Abstract
Background
The treatment and prognostic factors of intravenous leiomyomatosis (IVL) remain lacking systematic evidence.
Methods
A retrospective study was conducted on IVL patients from the Qilu Hospital of Shandong University, and IVL cases were published in PubMed, MEDLINE, Embase and Cochrane Library databases. Descriptive statistics were used for the basic characteristics of patients. The Cox proportional hazards regression analysis was used to assess the high-risk factors related to the progression-free survival (PFS). The comparison of survival curves was performed by Kaplan–Meier analysis.
Results
A total of 361 IVL patients were included in this study, 38 patients from Qilu Hospital of Shandong University, and 323 patients from the published literature. Age ≤45 years was observed in 173 (47.9%) patients. According to the clinical staging criteria, stage I/II was observed in 125 (34.6%) patients, and stage III/IV was observed in 221 (61.2%) patients. Dyspnea, orthopnea, and cough were observed in 108 (29.9%) patients. Completed tumor resection was observed in 216 (59.8%) patients, and uncompleted tumor resection was observed in 58 (16.1%) patients. Median follow-up period was 12 months (range 0–194 months), and 68 (18.8%) recurrences or deaths were identified. The adjusted multivariable Cox proportional hazard analysis showed age ≤45 years (vs. >45) (hazard ratio [HR] = 2.09, 95% confidence interval [CI] 1.15–3.80, p = 0.016), and uncompleted tumor resection (vs. completed tumor resection) (HR = 22.03, 95% CI 8.31–58.36, p < 0.001) were high-risk factors related to the PFS.
Conclusion
Patients with IVL have a high probability of recurrence after surgery and a poor prognosis. Patients younger than 45 years and with uncompleted tumor resection are at higher risk of postoperative recurrence or death.
Keywords: intravenous leiomyomatosis, prognostic factor, treatment, surgery, progression-free survival
Introduction
Intravascular leiomyomatosis (IVL) is a histologically-benign, rare mesenchymal tumor with a biological behavior similar to that of a malignant tumor. It is characterized by the growth of a benign leiomyoma along the intra- and extrauterine venous lumen forming a tumor thrombus that can extend to the inferior vena cava, right atrium, right ventricle, and pulmonary artery, and cause sudden death in severe cases (1, 2).
The incidence of IVL is low, with approximately 400 cases reported in the literature since it was first documented in 1896 (3). Clinical manifestations in early-stage patients are non-specific, with approximately 30% of the patients showing no symptoms or specific tumor markers, and the postoperative recurrence rate is approximately 14.0 − 27.8% (4, 5). In recent years, the emergence of imaging technologies such as computed tomography (CT), CT angiography, and magnetic resonance imaging (MRI) has provided an invaluable tool for the diagnosis of intravenous tumors and the evaluation of the scope of tumor involvement, improving the accuracy of surgical evaluation and the success rate of surgery (6, 7). However, the number of IVL cases remains underestimated due to its relatively obscure onset, and as current treatment is mainly derived from case reports, there is a lack of solid evidence to guide clinical treatment.
Therefore, we have conducted this retrospective study and systematic review to analyze the epidemiology, history, pathology, diagnosis, treatment, and prognosis of IVL, and the risk factors affecting the prognosis of patients were also analyzed.
Materials and methods
General information
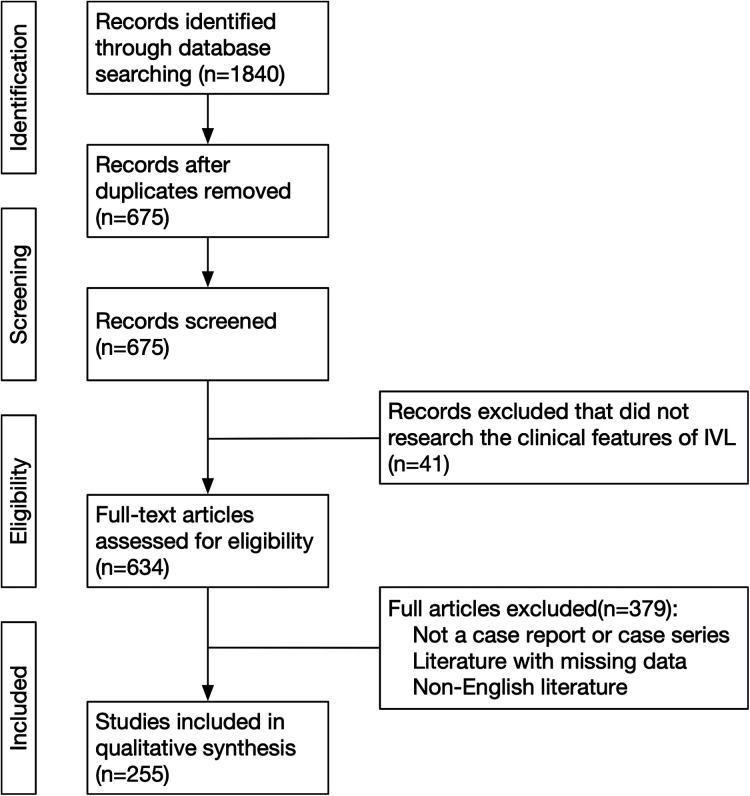
323 IVL patients in 255 studies were included in our study (Figure 1) (2, 7–260). Patients diagnosed with IVL in Qilu Hospital of Shandong University from January 1, 2007, to December 31, 2021 were enrolled in our study. The follow-up period ended on March 31, 2022. The PubMed, MEDLINE, Embase, and Cochrane Library databases were searched using the keywords “intravenous leiomyomatosis,” “intracardiac leiomyomatosis,” and “IVL.” The search period covered from January 1, 1959, to March 31, 2021. Of 1,840 studies screened, 323 IVL patients in 259 studies were included in our study (Figure 1) (8–242). Literature inclusion criteria were as follows: article type was a case report or case series, and the language was English; exclusion criteria included: irrelevant literature, duplicate case reports published by the same hospital or the same author, and literature with missing data. The following clinical characteristics were collected: age, gravidity, parity, history of hysteromyoma, clinical staging, tumor size, operation and pathological details, immunohistochemical characteristics [such as Ki-67, smooth muscle actin (SMA), desmin, estrogen receptor (ER), progesterone receptor (PR), and CD34], and follow-up time.
Figure 1.
Flow diagram of literatures searching. IVL, intravenous leiomyomatosis.
Clinical staging of IVL
Patients were classified as stage I−IV based on the clinical staging system proposed by Ma et al., which reflects the preoperative progression of the tumor (261). Stage I referred to tumors that had penetrated the uterine venous wall but were confined to the pelvic cavity; stage II referred to tumors that had extended into the abdominal cavity but had not reached the renal vein; stage III referred to tumors that had reached the renal vein and inferior vena cava and had further extended into the right atrium, but had not reached the pulmonary arteries; and finally, and stage IV referred to tumors that had reached the pulmonary arteries and/or lung metastases were observed.
Endpoints
The primary endpoint of this study was the progression-free survival (PFS). PFS is described as the time interval from primary treatment to the first evidence of tumor recurrence, death, or last follow-up.
Statistical analysis
Descriptive statistics were used to describe the basic characteristics, the grouped data was described as n (%), and the continuous data was described as the median (range). Univariable and adjusted multivariable Cox proportional hazards regression analysis were used to identify the high-risk factor affecting PFS, and results were described as hazard ratios (HR), associated 95% confidence intervals (CI), and p-values. The Kaplan-Meier method with the log-rank test was conducted to evaluate the effects of high-risk factors on PFS. Statistical analysis was conducted with SPSS (version 26.0), The p value <0.05 is considered significant.
Results
Patient characteristics are shown in Table 1. A total of 361 IVL patients were included in this study, 323 from the literature, and 38 from the Qilu Hospital of Shandong University. Age ≤45 years was observed in 173 (47.9%) patients. Dyspnea, orthopnea, and cough were observed in 108 (29.9%) patients. Completed tumor resection was observed in 216 (59.8%) patients, and uncompleted tumor resection was observed in 58 (16.1%) patients. According to the clinical staging criteria, stage I/II was observed in 125 (34.6%) patients, and stage III/IV was observed in 221 (61.2%) patients. The Median follow-up period was 12 months (range 0–194 months), and 68 (18.8%) recurrences or deaths were identified. The global regional distribution of all enrolled patients is shown in Supplementary Figure S1.
Table 1.
Clinical characteristics of IVL patients.
| Characteristics | Total (n = 361) | Patients from Qilu hospital (n = 38) | Patients from literature review (n = 323) |
|---|---|---|---|
| Age (years) | |||
| ≤45 | 173 (47.9) | 24 (63.2) | 149 (46.1) |
| >45 | 184 (51.0) | 14 (36.8) | 170 (52.6) |
| Unknown | 4 (1.1) | 0 (0.0) | 4 (1.2) |
| Gravidity | |||
| ≤2 | 70 (19.4) | 18 (47.4) | 52 (16.1) |
| >2 | 51 (14.1) | 20 (52.6) | 31 (9.6) |
| Unknown | 240 (66.5) | 0 (0.0) | 240 (74.3) |
| Parity | |||
| ≤1 | 61 (16.9) | 26 (68.4) | 35 (10.8) |
| >1 | 70 (19.4) | 12 (31.6) | 58 (18.0) |
| Unknown | 230 (63.7) | 0 (0.0) | 230 (71.2) |
| History of hysteromyoma | |||
| Yes | 149 (41.3) | 35 (92.1) | 114 (35.3) |
| No | 7 (1.9) | 2 (5.3) | 5 (1.5) |
| Unknown | 205 (56.8) | 1 (2.6) | 204 (63.2) |
| Clinical staging | |||
| I/II | 125 (34.6) | 29 (76.3) | 96 (29.7) |
| III/IV | 221(61.2) | 9 (23.7) | 212 (65.6) |
| Unknown | 15 (4.2) | 0 (0.0) | 15 (4.6) |
| Length of the tumor in vessel (cm) | |||
| ≤15 | 102 (28.2) | 28 (73.7) | 74 (22.9) |
| >15 | 92 (25.5) | 7 (18.4) | 85 (26.3) |
| Unknown | 167 (46.3) | 3 (7.9) | 164 (50.8) |
| Clinical symptom | |||
| Dyspnea / orthopnea / cough | 108 (29.9) | 3 (7.9) | 105 (32.5) |
| Menstrual changes | 57 (15.8) | 5 (13.2) | 52 (16.1) |
| Lower limb swelling or pain | 62 (17.2) | 2 (5.3) | 60 (18.6) |
| Abdominal pain / distention / discomfort | 87 (24.1) | 14 (36.8) | 73 (22.6) |
| Dizziness / syncope | 55 (15.2) | 2 (5.3) | 53 (16.4) |
| Palpitation / tachycardia | 39 (10.8) | 0 (0.0) | 39 (12.1) |
| Thoracalgia / chest tightness | 36 (10.0) | 1 (2.6) | 35 (10.8) |
| Hypodynamia | 15 (4.2) | 0 (0.0) | 15 (4.6) |
| Urinary frequency / incontinence / dysuria | 10 (2.8) | 4 (10.5) | 6 (1.9) |
| Others | 9 (2.5) | 0 (0.0) | 9 (2.8) |
| None | 100 (27.7) | 15 (39.5) | 85 (26.3) |
| Hysterectomy | |||
| Yes | 292 (80.9) | 30 (78.9) | 262 (81.1) |
| No | 60 (16.6) | 8 (21.1) | 52 (16.1) |
| Unknown | 9 (2.5) | 0 (0.0) | 9 (2.8) |
| Oopherectomy | |||
| Yes | 167 (46.3) | 20 (52.6) | 147 (45.5) |
| No | 155 (42.9) | 18 (47.4) | 137 (42.4) |
| Unknown | 39 (10.8) | 0 (0.0) | 39 (12.1) |
| Completed tumor resection | |||
| Yes | 216 (59.8) | 35 (92.1) | 181 (56.0) |
| No | 58 (16.1) | 3 (7.9) | 55 (17.0) |
| Unknown | 87 (24.1) | 0 (0.0) | 87 (26.9) |
| Surgical approach | |||
| One-stage | 55 (15.2) | 2 (5.3) | 53 (16.4) |
| Two-stage | 38 (10.5) | 2 (5.3) | 36 (11.1) |
| Others | 268 (74.2) | 34 (89.5) | 234 (72.4) |
| Follow-up time (months) | 12 (0-194) | 33 (0-178) | 9 (0-194) |
| Recurrence/death | 68 (18.8) | 7 (18.4) | 61 (18.9) |
Value are n (%) or median (range). One patient usually has multiple clinical manifestations, so the percentage of symptoms in all patients is more than 100%. IVL, intravenous leiomyomatosis.
