Summary
Mitochondrial metabolism is critical in hematopoietic stem cell maintenance and differentiation. Here, we present a step-by-step protocol to efficiently differentiate human induced pluripotent stem cells into myeloid progenitors by a robust feeder- and serum-free system. Furthermore, we provide a protocol to subsequently assess mitochondrial function in iPSC-derived myeloid progenitors. We comprehensively describe a protocol to analyze and to quantify key parameters of mitochondrial respiration of iPSC-derived myeloid progenitors by the Seahorse XFe96 Analyzer. Additionally, our protocol includes extensive troubleshooting suggestions.
For complete details on the use and execution of this protocol, please refer to Fan et al. (2022).1
Subject areas: Cell Biology, Immunology, Metabolism, Stem Cells, Cell Differentiation
Graphical abstract

Highlights
-
•
Protocol to generate myeloid progenitor cells from human iPSCs
-
•
Oxygen consumption analysis of iPSC-derived myeloid progenitors using Seahorse technology
-
•
Probe mitochondrial respiration in myeloid progenitors with neutrophil immunodeficiencies
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Mitochondrial metabolism is critical in hematopoietic stem cell maintenance and differentiation. Here, we present a step-by-step protocol to efficiently differentiate human induced pluripotent stem cells into myeloid progenitors by a robust feeder- and serum-free system. Furthermore, we provide a protocol to subsequently assess mitochondrial function in iPSC-derived myeloid progenitors. We comprehensively describe a protocol to analyze and to quantify key parameters of mitochondrial respiration of iPSC-derived myeloid progenitors by the Seahorse XFe96 Analyzer. Additionally, our protocol includes extensive troubleshooting suggestions.
Before you begin
Prepare reagents for iPSC cell culture and seahorse assay
Timing: 1 day
Here we utilize the HMGU1 human iPSC line generated from human foreskin fibroblasts.2
-
1.Prepare mTeSR complete medium.
-
a.Thaw the mTeSR Plus 5× Supplement (100 mL/bottle) overnight at 4°C.
-
b.Mix mTeSR Plus Basal Medium (400 mL) with mTeSR Plus 5× Supplement (100 mL).
-
c.Prepare mTeSR Plus complete medium aliquots in sterile 50 mL conical tubes (40 mL/tube) and store aliquots at −20°C.
-
a.
-
2.Prepare StemPro-34 complete medium.
-
a.Thaw the StemPro-34 Nutrient Supplement (13 mL) overnight at 4°C.
-
b.Combine StemPro-34 Serum Free Medium (500 mL) with StemPro-34 Nutrient Supplement (13 mL) and L-Glutamine (5 mL). Mix the StemPro-34 complete medium by inversion.Alternatives: GlutaMax can be used as an alternative of L-Glutamine.
-
c.Prepare StemPro-34 complete medium aliquots in sterile 50 mL conical tubes (40 mL/tube). Store aliquots at −20°C.
-
a.
-
3.Aliquot Matrigel and cytokines.
-
a.Thaw Matrigel (10 mL/vial) overnight on ice. Thawed Matrigel should be mixed well and divided into aliquots under sterile conditions. Store aliquots at −20°C.
 CRITICAL: Matrigel should be kept constantly on ice or 4°C to avoid gelation of the Matrigel.
CRITICAL: Matrigel should be kept constantly on ice or 4°C to avoid gelation of the Matrigel. -
b.Cytokines are reconstituted according to the instructions of the manufacturer under sterile conditions.Note: Except for CHIR99021 and SB431542 dissolved in sterile DMSO, the other cytokines are reconstituted with sterile PBS containing 0.1% BSA. Detailed information about solution concentrations and preparation are listed in the materials and equipment section. Cytokine aliquots are stored at −80°C after reconstitution.
-
a.
-
4.Prepare reagents for seahorse assay.
-
a.Reconstitute reagents related to seahorse assay based on the manufacturer instructions.Note: Detailed information about solution concentrations and preparation are listed in the materials and equipment section.
-
b.Filter reconstituted solutions through a 0.22 μm filter and aliquot solutions into sterile microtubes prior to the storage at −80°C.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Advanced DMEM/F-12 | Thermo Fisher Scientific | 12634028 |
| Agilent Seahorse XF RPMI Medium pH 7.4, no Phenol Red | Agilent | 103576-100 |
| Antimycin A from Streptomyces sp. | Sigma-Aldrich | A8674-25MG |
| Bambanker | Nippon Genetics Europe | BB01 |
| basic FGF | Peprotech | 100-18B |
| BMP4 | Peprotech | 120-05 |
| Carbonylcyanid-4-(trifluormethoxy)phenylhydrazon (FCCP) | Sigma-Aldrich | C2920-10MG |
| CHIR99021 | Millipore | 361571 |
| Collagenase type IV | STEMCELL Technologies | 7909 |
| D- (+)-Glucose | Sigma-Aldrich | G8270 |
| DPBS, no calcium, no magnesium | Thermo Fisher Scientific | 14190-094 |
| Essential 6 Medium | Thermo Fisher Scientific | A1516401 |
| Fetal bovine serum (FBS) | Thermo Fisher Scientific | 10270106 |
| Flt-3 | Peprotech | 300-19 |
| G-CSF | Peprotech | 300-23 |
| Hoechst 33342 Solution (20 mM) | Thermo Fisher Scientific | 62249 |
| IL-3 | Peprotech | 200-03 |
| L-Glutamine (200 mM) | Thermo Fisher Scientific | 25030081 |
| Matrigel growth factor-reduced basement membrane matrix | Corning | 356231 |
| mTeSR Plus | STEMCELL Technologies | 100-0276 |
| Oligomycin from Streptomyces diastatochromogenes | Sigma-Aldrich | O4876-5MG |
| Poly-L-lysine hydrobromide | Sigma-Aldrich | P6282-5MG |
| ReLeSR | STEMCELL Technologies | 5872 |
| Rotenone | Sigma-Aldrich | R8875-1G |
| SB431542 | Selleckchem | S1067 |
| SCF | Peprotech | 300-07 |
| StemPro-34 SFM (1×) | Thermo Fisher Scientific | 10639011 |
| TPO | Peprotech | 300-18 |
| Trypan blue solution (0.4%) | Thermo Fisher Scientific | 15250-061 |
| VEGF | Peprotech | 100-20 |
| Y-27632 (Dihydrochloride) | STEMCELL Technologies | 72304 |
| Cell line | ||
| HMGU1 human iPSC line | Dr. Drukker from the Institute of Stem Cell Research and the Induced Pluripotent Stem Cell Core Facility (Helmholtz Center Munich) | N/A |
| Software and algorithms | ||
| Gen5 Software for Imaging and Microscopy | Agilent | https://www.agilent.com/en/product/cell-analysis/cell-imaging-microscopy/cell-imaging-microscopy-software/biotek-gen5-software-for-imaging-microscopy-1623226 |
| Prism | Graphpad | https://www.graphpad.com/scientific-software/prism/ |
| Seahorse Wave Desktop Software | Agilent | https://www.agilent.com/en/product/cell-analysis/real-time-cell-metabolic-analysis/xf-software/seahorse-wave-desktop-software-740897 |
| Other | ||
| 10 mL pipettes | Sarstedt | 861.254.001 |
| 15 mL conical tubes | Sarstedt | 62.554.100 |
| 2 mL pipettes | Sarstedt | 861.252.001 |
| 25 mL pipettes | Sarstedt | 861.685.001 |
| 5 mL pipettes | Sarstedt | 861.253.001 |
| 50 mL conical tubes | Sarstedt | 62.547.254 |
| 6-well cell culture plate | Sarstedt | 833.920.005 |
| Counting chamber | Marienfeld Superior | N/A |
| Cryotubes | Greiner Bio-One | 121263 |
| Cytation 1 Cell Imaging Multimode Reader | BioTek/Agilent | N/A |
| Heraeus Hera cell 240 (cell culture incubator) | Thermo Fisher Scientific | 51032875 |
| Heraeus Multifuge X3R | Thermo Fisher Scientific | 50117498 |
| LSR Fortessa Cell Analyzer | BD Biosciences | N/A |
| Non-CO2, 37°C incubator | Benchmark | N/A |
| Seahorse XFe96 Analyzer | Agilent | N/A |
| Seahorse XFe96 FluxPaks | Agilent | 102601-100 |
| Sterile biosafety cabinets | Thermo Fisher Scientific | EN12469 |
| Syringe Filter Unit, 0.22 μm | Millipore | SLGP033RS |
| Water baths | GFL | N/A |
| Zeiss Inverted Phase Contrast Microscope | Zeiss | Z-AXIO40C |
Note: Sources of common cell-culture consumables and equipment mentioned in the table are optional.
