Abstract
Background:
Compensatory aids can help mitigate the impact of progressive cognitive impairment on daily living.
Objective:
We evaluate whether the learning and sustained use of an Electronic Memory and Management Aid (EMMA) application can be augmented through a partnership with real-time, activity-aware transition-based prompting delivered by a smart home.
Methods:
Thirty-two adults who met criteria for amnestic mild cognitive impairment (aMCI) were randomized to learn to use the EMMA app on its own (N = 17) or when partnered with smart home prompting (N = 15). The four-week, five-session manualized EMMA training was conducted individually in participant homes by trained clinicians. Monthly questionnaires were completed by phone with trained personnel blind to study hypotheses. EMMA data metrics were collected continuously for four months. For the partnered condition, activity-aware prompting was on during training and post-training months 1 and 3, and off during post-training month 2.
Results:
The analyzed aMCI sample included 15 EMMA-only and 14 partnered. Compared to the EMMA-only condition, by week four of training, participants in the partnered condition were engaging with EMMA more times daily and using more basic and advanced features. These advantages were maintained throughout the post-training phase with less loss of EMMA app use over time. There was little differential impact of the intervention on self-report primary (everyday functioning, quality of life) and secondary (coping, satisfaction with life) outcomes.
Conclusion:
Activity-aware prompting technology enhanced acquisition, habit formation and long-term use of a digital device by individuals with aMCI. (ClinicalTrials.gov NCT03453554).
Keywords: Alzheimer’s disease, assistive technology, dementia, functional status, memory training, quality of life, real-time systems, reminder system, smart health aids, supervised machine learning
INTRODUCTION
Declining memory, attention, and executive abilities can negatively impact the initiation and accurate completion of daily activities (e.g., cooking, managing medications) in individuals with amnestic mild cognitive impairment (aMCI). To mitigate these effects, research has shown that individuals with aMCI can learn to use paper-pencil and electronic calendars and memory notebooks to assist with scheduling, planning, and carrying out daily activities independently (e.g., [1–4]). However, teaching a memory-impaired individual to use a memory notebook takes time and using the notebook is itself a memory exercise [5]. Developing a habit of consistently using the notebook is usually accomplished through reminder prompts, which can come from a care-partner, a time-based alarm, or mental link with an aspect of the individual’s routine (e.g., meals). If an individual with memory impairments does not over-learn the process of using the memory notebook, the use of the compensatory tool will be forgotten or additional burden placed on a care-partner to prompt for notebook use. In this pilot study, we evaluate whether partnering a digital memory notebook application with a smart home that delivers context-aware reminder prompts can enhance acquisition and longer-term use of the aid in individuals with aMCI.
Change in ability to efficiently and independently complete everyday tasks is associated with disease progression in individuals with aMCI, and recent work suggests compensation use can delay transition to dementia [6, 7]. Although a variety of technology-assisted tools are being recommended as compensatory aids for individuals with aMCI (e.g., Penultimate, Touch Calendar, Cozi), these aids are not being widely adopted by the growing population of older individuals with cognitive deficits. A study by Collerton and colleagues (2014) found that, although older individuals living in supported accommodations were curious about electronic aids, the aids were viewed as too complicated and not easily adapted to their needs [8]. Furthermore, assistance from others was necessary to prompt compensatory aid use. Other obstacles that have been found to reduce adoption of technology-assisted tools by older individuals include complex interfaces, small font, poor contrast, and lack of training support [9–12]. It has also been argued that technology-assisted tools should support multiple functions, such as initiation of activities, recall of appointments, organization, goal-setting, and self-monitoring of progress [13, 14].
To tackle some of these challenges, we created a mobile application (app) that builds on practice-standard rehabilitation intervention methods. The app was created through iterative participatory design with older individuals with cognitive impairment and runs on a tablet with a keyboard attachment [2, 15, 16]. In earlier papers, the app was referred to as the Digital Memory Notebook [2, 15, 16]. The app name has been changed to EMMA, for Electronic Memory and Management Aid. The EMMA app is a comprehensive, “all-in-one” organizational tool and memory aid. EMMA’s tablet interface includes large font, simple icons, text support, simple color contrast, and consistency across features, making it user-friendly and intuitive to use for individuals with cognitive impairment and limited comfort with technology [15]. Prior recorded information can also be easily located with search features. The app records usage metrics facilitating real-time tracking and use analysis. Furthermore, the utility of the EMMA app and the manual-based intervention for improving everyday functioning and life satisfaction is supported by our earlier case study work, which demonstrated positive post-intervention effects on daily functioning, coping, and life satisfaction in individuals with memory impairment [2].
Data indicate individuals with memory impairment can perform daily tasks more consistently when prompted [17–19]. For example, prompting technologies (either alone or when combined with another aid) have been shown to decrease errors in daily living activities and increase independence, activity engagement, and adherence to instructions in individuals with MCI and cognitive impairment [19–21]. Most prompting systems require reminder prompts to be manually scheduled, typically based on time [22, 23]. Although hourly time-based reminders can support use of electronic devices like EMMA, they are only practical for short periods due to the frequent interruptions that may become annoying and occur at inconvenient times. Furthermore, a time-based prompt delivered when the person is taking a nap or actively engaged in an activity will likely be ineffective and can contribute to resistance to their use. Similarly, a prompt delivered after the individual has already completed the task may result in frustration. Although location-based prompting provides some contextual input, researchers have found that incorporating information about the current activities people are engaged in reduces the limitations of prompting systems [21, 24, 25]. For example, compared to time-based prompting, activity-based context-aware prompting helped people remember to take their medications and increased treatment adherence [26, 27]. Similarly, activity-aware transition-based timing of queries resulted in more responses by older adults to the queries compared to random prompting [28]. Notably, it has been argued that to provide adaptive prompting support, devices must incorporate activity recognition and be context-aware [21, 29].
To enhance use of the EMMA app, we pair it with smart home technology that provides real-time, context-aware, transition-based prompting. Most applications of smart technologies for health assistance have focused on a narrow set of tasks or have been performed in controlled laboratory settings and do not couple smart environment contributions with other technological healthcare innovations [30–32]. Our smart home in a box (CASAS SHiB) collects ambient sensor data in the home while residents perform normal daily routines [33]. We have designed machine learning methods to model and recognize daily activities (e.g., cooking, grooming) in real-time from streaming sensor data [34, 35]. We have also designed methods to detect change points that represent transitions between activities, allowing us to boost activity recognition and deliver prompts to individuals at opportune times that do not interrupt critical tasks [28, 36]. Receiving prompts during activity transitions also reduces cognitive load and disruption to an ongoing task and an individual’s normal routine for an activity.
In this pilot clinical trial, we evaluated a partnership between EMMA and a smart home. Half of the study participants with aMCI were randomly assigned to learn to use the EMMA app alone, while the other half had their homes turned into smart homes and learned to use the EMMA app in partnership with prompting from the smart home. The EMMA training protocol uses a structured interactive format, which was modeled after early memory notebook work by Sohlberg and Mateer (1989), and includes didactics, skills training, goal setting, problem-solving, and supportive bonding with a clinician [37]. Clinicians worked individually with each study participant in their own home over a period of five to six sessions within a one-month time frame. The training manual used exercises and images with gradual instructions to assist individuals with aMCI in learning to use the app. All participants received several days of hourly time-based prompts following training session two to begin to create an overlearned habit of using the EMMA app. Participants in the partnered condition then received context-aware transition-based prompts to use the EMMA app after periods of non-use during the remainder of the EMMA training phase (weeks 1–4). For the post-training phase, prompting was left on in month one (weeks 5–8), turned off in month two (weeks 8–12), and then turned back on in month three (weeks 13–16).
For aMCI participants in the partnered condition, the automated activity-aware prompting was expected to improve use of EMMA by utilizing the individual’s own routine to provide context for delivery of prompts in the real-world setting. Throughout the training and post-training phases, the EMMA app continuously collected data metrics about use. We hypothesized that participants who learned to use the EMMA app with the assistance of the smart home prompts would learn to use the notebook more quickly and use it more often than those who did not have access to the smart prompting technology. We further hypothesized that removal of the smart home/EMMA partnership in month two post-training would result in decreased EMMA use and entries and that reinstating the smart home/EMMA partnership in month three post-training would again increase EMMA use. Because prompting was expected to support frequent and regular notebook use, thereby reducing everyday retrospective and prospective memory difficulties, we also hypothesized that we would see greater post-intervention improvements in self-report ratings of functional independence, quality of life, coping, and life satisfaction in the partnered compared to the EMMA-only condition.
METHODS
Participants
Participants were community dwelling older adults, age 50+, who met criteria for aMCI. Participants were recruited in eastern Washington and northern Idaho between June 2018 and October 2019 using a variety of methods, including physician referrals, brochures/flyers hung in public spaces (e.g., doctors’ offices, libraries), advertisements placed in media (e.g., newspaper, websites), and from a pool of participants involved in prior studies. Participants initially completed a clinical telephone interview. The telephone interview included a brief cognitive screen (i.e., Telephone Interview of Cognitive Status [TICS]) and questions about medical history to rule out potential participants who were likely to meet criteria for dementia or for whom cognitive difficulties could be the result of an alternate medical or psychiatric condition (e.g., head injury, schizophrenia, substance abuse) [38]. Individuals who were too cognitively impaired to give their own informed consent or did not speak English fluently were also excluded following the interview. Furthermore, to move forward to testing, participants needed to be aware of their memory difficulties, express a desire for treatment, and have adequate visual, motor and auditory acuity to allow for memory skills training.
Figure 1 illustrates the flow of individuals through the study. Seventy-six individuals were screened. Forty-four individuals were tested to determine whether they met diagnostic criteria for aMCI. aMCI was defined based on Petersen (2004) criteria and included: (a) self- or informant-report of memory complaint for 6 or more months, (b) objective cognitive impairment in the memory domain, taking into account estimated premorbid ability; observed scores generally fell 1.5 standard deviations below appropriate norms, (c) did not meet criteria for the Diagnostic and Statistical Manual of Mental Disorders Major Neurocognitive Disorder (DSM-5), and (d) absence of severe depression at start of intervention (GDS < 10) [39]. Following standardized testing, 9 of the 44 participants did not meet criteria for aMCI and 3 additional participants decided not to pursue the intervention after eligibility was established due to either hospitalization (N = 1), hectic schedule (N = 1), or a nearing move from the area (N = 1). Due to an unexpected delay with elements of the smart home, the first 5 eligible participants were assigned in block to the EMMA-only condition and the next 5 eligible participants to the partnered condition. With the exception these participants, following a simple randomization allocation sequence generated by a computerized random number generator, the study lab manager assigned each of the remaining 22 participants who agreed to participate to either learn to use the EMMA app on its own (N = 12) or when partnered with smart home prompting (N = 10). All testing and intervention sessions were completed within the homes of study participants. Participants were either not taking nootropic medication or were on a stable dose of such medication throughout the duration of the study. The study was reviewed and approved by the Washington State University Institutional Review Board.