IVL patients from Qilu Hospital of Shandong University
Clinical characteristics of 38 IVL patients from Qilu Hospital of Shandong University are shown in Supplementary Table S1. From January 1, 2007, to December 31, 2021, A total of 31,594 patients with uterine fibroid were admitted to the Qilu Hospital of Shandong University, and 38 (1.2‰) IVL patients were diagnosed. The median age of the 38 IVL patients was 43 years (range 33−66). Abdominal discomfort (36.8%) was the most common clinical manifestation, followed by menstrual abnormalities (13.2%), urinary (10.5%), and cardiovascular symptoms (7.9%), which were mainly related to the site of the lesion involvement. In terms of clinical staging, stage I/II was observed in 29 (76.3%) patients, and stage III/IV was observed in 9 (23.7%) patients. All visible tumors were removed in 35 (92.1%) patients. One patient with stage IV disease died during surgery due to intraoperative left pulmonary artery hemorrhage. After a median follow-up period of 33 months (range 0–178 months), 6 recurrences and 1 death were identified.
IVL patients from literature
The median age at diagnosis was 46 years (range 21−81). Among the patients for whom clinical data could be obtained, 149 (46.1%) had a parturition number exceeded once. Stage I/II was observed in 96 (29.7%) patients, and stage III/IV was observed in 212 (65.6%) patients. The main clinical symptoms were respiratory discomfort (32.5%), and other common symptoms included abdominal discomfort (22.6%), lower limb edema (18.6%), abnormal menstruation (16.1%), and dizziness (16.4%). Remarkably, 26.3% of patients showed no symptoms. Among the patients who underwent surgical treatment, 181 patients (56.0%) had completed tumor resection. After a median follow-up period of 9 months (range 0–194 months), 61 recurrences and 11 deaths were identified (Supplementary Table S2).
Immunohistochemical characteristics of IVL patients
Immunohistochemical results are summarized in Table 2. A total of 19 patients underwent Ki-67 immunohistochemical analysis, with a positivity rate of less than 5% in 17 patients. Immunohistochemical analysis of SMA and desmin was performed in 45 and 41 patients, respectively, with a positivity rate of 100%. In addition, the positivity rate for the presence of ER was 97.4% (38/39), PR was 91.7% (33/36), and CD34 was 69.2% (9/13), respectively.
Table 2.
Immunohistochemical characteristics of IVL patients.
| Immunohistochemical characteristics | Total | Patients from Qilu hospital | Patients from literature review |
|---|---|---|---|
| Ki-67 | |||
| Positive rate ≤5% | 17 | 7 (100.0) | 10 (83.3) |
| Positive rate >5% | 2 | 0 (0.0) | 2 (16.7) |
| SMA | |||
| Positive | 45 | 9 (100.0) | 36 (100.0) |
| Negative | 0 | 0 (0.0) | 0 (0.0) |
| Desmin | |||
| Positive | 41 | 9 (100.0) | 32 (100.0) |
| Negative | 0 | 0 (0.0) | 0 (0.0) |
| ER | |||
| Positive | 38 | 4 (100.0) | 34 (97.1) |
| Negative | 1 | 0 (0.0) | 1 (2.9) |
| PR | |||
| Positive | 33 | 3 (75.0) | 30 (93.8) |
| Negative | 3 | 1 (25.0) | 2 (6.2) |
| CD34 | |||
| Positive | 9 | 5 (83.3) | 4 (57.1) |
| Negative | 4 | 1 (1.7) | 3 (2.9) |
Value are n (%). IVL, intravenous leiomyomatosis; SMA, smooth muscle actin; ER, estrogen receptor; PR, progesterone receptor.
High-risk factor analysis of PFS
The results of univariable and adjusted multivariable Cox proportional hazards regression analysis for PFS are shown in Table 3. After the adjusted multivariable Cox proportional hazard analysis, age ≤45 years (vs. > 45) (HR = 2.09, 95% CI 1.15–3.80, p = 0.016), and uncompleted tumor resection (vs. completed tumor resection) (HR = 22.03, 95% CI 8.31–58.36, p < 0.001) were selected as high-risk factors related to the PFS. Figure 2 shows the Kaplan–Meier curves of the high-risk factors for PFS in all 361 IVL patients, and Figure 3 shows the Kaplan–Meier curves of the high-risk factors for PFS in 38 IVL patients from Qilu Hospital of Shandong University.
Table 3.
Univariable and adjusted multivariable Cox proportional hazards regression analysis for PFS of 361 IVL patients.
| Univariable analysis | Adjusted multivariable analysis | |||
|---|---|---|---|---|
| Characteristics | HR (95%CI) | p value | HR (95%CI) | p value |
| Age (years) | 0.129 | 0.054 | ||
| ≤45 | 1.67 (1.02–2.73) | 0.043 | 2.09 (1.15–3.80) | 0.016 |
| >45 | Reference | Reference | ||
| Unknown | – | – | – | – |
| Gravidity | 0.543 | 0.068 | ||
| ≤2 | Reference | Reference | ||
| >2 | 1.26 (0.54–2.92) | 0.596 | 2.88 (1.08–7.71) | 0.035 |
| Unknown | 1.43 (0.76–2.70) | 0.273 | 0.60 (0.07–5.28) | 0.644 |
| Parity | 0.388 | 0.845 | ||
| ≤1 | Reference | Reference | ||
| >1 | 1.34 (0.59–3.04) | 0.486 | 0.81 (0.31–2.11) | 0.670 |
| Unknown | 1.58 (0.82–3.07) | 0.175 | 1.27 (0.13–12.17) | 0.837 |
| History of hysteromyoma | 0.899 | 0.664 | ||
| Yes | 0.75 (0.18–3.19) | 0.699 | 0.56 (0.11–2.86) | 0.481 |
| No | Reference | Reference | ||
| Unknown | 0.82 (0.20–3.40) | 0.783 | 0.49 (0.10–2.42) | 0.384 |
| Clinical staging | 0.100 | 0.092 | ||
| I/II | Reference | Reference | ||
| III/IV | 1.39 (0.81–2.39) | 0.228 | 1.08 (0.48–2.42) | 0.859 |
| Unknown | – | – | – | – |
| Length of the tumor in vessel (cm) | <0.001 | 0.082 | ||
| ≤15 | Reference | Reference | ||
| >15 | 1.04 (0.39–2.74) | 0.939 | 0.63 (0.23–1.75) | 0.377 |
| Unknown | 3.76 (1.90–7.43) | <0.001 | 1.66 (0.74–3.74) | 0.222 |
| Hysterectomy | 0.006 | 0.066 | ||
| Yes | 0.55 (0.31–0.95) | 0.032 | 0.68 (0.34–1.36) | 0.275 |
| No | Reference | Reference | ||
| Unknown | 2.65 (0.77–9.16) | 0.124 | 4.91 (0.93–25.86) | 0.060 |
| Oopherectomy | 0.016 | 0.132 | ||
| Yes | Reference | Reference | ||
| No | 2.09 (1.22–3.57) | 0.007 | 1.09 (0.55–2.18) | 0.805 |
| Unknown | 2.45 (1.07–5.62) | 0.034 | 0.34 (0.10–1.23) | 0.099 |
| Completed tumor resection | <0.001 | <0.001 | ||
| Yes | Reference | Reference | ||
| No | 14.69 (6.80–31.75) | <0.001 | 22.03 (8.31–58.36) | <0.001 |
| Unknown | 9.66 (4.64–20.10) | <0.001 | 21.62 (8.19–57.09) | <0.001 |
| Surgical approach | 0.543 | 0.129 | ||
| One-stage | Reference | Reference | ||
| Two-stage | 0.90 (0.29–2.84) | 0.858 | 0.73 (0.21–2.61) | 0.633 |
| Others | 1.36 (0.62–3.01) | 0.447 | 0.39 (0.15–1.01) | 0.052 |
PFS, progression-free survival; IVL, intravenous leiomyomatosis; HR, hazard ratio; CI, confidence interval.
Figure 2.

Kaplan–Meier analysis of high-risk factors for PFS in 361 IVL patients. PFS, progression-free survival; IVL, intravenous leiomyomatosis.
Figure 3.

Kaplan–Meier analysis of high-risk factors for PFS in 38 IVL patients from Qilu hospital of shandong university. PFS, progression-free survival; IVL, intravenous leiomyomatosis.
Discussion
The diagnosis and treatment of IVL have received increasing attention from clinicians. However, the available studies are still in the form of case reports and lack a cohort analysis. Our study included a total of 361 IVL patients, the clinical characteristics and pathological findings were described in detail. And the multivariable Cox proportional hazard regression analysis showed patients younger than 45 years and with uncompleted tumor resection were high-risk factors for PFS.
In this study, 27.7% of IVL patients showed no symptoms or signs. Common signs included pelvic pain, abdominal discomfort, and menorrhagia. In advanced-stage cases, when the inferior vena cava or the right heart and pulmonary artery were involved, some patients had dyspnea, chest pain, lower limb edema, and syncope, while some patients had no cardiopulmonary symptoms before the occurrence of cardiac insufficiency, pulmonary embolism, or sudden death (7, 13, 69, 244). Therefore, IVL has an insidious onset and complicated clinical manifestations, and lacks specificity, with patients usually noticing IVL only after the tumor has invaded the inferior vena cava or above, causing serious clinical symptoms that are difficult to diagnose.
In 2016, Ma et al. first proposed a clinical staging system that reflects the preoperative progression of tumors (261). Among the IVL patients admitted to the Qilu Hospital of Shandong University, stage III and IV patients only accounted for 23.7%, whereas in all the collected cases, they accounted for as high as 61.2% of the total number of patients. However, this did not represent the proportion of patients with IVL involving the inferior vena cava and cardiopulmonary system, as there might be many asymptomatic IVL patients; moreover, some early IVL patients might have been undiagnosed, or some early cases with relatively limited lesions might have been unreported due to the limitations of the medical treatment. Patients in stages III and IV have a higher risk of sudden or surgical deaths due to the possible tumor adhesion to the vascular wall, internal structure of the heart, or complete outflow tract obstruction (4, 13, 36). Eleven IVL-related deaths occurred in stage III and IV of all included cases, while no deaths occurred in stage I and II patients.
The exact pathogenesis of IVL is still unclear, and there are several theories about its origin. One theory held that IVL originated from the vessel wall (1); another theory postulated that IVL was the result of uterine leiomyoma invading the vascular muscle layer (262, 263); other researchers proposed that IVL might originate from the myometrium rather than fibroids (9, 238, 264). Kir et al. performed immunohistochemical ER and PR detection in tumor tissues and adjacent vascular walls of IVL patients, respectively, and the results showed that ER and PR were positively expressed to varying degrees (10%–60% and 10%–70%, respectively). However, ER and PR were absent or weakly expressed in the vascular endothelial and subendothelial cells. This confirmed that IVL originated from the uterus and not the blood vessel wall (262). In this study, immunohistochemical results were summarized, and the positive rates of ER and PR were 97.4% and 91.7%, respectively, confirming the hypothesis that IVL originates from the uterus to a certain extent. In addition, immunohistochemical analysis showed that the SMA and desmin positive rates were both 100%, suggesting that IVL has molecular cytogenetic characteristics similar to those of uterine leiomyoma (265). Ki-67 is widely used as a potential prognostic marker in the study of malignant diseases as it represents the degree of cell proliferation and is proportional to the degree of tumor malignancy (266, 267). Among the included IVL cases, 89.5% of patients had a Ki-67 positive rate of less than 5%. This indicated that although IVL shares certain biological behaviors with malignant tumors, the degree of malignancy was very low, and its mortality mainly resulted from complications caused by the cardiopulmonary metastasis of the tumors.
Surgical resection is the main treatment for IVL; however, there is still a lack of corresponding guidelines, and the current treatment is mainly based on limited case reports. Currently, the recommended surgical methods are radical tumor resection, including hysterectomy, bilateral salpingo-oophorectomy, and extrauterine tumor resection to remove all visible tumor and reduce the risk of future recurrence. Completed resection of all visible tumor can reduce the risk of recurrence, and several studies have reached this consistent conclusion (261, 268, 269). However, ovarian resection is still very cautiously applied, as the impact of oophorectomy on the endocrine function of women is crucial. As IVL is associated with high estrogen expression, it would be intuitive to assume that ovarian preservation is not recommended, and several studies have suggested bilateral oophorectomy to reduce recurrence (53, 261, 270); however, there is still insufficient evidence to confirm this conclusion. Peng J. et al. repoted a retrospective study of 166 IVL patients who accepted surgery treatment, similar to our findings, compared with total hysterectomy and bilateral salpingo-oophorectomy, total hysterectomy does not increase the risk of recurrence (odds ratio = 0.96, 95% CI 0.08–10.58, p = 0.96), but tumorectomy seems a high-risk factor of recurrence (odds ratio = 20.09, 95% CI 4.16–97.10, p < 0.01) (271).
Two surgical procedures were mainly performed in patients with lesions involving the cardiovascular system: one-stage surgery was a combined exploration of the chest, abdomen, and pelvis, with resection of the lesions in the pelvic and abdominal cavity and tumors involving the cardiovascular system, but conversely, two-stage surgery referred to the removal of lesions involving the cardiovascular system, followed by secondary surgery to remove the tumors in the abdominal and pelvic cavities. In a small number of cases, tumor resection of the cardiovascular system was performed only due to the patient's refusal to further surgery or the impossibility of removing the pelvic and intraperitoneal tumors. The two surgical procedures have advantages and disadvantages: extracorporeal circulation in one-stage surgery can effectively control intraoperative bleeding, protect vital organs, and provide better exposure to the surgical field and sufficient time for tumor resection. In addition, it can reduce the possibility of intraoperative embolism. However, one-stage surgery requires high anesthesia techniques and long operation times and increases the risk of coagulation dysfunction caused by heparin and the incidence of intraoperative and postoperative complications. Two-stage surgery avoids the shortcomings of one-stage surgery, but there is a risk of pulmonary embolism caused by postoperative residual tumor embolism, which increases pain and hospitalization costs (272). Furthermore, our research confirmed that there was no difference between one-stage and two-stage surgeries in the prognosis of the patients, and appropriate surgical methods could be selected according to their conditions.
