Abstract
Purpose
To examine histologic characteristics of macular Bruchś membrane defects (BMD) in axially elongated eyes.
Design
Histomorphometric study.
Methods
Using light microscopy, we examined enucleated human globes for BMDs.
Results
In 247 eyes, BMDs were detected in 15 (6.1%) eyes (axial length:27.0–36.0 mm), in 10 of them in the macular region. Prevalence and size of BMDs (mean:1.93 ± 1.62 mm; range:0.22mm–6.24 mm) correlated with longer axial length (OR:1.52; 95%CI:1.19,1.94; P = 0.001) and higher prevalence of scleral staphylomas (OR:16.3; 95%CI:2.67,99.3; P < 0.001). The BMDs were smaller than corresponding gaps in the retinal pigment epithelium (RPE) (1.93 ± 1.62 mm versus 2.61 mm ± 1.73 mm; P = 0.003), and larger than corresponding gaps in the inner nuclear layer (0.43 ± 0.76 mm; P = 0.008) and inner limiting membrane bridges (0.13 ± 0.33 mm; P = 0.001). Choriocapillaris thickness, BM thickness and RPE cell density did not vary (all P > 0.05) between the BDM border and adjacent areas. In the BMD, choriocapillaris and RPE were absent. The sclera was thinner in the BDM area than in adjacent areas (0.28 ± 0.19 mm versus 0.36 ± 0.13 mm; P = 0.006).
Conclusions
BMDs as hallmarks of myopic macular degeneration are characterized by longer gaps in the RPE and smaller gaps in the outer nuclear layer and inner nuclear layer, by localized scleral thinning, and by a spatial association with scleral staphylomas. Thickness of the choriocapillaris and density of the RPE cell layer, both absent within the BDMs, do not vary between the BMD border and adjacent regions. The results suggest an association between BDMs and absolute scotomas, stretching of the adjacent retinal nerve fiver layer, and an axial elongation-associated stretching effect on BM as etiology of the BDMs.
Keywords: Bruch’s membrane defects, Bruch’s membrane, Myopia, Myopic maculopathy, Myopic macular degeneration, Axial myopia
1. Introduction
Features of myopic maculopathy include an increased fundus tessellation (category I of myopic macular degeneration), diffuse chorioretinal atrophy (category II), extrafoveal “patchy atrophies” (category III), and patchy atrophies located in the foveal region (category IV), in addition to plus signs such as lacquer cracks and choroidal neovascularization [1]. The patchy atrophies are a hallmark of the more advanced stages of myopic macular degeneration [2]. According to recent histological and clinical studies, they include a defect in the retinal pigment epithelium (RPE) layer and in the Bruch’s membrane (BM) [2,3]. In a clinical study examining 210 patchy atrophies by swept-source optical coherence tomography, all patchy atrophies showed an RPE defect, and 174 (82.9%) patchy atrophies had a BM defect BMD). In 101 (82.8%) of 122 patchy atrophies with clearly detectable borders of the RPE defects and BMDs, the BMDs were smaller than the RPE defects [3]. In the population-based Beijing Eye Study, BMDs were detected in 17 out of 164 (10.4%) highly myopic eyes (defined as refractive error of ≤ -6 diopters or axial length of ≥26.5 mm) [4]. Since histological descriptions of the BMDs have been scarce so far and since the relationships between the macular BMDs and other morphological changes of the macula in myopic eyes have not fully been explored yet, we examined in this report the histologic features of macular BMDs in axially elongated eyes and their associations with myopic changes of other ocular tissues [5].
