Abstract
Earthworm has a variety of molecular biological characteristic, for example, growth promotion, antioxidant, and anti-bacteria. Thus, we decomposed earthworm by earthworm's own protease for preparing of earthworm hydrolysate. Muscovy ducks were fed with basal diet that formulated to contain 1.5% and 2.5% earthworm hydrolysate. Then, we investigated the influences of earthworm hydrolysate on growth performance in Muscovy ducks by performance terminology and measurement for poultry (NY/T 823-2020). The morphology of duodenum and number of intraepithelial lymphocytes were tested by HE staining and immunohistochemical method. Serum biochemical parameters and antioxidant capacity were also determined. High-throughput sequencing technology can sequence 16S rDNA of cecal contents from experimental Muscovy ducks. Results showed that 1.5% earthworm hydrolysate increased ADG (16–70 days old), ALB, HDL-C, T-AOC, CAT, SOD, GSH-PX, villi length, intestine thickness and surface area of villi (P < 0.05 or P < 0.01), and reduced FCR (16–70 days old), UREA, CRE, LDL-C, MDA (P < 0.05 or P < 0.01). Meanwhile, 2.5% improved ADG (16–70 days old), abdominal fat yield, breast muscle yield, heart index, spleen index, ALP, UA, T-AOC, CAT, SOD, GSH-PX, villi length, crypt depth, intestine thickness, surface area of villi, the percentage of intraepithelial lymphocytes (P < 0.05 or P < 0.01), and decreased FCR (42–70 days old and 16–70 days old), UREA, UA, MDA (P < 0.05 or P < 0.01). The sequencing results of gut flora demonstrated that earthworm hydrolysate improved variety of the gut flora in the V4 area of ducks immensely. In a word, our results provide the foundation for preliminary researching the potential principles of earthworm hydrolysate in promoting production performance, adjusting antioxidant function and intestinal functions in the Muscovy duck industry.
Key words: earthworm hydrolysate, production performance, serum biochemical parameters, antioxidant capacity and intestinal function
Abbreviations: BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio; TP, total protein; ALB, albumin; GLB, globulin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; UREA, urea; CRE, creatinine; UA, uric acid; LDH, lactate dehydrogenase; TG, three glycerol; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; T-AOC, total antioxidant capacity; MDA, malondialdehyde; CAT, catalase; SOD, superoxide dismutase; GSH-PX, glutathione peroxidase; IELs, intraepithelial lymphocytes; OTU, operational taxonomic unit; LDA, linear discriminant analysis; LEfSe, LDA EFfect Size; COG, Clusters of Orthologous Groups of proteins; KEGG, Kyoto Encyclopedia of Genes and Genomes
Graphical abstract
INTRODUCTION
The earthworm belongs to the phylum Annelida (Sharanpreet et al., 2016). They are acceptable sources of proteins, energy, vitamins, fat, and minerals (Doriana et al., 2020). In poultry, the provision of essential amino acids (EAAs) is a key factor to grow rapidly in a brief period (Dorigam et al., 2016). Having higher concentrations of EAAs, earthworms are a natural source of protein for poultry (Sun et al., 2020a). Additionally, literature showed that earthworm contains more than ten kinds of proteolytic enzymes (Akazawa et al., 2018). These hydrolases have strong proteolytic activity and can decompose earthworm protein itself to obtain hydrolysate of high nutritional value. The earthworm hydrolysate can be absorbed directly into the body of other animals and play its role in promoting growth and resisting disease (Sun et al., 2020b). Thus, earthworm hydrolysate hypothetically be likely to become a vital addition agent for basal diet of Muscovy ducks. Nevertheless, there is no study on the nutrient composition of earthworm hydrolysate from earthworms, as well as the effects of using earthworm hydrolysate from this species on production performance of Muscovy ducks. Therefore, we determined the body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR, ADFI/ADG) of Muscovy ducks for evaluating growth-promoting effect of using earthworm hydrolysate from Eisenia foetida as an additive agent for Muscovy ducks. Additionally, the levels of health and oxidation resistance were determined by the levels of serum biochemical parameters and antioxidant indices.
The immune system of earthworm is pretty various and abundant with substances in the coelomic fluid containing antimicrobial peptides, antimicrobial factors, and lysozyme that play an important part in immunity of earthworm (Ghosh, 2018). For some pathogenic bacteria, the earthworm coelomic fluid demonstrates vigoroso hemolytic, bacteriostatic, and antibacterial properties (Fiołka et al., 2019). To further explore whether feeding earthworm hydrolysate could affect health and immune state of Muscovy ducks, serum biochemical parameters and antioxidant capacity were also detected in this study.
Additionally, to investigate mechanisms of promoting growth and resisting disease of earthworm hydrolysate thoroughly the duodenal samples were collected at 70 days old. Tissue sections of duodenum were made for observation of intestinal morphological structure, measurement of intestinal morphologic parameters (villi length, crypt depth, villi width, ratio of villi length and crypt depth, intestine thickness, surface area of villi) and detection of intestinal barrier indicators (ratio of intraepithelial lymphocytes [IELs]). At the same time, microbial systems consisted of viruses, bacteria, and fungi in the gut of animals constitute a complicated ecosystem with the body. Complex mutual effects were existed among them (Zhu et al., 2021a). Generally, a symbiotic relationship was between the host and intestinal bacteria. As part of the intestinal barrier, intestinal bacteria of the host can play the roles of metabolic and immunomodulatory (Xie et al., 2021). The species composition and diversity differences of intestinal microecosystem can be uncovered by 16S rDNA sequencing (Dai et al., 2018). Thus, to investigate whether earthworm hydrolysate could affect the intestinal flora, cecum were collected at 70 days old for sequencing 16S rDNA by the technology of high-throughput sequence.
In brief, we researched the influence of earthworm hydrolysate on production performance based on the levels in ADG, ADFI, and FCR of Muscovy ducks. To further explore the state of health, the levels of biochemical parameters of serum, including total protein (TP), albumin (ALB), globulin (GLB), A/G, alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST/ALT, alkaline phosphatase (ALP), urea (UREA), creatinine (CRE), uric acid (UA), lactate dehydrogenase (LDH), three glycerol (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were also researched. Meanwhile, changes in total antioxidant capacity (T-AOC), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) in Muscovy ducks were analyzed for assessing antioxidant function of earthworm hydrolysate. Additionally, villi length, crypt depth, villi width, V/C ratio, intestine thickness, surface area of villi and percentage of IELs were detected by HE staining and immunohistochemistry. Changes of intestinal flora were measured by 16S rDNA sequencing. This research designed to close these study gaps in application of earthworm hydrolysate in Muscovy ducks.
MATERIALS AND METHODS
Earthworm Hydrolysate
All healthy earthworms were stably cultured in cattle manure or sheep manure in the laboratory for 1 wk. The fresh weight of mature earthworm was 0.3 to 0.5 g for the experiment. Fresh and clean earthworms were placed in biochemical incubator at 45°C and dissolved by earthworm's own digestive enzyme. After 24 h, the completely dissolved earthworm hydrolysate was obtained. Then hydrolysate was filtered with 4 layers of gauze for 3 times. Every 10 kg earthworm could produce 9 kg earthworm hydrolysate, and the extraction rate was 90%. The nutritional components of earthworm hydrolysate are exhibited in Table 1.
Table 1.
