Abstract
The degree of protonation of contact lens materials is affected by the surrounding pH environment, due to the different pKa values. The swelling of ionic contact lenses is generally controlled by these factors which determines physical properties of contact lenses. The purpose of this study was to evaluate the pH dependence of the physical properties of contact lenses. The ionic etafilcon A and non-ionic hilafilcon B contact lenses were used in this study. The diameter, refractive power, equilibrium water content (EWC), and the amounts of freezable-free water (Wff), freezable-bound water (Wfb) and non-freezable water (Wnf) in the contact lens at each pH condition were measured. The diameter, refractive power and EWC of etafilcon A decreased with decreasing pH below 7.0 or 7.4, whereas hilafilcon B showed relatively constant values. The quantity of Wfb tended to increase with increasing pH, showing a relatively constant value above 7.0, whereas Wnf decreased. Hilafilcon B did not show changes in EWC and specific trends in Wfb and Wnf. The significant change of etafilcon A at more acidic condition is derived from the presence of methacrylic acid (MA) which makes it vulnerable to pH. Additionally, though the EWC is composed of various states of water, (i) various states of water could response to surrounding environment in different way with EWC and (ii) Wfb could be the crucial factor that determines physical properties of contact lens.
Keywords: Contact lens, Ionic material, Water content, State of water, Physical properties
1. Introduction
The absorption of water by hydrogels depends on the hydrophilicity and ionicity of their constituent polymers. Specifically, the hydrophilicity of hydrogels with ionic pendant groups is largely controlled by the properties of the polymer and the swelling medium [1]. The common properties of polymer include the ionic charge, pKa value of the pendant group, and cross-linking density, which are important, especially for ionic polymers. The pKa value of pendant groups and the pH of the medium are crucial for regulating the swelling behavior of ionic polymers. In the case of the anionic polymer, when the pKa of the acidic pendant groups is lower than the pH of the medium, ionization of the acidic pendant groups occurs, generating negative charges on the polymer chains, which attracts positive charges in the medium [1,2]. Consequently, there is an increase in negative charges on the polymer chains, and the electrostatic repulsion between the chains leads to swelling of the polymer network [1,2].
The nature of water in polymers is significant not only in comprehending swelling behavior, but also in investigating solute transport and other diffusive properties of polymers [3,4,5]. The irregular behavior of water in polymers can be attributed to two factors: (i) the trapping of water molecules by the polymer network, capillary condensation, or (ii) the strong interactions between water molecules and the ionic groups of the polymer chain, either directly or via other water molecules [6,7,8].
The hydrated water in a polymer can be categorized into three types depending on the binding strength with the polymer chain: freezable-free water (Wff), freezable-bound water (intermediate water, Wfb), and non-freezable water (non-freezable-bound water, Wnf) [6,7]. The Wff is frozen at 0 °C and freely exchanged with bulk water whereas the Wfb molecules are frozen below 0 °C because of the weak interaction with the polymer chain and/or non-freezable water. On the other hand, the Wnf molecules interact strongly with the polymer chain, which is not frozen even at −100 °C.
In the case of contact lenses, the swelling media are the tear film, contact lens care solutions, and eye drops. Tear film is a thin layer of water that protects the ocular surface from the external environment and is somewhat vulnerable to it [9]. For example, the normal physiological pH is 7.4, while the pH of the tear film ranges from 5.9 to 7.9, which is affected by various factors such as diseases, contact lens wear, and air pollution [10,11,12,13]. The pH of contact lens care solutions or eye drops vary from 6.69 to 7.98 and from 6.36 to 7.99 respectively where the contact lenses are immersed in [14]. The fluctuation of the pH of swelling media could affect the physical properties of contact lenses, especially ionic contact lenses and the state of water.
The purpose of this study was to evaluate the effect of pH on the physical properties of contact lenses. Two types of contact lens materials, etafilcon A and hilafilcon B, were used. These contact lenses have the same polymer chain, poly (2-hydroxyethyl methacrylate) (pHEMA), while etafilcon A contains methacrylic acid (MA) and polyvinylpyrrolidone (PVP), and hilafilcon B contains N-vinyl pyrrolidone (NVP) (Table 1). At physiological pH, the methacrylate carboxylic acid groups are fully ionized, whereas PVP and NVP are not ionizable [15]. We hypothesized that due to the presence of MA, the state of water in the etafilcon A contact lens would be affected by pH due to the degree of ionization and hydration which result in the change of physical properties of contact lens.
Table 1.