Materials and equipment
mTeSR differentiation medium (day 0–1)
| Reagent | Final concentration | Volume (for 1 well of a 6-well plate) |
|---|---|---|
| mTeSR Plus complete medium | N/A | 2 mL |
| BMP4 80 μg/mL | 80 ng/mL | 2 μL |
| CHIR99021 6 mM | 3 μM | 1 μL |
| VEGF 80 μg/mL | 80 ng/mL | 2 μL |
| Total | N/A | 2 mL |
mTeSR Plus complete medium can be stored at 2°C–8°C for up to 2 weeks. Aliquots can be stored at −20°C for up to 6 months. The aliquoted mTeSR complete medium can be stored at 2°C–8°C for up to 2 weeks after thawing. Do not re-freeze. Upon cytokine reconstitution, mTeSR differentiation medium has to be used immediately.
Note: Add freshly thawed cytokine aliquots to the respective differentiation medium. All volumes described here are for one well of a 6-well plate. For other types of plates, volumes need to be adjusted accordingly.
Essential 6 differentiation medium (day 2–3)
| Reagent | Final concentration | Volume (for 1 well of a 6-well plate) |
|---|---|---|
| Essential 6 medium | N/A | 2 mL |
| VEGF 80 μg/mL | 80 ng/mL | 2 μL |
| Basic FGF 100 μg/mL | 25 ng/mL | 0.5 μL |
| SCF 100 μg/mL | 50 ng/mL | 1 μL |
| SB431542 20 mM | 2 μM | 0.2 μL |
| Total | N/A | 2 mL |
Essential 6 medium can be stored at 2°C–8°C for up to 6 months. Upon cytokine reconstitution, Essential 6 differentiation medium has to be used immediately.
StemPro-34 differentiation medium (day 4–5)
| Reagent | Final concentration | Volume (for 1 well of a 6-well plate) |
|---|---|---|
| StemPro-34 complete medium | N/A | 2 mL |
| VEGF 80 μg/mL | 80 ng/mL | 2 μL |
| Basic FGF 100 μg/mL | 25 ng/mL | 0.5 μL |
| SCF 100 μg/mL | 50 ng/mL | 1 μL |
| SB431542 20 mM | 2 μM | 0.2 μL |
| Total | N/A | 2 mL |
StemPro-34 complete medium can be stored at 2°C–8°C for up to one month. Aliquots can be stored at −20°C for up to 6 months. The aliquoted StemPro-34 complete medium can be stored at 2°C–8°C for up to one month after thawing. Do not re-freeze. Upon cytokine reconstitution, StemPro-34 differentiation medium has to be used immediately.
StemPro-34 differentiation medium (day 6–7)
| Reagent | Final concentration | Volume (for 1 well of a 6-well plate) |
|---|---|---|
| StemPro-34 complete medium | N/A | 2 mL |
| SCF 100 μg/mL | 50 ng/mL | 1 μL |
| IL-3 100 μg/mL | 50 ng/mL | 1 μL |
| Flt-3 100 μg/mL | 50 ng/mL | 1 μL |
| TPO 50 μg/mL | 5 ng/mL | 0.2 μL |
| VEGF 80 μg/mL | 50 ng/mL | 1.25 μL |
| Total | N/A | 2 mL |
StemPro-34 differentiation medium (day 6–7) is freshly prepared before usage.
StemPro-34 differentiation medium (day 8–17)
| Reagent | Final concentration | Volume (for 1 well of a 6-well plate) |
|---|---|---|
| StemPro-34 complete medium | N/A | 1 mL |
| SCF 100 μg/mL | 50 ng/mL | 0.5 μL |
| IL-3 100 μg/mL | 50 ng/mL | 0.5 μL |
| Flt-3 100 μg/mL | 50 ng/mL | 0.5 μL |
| TPO 50 μg/mL | 5 ng/mL | 0.1 μL |
| Total | N/A | 1 mL |
StemPro-34 differentiation medium (day 8–17) is freshly prepared before usage.
Note: From day 8 to day 17 of myeloid differentiation, half of the medium is removed and replaced with fresh Stempro-34 differentiation medium every 3–4 days.
Reconstituted seahorse RPMI medium
| Reagent | Final concentration | Volume |
|---|---|---|
| Seahorse RPMI medium | N/A | 30 mL |
| Glucose 2.5 M | 5 mM | 60 μL |
| L-Glutamine 200 mM | 2 mM | 0.3 mL |
| Total | N/A | 30 mL |
Seahorse RPMI medium can be stored at 2°C–8°C for up to 6 months. 30 mL of Seahorse RPMI medium are incubated in a 37°C water bath for 1 h prior to the reconstitution of glucose and L-Glutamine. Then the reconstituted seahorse RPMI medium is immediately used for the preparation of injection A-C solutions. The solution is freshly prepared before usage.