2. Methods
In this histological investigation, we examined human globes. The reasons which had led to their enucleation were disorders such as malignant melanomas of the choroid and ciliary body and painful secondary angle-closure glaucoma. The study had been approved by the Medical Ethics Committee II of the Medical Faculty Mannheim of the Heidelberg University. As already described in previous investigations, the enucleated globes had been prepared for the histological examination in a standardized manner [6,7]. These procedures included fixation in 4% formaldehyde/1% glutaraldehyde for one week, measurement of the globe diameters, removal of a middle part (about 8 mm thick), dehydration in alcohol, imbedding in paraffin, sectioning for light microscopy (approximate thickness: 5–8 μm), and staining by hematoxylin-eosin or applying the Periodic-Acid-Schiff method. The slides went through the center of the cornea and pupil and included the optic nerve head and the posterior fundus region. Using one histological section per eye, we searched for regions characterized by the presence of BMDs. We assessed the number and location of the BMDs and, using an in-built millimeter scale, we determined the distance between the BMDs and the optic disc border (defined by the end of the lamina cribrosa). We additionally measured the length of the BMDs, the length of a defect in the overlying RPE layer, retinal outer nuclear layer and retinal inner nuclear layer, the length of an inner limiting membrane bridge, the thickness of the BM and choriocapillaris and the density of the RPE cells at the edge of the BMDs and in a distance of 1 mm to the BMD, and the scleral thickness in the region of the BMD and in distance of about 5 mm to the center of the BMD. We defined an inner limiting membrane bridge by a region in which all retinal layers except for the inner limiting membrane were absent. We also measured the width of parapapillary beta zone, gamma zone and delta zone [5]. Parapapillary beta zone was defined as the parapapillary region characterized by the presence of BM and absence of RPE (“denuded BM”). Parapapillary gamma zone was defined as the region without BM, and parapapillary delta zone represented, within the region of gamma zone, the area of the elongated and thinned peripapillary scleral flange [8].
In the statistical analysis (statistical software package: SPSS for Windows, version 27.0, IBM-SPSS, Chicago, IL, USA), we calculated the mean values of the prevalence and length of the BMDs, and, in univariate analysis and in multivariable analysis, we assessed the relationships between these parameters and other ocular and systemic parameters. In the case of linear regression analyses, we determined the standardized regression coefficient beta, non-standardized regression coefficient B, and its 95% confidence interval (CI). In the case of binary regression analyses, we assessed the odds ratios (ORs) and their 95%CIs. Two-sided P-values were significant if < 0.05.
3. Results
The investigation consisted of 247 eyes (247 patients; 135 (54.7%) men). The mean age was 61.9 ± 14.0 years (median: 63.5 years; range: 24–89 years) and the mean axial length was 25.2 ± 3.1 mm (median: 24.0 mm; range: 20.0–37.0 mm). The axial length was ≥26.0 mm in 63 (25.5%) eyes, and it was ≥29.0 mm in 39 (15.6%).
BMDs were detected in 15 (6.1%) eyes with a mean age of 68.2 ± 18.2 years and a mean axial length of 30.6 ± 2.4 mm (range: 27.0–36.0 mm) (Figs. 1–3). The mean number of BMDs per eye with any BMD was 1.9 ± 0.9 defects/eye (median: 2; range: 1–3). The location of the BMDs was mostly in the macular region (in 10 out of 15 BMDs). The mean size of the largest BMD per eye was 1.93 ± 1.62 mm (median: 1.32 mm; range: 0.22 mm–6.24 mm), the mean length of the second largest BMD was 0.55 ± 0.59 mm (median: 0.34 mm; range: 0.03 mm–1.51 mm), and the mean diameter of the third largest BMDs was 0.29 ± 0.22 mm (median: 0.30 mm; range: 0.05 mm–0.62 mm).
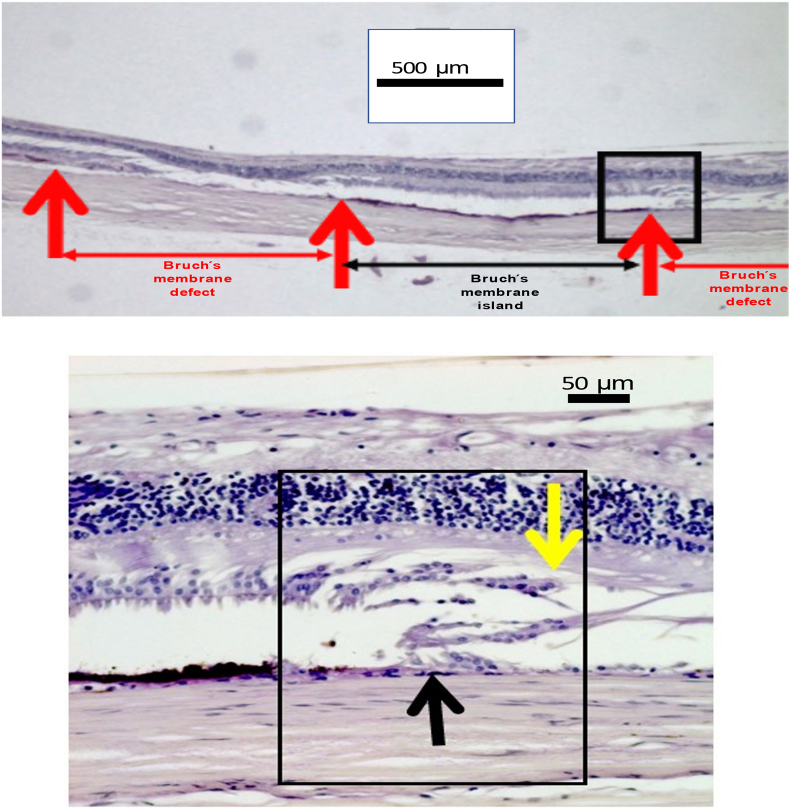
Fig. 2.
