Highlights
-
•
This motor classification system provides a reliable tool for disease subtyping.
-
•
MDS-UPDRS can be used to identify distinct motor subtypes with high sensitivity and specificity.
-
•
Motor subtypes could be predicted based on CSF HVA and 5-HIAA levels.
-
•
TD subtype is associated with lower motor scores and higher HVA levels.
-
•
AR subtype is associated with higher motor scores and lower 5-HIAA levels.
Abstract
Introduction
Motor classifications of Parkinson's Disease (PD) have been widely used. This paper aims to update a subtype classification using the MDS-UPDRS-III and determine if cerebrospinal neurotransmitter profiles (HVA and 5-HIAA) differ between these subtypes in a cohort from the Parkinson's Progression Marker Initiative (PPMI).
Methods
UPDRS and MDS-UPDRS scores were collected for 20 PD patients. Akinetic-rigid (AR), Tremor-dominant (TD), and Mixed (MX) subtypes were calculated using a formula derived from UPDRS, and a new ratio was developed for subtyping patients with the MDS-UPDRS. This new formula was subsequently applied to 95 PD patients from the PPMI dataset, and subtyping was correlated to neurotransmitter levels. Data were analyzed using receiver operating characteristic models and ANOVA.
Results
Compared to previous UPDRS classifications, the new MDS-UPDRS TD/AR ratios produced significant areas under the curve (AUC) for each subtype. The optimal sensitivity and specificity cutoff scores were ≥0.82 for TD, ≤0.71 for AR, and >0.71 and <0.82 for Mixed. Analysis of variance showed that the AR group had significantly lower HVA and 5-HIAA levels than the TD and HC groups. A logistic model using neurotransmitter levels and MDS-UPDRS-III could predict the subtype classification.
Conclusions
This MDS-UPDRS motor classification system provides a method to transition from the original UPDRS to the new MDS-UPDRS. It is a reliable and quantifiable subtyping tool for monitoring disease progression. The TD subtype is associated with lower motor scores and higher HVA levels, while the AR subtype is associated with higher motor scores and lower 5-HIAA levels.
1. Introduction
1.1. Background
Neurological disorders are the leading cause of disability worldwide, and Parkinson's disease (PD) is the fastest-growing [1]. Disease diagnosis remains clinical, focusing on motor symptoms using the UK Brain Bank criteria or MDS Clinical Diagnostic Criteria for PD. With no known biomarker for diagnosis or progression, subtyping has become a tool for understanding the rate of progression, medication response, and disease pathogenesis. Subtyping aims to enhance patient care and clinical trial design.
Different classifications have been proposed using hypothesis-driven empirical categorization or data-driven grouping schemes. Empirical classifications are determined by relevant clinical observations, with motor symptoms predominance being the most commonly used. The leading PD motor subtyping typically splits patients into either tremor-dominant or non-tremor dominant [with either bradykinesia, akinesia, rigidity, postural instability, and/or gait dysfunction as the prevailing symptoms] and intermediate or mixed phenotypes [2], [3]. This categorization has been described by our group [4] and others [5] using the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [6]. The different motor subtyping classifications seem to be congruent between them based on Cohen's kappa coefficient. Among these classification systems, the one proposed by our group appears to be the most stable [7]; however, these classification systems utilized different UPDRS components.
Our proposed scheme creates distinct groups that are easily and rapidly identified in a clinical setting; however, it excludes non-motor symptoms. On the other hand, cluster analyses include motor and non-motor features. Still, they are limited not only by the number of variables measured and the number of clusters pursued but also by the amount of time and tools needed to collect the data.
Multiple studies have tried to identify the difference between motor subtypes. The presence of akinesia, rigidity, postural instability, and gait dysfunction (PIGD) as initial symptoms is usually referred to as a malignant type describing a more rapid rate of progression [8], cognitive decline [9], higher prevalence of non-motor symptoms, and a shorter response to levodopa therapy. Additionally, it has been described that the non-tremor dominant subgroup has a higher mean of cortical Lewy bodies with more severe cell loss in the ventrolateral part of the substantia nigra pars compacta compared to the tremor-dominant type [10].