Still, due to the limitations inherent to a retrospective study, our research had some limitations. First, the publication year of the literature included in the study was relatively early, and there was inevitably some missing information. Second, although several published articles have been included, the number of patients in our study was relatively small. In future studies, increasing the sample size may compensate for the above research limitations.
Conclusion
The clinical manifestations of patients with IVL are not typical, especially in the early stages of the disease, and they are mainly related to the site of tumor involvement. The postoperative recurrence and death incidence was higher in young and uncomplete tumor resection patients.
Funding Statement
This work was supported by the Tai-Shan Scholar Program of Shandong Province (grant number ts20070743), and the Natural Science Foundation of Shandong Province (grant number ZR2021QH050).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JC, HB, ZZ and RC: collection and analysis, writing–initial draft, and accomplishing the final version; GQ, CZ, QW, XM, HW, and ZD: data analysis; HB and BK: surgeons of the patients; XW and BK: Study concept, design, supervision and revision of the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1020004/full#supplementary-material.
References
- 1.Norris HJ, Parmley T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis: a clinical and pathologic study of 14 cases. Cancer. (1975) 36:2164–78. 10.1002/cncr.2820360935 [DOI] [PubMed] [Google Scholar]
- 2.Timmis AD, Smallpeice C, Davies AC, Macarthur AM, Gishen P, Jackson G. Intracardiac spread of intravenous leiomyomatosis with successful surgical excision. N Engl J Med. (1980) 303:1043–4. 10.1056/NEJM198010303031806 [DOI] [PubMed] [Google Scholar]
- 3.Birch-Hirschfeld FV. Lehrbuch der pathologischen anatomie. Leipzig, Germany: Vogel; (1896). 226 p. [Google Scholar]
- 4.Wang J, Yang J, Huang H, Li Y, Miao Q, Lu X, et al. Management of intravenous leiomyomatosis with intracaval and intracardiac extension. Obstet Gynecol. (2012) 120:1400–6. 10.1097/AOG.0b013e31826ebb90 [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Zhang G, Lang J, Liu B, Zhao D. Factors associated with recurrence after surgical resection in women with intravenous leiomyomatosis. Obstet Gynecol. (2016) 128:1018–24. 10.1097/AOG.0000000000001718 [DOI] [PubMed] [Google Scholar]
- 6.Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiogr Rev Publ Radiol Soc N Am Inc. (2008) 28:1931–48. 10.1148/rg.287085095 [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Wang X-M, Liu C, Xv Z, Wang D, Sun X, et al. Intravenous leiomyomatosis: diagnosis and follow-up with multislice computed tomography. Am J Surg. (2010) 200:e41–3. 10.1016/j.amjsurg.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 8.Osawa H, Hosaka S, Akashi O, Furukawa H, Egi K. A case of intravenous leiomyomatosis of uterine origin, extending through the inferior vena cava to right atrium. Gen Thorac Cardiovasc Surg. (2013) 61:104–7. 10.1007/s11748-012-0108-1 [DOI] [PubMed] [Google Scholar]
- 9.Yaguchi C, Oi H, Kobayashi H, Miura K, Kanayama N. A case of intravenous leiomyomatosis with high levels of hyaluronan. J Obstet Gynaecol Res. (2010) 36:454–8. 10.1111/j.1447-0756.2009.01147.x [DOI] [PubMed] [Google Scholar]
- 10.Rosa P, Pidhorecky I. A case of intravenous leiomyomatosis with involvement of a renal vein. Ann Vasc Surg. (2018) 53(271):e11–271.e13. 10.1016/j.avsg.2018.05.061 [DOI] [PubMed] [Google Scholar]
- 11.Fukuyama A, Yokoyama Y, Futagami M, Shigeto T, Wada R, Mizunuma H. A case of uterine leiomyoma with intravenous leiomyomatosis—histological investigation of the pathological condition. Pathol Oncol Res. (2011) 17:171–4. 10.1007/s12253-010-9265-7 [DOI] [PubMed] [Google Scholar]
- 12.Itani Y, Otsuka Y, Deguchi F, Watanabe S, Masuda Y, Miyazaki A, et al. A case report of intravenous leiomyomatosis extending into the heart. Heart Vessels. (2000) 15:291–4. 10.1007/PL00007272 [DOI] [PubMed] [Google Scholar]
- 13.Shi T, Shkrum MJ. A case report of sudden death from intracardiac leiomyomatosis. Am J Forensic Med Pathol. (2018) 39:119–22. 10.1097/PAF.0000000000000377 [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Baek JH. A challenging case of intracardiac leiomyomatosis accompanied by Pseudo-meigs syndrome originating from uterine leiomyoma. Ann Vasc Surg. (2019) 55:309.e5–e8. 10.1016/j.avsg.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 15.Leitman M, Kuperstein R, Medalion B, Stamler A, Porat E, Rosenblatt S, et al. A highly unusual right atrial mass presented in two women. Eur Heart J Cardiovasc Imaging. (2008) 9:833–4. 10.1093/ejechocard/jen173 [DOI] [PubMed] [Google Scholar]
- 16.Chino Y, Tsuyoshi H, Tsujikawa T, Okazawa H, Yoshida Y. A novel diagnostic strategy using 16α-[18F]-fluoro-17-β-estradiol (18F-FES) PET/MRI to achieve complete resection of intravenous leiomyomatosis in reproductive-age women. Clin Nucl Med. (2017) 42:e335–6. 10.1097/RLU.0000000000001678 [DOI] [PubMed] [Google Scholar]
- 17.Rispoli P, Santovito D, Tallia C, Varetto G, Conforti M, Rinaldi M. A one-stage approach to the treatment of intravenous leiomyomatosis extending to the right heart. J Vasc Surg. (2010) 52:212–5. 10.1016/j.jvs.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 18.Kaur S, Tongaonkar H, Maheshwari A, Menon S. A rare case of recurrent intravenous leiomyomatosis: role of GnRH analogues? Indian J Cancer. (2015) 52:161. 10.4103/0019-509X.175577 [DOI] [PubMed] [Google Scholar]
- 19.Caldentey G, Flores E, San Antonio R, Caixal G, Sánchez P. A rare cause of intracardiac mass. Int J Cardiol. (2016) 223:91–2. 10.1016/j.ijcard.2016.08.140 [DOI] [PubMed] [Google Scholar]
- 20.Bender LC, Mitsumori LM, Lloyd KA, Stambaugh LE. AIRP Best cases in radiologic-pathologic correlation: intravenous leiomyomatosis. RadioGraphics. (2011) 31:1053–8. 10.1148/rg.314115013 [DOI] [PubMed] [Google Scholar]
- 21.Jan S, Dillon EH, Epstein NF. AJR Teaching file: right atrial mass in a woman with uterine fibroids. Am J Roentgenol. (2009) 192:S53–6. 10.2214/AJR.07.7080 [DOI] [PubMed] [Google Scholar]
- 22.Li M, Guo C, Lyu Y-H, Zhang M-B, Wang Z-L. An unusual case of intravenous leiomyomatosis involving the right atrium. Chin Med J. (2019) 132:474–6. 10.1097/CM9.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Özer N, Engin H, Akgül E, Şahiner L, Atalar E, Aksöyek S, et al. An unusual case of recurrent mass in the right atrium: intravenous leiomyomatosis. Echocardiography. (2005) 22:514–6. 10.1111/j.1540-8175.2005.04053.x [DOI] [PubMed] [Google Scholar]
- 24.Fang F, Lam Y-Y. An unusual cause of right heart failure in a patient with previous hysterectomy. J Ultrasound Med. (2010) 29:1647–50. 10.7863/jum.2010.29.11.1647 [DOI] [PubMed] [Google Scholar]
- 25.Oliveira L, Ramos S. Anesthetic approach for a clinical case of intravenous leiomyomatosis: case report. Braz J Anesthesiol Engl Ed. (2013) 63:504–7. 10.1016/j.bjane.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 26.Cornélis F, Belleannée G, Lederlin M. Cardiac extension of an intravascular leiomyomatosis 43 years after hysterectomy. J Thorac Cardiovasc Surg. (2012) 144:e3–5. 10.1016/j.jtcvs.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 27.Low G, Rouget AC, Crawley C. Case 188: intravenous leiomyomatosis with intracaval and intracardiac involvement. Radiology. (2012) 265:971–5. 10.1148/radiol.12111246 [DOI] [PubMed] [Google Scholar]
- 28.Lee E, LaBounty T, Romano M, Agarwal PP. Case of the season: intravenous leiomyomatosis: a rare cause of intracardiac mass. Semin Roentgenol. (2020) 55:226–9. 10.1053/j.ro.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 29.Cui Y, Li M, Guo H, Wang L, Zhang S. Case report of intravenous leiomyoma with intracaval and intracardiac extension. Int J Gynecol Obstet. (2017) 137:199–200. 10.1002/ijgo.12124 [DOI] [PubMed] [Google Scholar]
- 30.Gawne-Cain ML, Wilson AG, Corbishley C, Keating V, Joseph AE. Case report: intravenous leiomyomatosis, an unusual cause of intracardiac filling defect. Clin Radiol. (1995) 50:123–5. 10.1016/s0009-9260(05)82996-4 [DOI] [PubMed] [Google Scholar]
- 31.Little SJ, Van der Heusen F, Thornton KC. Complete intraoperative transesophageal echocardiogram imaging of the extent of an Inferior vena Cava mass guides surgical management. Anesth Analg. (2010) 111:1125–7. 10.1213/ANE.0b013e3181f1f919 [DOI] [PubMed] [Google Scholar]
- 32.Wei J-L, Ji X, Zhang P, Chen W-J, Zhao Y-N, Liu M. Complete intravenous leiomyomatosis: a case report and literature review. Ann Palliat Med. (2021) 10:12039–45. 10.21037/apm-21-3093 [DOI] [PubMed] [Google Scholar]
- 33.Li H, Xu D, Lu W, Wang C. Complete resection of intracardiac leiomyomatosis through an abdominal approach under peripheral cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2016) 152:e91–3. 10.1016/j.jtcvs.2016.06.037 [DOI] [PubMed] [Google Scholar]
- 34.Sun R, Guan H, Li H, Bai Y, Wang F, Li C. Computed tomography evaluation of extensive intravenous angioleiomyoma: a case report. BMC Med Imaging. (2020) 20:1–7. 10.1186/s12880-020-0417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan LB, Al-Nafussi A, Beattie G. Cotyledonoid hydropic intravenous leiomyomatosis: a new variant leiomyoma: cotyledonoid intravenous leiomyomatosis. Histopathology. (2002) 40:245–52. 10.1046/j.1365-2559.2002.01359.x [DOI] [PubMed] [Google Scholar]
- 36.Burke M, Opeskin K. Death due to intravenous leiomyomatosis extending to the right pulmonary artery. Pathology. (2004) 36:202–3. 10.1080/00313020410001672075 [DOI] [PubMed] [Google Scholar]
- 37.López FM B, Martínez-Enriquez A, Castrejón-Aivar FJ, Ruanova-León D, Yánez-Gutiérrez L. Echocardiographic study of an intravenous leiomyoma: case report and review of the literature. Echocardiogr Mt Kisco N. (2003) 20:723–5. 10.1111/j.0742-2822.2003.02152.x [DOI] [PubMed] [Google Scholar]
- 38.Li R, Zhang C, Yang Y, Song L, Wang Z, Luo X, et al. Echocardiographic study of intravenous leiomyomatosis with intracardiac extension: two case reports and review of the literature. Heart Lung Circ. (2013) 22:690–2. 10.1016/j.hlc.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 39.Bara C, Pi L, Haverich A, Winkler M, Pichlmaier M. Echocardiography in leiomyomatosis of the uterus: how to guide your surgeon. Clin Res Cardiol. (2008) 97:135–8. 10.1007/s00392-007-0613-x [DOI] [PubMed] [Google Scholar]
- 40.Tian X, Zhang R, Shen J, Ge X. Extension of uterine intravenous leiomyomatosis to the right heart. J Cardiovasc Comput Tomogr. (2016) 10:261–2. 10.1016/j.jcct.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 41.Deac MO, Sheppard MN, Moat N, Burke SJ, Christmas T, Mohiaddin RH. From uterus to pulmonary embolus: an uncommon association. Circulation. (2009) 120. 10.1161/CIRCULATIONAHA.108.826107 [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Miao Q, Liu J, Wang C, Zhang C, Zhai H. Giant intravenous leiomyomatosis with intracardiac extension. Ann Thorac Surg. (2012) 94:1013. 10.1016/j.athoracsur.2012.02.047 [DOI] [PubMed] [Google Scholar]