(a) Microphotograph showing Bruch’s membrane island, surrounded by Bruch’s membrane defects. In the defect, the outer nuclear layer is missing, the retina pigment epithelium and the choroid are missing, while the inner nuclear layer, the retinal nerve fiber layer and the inner limiting membrane are present in the region of the Bruch’s membrane defect. (b)Microphotograph (higher magnification of Fig. 2a) showing at the margin of the Bruch’s membrane defect the end of Bruch’s membrane (black arrow) and the end of the outer nuclear layer (yellow arrow).
Fig. 1.
Microphotograph showing a Bruch’s membrane defect, surrounded by a larger defect in the retinal pigment epithelium layer, and capped by a smaller defect in the outer nuclear layer. The inner nuclear layer, the retinal nerve fiber layer and the inner limiting membrane are intact in the region of the Bruch’s membrane defect.
Fig. 3.
(a) Microphotograph showing a Bruchś membrane defect (red arrow). (b) Microphotograph (higher magnification of Fig. 3a#) showing at both margins of the Bruch’s membrane defect the ends of Bruch’s membrane (red arrows) and the end of the outer nuclear layer. The defect is covered by inner retinal layers and the inner limiting mmebrane.
The length of the BMDs (1.93 ± 1.62 mm) was significantly (P = 0.003) smaller than the length of the gap in the RPE layer on top of the BMD (2.61 mm ± 1.73 mm (median: 2.54 mm; range: 0.52 mm–6.34 mm), and it was smaller, however not significantly (P = 0.11) smaller, than the length of the gap in the adjacent outer nuclear layer (2.43 ± 1.51 mm; median: 2.98 mm; range: 0.62 mm–5.00 mm). The length of the BMDs (1.93 ± 1.62 mm) was significantly (P = 0.008) longer than the length of a gap in the adjacent inner nuclear layer (present in 4/15 eyes; 0.43 ± 0.76 mm (median: 0; range: 0.00 mm–2.30 mm), and it was longer (P = 0.001) than the length of an inner limiting membrane bridge (0.13 ± 0.33 mm; median: 0 mm; range: 0.00–1.06 mm). Correspondingly, the defect in the retinal outer nuclear layer was significantly (P = 0.001) longer than the defect in the retinal inner nuclear layer which was longer than the length of the inner limiting membrane bridge in both eyes with an inner limiting membrane bridge. Fitting with the defects in the retinal outer nuclear layer and retinal inner nuclear layer, the retinal thickness in the BMD region was significantly lower than in the neighboring regions (76.5 ± 83.0 μm versus 139.9 ± 69.1 μm; P = 0.005).
Thickness of the choriocapillaris and of BM did not differ significantly between the BMD margins and regions in a distance of 1 mm to the BMD margin (choriocapillaris: 8.8 ± 5.0 μm versus 7.9 ± 3.2 μm; P = 0.94; and BM thickness: 3.1 ± 0.6 μm versus 3.0 ± 0.5 μm; P = 0.43). In a similar manner, the RPE cell density at the BMD margins did not vary from the RPE cell density in the BMD vicinity (31.6 ± 8.4 cells/480 μm length versus 27.4 ± 8.8 cells/480 μm length; P = 0.051) nor from the RPE cell density at similar locations in other highly myopic eyes without BMDs. In the BMD region, the choriocapillaris and the RPE layer were absent.