IMP-SPECT and PET studies suggested regional cerebral blood flow and metabolic pattern differences. Functional MRI demonstrated that non-tremor-dominant PD patients have striatal-thalamocortical circuitry deficits. In contrast, tremor-dominant PD patients tend to have more significant cerebellar-thalamocortical circuitry dysfunction [11].
The relationship between MDS-UPDRS motor phenotypes and neurotransmitters in cerebrospinal fluid (CSF) is unknown. CSF 5-Hydroxyindoleacetic acid (5-HIAA) levels but not Homovanillic acid (HVA) could differentiate UPDRS Mixed and AR subgroups from healthy controls (HC) using our previous classification system [4]. However, others have shown that CSF's HVA levels are significantly lower in both PIGD and TD groups compared to healthy controls. Additionally, they described a negative correlation between PIGD severity and HVA levels in CSF [12].
This paper updated our previous motor classification by proposing a new ratio using the MDS-UPDRS and compared its congruency with the UPDRS. We also examined whether 5-HIAA and HVA levels differed among the different subtypes and between subtypes and HC.
2. Methods
2.1. Study participants
2.1.1. UTMove
Twenty immunocompetent patients, 45 to 78 years old, with mild to moderate PD, were recruited, Table 1. Critical enrollment criteria included: a) UK Brain Bank criteria, b) OFF-state Hoehn and Yahr scale (H&Y) of ≤ 3, c) robust response to dopaminergic therapy defined as ≥ 33% reduction of the UPDRS motor score in the OFF vs ON state, d) stable dose of dopaminergic replacement at baseline (≥60 days), and e) onset of motor symptoms of ≥ 4 and ≤ 10 years. Subjects were excluded if they had atypical or secondary parkinsonism features, a history of DBS or ablative brain surgery, or a Montreal Cognitive Assessment (MoCA) of <23. Neurotransmitter information for these patients was unavailable, so these patients' data was utilized to validate the MDS-UPDRS for motor subtyping alone. All participants provided written informed consent. The study was conducted according to the International Council for Harmonization Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
Table 1.
Demographics and baseline characteristics.
| PPMI PD patients | PPMI Controls | UTMOVE PD patients | |
|---|---|---|---|
| Number of subjects | 95 | 56 | 20 |
| Gender, Male: Female ratio | 63:32 | 36:20 | 9:11 |
| Age (yrs) | 62.61 (9.78) | 73.10 (10.76) | 66.51 (7.07) |
| Disease duration (yrs) | 0.65 (0.57) | NA | 5.6 (1.31) |
| Motor subtypes:Akinetic-rigid dominant (%)Tremor-dominant (%) *Mixed (%) |
69 (72.63%)22 (23.16%)4 (4.21%) |
NA | 11 (55%)8 (40%)1 (5%) |
| MDS-UPDRS Total | 33.69 (13.47) | NA | 38.45 (14.7) |
| MDS-UPDRS-III | 21 (12.73) | NA. | 67.7 (22.33) |
| LEDD | 0 | NA | 669.22 (295.1) |
| The table shows the mean and SD for each category. MDS-UPDRS- III, Movement disorder society Unified Parkinson's Disease Rating Scale motor score; MDS-UPDRS- T, Movement disorder society Unified Parkinson's Disease Rating Scale Section I-IV; LEDD, Levodopa equivalent daily dosage. | |||
2.1.2. Ppmi
The Parkinson's Progression Markers Initiative (PPMI) is a longitudinal, observational, multi-center natural history study that assesses the progression of clinical features, imaging outcomes, biologic and genetic markers, and digital outcomes of Parkinson's across all stages of PD from prodromal to moderate disease. Ninety-five naïve PD patients and 56 Healthy controls (HC) were selected from the PPMI dataset based on the availability of Homovanillic acid (HVA) and 5-Hydroxyindoleacetic acid (5-HIAA) information, Table 1. PD patient's criteria were a) age over 30 years, b) presence of 2 of the following: bradykinesia, rigidity, and resting tremor, or presence of asymmetric resting tremor, or asymmetric bradykinesia, c) within two years of diagnosis, d) PD drug naivety, and e) dopamine transporter deficit in the putamen on the DaTscan by central reading.