Seahorse injection C solution
| Reagent | Final solution concentration | Volume |
|---|---|---|
| RPMI reconstituted medium | N/A | 2.5 mL |
| Rotenone 2.5 mM | 5 μM | 5 μL |
| Antimycin A 10 mM | 5 μM | 1.25 μL |
| Hoechst 20 mM | 80 μM | 10 μL |
| Total | N/A | 2.5 mL |
Seahorse injection C solution is freshly prepared before usage. Solve 1 mg Rotenone with 2.55 mL DMSO to get 100 mM Rotenone solution, which is further diluted into 2.5 mM with DMSO. Rotenone solution (2.5 mM) is aliquoted into 10 μL/tube and stored at −80°C for up to 12 months. 10 mM Antimycin is prepared by solving 25 mg Antimycin powder with 4.7 mL DMSO. Antimycin solution (10 mM) is aliquoted into 5 μL/tube and stored at −80°C for up to 12 months. 20 mM Hoechst solution can be stored at 2°C–8°C for up to 6 months.
-
•
Seahorse injection A solution: solve 5 mg Oligomycin into 2.53 mL DMSO (2.5 mM stock solution), which is further aliquoted into 20 μL/tube and stored at −80°C for up to 12 months Seahorse injection A solution is freshly prepared by adding 15 μL Oligomycin stock solution into 2.5 mL RPMI reconstituted medium before usage.
-
•
Seahorse injection B solution: solve 10 mg FCCP with 1.57 mL DMSO to obtain 25 mM FCCP solution and further dilute 25 mM FCCP into 2.5 mM FCCP solution with DMSO. FCCP solution (2.5 mM) is aliquoted into 15 μL/tube and stored at −80°C for up to 6 months. Seahorse injection B solution is freshly prepared by adding 10 μL FCCP stock solution (2.5 mM) into 2.5 mL RPMI reconstituted medium before usage.
Note: Do not re-freeze reagent aliquots stored at −80°C.
Alternatives: CCCP and Piericidin A can be potential alternatives of FCCP and rotenone respectively. However, users need to test the optimal drug concentrations prior to the assay.
Note: Hoechst is used for visualization and counting of cell nuclei. When methods other than cell imaging are adopted for cell normalization, Hoechst is optimal for the recipe.
CRITICAL: Powder of oligomycin, rotenone, antimycin A (inhibitors of mitochondrial complexes) and FCCP (uncoupler reagent of mitochondrial transfer chain) are harmful to the respiratory tract and can cause skin and eye irritation. To avoid the exposure to these hazardous chemicals, please handle them under a fume hood and wear suitable protective equipment and clothing.
Step-by-step method details
Part 1. The feeder-free maintenance of iPSCs
Timing: up to 2 weeks
This section describes the procedure for thawing, passaging and freezing human iPSCs (hiPSCs). We cultivate hiPSCs using mTeSR plus complete medium on growth factor-reduced Matrigel-coated 6-well plates. Routinely, we passage the cells every 3–4 days using ReLeSR.
Note: All cell culture procedures are performed under a sterile hood. We utilize WT iPSC clones and HAX1-KO iPSC clones generated by CRISPR-Cas9-mediated genome editing.1 Because of clonal variations, we suggest at least two clones for each genotype to be included in the assay.
-
1.Cell thawing.
-
a.Experimental setup.
-
i.Thaw one aliquot of mTeSR plus complete medium (40 mL) at 4°C overnight and prewarm it at room temperature (RT) for at least 30 min before usage.
-
ii.Dilute 100× Matrigel stock into ice-cold DMEM/F12 medium and quickly dispense the Matrigel dilution into 6-well plates (1 mL/well).
-
iii.Incubation 6-well plates with Matrigel solution in a 5% CO2 tissue culture incubator for at least 30 min at 37°C.Note: Plates can also be coated with Matrigel overnight in 37°C incubator before usage. Additional coated plates can be sealed with parafilm prior to the storage at 4°C for up to 2 weeks.
-
iv.Fill each 15 mL conical tube with 10 mL plain Advanced DMEM/F12 medium and warm conical tubes with medium in water bath at 37°C for at least 15 min till usage.
-
v.Transfer a frozen vial with 1 million cells from liquid nitrogen on dry ice.
-
vi.Wash the Matrigel coated 6-well plate with PBS and add mTeSR plus complete medium supplemented with rho-associated protein kinase inhibitor referred as ROCK inhibitor (Y-27632) at a final concentration of 10 μM into each well (1.5 mL/well).
-
i.
-
b.Transfer a vial of cryopreserved hiPSCs from dry ice into a 37°C water bath and keep cells into the water bath for 2 min until only a sliver of ice remains.
-
c.Use a 2 mL pipette to remove the contents from the vial and transfer it into a conical tube containing pre-warmed Advanced DMEM/F12 medium (10 mL/tube).
-
d.Harvest hiPSCs by centrifugation at 200 × g for 3 min at RT.
-
e.Aspirate supernatant and gently resuspend cell pellets with 500 μL pre-warmed mTeSR plus complete medium +ROCK inhibitor by pipetting up and down for 5 times.
-
f.Distribute cell suspension onto the prewarmed 6-well plate equally in a drop-wise manner. Carefully, shake plate to further distribute cells evenly.Note: Gently resuspend the cell suspension to preserve cell clonal size and structure. Y-27632 acts as a cell-permeable, highly potent and selective inhibitor of ROCK1 and ROCK2 limiting cell death.
-
g.Examine shape and distribution of newly thawed hiPSCs under the microscope and return the 6-well plate back to incubator.
-
h.Replace medium with mTeSR plus complete medium (2 mL/well) the next day.
-
i.Replace medium every second day until single colonies are visible and wells become 70%–80% confluent.
-
a.
-
2.Cell passaging.
-
a.Grow hiPSCs in mTeSR plus complete medium to 70%–80% confluency in a humidified incubator at 37°C and 5% CO2.
-
b.Wash hiPSCs twice with PBS by removing mTeSR plus complete medium and replacing it with 1 mL of PBS per well of a 6-well plate.
-
c.Aspirate PBS, add 0.4 mL of ReLeSR per well and incubate for 3–5 min at 37°C.Note: Closely monitor cells under the tissue culture microscope until the cells start to dissociate.Alternatives: Collagenase IV can be a milder substitute of ReLeSR with longer detachment duration (around 30 min–60 min).
-
d.Carefully aspirate the ReLeSR solution, add 1 mL of mTeSR plus complete medium and gently resuspend cells using a 2 mL pipette 2–3 times.
-
e.Transfer cell suspension into a conical tube and harvest cells by centrifugation at 200 × g for 3 min at RT.
-
f.Aspirate supernatant and resuspend cell pellet with 1 mL mTeSR plus complete medium.
-
g.Transfer cell suspension at different ratios (e.g., 1:15 to 1:20) into wells of the Matrigel-coated 6-well plate to a final volume of 1.5 mL.Note: Ideally, cells should reach 70%–80% confluence in 3–4 days. Adjust the split ratio to 1:15 to 1:20 to achieve this result, as higher split ratios are associated with more uniform distribution of cells across the well and result in more efficient differentiations into myeloid progenitors.Note: Ratios from 1:15 to 1:20 are typically for cell passaging of WT clones, while ratios for KO clones should be optimized based on the cell growth rate of KO cells. For our KO clones, we split cells in the ratio of 1:8 to 1:10 so as to synchronize cell growths between WT and KO before the start of myeloid differentiation.