Fig. 1.
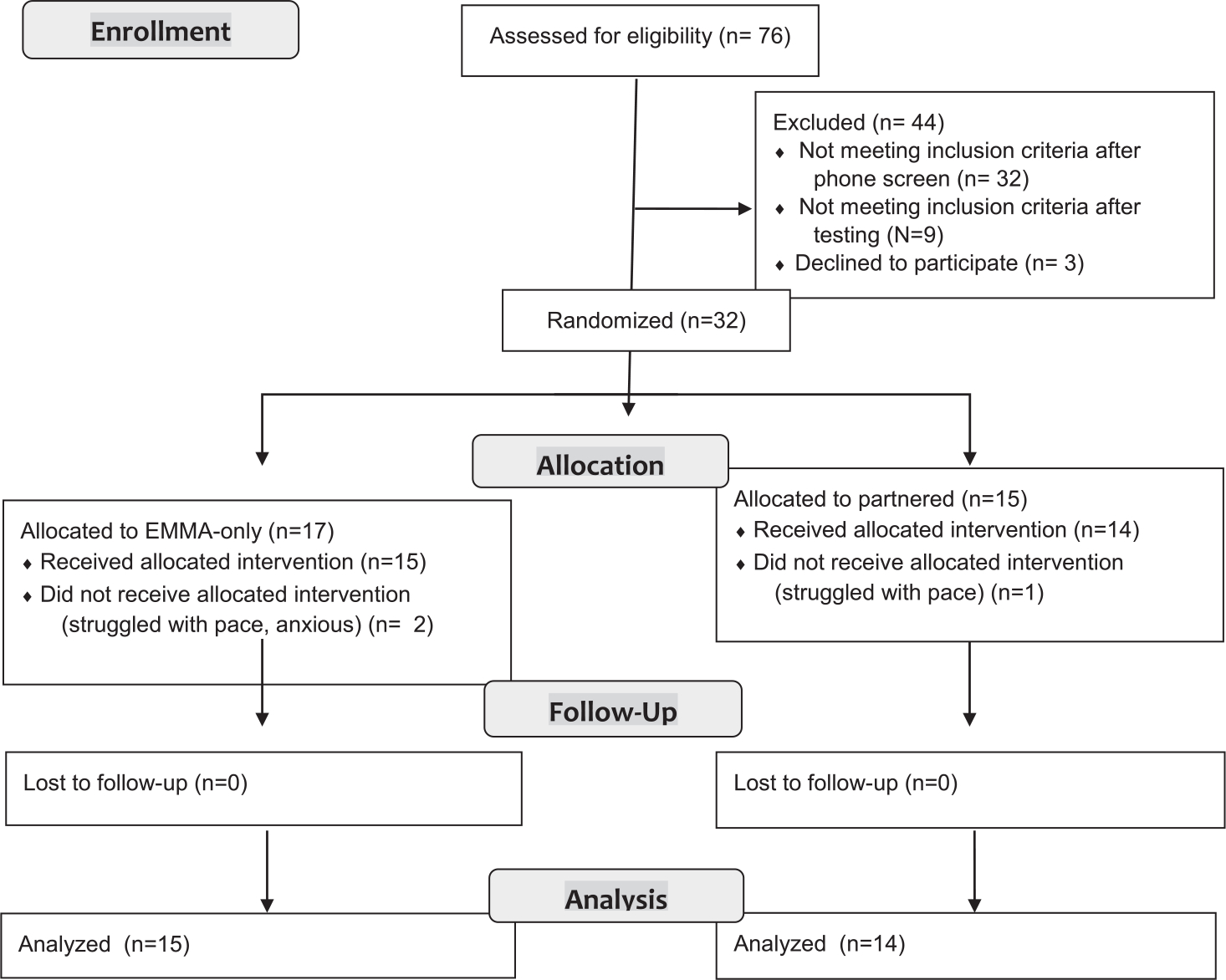
CONSORT Flow Diagram.
Measures
Cognitive assessment
Participants were administered the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), a screening tool that assesses five cognitive domains: Immediate Memory, Visuospatial/Constructional, Language, Attention, and Delayed Memory [40]. The RBANS provides a standard score for each cognitive domain and for global cognitive status. Premorbid ability level was estimated using the Weschler Test of Adult Reading [41]. Executive functioning was assessed with the Delis-Kaplan Executive Function System (DKEFS) Letter Fluency and Design Fluency subtests [42]. The Timed Up and Go task was administered as a mobility screen [43]. For a complete description of the administered measures, see Table 1. All measurement instruments have demonstrated reliability and validity [44].
Table 1.
Cognitive assessment battery
| Indices/Test | Description |
|---|---|
|
| |
| RBANS | |
| Immediate Memory | Subtests require immediate recall of 10 words repeated over 4 trials and a story containing two sentences repeated over 2 trials. |
| Visuospatial/Constmctional | Subtests require accurate copy of a figure and judgement of orientation of various lines. |
| Language | Subtests requires participants to name pictures of common figures and generate words belonging to a specified category for 60 seconds. |
| Attention | Subtests assess ability to repeat increasing strings of orally presented numbers and to decode various marks, by recording what number goes with each mark according to a key. |
| Delayed Memory | Subtests include delayed recall and recognition of a word list, and delayed recall of a story and a figure. |
| Total | This score is a global measure of overall cognitive status. |
| DKEFS | |
| Letter Fluency | Participants generate words starting with a given letter for 60 seconds. |
| Design Fluency | Participant are given 60 seconds to develop unique designs following specific rules and using only four straight lines. |
| WTAR | Participants read aloud 50 irregular words. |
| TUG | Participants rise from a chair, walk 10 feet at their normal pace, and then return to and sit down at the chair they started in. |
RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; DKEFS, Delis-Kaplan Executive Function System; WTAR, Weschler Test of Adult Reading; TUG, Timed “Up and Go” task.
Scheduling tools and technology use
The 5-item Scheduling Tool Use Questionnaire (STUQ) was developed for this study (Cronbach’s α = 0.93) and assesses participants’ agreement with statements related to the use of scheduling tools and reminders (e.g., “I feel that scheduling tools and reminders help me stay organized”). Questions are rated on a Likert rating scale from 1 (“Strongly Disagree”) to 7 (“Strongly Agree”), with higher scores indicating more positive perceptions of scheduling tools. The 5-item Technology Comfort Questionnaire (TCQ) was administered to examine technology self-efficacy [45]. Participants rate statements related to technology use (e.g., “I am generally comfortable with technology”) on a Likert Scale from 1 (“Strongly Disagree”) to 7 (“Strongly Agree”). Higher scores on the TCQ (Cronbach’s α = 0.88) reflect greater technology comfort.
Primary outcomes: EMMA data metrics
Primary outcome measures included daily metrics captured by the EMMA app. For data analyses, the daily data metrics were averaged across each of the 16 weeks of the study.
Distinct uses.
This variable represents the number of different times each day that the participant interacted with the EMMA app. At least 5 minutes of no interaction had to occur for an interaction with EMMA to be considered a distinct use.
Basic uses.
This variable represents user interactions with aspects of the EMMA app that would be considered basic use features. These included interactions (i.e., create, view, edit, and delete) participants engaged in with functions related to the to-do list, calendar, note and journal sections, help, event scheduling, profile page and when checking off completed events.
Advanced uses.
This variable represents interactions with aspects of the EMMA app that would be considered advanced features. These included using the search and picture features, setting reminders and high priority events, and setting repeated events.
Primary outcomes: Self-report measures
Quality of Life – Alzheimer’s Disease (QoL-AD).
The QoL-AD is a 13-item measure that asks participants to rate different aspects of their life (e.g., physical health, memory) on a Likert scale from 1 (“poor”) to 4 (“excellent”), with higher scores reflecting better quality of life [46]. While the QoL-AD measure has been used in several MCI intervention studies [1].
Instrumental Activities of Daly Living – Compensation (IADL-C).
The IADL-C is a 27-item self-report measure of everyday functioning [47]. Respondents rate the degree to which they can accomplish various IADLs on an 8-point Likert scale ranging from 1 (“independent, no aid”) to 8 (“cannot complete this activity anymore”). Higher scores on this measure reflect greater difficulty with completing everyday tasks. To reduce the potential for negatively penalizing participants for using an aid as a result of the intervention to improve everyday functioning, the scale was recoded from 1–7, with a “1” being coded for both “independent, no aid” and “independent; use and aid to assist”. The IADL-C can differentiate healthy older adults from older adults with MCI, has good internal consistency (Rasch reliability 0.80–0.93), and exhibits test-retest reliability (Spearman Coefficient 0.91) [47].
Secondary outcomes
These measures assess more specific aspects related to quality of life.
Coping Self-Efficacy Scale (CSE).
The CSE is a 13-item measure in which respondents’ rate how confident they are that they can do things to cope when they are having problems [48]. Ratings are on an 11-point Likert scale, ranging from 0 (“cannot do at all”) to 10 (“certain can do”), with higher scores reflect greater self-efficacy in coping abilities. Internal consistency and test-retest reliability are strong [48].
Satisfaction with Life Scale (SWLS).
The five-item SWLS asks participants to rate their agreement with questions that address satisfaction with life (e.g., “the conditions of my life are excellent”) on a 7-point Likert scale, ranging from 1 (“extremely dissatisfied”) to 7 (“extremely satisfied”) [49]. The SWLS has been shown to be a sensitive measure for detecting change in life satisfaction following an intervention and has demonstrated good test-retest reliability (0.85) [50].