The sclera was thinner in the BMD area than in the areas close to the BMDs (0.28 ± 0.19 mm (median: 0.24 mm; range: 0.06 mm–0.62 mm) versus 0.36 ± 0.13 mm (median: 0.33 mm; range: 0.21 mm–0.60 mm; P = 0.006). The length of the scleral thinning was 2.14 ± 2.61 mm (median: 0.75 mm; range: 0–6.67 mm). A scleral staphyloma was detected in 10 out of the 247 eyes, 7 of which (70%) showed a BMD. Out of the 15 eyes with a BMD, 7 eyes showed a staphyloma. A higher prevalence of BMDs was significantly (P < 0.001) associated with a higher staphyloma prevalence.
In univariable analysis, the BMD length increased with longer axial length (beta: 0.42; P < 0.001), longer length of parapapillary gamma zone (beta: 0.67; P < 0.001) and parapapillary delta zone (beta: 0.57; P < 0.001), longer RPE defect length (beta: 0.74; P = 0.002), longer defect length of the retinal outer nuclear defect (beta: 0.60; P = 0.03), higher scleral staphyloma prevalence (beta: 0.46; P < 0.001), and marginally significantly with a longer length of a scleral thinning (beta: 0.56; P = 0.06). The BMD length was not significantly associated with the BM thickness at the edge of the BMD (beta: 0.14; P = 0.62) or the BM thickness in the vicinity of the BMD (beta: 0.14; P = 0.63), the distance to the optic disc (beta: −0.05; P = 0.85), the retinal thickness in the BMD (beta: −0.10; P = 0.74), length of a retinal inner nuclear defect (beta: 0.33 P = 0.27), length of an inner limiting membrane bridge (beta: 0.03; P = 0.91), the scleral thickness within the BMD (beta: −0.05; P = 0.86) or in its vicinity, and the length of parapapillary beta zone (beta: −0.14; P = 0.34). In multivariable analysis, a longer BMD length remained to be significantly associated only with a longer axial length (beta: 0.27; B: 52.4; 95%CI: 26.9, 77.8; P < 0.001) and higher prevalence of scleral staphyloma (beta: 0.33; B: 888; 95%CI: 538, 1238; P < 0.001).
In a similar manner, a higher prevalence of a BMD was associated with longer axial length (OR: 1.52; 95%CI: 1.19, 1.94; P = 0.001) and higher prevalence of scleral staphyloma (OR: 16.3; 95%CI: 2.67, 99.3; P < 0.001), while it was not significantly related to the length of parapapillary gamma zone (P = 0.09) and parapapillary delta zone (P = 0.72), age (P = 0.92).
4. Discussion
In our histological investigation on human eyes, the prevalence of secondary BMDs was related with longer axial length and a higher scleral staphyloma prevalence. Similar relationships were detected for the BMD length per eye. Most of the BMDs (mean size: 1.93 ± 1.62 mm) were located in the macular region. The BMDs were smaller than the gaps in the RPE layer on top of BM, and they were marginally smaller than the gaps in the outer nuclear layer on top of the RPE layer. The BMDs were also larger than the gaps in the inner nuclear layer which were longer than corresponding inner limiting membrane bridges. The thickness of the choriocapillaris and of BM and the RPE cell density did not vary significantly between the area close to the BMD margin and other neighboring regions. The BMD area did not have any choriocapillaris or RPE layer. With the sclera being thinner in the BMDs areas as compared to the neighboring regions, scleral staphylomas were spatially associated with BMDs.
These results confirm the findings of a previous histologic examination of a similar set of enucleated human eyes in which a higher BMD prevalence correlated mainly with longer axial length, and in univariable analysis, with a wider parapapillary gamma zone and delta zone [5]. The results of the present study extend the findings of the previous investigation by the observations of the spatial relationships between the BMDs and gaps in the adjacent RPE and retinal layers, the association between the BMDs and scleral thinning and scleral staphylomas, and the unremarkable thickness of the choriocapillaris thickness and BM and the unremarkable RPE cells density at the BMD borders.