-
a.
-
3.Cell freezing.
-
a.Follow the procedure 2a-2e.
-
b.Aspirate supernatant and use 1 mL of freezing solution (Bambanker) to resuspend the cell pellet of one well of a 6-well plate.
-
c.Transfer each cell solution to a cryovial and place the cryovial(s) in a cell-freezing container.
-
d.Place the cell-freezing container in a −80°C freezer as fast as possible and the next day store the cryovial(s) in liquid nitrogen.
-
a.
Note: Make a clear record of cell passage number, which is of importance for iPSC differentiation.
Part 2. iPSC differentiation into myeloid progenitors
Timing: 21 days
We here describe the protocol to generate myeloid progenitor cells from human iPSCs (hiPSCs) by stepwise tuning of cytokines and small chemicals in vitro.
CRITICAL: Proper maintenance of hiPSCs is critical to efficient neutrophil granulocyte differentiation. Seeding density and colony size must be closely monitored and spontaneous background differentiation must be kept as low as possible. All cell culture steps should be performed in a Class II biosafety cabinet. All solutions should be kept sterile and proper sterile technique should be used throughout.
Note: We describe a refined protocol from this previous protocol.3
-
4.Seeding iPSC colonies for the myeloid progenitor differentiation.
-
a.Wash each well of a 6-well plate at a cell confluency of 60%–80% with PBS.
-
b.Aspirate PBS and add 1 mL Collagenase IV (1 mg/mL) to one well of a 6-well plate for generation of iPSC cell clumps.
-
c.Return the 6-well plate into the cell incubator and closely monitor hiPSCs under the tissue culture microscope between the 45 min incubation time.Note: The incubation times may vary among different batches of Collagenase IV; therefore, examination of the colonies is needed to determine the appropriate incubation time.Note: Passage numbers lower than 40 are optimal and robust for the iPSC differentiation.
-
d.Stop the incubation when the edges of the colonies have pulled away from the plate by adding 1 mL of mTeSR plus complete medium.
-
e.Carefully collect iPSC colonies with a 2 mL pipette and transfer cell suspension into a 15 mL conical tube.
-
f.Harvest iPSC colonies by centrifugation at 10 × g for 1 min.
-
g.Discard supernatant and carefully resuspend iPSC colonies with 1 mL mTeSR plus complete medium using a 2 mL pipette.Note: Using a 2 mL pipette and limited pipetting times can minimize colony fragmentation.
-
h.Drop 10 μL cell suspension on a cell culture dish and carefully examine the size and count the number of iPSC colonies (clumps) within the 10 μL suspension under the microscopy.
 CRITICAL: Take the colony clumps whose diameters are between 0.1 mm and 0.2 mm into account, as shown in Figure 1.
CRITICAL: Take the colony clumps whose diameters are between 0.1 mm and 0.2 mm into account, as shown in Figure 1. -
i.Seed 6–10 iPSC colonies into a new Matrigel-coated well of a 6-well plate with 1.5 mL prewarmed mTeSR plus complete medium.Note: Because of the frequent random differentiation in KO clones, 10–15 KO colonies are optimal for seeding.
-
j.Replace mTeSR plus complete medium every 2 days until the area of colonies reaches 0.5 × 0.5 mm2 to 1.0 × 1.0 mm2.Note: Typically, 3–4 days after seeding, iPSC colonies have reached the appropriate size and round shape critical for differentiation start. During this time, 6 homogenous iPSC colonies characterized by an appropriate size and round morphology/shape are selected by microscopic examination and labeled with a pen at the bottom of the 6-well plate. Additional iPSC colonies will be carefully removed with a pipet tip. Wash shortly with PBS and add mTeSR plus complete medium. Return 6-well plate to the incubator.
-
a.
-
5.
On day 0, replace medium with mTeSR differentiation medium (as indicated in the materials and equipment section) to start myeloid differentiation (2 mL/well).
-
6.
On day 2, replace medium with prewarmed Essential 6 differentiation medium (2 mL/well).
-
7.
On day 4, replace medium with prewarmed StemPro-34 differentiation medium supplemented with SB431542, SCF, bFGF and VEGF (2 mL/well).
-
8.
On day 6, replace StemPro-34 differentiation medium supplemented with SCF, IL-3, Flt-3, VEGF and TPO (2 mL/well).
-
9.
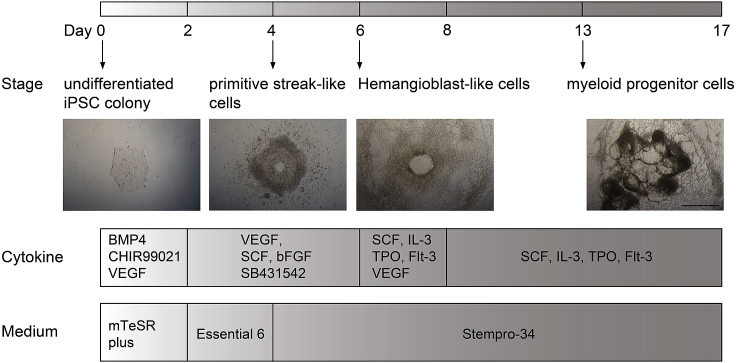
On day 8 to day 17, replace half of the medium with Stempro-34 differentiation medium supplemented with SCF, IL-3, Flt-3 and TPO every 3–4 days (1 mL/well). The scheme of iPSC differentiation into myeloid progenitors is shown in Figure 3.
-
10.
For cell counting at day 17, mix 10 μL of cell suspension and 40 μL of 0.1% Trypan blue solution (5×) and pipette 10 μL of the mixture onto a counting chamber slide. Count viable cells under the light microscope.
Note: Mix the cell suspension thoroughly before taking the counting aliquot to achieve accurate and consistent cell number measurements.
-
11.
Harvest floating cells on day 17 for functional assays such as Seahorse of myeloid progenitors. Details are shown in Part 3 session.
Figure 1.
Cell suspension with iPSC colonies (clumps) before iPSC seeding for myeloid differentiation
(A) 10 μL cell suspension was dropped on a cell culture dish and observed under the light microscope. Scale bar: 1.0 mm.
(B) Cell colonies/clumps at optimal size (black circles) were taken into account for clump seeding. Scale bar: 1.0 mm.
Figure 2.
Representative images showing colonies with optimal sizes before the start of iPSC differentiation
(A and B) iPSC colonies were grown till the optimal sizes reaching 60%–80% confluences of the vision observed by light microscope with 20× magnification. Scale bar: 200 μm.
Figure 3.
Scheme of hiPSC-differentiation into myeloid progenitor cells
Scale bar is 500 μm.