Additive compounds in contact lens material.
| Chemical Name | Abbreviation | Chemical Structure |
|---|---|---|
| Poly (2-hydroxyethyl methacrylate) | pHEMA | 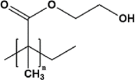 |
| Methacrylic acid | MA | 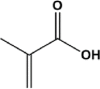 |
| Polyvinylpyrrolidone | PVP | 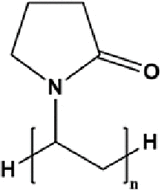 |
| N-Vinyl-2-pyrrolidone | NVP | 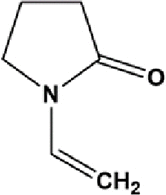 |
2. Materials and methods
2.1. Materials
The 1-Day ACUVUE MOIST (Johnson & Johnson Inc.) and SofLens daily disposable (Bausch + Lomb Inc.) were used (Table 2). PBS buffer solutions with pH from 6.2 to 8.2 (0.4 step) were used throughout the study. All lenses were soaked in each buffer solution for 48 h to remove the residual blister solution and reach an equilibrium state. The EWC, diameter, and DSC (Differential scanning calorimetry) of the contact lenses under each pH condition were measured.
Table 2.
Contact lens material types and classification.
| Proprietary name | 1-Day ACUVUE® MOIST | SofLens daily disposable |
|---|---|---|
| Manufacturer | Johnson & Johnson Vision Care | Bausch + Lomb Inc. |
| FDA Group | IV | II |
| USAN* | etafilcon A | hilafilcon B |
| Principal monomers | pHEMA–methacrylic acid + polyvinylpyrrolidone | pHEMA + N-Vinyl-2-pyrrolidone |
| Water content (%) | 58 | 59 |
| Diameter (mm) | 14.2 | 14.2 |
* United States Adopted Name.
2.2. Measurements of equilibrium water contents of contact lenses
In order to measure the equilibrium water content (EWC) of the contact lenses at each pH condition, a gravimetric determination method was used [16]. The weights of the contact lenses in the dry (Wdry) and hydrated (Whydrated) states were measured after they had reached equilibrium in PBS solutions with a pH of 6.2–8.2. The EWC was calculated using equation (1).
| (1) |
2.3. Measurements of state of water in the contact lens
The melting endotherms of water in the contact lenses were measured using DSC (DSC-250, TA Instruments, United States). The residual water was removed by blotting the contact lenses with tissue. The contact lenses were sealed in a DSC cell with a lid, and promptly placed in the instrument to minimize the evaporation of water in the contact lens.
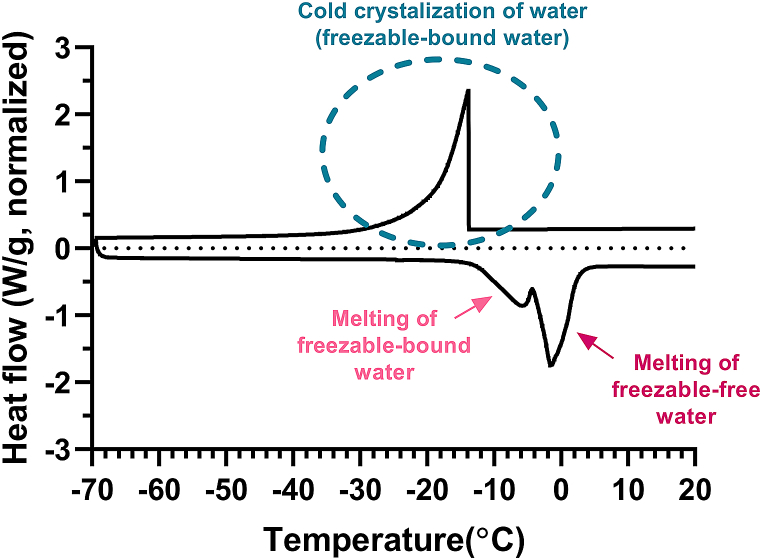
Subsequently, the pan was cooled to −70 °C from room temperature at a rate of 5 °C/min under nitrogen gas, and the sample was then heated at the same rate up to 30 °C. When the contact lens sample is cooled to −70 °C, the freezable water freezes, but the non-freezable water remains unfrozen. On the other hand, when the contact lens sample is heated in a DSC cell, the frozen water in the contact lens starts to melt, and the energy required to melt the frozen water is measured (Fig. 1).
Fig. 1.
DSC heating curve of the contact lens.
The amount of Wff, Wfb, and Wfb was calculated by direct integration of the melting endotherm and cold crystallization, assuming that the melting enthalpies for both Wff and Wfb are the same as those of bulk water (). The amount of Wff and Wfb was calculated using the following equations (2) and (3) [[17], [18], [19]].
| (2) |
| (3) |
The and are the enthalpy of melting freezable water and cold crystallization in the contact lens measured from the DSC experiment, and is the enthalpy of melting pure water. The EWC is the sum of Wff, Wfb, and Wfb. Therefore, the amount of Wnf was determined by subtracting the sum of Wff and Wfb from the EWC using the following equation (4) [17–19].
| (4) |
2.4. Measurements of diameters of the contact lenses
The diameters of the hydrated contact lenses were measured while they were soaked in PBS solutions at pH of 6.2–8.2 using an Optimec Soft Contact Lens Analyzer (Optimec JCF, Optimec Ltd., United Kingdom). The measurement was performed using −3.00D contact lens.