In all eyes with BMDs, the adjacent RPE gap was longer than the BMD. It fits with clinical descriptions of patchy atrophies for which OCT-based examinations revealed an RPE defect with a smaller BMD in its center [2,3]. Since a histological examination is not based on longitudinal histological observations, it cannot determine risk factors or the longitudinal development of changes. If one speculates about the development of patchy atrophies, one may discuss that first the RPE defects occurred, eventually followed by BMDs in the center of the patchy atrophies. Reason for such a notion is that highly myopic eyes can show RPE defects in the macular region in the absence of BMDs, however all BMDs are accompanied by a (larger) RPE defect. It has remained unclear whether the RPE defects led to the BMDs or whether both, the RPE defects and the BMDs, were the results of the same pathomechanism which could be an axial elongation-induced increased strain in the RPE layer and in BM. Since the basal membrane of the RPE is considerably thinner than BM, one may assume that the RPE basal membrane (together with the RPE cell layer) is biomechanically less resistant and undergoes a rupture first, before a BDM develops. In a first step of an RPE rupture, a lacquer crack may develop. On OCT images, lacquer cracks show an increased transmission of laser rays into the choroidal and scleral compartment, corresponding to a RPE layer lesion. These areas usually do not show linear defects in BM [9]. If the axial elongation leads to an RPE gap, first starting with a lacquer crack and subsequently changing into a more circular lesion (i.e., a patchy atrophy), the question arises if the region adjacent the RPE gap had an RPE cell loss or if the RPE layer gap developed as an expansion hole, with the RPE layer slipping away. The theory of the RPE slipping away is strengthened by findings made in patients with age-related macular degeneration with an RPE detachment, which may more precisely be described as separation of the RPE basal membrane from the adjacent BM. It may be unlikely, that the RPE cells separate from their basal membrane since cells of ectodermal origin, such as RPE cells, are usually strongly connected to their basal membranes and may otherwise get dissociated. Another observation is that pronounced increase in intraocular pressure (IOP) can be associated with RPE rolls at the parapapillary BM margin. The RPE rolls regress after IOP reduction [10]. A further hint in favor of the notion of an RE slippage is the finding of a flattening of macular RPE detachments in relationship with an IOP increase [11]. According to the notion of a possibility of an RPE slippage, myopic axial elongation may elevate the strain within the RPE layer in the posterior fundus region, with the first sequel of a development of a parapapillary myopic beta zone. In beta zone, BM is present and the RPE is absent. The myopic beta zone can thus be regarded as an enlargement of the RPE opening forming one of the layers of the optic nerve head. If the extension of the RPE layer opening by the developing myopic beta zone does not sufficiently reduce the strain within the RPE basal membrane, additional (secondary) expansion gaps in the RPE layer in the macular region may form. In that context, an RPE cell loss (as it is discussed to be the case in glaucomatous parapapillary beta zone) may not be the cause of macular RPE defects. Instead, an enlarged strain in the RPE basal membrane may cause first a linear RPE defect (e.g., lacquer crack), which then enlarges to a patchy atrophy. Interestingly, the lacquer cracks usually run in a right-angled direction to the smallest optic disc diameter and to the largest width of parapapillary gamma zone [12,13]. Clinical studies have described the change from lacquer cracks to patchy atrophies [9]. The finding that the gap in the photoreceptor layer on top of the BMD was smaller than the RPE layer gap agrees with the notion that if the slippery RPE layer beneath the photoreceptors is drawn away, the photoreceptors are less movable since they are attached to the inner layers of the retina.
Interestingly, the choriocapillaris was fully present in the ring-like region surrounding the BMD and did not differ in its thickness from the choriocapillaris in a distance of 1 mm to the BM border. This finding supports the notion of a strain-associated rupture and sliding of the RPE layer, and speaks against the possibility of an RPE cell loss due to an insufficiency of the choriocapillaris. In a similar manner, the RPE cell density did not vary between the RPE defect margin and an area in a distance of 1 mm. BM thickness at the BMD margin did not vary significantly from BM thickness in the adjacent areas. It fits with observations that the BM thickness in general was not related with axial length [14]. It contradicts the possibility, that the BMD started with a gradual thinning of BM.