Part 3. Seahorse assay of myeloid progenitors derived from iPSCs
Timing: 2 days
Here we describe the mitochondrial stress protocol optimized for the full-plate seeding of a single Seahorse XFe96 set. For other Seahorse XF Analyzers (8-well, 24-well) provided by Agilent, the total buffer volumes and total cell numbers require proportionately adjusted. Details can be referred to other seahorse protocols.4,5
Note: General manufacturer’s instructions for performing a mitochondrial stress test can be found at https://www.agilent.com/cs/library/usermanuals/public/XF_Cell_Mito_Stress_Test_Kit_User_Guide.pdf.
Note: There are two sets of plates included in the seahorse assay. The set of cell culture microplate is used for seeding cells of interest. The set of utility plate is used for incubating the sensor cartridge with water or calibrant buffer (included in Seahorse FluxPaks). On the top of the sensor cartridge, there are four cavities/ports for drug loading.
-
12.One day before performing the seahorse assay (on day 16 of iPSC differentiation), prepare the following experimental setup in advance.
-
a.Coat seahorse cell culture microplate with 0.05 mg/mL poly-L-lysine (18 μL/well) for 1 h at RT.
-
b.Wash plate with dH2O twice (200 μL/well) and dry cell culture microplate under the hood overnight.Note: Make sure there are no bubbles when the cell culture microplate is coated with poly-L-lysine. Alternatively, poly-D-lysine can be used for the plate coating of seahorse assay.
-
c.Incubate the sensor cartridge with dH2O (200 μL/well) in the utility plate and place the cartridge/utility plate in a non-CO2, 37°C incubator for 12–18 h.
-
d.Incubate 20 mL calibrant solution in a non-CO2, 37°C incubator for 12–18 h.
-
e.Transfer aliquoted chemicals and reagents (2.5 M D-Glucose, 200 mM L-Glutamine, 2.5 mM Oligomycin, 2.5 mM FCCP, 2.5 mM Rotenone and 10 mM Antimycin A) from −80°C to 4°C.
-
f.Turn on the seahorse machine one day before the seahorse assay.
-
a.
-
13.
Incubate 30 mL seahorse XF RPMI medium in a 37°C water bath for 1 h prior to step 15.
-
14.
Discard dH2O and subsequently incubate sensor cartridge with prewarmed calibrant solution in the utility plate and incubate the cartridge/utility plate in a non-CO2, 37°C incubator for at least 30 min.
-
15.
Reconstitute prewarmed seahorse XF RPMI medium (30 mL) with Glucose (fc. 5 mM) and L-Glutamine (fc. 2 mM) and directly take 2.5 mL reconstituted RPMI medium for chemical dilution of port A, B and C respectively.
-
16.
Further reconstituted the rest 22.5 mL RPMI medium with FBS (1%) and incubate RPMI medium with FBS in a non-CO2, 37°C incubator until usage.
-
17.Injection cavities preparation using reconstituted RPMI medium prepared at step 15.
-
a.Buffer A: Add 15 μL Oligomycin (2.5 mM) into 2.5 mL reconstituted RPMI medium.
-
b.Buffer B: Add 10 μL FCCP (2.5 mM) into 2.5 mL reconstituted RPMI medium.
-
c.Buffer C: Add 5 μL Rotenone (2.5 mM), 1.25 μL Antimycin A (10 mM) and 10 μL Hoechst (20 mM) into 2.5 mL reconstituted RPMI medium.
-
a.
Note: Alive cells are stained with Hoechst after the last injection of rotenone and antimycin and quantified by multi-imager Cytation 1 (BioTeK Instruments/Agilent). Details are shown in session “Quantification and statistical analysis”.
-
18.
Harvest floating cells from each well of iPSC culture by centrifugation at 200 × g for 3 min at RT.
-
19.
Resuspend cell pellets with reconstituted RPMI medium with FBS (1%) prepared at step 16.
-
20.
Count cell number in each sample and prepare cell suspension at 100,000 cells per 50 μL.
CRITICAL: To obtain a 90%–100% confluence of cell monolayer in the well of a seahorse cell culture microplate, it is critical to titrate the number of cells based on the cell size of each cell line before the assay. 90,000–100,000 cells are optimal for the cell seeding of iPSC-derived myeloid progenitors in the seahorse assay. For the initial setup assay of other cell lines, recommended cell seeding density per well can be found at “Agilent Life Science Publication Database” (http://www.agilent.com/cell-reference-database). 0.5-fold, 1-fold and 2-fold of recommended cell numbers per well can be seeded as references respectively, in order to define the optimal cell seeding density.
Note: Addition of FBS (1%) is necessary to ensure the cell viability of suspension cells derived from iPSCs.
-
21.
Seed 50 μL well-mixed cell suspension into each well of seahorse cell culture microplate precoated with poly-L-lysine.
-
22.
Centrifuge the cell culture microplate at 300 × g for 1 min with 7/9 acceleration and 2/9 deceleration.
Note: Include at least 4 wells as technical replicates for the seahorse assay of each sample. 4 wells at corners of the plate are normally served as control wells, where 50 μL RPMI medium with 1% FBS instead of cell suspension is loaded.
-
23.
After the centrifugation, take out the microplate and carefully add 130 μL RPMI medium with 1% FBS into each well using a multichannel pipette.
-
24.
Perform the brightfield imaging of all wells of the microplate in Cytation1 machine to get an overview of the cell distribution in each well before performing the seahorse assay.
Alternatives: The brightfield imaging of all wells of the microplate by Cytation 1 machine is optional. Alternatively, the cell seeding per well can be visually monitored and confirmed by light microscopy.
-
25.
Degas the microplate after imaging for 30 min in Cytation1 machine (37°C, without CO2).
CRITICAL: The non-CO2 incubation is important and necessary for the further cell seeding and attachment.
-
26.
After calibrating the sensor cartridge at step 14, add 20 μL buffer A, 22 μL buffer B and 24.4 μL buffer C into port A, B and C of utility/cartridge plate using a multichannel pipette.
Note: Since the experimental design requires no injection for port D, each port D is left empty.
Note: Tips should enter into the port and attach the wall before loading solutions.6 Because the bottom of each port is not sealed, thus avoid touching the very bottom of the port during loading. To avoid drug leaking in ports resulting from air pressure, do not add the plate lid to the utility/cartridge plate after drug loading. More details about loading the injection ports can be found at https://www.agilent.com/cs/library/usermanuals/public/DAY OF LOADING CARTRIDGE XFe96-XF96.pdf.
Note: The final working concentrations of solution A-C in each well after being injected into cell microplate are 1.5 μM (Oligomycin), 1 μM (FCCP) and 0.5 μM (Antimycin/Rotenone) respectively. For most human cell lines, the concentration ranges of solution A (Oligomycin) and solution C (Antimycin and Rotenone) are normally 0.25–2 μM and 0.5–2 μM respectively. The concentration range of solution B (FCCP) may vary from 1 to 10 μM. Optimal solution concentrations, especially for solution B (FCCP), need be titrated for individual cell type prior to the assay.