2.5. Measurements of refractive power of contact lenses
The refractive power of contact lenses was measured using automated lensmeter (HLM-90000, Huvitz, South Korea) with a contact lens holder to hydrate them in PBS solutions at pH of 6.2–8.2. In order to compare the change of refractive power of contact lenses, the measurement was performed using −3.00D contact lens.
2.6. Statistical analysis
The IBM SPSS Statistics (for Windows, Version 29.0.0., SPSS Inc) was used as analytical tool. The raw data from each experimental groups did not show a normal distribution (Kolmogorov-Smirnov test). Further statistical analysis was performed with Kruskal-Wallis test. The level of significance was set at P < 0.05.
3. Results
3.1. Equilibrium water contents of the contact lenses
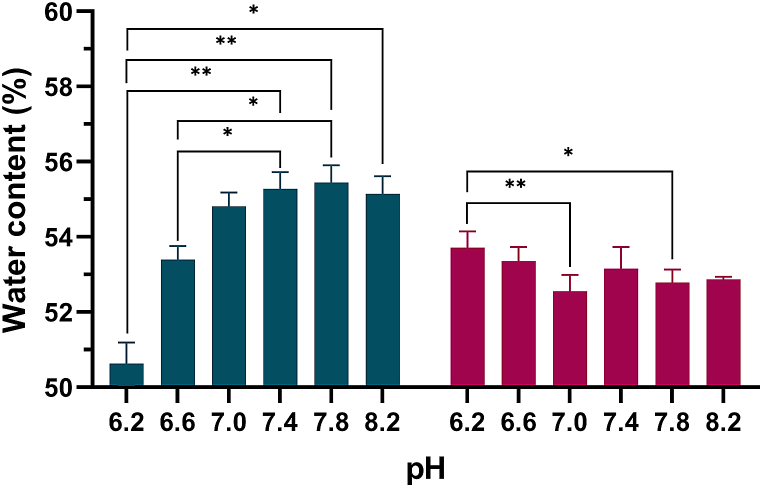
The EWC (mean ± standard deviation) of etafilcon A and hilafilcon B is shown in Fig. 2 and Table 3 as a function of pH. The EWC of etafilcon A decreased with decreasing pH below 7.0, whereas hilafilcon B showed a relatively constant value.
Fig. 2.
Equilibrium water contents of etafilcon A ( ) and hilafilcon B (
) and hilafilcon B ( ) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
Table 3.
Kruskal-Wallis H test results of association between equilibrium water content and pH.
| Contact lens material | pH | n | mean rank | χ2 | p value |
|---|---|---|---|---|---|
| etafilcon A | 6.2 | 3 | 2.00 | 13.386 | 0.020* |
| 6.6 | 3 | 5.00 | |||
| 7.0 | 3 | 9.83 | |||
| 7.4 | 3 | 13.83 | |||
| 7.8 | 3 | 14.67 | |||
| 8.2 | 3 | 11.67 | |||
| hilafilcon B | 6.2 | 3 | 15.83 | 9.521 | 0.090 |
| 6.6 | 3 | 12.33 | |||
| 7.0 | 3 | 4.33 | |||
| 7.4 | 3 | 10.67 | |||
| 7.8 | 3 | 6.50 | |||
| 8.2 | 3 | 7.33 |
*p < 0.05.
3.2. State of water in the contact lenses
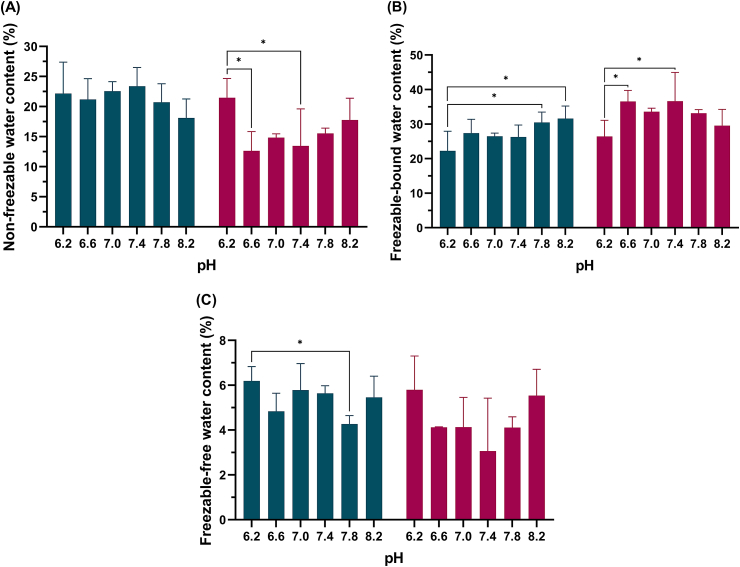
The values of Wff, Wfb, and Wnf are shown in Fig. 3(A-C) and Table 4. For etafilcon A, the quantity of Wfb tended to increase with pH, showing a relatively constant value above 7.0, whereas Wnf decreased. Hilafilcon B did not show changes in water content or specific trends in Wff, Wfb, and Wnf.