HC were required to be > 30 years old without any neurological disorder or a first-degree relative with PD.
CSF levels of HVA and 5-HIAA were measured using high-performance liquid chromatography-mass spectrometry [13].
2.1.3. PD subtyping
Based on our previously proposed classification, patients were initially classified into TD, AR, or MX motor subgroups using the UPDRS [4]. The ratio of the UPDRS tremor subscore (9 items) to the mean UPDRS AR subscore (12 items) was used to define TD patients (ratio ≥ 0.97), AR patients (ratio ≤ 0.80), and Mixed patients (ratios > 0.80 and < 0.97). Afterwards, patients were placed into TD, AR, or MX motor subgroups using the MDS-UPDRS. Subsequently, MDS-UPDRS items comparable to the TD and AR items on the UPDRS (11 items for TD and 15 items for AR) were selected and developed TD/AR ratio scores for all patients. The items used for each subscore and the equations are shown in Table 2. Items included in the MDS-UPDRS were based on the notion of representative assessments for bradykinesia, tremor, and muscular rigidity. The MDS-UPDRS has considerably more elements for tremor details and constancy of tremor, as well as more items assessing lower extremities (leg agility and toe-tapping). Additionally, based on correlation analyses, certain items were associated with each other, indicating that if a patient had worse scores on one of the items added to the TD or AR group, they also had worse scores on different items in the TD or AR group. For example, facial expression correlated with bradykinesia, pronation/supination associated with finger tapping, finger tapping correlates with toe-tapping, and leg agility correlates with finger tapping such that facial expression, pronation/supination, toe-tapping, and leg agility were items added to the AR group. All assessments were done in the conventionally defined “off” state; for the UTMove study, it was >12 h after the last dose of dopaminergic medication, and for PPMI, it was a baseline assessment with no prior dopaminergic treatment.
Table 2.
Ratio calculation for PD motor subtypes.
| Scale | UPDRS | MDS-UPDRS |
|---|---|---|
| Formula |
TD/AR ratio = TD score/ AR score AR score = sum of rigidity items/12 TD score = sum of tremor items/9 |
TD/AR ratio = TD score/ AR score AR score = sum of rigidity items/15 TD score = sum of tremor items/11 |
| Cutoff score | Tremor Dominant ≥ 0.97 Akinetic Rigid ≤ 0.80 Mixed > 0.80 and < 0.97 |
Tremor Dominant ≥ 0.82 Akinetic Rigid ≤ 0.71 Mixed > 0.71 and < 0.82 |
| Tremor Items | Part II 2.16 Tremor Part III 3.20. Rest tremor face 3.20. Rest tremor RUE 3.20. Rest tremor LUE 3.20. Rest tremor RLE 3.20. Rest tremor LLE 3.21. Action tremor RUE 3.21. Action tremor LUE |