Nutritional composition of earthworm hydrolysate.
| Items | Contents |
|---|---|
| Conventional nutrients | |
| CP, % | 9.67 |
| EE, % | 0.40 |
| Ash, % | 0.84 |
| Trace element | |
| Ca, mg/kg | 682.87 |
| P, % | 0.098 |
| Zn, % | 0.000 72 |
| Fe, % | 0.013 |
| Se, mg/kg | 0.46 |
| Mn, % | 0.000 96 |
| Cu, % | 0.000 06 |
| Amino acids | |
| Asp#, % | 0.19 |
| Gly#, % | 0.46 |
| Ser#, % | 0.10 |
| Met*, % | 0.12 |
| Thr#*, % | 0.12 |
| Ala#, % | 0.66 |
| Cys-Cys, % | 0.66 |
| Leu*, % | 0.71 |
| Glu#, % | 0.67 |
| Ile*, % | 0.43 |
| Val, % | 0.54 |
| Tyr, % | 0.05 |
| Lys*, % | 0.06 |
| His, % | 0.35 |
| Phe*, % | 0.26 |
| Arg, % | 0.05 |
| Pro#, % | 0.08 |
| Trp*, % | 0.10 |
| Total amino acids | 5.61 |
| Heavy metal | |
| As, mg/kg | 0.420 |
| Hg, mg/kg | 0.010 |
| Pb, mg/kg | 0.066 |
| Cd, mg/kg | 0.016 |
| Cr, mg/kg | 0.450 |
represents for essential amino acid.
represents for nonessential amino acid.
Experimental Design
Two hundred and seventy 16-day-old healthy male Muscovy ducks (Ganzhou, Jiangxi, China) were grouped into 3 groups (n = 90/group) randomly. Control group was fed with basal diet (Table 2). Group Ⅰ was fed with basal diet that added with 1.5% earthworm hydrolysate. Group Ⅱ was fed with basal diet that added with 2.5% earthworm hydrolysate. All Muscovy ducks’ trial protocols of this study and all steps of experiments were accomplished in accordance with the requirements of the Institutional Animal Care and Use Committee. Serum and tissues from 6 ducks in each group were collected at 70 days old for the following experiments.
Table 2.
Composition and nutrient levels of basal diets (air-dry basis).
| Items | Contents |
|
|---|---|---|
| 15–21 days old | 22–70 days old | |
| Ingredients | ||
| Corn, % | 61.80 | 62.0 |
| Soybean meal, % | 26.00 | 20.00 |
| Fish meal, % | 4.20 | 0 |
| Wheat bran, % | 4.00 | 14.00 |
| Met, % | 0.13 | 0.15 |
| Limestone, % | 1.22 | 1.50 |
| CaHPO4, % | 0.95 | 1.07 |
| NaCl, % | 0.20 | 0.28 |
| Mineral/vitamin premix1, % | 1.50 | 1.50 |
| Total | 100 | 100 |
| Nutrient levels2 | ||
| Metabolizable energy, MJ/kg | 11.60 | 11.29 |
| Crude protein, % | 20.00 | 15.50 |
| Crude fiber, % | 2.80 | 3.12 |
| Ca, % | 1.25 | 1.74 |
| Met, % | 0.45 | 0.40 |
| Met+Cys-Cys, % | 0.80 | 0.68 |
| Lys, % | 1.05 | 0.75 |
The premix offered the following per kg of diets: vitamin 8,000 IU; vitamin D3 3,000 IU; vitamin E: 20 IU, vitamin B1: 4 mg, vitamin B2: 3.6 mg, vitamin B5: 40 mg, vitamin B6: 4 mg, vitamin B12: 0.02 mg, vitamin K3: 2 mg, biotin: 0.15 mg, folic acid: 1.0 mg, D-pantothenic acid: 11 mg, nicotinic acid: 10 mg, antioxidant: 100 mg, Cu (as copper sulfate): 10 mg, Fe (as ferrous sulfate): 80 mg, Mn (as manganese sulfate): 80 mg, Zn (as zinc sulfate): 75 mg, I (as potassium iodide): 0.40 mg, Se (as sodium selenite): 0.30 mg.
Criteria for evaluating basal diets obtained through measurement and calculation.
Collection of Samples and Measurement of Production Performance
The BW of the ducks was measured before feeding in the morning at 16, 21, 42, and 70 days old. Daily feed consumption was recorded. ADG, ADFI, and FCR were calculated. The serum from 6 ducks in each group was separated out from blood samples and preserved at −20°C for later tests. After the slaughter, the determination of carcass traits, containing carcass yield, semi-eviscerated yield, eviscerated yield, and abdominal fat yield, was performed according to NY/T 823-2020. The measurement of visceral organ parameters, consisting of heart and liver indices, was executed to as one of evaluating production performance of ducks. The immune organs indices, including spleen and bursa of fabricius indices, were also detected. And the organ parameters (visceral organ parameters and immune organs indices) = the weight of organ (mg)/live BW (g). After determination of production performance, 3.0 to 5.0 cm duodenum samples and cecal contents from 6 ducks in each group were collected for intestinal morphology detection and high-throughput sequencing of gut flora.
Serum Biochemical Parameters
Sixteen serum biochemical parameters of 18 serum samples (n = 6/group), including TP, ALB, GLB, A/G, ALT, AST, AST/ALT, ALP, UREA, CRE, UA, LDH, TG, TC, HDL-C, and LDL-C, were analyzed through automatic biochemical analyzer (Hitachi, Tokyo, Japan).
Antioxidant Capacity Indicators and Immune Status
The levels of T-AOC, MDA, CAT, SOD, and GSH-PX in serum were respectively determined by the method of chemical colorimetry, thiobarbituric acid (TBA) method, ammonium molybdate photometry method, WST-1 method, and biuret method in line with the kits’ instructions. The kits of above assays were from Ganzhou Beisite Biological Co. Ltd. (Ganzhou, China).
Histology
Eighteen frozen sections were re-warmed and dried, fixed with 4% paraformaldehyde for 15 min, differentiated using 75% alcohol slightly, redyed for 3 to 5 min by hematoxylin, differentiated using hydrochloric acid alcohol rapidly, and blued by ammonia solution. Subsequently, the stained sections were panoramically scanned by Pannoramic Scan (3Dhistech, The Digital Pathology Company, Budapest, Hungary). The measurement of villi length (V), crypt depth (C), villi width, villi length/crypt depth (V/C) ratio, and intestinal thickness were performed by Caseviewer Systems. Surface area of villi calculated as follows: Surface area of villi = π·w·h (w is width; h is villi length).
Immunohistochemistry
All formalin-fixed, paraffin-embedded duodenal sections (5–6 µm) were placed on coated object-slides. Thereafter, the sections were deparaffinized in light of normal steps and subjected to immunohistochemistry by the procedures of routine immunohistochemical method. Rabbit anti-human CD3 monoclonal antibody (1: 200; Proteintech, Chicago, Illinois) was used as primary antibody. HRP-Goat anti-rabbit IgG (1:200; Solaribio, China) was used as secondary antibody. Subsequently, the sections were washed, stained with 0.05% 3’, 3-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) for color rendering and counter stained using hematoxylin. Yellow brown staining appeared in immunoreactive cells. The positive cells were counted in 25 random fields from 5 cross-sections in each sample. Positive cells were determined and calculated by mean integral optical density.
Gut Microbiota Analysis
DNA Extraction, PCR, and Sequencing
Library construction and sequencing: through extracting total DNA of samples by the E.Z.N.ATM Mag-Bind soil DNA kit (OMEGA, Norcross, Georgia), primers (515F /GTGCCAGCMGCCGCGGTAA and 806R/GGACTACHVGGGTWTCTAAT) were designed according to the V4 region of 16S rDNA gene. Sequencing adapters were appended to the end of primers. PCR was performed to amplify the target sequences. Products of PCR were further purified by Agencourt Ampure XP Beads (Beckman Coulter, Bria, California). Then they were quantified by Qubit 2.0 DNA assay kit (Life Technologies, Carlsbad, Chicago, Illinois) and homogenized to get a sequencing library. Library QC was executed to construct libraries. Illumina Novaseq 6000 was used to sequence qualified libraries. Base calling analysis was applied to convert the original image data files obtained by high-throughput sequencing into Sequenced Reads. Results were preserved with FASTQ format file. Reads’ sequence messages and their qualities of corresponding sequencing information were contained.
Processing of Sequencing Data
On the basis of overlapping sequences, the software of FLASH v1.2.7 was used to assemble high-quality reads. Quality filtering on the raw tags was performed under specific filtering conditions to obtain the high-quality clean tags according to the QIIME V1.9.1 software quality controlled process. UCHIME v4.2 was performed to identify and remove the chimeric sequences, and effective reads were generated. Bioinformatic analysis was performed for further analysis.