Procedure
Table 2 provides a timeline of methods and data collection for participants in both conditions. Clinical testing to establish eligibility utilized a battery of cognitive and motor tests along with questionnaires (see Cognitive Assessment section) that took approximately 1.5 to 2 hours to complete. The clinical battery was re-administered approximately 5 months later to assess for changes in cognition across time. Testers who collected the clinical assessment data were blind to study hypotheses and condition. After completing the initial clinical assessment, participants who met study eligibility, agreed to participate, and were allocated to the partnered condition had their homes turned into smart homes using our smart home in a box (SHiB) [33, 51]. Ambient sensors were installed throughout the house, including narrow and wide-area infrared motion, magnetic (door) contact, light level, and temperature level sensors. These sensors provide information about movement throughout the home, opening and closing of doors and cabinets, and heat and light level changes.
Table 2.
Timeline of Data Collection Methods and Intervention Techniques
| Data Collection Events and Time Points | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Assessments Completed | To qualify | Pre-training | Post-training | Post-training Month 1 |
Post-training Month 2 |
Post-training Month 3 |
| (Day 1) | (Day 30) | (Day 60) | (Day 90) | (Day 120) | (Day 150) | |
| Cognitive Assessment (Baseline) | Cognitive Assessment (End of Intervention) | |||||
| Questionnaires | Questionnaires | Questionnaires | Questionnaires | Questionnaires | Questionnaires | |
|
| ||||||
| EMMA Data Metrics Collection | Training | Post-training Phase |
Post-training Phase |
Post-training Phase |
||
| Month 1 | Month 2 | Month 3 | ||||
| (Weeks 1–4) daily data | (Weeks 5–8) daily data | (Weeks 9–12) daily data | (Weeks 13–16) daily data | |||
|
| ||||||
| Partnered Condition | Smart home learning of activity models for one-month pre-training | EMMA training with prompt support (on after Session 3) | EMMA use with prompt support | EMMA use only | EMMA use with prompt support | |
|
| ||||||
| EMMA-only Condition | EMMA training | EMMA use only | EMMA use only | EMMA use only | ||
EMMA, Electronic Memory and Management Aid; EMMA data metrics documented continuously throughout weeks 1–16.
To provide the smart home time to capture activity level, learn participant routines, and provide accurate labels for everyday behaviors (e.g., cooking, grooming), one month of data monitoring occurred prior to the start of EMMA training. We used our activity recognition (CASAS-AR) and discovery (CASAS-AD) machine learning algorithms to recognize activities in real-time and provide context for prompting EMMA use [52, 53]. In addition to activities that were automatically discovered and then tracked, we monitored the following 32 predefined activities: enter home, personal hygiene, cook (breakfast, lunch, dinner), eat (breakfast, lunch, dinner), exercise, hydrate, wash dishes (breakfast, lunch, dinner), relax, read, phone, take medicine, watch TV, housekeeping, entertain guests, toilet, work at desk, work at table, leave home, bed toilet transitions, bathe, shower, sleep in bed, nap, work on computer, play music, and dress. We also used our activity forecasting (CASAS-AP) and transition-detection algorithms to provide prompts at opportune times (i.e., during activity transitions) [54–56]. No video or audio data was collected. Data were encrypted before being transmitted over a network and stored in a password-protected database to protect privacy. Similar to participants in the partnered condition, participants in the EMMA-only condition also waited one month after clinical testing to begin EMMA training, but no monitoring occurred.
After one month, participants in both the EMMA-only and partnered conditions received the same manualized training in EMMA use. Table 3 provides a description of the EMMA training sessions and their purposes. Each training session was led by a trained clinician who was in a clinical psychology doctoral program. The sessions were manualized and a workbook was created for study participants. Each clinician read the clinician manual, reviewed the workbook, which was created for study participants, and participated in a two-hour training session. Treatment administration was closely supervised by a licensed psychologist and experienced doctoral students, and included videotape review to monitor fidelity of content and process.
Table 3.
Digital Memory Notebook Training
| Session 1 (Anticipation) |
| Foundational session that introduces the EMMA app features and format of intervention sessions; discusses basic tablet technology (i.e., charging); provides psychoeducation about memory; and identifies participants everyday struggles to help tailor specific uses of EMMA. Participants also learn to use the Profile page; select a prime central location for EMMA in home (i.e., near outlet, easily accessible); and develop behavioral routines to help keep EMMA with them when leave house and to put EMMA back in central location when return. |
| Session 2 (Acquisition) |
| Focus is on using the EMMA app to support retrospective memory; learn to create detailed entries in the Time Schedule on the Today page to aid recall of past events; develop behavioral routines to establish habitual use of EMMA, including the use of hourly training alarms. Problem-solve ways to minimize stumbling blocks to EMMA app use (e.g., forget to use it). |
| Session 3 (Application) |
| Focus is on using EMMA app to support prospective memory and daily activity completion through time management; learn to schedule upcoming events/appointments; effectively use to-do list; make appointment with oneself; mark events as ‘High Priority’; and set alarms for upcoming events. Problem-solve plan for managing daily tasks by generating daily ‘to do’ list. |
| Session 4 (Adaptation) |
| Teaches expanded utility and individualization of EMMA through Notes pages; typical uses of Notes pages (e.g., pill regime, shopping list); how to create effective and organized notes and to use the journaling feature; and problem-solve how to develop a plan for using the Notes page to complete a project. Also teaches how to identify and reschedule unfinished activities. |
| Session 5 (Adaptation) |
| Focus is on maintaining consistent use of EMMA; reviews motivational features of the app and other motivating strategies to support long-term use; teaches methods for using the EMMA app to support long-term goals. This session also helps participants problem-solve how to use the app to support adoption of a positive brain health behavior. |
| Session 6 |
| Only for participants who want additional training, during which EMMA functions and features are reviewed; participant specific questions answered; and relevant exercises to bolster comfort with EMMA features and functions completed. Session 6 lasts between 1—1.5 hours and is scheduled in the same week as session 5 (i.e., week 4 of the intervention period). |
EMMA training was designed to be a four-week, five session training intervention with the first two sessions occurring within the same week and subsequent sessions spread one week apart. Three study participants (2 EMMA-only; 1 partnered) required a sixth session to fully cover all material (also completed within the 4-week time frame). Each training session maintained the following format: 1) review of prior lesson, homework, and discussion of any difficulties the participant may have experienced; 2) overview of the topic for the current lesson 3) didactics and exercises for the current lesson’s concepts; and 4) goal setting, planning, and problem solving for assignments to be completed before the next session. The design of the EMMA intervention was modeled after early work by Sohlberg and Mateer (1989) and additional details can be found in Chudoba et al. (2019) [2, 37].
To help participants begin to establish an overlearned habit of looking at and using the EMMA app., all participants received hourly training alarms during waking hours (agreed upon with each study participant) on the iOS tablet for several days between training Sessions 2 and 3. Participants were told to respond to each hourly alarm by entering information into the Time Schedule about recent activities. The following prompt appeared on the tablet: “Please add recently completed activities into your Time Schedule”. The alarm (i.e., a 30-s ringtone) was repeated every two minutes for up to 10 minutes if the participant did not respond. Along with habit formation, this activity was designed to create a chronicle of past events that the participants could refer to later to assist with retrospective memory difficulties.
Following Session 3, participants in the partnered condition received context-aware transition-based prompts reminding them to use the EMMA app for the remainder of the one-month duration of training. Specifically, after one-hour of EMMA app non-use, participants were prompted to use the EMMA app at the next activity transition point as detected by the smart home algorithm or after 30 additional minutes of nonuse, whichever came first. (Note: Initially our plan was to prompt at every activity transition. However, this resulted in a very high prompting rate for one of our first study participants causing us to add the 60 minutes of nonuse prior to identifying an activity transition point. Furthermore, in multiple resident homes, the detected transition point between activities may have been associated with the behavior of another house resident (see Table 4). The prompt to use the EMMA app (“Is now a good time to use your ‘EMMA’ app”) was delivered on the iOS tablet and was preceded by an alarm (i.e., a 3 sec chime). The alarm was repeated every two minutes for up to 10 minutes if the participant did not respond to the prompt. In contrast, participants in the EMMA-only condition had to rely on more traditional methods to cue use of EMMA (e.g., post-it notes, care-partner cueing).
Table 4.
Demographic Data for the Partnered and EMMA-only conditions (standard deviations in parentheses)
| Partnered (N =14) |
EMMA-only (N = 15) |
t statistic | Cohen’s d | |
|---|---|---|---|---|
|
| ||||
| Age (y) | 74.4 (5.6) | 70.6 (6.3) | 1.71 | 0.64 |
| Education (y) | 17.1 (2.2) | 15.6 (2.0) | 1.89 | 0.71 |
| % Female | 71% | 33% | ||
| % White/Not Hispanic or Latino | 100% | 100% | ||
| % living alone | 50% | 27% | ||
| TICS total score | 32.4 (3.0) | 32.4 (3.1) | −0.04 | 0.02 |
| 15-item GDS | 4.0 (3.3) | 3.2 (2.2) | 0.79 | 0.29 |
| Scheduling Tool Usea | 6.6 (0.5) | 6.0 (1.3) | 1.50 | 0.61 |
| Technology Comforta | 3.6 (1.0) | 4.7 (1.2)^ | −2.57* | 0.99 |
EMMA, Electronic Memory and Management Aid; TICS, Telephone Interview of Cognitive Status, lower values indicate more cognitive impairment; GDS, Geriatric Depression Scale.
Higher values indicate greater comfort with or perceptions of tool.
N = 13.
p < 0.05
Following training (weeks 1–4), there were three additional post-training months of monitoring. For participants in the partnered condition, the smart home prompting was left on during post-training months 1 (weeks 5–8) and 3 (weeks 13–16) and turned off during post-training month 2 (weeks 9–12). Participants (and care-partners when available; data not described here) completed the self-report questionnaire measures described in the Methods section at clinical testing and at one-month intervals throughout the 5-month duration of the study via phone with research personnel. EMMA data metrics were captured throughout the training and post-training months. The number of training alarms delivered and responded to between Sessions 2 and 3 by participants in both conditions, as well as the number of smart-home prompts delivered and responded to by participants in the partnered condition, were also recorded.
Statistical analyses
This pilot clinical trial was powered for 28 participants to provide 80% power to detect a clinically meaningful, medium effect size (f = 0.28) for a condition by time interaction at p < 0.05 (critical F = 4.22) in cases with two time points, with greater power for analyses that involved more than two within-subject time points. To examine the success of randomization, t-tests and/or chi-squared analysis were used to assess for differences in the demographic and cognitive performance data of the EMMA-only and partnered conditions at the start of the study. T-tests were also used to compare the total number of hourly training alarms received between Sessions 2 and 3 by participants in the EMMA-only and partnered conditions. As there was an early issue with data capture for this variable, training alarm data was available for nine participants in the partnered and eight in the EMMA-only condition. A repeated measures ANOVA was used to examine the average number of daily smart home prompts delivered to participants in the partnered condition across the 16 weeks that EMMA data metrics were gathered.