- 43.Matos AP, Ramalho M, Palas J, Herédia V. Heart extension of an intravenous leiomyomatosis. Clin Imaging. (2013) 37:369–73. 10.1016/j.clinimag.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 44.Hinojosa CA, Medina-Franco H, Orozco-Zepeda V, Martinez Mijangos O, Valdes KA, Aragon Han P. Infrarenal transcaval extraction of intracardiac leiomyomatosis. Ann Vasc Surg. (2013) 27:238.e1–e4. 10.1016/j.avsg.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 45.Skripochnik E, Terrana LM, Labropoulos N, Henretta M, Griffin T, Loh SA. Inguinal pain and fullness due to an intravascular leiomyoma in the external iliac vein. J Vasc Surg Cases Innov Tech. (2017) 3:102–4. 10.1016/j.jvscit.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizuno T, Mihara A, Arai H. Intracardiac and intravascular leiomyomatosis associated with a pelvic arterio-venous fistula. Ann Transl Med. (2014) 2:48. 10.3978/j.issn.2305-5839.2014.04.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spanuchart I, Satitthummanid S, Cheanvechai C, Chantranuwatana P, Trivijitsilp P, Chattranukulchai P, et al. Intracardiac and intravenous leiomyomatosis. J Am Coll Cardiol. (2012) 60:e27. 10.1016/j.jacc.2012.02.088 [DOI] [PubMed] [Google Scholar]
- 48.Kaszar-Seibert DJ, Gauvin GP, Rogoff PA, Vittimberga FJ, Margolis S, Hilgenberg AD, et al. Intracardiac extension of intravenous leiomyomatosis. Radiology. (1988) 168:409–10. 10.1148/radiology.168.2.3393658 [DOI] [PubMed] [Google Scholar]
- 49.Price JD, Anagnostopoulos C, Benvenisty A, Kothuru RK, Balaram SK. Intracardiac extension of intravenous leiomyomatosis. Ann Thorac Surg. (2017) 103:e145–7. 10.1016/j.athoracsur.2016.07.037 [DOI] [PubMed] [Google Scholar]
- 50.Nithiyanandhan P, Suneel PR, Azeez AM, Pillai VV, Pitchai S. Intracardiac extension of intravenous leiomyomatosis in a patient with vascular pelvic tumor and prior hysterectomy: a case report. Ann Card Anaesth. (2021) 24:483–6. 10.4103/aca.ACA_25_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling FT, David TE, Merchant N, Yu E, Butany JW. Intracardiac extension of intravenous leiomyomatosis in a pregnant woman: a case report and review of the literature. Can J Cardiol. (2000) 16:73–9. [PubMed] [Google Scholar]
- 52.Abdelbar A, Schmitt M, Jenkins P, Hoschtitzky JA. Intracardiac extension of uterine leiomyomatosis. BMJ Case Rep. (2015) 2015:bcr2014209278. 10.1136/bcr-2014-209278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stilidi I, Paianidi J, Bokhian V, Andreeva J, Shevchuk A, Ramirez PT. Intracardiac intravenous leiomyomatosis: diagnosis and management. Int J Gynecol Cancer. (2020) 30:1243–7. 10.1136/ijgc-2020-001614 [DOI] [PubMed] [Google Scholar]
- 54.Kullo IJ, Oh JK, Keeney GL, Khandheria BK, Seward JB. Intracardiac leiomyomatosis. Chest. (1999) 115:587–91. 10.1378/chest.115.2.587 [DOI] [PubMed] [Google Scholar]
- 55.Fogarty SJ, Hart G, Nicklin J, Venkatesh B, Boyne N, Wong DC, et al. Intracardiac leiomyomatosis—an unusual cause of syncope in a middle-aged woman. Heart Lung Circ. (2017) 26:e22–5. 10.1016/j.hlc.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro V, Almeida J, Madureira AJ, Lopez E, Machado L, Albuquerque R, et al. Intracardiac leiomyomatosis complicated by pulmonary embolism: a multimodality imaging case of a rare entity. Can J Cardiol. (2013) 29:1743.e1–.e3. 10.1016/j.cjca.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 57.Alves AJT, Ferreira MA, Gallego-Poveda J, Matos A, Costa-Silva A, Nobre Â, et al. Intracardiac leiomyomatosis presenting as an intraoperative consultation. Pathol Res Pract. (2016) 212:578–81. 10.1016/j.prp.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 58.Politzer F, Kronzon I, Wieczorek R, Feiner H, De Marco LE, Weintraub PR, et al. Intracardiac leiomyomatosis: diagnosis and treatment. J Am Coll Cardiol. (1984) 4:629–34. 10.1016/s0735-1097(84)80113-8 [DOI] [PubMed] [Google Scholar]
- 59.Bennett ES, Arora NS, Kay M, Robinson TT, Fergus I. Intracardiac leiomyomatosis: iliac vein to right-ventricular outflow tract. Nat Clin Pract Cardiovasc Med. (2005) 2:369–72. 10.1038/ncpcardio0250 [DOI] [PubMed] [Google Scholar]
- 60.Vaideeswar P, Kulkarni DV, Karunamurthy A, Hira P. Intracardiac leiomyomatosis: report of two cases. Indian J Pathol Microbiol. (2011) 54:158–60. 10.4103/0377-4929.77388 [DOI] [PubMed] [Google Scholar]
- 61.Lo KW, Lau TK. Intracardiac leiomyomatosis. Case report and literature review. Arch Gynecol Obstet. (2001) 264:209–10. 10.1007/s004040000115 [DOI] [PubMed] [Google Scholar]
- 62.Stoleriu C, Rizas K, Gawaz M, Geisler T. Intracaval and intracardiac leiomyomatosis of uterine origin. BMJ Case Rep. (2013) 2013::bcr2012008368. 10.1136/bcr-2012-008368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarbay B C, Kır G, Gursoy F. Intravascular adenomyomatosis: a rare variant of ıntravascular leiomyomatosis. J Obstet Gynaecol. (2017) 37:118–20. 10.1080/01443615.2016.1225020 [DOI] [PubMed] [Google Scholar]
- 64.Bahary C, Gorodeski I, Nilly M, Neri A, Avidor I, Garti I. Intravascular leiomyomatosis. Obstet Gynecol. (1982) 59:S73–7. [PubMed] [Google Scholar]
- 65.Arif S, Ganesan R, Spooner D. Intravascular leiomyomatosis and benign metastasizing leiomyoma: an unusual case. Int J Gynecol Cancer. (2006) 16:1448–50. 10.1111/j.1525-1438.2006.00607.x [DOI] [PubMed] [Google Scholar]
- 66.Grove A, Jørgensen A. Intravascular leiomyomatosis of the uterus. Pathol Res Pract. (1996) 192:949–56. 10.1016/S0344-0338(96)80078-1 [DOI] [PubMed] [Google Scholar]
- 67.Aydin E, Köse O, Kocaaslan C, Aldağ M, MŞ B. Intravascular leiomyomatosis with extension into the pulmonary artery. J Card Surg. (2018) 33:453–4. 10.1111/jocs.13746 [DOI] [PubMed] [Google Scholar]
- 68.Harnoy Y, Rayar M, Levi Sandri GB, Zamreek A, Turner K, Sulpice L, et al. Intravascular leiomyomatosis with intracardiac extension. Ann Vasc Surg. (2016) 30:306.e13–e15. 10.1016/j.avsg.2015.06.094 [DOI] [PubMed] [Google Scholar]
- 69.Miro A, Coppola Bottazzi E, Vanella S, Palma T, Noviello A, Apicella I, et al. Intravascular leiomyomatosis with intracardiac extension: a toraco-abdominal approach. J Surg Case Rep. (2021) 2021:rjab249. 10.1093/jscr/rjab249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashiguchi J, Ito M, Kishikawa M, Sekine I, Kase Y. Intravascular leiomyomatosis with uterine lipoleiomyoma. Gynecol Oncol. (1994) 52:94–8. 10.1006/gyno.1994.1018 [DOI] [PubMed] [Google Scholar]
- 71.Simon AJ, Parry-Smith WR, Redman CWE, Kodampur M, Todd R, Satur C, et al. Intravascular leiomyomatosis: a case report and review of the literature. J Obstet Gynaecol. (2015) 35:539–40. 10.3109/01443615.2014.978847 [DOI] [PubMed] [Google Scholar]
- 72.Brunel I, Iacoponi S, Hernandez A, Diestro MD, De Santiago J, Zapardiel I. Intravascular leiomyomatosis: an exceptional entity. Clin Exp Obstet Gynecol. (2016) 43:443–5. 10.12891/ceog2141.2016 [DOI] [PubMed] [Google Scholar]
- 73.Stegmann T, Garcia-Gallont R, Döring W. Intravascular leiomyomatosis: report of a case and review of the literature. Thorac Cardiovasc Surg. (1987) 35:157–60. 10.1055/s-2007-1020220 [DOI] [PubMed] [Google Scholar]
- 74.Brescia RJ, Tazelaar HD, Hobbs J, Miller AW. Intravascular lipoleiomyomatosis: a report of two cases. Hum Pathol. (1989) 20:252–6. 10.1016/0046-8177(89)90132-9 [DOI] [PubMed] [Google Scholar]
- 75.Anichini C, Calamai G, Pedemonte E, Moroni M, Tozzini S, Nesi G. Intravenous leiomyoma with cardiac involvement. Int Angiol J Int Union Angiol. (2001) 20:345–7. [PubMed] [Google Scholar]
- 76.Ahmed M, Zangos S, Bechstein WO, Vogl TJ. Intravenous leiomyomatosis. Eur Radiol. (2004) 14. 10.1007/s00330-003-2186-z [DOI] [PubMed] [Google Scholar]
- 77.Diakomanolis E, Elsheikh A, Sotiropoulou M, Voulgaris Z, Vlachos G, Loutradis D, et al. Intravenous leiomyomatosis. Arch Gynecol Obstet. (2003) 267:256–7. 10.1007/s00404-002-0443-z [DOI] [PubMed] [Google Scholar]
- 78.Grella L, Arnold TE, Kvilekval KH, Giron F. Intravenous leiomyomatosis. J Vasc Surg. (1994) 20:987–94. 10.1016/0741-5214(94)90237-2 [DOI] [PubMed] [Google Scholar]
- 79.Konrad P, Mellblom L. Intravenous leiomyomatosis. Acta Obstet Gynecol Scand. (1989) 68:371–6. 10.3109/00016348909028675 [DOI] [PubMed] [Google Scholar]
- 80.Mariyappa N, Manikyam UK, Krishnamurthy D, Preeti K, Agarwal Y, Prakar U. Intravenous leiomyomatosis. Niger J Surg. (2012) 18:105–6. 10.4103/1117-6806.103122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scharfenberg JC, Geary WL. Intravenous leiomyomatosis. Obstet Gynecol. (1974) 43:909–14. [PubMed] [Google Scholar]
- 82.Kuklik E, Drelich-Zbroja A, Kuczyńska M, Szmygin M, Grzycka-Kowalczyk L, Jargiełło T. Intravenous leiomyomatosis as an unusual cause of the misdiagnosis of deep vein thrombosis. Pol Arch Intern Med. (2021) 131:75–7. 10.20452/pamw.15654 [DOI] [PubMed] [Google Scholar]
- 83.Kuenen BC, Slee PH, Seldenrijk CA, Wagenaar SS. Intravenous leiomyomatosis complicated by budd-chiari syndrome. Postgrad Med J. (1996) 72:686–8. 10.1136/pgmj.72.853.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanaka YO, Jikuya T, Iijima T, Sakakibara Y, Itai Y. Intravenous leiomyomatosis diagnosed by plain radiographs. Clin Radiol. (2002) 57:1037–40. 10.1053/crad.2002.1078 [DOI] [PubMed] [Google Scholar]
- 85.Gunderson CC. Intravenous leiomyomatosis disguised as a large deep vein thrombosis. J Radiol Case Rep. (2016) 10:29–35. 10.3941/jrcr.v10i5.2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Virzì G, Ragazzi S, Bussichella F, D’Agati P, Caputo S, Scaravilli F, et al. Intravenous leiomyomatosis extending from the inferior caval vein to the pulmonary artery. J Thorac Cardiovasc Surg. (2007) 133:831–2. 10.1016/j.jtcvs.2006.10.050 [DOI] [PubMed] [Google Scholar]
- 87.Koter SH, Tiesenhausen K. Intravenous leiomyomatosis extending from the pelvic veins to the heart. Eur J Vasc Endovasc Surg. (2017) 54:219. 10.1016/j.ejvs.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 88.Qiuying L. Intravenous leiomyomatosis extending into right heart: a case report. Turk J Thorac Cardiovasc Surg. (2020) 28:386–9. 10.5606/tgkdc.dergisi.2020.18916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Topcuoglu MS, Yaliniz H, Poyrazoglu H, Tokcan A, Demir SC, Bozkurt A, et al. Intravenous leiomyomatosis extending into the right ventricle after subtotal hysterectomy. Ann Thorac Surg. (2004) 78:330–2. 10.1016/S0003-4975(03)01371-7 [DOI] [PubMed] [Google Scholar]
- 90.Nishizawa J, Matsumoto M, Sugita T, Matsuyama K, Tokuda Y, Yoshida K, et al. Intravenous leiomyomatosis extending into the right ventricle association with pulmonary metastasis and extensive arteriovenous fistula. J Am Coll Surg. (2004) 198:842–3. 10.1016/j.jamcollsurg.2003.06.009 [DOI] [PubMed] [Google Scholar]