Data Analysis
All data were analyzed using SPSS17.0 software and were expressed as the mean ± standard deviation (SD). Multiple t tests were performed to determine the significance of differences among 3 groups. P < 0.05 was statistically significant. P < 0.01 was extremely significant.
RESULTS
Production Performance
According to the Table 3, there was no significant difference in BW, ADFI among 3 groups. ADG and FCR of Muscovy ducks were affected by earthworm hydrolysate. Compared with Control group, ADG (16–70 days old) in Group Ⅰ was observably increased (P < 0.01), whereas FCR (16–70 days old) in Group Ⅰ was significantly decreased (P < 0.01); ADG (16–70 days old) in Group Ⅱ was memorably accelerated (P < 0.01), whereas FCR (42–70 and 16–70 days old) were significantly decreased (P < 0.01).
Table 3.
Changes in growth performance of Muscovy ducks (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| Body weight, g | ||||
| 16 d | 267.33 ± 9.50 | 269.33 ± 8.39 | 277.67 ± 4.62 | 0.28 |
| 21 d | 386.69 ± 19.54 | 375.64 ± 12.79 | 383.63 ± 18.15 | 0.59 |
| 42 d | 1089.25 ± 49.16 | 1080.48 ± 28.78 | 1087.88 ± 62.47 | 0.91 |
| 70 d | 2220.00 ± 82.46 | 2263.00 ± 42.14 | 2271.88 ± 52.98 | 0.22 |
| Average daily gain, g | ||||
| 16–21 d | 21.68 ± 4.36 | 22.38 ± 3.87 | 22.30 ± 5.50 | 0.98 |
| 21–42 d | 31.95 ± 0.54 | 34.49 ± 0.78 | 34.48 ± 0.87 | P < 0.01 |
| 42–70 d | 40.06 ± 3.25 | 42.16 ± 1.95 | 42.47 ± 3.28 | 0.22 |
| 16–70 d | 34.50 ± 3.50Bb | 37.01 ± 3.33Aa | 36.80 ± 3.32Aa | P < 0.01 |
| Average daily feed intake, g | ||||
| 16–21 d | 43.22 ± 2.65 | 45.76 ± 0.44 | 45.21 ± 0.89 | 0.21 |
| 21–42 d | 89.11 ± 1.05 | 89.45 ± 1.41 | 91.33 ± 1.03 | 0.76 |
| 42–70 d | 160.95 ± 1.77 | 164.84 ± 2.84 | 161.57 ± 1.50 | 0.91 |
| 16–70 d | 120.68 ± 2.37 | 123.06 ± 0.89 | 122.06 ± 1.01 | 0.13 |
| Feed conversion ratio | ||||
| 16–21 d | 2.05 ± 0.40 | 2.08 ± 0.34 | 2.12 ± 0.59 | 0.98 |
| 21–42 d | 2.79 ± 0.04 | 2.59 ± 0.06 | 2.65 ± 0.07 | 0.02 |
| 42–70 d | 3.94 ± 0.22a | 3.71 ± 0.03ab | 3.59 ± 0.03b | 0.04 |
| 16–70 d | 3.49 ± 0.12Aa | 3.33 ± 0.06Bb | 3.32 ± 0.02Bb | 0.05 |
Note: In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05). Different capital letter superscripts: significant difference (P < 0.01).
Additionally, as shown in Table 4, abdominal fat yield, breast muscle yield, heart index, and spleen index in Group Ⅱ were extremely elevated, compared with Control group (P < 0.01).
Table 4.
Changes in carcass traits of Muscovy ducks (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| Dressed percentage, % | 82.77 ± 4.39 | 84.15 ± 6.61 | 87.75 ± 2.22 | 0.31 |
| Percentage of half-eviscerated yield with giblet, % | 75.50 ± 4.57 | 77.00 ± 6.09 | 80.34 ± 2.37 | 0.26 |
| Percentage of eviscerated yield, % | 68.54 ± 4.22 | 70.67 ± 5.84 | 72.80 ± 2.09 | 0.40 |
| Percentage of abdominal fat yield, % | 1.30 ± 0.43B | 0.93 ± 0.49B | 2.25 ± 0.56A | P < 0.01 |
| Percentage of breast muscle yield, % | 9.34 ± 1.84B | 8.98 ± 1.62B | 13.31 ± 1.04A | P < 0.01 |
| Percentage of leg muscle yield, % | 14.16 ± 1.41 | 14.68 ± 3.73 | 13.62 ± 1.17 | 0.67 |
| Heart index | 4.82 ± 0.35B | 4.81 ± 0.31B | 5.29 ± 0.64A | P < 0.01 |
| Liver index | 20.28 ± 2.27 | 20.36 ± 2.18 | 20.00 ± 2.96 | 0.81 |
| Spleen index | 0.61 ± 0.12B | 0.70 ± 0.16B | 0.82 ± 0.22A | P < 0.01 |
| Bursal index | 0.78 ± 0.27 | 0.86 ± 0.36 | 0.56 ± 0.27 | 0.24 |
Note: In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05). Different capital letter superscripts: significant difference (P < 0.01).
Serum Biochemical Parameters and Antioxidant Capacity Indicators
As shown in Table 5, the serum ALB and HDL-C levels in Group Ⅰ were significantly or extremely significantly elevated (P < 0.05 or P < 0.01), whereas the serum UREA, CRE, and LDL-C levels in Group Ⅰ were extremely depressed (P < 0.01), in comparison with the Control group. The serum ALP level in Group Ⅱ was observably increased (P < 0.01), whereas the serum UREA and UA levels in Group Ⅱ were dramatically reduced (P < 0.05), in comparison with the Control group.
Table 5.
Changes in serum biochemical indices of Muscovy ducks (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| TP, g/L | 37.25 ± 1.33 | 42.38 ± 7.44 | 36.18 ± 3.88 | 0.10 |
| ALB, g/L | 13.47 ± 0.87B | 15.63 ± 0.92A | 13.35 ± 0.86B | P < 0.01 |
| GLO, g/L | 23.78 ± 1.07 | 26.75 ± 6.96 | 22.83 ± 2.95 | 0.30 |
| A/G | 0.57 ± 0.04 | 0.62 ± 0.15 | 0.60 ± 0.02 | 0.60 |
| ALT, U/L | 17.00 ± 4.56 | 25.50 ± 18.78 | 14.03 ± 3.89 | 0.23 |
| AST, U/L | 45.85 ± 20.32 | 77.00 ± 86.56 | 46.52 ± 6.20 | 0.50 |
| AST/ALT | 2.73 ± 1.23 | 2.79 ± 0.95 | 3.53 ± 0.96 | 0.37 |
| ALP, U/L | 202.88 ± 99.38B | 329.40 ± 95.10B | 409.23 ± 42.33A | P < 0.01 |
| UREA, mmo/L | 1.15 ± 0.34Aa | 0.33 ± 0.17Bb | 0.75 ± 0.14Ab | P < 0.01 |
| CRE, μmol/L | 40.02 ± 12.46A | 9.50 ± 4.11B | 24.82b ± 8.08A | P < 0.01 |
| UA, μmol/L | 222.55 ± 49.55a | 228.00 ± 120.65ab | 136.55 ± 52.63b | 0.13 |
| LDH, U/L | 546.03 ± 119.39 | 886.88 ± 772.00 | 469.42 ± 79.96 | 0.27 |
| TG, mmol/L | 0.983 ± 0.223 | 0.85 ± 0.35 | 0.88 ± 0.21 | 0.67 |
| TC, mmol/L | 4.38 ± 0.16 | 5.37 ± 1.10 | 4.46 ± 0.18 | 0.03 |
| HDL-C, mmo/L | 2.22 ± 0.19B | 3.96 ± 1.05A | 2.21 ± 0.10B | P < 0.01 |
| LDL-C, mmol/L | 1.18 ± 0.07ABa | 1.03 ± 0.12Bb | 1.35 ± 0.21Aa | P < 0.01 |
Note: In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05). Different capital letter superscripts: significant difference (P < 0.01).