More details can be found in the Basic Procedures on Agilent Cell Analysis Learning Center and the Cell Line Reference Database at https://www.agilent.com/en/product/cell-analysis/how-to-run-an-assay; http://www.agilent.com/cell-reference-database.
-
27.Calibrate the utility/cartridge plate after drug loading in the seahorse machine (preheated at 37°C).
-
a.Choose the program “XF Cell Mito Stress Test” on the Templates window and edit the group information of samples in the option “Plate Map”.
-
b.Set parameters of seahorse assay in the option “Protocols” as shown in Figure 4.
-
c.Click “Start Run” at the Run Assay page and save the assay result file in a folder.
-
d.Set the utility/cartridge plate without the lid into the seahorse machine.
-
e.The calibration process takes around 20 min prior to an automatic degassing procedure.
-
a.
-
28.
Set sensor cartridge calibrated at step 27 onto the cell culture microplate degassed at step 25 and load the complete plate into the seahorse machine and start the assay.
Note: To avoid the damage of machine, the plate lid must be taken away before the whole plate entering the seahorse analyzer.
-
29.
After finishing the seahorse assay, perform fluorescent imaging of all wells of the cell culture microplate by Cytation 1 machine for alive cell quantification.
Note: The cell counts quantified by Cytation 1 machine are used for normalization of seahorse assay. Details are shown in Quantification and statistical analysis.
Figure 4.
Protocol of Seahorse assay on the Cell Mito Stress Test
Expected outcomes
The described protocol allows the generation of myeloid progenitor cells from induced pluripotent stem cells. Approximately 80% of suspension cells derived from WT iPSCs at day 17 are promyelocytes/myelocytes (Figure 5).
Figure 5.
Analysis of myeloid progenitors derived from WT iPSCs
(A) Quantification of the distribution of suspension cells derived from WT iPSCs at differentiation day 17. Seg: segmented neutrophils, Band: band neutrophils, Unclassified: macrophage, monocyte or dendritic cell lineages.
(B) Light microscopy of WT iPSC-derived suspension cells at day 17 stained with May-Gruenwald Giemsa. Scale bar: 10 μm.
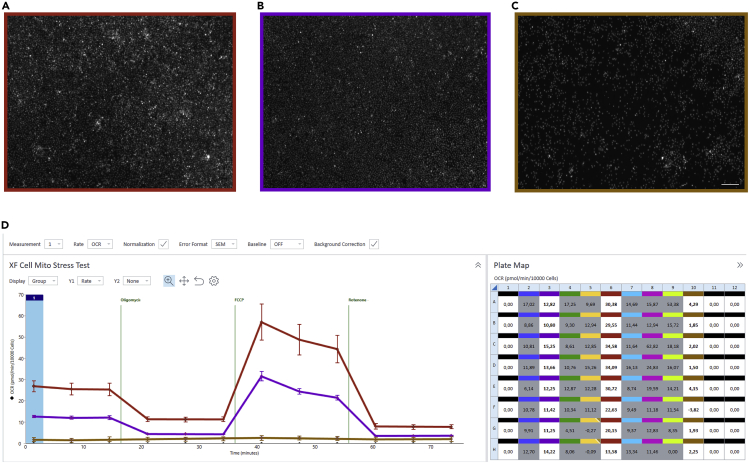
Seahorse Cell Mito Stress test is applied to determine the mitochondrial respiration with alive cells in real-time manner. As shown in Figure 6A, the basal respiration of myeloid progenitors is first measured before the inhibition of ATP synthase by oligomycin (1.5 μM). The difference of OCR before and after oligomycin injection demonstrates the amount of O2 consumed for mitochondrial ATP production.7 Oligomycin blocks the proton translocation back to the matrix and thereby generates an unexploited gradient, which further leads to the prevention of electron transport through electron transfer chain (ETC) (complex I-IV). FCCP, as a protonophore, facilitates the translocation of protons back into the matrix independent of the ATP synthase. As a result, ETC is re-activated and pumps protons to the intermembrane space (IMS) so as to maintain the electrochemical proton gradient upon FCCP injection (1 μM). Thus, the maximum mitochondrial respiration is indicated after FCCP injection. The ETC is fully blocked after the subsequent inhibition of complex I by rotenone (0.5 μM) and complex III by antimycin (0.5 μM). The rest OCR is indicated as non-mitochondrial oxygen consumption. The difference between maximal respiration and basal respiration represents the spare respiration capacity, indicating the mitochondrial capacity of reaching its bioenergetic limit.8
Figure 6.
Oxygen consumption rate measurement with myeloid progenitors derived from WT and HAX1-KO iPSCs
(A) Scheme of seahorse mitochondrial stress test. Measurement of oxygen consumption rate (OCR) in myeloid progenitor cells derived from WT iPSCs following a sequential addition of Oligomycin, FCCP, and Rotenone (Rot) and Antimycin A (AA).
(B) Measurement of oxygen consumption rate (OCR) in myeloid progenitor cells derived from WT and HAX1-KO iPSCs following a sequential addition of Oligomycin, FCCP, and Rotenone (Rot) and Antimycin A (AA).
(C) Analysis of basal respiration, maximal respiratory, ATP production and spare respiratory capacity of myeloid progenitors derived from WT and HAX1-KO iPSCs (n = 8, ∗∗∗∗p < 0.0001, Sidak’s multiple comparisons test). In detail, Basal respiration = (Last rate measurement before first injection) – (Non-mitochondrial respiration rate), Maximal respiratory capacity = (Maximum rate measurement after FCCP injection) – (Non-mitochondrial respiration rate), ATP production = (Last rate measurement before Oligomycin injection) – (Minimum rate measurement after Oligomycin injection), Spare respiratory capacity = (Maximal respiration) – (Basal respiration).
We analyze the mitochondrial respiration/oxygen consumption rate of myeloid progenitors derived from either WT or HAX1-KO iPSCs (Figure 6B). HAX1 is a mitochondrial protein, whose deficiency results in a perturbed mitochondrial proteostasis in the PLB-985 myeloid cell line.1 Compared to WT myeloid progenitors, maxi respiration and spare respiratory capacity are significantly reduced in the absence of HAX1 (Figure 6C). Data are analyzed with Wave 2.6.3 software and exported as GraphPad Prism or Seahorse XF Cell Mito Stress Test Report Generator.
Quantification and statistical analysis
To get an overview of the cell distribution in each well of the cell culture microplate before the seahorse assay, all wells are imaged in Brightfield mode by the multi-imager Cytation 1 (BioTeK Instruments/Agilent). Hoechst-positive cells (alive cells) are quantified by Cytation 1 directly after the seahorse assay.
Hoechst-positive cells detected by Cytation1 and shown in the Gen5 software9,10 can be directly imported into the corresponding seahorse experiment recorded by Wave software (Figure 7A). Subsequently, in the Edit Normalization Mode of the seahorse assay shown in the Wave software, the normalization unit can be set into 10,000 cells and the normalization values are automatedly calculated (Figure 7B), while the control wells (without cells) show as blanks. As a result, the unit of oxidative consumption rate (OCR) for each sample is normalized into pmol/min/10,000 cells (Figure 7C).