Part II 2.10 Tremor Part III 3.15a. Postural tremor– Right hand 3.15b. Postural tremor– Left hand 3.16a. Kinetic tremor– Right hand 3.16b. Kinetic tremor– Left hand 3.17a. Rest tremor amplitude– RUE 3.17b. Rest tremor amplitude– LUE 3.17c. Rest tremor amplitude– RLE 3.17d. Rest tremor amplitude– LLE 3.17e. Rest tremor amplitude– Lip/jaw 3.18. Constancy of rest tremor |
| Rigidity Items | Part III 3.22. Rigidity-Neck 3.22. Rigidity-RUE 3.22. Rigidity-LUE 3.22. Rigidity-RLE 3.22. Rigidity-LLE 3.23. Finger taps-RUE 3.23. Finger taps-LUE 3.24. Hand movements-RUE 3.24. Hand movements-LUE 3.27. Arising from Chair 3.28. Posture 3.29. Gait 3.30. Postural instability 3.31. Body Bradykinesia |
Part III 3.2. Facial expression 3.3a. Rigidity-Neck 3.3b. Rigidity-RUE 3.3c. Rigidity-LUE 3.3d. Rigidity-RLE 3.3e. Rigidity-LLE 3.4a. Finger tapping-RUE 3.4b. Finger tapping-LUE 3.6a. Pronation-supination-RUE 3.6b. Pronation-supination-LUE 3.7a. Toe tapping-Right 3.7b. Toe tapping-Left 3.8a. Leg agility-Right 3.8b. Leg agility-Left 3.14. Global spontaneity of movement |
2.2. Statistical analysis
Using the previous UPDRS subtypes (TD, AR, and MX) as labels and the new MDS-UPDRS subtypes as predictors, we applied receiver operating characteristics (ROC) analyses to determine the area under the curve (AUC) and ideal cutoff score for each subtype with sensitive and specific group identification. We performed ANOVAs to compare 5-HIAA and HVA levels among subtypes and HC. Logistic models were also used in ROC analyses. All analyses were performed using R ROC packages.
3. Results
3.1. Cutoff scores
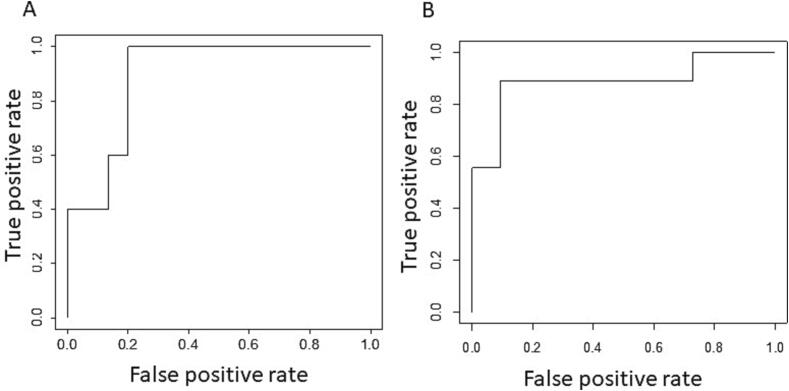
UPDRS and MDS-UPDRS data sets for TD and AR items were available for 20 PD patients (UTMove dataset). Four (25%) met the criteria for TD, 11 (55%) met the criteria for AR, and five (35%) met the criteria for MX based on UPDRS definitions. The new MDS-UPDRS TD/AR ratio for TD had an AUC of 89% (0.89; p = 0.0057; 95% confidence interval, 0.75–1), and for AR demonstrated an AUC of 89% (0.89; p = 0.0015; 95% confidence interval, 0.72–1), Fig. 1.
Fig. 1.
UTMove ROC. ROC curve using MDS-UPDRS as a predictor and UPDRS as a label. A. ROC curve showing false positive versus true positive rate for classification into TD subtype using MDS-UPDRS TD subtype as predictor and UPDRS TD subtype as label. B. ROC curve showing false positive versus true positive rate for classification into AR subtype.
Analysis of the ROC curve revealed the optimum cutoff levels for the MDS-UPDRS TD/AR ratio of ≥ 0.82 for TD classification (sensitivity = 1; specificity = 0.8; positive predictive value = 0.63; negative predictive value = 1) and ≤ 0.71 for AR classification (sensitivity = 0.91; specificity = 0.89; positive predictive value = 0.91; negative predictive value = 0.89).