- 91.Okamoto H, Itoh T, Morita S, Matsuura A, Yasuura K. Intravenous leiomyomatosis extending into the right ventricle: one-stage radical excision during hypothermic circulatory arrest. Thorac Cardiovasc Surg. (1994) 42:361–3. 10.1055/s-2007-1016525 [DOI] [PubMed] [Google Scholar]
- 92.Wakiyama H, Sugimoto T, Ataka K, Yamashita C, Tsuji Y, Nakagiri K, et al. Intravenous leiomyomatosis extending into the right ventricular cavity: one-stage radical operation using cardiopulmonary bypass–a case report. Angiology. (2000) 51:505–9. 10.1177/000331970005100608 [DOI] [PubMed] [Google Scholar]
- 93.Bernal JM, Gutiérrez F, Arnaiz E, Pontón A. Intravenous leiomyomatosis extending to both pulmonary arteries: the longest tumour. Eur J Cardiothorac Surg. (2009) 35:361. 10.1016/j.ejcts.2008.10.054 [DOI] [PubMed] [Google Scholar]
- 94.Khayata GM, Thwaini S, Aswad SG. Intravenous leiomyomatosis extending to the heart. Int J Gynaecol Obstet. (2003) 80:59–60. 10.1016/s0020-7292(02)00336-3 [DOI] [PubMed] [Google Scholar]
- 95.Polizzi V, Pergolini A, Zampi G, Lo Presti M, Pino PG, Cartoni D, et al. Intravenous leiomyomatosis extending to the heart: a multimodality imaging approach. Herz. (2014) 39:720–1. 10.1007/s00059-013-3862-7 [DOI] [PubMed] [Google Scholar]
- 96.Chan MG, Huang K-G. Intravenous leiomyomatosis in the parametrium. J Minim Invasive Gynecol. (2016) 23:849–50. 10.1016/j.jmig.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 97.Wang L, He Y-Y, Ren X-D, Zhang J-Y. Intravenous leiomyomatosis involving the right atrium: a case report. Asian J Surg. (2021) 44:904–5. 10.1016/j.asjsur.2021.03.040 [DOI] [PubMed] [Google Scholar]
- 98.Murphy AN, Byrne D, Salati U, Lawler L. Intravenous leiomyomatosis manifesting as saddle embolism. BMJ Case Rep. (2019) 12:e228267. 10.1136/bcr-2018-228267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han Y, Chung Y-J, Shin I, Park JY, Shim S, Hijazi A, et al. Intravenous leiomyomatosis misdiagnosed with large thrombosis in inferior vena cava. Taiwan J Obstet Gynecol. (2021) 60:367–9. 10.1016/j.tjog.2021.01.019 [DOI] [PubMed] [Google Scholar]
- 100.Shida T, Yoshimura M, Chihara H, Nakamura K. Intravenous leiomyomatosis of the pelvis with reextension into the heart. Ann Thorac Surg. (1986) 42:104–6. 10.1016/S0003-4975(10)61850-4 [DOI] [PubMed] [Google Scholar]
- 101.Fang H, You Y, Cai F, Yang Y, Yang C, Lv P. Intravenous leiomyomatosis of the subclavian vein. J Vasc Surg Venous Lymphat Disord. (2017) 5:254–6. 10.1016/j.jvsv.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 102.Bodner K, Bodner-Adler B, Wierrani F, Mayerhofer K, Grünberger W. Intravenous leiomyomatosis of the uterus. Anticancer Res. (2002) 22:1881–3. [PubMed] [Google Scholar]
- 103.Matsumoto K, Nouga K, Yokoyama I, Ishii S, Wakabayashi G, Yoshida Y. Intravenous leiomyomatosis of the uterus. Eur J Vasc Surg. (1994) 8:377–8. 10.1016/s0950-821x(05)80163-x [DOI] [PubMed] [Google Scholar]
- 104.Garcia F, Villanueva R, Narciso F, Aventura A. Intravenous leiomyomatosis of the uterus and pelvis presenting as a cardiac tumor. Ann Thorac Surg. (1986) 42:S41–3. 10.1016/S0003-4975(10)64641-3 [DOI] [PubMed] [Google Scholar]
- 105.Marshall JF, Morris DS. Intravenous leiomyomatosis of the uterus and pelvis: case report. Ann Surg. (1959) 149:126–34. 10.1097/00000658-195901000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tierney W, Ehrlich C, Bailey J, King R, Roth L, Wann L. Intravenous leiomyomatosis of the uterus with extension into the heart. Am J Med. (1980) 69:471–5. 10.1016/0002-9343(80)90022-4 [DOI] [PubMed] [Google Scholar]
- 107.Lou Y-F, Shi X-P, Song Z-Z. Intravenous leiomyomatosis of the uterus with extension to the right heart. Cardiovasc Ultrasound. (2011) 9:25. 10.1186/1476-7120-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsumoto K, Yamamoto T, Hisayoshi T, Asano G. Intravenous leiomyomatosis of the uterus with multiple pulmonary metastases associated with large bullae-like cyst formation. Pathol Int. (2001) 51:396–401. 10.1046/j.1440-1827.2001.01205.x [DOI] [PubMed] [Google Scholar]
- 109.Konishi I, Fujii S, Takakura K, Ukita M, Mori T. Intravenous leiomyomatosis of the uterus: a light and electron microscopic study. Asia Oceania J Obstet Gynaecol. (1987) 13:417–26. 10.1111/j.1447-0756.1987.tb00285.x [DOI] [PubMed] [Google Scholar]
- 110.Andrade LA, Torresan RZ, Sales JF, Vicentini R, De Souza GA. Intravenous leiomyomatosis of the uterus. A report of three cases. Pathol Oncol Res POR. (1998) 4:44–7. 10.1007/BF02904695 [DOI] [PubMed] [Google Scholar]
- 111.Edwards DL, Peacock JF. Intravenous leiomyomatosis of the uterus. Report of 2 cases. Obstet Gynecol. (1966) 27:176–81. [PubMed] [Google Scholar]
- 112.Harper R, Scully R. Intravenous leiomyomatosis of uterus—report of four cases. Obstet Gynecol. (1961) 18:519. [PubMed] [Google Scholar]
- 113.Barksdale J, Abolhoda A, Saremi F. Intravenous leiomyomatosis presenting as acute budd-chiari syndrome. J Vasc Surg. (2011) 54:860–3. 10.1016/j.jvs.2011.03.261 [DOI] [PubMed] [Google Scholar]
- 114.Carr RJ, Hui P, Buza N. Intravenous leiomyomatosis revisited: an experience of 14 cases at a single medical center. Int J Gynecol Pathol. (2015) 34:169–76. 10.1097/PGP.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 115.Biri A, Korucuoglu U, Zumrutbas N, Tiras B, Guner H. Intravenous leiomyomatosis treated with aromatase inhibitor therapy. Int J Gynecol Obstet. (2008) 101:299–300. 10.1016/j.ijgo.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 116.Mizoguchi C, Matsumoto H, Nasu K, Arakane M, Kai K, Narahara H. Intravenous leiomyomatosis treated with radical hysterectomy and adjuvant aromatase inhibitor therapy: surgical resection for IVL. J Obstet Gynaecol Res. (2016) 42:1405–8. 10.1111/jog.13063 [DOI] [PubMed] [Google Scholar]
- 117.Lam PM, Lo KWK, Yu MMM, Lau TK, Cheung TH. Intravenous leiomyomatosis with atypical histologic features: a case report. Int J Gynecol Cancer. (2003) 13:83–7. 10.1046/j.1525-1438.2003.13008.x [DOI] [PubMed] [Google Scholar]
- 118.Ayling O, Roy T, Cusimano RJ, McGilvray I, Roche-Nagle G. Intravenous leiomyomatosis with cardiac extension. VASA Z Gefasskrankheiten. (2015) 44:151–5. 10.1024/0301-1526/a000422 [DOI] [PubMed] [Google Scholar]
- 119.Saitoh M, Hayasaka T, Nakahara K, Ohmichi M, Shimazaki Y, Kurachi H. Intravenous leiomyomatosis with cardiac extension. Gynecol Obstet Invest. (2004) 58:168–70. 10.1159/000079658 [DOI] [PubMed] [Google Scholar]
- 120.Suginami H, Kaura R, Ochi H, Matsuura S. Intravenous leiomyomatosis with cardiac extension: successful surgical management and histopathologic study. Obstet Gynecol. (1990) 76:527–9. [PubMed] [Google Scholar]
- 121.Harris LM, Karakousis CP. Intravenous leiomyomatosis with cardiac extension: tumor thrombectomy through an abdominal approach. J Vasc Surg. (2000) 31:1046–51. 10.1067/mva.2000.104601 [DOI] [PubMed] [Google Scholar]
- 122.Marom D, Pitlik S, Sagie A, Ovadia Y, Bishara J. Intravenous leiomyomatosis with cardiac involvement in a pregnant woman. Am J Obstet Gynecol. (1998) 178:620–1. 10.1016/S0002-9378(98)70453-3 [DOI] [PubMed] [Google Scholar]
- 123.Bertrand P, Amabile P, Hardwigsen J, Campan P, Le Treut YP. Intravenous leiomyomatosis with caval involvement: report of a case with radical resection and venous replacement. Arch Surg. (1998) 133:460–2. 10.1001/archsurg.133.4.460 [DOI] [PubMed] [Google Scholar]
- 124.He J, Chen Z-B, Wang S-M, Liu M-B, Li Z-G, Li H-Y, et al. Intravenous leiomyomatosis with different surgical approaches: three case reports. World J Clin Cases. (2019) 7:347–56. 10.12998/wjcc.v7.i3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fang B-R, Ng Y-T, Yeh C-H. Intravenous leiomyomatosis with extension to the heart: echocardiographic features: a case report. Angiology. (2007) 58:376–9. 10.1177/0003319707302504 [DOI] [PubMed] [Google Scholar]
- 126.Kocica MJ, Vranes MR, Kostic D, Kostic NK, Lackovic V, Mihajlovic VB, et al. Intravenous leiomyomatosis with extension to the heart: rare or underestimated? J Thorac Cardiovasc Surg. (2005) 130:1724–6. 10.1016/j.jtcvs.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 127.Qin X, Liang W, Yue H, Zhang T, Bian L, Wen X, et al. Intravenous leiomyomatosis with extension to the pulmonary artery associated with syncope. J Card Surg. (2018) 33:753–5. 10.1111/jocs.13933 [DOI] [PubMed] [Google Scholar]
- 128.Zhang G, Yu X, Lang J. Intravenous leiomyomatosis with inferior vena cava or intracardiac extension and concurrent bilateral multiple pulmonary nodules: a report of 2 cases. Medicine. (2016) 95:e4722. 10.1097/MD.0000000000004722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ricci MA, Cloutier LM, Mount S, Welander C, Leavitt BJ. Intravenous leiomyomatosis with intracardiac extension. Cardiovasc Surg Lond Engl. (1995) 3:693–6. 10.1016/0967-2109(96)82871-7 [DOI] [PubMed] [Google Scholar]
- 130.Song BG, Park YH, Kang GH, Chun WJ, Oh JH. Intravenous leiomyomatosis with intracardiac extension. Asian Cardiovasc Thorac Ann. (2011) 19:179. 10.1177/0218492311400100 [DOI] [PubMed] [Google Scholar]
- 131.Clay TD, Dimitriou J, McNally OM, Russell PA, Newcomb AE, Wilson AM. Intravenous leiomyomatosis with intracardiac extension—a review of diagnosis and management with an illustrative case. Surg Oncol. (2013) 22:e44–52. 10.1016/j.suronc.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 132.Yu L, Shi E, Gu T, Xiu Z, Fang Q, Wang C. Intravenous leiomyomatosis with intracardiac extension: a report of two cases. J Card Surg. (2011) 26:56–60. 10.1111/j.1540-8191.2010.01148.x [DOI] [PubMed] [Google Scholar]
- 133.Lee PK, David TE, Sloggett C, Ross JR. Intravenous leiomyomatosis with intracardiac extension: an unusual cause of cardiac syncope. CMAJ. (1990) 142:1257–9. [PMC free article] [PubMed] [Google Scholar]
- 134.Jain N, Rissam HK, Mittal UK, Sharma A. Intravenous leiomyomatosis with intracardiac extension: an unusual presentation of uterine leiomyoma and evaluation with 256-slice dual-source multidetector CT and cardiac MRI. BMJ Case Rep. (2015):bcr2015211712. 10.1136/bcr-2015-211712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh T, Lamont PM, Otton GR, Thomson DS. Intravenous leiomyomatosis with intracardiac extension: first reported case in Australia. Heart Lung Circ. (2010) 19:50–2. 10.1016/j.hlc.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 136.To WW, Ngan HY, Collins RJ. Intravenous leiomyomatosis with intracardiac involvement. Int J Gynaecol Obstet. (1993) 42:37–40. 10.1016/0020-7292(93)90443-z [DOI] [PubMed] [Google Scholar]
- 137.Xia M, Liu J, Xiang X, Xu M, He M. Intravenous leiomyomatosis with intracardiac involvement. Arch Gynecol Obstet. (2014) 290:595–9. 10.1007/s00404-014-3278-5 [DOI] [PubMed] [Google Scholar]