Fig. 3.
Contents of the (A) non-freezable (Wnf), (B) freezable-bound (Wfb) and (C) freezable-free (Wff) water content of etafilcon A ( ) and hilafilcon B (
) and hilafilcon B ( ) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
Table 4.
Kruskal-Wallis H test results of association between state of water and pH.
| Contact lens material | pH | n | mean rank | χ2 | p value | |
|---|---|---|---|---|---|---|
| Wnf | etafilcon A | 6.2 | 3 | 10.67 | 4.415 | 0.491 |
| 6.6 | 3 | 9.67 | ||||
| 7.0 | 3 | 12.00 | ||||
| 7.4 | 3 | 12.00 | ||||
| 7.8 | 3 | 8.33 | ||||
| 8.2 | 3 | 4.33 | ||||
| hilafilcon B | 6.2 | 3 | 16.00 | 7.971 | 0.158 | |
| 6.6 | 3 | 5.33 | ||||
| 7.0 | 3 | 7.67 | ||||
| 7.4 | 3 | 6.67 | ||||
| 7.8 | 3 | 9.67 | ||||
| 8.2 | 3 | 11.67 | ||||
| Wfb | etafilcon A | 6.2 | 3 | 4.33 | 8.485 | 0.131 |
| 6.6 | 3 | 9.00 | ||||
| 7.0 | 3 | 6.67 | ||||
| 7.4 | 3 | 8.67 | ||||
| 7.8 | 3 | 13.33 | ||||
| 8.2 | 3 | 15.00 | ||||
| hilafilcon B | 6.2 | 3 | 3.67 | 8.228 | 0.144 | |
| 6.6 | 3 | 14.33 | ||||
| 7.0 | 3 | 10.33 | ||||
| 7.4 | 3 | 12.67 | ||||
| 7.8 | 3 | 9.67 | ||||
| 8.2 | 3 | 6.33 | ||||
| Wff | etafilcon A | 6.2 | 3 | 14.33 | 8.289 | 0.141 |
| 6.6 | 3 | 6.67 | ||||
| 7.0 | 3 | 11.33 | ||||
| 7.4 | 3 | 11.33 | ||||
| 7.8 | 3 | 3.17 | ||||
| 8.2 | 3 | 10.17 | ||||
| hilafilcon B | 6.2 | 3 | 15.00 | 8.219 | 0.145 | |
| 6.6 | 3 | 7.00 | ||||
| 7.0 | 3 | 7.00 | ||||
| 7.4 | 3 | 7.17 | ||||
| 7.8 | 3 | 6.67 | ||||
| 8.2 | 3 | 14.17 |
3.3. Diameters of the contact lenses
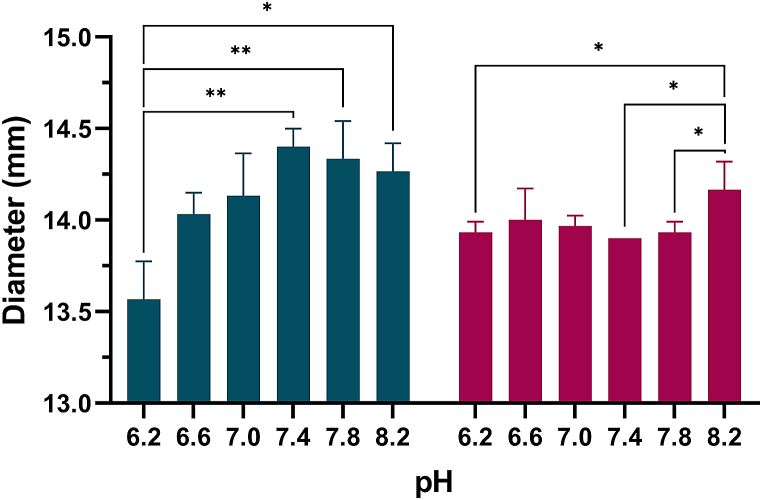
The diameter (mean ± standard deviation) of etafilcon A and hilafilcon B are shown in Fig. 4, Fig. 5(A and B) and Table 5 as a function of pH. Etafilcon A showed a decreased value with decreasing pH below 7.4, whereas hilafilcon B showed a relatively constant value.
Fig. 4.