3.2. Subtypes and neurotransmitters
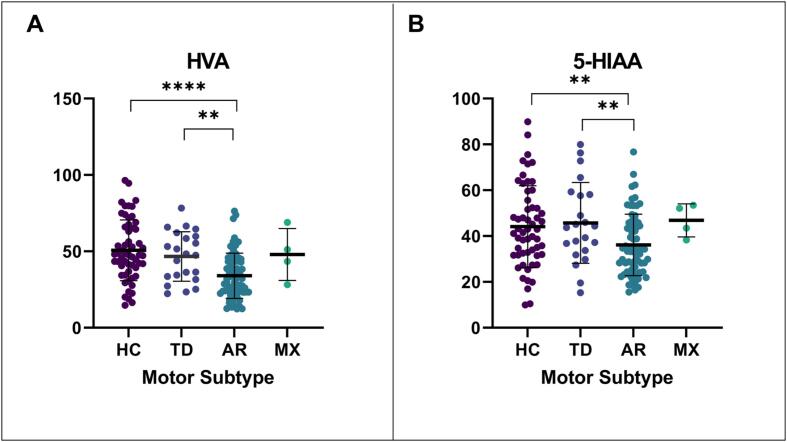
Ninety-five PD patients from the PPMI cohort were analyzed. Of this cohort, 22 (23.16%) met the criteria for TD, 69 (72.63%) for AR, and four (4.21%) for mixed based on MDS-UPDRS definitions. There was a significant difference between group levels for HVA (p < 0.0001) and 5-HIAA (p = 0.0121), Fig. 2. AR patients had significantly lower HVA levels than TD patients (p = 0.010) and HC (p < 0.0001). Additionally, AR patients had substantially lower 5-HIAA levels than TD (p = 0.0081) and HC (p = 0.0051).
Fig. 2.
CSF neurotransmitters. The graph depicts the mean and SD for each motor subtype. HVA, Homovanillic acid; 5-HIAA, 5-hydroxyindoleacetic acid. P-value represents a t-test between groups. p-value ** ≤ 0.01, *** ≤ 0.001, **** ≤ 0.0001.
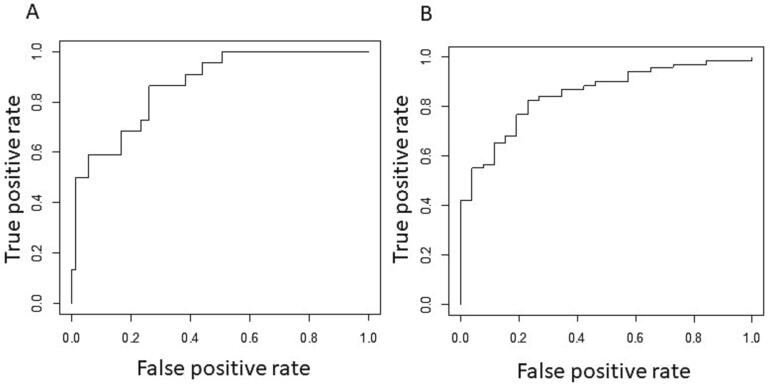
A linear model with HVA (5.3% odds, p = 0.007), MDS-UPDRS motor (-16.7% odds, 0.0014), and age (9.5% odds, p = 0.006) was used to predict classification for TD. This model associated lower MDS-UPDRS motor scores and higher HVA levels with the TD subtype. The model had an AUC of 87% (0.87, p = 9.02*10-8, 95% confidence interval, 0.79–0.95), Fig. 3. The cutoff probability was > 0.19 for TD classification (sensitivity = 0.86, specificity = 0.74, positive-predictive value = 0.5, negative-predictive value = 0.95).
Fig. 3.