Figure 7.
Cell normalization according to cell counts detected by Cytation 1 imager in the Wave software
(A) All wells of the cell culture microplate were chosen and imported into the corresponding seahorse assay file in wave software by clicking “Import Selected” (red mark). Once the import is finished, it will demonstrate “Image imported and embedded in assay file” (red mark).
(B) In the toolbar of the Wave software, chose “Normalize” and set the normalization unit into 10,000 cells in the “Edit Normalization Mode” then click the “Apply” button.
(C) An example of the seahorse Cell Mito Stress test after cell normalization by Cytation 1 imager. Of note, the unit of the OCR on the Y axis was normalized into “pmol/min/10000cells” (red mark).
In addition to cell number, protein content and DNA content can also be adopted for cell normalization of seahorse assay. To determine the protein content of cells in each well after seahorse assay, medium is gently removed and cells are mixed/lysed with cell lysis buffer (i.e., RIPA buffer). The total protein content/well is measured by standard protein measurement protocols and total protein measurement per well can be updated in the wave software (Edit Normalization Mode). As a result, the final unit of OCR for each sample is normalized into pmol/min/μg.5 In case that cell number and protein content can hardly be measured due to cell culture limitation or cell 3D structure (i.e., organoids), genomic DNA can be isolated from samples and gDNA content per well can thus be utilized for OCR normalization.11
Limitations
Here we describe a refined protocol allowing us to generate myeloid progenitors from induced human pluripotent stem cells (hiPSCs) in a serum and feeder-free system. The protocol enables us to achieve a reproducible and robust generation of iPSC-derived myeloid progenitors (7–9 × 105 cells /mL for WT clones) in vitro. We have applied the described protocol with studies of different human iPSC cell lines (human fibroblasts-derived, CD34+ blood cells-derived iPSCs) and iPSC clones with various myeloid deficiencies.1,12,13 Even though, iPSC-derived neutrophil-progenitors and differentiated neutrophil granulocytes resemble phenotypically and functionally primary human cells, certain differences remain (e.g., morphology of nucleus, expression level of proteins). To obtain consistent differentiation efficiencies among individual clones and different batches of experiments, careful bench practice is mandatory. Besides myeloid progenitors, hiPSC can be differentiated into other blood lineages with specific combinations of cytokines.14,15,16,17,18,19
We further make use of the seahorse assay to measure the mitochondrial respiration of suspension myeloid progenitors derived from iPSCs. The seahorse assay is very sensitive with a working chamber volume less than 3 μL in each XFe96 well. Therefore, accurate cell counting and cell operations are critical. The appropriate cell number that covers the bottom of seahorse 96-well plate with 90%–100% confluency in a monolayer configuration is important for precise measurement of the mitochondrial oxidative consumption rate by the Seahorse Xfe96 Analyzer. The working concentrations of substrates (glucose, glutamine and pyruvate) and drugs (oligomycin, FCCP, rotenone and antimycin) might differ for various cell lines. Titrations of substrate/drug concentrations for a specific cell line are necessary for obtaining a reliable and robust readout. For cell lines deficient in mitochondrial ATP production, their susceptibilities to cell death after ETC inhibition should be taken into consideration (e.g., by titrating drug concentrations).
Troubleshooting
Problem 1
Low yield of hematopoietic/myeloid progenitor cells derived from iPSCs (step 11).
Potential solution
Make sure that the status of iPSCs before the start of iPSC differentiation remains undifferentiated as shown in Figure 3 (step 4). This requires a proper maintenance of iPSCs with a low passage number (less than 40). In order to get rid of randomly differentiated iPSCs, shorten the digestion time of ReLeSR or Collengenase IV during cell passaging can obtain a high percentage of undifferentiated cells (step 2). In addition, the optimal size of colony before the start of iPSC differentiation is between 0.5 × 0.5 mm2 to 1.0 × 1.0 mm2 as shown in Figure 2 (step 4).
Problem 2
Variations of iPSC differentiation efficiencies between clones of each genotype (step 4).
Potential solution
We do observe variable myeloid differentiation rates among clones with the same genotype. Therefore, at least two-three clones for each genotype should be picked up after single cell sorting/gene editing and included in each assay. Some gene-deficient clones grow slower than WT clones during the cell maintenance. To synchronize the start timepoint of myeloid differentiation for WT and gene-deficient clones, the ratio of cell splitting should be adjusted according to cell growths of different clones respectively (step 2).
Problem 3
Cell distribution is not equal in wells of the cell culture microplate of Seahorse assay (steps 19–22).
Potential solution
To obtain a 90%–100% confluence of cell monolayer in each well of a cell culture microplate, it is critical to titrate the number of cells seeded in each well based on the cell size of each cell line before the assay (step 20). Mix samples well and prepare technical duplicates for cell counting (step 20).
Problem 4
Cells do not robustly response to drug injections in the seahorse assay (step 26).
Potential solution
Make sure the cell status prior to assay is optimal. The basal respiration is normally around 10–20 pmol/min/10000cells. When each well is covered with 90%–100% confluence of cell monolayer but basal respiration is still below 5 pmol/min/10000cells, this poor cell status could due to an over-incubation of cell microplate in the non-CO2 incubator. Overall non-CO2 incubation should not exceed 60 min after cell seeding (step 25). In addition, concentrations of drugs need be titrated to find their optimal working concentrations for individual cell line before the assay (step 26). Make sure storage conditions of drugs are appropriate and drugs are functional within their shelf lives (materials and equipment).
Problem 5
High variability in the OCR measurement within technical replicates and between different batches of experiments (steps 20–29).
Potential solution
Make sure Poly-L-Lysine is evenly coated on the culture surface of each well (step 12). When cartridges are incubated in either dH2O or calibrant buffer, make sure there are no bubbles between sensor cartridge and the cell culture microplate (steps 12, 14 and 28). During experimental operations, the sensor cartridge should not touch any other surfaces (steps 12, 14 and 28). To avoid drug leaking in ports resulting from the air pressure by adding the plate lid on top, set the cartridge plate into the seahorse machine for calibration after filling the injection ports without the plate lid (steps 27 and 28). Once the proper cell number for cell seeding has been set up, cell counting should be strictly well-preformed among cell clones (step 20). Lower or higher cell density will lead to not only high variability among seahorse assays but also inaccurate experimental interpretation (Figure 8). From an example of optimal cell density shown in Figure 8B, cells marked in purple are uniformly and evenly seeded as a monolayer configuration. Instead, a higher cell density leads to cell cluster formation (Figure 8A) and inaccurate measurement of OCR (Figure 8D, dark red). A lower cell density with less than 50% confluence of the well center results in a failed OCR measurement (Figures 8C and 8D, dark yellow).
Figure 8.