Changes in Antioxidant Indices of Muscovy Ducks
As shown in Table 6, the levels of T-AOC, CAT, SOD, and GSH-PX in Group Ⅰ and Group Ⅱ were markedly accelerated (P < 0.01), and the levels of MDA in Group Ⅰ and Group Ⅱ were clearly reduced (P < 0.01).
Table 6.
Changes in antioxidant indices of Muscovy ducks (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| T-AOC, U/mL | 4.15 ± 0.046B | 6.37 ± 1.27A | 6.84 ± 1.41A | P < 0.01 |
| MDA, nmol/mL | 4.06 ± 0.043A | 2.97 ± 0.50B | 2.82 ± 0.33B | P < 0.01 |
| CAT, U/mL | 15.50 ± 2.37B | 45.10 ± 2.85A | 49.88 ± 6.90A | P < 0.01 |
| SOD, U/mL | 16.39 ± 4.77B | 51.67 ± 4.70A | 57.14 ± 6.87A | P < 0.01 |
| GSH-PX, U/mL | 453.37 ± 46.70B | 876.00 ± 53.50A | 903.46 ± 27.40A | P < 0.01 |
Note: In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05). Different capital letter superscripts: significant difference (P < 0.01).
Changes in the Intestinal Parameters of Duodenum
As shown in Figure 1, the intestinal mucosa in each group was structurally intact, with orderly villi and without edema, evidence of congestion, unusual epithelial cells, or infiltration by inflammatory cells. Meanwhile, the intestinal mucosal villi of Group Ⅰ and Group Ⅱ were more dense and longer compared to Control group. Collectively, the results suggest that earthworm hydrolysate enhance intestinal morphological structure of duodenal mucosa. Important indicators of intestinal function, namely villi length, villi width, V/C ratio, surface area of villi, and thickness of the duodenum in Group Ⅰ and Group Ⅱ were all higher than these in Control group. Compared with Control group, the villi length, intestine thickness, surface area of villi of Group Ⅰ was improved prominently (P < 0.05 or P < 0.01), and the villi length, crypt depth, intestine thickness, and surface area of villi of Group Ⅱ were significantly boosted (P < 0.05 or P < 0.01). To further explore if the earthworm hydrolysate affect the immune function of intestine, we test lymphocyte percentage by immunohistochemistry. Picture of immunohistochemistry is shown in Figures 1D to 1F. Result of measured positive IELs in Table 7 showed that 2.5% earthworm hydrolysate obviously increased the percentage of IELs (P < 0.05). These results imply that earthworm hydrolysate remarkably increased the intestinal digestion and absorption function (morphological parameter) and mechanical barrier of mucosa (IELs) in Muscovy ducks.
Figure 1.
Morphology and IELs’ immunohistochemistry of duodenum in Muscovy ducks. Duodenum was obtained at 70 days old were sectioned, stained, and scanned by Pannoramic Scan (3Dhistech, The Digital Pathology Company, Hungary). (A) Hematoxylin and eosin staining for observation of morphology in Control group (400×). (B) Hematoxylin and eosin staining for observation of morphology in Group Ⅰ (400×). (C) Hematoxylin and eosin staining for observation of morphology in Group Ⅱ (400×). (D) Immunohistochemistry for observation of IELs in Control group (200×). (E) Immunohistochemistry for observation of IELs in Group Ⅰ (200×). (F) Immunohistochemistry for observation of IELs in Group Ⅱ (200×). Abbreviation: IELs, intraepithelial lymphocytes.
Table 7.
Changes in the intestinal parameters of duodenum (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| Villi length, μm | 1,132.78 ± 143.44Bc | 1,417.05 ± 64.20ABb | 1,870.30 ± 115.93Aa | 0.01 |
| Crypt depth, μm | 247.92 ± 52.18b | 285.19 ± 40.29ab | 337.14 ± 34.22a | 0.11 |
| Vilii width, μm | 244.04 ± 61.30 | 261.31 ± 52.51 | 272.64 ± 83.63 | 0.87 |
| V/C ratio, μm | 4.66 ± 0.80 | 5.05 ± 0.88 | 5.61 ± 0.96 | 0.47 |
| Intestine thickness, μm | 274.82 ± 26.12Bb | 380.61 ± 52.07Aa | 409.34 ± 20.14Aa | P < 0.01 |
| Surface area of villi, mm2 | 0.87 ± 0.25Bc | 1.26 ± 0.12Bb | 1.97 ± 0.09Aa | P < 0.01 |
| Intraepithelial lymphocytes, % | 3.44 ± 0.87Bb | 5.05 ± 0.42ABb | 7.56 ± 1.00Aa | 0.02 |
Note: In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05). Different capital letter superscripts: significant difference (P < 0.01).
16S rDNA Sequencing Results
16S rDNA Sequencing Data Statistics
Total 638,696 raw reads were generated from 9 samples. Upon that, we acquired 590,790 clean reads through quality control and assembly of reads. Minimum of 59,869 and average of 65,643 clean reads were generated for each sample. The results of raw data processing, including raw reads, clean reads, effective reads, avgLen, GC, Q20, Q30, and Effective of each group, were shown in Table 8.
Table 8.
Summary on raw data processing (n = 6/group).
| Items | Control group | Group Ⅰ | Group Ⅱ | P value |
|---|---|---|---|---|
| Raw Reads | 71,796.33 ± 6,946.93 | 73,034.00 ± 7,595.15 | 68,068.33 ± 4,602.79 | 0.65 |
| Clean Reads | 66,592.33 ± 6,416.54 | 67,540.67 ± 7,092.84 | 62,797 ± 4,285.66 | 0.62 |
| Effective Reads | 62,356.33 ± 6,123.90 | 63,175.00 ± 6,723.42 | 58,859.33 ± 3,881.90 | 0.64 |
| AvgLen(bp) | 252.00 | 252.00 | 252.00 | |
| GC, % | 53.22 ± 0.15b | 53.43 ± 0.25ab | 53.71 ± 0.31a | 0.12 |
| Q20, % | 99.82 ± 0.00b | 99.84 ± 0.02ab | 99.85 ± 0.01a | 0.49 |
| Q30, % | 99.09 ± 0.01b | 99.14 ± 0.04a | 99.85 ± 0.01a | 0.03 |
| Effective, % | 86.84 ± 0.20 | 86.49 ± 0.45 | 86.48 ± 0.15 | 0.30 |
Note: Data estimation on each sample were shown in the following table, where Sample ID: Sample ID; Raw Reads: Counts of raw reads; Clean Reads: Counts of clean reads (post quality control and assembly); Effective Reads: Counts of effective reads after chimeric reads removal; AvgLen (bp): Average read length of each sample; GC(%): GC content, i.e., proportion of G and C in all bases; Q20(%): Percentage of bases with Q-score larger or equal to Q20; Q30(%): Percentage of bases with Q-score larger or equal to Q30; Effective(%): Percentage of effective reads in raw reads. In the same row, no letter or the same letter superscripts: no significant difference (P > 0.05). Different small letter superscripts: significant difference (P < 0.05).
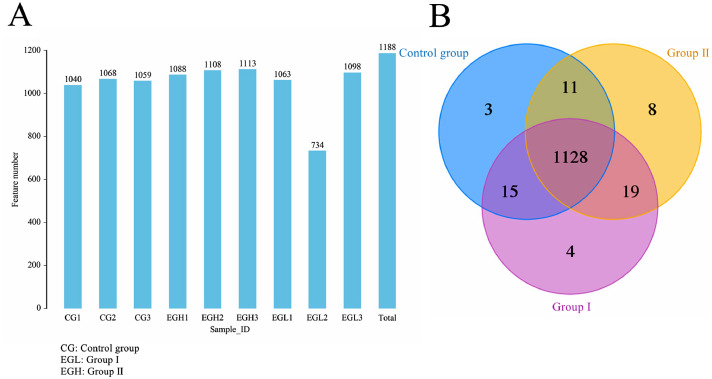
Operational taxonomic unit (OTU) refers to a cluster of sequences used to define a group (e.g., species, genus, strain, etc.) in phylogenetic studies or population genetic studies. Reads were clustered with similarity above 97% by Usearch, generating OTUs. Statistics of OTUs in each sample was shown in Figure 2A, with OTU counts marked on top of each bar. Common and unique features among samples were visualized by Venn diagram, from which common microbial among groups can be identified depending on common features (Figure 2B). Three (0.25%), 4 (0.34%), and 8(0.67%) features were discovered to be extremely significant and could be matched with noted features in the Clusters of Orthologous Groups of proteins (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, respectively. A total of 1,188 noted features were ultimately acquired (Figure 2B).