PPMI ROC. ROC curve using probabilities derived from a logistic regression model as predictors (which combine neurotransmitter levels) and MDS-UPDRS subtypes as labels. A. ROC curve showing false positive versus true positive rate for classification into TD subtype using TD probabilities derived from a logistic regression model as predictors and MDS-UPDRS TD subtypes as labels. B. ROC curve showing false positive versus true positive rate for classification into AR subtype using AR probabilities derived from a logistic regression model as predictors and MDS-UPDRS AR subtypes as labels.
A linear model with 5-HIAA (−4.9% odds, p = 0.013), MDS-UPDRS motor (12.6% odds, p = 0.003), and age (−8.8% odds, p = 0.006) was used to predict classification for AR. In this model, higher MDS-UPDRS scores and lower 5-HIAA were related to the AR subtype. The model had an AUC of 85% (0.85, p = 7.76*10-8, 95% confidence interval, 0.77–0.93), Fig. 3. The cutoff probability was > 0.66 for AR classification (sensitivity = 0.83, specificity = 0.77, positive-predictive value = 0.90, negative-predictive value = 0.63).
4. Discussion
A heterogeneous phenotypic expression and pathological underpinning characterize PD. Clinicians, therefore, have widely used motor subtyping to identify features that influence the rate of progression, dopaminergic response, and prevalence of non-motor symptoms that correlate with neural and biochemical substrates of disease pathogenesis. In this paper, we update our previously published motor subtyping using the MDS-UPDRS and compare the levels of both HVA and 5-HIAA between subtypes.
The optimal sensitivity and specificity for the MDS-UPDRS cutoff scores were ≥ 0.82 for TD and ≤ 0.71 for AR and between 0.71 and 0.82 for Mixed classifications. Further, these subtypes could be predicted based on CSF HVA and 5-HIAA levels. A logistic model consistent with previous studies found that lower MDS-UPDRS motor scores and higher HVA levels were associated with the TD subtype in the PPMI cohort. Furthermore, a second logistic model found that higher MDS-UPDRS motor scores and lower 5-HIAA levels were related to the AR subtype in this cohort.
HVA, the primary terminal metabolite of dopamine and product of monoamine oxidase and catechol-O-methyltransferase activity, is thought to represent dopamine turnover in the brain [14]. HVA was elevated in TD compared to AR patients, and it appears to be a more specific biomarker for the TD disease subtype. Decreased DA levels in the striatum may correlate to reduced levels of HVA in the CSF. Other groups have found that TD patients had elevated levels of striatal DA compared to AR patients [15], which may be secondary to decreased intracerebral connectivity between their STN and putamen, leading to suppression of the basal ganglia-thalamocortical feedback loop [16]. In contrast to our previous paper, the HVA levels differed significantly between groups. A possible explanation for the difference in HVA levels may be that the PPMI subjects were naïve to PD medications and early in the disease process. In contrast, our initial population, which was used to define motor subtypes by the UPDRS, received dopaminergic medications and represented a more advanced stage of the disease with a mean disease duration of 9.2 years [4].
Serotonin is known to help mediate DA release and impact GABA and glutamate functioning, which are implicated in the basal ganglia and motion initiation [17]. It is noteworthy that only 12.6% of the patients in the study were on an SSRI or SNRI. Additionally, the proportion of TD patients (3.2%) versus AR patients (9.4%) and MX patients (0%) on the medications matched with the frequency of each subtype.
Multiple studies have shown decreased CSF levels of 5-HIAA, the primary metabolite of serotonin, in PD patients [18], [19], [20] and have linked serotoninergic dysfunction with tremor and non-motor symptoms [21], [22], [23]. Interestingly, in this study, AR patients had the lowest levels of 5-HIAA, which goes along with our previously published findings [4]. This may be explained by the fact that PPMI patients are recruited within two years of diagnosis with a positive DaTscan, so they may represent a population characterized beyond a Braak stage 3 (raphe complex is affected) [24], and it might be the case that based on the more rapid progression in the AR subtype [8], their serotoninergic system is more involved, which can also explain the higher prevalence of depression in this subtype [25]. Other groups have shown that poor levodopa responders have low CSF 5-HIAA levels [19], which is the case for AR patients; furthermore, a negative correlation between levels of 5-HT and rigidity, akinesia, and gait freezing has been described [26].