- 138.Mullings AM, Char G, Martin MP, Frederick J, Walton N, Pickering K. Intravenous leiomyomatosis with massive ascites. Int J Gynaecol Obstet. (1992) 37:285–8. 10.1016/0020-7292(92)90331-c [DOI] [PubMed] [Google Scholar]
- 139.Kong L-Y, Chen L-L, Xiang W, Liu F. Intravenous leiomyomatosis with paradoxical embolism: unusual presentation of uterine leiomyoma. Circ Cardiovasc Imaging. (2020) 13:e009930. 10.1161/CIRCIMAGING.119.009930 [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Mei F, Yang C, Lv P, Ouyang C, Jin B. Intravenous leiomyomatosis with right heart involvement—a report of 4 cases and literature review. J Huazhong Univ Sci Technolog Med Sci. (2011) 31:586–8. 10.1007/s11596-011-0494-0 [DOI] [PubMed] [Google Scholar]
- 141.Nishida N, Nonoshita A, Kojiro S, Takemoto Y, Kojiro M. Intravenous leiomyomatosis with uterine leiomyoma and adenomyosis: a case presentation and brief comment on the histogenesis. Kurume Med J. (2003) 50:173–5. 10.2739/kurumemedj.50.173 [DOI] [PubMed] [Google Scholar]
- 142.Mata RP, Urzal C, Belo AI, Guerreiro F. Intravenous leiomyomatosis without extrapelvic involvement. Bmj Case Rep. (2020) 13:e234864. 10.1136/bcr-2020-234864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zaidi AZ, Hawley I, Zaidi J. Intravenous leiomyomatosis—a case report. J Obstet Gynaecol. (2021) 41:996–7. 10.1080/01443615.2020.1738362 [DOI] [PubMed] [Google Scholar]
- 144.Ohmori T, Uraga N, Tabei R, Abe M, Sumimoto T, Hamada M, et al. Intravenous leiomyomatosis: a case report emphasizing the vascular component. Histopathology. (1988) 13:470–2. 10.1111/j.1365-2559.1988.tb02066.x [DOI] [PubMed] [Google Scholar]
- 145.Cruz I, João I, Stuart B, Iala M, Bento L, Cotrim C, et al. Intravenous leiomyomatosis: a rare cause of intracardiac mass. Rev Port Cardiol. (2014) 33:735.e1–e5. 10.1016/j.repc.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 146.Rosenberg JM, Marvasti MA, Obeid A, Johnson LW, Bonaventura M. Intravenous leiomyomatosis: a rare cause of right sided cardiac obstruction. Eur J Cardio Thorac Surg. (1988) 2:58–60. 10.1016/1010-7940(88)90099-1 [DOI] [PubMed] [Google Scholar]
- 147.Florou V, Jarboe E, Deftereos G. Intravenous leiomyomatosis: a rare diagnosis with aggressive potential. JCO Oncol Pract. (2021) 17:206–8. 10.1200/OP.20.00666 [DOI] [PubMed] [Google Scholar]
- 148.Kloska M, Patel P, Soliman A, Musco K, Rovella J. Intravenous leiomyomatosis: an uncommon cause of pulmonary embolism. Am J Case Rep. (2021) 22:e931386. 10.12659/AJCR.931386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hughes PF. Intravenous leiomyomatosis: case report and review. Asia Oceania J Obstet Gynaecol. (1988) 14:421–5. 10.1111/j.1447-0756.1988.tb00127.x [DOI] [PubMed] [Google Scholar]
- 150.Liu N, Long Y, Liu Y. Intravenous leiomyomatosis: case series and review of the literature. J Int Med Res. (2020) 48:300060519896887. 10.1177/0300060519896887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wong YY, Chu WCW, Lam WWM. Intravenous leiomyomatosis: computed tomography diagnosis. Hong Kong Med J Xianggang Yi Xue Za Zhi. (2006) 12:239–40. [PubMed] [Google Scholar]
- 152.Quade BJ, Dal Cin P, Neskey DM, Weremowicz S, Morton CC. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol. (2002) 15:351–6. 10.1038/modpathol.3880529 [DOI] [PubMed] [Google Scholar]
- 153.Corbett GA, O’Gorman C, Kamran W. Intravenous leiomyomatosis: the first reported case of intraoperative intracaval embolisation of tumour to the right atrium. BMJ Case Rep. (2020) 13:e233341. 10.1136/bcr-2019-233341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogino M, Urushibata H, Takada H, Okinaga S, Mori H, Tanaka F, et al. Intravenous leiomyomatosis: three case-reports. J Obstet Gynaecol Tokyo Jpn. (1995) 21:241–7. 10.1111/j.1447-0756.1995.tb01004.x [DOI] [PubMed] [Google Scholar]
- 155.Lam PM, Lo KWK, Yu MY, Wong WS, Lau JYW, Arifi AA, et al. Intravenous leiomyomatosis: two cases with different routes of tumor extension. J Vasc Surg. (2004) 39:465–9. 10.1016/j.jvs.2003.08.012 [DOI] [PubMed] [Google Scholar]
- 156.Bilyeu SP, Bilyeu JD, Parthasarathy R. Intravenous lipoleiomyomatosis. Clin Imaging. (2006) 30:361–4. 10.1016/j.clinimag.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 157.Vural Ç, Özen Ö, Demirhan B. Intravenous lipoleiomyomatosis of uterus with cardiac extension: a case report. Pathol Res Pract. (2011) 207:131–4. 10.1016/j.prp.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 158.Oehler MK, Scopacasa L, Brown M, Kumar G, Edwards J. Intravenous uterine leiomyomatosis extending into the right heart: case reports. Aust N Z J Obstet Gynaecol. (2011) 51:92–4. 10.1111/j.1479-828X.2010.01249.x [DOI] [PubMed] [Google Scholar]
- 159.Castelli P, Caronno R, Piffaretti G, Tozzi M. Intravenous uterine leiomyomatosis with right heart extension: successful two-stage surgical removal. Ann Vasc Surg. (2006) 20:405–7. 10.1007/s10016-006-9024-0 [DOI] [PubMed] [Google Scholar]
- 160.Jin X, Li F, Lu Z, Cheng W. IV Leiomyomatosis on FDG PET/CT. Clin Nucl Med. (2016) 41:580–2. 10.1097/RLU.0000000000001185 [DOI] [PubMed] [Google Scholar]
- 161.Morice P, Chapelier A, Dartevelle P, Castaigne D, Lhommé C. Late intracaval and intracardiac leiomyomatosis following hysterectomy for benign myomas treated by surgery and GnRH agonist. Gynecol Oncol. (2001) 83:422–3. 10.1006/gyno.2001.6389 [DOI] [PubMed] [Google Scholar]
- 162.Mahmoud MS, Desai K, Nezhat FR. Leiomyomas beyond the uterus; benign metastasizing leiomyomatosis with paraaortic metastasizing endometriosis and intravenous leiomyomatosis: a case series and review of the literature. Arch Gynecol Obstet. (2015) 291:223–30. 10.1007/s00404-014-3356-8 [DOI] [PubMed] [Google Scholar]
- 163.Thukkani N, Ravichandran PS, Das A, Slater MS. Leiomyomatosis metastatic to the tricuspid valve complicated by pelvic hemorrhage. Ann Thorac Surg. (2005) 79:707–9. 10.1016/j.athoracsur.2003.08.038 [DOI] [PubMed] [Google Scholar]
- 164.Chen B-B, Chen C-A, Liu K-L. Leiomyomatosis with extension to the left gluteal muscle, Inferior vena cava, and right atrium. Am J Roentgenol. (2006) 187:W546–7. 10.2214/AJR.06.0112 [DOI] [PubMed] [Google Scholar]
- 165.Canzonieri V, D’Amore ES, Bartoloni G, Piazza M, Blandamura S, Carbone A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch Int J Pathol. (1994) 425:541–5. 10.1007/BF00197559 [DOI] [PubMed] [Google Scholar]
- 166.Tresukosol D, Kudelka AP, Malpica A, Varma DG, Edwards CL, Kavanagh JJ. Leuprolide acetate and intravascular leiomyomatosis. Obstet Gynecol. (1995) 86:688–92. 10.1016/0029-7844(95)00138-h [DOI] [PubMed] [Google Scholar]
- 167.Agathos EA, Tafralis D, Kafetsis G, Gloustianou G, Lachanas E. Local cardiac recurrence of intravenous leiomyomatosis. Ann Thorac Surg. (2014) 97:1084. 10.1016/j.athoracsur.2013.07.101 [DOI] [PubMed] [Google Scholar]
- 168.Liu H, Pan L, Shen K, Lang J, Shi J, Cui Q, et al. Magnetic resonance imaging is useful for diagnosis and evaluation of recurrent intravenous leiomyomatosis before surgery. Fertil Steril. (2009) 92:1150–2. 10.1016/j.fertnstert.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 169.Lai TKK, Huang HYH, Chan RYY, Chin ACW, Wong WC, Sit CY, et al. Magnetic resonance venogram of intravenous leiomyomatosis. Hong Kong Med J Xianggang Yi Xue Za Zhi. (2005) 11:524–6. [PubMed] [Google Scholar]
- 170.Lakhi N, Serur E, Chi DS. Management of intravascular leiomyomatosis. Am J Obstet Gynecol. (2013) 208:333.e1–e2. 10.1016/j.ajog.2013.02.044 [DOI] [PubMed] [Google Scholar]
- 171.Alizade K, Maddah G, Jafarian AH, Bagheri AK, Esfehani RJ, Mirzaeian S. Management of intravenous leiomyomatosis of uterus with extension to heart. Arch Iran Med. (2016) 19:147–9. [PubMed] [Google Scholar]
- 172.Gissey L C, Mariano G, Musleh L, Lepiane P, Colasanti M, Meniconi RL, et al. Massive pelvic recurrence of uterine leiomyomatosis with intracaval-intracardiac extension: video case report and literature review. BMC Surg. (2017) 17:118. 10.1186/s12893-017-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Parker NA, Dakhil CSR, Dakhil SR, Lalich D. Metastasis of benign leiomyomas outside the uterus. Kans J Med. (2018) 11:1–11. 10.17161/kjm.v11i2.8689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilson M, Evans F, Mylona E, Murray C, Govind A. Microscopic intravenous leiomyomatosis: an incidental finding at myomectomy. J Obstet Gynaecol. (2011) 31:96–7. 10.3109/01443615.2010.513458 [DOI] [PubMed] [Google Scholar]
- 175.Altinok D, Tasci Yildiz Y, Tacal T, Karapinar K, Eryilmaz M. MRI Of intravascular leiomyomatosis extending to the heart. Eur Radiol. (2000) 10:871. 10.1007/s003300051023 [DOI] [PubMed] [Google Scholar]
- 176.Marrone G, Crinò F, Morsolini M, Caruso S, Miraglia R. Multidisciplinary approach in the management of uterine intravenous leiomyomatosis with intracardiac extension: case report and review of literature. J Radiol Case Rep. (2019) 13:1–13. 10.3941/jrcr.v13i7.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thannickal A, Shafa A, Maharaj J, Schoolmeester JK, Heimbach J, DeMartino R, et al. Multidisciplinary management of extensive intravenous leiomyomatosis: a coordinated effort of a single institution. Gynecol Oncol Rep. (2020) 32:100557. 10.1016/j.gore.2020.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fornaris RJ, Rivera M, Jiménez L, Maldonado J. Multimodality evaluation of intravenous leiomyomatosis: a rare, benign but potentially life-threatening tumor. Am J Case Rep. (2015) 16:794–800. 10.12659/AJCR.894939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Y, Zhu J, Wang C, Tu R, Jiang J, Lu W. Multimodality treatment of two cases of intracardiac leiomyomatosis with enormous mass in the abdominopelvic cavity. Expert Rev Anticancer Ther. (2013) 13:137–41. 10.1586/era.12.145 [DOI] [PubMed] [Google Scholar]
- 180.Barnaś E, Raś R, Skręt-Magierło J, Wesecki M, Filipowska J, Książek M, et al. Natural history of leiomyomas beyond the uterus. Medicine. (2019) 98:e15877. 10.1097/MD.0000000000015877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu Y-H, Lee Y-T, Lee C-I, Tzeng Y-H, Wei J. Nonthrombotic pulmonary embolism caused by intravenous leiomyomatosis: a case report. Medicine. (2019) 98:e14118. 10.1097/MD.0000000000014118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Park S-Y, Yeo IH, Kim YJ, Kim JK. Obstruction of the hepatic venous flow caused by intravenous leiomyomatosis. Medicina (Mex). (2020) 56:696. 10.3390/medicina56120696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Butler MW, Sanders A. Obstructive shock in a 47 year old female with a deep venous thrombosis due to intravascular leiomyomatosis: a case report. Cases J. (2009) 2:8159. 10.4076/1757-1626-2-8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li B, Li R-Y, Chen X, Xu L-J, You Q-H, Ni Y-M, et al. One-stage complete removal of intracardiac leiomyomatosis without cardiac arrest. Thorac Cardiovasc Surg. (2012) 61:88–90. 10.1055/s-0032-1324712 [DOI] [PubMed] [Google Scholar]