Figure 2.
OTU/ASV of intestinal microbiome of Muscovy ducks in each group. (A) Statistics on feature number in each sample. Note: X-axis: Sample ID; Y-axis: Feature number. (B) Venn diagram on features. Note: The number in each independent or overlapped area stands for number of unique or common features in each corresponding collection. Abbreviations: ASV, amplicon sequence variant; IELs, intraepithelial lymphocytes; OTU, operational taxonomic unit.
Alpha Diversity Analysis
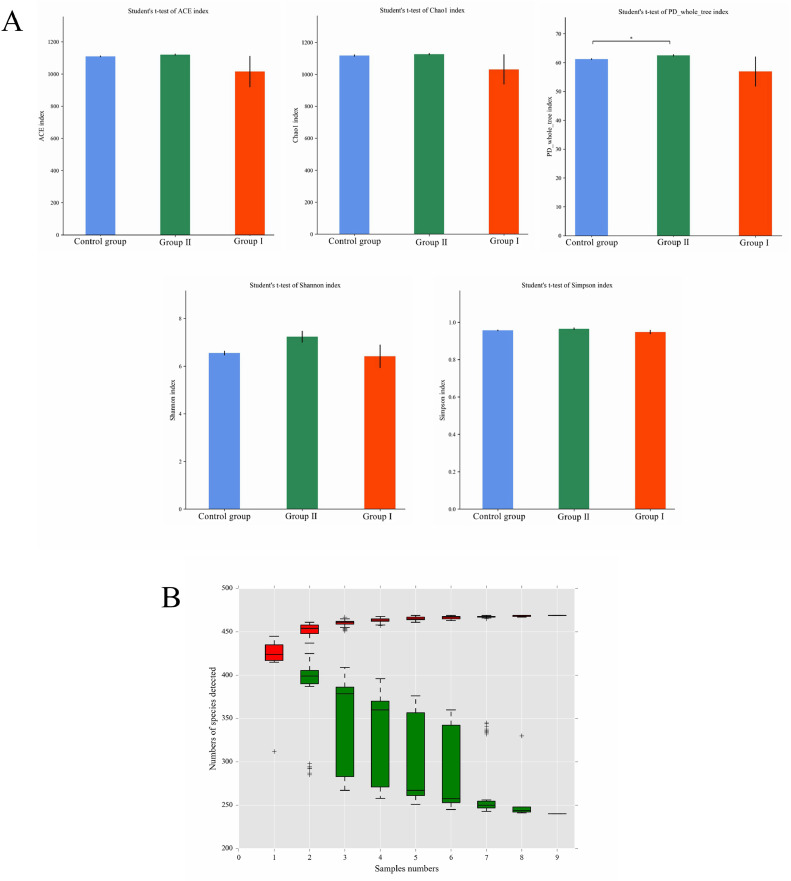
Alpha diversity mirrors richness and diversity of species within single sample. It can be accessed by several metrics, including Chao1, Ace, Shannon, Simpson, Coverage, PD_whole_tree. T test was processed for differential analysis among groups on alpha diversity metrics. Alpha diversity indices showed higher diversity and evenness (P < 0.05) in cecal microbe of Muscovy ducks that fed 2.5% earthworm hydrolysate in comparison with Control group in the PD_whole_tree (Figure 3A). In addition, species accumulation curve (Figure 3B) is designed to record the accumulation of annotated species with growing number of samples. The red boxes represent species numbers annotated in corresponding number of samples. The green boxes present the number of species shared by all picked samples. As shown in Figure 3B, 2 curves tend to be flat, which means there are enough samples to represent corresponding microbial community.
Figure 3.
Alpha diversity metrics of intestinal microbiome of Muscovy ducks in each group. (A) Histogram of alpha diversity metrics. Note: X-axis: Group name; Y-axis: Alpha diversity metrics. (B) Genus level species accumulation curve. Note: X-axis represents sample amount; Y-axis represents species number after sampling; red boxes form accumulation curve; green boxes form shared amount curve.
Beta Diversity Analysis
QIIME software was processed for beta diversity analysis, comparing species diversity between different samples. A significant separation was presented in PCoA based on unweighted UniFrac distances among Control group, Group Ⅰ, and Group Ⅱ (Figure 4A), suggesting an extremely significant in the structure of gut microbes after feeding earthworm hydrolysate. Anosim analysis in Figure 4B verified the above results (P < 0.05). This diagram in Figure 4C combines UPGMA clustering tree and histogram of species composition. Sample clustering trees on the left (same as UPGMA clustering tree above) were constructed based on four distance algorithms, which shown the similarity between samples. There are large intra-group similarity and small extra-group similarity among 3 groups. The results indicated that earthworm hydrolysate could change the diversity of intestinal flora. The results of Figure 4C are consistent with the above results. Additionally, the dominant bacterial genuses in cecum of the ducks in this study were Bacteroides and Desulfovibrio.
Figure 4.
Beta diversity analysis of intestinal microbiome of Muscovy ducks in each group. (A) Principal component analysis of intestinal microbiome of Muscovy ducks in each group. Note: Each dot represents a sample. Samples in different groups are presented in different color. The influence of each eigenvalue was measured in percentage. (B) PERMANOVA/Anosim analysis box plot. Note: Y-axis: Beta distance. The box above “All between” represents the Beta distance data of samples between all groups, whereas the box above “All within” represents the Beta distance data of samples within all groups. The box below represents the Beta distance data of samples within different groups. (C) Combining drawing of clustering tree and histogram. Note: The figure legend in the bottom left represents the color of each group in which the cluster tree samples are grouped. The legend in the top right represents the top 10 species in abundance. The rest are classified as Others. Unannotated species are classified as Unclassified.
Analysis of Differences in Abundance of Microbiota
Figure 5A shows changes of intestinal microbial abundance of Muscovy ducks in each group. As can be seen from the Figure 5A, 1.5% and 2.5% earthworm hydrolysate observably increased the levels of Rikenellaceae, Muribaculaceae, Erysipelotrichaceae, Christensenellaceae, Eubacterium coprostanoligenes, Ruminococcaceae, Prevotellaceae-Ga6A1-group, Labilithrix, Fournierella, Blastococcus (P < 0.05). Additionally, 1.5% and 2.5% earthworm hydrolysate dramatically reduced the levels of Rhodospirillales, Bacteroidales, Desulfovibrionaceae, Barnesiellaceae, Succinatimonas, Sphaerochaeta, Mucispirillum, Methanobrevibacter, Candidatus-Saccharimonas (P < 0.05).
Figure 5.
Intestinal microbial abundance of Muscovy ducks in each group. (A) Histogram of analysis of variance between groups. Note: X-axis: species (The top 20 species with the lowest P values are shown); Y-axis: relative richness of species; Columns with different colors represent samples, **P < 0.01; *P < 0.05. (B) Linear discriminant analysis (LDA) value distribution histogram. (C) Cladogram based on LDA EFfect Size (LEfSe) analysis.
Linear discriminant analysis Effect Size (LEfSe) analysis were further performed for chasing down the microbial taxa that best explain the differences among 3 groups, as shown in Figures 5B and 5C. The results showed that Fusobacteriia, Fusobacteriaceae and Fusobacterium were enriched in Group Ⅰ (1.5% earthworm hydrolysate). Clostridia, Clostridiale, Desulfovibrionaceae, and Bacteroidales were widespread in control group.