Diameters of etafilcon A ( ) and hilafilcon B (
) and hilafilcon B ( ) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
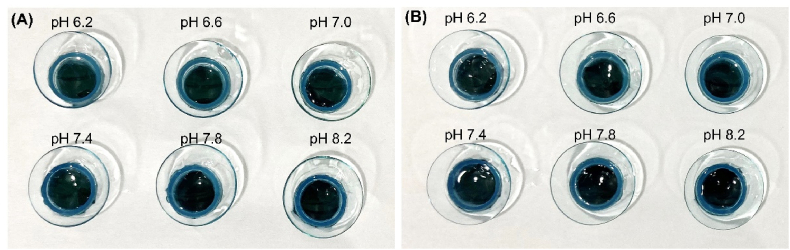
Fig. 5.
The image of swollen (A) etafilcon A and (B) hilafilcon B in the series of pH ranges.
Table 5.
Kruskal-Wallis H test results of association between diameter and pH.
| Contact lens material | pH | n | mean rank | χ2 | p value |
|---|---|---|---|---|---|
| etafilcon A | 6.2 | 3 | 2.00 | 11.699 | 0.039* |
| 6.6 | 3 | 7.00 | |||
| 7.0 | 3 | 8.50 | |||
| 7.4 | 3 | 14.50 | |||
| 7.8 | 3 | 13.50 | |||
| 8.2 | 3 | 11.50 | |||
| hilafilcon B | 6.2 | 3 | 8.00 | 8.04 | 0.154 |
| 6.6 | 3 | 9.17 | |||
| 7.0 | 3 | 10.50 | |||
| 7.4 | 3 | 5.50 | |||
| 7.8 | 3 | 8.00 | |||
| 8.2 | 3 | 15.83 |
*p < 0.05.
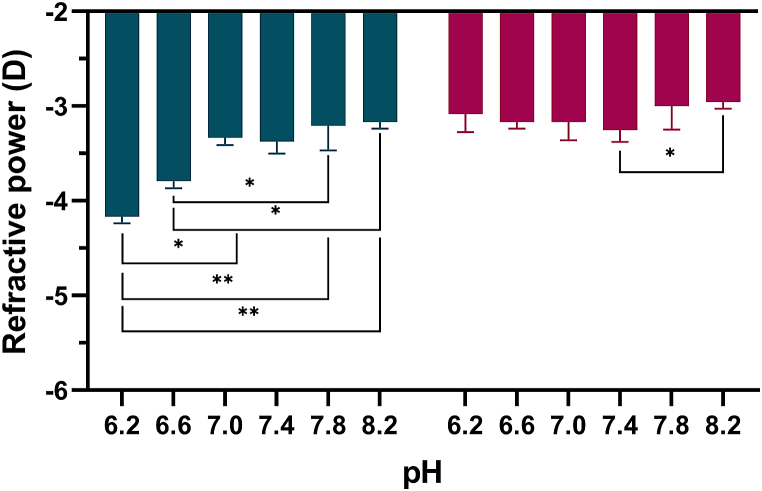
3.4. Refractive powers of the contact lenses
The refractive power (mean ± standard deviation) of etafilcon A and hilafilcon B are shown in Fig. 6 and Table 6. Etafilcon A showed a more negative refractive power with decreasing pH below 7.0. Hilafilcon B showed a relatively constant refractive power.
Fig. 6.
Refractive powers of etafilcon A ( ) and hilafilcon B (
) and hilafilcon B ( ) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
) in the series of pH ranges. Repeated measures Kruskal-Wallis test was used for statistical analysis; *p < 0.05; **p < 0.01.
Table 6.
Kruskal-Wallis H test results of association between refractive power and pH.
| Contact lens material | pH | n | mean rank | χ2 | p value |
|---|---|---|---|---|---|
| etafilcon A | 6.2 | 3 | 2.00 | 13.709 | 0.018* |
| 6.6 | 3 | 5.00 | |||
| 7.0 | 3 | 11.00 | |||
| 7.4 | 3 | 10.17 | |||
| 7.8 | 3 | 13.83 | |||
| 8.2 | 3 | 15.00 | |||
| hilafilcon B | 6.2 | 3 | 10.00 | 6.382 | 0.271 |
| 6.6 | 3 | 7.50 | |||
| 7.0 | 3 | 8.00 | |||
| 7.4 | 3 | 5.00 | |||
| 7.8 | 3 | 12.00 | |||
| 8.2 | 3 | 14.50 |
*p < 0.05.
4. Discussion
The pH-dependent physical properties of two types of contact lens materials, etafilcon A and hilafilcon B, were analyzed in this study. The etafilcon A and hilafilcon B are involved in an FDA group Ⅳ and Ⅱ, respectively. Though both etafilcon A and hilafilcon B have high water content, they are distinguished by surface ionicity; etafilcon A has ionic surface whereas hilafilcon B has non-ionic surface. The presence of methacrylic acid (MA) in etafilcon A contributes the ionicity of it since the acid group of MA became fully ionized at physiological pH and less ionized at lower pH condition. The NVP in hilafilcon B are less ionizable which result in the non-ionic surface of hilafilcon B.