Due to a connection issue, collection of EMMA data metrics (i.e., distinct, basic, and advanced uses) was delayed one day for two participants and several days for a third participant. For these participants, EMMA data metric values were replaced by the mean for all participants given that training was just beginning and most participants were performing similarly. A mixed-model analysis of variance (ANOVA) was run separately for each of the three EMMA data metrics with condition (EMMA-only, partnered) as the between subjects factor and time (weeks 1–16) as the within subjects factor. Because the EMMA data metrics were not normally distributed, a square root transformation was applied prior to analyses. In addition, when the condition of sphericity was not met, we checked to determine whether the more conservative Greenhouse-Geisser correction was significant. In all cases it was also significant, suggesting no increased risk of type 1 error, therefore, we report the standard univariate analysis data for all analyses [57].
For each of the self-reported primary (i.e., QOL-AD, IADL-C) and secondary (i.e., CSES, SWLS) outcome variables, condition (EMMA-only, partnered) by time (pre-training, post-training, post-training month 1, post-training month 3) ANOVAs were conducted. As there were no differences between self-report data collected at baseline cognitive testing and prior to training, ts < 1.10, ps > 0.29, we used the data point most proximal to the start of training in these analyses. We also removed time 5 questionnaire data collection from the analyses as time 5 data collection followed the post-training month (month 2) when we removed the smart home partnership by turning off the context-aware transition-based prompting. There were very few missing self-report (i.e., questionnaires) data collection points (3 total). These occurred due to an inability to schedule a phone call with the study participant close to the data collection time point. Linear interpolation was used to replace the questionnaire missing values. Finally, to examine for changes in cognition, a condition (EMMA-only, partnered) by time (baseline, end of intervention) ANOVA was conducted. For one participant, the end of intervention cognitive data had to be gathered remotely due to COVID-19 and, therefore, this participant could not complete all cognitive tests. Given the small sample size and pilot aspects of this work, a p-value < 0.05 was used to determine significance for all analyses. Effect sizes are included with the analyses so the reader can better judge the practical significance of the findings.
RESULTS
Program feasibility and acceptance
A total of three participants dropped from the intervention in consultation with the training clinician after completing two training sessions. All three participants struggled with the pace of the intervention. One participant became anxious and the other two appeared to lack motivation, being encouraged primarily by the care-partner to participate. Examination of cognitive testing data revealed that all three participants exhibited no delayed recall on the RBANS list learning subtest, suggesting that they were experiencing significant difficulty retaining new information across time. However, two additional participants who scored zero on the list learning delayed recall test successfully completed training. The final analyzed sample consisted of 29 participants (EMMA-only: n = 15; partnered: n = 14). All 29 participants successfully completed the sequence of training sessions within the one-month time frame. The smart home sensors remained in the homes of those in the partnered condition throughout the duration of the intervention without any significant issues.
Participant information and randomization tests
As seen in Table 4, which provides the means, standard deviations, t-test statistics, and Cohen’s d data, t-tests revealed that the age and education of study participants did not differ across conditions. A chi-square analysis revealed that there was a higher percentage of females in the partnered condition than in the EMMA-only condition, χ2 (1, N = 29) = 4.21, p = 0.04. The sample was entirely white and not Hispanic or Latino. There were no differences between conditions on a screening measure of global cognitive status (TICS), with mean scores for both conditions falling in the ambiguous interpretive range. The conditions also did not differ in self-reported symptoms of depression (GDS), with mean scores for both conditions considered normal. Participants in both conditions endorsed high levels of confidence in and use of scheduling tools (i.e., agree to strongly agree). Participants in the EMMA-only condition endorsed significantly higher confidence and comfort with technology (somewhat agree) than participants in the partnered condition (neutral). This finding was no longer significant after controlling for sex, F = 3.15, p = 0.09, Cohen’s d = 0.65.
As seen in Table 5, cognitive testing indicated that the estimated premorbid IQ for participants in both conditions fell within the High Average range and did not differ significantly. Furthermore, there were no differences between conditions on any of the cognitive measures. Consistent with participants meeting criteria for aMCI, the RBANS immediate and delayed memory composites fell within the Low Average range for both conditions. Performances on the remaining RBANS composite scores and executive measures fell within the Average range for both conditions.
Table 5.
Baseline Cognitive Data for the Partnered and EMMA-only conditions (standard deviations in parentheses)
| Partnered (N = 14) |
EMMA-only (N = 15) |
t statistic | Cohen’s d | |
|---|---|---|---|---|
|
| ||||
| WTAR efsiq | 112.9 (11.6) | 110.3 (9.6) | 0.66 | 0.24 |
| RBANS | ||||
| Immediate Memory | 86.1 (14.8) | 83.3 (14.8) | 0.51 | 0.19 |
| Visuospatial | 98.6 (21.1) | 101.7 (17.0) | −0.44 | 0.16 |
| Language | 98.4 (10.9) | 90.4 (14.5) | 1.67 | 0.62 |
| Attention | 98.5 (21.4) | 93.6 (15.6) | 0.71 | 0.26 |
| Delayed Memory | 82.9 (17.5) | 84.4 (17.5) | −0.23 | 0.09 |
| Total | 90.4 (13.7) | 87.4 (12.7) | 0.62 | 0.23 |
| DKEFS | ||||
| Letter Fluency SS | 11.6 (3.5) | 10.5 (2.9) | 0.98 | 0.34 |
| Design Fluency SS | 10.7 (2.8) | 10.7 (2.7) | 0.04 | 0.00 |
| Timed Up and Go (s) | 10.4 (2.0) | 11.1 (2.7) | −0.88 | 0.29 |
EMMA, Electronic Memory and Management Aid; WTAR, Weschler Test of Adult Reading; efsiq, estimated full scale intelligence quotient; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; DKEFS, Delis-Kaplan Executive Function System; SS, Standard Score.
Training alarms
There was no difference between the total number of hourly training alarms received between Sessions 2 and 3 by participants in the partnered condition (M = 41.55, SD = 18.70, range = 18–62) compared to the EMMA-only condition (M = 31.13, SD = 15.06, range = 15–58), t(15) = 1.26, p = 0.23, Cohen’s d = 0.61. Similarly, percent response rate to the training alarms did not differ between the partnered (70%) and EMMA-only (61%) conditions, t(15) = 0.96, p = 0.35, Cohen’s d = 0.46.
Smart home prompting
Figure 2 shows the mean number of daily context-aware transition-based smart home prompts received by participants in the partnered condition along with the mean number of daily prompts responded to across the 16 weeks. There was a significant change across time in daily prompts delivered, F(15, 195) = 5.47, p < 0.001, η2 = 0.30. As expected, prompting was near zero during the first week of training and during weeks 9–12, as smart home prompting did not start until Session 3 and was turned off during post-testing month 2. As months (28–31 days) dictated questionnaire collection and when prompts were turned on and off, there was some bleed over of prompts as plotted by weeks into weeks 9 and 10 and a ramp up of prompting again in weeks 13 and 14. Participants in the partnered condition received an average of 3.27 daily prompts (SD = 2.82, range = 0.29–11.14, median = 2.69) during the last two weeks of the training phase (weeks 3 and 4). The average daily prompts delivered during post-training month 1 (weeks 5–8) was 4.26 (SD = 3.19, range = 0.39–12.14, median = 3.34) and during the last 2 weeks of post-training month 3 (weeks 15 and 16) was 3.29 (SD = 2.92, range = 0–9.93, median = 2.82). With the exception of one person, there was no prompting the last two weeks (weeks 11–12) of post-training month 2 (M = 0.07, SD = 0.26, range = 0–0.96, median = 0). The percentage of weekly context-aware transition-based smart home prompts that participants successfully responded to within 10 minutes across the 16 weeks ranged from 63% to 77% (see Fig. 2).
Fig. 2.
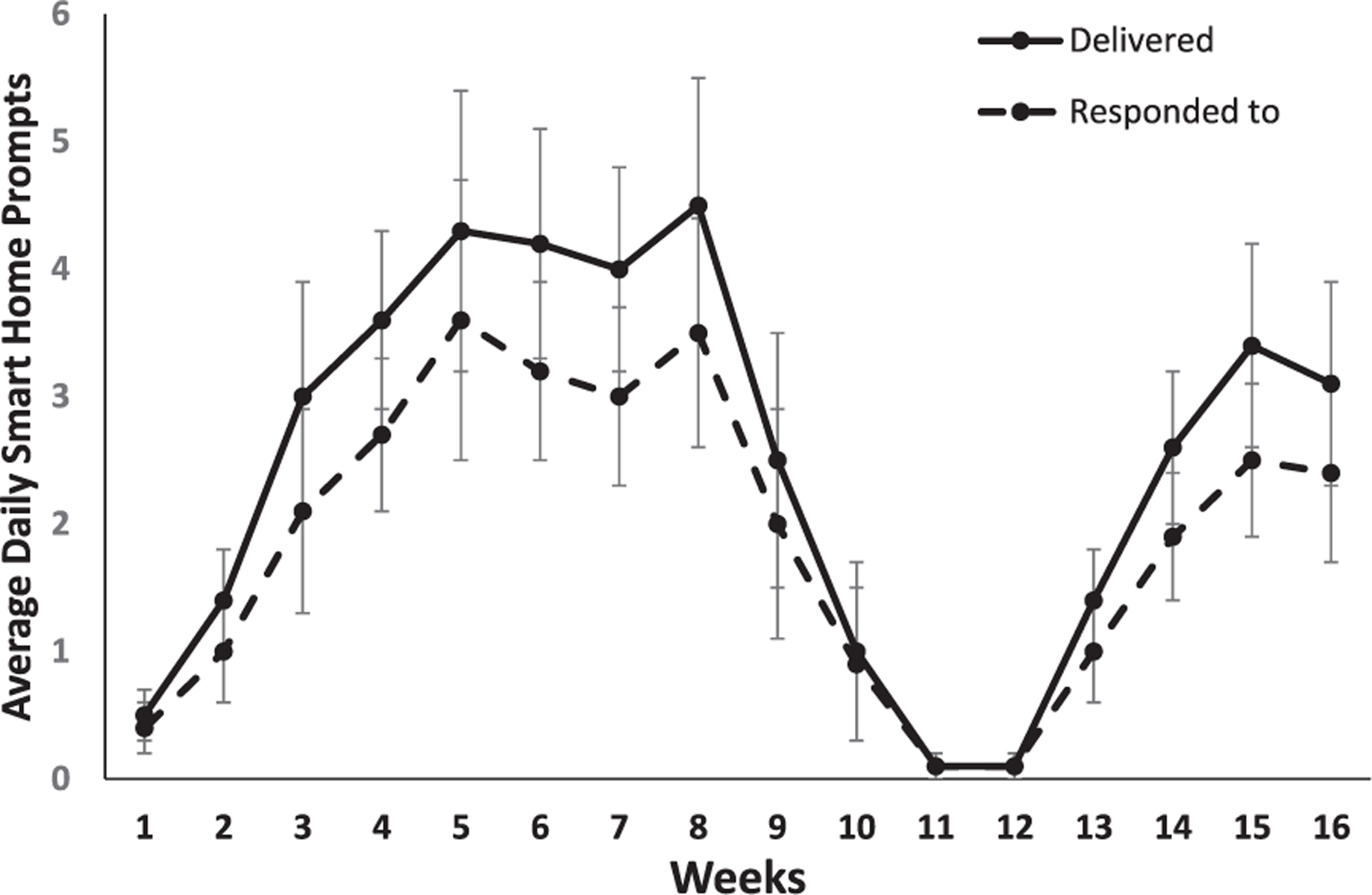
Mean number of daily context-aware transition-based smart home prompts received and responded to by participants in the partnered condition plotted by week (error bars represent standard error). Training = weeks 1–4; post-training: month 1 = weeks 5–8 (prompts on); month 2 = 9–12 (prompts off); month 3 = 13–16 (prompts on).