PICRUSt Function Analysis
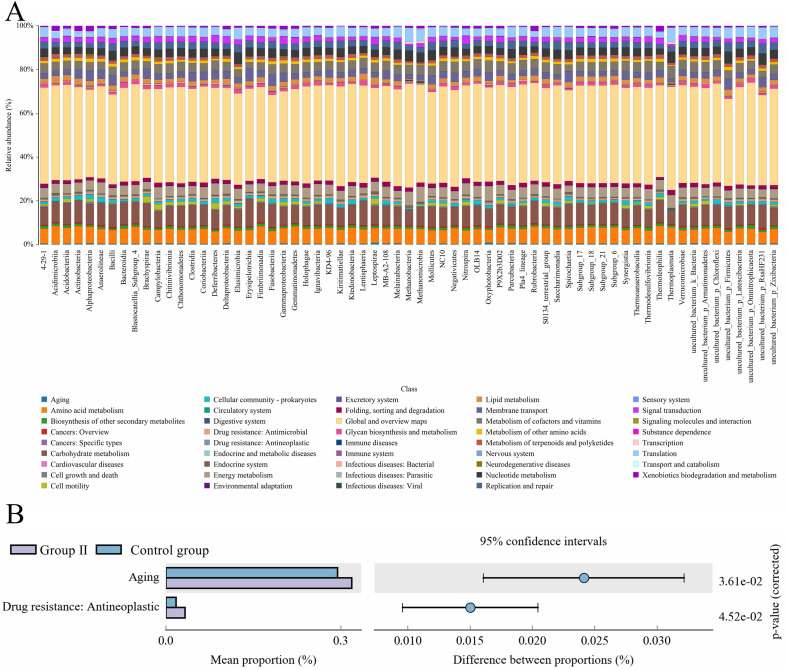
Based on reference phylogenetic tree, PICRUSt was applied to perform the annotation of species on feature sequences, which can further revealed the difference in functional genes of gut bacteria on the basis of Integrated Microbial Genomes (IMG) database. KEGG-based function prediction is a strong evidence to discover that how metabolic functions changed in an animal for adaptation to the living environment of their own. Results showed that KEGG pathways in this experiment were primarily enriched in Global and overview maps, Amino acid metabolism, Carbohydrate metabolism, Energy metabolism, and so on (Figure 6A). And different abundance (P < 0.05) of bacterial functional gene in control group and test group was mainly associated with “Aging” and “Drug resistance: Antineoplastic” in KEGG level (Figure 6B). COGs are frequently applied functional classification database of proteins in prokaryotes. Amino acid transport and metabolism, Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis, Energy production and conversion, Signal transduction mechanism, General function prediction, Translation, ribosomal structure and biogenesis enriched in cecum of Muscovy ducks in COG level (Figure 7A). RNA processing and modification, Nucleotide transpory and metabolism of Group Ⅱ were markedly lower than Control group. Posttranslational modification, Protein turnover, chaperones and Cell motility were extremely elevated in Group Ⅱ, in comparison with the Control group (Figure 7B).
Figure 6.
KEGG-based function prediction. (A) Histogram on KEGG pathways. Note: X-axis: Species; Y-axis: Relative abundance of pathway in percentage. (B) Figure of analysis of KEGG metabolic pathways difference between groups. Note: Abundance proportion of different functions in two groups of samples was shown in the left part of the figure. The difference proportion of functional abundance within the 95% confidence interval was shown in the middle part of the figure and the value on the far right is P value. Abbreviation: KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 7.
COG function prediction. (A) Histogram on COG pathways. Note: X-axis: Species; Y-axis: Relative abundance of pathway in percentage. (B) Statistical graph of COG function classification. Note: Left figure: abundance proportion of different functions in among three groups of samples. Middle figure: difference proportion of functional abundance within the 95% confidence interval. P value is on the far right of the figure. Abbreviation: COG, Clusters of Orthologous Groups of proteins.
DISCUSSION
Feeding is a necessary life activity to guarantee subsistence and production of animals (Dixon et al., 2022). Adequate feed intake (FI) is significant for enhancing animal subsistence and production potential (Ribadiya et al., 2015). Research indicated that earthworm meal can reduce palatability and feed intake (Bahadori et al., 2017). Nevertheless, ADFI of 3 groups in this study has no significant difference. This may be due to the better palatability of earthworm hydrolysate. Meanwhile, the results of growth performance show remarkable increase when Muscovy ducks are fed on a earthworm hydrolysate. The reduced FCR may be one of the reasons for the promoted growth in groups fed on earthworm hydrolysate.
Carcass trait is an important index to reflect the carcass quality (Gungor E. and Erener G., 2020). It is generally acknowledged that meat performance is well when carcass yield and eviscerated yield are over 80% and 60%, respectively (Park et al., 2020). As shown in Table 4, the meat performance in each group is excellent as the carcass yield and eviscerated yield exceed the above standards. The improved abdominal fat yield in Group Ⅱ means that the taste of duck is boosted. Additionally, the increased breast muscle yield in Group Ⅱ also indicates that meat performance is enhanced. Organs are the essential "installations" of animal life process and the material foundation of their physiological functions, affecting the velocity of weight gain, the health condition of the animal, and even the ability to adapt to their environment directly (Choi et al., 2021). In this study, there is an increase in heart index of Group Ⅱ, which implies earthworm hydrolysate can enhance the function of cardiac pump. Thus adequate blood flow is provided to organs and tissues to supply oxygen and various nutrients, which further improves its production performance. That can improve the utilization rate of feed, which is one of the reasons why the feed conversation ratio of Muscovy ducks decreased without increasing body weight in this study. Commonly, immune organ indices are applied to assess the organs’ growth and development (Zhang et al., 2021b). The improvement of immunity and disease resistance were resulted from rapid development of immune organs in Muscovy ducks. Therefore, the level of the body's immune response was reflected by immune organ indices primarily (Xing et al., 2020). The boosted spleen index implies that earthworm hydrolysate can strengthen the immune function of Muscovy ducks.
Serum biochemical indices can give an index to the metabolism and health condition of the organism, as well as the responses of body to external and internal stressors and stimuli (Yang et al., 2022). Albumin is a small molecule protein, which is an important protein of the body and is produced by the liver mostly. It is of great significance in maintaining the body osmotic pressure, improving the body immunity and maintaining acid–base balance. Results show that ALB in Group I is clearly boosted, which indicated that 1.5% earthworm hydrolysate could improve immune function and maintain body osmotic pressure and acid-base balance. Metabolic condition of protein in the body can be reflected by the levels of UREA and UA to a certain extent. UREA and UA of Group Ⅱ are reduced in this study, which indicated that improvement of the metabolic status of protein. This may be in connection with the higher improvement of growth performance. ALP, which is a ubiquitous enzyme, can catalyze the hydrolysis of phosphate monoesters, and can be used as a general indicator of skeletal development (Barshan et al., 2019). The serums of 70-day-old Muscovy ducks were collected for the determination of ALP, and the increase of ALP activity in Group Ⅱ was conducive to osteogenesis in this growth-boom period. Therefore, we speculate that 2.5% earthworm hydrolysate can promote skeletal development to a certain extent. Additionally, results show that 1.5% earthworm hydrolysate exhibit certain regulatory activity on several serum lipid values, through reducing of LDL-C and increasing of HDL-C. Further researches of underlined mechanism of earthworm hydrolysate as a natural lipid-lowering agent in poultry ought to be validated.
Next, we determined the antioxidant indices, including T-AOC, MDA, CAT, SOD, and GSH-PX, to investigate the influences of earthworm hydrolysate on the extent of oxidative state in ducks (Liang et al., 2019). In non-enzymatic and enzymatic systems, T-AOC represents the total capacity of all kinds of antioxidants eliminating oxygen free radicals (Cecchini et al., 2020). Results of T-AOC indicated that long-term usage of earthworm hydrolysate can decreased oxidative stress, keeping the antioxidant defense system's balance and leading to an enhancement in antioxidant function. Furthermore, worked as an oxidized lipid metabolite, MDA can be applied to measure the level of oxidative stress of organism for the reason that free radicals will boost MDA production (Aktas et al., 2017). There are activity in antioxidant defense systems with CAT and SOD, for example, the CAT can catalyze the decomposition of H2O2 to H2O (Li et al., 2020). In the present study, the activities of CAT and SOD were depressed in earthworm hydrolysate-treated groups, indicating that earthworm hydrolysate could reduce oxidative damage. The activity of GSH-PX is also crucial indices of the duck's antioxidant status. The improved GSH-PX in earthworm hydrolysate group means the enhancement of antioxidant capacity. In brief, earthworm hydrolysate could improve the antioxidant capacity of Muscovy duck.