The dehydration of etafilcon A at low pH is attributed to the presence of MA, a hydrophilic monomer that has a pKa of 4.65, which makes it ionized in a range of pH values investigated in this study [20]. In a physiological environment, MA became fully ionized reaching the maximum EWC of etafilcon A. At higher pH, higher than the physiological environment, around 7.0 or 7.4, the EWC of etafilcon A contact lens became constant due to the fully ionized MA [14,20]. However, under the physical pH, the MA is hardly fully ionized resulting in the reduced EWC where they showed significantly reduced EWC. The fact that the constant physical properties of contact lens, EWC, diameter, and refractive power, in the pH higher than the 7.0 or 7.4 and that the statistically significant difference of those properties between acidic and basic conditions support that the presence of MA in etafilcon A makes it vulnerable to pH while hilafilcon B rarely responsive to pH change.
The EWC is composed of various states of water which could response to surrounding environment in different way with EWC. The physiological environment surrounding the contact lens effects the ionization state of the polymer or additives contained in the contact lens which regulates the state of water in contact lens. The state of water of etafilcon A had distinct trend differ from that of the EWC; the EWC increased as the pH increased, but Wnf was slightly reduced while Wfb was increased with statistically significant difference between most acidic and basic conditions. There has been debate about the formation of Wnf whether it is attributed from (i) a ‘nanocavity’ of polymer network or (ii) a hydrogen bond with polymer or additives. According to the previous study, the amount of Wnf and Wfb is increased with the EWC until it reaches its maximum value. After a network exceeds its critical EWC, the amount of Wnf is decreased and that of Wfb is increased. This phenomenon is derived from the reduced ‘nanocavity’ of network. Liu et al. (2001) reported that under the critical EWC, the amount of Wnf and Wfb is increased with the EWC [6]. But after exceeding the critical EWC, some of the neighboring ‘nanocavities’ are linked together reducing the cavity of polymeric network. We hypothesized that the etafilcon A material already exceeded its critical EWC even at lower pH condition. Therefore, the amount of Wnf got decreased with increasing pH and EWC replaced by Wfb. Unlike etafilcon A, hilafilcon B showed relatively constant value of EWC and state of water. This is derived from the temperature sensitive property of NVP [21]. In this study, the temperature was kept at room temperature. Therefore, hilafilcon B containing NVP rarely changed its physical properties.
The diameter of etafilcon A was decreased with pH, whereas the refractive power of it became more negative. The reduced diameter of the contact lens implies a contraction that results from the dehydration of the contact lens [22,23]. This also attributes the change of refractive power since the shape and refractive index are affected by hydration state of material [24,25]. In previous literatures, there has been suggested that the total EWC is largely associated with general physical properties of contact lens including diameter, refractive power [22,23,24,25]. In this study, more specifically, the dehydration of Wfb could contribute these phenomenon. As shown in Fig. 3(A), the amount of Wfb in etafilcon A got largely reduced with pH which showed similar pattern with diameter, refractive power, and total EWC. This similar pattern of change supports that they are closely related to each other.
The EWC has traditionally been regarded as one of the important properties of contact lenses, and several previous studies have shown that it affects diameter, shape, and refractive index of contact lens [22,23,24,25]. However, the results of this study showed that the trends of EWC and state of water could be different; though the EWC increases, various state of water of contact lens could decrease and/or increase. Regarding this inconsistency, there should be a specific type of state of water that determines the physical properties of contact lens. Therefore, we should study which water in contact lens, Wnf, Wfb, or Wff, affects the physical properties of contact lens. For example, EWC also affects the oxygen permeability of contact lens materials [26,27]. Although the relationship between EWC and oxygen permeability of contact lenses has been investigated, the type of water in the contact lens that affects oxygen permeability has yet to be studied. Previous studies mainly studied the state of water that varies depending on the type and/or ratio of polymer and additive [26,28,29]. When the developed materials are applied to ocular surface or immersed in contact lens care solutions or eye drops, the content of state of water could be changed and affect various physical properties including not only diameter, refractive power that examined in this study but also oxygen permissibility [30,31,32]. In addition, this study only includes conventional hydrogel contact lenses while the most prescribed or used are silicon hydrogel contact lenses [33]. Therefore, more advanced research is needed to investigate the state of water in various types of contact lens materials and how it affects the physical properties of contact lens.