Primary outcomes
EMMA data metrics
Figures 3 and 4 present graphs showing the pattern of data for distinct, basic, and advanced uses of the EMMA app for both the EMMA-only and partnered conditions plotted using the mean rather than the transformed data for ease of interpretation. All significant main effects and interactions remained significant when sex was treated as a covariate.
Fig. 3.
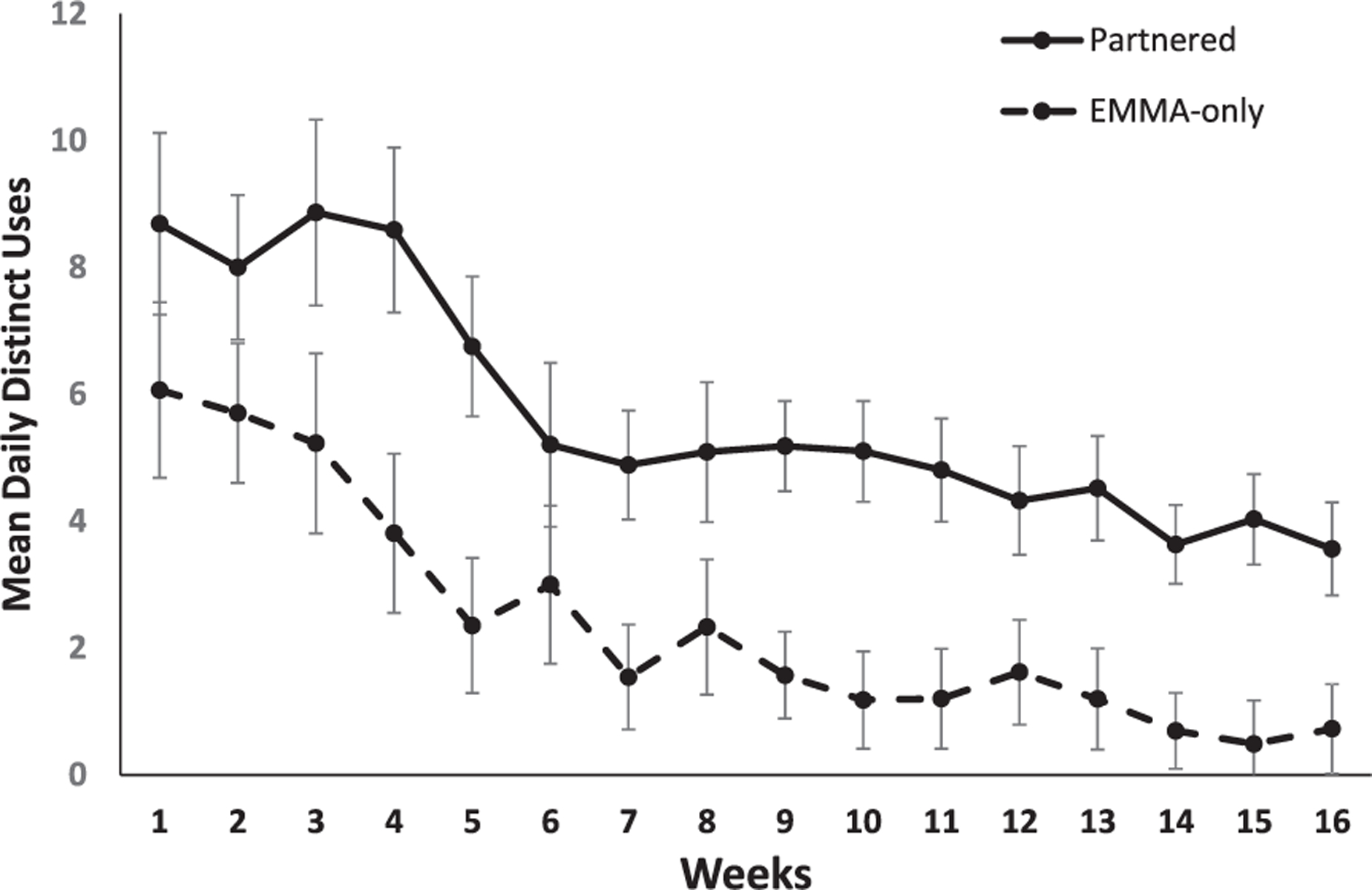
Mean daily distinct uses of the EMMA app plotted as a function of weeks (x-axis) for participants in the partnered (solid) and EMMA-only (dotted) conditions (error bars represent standard error). Training = weeks 1–4; post-training: month 1 = weeks 5–8 (prompts on); month 2 = 9–12 (prompts off); month 3 = 13–16 (prompts on).
Fig. 4.
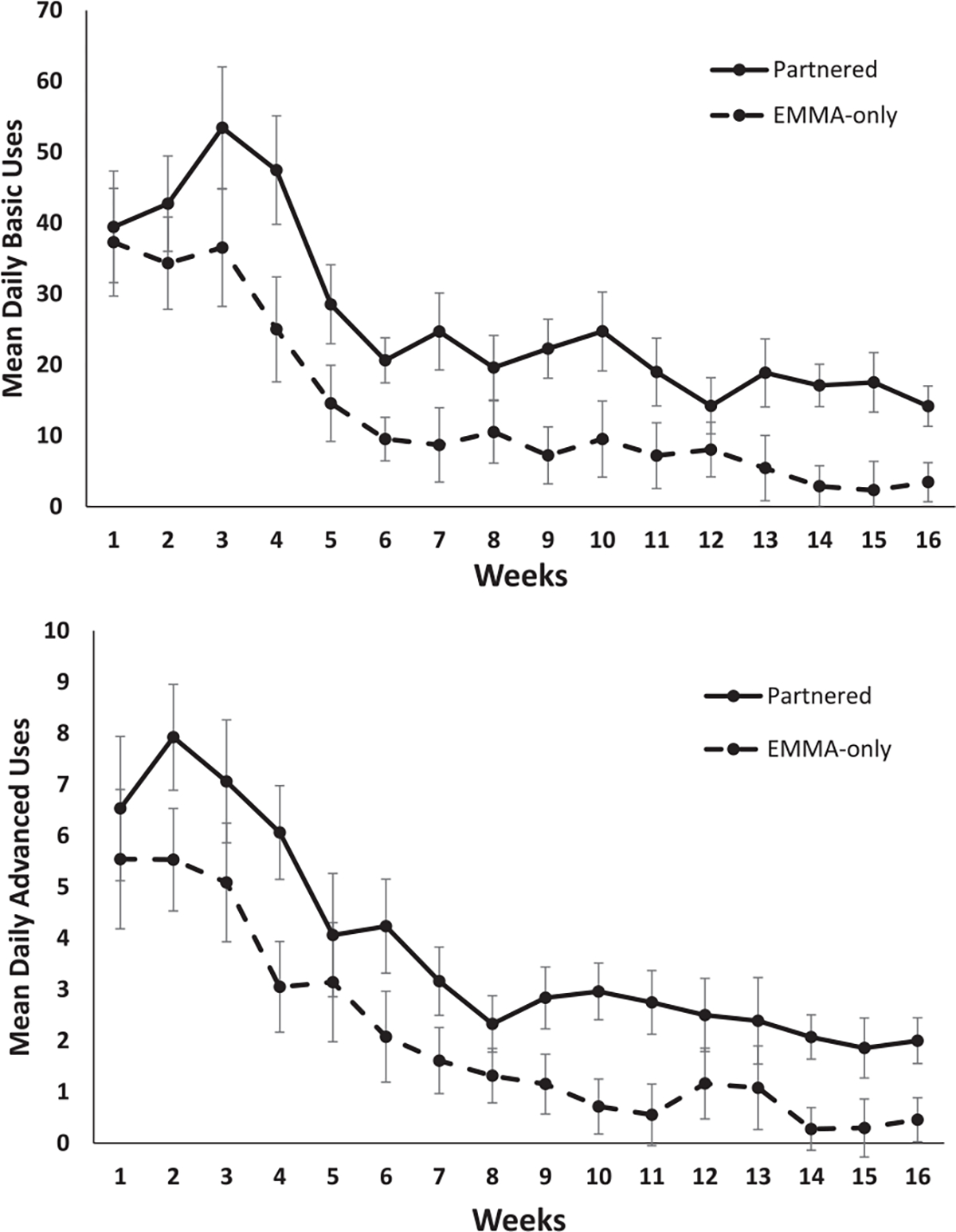
Mean daily for basic (top) and advanced (bottom) uses of the EMMA app plotted as a function of weeks (x-axis) for participants in the partnered (solid) and EMMA-only (dotted) conditions (error bars represent standard error). Training = weeks 1–4; post-training: month 1 = weeks 5–8 (prompts on); month 2 = 9–12 (prompts off); month 3 = 13–16 (prompts on).