- 185.Li X, Xiao F, Yang Y, He Y, Zhang S. One-stage complete resection of giant intracardiac leiomyomatosis with moderate hypothermia extracorporeal circulation and beating heart technique with 36 months follow-up—a case report. J Cardiothorac Surg. (2016) 11:64. 10.1186/s13019-016-0445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chiang C-S, Chen P-L, Kuo T-T, Chen I-M, Wu N-Y, Chang H-H. One-stage surgery for removal of intravascular leiomyomatosis extending to right ventricle. Medicine. (2018) 97:e0051. 10.1097/MD.0000000000010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vujić G, Škopljanac Mačina A, Mikuš M, Đurić Ž, Pedišić I, Korda AZ. One-stage surgical removal of post-hysterectomy intravenous leiomyomatosis with inferior vena cava and heart extension using normothermic cardiopulmonary bypass. J Obstet Gynaecol. (2021) 41:326–8. 10.1080/01443615.2020.1832974 [DOI] [PubMed] [Google Scholar]
- 188.Li Y-Q, Yin X-P, Xu Z-W. Orthostatic hypotension and right heart failure as the initial manifestation of intravenous leiomyomatosis. Cardiol Young. (2016) 26:586–8. 10.1017/S1047951115001407 [DOI] [PubMed] [Google Scholar]
- 189.Knight J, Phillips DP, Esper SA, Zeh HJ, Badhwar V, Subramaniam K. Paradoxical tumor embolism and recurrent intracardiac mass from uterine intravenous leiomyomatosis. J Cardiothorac Vasc Anesth. (2017) 31:642–5. 10.1053/j.jvca.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 190.Okamura H, Yamaguchi A, Kimura N, Adachi K, Adachi H. Partial resection of intravenous leiomyomatosis with cardiac extension. Gen Thorac Cardiovasc Surg. (2011) 59:38–41. 10.1007/s11748-010-0620-0 [DOI] [PubMed] [Google Scholar]
- 191.Correia P, Castro A, Rocha A, Freitas D, Carnide C, Moutinho O. Pelvic intravenous leiomyomatosis—case report. Rev Bras Ginecol Obstet. (2016) 38:412–5. 10.1055/s-0036-1588002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Izzat MB, Bayazid S, Shuaibi I. Pelvic intravenous leiomyomatosis with trans-caval extension to the heart and pulmonary arteries. J Card Surg. (2011) 26:630–1. 10.1111/j.1540-8191.2011.01316.x [DOI] [PubMed] [Google Scholar]
- 193.Katsumata T, Shinfeld A, Houel R, Westaby S. Pelvic leiomyoma in the right atrium. Ann Thorac Surg. (1998) 66:2095–6. 10.1016/S0003-4975(98)01075-3 [DOI] [PubMed] [Google Scholar]
- 194.Nam MS, Jeon MJ, Kim YT, Kim JW, Park KH, Hong YS. Pelvic leiomyomatosis with intracaval and intracardiac extension: a case report and review of the literature. Gynecol Oncol. (2003) 89:175–80. 10.1016/S0090-8258(02)00138-5 [DOI] [PubMed] [Google Scholar]
- 195.Schultz KL, Quinn SM, Miller AH, Fieman RE, Cipolle MD, Misselbeck TS, et al. Point of care ultrasound facilitated diagnosis of right ventricular mass as the etiology of syncope; A case report of intravenous leiomyomatosis. Radiol Case Rep. (2021) 16:1288–93. 10.1016/j.radcr.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kokawa K, Yamoto M, Yata C, Mabuchi Y, Umesaki N. Postmenopausal intravenous leiomyomatosis with high levels of estradiol and estrogen receptor. Obstet Gynecol. (2002) 100:1124–6. 10.1016/s0029-7844(02)02194-4 [DOI] [PubMed] [Google Scholar]
- 197.Miller J. Pregnancy complicated by intravenous leiomyomatosis. Am J Obstet Gynecol. (1975) 122:485–9. 10.1016/S0002-9378(16)33541-4 [DOI] [PubMed] [Google Scholar]
- 198.Tai Y-H, Lin S-M, Hsu C-P, Yu W-C, Sung C-S. Preoperative echocardiography first diagnosed and intraoperative echocardiography altered the surgical plan in intravenous leiomyomatosis. J Cardiothorac Vasc Anesth. (2015) 29:e56–8. 10.1053/j.jvca.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 199.Gao B, Zhou D, Qian X, Zhang W, Ying L, Wang W. Primary leiomyoma of the inferior vena cava mimicking a cystic neoplasm of the pancreas: a case report. Cardiovasc Pathol. (2020) 46:107097. 10.1016/j.carpath.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 200.Lee S, Kim D-K, Narm KS, Cho S-H. Pulmonary artery embolization of intravenous leiomyomatosis extending into the right atrium. Korean J Thorac Cardiovasc Surg. (2011) 44:243–6. 10.5090/kjtcs.2011.44.3.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rajaii-Khorasani A, Kahrom M, Hashemzadeh M, Tayebi S, Ghazi M, Hamedanchi A. Pulmonary artery extension of uterine leiomyoma. J Card Surg. (2012) 27:466–9. 10.1111/j.1540-8191.2012.01469.x [DOI] [PubMed] [Google Scholar]
- 202.Marcus SG, Krauss T, Freedberg RS, Culliford AT, Weinreich DJ, Kronzon I. Pulmonary embolectomy for intravenous uterine leiomyomatosis. Am Heart J. (1994) 127:1642–5. 10.1016/0002-8703(94)90404-9 [DOI] [PubMed] [Google Scholar]
- 203.Zhang C, Liu X, Ma G, Zhang H, Wang C, Liu J, et al. Pulmonary embolization as the primary clinical manifestation of intravenous leiomyomatosis with intracardiac extension. Ann Thorac Surg. (2012) 94:1012. 10.1016/j.athoracsur.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 204.Jalaguier-Coudray A, Allain-Nicolai A, Thomassin-Piana J, Villard-Mahjoub R, Delarbre B, Rua S, et al. Radio-surgical and pathologic correlations of pelvic intravenous leiomyomatosis. Abdom Radiol. (2017) 42:2927–32. 10.1007/s00261-017-1225-1 [DOI] [PubMed] [Google Scholar]
- 205.Zhang G, Feng F, Wang W, Zhu L. Rapamycin (sirolimus) in treatment of recurrent intravenous leiomyomatosis: a case report. BJOG Int J Obstet Gynaecol. (2020) 127:768–71. 10.1111/1471-0528.16156 [DOI] [PubMed] [Google Scholar]
- 206.Gehr NR, Lund O, Alstrup P, Nielsen JS, Villadsen AB, Bartholdy NJ. Recurrence of uterine intravenous leiomyomatosis with intracardiac extension. Diagnostic considerations and surgical removal. Scand Cardiovasc J SCJ. (1999) 33:312–4. 10.1080/14017439950141597 [DOI] [PubMed] [Google Scholar]
- 207.Esmaeilzadeh M, Tavakolli A, Safaei A. Recurrent intracardiac leiomyomatosis. Can J Cardiol. (2007) 23:1085–6. 10.1016/s0828-282x(07)70879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cooper MM, Guillem J, Dalton J, Marboe CC, Corwin S, Todd GJ, et al. Recurrent intravenous leiomyomatosis with cardiac extension. Ann Thorac Surg. (1992) 53:139–41. 10.1016/0003-4975(92)90773-w [DOI] [PubMed] [Google Scholar]
- 209.Elkington NM, Carlton M. Recurrent intravenous leiomyomatosis with extension up the inferior vena cava. Aust N Z J Obstet Gynaecol. (2005) 45:167. 10.1111/j.1479-828X.2005.00355.x [DOI] [PubMed] [Google Scholar]
- 210.Evans A, Symmonds R, Gaffey T. Recurrent pelvic intravenous leiomyomatosis. Obstet Gynecol. (1981) 57:260–4. [PubMed] [Google Scholar]
- 211.Schindler N, Babrowski T, DeSai T, Alexander JC. Resection of intracaval leiomyomatosis using abdominal approach and venovenous bypass. Ann Vasc Surg. (2012) 26:109.e7–109.e11. 10.1016/j.avsg.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 212.Torres-de la Roche LA, Devassy R, Makhlouf G, San Juan J, Eidswick J, De Wilde RL. Retroperitoneal angioleiomyomatosis. J Obstet Gynecol India. (2021) 71:337–41. 10.1007/s13224-020-01404-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sakamoto H, Jikuya T, Sasaki A, Satoh M, Sakakibara Y. Severely calcified intravenous leiomyomatosis with cardiac extension. Jpn J Thorac Cardiovasc Surg. (2004) 52:148–51. 10.1007/s11748-004-0132-x [DOI] [PubMed] [Google Scholar]
- 214.Pesi B, Moraldi L, Antonuzzo L, Meoni G, Addasi R, Montesi G, et al. Single-stage operation using hypothermic circulatory arrest to remove uterine intravenous leiomyomatosis extended to the vena cava and right atrium. Int J Gynecol Obstet. (2015) 129:87–8. 10.1016/j.ijgo.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 215.Gul P, Gul K, Gul P, Jogezai S. Subserosal leiomyoma with intravenous leiomyomatosis extending into Inferior vena Cava and right-sided cardiac chambers. J Coll Physicians Surg Pak. (2019) 29:775–7. 10.29271/jcpsp.2019.08.775 [DOI] [PubMed] [Google Scholar]
- 216.Matsuo K, Fleischman F, Ghattas CS, Gabrielyan AS, Ballard CA, Roman LD, et al. Successful extraction of cardiac-extending intravenous leiomyomatosis through gonadal vein. Fertil Steril. (2012) 98:1341–1345.e1. 10.1016/j.fertnstert.2012.07.1121 [DOI] [PubMed] [Google Scholar]
- 217.Yoshida H, Wakabayashi R, Ishikawa R, Furugori M, Shigeta H. Successful live birth after myomectomy for intravenous leiomyomatosis. J Obstet Gynaecol. (2022) 42:171–2. 10.1080/01443615.2021.1904229 [DOI] [PubMed] [Google Scholar]
- 218.Atalay A, Poyrazoglu HH, Göçen U, Yüksel M, Eray IC, Guzel AB, et al. Successful one stage surgical removal of intravenous leiomyomatosis with on pump beating heart technique. Heart Lung Circ. (2016) 25:e72–4. 10.1016/j.hlc.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 219.Arinami Y, Kodama S, Kase H, Tanaka K, Okazaki H, Maruyama Y. Successful one-stage complete removal of an entire intravenous leiomyomatosis in the heart, vena cava, and uterus. Gynecol Oncol. (1997) 64:547–50. 10.1006/gyno.1996.4604 [DOI] [PubMed] [Google Scholar]
- 220.Sun H-R, Song H-B, Zhang Y-Z, Lin K, Liu J. Successful one-stage extraction of an intracardiac and intravenous leiomyoma through the right atrium under transesophageal ultrasound monitoring. Can J Anesth Can Anesth. (2014) 61:446–51. 10.1007/s12630-014-0130-0 [DOI] [PubMed] [Google Scholar]
- 221.Uchida H, Hattori Y, Nakada K, Iida T. Successful one-stage radical removal of intravenous leiomyomatosis extending to the right ventricle. Obstet Gynecol. (2004) 103:1068–70. 10.1097/01.AOG.0000127947.14387.01 [DOI] [PubMed] [Google Scholar]
- 222.Okada M, Miyoshi Y, Kato G, Ochi Y, Shimizu S, Nakai M. Successful one-stage surgical removal of intravenous leiomyomatosis with cardiac extension in an elderly patient. Gen Thorac Cardiovasc Surg. (2012) 60:153–6. 10.1007/s11748-011-0791-3 [DOI] [PubMed] [Google Scholar]
- 223.Fujiwara K, Haba M, Noguchi Y, Yamamoto S, Iwasaki M. Successful one-stage surgical removal of intravenous uterine leiomyomatosis with right heart extension. Jpn J Thorac Cardiovasc Surg. (2003) 51:462–5. 10.1007/BF02719605 [DOI] [PubMed] [Google Scholar]
- 224.Kikuchi C, Asami F, Hanzawa K, Tsuchida M. Successful surgical removal of an intravenous leiomyoma extending to the right atrium 4 years after hysterectomy. Eur J Cardiothorac Surg. (2013) 43:1262. 10.1093/ejcts/ezs610 [DOI] [PubMed] [Google Scholar]
- 225.Gaudino M, Spatuzza P, Snider F, Luciani N, Cina G, Possati G. Surgical management of a uterine leiomyoma extending through the inferior vena cava into the right heart. Heart Vessels. (2002) 17:80–2. 10.1007/s003800200049 [DOI] [PubMed] [Google Scholar]
- 226.Baboci A, Prifti E, Xhabija N, Alimehmeti M. Surgical removal of an intravenous leiomyoma with intracardiac extension and pulmonary benign metastases. Heart Lung Circ. (2014) 23:174–6. 10.1016/j.hlc.2013.10.058 [DOI] [PubMed] [Google Scholar]
- 227.Andrade D, Vinck EE, Torres LN, Citarella D. Surgical removal of intravascular leiomyomas with intracardiac extension: a two-case report. J Card Surg. (2020) 35:1094–7. 10.1111/jocs.14525 [DOI] [PubMed] [Google Scholar]