Morphological structure and mechanical barrier of intestine directly affect absorptive and barrier functions (Chen et al., 2022). Here, we explored the effects of earthworm hydrolysate on the morphological structure (villi length, crypt depth, villi width, V/C ratio, intestine thickness, surface area of villi) and intestinal barrier (IELs). Results showed that 1.5% earthworm hydrolysate markedly increased villi length, intestine thickness, surface area of villi, and 2.5% earthworm hydrolysate clearly increased the villi length, crypt depth, intestinal thickness and villi surface area, causing enhancement of absorptive functions. IELs are accurately located in the intestinal epithelium, offering the first line of protection in mucosal defense against luminal microbes or rapidly react to epithelial damages. In our study, 2.5% earthworm hydrolysate obviously promoted the percentage of IELs. In a word, earthworm hydrolysate could increase barrier and absorptive functions.
Microbial diversity study aims at revealing species abundance and taxonomic diversity in a microbial community based on investigation of genetic materials (Hou et al., 2020a). This study is mainly processed on Illumina Novaseq platform by generating PE reads targeted regions. These short reads are assembled, clustered, and de-noised to obtain tags, which can be used for species annotation and abundance analysis. Further analysis includes alpha diversity, beta diversity, differential analysis among groups, correlation analysis, function prediction, etc. Results of alpha diversity and beta diversity show that the data in this study are reliable. Results of differential analysis among groups show that 1.5% and 2.5% earthworms hydrolysate extremely increased Rikenellaceae, Muribaculaceae, Erysipelotrichaceae, Christensenellaceae, Eubacterium coprostanoligenes, Ruminococcaceae, Prevotellaceae-Ga6A1-group, Labilithrix, Fournierella, Blastococcus, and significantly decreased Rhodospirillales, Bacteroidaceae, Desulfovibrionaceae, Barnesiellae, Succindimonas, Sphaerocheta, Mucoidales, methanobrevibacter, and Candidatus-Saccharimonas in Muscovy ducks compared with controls. There are no direct information members of the Rikenellaceae and Labilithrix association with disease. Recent research have demonstrated that the family of Muribaculaceae, also known as family S24-7, is strongly linked with the degradation of carbohydrate (Zou et al., 2021c). Additionally, there is strong evidence supporting an association between Erysipelotrichaceae and host lipid metabolism, and this fecal sterol-producing eubacteria can decompose cholesterol into unobservable fecal sterols for excretion (Li et al., 2021). Christensenellaceae is significantly negatively correlated with metabolic diseases such as BMI and inflammation (Mazier et al., 2021a). Eubacterium coprostanoligenes could turn cholesterol into coprostanol (Freier et al., 1994). Ruminococcaceae transforms primary bile acids into major microorganisms of secondary bile acids (Qu et al., 2022). Prevotellaceae-Ga6A1-group are capable of breaking down proteins and carbohydrates (Guo et al., 2019). The enhancement of these gut flora indicated that earthworm hydrolysate can promote the improvement in the abundance of some beneficial bacteria, increasing metabolic function of lipid, proteins and carbohydrates, improving the degradation effect of intestinal microorganisms on the contents, and causing the enhancement of the nutrient absorption of Muscovy ducks. Additionally, it is reported that Fournierella is considerably positively concerned to the expression levels of tight junction barrier genes and the increased Fournierella in Group Ⅱ indicate the function of intestinal barrier is enhanced. Rhodospirillales are driven by amino acid metabolism and are associated with functions in connection with vitamin and cofactor metabolism (McMullen et al., 2021b). Bacteroidaceae is related with many kinds of disease, such as thromboembolic disease, bacteremia, eczema and chronic inflammatory bowel disease (Humbel et al., 2020b). Simultaneously, Desulfovibrionaceae, Barnesiellae, Succindimonas, Sphaerocheta, Mucoidales, methanobrevibacter, and Candidatus-Saccharimonas are also been linked to some diseased and dysfunction, such as gut barrier dysfunction, immune dysfunction, metabolic dysfunction, systemic inflammation, and so on. In short, the depression of above gut flora showed that earthworm hydrolysate can reduce the risk of disease in Muscovy ducks, which is related to regulation of intestinal flora of earthworm hydrolysate.
CONCLUSION
In brief, our outcomes indicate that earthworm hydrolysate results in great improvement in production performance (increased ADG and decreased FCR), regulating the balance of immune and metabolic function (improved ALB, ALP, and HDL-C; declined UREA, CRE, UA, and LDL-C), contributing to the boosting of oxidation resistance (elevated T-AOC, CAT, CAT, SOD, and GSH-PX; depressed MDA); improving absorptive function (boosted villi length, crypt depth, intestinal thickness, and villi surface area) and immuno-barrier function (increased IELs). Meanwhile, earthworm hydrolysate can increase intestinal barrier function by promoting the abundance of intestinal flora, increase the number of beneficial and functional bacteria and decrease the number of pathogenic bacteria. This research will establish a foundation for the clinical application of earthworm hydrolysate in Muscovy duck industry in the future.
ACKNOWLEDGMENTS
This study was funded in part by the grant from Municipal Party Committee Talent Project of Ganzhou City (grant number: JXXTCXQN202009), Science and Technology Project of Ganzhou City (grant number: 2019GSKF), and Technical System of Poultry Industry in Jiangxi Province (grant number: JXARS-09).
DISCLOSURES
All authors declare they have no conflicts of interest.