EMMA distinct uses
The ANOVA indicated that aMCI participants in the partnered condition used the EMMA app more times daily than participants in the EMMA-only condition, F(1, 27) = 17.04, MSE = 0.58, p < 0.001, η2 = 0.39. There was also a significant main effect of time, F(15, 405)=25.65, MSE = 0.32, p < 0.001, η2 = 0.49, that was modified by a significant condition by time interaction, F(15, 405) = 2.65, MSE = 0.32, p = 0.001, η2 = 0.09. As can be seen in Fig. 3, breakdown of the interaction showed that number of average daily uses of the EMMA app did not differ between the EMMA-only and partnered conditions during weeks 1 and 2, ts < 1.74, ps > 0.09. For the remaining weeks, daily distinct uses were greater in the partnered condition compared to the EMMA-only condition ts > 2.10, ps > 0.05. As seen in Fig. 3, during the third month post-intervention, participants in the partnered condition were using the EMMA app on average 3.9 times per day (range = 0.1–11.9, median =2.8) compared to 0.8 times daily (range = 0.0–4.4, median = 0.0) for the EMMA-only condition. Examination of the data at the person level, revealed that during the third month post-intervention, 93% of participants in the partnered condition were regularly engaging with EMMA compared to 27% in the EMMA-only condition.
EMMA basic uses
The ANOVA conducted on the square root transformation of the basic uses score revealed significant main effects of condition, F(1, 27) = 10.00, MSE = 2.98, p = 0.004, η2 = 0.27, and time, F(15, 405) = 38.07, MSE = 1.62, p < 0.001, η2 = 0.59, that were modified by a group by time interaction, F(15, 405) = 2.76, MSE = 1.62, p < 0.001, η2 = 0.09. As seen in Fig. 4, breakdown of the interaction revealed that average daily basic uses by participants in the EMMA-only and partnered conditions did not differ during weeks 1–3 of learning, ts < 1.50, ps > 0.14. In contrast, during weeks 4–16, individuals in the partnered condition completed more daily basic use interactions with the EMMA app compared to participants in the EMMA-only condition, ts > 2.11, ps < 0.05. During the third month post-training (weeks 13–16), there was an average of 16.9 basic use interactions per day (range = 0.8–66.0, median = 10.3) in the partnered condition compared to 3.5 (range = 0–15.9, median = 0) in the EMMA-only condition (see Fig. 4).
EMMA advanced uses
The ANOVA indicated that participants in the partnered condition made daily use of the EMMA advanced features at a higher rate than participants in the EMMA-only condition, F(1, 27) = 9.34, MSE = 0.38, p = 0.005, η2 = 0.26. Examination of the within-subject contrasts for the main effect of time, F(15, 405) = 33.87, MSE = 0.32, p < 0.001, η2 = 0.56, indicated that use of advanced features did not differ between conditions across the first 3 weeks of training when these features were being taught, Fs < 1.63, ps > 0.12, and week 5, F = 1.58, p = 0.13. During month 3 post-training (weeks 13–16), the average advanced use interactions per day was 2.1 (range = 0.0–10.4, median = 1.4) for the partnered group and 0.5 (range = 0.0–3.3, median = 0.0) for the EMMA-only group. There was no significant condition by time interaction, F(15, 405) = 1.28, MSE = 0.32, p = 0.21, η2 = 0.05 (see Fig. 4).
Self-report measures
Table 6 presents the means and standard deviations for the primary and secondary self-report outcome measures across time and conditions.
Table 6.
Primary and Secondary Self-report Outcome Measures for the EMMA-only and partnered Condition (standard deviations in parentheses)
| Time |
||||
|---|---|---|---|---|
| Outcome Measure | Pre-training | Post-training | Post-training Month 1 |
Post-training Month 3 |
|
| ||||
| QOL-AD | ||||
| Partnered | 38.7 (6.1) | 38.6 (6.9) | 39.2 (6.1) | 38.5 (7.3) |
| EMMA-only | 38.1 (5.3) | 38.9 (4.9) | 39.0 (4.5) | 39.1 (4.8) |
| IADL-Ca | ||||
| Partnered | 2.0 (0.7) | 2.1 (0.9) | 1.9 (0.6) | 1.9 (0.8) |
| EMMA-only | 1.9 (0.5) | 2.0 (0.6) | 2.3 (0.8) | 2.0 (0.8) |
| CSE | ||||
| Partnered | 86.8 (20.5) | 94.2 (19.1) | 93.5 (19.1) | 95.5 (18.4) |
| EMMA-only | 91.7 (14.3) | 95.4 (14.7) | 97.2 (18.4) | 94.0 (20.9) |
| SWLS | ||||
| Partnered | 22.9 (8.4) | 25.6 (7.6) | 24.7 (8.4) | 25.2 (6.5) |
| EMMA-only | 25.3 (7.2) | 26.9 (5.6) | 24.3 (6.2) | 24.8 (7.2) |
EMMA, Electronic Memory and Management Aid; QOL-AD, Quality of Life – Alzheimer’s Disease; IADL-C, Instrumental Activities of Daly Living –Compensation; CSE, Coping Self-Efficacy Scale; SWLS, Satisfaction with Life Scale. Follow-up month 2 data was not included because prompts were removed for the partnered condition.
A lower score represents better self-reported everyday functioning.
Quality of life
The ANOVA on the QOL measure revealed no significant main effect of condition, F(1, 27) = 0.00, MSE = 30.20, p = 0.99, η2 = 0.00, or time, F(3, 81) = 0.59, MSE = 4.42, p = 0.63, η2 = 0.02, and no interaction, F(3, 81) = 0.45, MSE = 4.42, p = 0.72, η2 = 0.02. Overall, this measure changed very little across time.
Instrumental Activities of Daily Living
The ANOVA on the IADL-C measure revealed no significant main effect of condition, F(1, 27) = 0.07, MSE = 0.50, p = 0.80, η2 = 0.01, or time, F(3, 81) = 0.40, MSE = 0.11, p = 0.75, η2 = 0.02. The interaction was significant, F(3, 81) = 3.22, MSE = 0.11, p = 0.03, η2 = 0.11. Examination of each time point against the pre-training time point revealed an interaction at post-training month 1, F = 5.80, p = 0.02, reflecting an increase in self-report functional difficulties from pre-training (M = 1.94) to post-training month 1 (M = 2.26) in the EMMA-only condition and a decrease in functional difficulties from pre-training (M = 2.05) to post-training month 1 (M = 1.89) in the partnered condition.
Secondary outcomes
Self-report measures
Coping self-efficacy.
The mixed model ANOVA on the coping abilities measure revealed a significant main effect of time, F(3, 81) = 2.90, MSE = 82.06, p = 0.04., η2 = 0.10. Within-subjects contrasts revealed that self-reported coping abilities were higher post-training (M = 94.83) and at post-training month 1 (M = 95.40) and post-training month 3 (M = 94.72) compared to pre-training (M = 89.34), Fs > 4.77, ps < 0.038. There was no main effect of condition, F(1, 27) = 0.12, MSE = 272.20, p = 0.74, η2 = 0.00, or interaction, F(3, 81) = 0.72, MSE = 82.06, p = 0.55, η2 = 0.03.
Satisfaction with life.
Analysis of the satisfaction with life measure showed a significant main effect of time, F(3, 81)=3.59, MSE = 6.98, p = 0.02., η2 = 0.12. Within-subjects contrasts revealed that self-reported satisfaction with life was higher post-training (M = 26.28) compared to pre-training (M = 24.17), F = 11.78, p = 002, but not at month 1 (M = 24.47) or month 3 (M = 25.00) post-training, Fs < 1.24, ps > 0.27. There was no main effect of condition, F(1, 27) = 0.08, MSE = 46.60, p = 0.77, η2 = 0.00, or condition by time interaction, F(3, 81) = 2.01, MSE = 6.98, p = 0.12, η2 = 0.07.
Cognition
Global cognition.
The ANOVA on the RBANS total score revealed no main effect of condition, F(1, 26) = 0.66, MSE = 185.85, p = 0.43, η2 = 0.03, or time, F(1, 26) = 0.84, MSE = 23.27, p = 0.37, η2 = 0.03, and there was no significant interaction, F(1, 27) = 0.22, MSE = 23.27, p = 0.64, η2 = 0.01. Global cognitive scores did not differ between testing sessions for either the EMMA-only (baseline: M = 86.9, SD = 13.0, end of intervention: M = 87.4, SD = 16.3), t(14) = −0.30, or partnered (baseline: M = 90.4, SD = 13.7, end of intervention: M = 92.2, SD = 12.9), t(14) = −1.0, conditions.
DISCUSSION
Effective use of compensatory strategies and organizational aids can help individuals maintain their functional independence, thereby improving quality of life. Teaching individuals with memory impairment to use such aids can be challenging. In this study, we evaluated the potential benefits of partnering an electronic device with activity-aware transition-based prompting to use the device.
Consistent with our hypothesis, aMCI participants who learned to use the EMMA app partnered with the smart home prompts used the app more often and learned to use it to a higher level of proficiency earlier in training than participants in the EMMA-only condition. This was evidenced by the real-time EMMA data collection metrics showing that, starting in week three, participants in the partnered condition were engaging with EMMA more throughout the day compared to participants in the EMMA-only condition. Furthermore, by week four, participants in the partnered condition were engaging in a greater number of basic and advanced interactions with EMMA compared to the EMMA-only condition. This suggests that prompting beyond the time-based hourly training alarms, which were used to cue the recording of recent events into the Time Schedule for several days after Session 2 (week 1), was required to establish an overlearned routine. The transition-based activity aware prompts in the partnered condition enabled most participants to establish and overlearned behavior of frequently looking at and using the EMMA app.