A BMD leads to a localized enlargement of the fundament of the retina. It may lead to a strain in the retinal tissues, resulting in defects in the outer nuclear layer, and to a minor degree, in the inner retinal nuclear layer. The strain will also affect the retinal nerve fibers running over the BMD. Although we did not measure the retinal nerve fiber layer thickness in the region of the BMDs, one may discuss that large BMDs with a localized strain in the retinal nerve fiber layer may cause a stretching and potential damage and loss of these retinal nerve fibers. It may be one of the reasons for an increased prevalence of non-glaucomatous optic nerve damage in highly myopic eyes [15,16]. Besides the retinal nerve fiber layer, the inner limiting membrane is the other structure connecting the retina with the optic nerve head and which may get locally stretched in the BMD region. It has been discussed that an increased strain in the inner limiting membrane may lead to a “washing line effect” with a partial intraretinal detachment of the inner limiting membrane and secondary formation of a myopic maculoschisis [17,18].
The localized thinning of the sclera in the region of the BMD fits with the observation of a spatial association between scleral staphylomas and BMDs [19]. It supports the notion of a biomechanical role of BM [[18], [20]].
The limitations of our study should be considered: Since we did not have histological serial sections, the BMD count per eye might have been higher than we found. The data on BMDs cannot be transferred onto the general population, since the study participants were not included into the study in a population-based fashion. In addition, the diseases which led to the enucleation of the eyes might have influenced the BMDs. Artefacts due to the processing of the histological slides including ischemia-related tissue swelling, mechanically induced changes and fixation-induced tissue shrinkage may have had an influence on the dimensions of the tissues. The influence of these parameters on the tissues might however have been comparable between the various tissues so that the spatial relationship between the tissue might not have been markedly affected. Finally, the enucleated eyes came from individuals of European descent, so that the study findings cannot directly be transferred on individuals of non-European ethnicity.
In conclusion, BMDs as hallmark of myopic macular degeneration are characterized by a longer defect in the adjacent RPE layer, smaller defects in the adjacent retinal outer nuclear layer and inner nuclear layer, localized scleral thinning and an association with scleral staphylomas. Thickness of the choriocapillaris and density of the RPE cells, both absent within the BDMs, do not vary between the BMD margin and adjacent areas. The results suggest an association between BDMs and absolute scotomas, stretching of the adjacent retinal nerve fiver layer, and an axial elongation-associated stretching effect as etiology of the BDMs.
References
- 1.Ohno-Matsui K., Kawasaki R., Jonas J.B., Cheung C.M., Saw S.M., Verhoeven V.J., Klaver C.C., Moriyama M., Shinohara K., Kawasaki Y., Yamazaki M., Meuer S., Ishibashi T., Yasuda M., Yamashita H., Sugano A., Wang J.J., Mitchell P., Wong T.Y. META-analysis for Pathologic Myopia (META-PM) Study Group. International photographic classification and grading system for myopic maculopathy. Am. J. Ophthalmol. 2015;159(5):877. doi: 10.1016/j.ajo.2015.01.022. 83.e7. [DOI] [PubMed] [Google Scholar]
- 2.Ohno-Matsui K., Jonas J.B., Spaide R.F. Macular Bruch membrane holes in highly myopic patchy chorioretinal atrophy. Am. J. Ophthalmol. 2016;166:22–28. doi: 10.1016/j.ajo.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Du R., Fang Y., Jonas J.B., Yokoi T., Takahashi H., Uramoto K., Kamoi K., Yoshida T., Ohno-Matsui K. Clinical features of patchy chorioretinal atrophy in pathological myopia. Retina. 2020;40(5):951–959. doi: 10.1097/IAE.0000000000002575. [DOI] [PubMed] [Google Scholar]