REFERENCES
- Aktas M.S., Kandemir F.M., Kirbas A., Hanedan B., Aydin M.A. Evaluation of oxidative stress in sheep infected with psoroptes ovis using total antioxidant capacity, total oxidant status, and malondialdehyde level. J. Vet. Res. 2017;61:197–201. doi: 10.1515/jvetres-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa S.I., Tokuyama H., Sato S., Watanabe T., Shida Y., Ogasawara W. High-pressure tolerance of earthworm fibrinolytic and digestive enzymes. J. Biosci. Bioeng. 2018;125:155–159. doi: 10.1016/j.jbiosc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Bahadori Z., Esmaielzadeh L., Karimi-Torshizi M.A., Seidavi A., Olivares J., Rojas S., Salem A.Z.M., Khusro A., López S. The effect of earthworm (Eisenia foetida) meal with vermi-humus on growth performance, hematology, immunity, intestinal microbiota, carcass characteristics, and meat quality of broiler chickens. Livestock Sci. 2017;202:74–81. [Google Scholar]
- Barshan S., Khalaji S., Hedayati M., Yari M. Influence of bone meal degelatinisation and calcium source and particle size on broiler performance, bone characteristics and digestive and plasma alkaline phosphatase activity. Br. Poult. Sci. 2019;60:297–308. doi: 10.1080/00071668.2019.1587151. [DOI] [PubMed] [Google Scholar]
- Cecchini S., Fazio F. Assessment of total antioxidant capacity in serum of heathy and stressed hens. Animals (Basel) 2020;10:2019. doi: 10.3390/ani10112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen W.W., Ci W.J., Zheng Y.Y., Han X.Y., Huang J.P., Zhu J.J. Effects of dietary supplementation with lactobacillus acidophilus and bacillus subtilis on mucosal immunity and intestinal barrier are associated with its modulation of gut metabolites and microbiota in late-phase laying hens. Probiotics. Antimicrob. Proteins. 2022;1:1–13. doi: 10.1007/s12602-022-09923-7. [DOI] [PubMed] [Google Scholar]
- Choi W.J., Kim J.H., Han G.P., Kwon C.H., Kil D.Y. Effects of dietary hatchery by-products on growth performance, relative organ weight, plasma measurements, immune organ index, meat quality, and tibia characteristics of broiler chickens. Anim. Biosci. 2021;34:1181–1192. doi: 10.5713/ab.20.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigam J.C.P., Sakomura N.K., de Lima M.B., Sarcinelli M.F., Suzuki R.M. Establishing an essential amino acid profile for maintenance in poultry using deletion method. Clin. Trial. 2016;100:884–892. doi: 10.1111/jpn.12403. [DOI] [PubMed] [Google Scholar]
- Dai S.J., Zhang K.Y., Ding X.M., Bai S.P., Luo Y.H., Wang J.P., Zeng Q.F. Effect of dietary non-phytate phosphorus levels on the diversity and structure of cecal microbiota in meat duck from 1 to 21 d of age. Poult. Sci. 2018;97:2441–2450. doi: 10.3382/ps/pey090. [DOI] [PubMed] [Google Scholar]
- Doriana E.A.T., Marta C., Aldo T., Sara P., Claudia M.B. From a food safety prospective: the role of earthworms as food and feed in assuring food security and in valuing food waste. Insects. 2020;11:293. doi: 10.3390/insects11050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L.M, Dunn I.C, Brocklehurst S., Baker L., Boswell T., Caughey S.D, Reid A., Sandilands V., Wilson P.W, D'Eath R.B. The effects of feed restriction, time of day, and time since feeding on behavioral and physiological indicators of hunger in broiler breeder hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier T.A., Beitz D.C., Li L., Hartman P.A. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int. J. Syst. Bacteriol. 1994;44:137–142. doi: 10.1099/00207713-44-1-137. [DOI] [PubMed] [Google Scholar]
- Fiołka M.J., Czaplewska P., Macur K., Buchwald T., Kutkowska J., Paduch R., Kaczyński Z., Wydrych J., Urbanik-Sypniewska T. Anti-Candida albicans effect of the protein-carbohydrate fraction obtained from the coelomic fluid of earthworm Dendrobaena veneta. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. Environmental pollutants, pathogens and immune system in earthworms. Environ. Sci. Pollut. Res. Int. 2018;25:6196–6208. doi: 10.1007/s11356-017-1167-8. [DOI] [PubMed] [Google Scholar]
- Gungor E., Erener G. Effect of dietary raw and fermented sour cherry kernel (Prunus cerasus L. on growth performance, carcass traits, and meat quality in broiler chickens. Poult. Sci. 2020;99:301–309. doi: 10.3382/ps/pez490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B.D., Li D.H., Zhou B.B., Jiang Y., Bai H., Zhang Y., Xu Q., Zhao W.M., Chen G.H. Comparative characterization of bacterial communities in geese consuming of different proportions of ryegrass. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L.Y., Sun B.S., Yang Y. Effects of added dietary fiber and rearing system on the gut microbial diversity and gut health of chickens. Animals (Basel) 2020;10:107. doi: 10.3390/ani10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel F., Rieder J.H., Franc Y., Juillerat P., Scharl M., Misselwitz B., Schreiner P., Begré S., Rogler G., Käne R., Yilmaz B., Biedermann L. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 2020;18:2019–2029. doi: 10.1016/j.cgh.2019.09.022. [DOI] [PubMed] [Google Scholar]
- Liang J.R., Dai H., Yang H.M., Yang Z., Wang Z.Y. The effect of dietary vitamin A supplementation in maternal and its offspring on the early growth performance, liver vitamin A content, and antioxidant index of goslings. Poult. Sci. 2019;98:6849–6856. doi: 10.3382/ps/pez432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao X.L., Jiang X.M., Chen L., Hong L., Zhuo Y., Lin Y., Fang Z.F., Che L.Q., Feng B., Xu S.Y., Li J., Wu D. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2020;98:skaa067. doi: 10.1093/jas/skaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.T., Huang Z.R., Jia R.B., Lv X.C., Zhao C., Liu B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021;147 doi: 10.1016/j.foodres.2021.110530. [DOI] [PubMed] [Google Scholar]
- Mazier W., Corf K.L., Martinez C., Tudela H., Kissi D., Kropp C., Coubard C., Soto M., Elustondo F., Rawadi G., Claus S.P. A new strain of christensenella minuta as a potential biotherapy for obesity and associated metabolic diseases. Cells. 2021;10:823. doi: 10.3390/cells10040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen J.G., Bueno E., Blow F., Douglas A.E. Genome-inferred correspondence between phylogeny and metabolic traits in the wild drosophila gut microbiome. Genome Biol. Evol. 2021;13:evab127. doi: 10.1093/gbe/evab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Kim I.H. Effects of dietary Achyranthes japonica extract supplementation on the growth performance, total tract digestibility, cecal microflora, excreta noxious gas emission, and meat quality of broiler chickens. Poult. Sci. 2020;99:463–470. doi: 10.3382/ps/pez533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y.C., Su C.J., Zhao Q.H., Shi A.M., Zhao F.L., Tang L.X., Xu D.L., Xiang Z., Wang Y., Wang Y.Y., Pan J., Yu Y.L. Gut microbiota-mediated elevated production of secondary bile acids in chronic unpredictable mild stress. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.837543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribadiya N.K., Savsani H.H., Patil S.S., Garg D.D., Gadariya M.R., Karangiya V.K., Gajera A.P. Effect of feeding varying levels of groundnut haulms on feed intake and growth performance in broiler chickens. Vet. World. 2015;8:139–142. doi: 10.14202/vetworld.2015.139-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharanpreet S., Jaswinder S., Adarsh P.V. Earthworm as ecological engineers to change the physico-chemical properties of soil: soil vs vermicast. Ecol. Eng. 2016;90:1–5. [Google Scholar]
- Sun X.F., Yue S.Z., Qiao Y.H., Sun Z.J., Wang C., Li H.F. Dietary supplementation with selenium-enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poult. Sci. 2020;99:5344–5349. doi: 10.1016/j.psj.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.S., Yu G.H., Ning J.Y., Zhu X.D., Goodman B.A, Wu J. Biological removal of cadmium from biogas residues during vermicomposting, and the effect of earthworm hydrolysates on Trichoderma guizhouense sporulation. Bioresour. Technol. 2020;312 doi: 10.1016/j.biortech.2020.123635. [DOI] [PubMed] [Google Scholar]
- Xing R., Yang H., Wang X., Yu H., Liu S., Li P. Effects of calcium source and calcium level on growth performance, immune organ indexes, serum components, intestinal microbiota, and intestinal morphology of broiler chickens. J. Appl. Poult. Res. 2020;29:106–120. [Google Scholar]
- Xie Y.Q., Wen M., Zhao H., Liu G.M., Chen X.L., Tian G., Cai J.Y., Jia G. Effect of zinc supplementation on growth performance, intestinal development, and intestinal barrier function in Pekin ducks with lipopolysaccharide challenge. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tang X.W., Liu X., Yang H., Bin D.M., Liu H.J, Tang Q.H., Tang J.Y. Effects of dietary oligosaccharides on serum biochemical index, intestinal morphology, and antioxidant status in broilers. Anim. Sci. J. 2022;93:e13679. doi: 10.1111/asj.13679. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Liao L.Y., Su J.W., Liu Z.N., Pan S.L., Huang Y.F., Wu Y.J. Interactions of Muscovy duck reovirus, gut microbiota, and host innate immunity: transcriptome and gut microbiota analysis. Vet. Microbiol. 2021;264 doi: 10.1016/j.vetmic.2021.109286. [DOI] [PubMed] [Google Scholar]
- Zhang Q.Y., Zhang S., Cong G.L., Zhang Y.J., Madsen M.H., Tan B.J., Shi S. Effects of soy protein concentrate in starter phase diet on growth performance, blood biochemical indices, carcass traits, immune organ indices and meat quality of broilers. Animals (Basel) 2021;11:281. doi: 10.3390/ani11020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Liang N.N., Zhang X.X., Han C.X., Nan X.N. Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet. Res. 2021;17:216. doi: 10.1186/s12917-021-02911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]