- 228.He H, Li Q, Shu C. Surgical treatment of intravenous leiomyomatosis with inferior vena cava and intracardiac extension. J Vasc Surg Venous Lymphat Disord. (2020) 8:1102–3. 10.1016/j.jvsv.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 229.Podolsky LA, Jacobs LE, Ioli A, Kotler MN. TEE In the diagnosis of intravenous leiomyomatosis extending into the right atrium. Am Heart J. (1993) 125:1462–4. 10.1016/0002-8703(93)91035-d [DOI] [PubMed] [Google Scholar]
- 230.Subramaniam B, Pawlowski J, Gross BA, Kim YB, LoGerfo FW. TEE-Guided One-Stage excision of intravenous leiomyomatosis with cardiac extension through an abdominal approach. J Cardiothorac Vasc Anesth. (2006) 20:94–5. 10.1053/j.jvca.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 231.Han HS, Park IA, Kim SH, Lee HP. The clear cell variant of epithelioid intravenous leiomyomatosis of the uterus: report of a case. Pathol Int. (1998) 48:892–6. 10.1111/j.1440-1827.1998.tb03857.x [DOI] [PubMed] [Google Scholar]
- 232.Wang B, Wang R, Zhang L, Xie M. The crawling tumour: intravenous leiomyomatosis involving inferior vena cava and heart. Eur Heart J. (2020) 41:1216. 10.1093/eurheartj/ehz795 [DOI] [PubMed] [Google Scholar]
- 233.Galajda Z, Copotoiu C, Suciu H, Tint D, Glasz T, Deac R. The diagnosis, morphological particularities, and surgical technique in a case of intravascular leiomyoma extended to the right heart chambers. J Vasc Surg. (2010) 51:1000–2. 10.1016/j.jvs.2009.09.061 [DOI] [PubMed] [Google Scholar]
- 234.Xiao J, Liu G, Cheng D, Wang H, Shi H. The value of 18F-FDG PET/CT in the diagnosis of intravenous leiomyomatosis of the uterus extended into the right atrium. Hell J Nucl Med. (2016) 19:179–81. 10.1967/s002449910377 [DOI] [PubMed] [Google Scholar]
- 235.Deng Y, Song B. Three case reports of intravenous leiomyomatosis with intracardiac extensions. Thorac Cardiovasc Surg Rep. (2020) 09:e40–3. 10.1055/s-0040-1715183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Basso LV, Gradman M, Finkelstein S, Gonzalez-Lavin L. Tricuspid valve obstruction due to intravenous leiomyomatosis. Clin Nucl Med. (1984) 9:152–5. 10.1097/00003072-198403000-00010 [DOI] [PubMed] [Google Scholar]
- 237.Gonzalez-Lavin L, Lee RH, Falk L, Gradman MD, McFadden PM, Basso LV, et al. Tricuspid valve obstruction due to intravenous leiomyomatosis. Am Heart J. (1984) 108:1544–6. 10.1016/0002-8703(84)90705-1 [DOI] [PubMed] [Google Scholar]
- 238.Konishi H, Koh I, Shiroma N, Kidani Y, Urabe S, Tanaka N, et al. Two case reports of intravenous leiomyomatosis with hyaluronan expression. Case Rep Obstet Gynecol. (2018) 2018:1–5. 10.1155/2018/4039183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Taşdelen A, Mercan AS, Sezgin A, Karapinar K, Yaveri A, Aslamaci S. Two discrete masses of leiomyomatosis in a patient, one extending to the right atrium. Thorac Cardiovasc Surg. (2000) 48:161–3. 10.1055/s-2000-9634 [DOI] [PubMed] [Google Scholar]
- 240.Maurer G, Nanda N. Two-Dimensional echocardiographic identification of intracardiac leiomyomatosis. Am Heart J. (1982) 103:915–7. 10.1016/0002-8703(82)90409-4 [DOI] [PubMed] [Google Scholar]
- 241.Bayramoglu D, Orhan A, Gul A, Sahin G, Celik ZE, Koplay M, et al. Two-stage surgery for extra pelvic intravenous leiomyomatosis: report of a case. J Obstet Gynaecol. (2020) 40:731–2. 10.1080/01443615.2019.1624950 [DOI] [PubMed] [Google Scholar]
- 242.Ge Z, Wang Y, Qi Z, Zhang Q, Jin J, Li J. Ultrasound appearance of intravenous leiomyomatosis: a case report. Medicine. (2019) 98:e16913. 10.1097/MD.0000000000016913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Stolf NAG, dos Santos GG, Haddad VLS. Unusual abdominal tumors with intracardiac extension. Two cases with successful surgical resection. Rev Hosp Clínicas. (1999) 54:159–64. 10.1590/S0041-87811999000500006 [DOI] [PubMed] [Google Scholar]
- 244.Pfenniger A, Silverberg RA, Lomasney JW, Churyla A, Maganti K. Unusual case of right ventricular intravenous leiomyoma. Circ Cardiovasc Imaging. (2021) 14:e010363. 10.1161/CIRCIMAGING.119.010363 [DOI] [PubMed] [Google Scholar]
- 245.Dong L, Wang Y, Zuo W, Kong D, Shu X. Unusual intravascular leiomyomatosis arising from the pulmonary artery. Eur Heart J Cardiovasc Imaging. (2016) 17:844. 10.1093/ehjci/jew072 [DOI] [PubMed] [Google Scholar]
- 246.Devesa V V, Conley CR, Stone WM, Collins JM, Magrina JF. Update on intravenous leiomyomatosis: report of five patients and literature review. Eur J Obstet Gynecol Reprod Biol. (2013) 171:209–13. 10.1016/j.ejogrb.2013.09.031 [DOI] [PubMed] [Google Scholar]
- 247.Nogales F, Navarro N, Devictoria J, Contreras F, Redondo C, Herraiz M, et al. Uterine intravascular leiomyomatosis—an update and report of 7 cases. Int J Gynecol Pathol. (1987) 6:331–9. 10.1097/00004347-198712000-00005 [DOI] [PubMed] [Google Scholar]
- 248.Cea-Calvo L, Lozano F, Pombo M, Serrano A, Rodríguez E, Porto J, et al. Uterine intravenous leiomyomatosis extending through the Inferior vena Cava into the right cardiac cavities. Circulation. (2000) 101:581–3. 10.1161/01.CIR.101.5.581 [DOI] [PubMed] [Google Scholar]
- 249.Yano M, Katoh T, Nakajima Y, Iwanaga S, Kin R, Kozawa E, et al. Uterine intravenous leiomyomatosis with an isolated large metastasis to the right atrium: a case report. Diagn Pathol. (2020) 15:4. 10.1186/s13000-019-0913-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xu Z-F. Uterine intravenous leiomyomatosis with cardiac extension: imaging characteristics and literature review. World J Clin Oncol. (2013) 4:25. 10.5306/wjco.v4.i1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Masood I, Duran C, Malik K, Frank L. Uterine intravenous leiomyomatosis with cardiac involvement. Radiol Case Rep. (2020) 15:1389–93. 10.1016/j.radcr.2020.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Akinseye OA, Nayyar M, Das P. Uterine intravenous leiomyomatosis with femoral vein, intracaval, intracardiac and pulmonary artery extension. Future Cardiol. (2020) 16:27–32. 10.2217/fca-2019-0002 [DOI] [PubMed] [Google Scholar]
- 253.Wang H-C, Wang Y-B, Chen X-H, Cui L-L. Uterine intravenous leiomyomatosis with intracardiac extension and pulmonary benign metastases on FDG PET/CT: a case report. Korean J Radiol. (2016) 17:289. 10.3348/kjr.2016.17.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sogabe M, Kawahito K, Aizawa K, Sato H, Misawa Y. Uterine intravenous leiomyomatosis with right ventricular extension. Ann Thorac Cardiovasc Surg. (2014) 20:933–6. 10.5761/atcs.cr.13-00309 [DOI] [PubMed] [Google Scholar]
- 255.Xu H-S, Firoj KM, Inamdar KY, Zhao W-Z. Uterine leiomyoma extension into right atrium. J Card Surg. (2013) 28:702. 10.1111/jocs.12130 [DOI] [PubMed] [Google Scholar]
- 256.Thompson AT, Desai A, Ford SJ, Gourevitch D. Uterine leiomyomatosis with intracardiac extension. BMJ Case Rep. (2016) 2016:bcr2016218234. 10.1136/bcr-2016-218234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vaquero ME, Magrina JF, Leslie KO. Uterine smooth-muscle tumors with unusual growth patterns. J Minim Invasive Gynecol. (2009) 16:263–8. 10.1016/j.jmig.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 258.Steinmetz OK, Bedard P, Prefontaine ME, Bourke M, Barber GG. Uterine tumor in the heart: intravenous leiomyomatosis. Surgery. (1996) 119:226–9. 10.1016/s0039-6060(96)80174-7 [DOI] [PubMed] [Google Scholar]
- 259.Kocaoglu M, Bulakbasi N, Ugurel MS, Ors F, Tayfun C, Ucoz T. Value of magnetic resonance imaging in the depiction of intravenous leiomyomatosis extending to the heart. J Comput Assist Tomogr. (2003) 27:630–3. 10.1097/00004728-200307000-00033 [DOI] [PubMed] [Google Scholar]
- 260.Maneyama H, Miyasaka N, Wakana K, Nakamura M, Kitazume Y, Kubota T. Vanishing intravenous leiomyomatosis after hysterectomy: assessment of the need to perform complete resection: vanishing intravenous leiomyomatosis. J Obstet Gynaecol Res. (2016) 42:1058–62. 10.1111/jog.13012 [DOI] [PubMed] [Google Scholar]
- 261.Ma G, Miao Q, Liu X, Zhang C, Liu J, Zheng Y, et al. Different surgical strategies of patients with intravenous leiomyomatosis. Medicine. (2016) 95:e4902. 10.1097/MD.0000000000004902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kir G, Kir M, Gurbuz A, Karateke A, Aker F. Estrogen and progesterone expression of vessel walls with intravascular leiomyomatosis; discussion of histogenesis. Eur J Gynaecol Oncol. (2004) 25:362–6. [PubMed] [Google Scholar]
- 263.Cin P D, Quade BJ, Neskey DM, Kleinman MS, Weremowicz S, Morton CC. Intravenous leiomyomatosis is characterized by a der(14)t(12;14)(q15;q24). Genes Chromosomes Cancer. (2003) 36:205–6. 10.1002/gcc.10159 [DOI] [PubMed] [Google Scholar]
- 264.Chen M-J, Peng Y, Yang Y-S, Huang S-C, Chow S-N, Torng P-L. Increased hyaluronan and CD44 expressions in intravenous leiomyomatosis. Acta Obstet Gynecol Scand. (2005) 84:322–8. 10.1111/j.0001-6349.2005.00707.x [DOI] [PubMed] [Google Scholar]
- 265.Bu H, Jin C, Fang Y, Ma Y, Wang X, Chen J, et al. Successful pregnancy after complete resection of leiomyomatosis peritonealis disseminate without recurrence:a case report with next-generation sequencing analysis and literature review. World J Surg Oncol. (2020) 18:85. 10.1186/s12957-020-01857-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rioux-Leclercq N, Turlin B, Bansard J, Patard J, Manunta A, Moulinoux JP, et al. Value of immunohistochemical Ki-67 and p53 determinations as predictive factors of outcome in renal cell carcinoma. Urology. (2000) 55:501–5. 10.1016/s0090-4295(99)00550-6 [DOI] [PubMed] [Google Scholar]
- 267.Visapää H, Bui M, Huang Y, Seligson D, Tsai H, Pantuck A, et al. Correlation of Ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology. (2003) 61:845–50. 10.1016/s0090-4295(02)02404-4 [DOI] [PubMed] [Google Scholar]
- 268.Li B, Chen X, Chu Y-D, Li R-Y, Li W-D, Ni Y-M. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg. (2013) 17:132–8. 10.1093/icvts/ivt117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gan H-L, Zhang J-Q, Bo P. The classification and surgical strategy of intracardiac leiomyomatosis. Asian J Surg. (2009) 32:129–36. 10.1016/S1015-9584(09)60383-3 [DOI] [PubMed] [Google Scholar]
- 270.Du J, Zhao X, Guo D, Li H, Sun B. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol. (2011) 42:1240–6. 10.1016/j.humpath.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 271.Peng J, Zhong F, Zhu Y, Zhang M, Zhang M, Lu C, et al. Clinical analysis of uterine intravenous leiomyomatosis: a retrospective study of 260 cases. J Obstet Gynaecol Res. (2021) 47:4357–64. 10.1111/jog.15013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vallejo JM, Ballester C, Sorribas F. Intravascular leiomyomatosis: the surgical challenge of tumors with extension. Rev Esp Cardiol. (2005) 58:1246–7. 10.1016/S1885-5857(06)60407-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.