Some of the study limitations include the sample size used for validation and the concomitant use of medication that might influence the serotonin metabolic pathway, even though this was in a small percentage of patients.
5. Conclusions
This MDS-UPDRS motor classification system provides a method to transition from the original UPDRS to the new MDS-UPDRS and is a reliable tool for disease subtyping. The TD subtype is associated with lower motor scores and higher HVA levels, while the AR subtype is associated with higher motor scores and lower 5-HIAA levels.
6. Funding Sources for study
This research received support from the Huffington Foundation and the Adriana Blood Endowed Chair.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/access-data-specimens/download-data). For up-to-date information on the study, visit ppmi-info.org. PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson's Research funding partners 4D Pharma, Abbvie, Acurex Therapeutics, Allergan, Amathus Therapeutics, ASAP, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, Dacapo Brain Science, Denali, The Edmond J. Safra Foundation, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.
Authors' Roles
C.A, M.C.S, and J.S designed the study, were responsible for data interpretation and wrote the manuscript. C.A was in charge of data acquisition and data analysis. A.H was involved in data collection. K.B and T.E assisted in the interpretation of data. All authors reviewed and edited the manuscript and decided to submit the paper for publication.
References
- 1.Dorsey E.R., Sherer T., Okun M.S., Bloem B.R., Brundin P., Langston J.W., Bloem B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018;8(s1):S3–S8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zetusky W.J., Jankovic J., Pirozzolo F.J. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology. 1985;35(4):522–526. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W., Gerstenbrand F. Clinical subtypes of Parkinson disease. Wien. Med. Wochenschr. 1986;136(15–16):384–387. [PubMed] [Google Scholar]
- 4.Schiess M.C., Zheng H., Soukup V.M., Bonnen J.G., Nauta H.J.W. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat. Disord. 2000;6(2):69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J., McDermott M., Carter J., Gauthier S., Goetz C., Golbe L., Huber S., Koller W., Olanow C., Shoulson I., Stern M., Tanner C., Weiner W. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 6.Stebbins G.T., Goetz C.G., Burn D.J., Jankovic J., Khoo T.K., Tilley B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov. Disord. 2013;28(5):668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 7.Ren J., et al. Consistency and stability of motor subtype classifications in patients with de novo Parkinson's disease. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.637896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleksovski D., Miljkovic D., Bravi D., Antonini A. Disease progression in Parkinson subtypes: the PPMI dataset. Neurol. Sci. 2018;39(11):1971–1976. doi: 10.1007/s10072-018-3522-z. [DOI] [PubMed] [Google Scholar]
- 9.Burn D.J., Rowan E.N., Minett T., Sanders J., Myint P., Richardson J., Thomas A., Newby J., Reid J., O'Brien J.T., McKeith I.G. Extrapyramidal features in Parkinson's disease with and without dementia and dementia with Lewy bodies: A cross-sectional comparative study. Mov. Disord. 2003;18(8):884–889. doi: 10.1002/mds.10455. [DOI] [PubMed] [Google Scholar]
- 10.Eggers C., Kahraman D., Fink G.R., Schmidt M., Timmermann L. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov. Disord. 2011;26(3):416–423. doi: 10.1002/mds.23468. [DOI] [PubMed] [Google Scholar]