5. Conclusion
In this study, we investigated effect of pH on the physical properties of ionic (etafilcon A) and non-ionic (hilafilcon B) contact lens. The degree of ionization of MA affects the state of water in etafilcon A contact lens which leads to the change of their physical properties. At acidic pH condition, the MA in etafilcon A became less ionized affecting the EWC, state of water, diameter, and refractive power of contact lens. At physiological pH and above it, the MA in etafilcon A became fully ionized resulting in the constant those of contact lens. The hilafilcon B which contains NVP, temperature-sensitive additive, rarely responded to the pH condition. In addition, it was found (i) that the state of water in the contact lens could show different compositions apart from its EWC and (ii) that the Wfb could contribute the change of physical properties of contact lens.
Author contribution statement
Jihye Ahn: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Moonsung Choi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Research Program funded by the SeoulTech (Seoul National University of Science and Technology).
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
References
- 1.Rizwan M., Yahya R., Hassan A., Yar M., Azzahari A.D., Selvanathan V., Sonsudin F., Abouloula C.N. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polym. Bull. (Berlin) 2017;9:137. doi: 10.3390/polym9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kocak G., Tuncer C., Butun V. pH-Responsive polymers. Polym Chem-Uk. 2016;8:144–176. doi: 10.1039/c6py01872f. [DOI] [Google Scholar]
- 3.Hwang B.-J., Joseph J., Zeng Y.-Z., Lin C.-W., Cheng M.-Y. Analysis of states of water in poly (vinyl alcohol) based DMFC membranes using FTIR and DSC. J. Membr. Sci. 2011;369:88–95. doi: 10.1016/j.memsci.2010.11.031. [DOI] [Google Scholar]
- 4.Vieira E.F.S., da Costa L.P., Cestari A.R. Preparation and characterization of polyalginate–glutaraldehyde membranes—swelling analysis by microcalorimetry and adsorption kinetics of cationic dye. J. Appl. Polym. Sci. 2010;118:857–865. doi: 10.1002/app.32408. [DOI] [Google Scholar]
- 5.Kim Y.S., Dong L., Hickner M.A., Glass T.E., Webb V., McGrath J.E. State of water in disulfonated poly(arylene ether sulfone) copolymers and a perfluorosulfonic acid copolymer (nafion) and its effect on physical and electrochemical properties. Macromolecules. 2003;36:6281–6285. doi: 10.1021/ma0301451. [DOI] [Google Scholar]
- 6.Liu W.G., Yao K.D. What causes the unfrozen water in polymers: hydrogen bonds between water and polymer chains? Polymer. 2001;42:3943–3947. doi: 10.1016/s0032-3861(00)00726-6. [DOI] [Google Scholar]
- 7.Tanaka M., Hayashi T., Morita S. The roles of water molecules at the biointerface of medical polymers. Polym. J. 2013;45:701–710. doi: 10.1038/pj.2012.229. [DOI] [Google Scholar]
- 8.Tanaka M., Motomura T., Ishii N., Shimura K., Onishi M., Mochizuki A., Hatakeyama T. Cold crystallization of water in hydrated poly(2-methoxyethyl acrylate) (PMEA) Polym. Int. 2000;49:1709–1713. doi: 10.1002/1097-0126. (200012)49:12<1709::aid-pi601>3.0.co;2-l. [DOI] [Google Scholar]
- 9.Willcox M.D.P., Argueso P., Georgiev G.A., Holopainen J.M., Laurie G.W., Millar T.J., Papas E.B., Rolland J.P., Schmidt T.A., Stahl U., Suarez T., Subbaraman L.N., Ucakhan O.O., Jones L. TFOS DEWS II tear film report. Ocul. Surf. 2017;15:366–403. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abelson M.B., Udell I.J., Weston J.H. Normal human tear pH by direct measurement. Arch Ophthalmol-Chic. 1981;99:301. doi: 10.1001/archopht.1981.03930010303017. 301. [DOI] [PubMed] [Google Scholar]
- 11.Norn M.S. Tear fluid pH in normals, contact lens wearers, and pathological cases. Acta Ophthalmol. 1988;66:485–489. doi: 10.1111/j.1755-3768.1988.tb04368.x. [DOI] [PubMed] [Google Scholar]
- 12.Andres S., Garcia M.L., Espina M., Valls J. Valero O. Tear pH, air pollution, and contact lenses. Optom. Vis. Sci. 1988;65:627–631. doi: 10.1097/00006324-198808000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Coles W.H., Jaros P.A. Dynamics of ocular surface pH. Br. J. Ophthalmol. 1984;68:549. doi: 10.1136/bjo.68.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pena-Verdeal H., Garcia-Queiruga J., Garcia-Resua C., Yebra-Pimentel E., Giraldez M.J. Osmolality and pH of commercially available contact lens care solutions and eye drops. Contact Lens Anterior Eye. 2021;44 doi: 10.1016/j.clae.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Garrett Q., Chatelier R.C., Griesser H.J., Milthorpe B.K. Effect of charged groups on the adsorption and penetration of proteins onto and into carboxymethylated poly(HEMA) hydrogels. Biomaterials. 1998;19:2175–2186. doi: 10.1016/s0142-9612(98)00125-2. [DOI] [PubMed] [Google Scholar]