- 4.You Q.S., Peng X.Y., Xu L., Chen C.X., Wei W.B., Wang Y.X., Jonas J.B. Macular Bruch's membrane defects in highly myopic eyes. The Beijing Eye Study. Retina. 2016;36(3):517–523. doi: 10.1097/IAE.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 5.Jonas J.B., Ohno-Matsui K., Spaide R.F., Holbach L., Panda-Jonas S. Macular Bruch's membrane defects and axial length: association with gamma zone and delta zone in peripapillary region. Invest. Ophthalmol. Vis. Sci. 2013;54(2):1295–1302. doi: 10.1167/iovs.12-11352. [DOI] [PubMed] [Google Scholar]
- 6.Panda-Jonas S., Holbach L., Jonas J.B. Choriocapillaris thickness and density in axially elongated eyes. Acta Ophthalmol. 2021;99(1):104–110. doi: 10.1111/aos.14486. [DOI] [PubMed] [Google Scholar]
- 7.Jonas J.B., Jonas S.B., Jonas R.A., Holbach L., Dai Y., Sun X., Panda-Jonas S. Parapapillary atrophy: histological gamma zone and delta zone. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y.X., Panda-Jonas S., Jonas J.B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: histology and clinical features. Prog. Retin. Eye Res. 2021;83 doi: 10.1016/j.preteyeres.2020.100933. [DOI] [PubMed] [Google Scholar]
- 9.Xu X., Fang Y., Uramoto K., Nagaoka N., Shinohara K., Yokoi T., Tanaka N., Ohno-Matsui K. Clinical features of lacquer cracks in eyes with pathological myopia. Retina. 2019;39(7):1265–1277. doi: 10.1097/IAE.0000000000002168. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y.X., Jiang R., Wang N.L., Xu L., Jonas J.B. Acute peripapillary retinal pigment epithelium changes associated with acute intraocular pressure elevation. Ophthalmology. 2015;122(10):2022–2028. doi: 10.1016/j.ophtha.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.X., Ran J., Yang L.H., Xu L., Jonas J.B. Reversibility of retinal pigment epithelium detachment parallel to acute intraocular pressure rise. J. Glaucoma. 2015;24(3):e16–e18. doi: 10.1097/IJG.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 12.Jonas R.A., Wei C.C., Jonas J.B., Wang Y.X. Decreasing myopic lacquer crack and widening parapapillary gamma zone: case report. BMC Ophthalmol. 2021 24;21(1):443. doi: 10.1186/s12886-021-02216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas J.B., Xu L., Wei W.B., Jonas R.A., Wang Y.X. Progression and associated factors of lacquer cracks/patchy atrophies in high myopia: the Beijing Eye Study 2001-2011. Graefes Arch. Clin. Exp. Ophthalmol. 2022 doi: 10.1007/s00417-022-05705-7. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Jonas J.B., Holbach L., Panda-Jonas S. Bruchś membrane thickness in high myopia. Acta Ophthalmol. 2014 Sep;92(6):e470–e474. doi: 10.1111/aos.12372. [DOI] [PubMed] [Google Scholar]
- 15.Bikbov M.M., Gilmanshin T.R., Kazakbaeva G.M., Zainullin R.M., Rakhimova E.M., Rusakova I.A., Bolshakova N.I., Safiullina K.R., Zaynetdinov A.F., Zinatullin A.A., Nuriev I.F., Khalimov T.A., Panda-Jonas S., Arslangareeva, Bikbova G.M., Yakupova D.F., Uzianbaeva Y.V., Jonas J.B. Prevalence of myopic maculopathy among adults in a Russian population. JAMA Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas J.B., Wang Y.X., Dong L., Panda-Jonas S. High myopia and glaucoma-like optic neuropathy. Asia Pac. J. Ophthalmol. (Phila) 2020;9(3):234–238. doi: 10.1097/APO.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas J.B., Ohno-Matsui K., Panda-Jonas S. Myopia: anatomic changes and consequences for its etiology. Asia Pac. J. Ophthalmol. (Phila) 2019;8(5):355–359. doi: 10.1097/01.APO.0000578944.25956.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara K., Tanaka N., Jonas J.B., Shimada N., Moriyama M., Yoshida T., Ohno-Matsui K. Ultrawide-field OCT to investigate relationships between myopic macular retinoschisis and posterior staphyloma. Ophthalmology. 2018;12510:1575–1586. doi: 10.1016/j.ophtha.2018.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Jonas J.B., Ohno-Matsui K., Holbach L., Panda-Jonas S. Histology of myopic posterior scleral staphylomas. Acta Ophthalmol. 2020;98(7):e856–e863. doi: 10.1111/aos.14405. [DOI] [PubMed] [Google Scholar]
- 20.Jonas J.B., Ohno-Matsui K., Jiang W.J., Panda-Jonas S. Bruch membrane and the mechanism of myopization: a new theory. Retina. 2017;37(8):1428–1440. doi: 10.1097/IAE.0000000000001464. [DOI] [PubMed] [Google Scholar]