- 11.Boonstra J.T., Michielse S., Temel Y., Hoogland G., Jahanshahi A. Neuroimaging Detectable differences between Parkinson's disease motor subtypes: a systematic review. Mov. Disord. Clin. Practice. 2021;8(2):175–192. doi: 10.1002/mdc3.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo L.J., et al. Phenotype of postural instability/gait difficulty in Parkinson disease: relevance to cognitive impairment and mechanism relating pathological proteins and neurotransmitters. Sci. Rep. 2017;7:44872. doi: 10.1038/srep44872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada H., Yamahara A., Yasuda S., Abe M., Oguri K., Fukushima S., Ikeda-Wada S. Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J. Anal. Toxicol. 2002;26(1):17–22. doi: 10.1093/jat/26.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Bernheimer H. Distribution of homovanillic acid in the human brain. Nature. 1964;204(4958):587–588. doi: 10.1038/204587b0. [DOI] [PubMed] [Google Scholar]
- 15.Huertas I., Jesús S., Lojo J.A., García-Gómez F.J., Cáceres-Redondo M.T., Oropesa-Ruiz J.M., Carrillo F., Vargas-Gonzalez L., Martín Rodríguez J.F., Gómez-Garre P., García-Solís D., Mir P., Toft M. Lower levels of uric acid and striatal dopamine in non-tremor dominant Parkinson's disease subtype. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Chen H., Ma H., Ma L., Wu T., Feng T. Resting-state functional connectivity of subthalamic nucleus in different Parkinson's disease phenotypes. J. Neurol. Sci. 2016;371:137–147. doi: 10.1016/j.jns.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Fox S.H., Chuang R., Brotchie J.M. Serotonin and Parkinson's disease: On movement, mood, and madness. Mov. Disord. 2009;24(9):1255–1266. doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- 18.Chase T.N. Cerebrospinal fluid monoamine metabolites and peripheral decarboxylase inhibitors in parkinsonism. Neurology. 1970;20(12) p. Suppl:36–40. [PubMed] [Google Scholar]
- 19.Davidson D.L., Yates C.M., Mawdsley C., Pullar I.A., Wilson H. CSF studies on the relationship between dopamine and 5-hydroxytryptamine in Parkinsonism and other movement disorders. J. Neurol. Neurosurg. Psychiatry. 1977;40(12):1136–1141. doi: 10.1136/jnnp.40.12.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guldberg H.C., Turner J.W., Hanieh A., Ashcroft G.W., Crawford T.B.B., Perry W.L.M., Gillingham F.J. On the occurrence of homovanillic acid and 5-hydroxyindol-3-ylacetic acid in the ventricular C.S.F. of patients suffering from parkinsonism. Confin. Neurol. 1967;29(2-5):73–77. doi: 10.1159/000103680. [DOI] [PubMed] [Google Scholar]
- 21.Politis M., Niccolini F. Serotonin in Parkinson's disease. Behav. Brain Res. 2015;277:136–145. doi: 10.1016/j.bbr.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Huot P., Fox S.H., Brotchie J.M. The serotonergic system in Parkinson's disease. Prog. Neurobiol. 2011;95(2):163–212. doi: 10.1016/j.pneurobio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Vermeiren Y., De Deyn P.P. Targeting the norepinephrinergic system in Parkinson's disease and related disorders: The locus coeruleus story. Neurochem. Int. 2017;102:22–32. doi: 10.1016/j.neuint.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Braak H., Tredici K.D., Rüb U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 25.Mayeux R., Stern Y., Cote L., Williams J.B.W. Altered serotonin metabolism in depressed patients with parkinson's disease. Neurology. 1984;34(5):642. doi: 10.1212/wnl.34.5.642. [DOI] [PubMed] [Google Scholar]
- 26.Tohgi H., Abe T., Takahashi S., Takahashi J., Hamato H. Concentrations of serotonin and its related substances in the cerebrospinal fluid of Parkinsonian patients and their relations to the severity of symptoms. Neurosci. Lett. 1993;150(1):71–74. doi: 10.1016/0304-3940(93)90111-w. [DOI] [PubMed] [Google Scholar]