- 16.International Standards Organization . 2017. Ophthalmic Optics—Contact Lenses—Part4: Physicochemical Properties of Contact Lens Materials.https://www.iso.org/standard/66341.html ISO 18369-4:2017) [Google Scholar]
- 17.Ping Z.H., Nguyen Q.T., Chen S.M., Zhou J.Q., Ding Y.D. States of water in different hydrophilic polymers — DSC and FTIR studies. Polymer. 2001;42:8461–8467. doi: 10.1016/s0032-3861(01)00358-5. [DOI] [Google Scholar]
- 18.Ostrowska-Czubenko J., Gierszewska-Druzynska M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr. Polym. 2009;77:590–598. doi: 10.1016/j.carbpol.2009.01.036. [DOI] [Google Scholar]
- 19.Thankam F.G., Muthu J., Sankar V., Gopal R.K. Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf., B. 2013;107:137–145. doi: 10.1016/j.colsurfb.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 20.Serjeant E.P., Dempsey B. Pergamon; New York: 1979. Ionisation Constants of Organic Acids in Aqueous Solution. IUPAC Chem Data Ser No.23. [Google Scholar]
- 21.Efron N., Maldonado-Codina C. second ed. 2017. Comprehensive Biomaterials; pp. 686–714. [DOI] [Google Scholar]
- 22.Tranoudis I., Efron N. Parameter stability of soft contact lenses made from different materials. Contact Lens Anterior Eye. 2004;27:115–131. doi: 10.1016/j.clae.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Ramamoorthy P., Sinnott L.T., Nichols J.J. Contact lens material characteristics associated with hydrogel lens dehydration. Ophthal. Physiol. Opt. 2010;30:160–166. doi: 10.1111/j.1475-1313.2009.00705.x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Meijome J.M., Lira M., Lopez-Alemany A., Almeida J.B., Parafita M.A., Refojo M.F. Refractive index and equilibrium water content of conventional and silicone hydrogel contact lenses. Ophthal. Physiol. Opt J Br Coll Ophthal. Opt. Optomet. 2006;26:57–64. doi: 10.1111/j.1475-1313.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Meijome J.M., Lopez-Alemany A., Lira M., Almeida J.B., Oliveira M.E.C.D.R., Parafita M.A. Equivalences between refractive index and equilibrium water content of conventional and silicone hydrogel soft contact lenses from automated and manual refractometry. J. Biomed. Mater. Res. Part B Appl Biomat. 2006;80:184–191. doi: 10.1002/jbm.b.30583. [DOI] [PubMed] [Google Scholar]
- 26.Efron N., Morgan P.B., Cameron I.D., Brennan N.A., Goodwin M. Oxygen permeability and water content of silicone hydrogel contact lens materials. Optom. Vis. Sci. 2007;84:E328–E337. doi: 10.1097/opx.0b013e31804375ed. [DOI] [PubMed] [Google Scholar]
- 27.Young M.D., Benjamin W.J. Calibrated oxygen permeability of 35 conventional hydrogel materials and correlation with water content. Eye Contact Lens. 2003;29:126–133. doi: 10.1097/01.icl.0000062463.64717.86. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Tan G., Zhang S., Guang Y. Influence of water states in hydrogels on the transmissibility and permeability of oxygen in contact lens materials. Appl. Surf. Sci. 2008;255:604–606. doi: 10.1016/j.apsusc.2008.06.178. [DOI] [Google Scholar]
- 29.Sun D., Zhou J. Effect of water content on microstructures and oxygen permeation in PSiMA–IPN–PMPC hydrogel: a molecular simulation study. Chem. Eng. Sci. 2012;78:236–245. doi: 10.1016/j.ces.2011.11.020. [DOI] [Google Scholar]
- 30.Alves M., Castanheira E.M.S., Lira M. Interactions between contact lenses and lens care solutions: influence in optical properties. Contact Lens Anterior Eye. 2021;44 doi: 10.1016/j.clae.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Lira M., Silva R. Effect of lens care systems on silicone hydrogel contact lens hydrophobicity. Eye Contact Lens. 2017;43:89–94. doi: 10.1097/icl.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 32.Lira M., Franco S., Vazquez-Dorrio J.B., Oliveira M.E.C.D.R., Costa M.F.M. Surface roughness and refractive index changes in contact lens induced by lens care systems. Eye Contact Lens. 2014;40:140–147. doi: 10.1097/icl.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 33.Orsborn G., Dumbleton K. Eye care professionals' perceptions of the benefits of daily disposable silicone hydrogel contact lenses. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2019;42:373–379. doi: 10.1016/j.clae.2019.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.