The earlier learning and increased use of the EMMA app during training by participants also appeared to support a higher rate of sustained use of the device by individuals with aMCI through follow-up month 3. Removal of the smart home prompts during post-training month 2 did not lead to decreased use of EMMA by aMCI participants in the partnered group. Although this was not hypothesized, it is an exciting finding that underscores the importance of building an overlearned and automatic habit for using such devices early in training. For the aMCI participants in this study, it appeared that once the habit was formed, looking at and using EMMA multiple times per day became part of their daily activities, and the additional prompting did not appear to significantly decrease their engagement with EMMA during post-training month 2. Early work with the Neuropage device, a pager system used to remind individuals with brain injuries to complete activities, similarly found that three months with the device was enough to establish routines (e.g., using checklists, taking medication on time) that were maintained after the pager was withdrawn [58]. It is possible that with a longer post-training follow-up period, continued prompting by the smart home would be necessary to keep levels of EMMA app use high, especially as cognitive functions decrease across time. It is also possible that for individuals with more significant levels of cognitive impairment, such as mild dementia, removal of prompts would lead to decreased use of the app. Future studies will be needed to better understand how prompting can best be used to support acquisition and long-term use of devices like EMMA that are designed to support everyday functioning.
A novel aspect of the prompting used in this study is the activity-aware and transition-based design. Past studies that have used watch alarms to cue participants to use aids, such as a memory notebook, have relied on time-based alarms [4]. Unlike time-based alarms that may ring at inopportune times and must be set ahead of time, typically by a caregiver, prompts were issued in this study by the smart home only after periods of EMMA non-use and using algorithms that targeted transitions between activities. Such prompts should decrease frustration with the reminders by only prompting when prompts are needed and by targeting reminders to times that should decrease interference with other activities. It currently remains unclear whether prompting formats, such as receiving prompts to engage with EMMA on one’s phone or computer after periods of nonuse, might be as effective as using a smart home to promote context-aware prompting. Based on recent advances, in the future, it may be possible to use wearable technologies to provide real-time context-aware prompting [54, 59].
Given we identified higher levels of use of the EMMA device by aMCI participants in the partnered condition, we expected this to translate into improved self-report ratings on measures of quality of life and functional independence compared to participants in the EMMA-only condition. We found no significant change in our self-reported primary outcome measure that assessed IADLs for either condition. At post-training month 1, a significant interaction for the functional measure reflected increased self-report of functional difficulties in the EMMA-only condition and decreased self-report of functional difficulties in the partnered condition, consistent with the greater use of the EMMA device by participants in the partnered condition. This was not found at post-training month 3. When a goal of an intervention is to improve functional status, identifying outcome measures that accurately capture everyday functioning in the real-world is challenging. Self-report measures can be impacted by bias, including lack of insight [60]. Numerous studies have also shown that objective and self-report measures do not always correlate well with each other [61, 62]. The continued use of EMMA during post-training month 3, up to 10–11 times per day by some study participants, suggests that participants were finding the tool to be helpful in their daily life. Further suggestive of positive functional impacts were participant statements indicating that they had not forgotten things like taking medications since putting reminders in EMMA, and our direct observation of participants foregoing reminder calls for questionnaire appointments because they were recording the appointments in EMMA.
Although the impact of the intervention was also not evident on the primary outcome measure assessing quality of life, secondary measures that examined aspects of well-being including coping self-efficacy and satisfaction with life did show positive change. This change was not specific to the partnered condition. Rather, when compared to self-reported coping at pre-training, study participants reported higher coping self-efficacy at post-training and at post-training months 1 and 3. Self-reported life satisfaction was higher at post-training but was not maintained at post-training months 1 and 3. This suggests that there were some positive benefits to learning to use the EMMA app on coping and life satisfaction, even when the app was not partnered with the smart home prompts. In terms of intervention impact, prior work indicates that quality of life is ranked as the highest priority by individuals with aMCI and their partners, followed by patient self-efficacy and functional status [63]. The current quality of life measure assessed a broad array of outcomes (e.g., living situation, marriage), some of which may not have been easily impacted by the current intervention. The secondary outcome variables assessing coping abilities and life-satisfaction can also impact life quality and in future work it will be important to understand how changes in such dimensions are perceived by individuals with aMCI and their care-partners.
The intervention, including the smart home prompting and learning to use the EMMA app, was feasible to deliver and acceptable to study participants. A total of three participants who had difficulty with the pace of the intervention did not complete the training phase. Although all three of these participants exhibited no delayed recall on a list learning task, two additional participants with this profile successfully completed the training. Some participants may require a greater number of training sessions and a slower presentation of information. Future work will be needed to determine whether individuals with mild dementia can learn to use the EMMA app and what other person characteristic (e.g., personality) may impact long-term use of the app. EMMA could also benefit from an improved help feature as the current EMMA app required that participants hold information in memory as they maneuvered between viewing help information and completing an EMMA-related function. Providing feedback on actions may also be useful for promoting learning. A recent systematic review of assistive technologies, including memory aids, for use by individuals with dementia suggested that few conclusions could be drawn due to small sample sizes, unsophisticated statistics and issues that arose with the technology during the clinical trials [64]. Designing well-powered studies that can appropriately evaluate the efficacy of devices such as electronic memory aids is an important goal. Promoting the use of beneficial technologies will also require that clinicians be well versed about these devices and be able to recommend and support individuals with cognitive impairment in their use of such devices. New methods for scaling of training will also be important.
There are limitations to this work which must be considered. This was a pilot clinical trial and as such the sample size was small and powered to identify medium effect sizes. The sample was 100% White, highly educated and endorsed high confidence with and use of scheduling tools, which limits generalizability of the findings. In addition, there was some bleed over of prompts into no prompting weeks, which may have impacted the findings interpreted as indicating that reduction in prompting did not impact use of EMMA once a habit was established. Due to the pilot nature of this study and small sample size, many analyses were completed with no adjustment to the p-value, increasing risk of Type I error; future work will be required to replicate the findings. In addition, we did not obtain information about whether participants in the EMMA-only condition used other methods (e.g., care-partner, set alarms on other devices) to remind them to use their EMMA app. Furthermore, at the end of post-training month 3, some of the participants with aMCI were no longer actively using the EMMA app. We plan to analyze the continuous data collected from EMMA to identify patterns of use that may lead to non-adherence of the entire EMMA app or specific app features (e.g., notes section, search function) so that a booster session could be provided automatically when the booster is needed to help support long-term maintenance and use of the tool. In future work, it will also be important to understand what characteristics of the person and the training result in an individual becoming a long-term adopter of an aid as such insights are expected to improve future interventions.
We found that activity-aware transition-based prompting delivered by a smart home enhanced acquisition, habit formation and long-term use of a digital app. Although there was little differential intervention impact on primary and secondary self-report outcomes, training in use of the EMMA app was associated with positive changes in the secondary outcome of coping self-efficacy at all time points post-training and life satisfaction immediately following training. The daily data metrics collected by the app allowed us to examine participants’ use of EMMA throughout the training and three-month post-training phase. The availability of such data will also allow for exploration of other important questions (e.g., non-adherence patterns, characteristics of adopters) that could improve future interventions aimed at extending the functional independence and quality of life of older individuals experiencing progressive cognitive decline.
ACKNOWLEDGMENTS
This work was supported in part by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Alzheimer’s Research Program, Quality of Life Research Award, Funding Opportunity Number: W81XWH-15-PRA RP-QUAL under Award No. AZ150096. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. We would like to thank members of the Neuropsychology and Aging Laboratory and the CASAS computer science team for their assistance in completing this work.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5022r1).
REFERENCES
- [1].Chandler MJ, Locke DEC, Duncan NL, Hanna SM, Cuc AV, Fields JA, Hoffman Snyder CR, Lunde AM, Smith GE (2017) Computer versus compensatory calendar training in individuals with mild cognitive impairment: Functional impact in a pilot study. Brain Sci 7, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chudoba L, Sawaqdeh A, Dahmen J, Brown K, Schmitter-Edgecombe M (2020) The development of a manual-based Digital Memory Notebook intervention with case illustrations. Neuropsychol Rehabil 30, 1829–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Greenaway MC, Duncan NL, Smith GE (2013) The memory support system for mild cognitive impairment: Randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry 28, 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schmitter-Edgecombe M, Dyck D (2014) A cognitive rehabilitation multi-family group intervention forindividuals with mild cognitive impairment and their care-partners. J Int Neuropsychol Soc 20, 897–908. [DOI] [PubMed] [Google Scholar]
- [5].Schmitter-Edgecombe M, Pavawalla S, Howard JT, Howell L, Rueda A (2009) Dyadic interventions for persons with early-stage dementia: A cognitive rehabilitative focus. New Directions in Aging Research: Health and Cognition, pp. 39–56. [Google Scholar]
