadkar K (2020) gQSPSim: a SimBiology-based GUI for standardized QSP model development and application. CPT: Pharmacomet Syst Pharmacol 9(3):165–176. 10.1002/psp4.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helmlinger G, Sokolov V, Peskov K, Hallow KM, Kosinsky Y, Voronova V, Chu L, Yakovleva T, Azarov I, Kaschek D, Dolgun A, Schmidt H, Boulton DW, Penland RC (2019) Quantitative systems pharmacology: an exemplar model-building workflow with applications in cardiovascular, metabolic, and oncology drug development. CPT: Pharmacomet Syst Pharmacol 8(6):380–395. 10.1002/psp4.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson MC, Riggs MM (2015) FDA advisory meeting clinical pharmacology review utilizes a quantitative systems pharmacology (QSP) model: a watershed moment? CPT Pharmacomet Syst Pharmacol 4(3):e00020. 10.1002/psp4.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.FDA (2017) US FDA regulatory science priorities (FY 2017).
- 65.FDA (2011) Advancing regulatory science at FDA. A strategic plan. [Google Scholar]
- 66.FDA (2020) The use of physiologically based pharmacokinetic analyses — biopharmaceutics applications for oral drug product development, manufacturing changes, and controls. Guidance for Industry. [Google Scholar]
- 67.Morrison TM, Pathmanathan P, Adwan M, Margerrison E (2018) Advancing regulatory science with computational modeling for medical devices at the FDA’s office of science and engineering laboratories. Front Med (Lausanne) 5:241. 10.3389/fmed.2018.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai JPF, Schmidt BJ, Gadkar KG, Damian V, Earp JC, Friedrich C, van der Graaf PH, Madabushi R, Musante CJ, Naik K, Rogge M, Zhu H (2021) FDA-industry scientific exchange on assessing quantitative systems pharmacology models in clinical drug development: a meeting report, summary of challenges/gaps, and future perspective. AAPS J 23(3):60. 10.1208/s12248-021-00585-x [DOI] [PubMed] [Google Scholar]
- 69.Bradshaw EL, Spilker ME, Zang R, Bansal L, He H, Jones RDO, Le K, Penney M, Schuck E, Topp B, Tsai A, Xu C, Nijsen M, Chan JR (2019) Applications of quantitative systems pharmacology in model-informed drug discovery: perspective on impact and opportunities. CPT Pharmacomet Syst Pharmacol 8(11):777–791. 10.1002/psp4.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zineh I (2019) Quantitative systems pharmacology: a regulatory perspective on translation. CPT Pharmacomet Syst Pharmacol 8(6):336–339. 10.1002/psp4.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leil TA, Bertz R (2014) Quantitative systems pharmacology can reduce attrition and improve productivity in pharmaceutical research and development. Front Pharmacol 5:247. 10.3389/fphar.2014.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]