Cell intensity plays a key role in the OCR measurement of Seahorse assay
(A–C) Cell intensity is too high so that cells form clusters (A) or too low with less than 50% confluency in the well center (C). Optimal cell intensity is shown as a nearly 100% confluent monolayer (B). Cells were stained with Hoechst after the third injection of OCR measurement and directly detected by Cytation1 imager. Scale bar: 200 μm.
(D) OCR measurement of groups with high cell intensity (dark red), optimal cell intensity (purple) and low cell intensity (dark yellow).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Christoph Klein (christoph.klein@med.uni-muenchen.de).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Dr. Drukker from the Institute of Stem Cell Research and the Induced Pluripotent Stem Cell Core Facility (Helmholtz Center Munich) for kindly providing us with healthy control fibroblast-derived iPS cells. We thank the group of Prof. Theurich from Gene Center of LMU for kindly providing us with introduction and technical support of Seahorse assay. This work has been funded by the German Research Society (Gottfried Wilhelm Leibniz Program), the BMBF (PID-NET), the German Center for Infection Research (DZIF), the Care-for-Rare Foundation, and China Scholarship Council.
Author contributions
Conceptualization and investigation, Y.F., Y.M., M.I.L.; writing - original draft and preparing figures, Y.F.; iPSC protocol development, Y.M., M.T., S.F.; Seahorse protocol development, Y.F., J.C.; graphical abstract, M.T.; writing - review and editing, all authors; funding acquisition and supervision, C.K.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Yanxin Fan, Email: yanxin.fan@med.uni-muenchen.de.
Christoph Klein, Email: christoph.klein@med.uni-muenchen.de.
Data and code availability
This study did not generate/analyze datasets and code.
References
- 1.Fan Y., Murgia M., Linder M.I., Mizoguchi Y., Wang C., Łyszkiewicz M., Ziȩtara N., Liu Y., Frenz S., Sciuccati G., et al. HAX1-dependent control of mitochondrial proteostasis governs neutrophil granulocyte differentiation. J. Clin. Invest. 2022;132:e153153. doi: 10.1172/JCI153153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunze C., Börner K., Kienle E., Orschmann T., Rusha E., Schneider M., Radivojkov-Blagojevic M., Drukker M., Desbordes S., Grimm D., Brack-Werner R. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia. 2018;66:413–427. doi: 10.1002/glia.23254. [DOI] [PubMed] [Google Scholar]
- 3.Niwa A., Heike T., Umeda K., Oshima K., Kato I., Sakai H., Suemori H., Nakahata T., Saito M.K. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS One. 2011;6:e22261. doi: 10.1371/journal.pone.0022261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotoh K., Takata Y., Nakashima Y., Mizuguchi S., Komori K., Kang D. Metabolic analysis of mouse bone-marrow-derived dendritic cells using an extracellular flux analyzer. STAR Protoc. 2021;2:100401. doi: 10.1016/j.xpro.2021.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandker L., Wood T.L. Live-cell metabolic analysis of oligodendroglia isolated from postnatal mouse brain and spinal cord. STAR Protoc. 2022;3:101655. doi: 10.1016/j.xpro.2022.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wettmarshausen J., Perocchi F. Assessing calcium-stimulated mitochondrial bioenergetics using the seahorse XF96 analyzer. Methods Mol. Biol. 2019;1925:197–222. doi: 10.1007/978-1-4939-9018-4_18. [DOI] [PubMed] [Google Scholar]
- 7.van der Windt G.J., Chang C., Pearce E.L. Measuring bioenergetics in T cells using a seahorse extracellular flux analyzer. Curr. Protoc. Immunol. 2016;113:3.16B.1–3.16B.14. doi: 10.1002/0471142735.im0316bs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsen J., Cömert C., Bross P., Palmfeldt J. Optimized high-contrast brightfield microscopy application for noninvasive proliferation assays of human cell cultures. Assay Drug Dev. Technol. 2020;18:215–225. doi: 10.1089/adt.2020.981. [DOI] [PubMed] [Google Scholar]
- 10.Truong V., Viken K., Geng Z., Barkan S., Johnson B., Ebeling M.C., Montezuma S.R., Ferrington D.A., Dutton J.R. Automating human induced pluripotent stem cell culture and differentiation of iPSC-derived retinal pigment epithelium for personalized drug testing. SLAS Technol. 2021;26:287–299. doi: 10.1177/2472630320972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludikhuize M.C., Meerlo M., Burgering B.M.T., Rodríguez Colman M.J. Protocol to profile the bioenergetics of organoids using Seahorse. STAR Protoc. 2021;2:100386. doi: 10.1016/j.xpro.2021.100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey L., Ziętara N., Łyszkiewicz M., Marquardt B., Mizoguchi Y., Linder M.I., Liu Y., Giesert F., Wurst W., Dahlhoff M., et al. Mammalian VPS45 orchestrates trafficking through the endosomal system. Blood. 2021;137:1932–1944. doi: 10.1182/blood.2020006871. [DOI] [PubMed] [Google Scholar]
- 13.Linder M.I., Mizoguchi Y., Hesse S., Csaba G., Tatematsu M., Łyszkiewicz M., Zietara N., Jeske T., Hastreiter M., Rohlfs M., et al. Human genetic defects in SRP19 and SRPRA cause severe congenital neutropenia with distinctive proteome changes. Blood. 2022 doi: 10.1182/blood.2022016783. blood.2022016783. [DOI] [PubMed] [Google Scholar]
- 14.Byrska-Bishop M., VanDorn D., Campbell A.E., Betensky M., Arca P.R., Yao Y., Gadue P., Costa F.F., Nemiroff R.L., Blobel G.A., et al. Pluripotent stem cells reveal erythroid-specific activities of the GATA1 N-terminus. J. Clin. Invest. 2015;125:993–1005. doi: 10.1172/JCI75714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi K.D., Vodyanik M.A., Slukvin I.I. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J. Clin. Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French A., Yang C.T., Taylor S., Watt S.M., Carpenter L. Human induced pluripotent stem cell-derived B lymphocytes express sIgM and can be generated via a hemogenic endothelium intermediate. Stem Cells Dev. 2015;24:1082–1095. doi: 10.1089/scd.2014.0318. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans F., Velghe I., Vanwalleghem L., De Smedt M., Van Coppernolle S., Taghon T., Moore H.D., Leclercq G., Langerak A.W., Kerre T., et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J. Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 18.Watarai H., Fujii S.i., Yamada D., Rybouchkin A., Sakata S., Nagata Y., Iida-Kobayashi M., Sekine-Kondo E., Shimizu K., Shozaki Y., et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J. Clin. Invest. 2010;120:2610–2618. doi: 10.1172/JCI42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagimachi M.D., Niwa A., Tanaka T., Honda-Ozaki F., Nishimoto S., Murata Y., Yasumi T., Ito J., Tomida S., Oshima K., et al. Robust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions. PLoS One. 2013;8:e59243. doi: 10.1371/journal.pone.0059243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets and code.