- [6].Amofa PA, DeFeis B, De Wit L, O’Shea D, Mejia A, Chandler M, Locke DEC, Fields J, Phatak V, Dean PM, Crook J, Smith G (2020) Functional ability is associated with higher adherence to behavioral interventions in mild cognitive impairment. Clin Neuropsychol 34, 937–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Farias S, Gravano J, Weakley A, Schmitter-Edgecombe M, Harvey D, Mungas D, Chan M, Giovannetti T (2020) The Everyday Compensation (EComp) questionnaire: Construct validity and associations with diagnosis and longitudinal change in cognition and everyday function. J Int Neuropsychol Soc 26, 303–313. [DOI] [PubMed] [Google Scholar]
- [8].Collerton D, Forster E, Packham D (2014) An exploratory study of the effectiveness of memory aids for older people living in supported accommodation. J Appl Gerontol 33, 963–981. [DOI] [PubMed] [Google Scholar]
- [9].Boman I, Persson A, Bartfail A (2016) First steps in designing an all-in-one ICTbased device for persons with cognitive impairment: Evaluation of the first mock-up. BMC Geriatr 16, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Joode E, van Heugten C, Verhey F, van Boxtel M (2010) Efficacy and usability of assistive technology for patients with cognitive deficits: A systematic review. Clin Rehabil 24, 701–714. [DOI] [PubMed] [Google Scholar]
- [11].Mayhorn CB, Stronge AJ, McLaughlin AC, Rogers WA (2004) Older adults, computer training, and the systems approach: A formula for success. Educ Gerontol 30, 185–203. [Google Scholar]
- [12].Inglis EA, Szymkowiak A, Gregor P, Newell AF, Hine N, Wilson BA, Evans JJ, Shah P (2004) Usable technology? Challenges in designing a memory aid with current electronic devices. Neuropsychol Rehabil 14, 77–87. [Google Scholar]
- [13].Chu Y, Brown P, Harniss M, Kautz H, Johnson K (2013) Cognitive support technologies for people with TBI: Current usage and challenges experienced. Disabil Rehabil Assist Technol 9, 279–285. [DOI] [PubMed] [Google Scholar]
- [14].Charters E, Gillett L, Simpson GK (2015) Efficacy of electronic portable assistive devices for people with acquired brain injury: A systematic review. Neuropsychol Rehabil 25, 82–121. [DOI] [PubMed] [Google Scholar]
- [15].Raghunath N, Dahmen J, Brown K, Cook D, Schmitter-Edgecombe M (2020) Creating a Digital Memory Notebook application for individuals with mild cognitive impairment to support everyday functioning. Disabil Rehabil Assist Technol 15, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dahmen J, Minor B, Cook D, Vo T, Schmitter-Edgecombe M (2018) Smart home-driven digital memory notebook support of activity self-management for older adults. Gerontechnology 17, 113–125. [Google Scholar]
- [17].Wilson BA, Emslie HC, Quirk K, Evans JJ (2001) Reducing everyday memory and planning problems by means of a paging system: A randomized controlled crossover study. J Neurol Neurosurg Psychiatry 70, 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dowds MM, Lee PH, Sheer JB, O’Neil-Pirozzi TM, Xenopoulos-Oddsson A, Goldstein R, Zainea KL, Glenn MB (2011) Electronic reminding technology following traumatic brain injury: Effects on timely task completion. J Head Trauma Rehabil 26, 339–347 [DOI] [PubMed] [Google Scholar]
- [19].Hackett K, Lehman S, Divers R, Ambrogi M, Gomes L, Tan CC, Giovannetti T (2020) Remind Me To Remember: A pilot study of a novel smartphone reminder application for older adults with dementia and mild cognitive impairment. Neuropsychol Rehabil, doi: 10.1080/09602011.2020.1794909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bosa HR, Babbageb DR, Leathema JM (2017) Efficacy of memory aids after traumatic brain injury: A single case series. Neurorehabilitation 41, 463–481. [DOI] [PubMed] [Google Scholar]
- [21].Seelye AM, Schmitter-Edgecombe M, Cook DJ, Crandall A (2013) Naturalistic assessment of everyday activities and prompting technologies in mild cognitive impairment. J Int Neuropsychol Soc 19, 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hart T, Buchhofer R, Vaccaro M. (2004) Portable electronic devices as memory and organizational aids after traumatic brain injury: A consumer survey study. J Head Trauma Rehabil 19, 351–65. [DOI] [PubMed] [Google Scholar]
- [23].Yang X, Ma L, Zhao X, Kankanhalli A (2020) Factors influence user’s adherence to physical activity applications: A scoping literature review and future directions. Int J Med Inform 134, 104039. [DOI] [PubMed] [Google Scholar]
- [24].Barbeau SJ, Georggi NL, Winters PL (2010) Global positioning system integrated with personalized real-time transit information from automatic vehicle location. Transp Res Rec 2143, 168–176. [Google Scholar]
- [25].Sohn T, Li KA, Lee G, Smith I, Scott J, Griswold WG (2005) Place-Its: A study of location-based reminders on mobile phones. In UbiComp 2005: Ubiquitous Computing. UbiComp 2005. Lecture Notes in Computer Science, vol 3660, Beigl M, Intille S, Rekimoto J, Tokuda H, eds. Springer, Berlin, Heidelberg. [Google Scholar]
- [26].Hayes TL, Cobbinah K, Dishongh T, Kaye JA, Kimel J, Labhard M, Leen T, Lundell J, Ozertem U, Pavel M, Philipose M, Rhodes K, Vurgun S (2009) A study of medication-taking and unobtrusive, intelligent reminding. Telemed J E Health 15, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lundell J, Hayes T, Vurgun S, Ozertem U, Kimel J, Kaye J, Guilak F, Pavel M (2007) Continuous activity monitoring and intelligent contextual prompting to improve medication adherence. 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 6286–6289. [DOI] [PubMed] [Google Scholar]
- [28].Aminikhanghahi S, Schmitter-Edgecombe M, Cook D (2020) Context-aware delivery of ecological momentary assessment. IEEE J Biomed Health Inform 24, 1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsui K, Yanco H (2010) Prompting devices: A survey of memory aids for task sequencing. International Symposium on Quality of Life Technologies: Intelligent Systems for Better Living, held in conjunction with RESNA 2010. [Google Scholar]
- [30].Acampora G, Cook DJ, Rashidi P, Vasilakos AV (2013) A survey on ambient intelligence in health care. Proc IEEE Inst Electr Electron Eng 12, 2470–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barger TS, Brown DE, Alwan M (2005) Health-status monitoring through analysis of behavioral patterns. IEEE Trans Syst Man Cybern A Syst Hum 35, 22–27. [Google Scholar]
- [32].Hoey J, Poupart P, Bertoldi AV, Craig T, Boutilier C, Mihailidis A (2010) Automated handwashing assistance for persons with dementia using video and a partially observable Markov decision process. Comput Vis Image Underst 114, 503–519. [Google Scholar]
- [33].Hu Y, Tilke D, Adams T, Crandall AS, Cook DJ, Schmitter-Edgecombe M (2016) Smart home in a box: Usability study for a large scale self-installation of smart home technologies. J Reliab Intell Environ 2, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cook DJ, Krishnan N (2015) Activity Learning: Discovering, Recognizing, and Predicting Human Behavior from Sensor Data. Wiley. [Google Scholar]
- [35].Cook DJ, Krishnan NC, Rashidi P (2013) Activity discovery and activity recognition: A new partnership. IEEE Trans Cybern 43, 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Feuz K, Robertson K, Rosasco C, Cook D, Schmitter-Edgecombe M (2013) Automated detection of activity transitions via decision trees and density-ratio estimation. Gerontechnology. IEEE Trans Hum Mach Syst 45, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sohlberg MM, Mateer CA (1989) Introduction to cognitive rehabilitation: Theory and practice, Gilford Press. [Google Scholar]
- [38].Brandt J, Folstein M (2003) Telephone Interview for Cognitive Status, Psychological Assessment Resources, Inc. [Google Scholar]
- [39].Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, Mielke M, Pankratz VS, Roberts R, Rocca WA, Weigand S, Weiner M, Wiste H, Jack CR (2013) Criteria for mild cognitive impairment due to Alzheimer’s disease in the community. Ann Neurol 74, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Randolph C, Tierney MC, Mohr D, Chase TN (1988) The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol 20, 310–319. [DOI] [PubMed] [Google Scholar]
- [41].Wechsler D (2001) Wechsler test of adult reading (WTAR), The Psychological Corporation. [Google Scholar]
- [42].Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System: Examiner’s Manual, The Psychological Corporation. [Google Scholar]
- [43].Podsiadlo D, Richadson S (1991) The timed ‘Up and Go’ test: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39, 142–148. [DOI] [PubMed] [Google Scholar]
- [44].Strauss E, Sherman EMS, Spreen O (2006) A compendium of neuropsychological tests: Administration, norms, and commentary (3rd ed.). Oxford University Press. [Google Scholar]
- [45].Tam JW, Son CV, Dyck D, Schmitter-Edgecombe M (2017) An educational video program to increase aging services technology awareness among older adults. Patient Educ Couns 100, 1564–1571. [DOI] [PubMed] [Google Scholar]
- [46].Logsdon RG, Gibbons LE, McCurry SM, Teri L (1999) Quality of life in Alzheimer’s disease: Patient and caregiver reports. Aging Ment Health 5, 21–32. [Google Scholar]
- [47].Schmitter-Edgecombe M, Parsey CM, Lamb R (2014) Development and psychometric properties of the instrumental activities of daily living – compensation scale (IADL-C). Arch Clin Neuropsychol 29, 776–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chesney MA, Neilands TB, Chambers DB, Taylor JM, Folkman S (2006) A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol 11, 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Diener E, Emmons RA, Laresen RJ, Griffin S (1985) The satisfaction with life scale. J Person Assess 49, 71–75. [DOI] [PubMed] [Google Scholar]
- [50].Pavot W, Diener E (1993) Review of the satisfaction with life scale. Psychol Assess 5, 164–172. [Google Scholar]
- [51].Cook DJ, Crandall AS, Thomas BL, Krishnan NC (2013) CASAS: A smart home in a box. Computer (Long Beach Calif) 46, 10.1109/MC.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cook DJ (2010) Learning setting-generalized activity models for smart spaces. IEEE Intell Syst 2010, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rashidi P, Cook DJ, Holder LB, Schmitter-Edgecombe M (2011) Discovering activities to recognize and track in a smart environment. IEEE Trans Knowl Data Eng 23, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Minor B, Doppa J, Cook D (2017) Learning activity predictors from sensor data: Algorithms, evaluation, and applications. IEEE Trans Knowl Data Eng 29, 2744–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Aminikhanghahi S, Wang T, Cook, D (2019) Real-time change point detection with application to smart home time series data. IEEE Trans Knowl Data Eng 31, 1010–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Aminikhanghahi S, Cook D (2019) Enhancing activity recognition using CPD-based activity segmentation. Pervasive Mob Comput 53, 75–89. [Google Scholar]
- [57].Myers JL, Well AD (2003) Research Design and Statistical Analysis. 2nd Edition, Lawrence Erlbaum Associates, Mahwah. [Google Scholar]
- [58].Wilson BA, Evans JJ, Emslie H, Malinek V (1997) Evaluation of NeuroPage: A new memory aid. J Neurol Neurosurg Psychiatry 63, 113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Culman C, Aminikhanghahi S, Cook D (2020) Easing power consumption of wearable activity monitoring with change point detection. Sensors 20, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fieo R, Zahodne L, Tang MX, Manly JJ, Cohen R, Stern Y (2018) The historical progression from ADL scrutiny to IADL to Advanced ADL: Assessing functional status in the earliest stages of dementia. J Gerontol A Biol Sci Med Sci 73, 1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schmitter-Edgecombe M, Parsey C, Cook D (2011) Cognitive correlates of functional performance in older adults: Comparison of self-report, direct observation and performance-based measures. J Int Neuropsychol Soc 17, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Burton CA, Strauss E, Bunce D, Hunter MA, Hultsch DF (2009) Functional abilities in older adults with mild cognitive impairment. Gerontology 55, 570–581. [DOI] [PubMed] [Google Scholar]
- [63].Smith GE, Chandler M, Fields JA, Aakre J, Locke DEC (2018) A survey of patient and partner outcome and treatment preferences in mild cognitive impairment. J Alzhei mers Dis 63, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].King AC, Dwan C (2019) Electronic memory aids for people with dementia experiencing prospective memory loss: A review of empirical studies. Dementia 18, 1994–2007. [DOI] [PubMed] [Google Scholar]


