Abstract
Primary hyperparathyroidism (PHPT) is classically characterized by hypercalcemia with elevated or inappropriately normal parathyroid hormone (PTH) levels. Elevated PTH levels in the presence of normal calcium levels are not infrequently found during the evaluation of metabolic bone disorders or kidney stone disease. This can be caused by secondary hyperparathyroidism (SHPT) or normocalcemic primary hyperparathyroidism (NPHPT). NPHPT is due to autonomous parathyroid function whereas SHPT is caused by a physiologic stimulation to PTH secretion. Many medical conditions and medications can contribute to SHPT, and differentiation between SHPT and NPHPT may be difficult. Cases are presented to illustrate examples. In this paper, we review the distinction between SHPT and NPHPT as well as end organ effects of NPHPT and outcomes of surgery in NPHPT. We suggest that the diagnosis of NPHPT be made only after careful exclusion of causes of SHPT and consideration of medications that can increase PTH secretion. Further, we advise a conservative approach to surgery in NPHPT.
Keywords: hyperparathyroidism, primary hyperparathyroidism, normocalcemic primary hyperparathyroidism, secondary hyperparathyroidism
Primary hyperparathyroidism (PHPT) is a relatively common disorder. An estimate of prevalence in the United States was 233 per 100 000 in women and 85 per 100 000 in men [1], and PHPT is more common after menopause [2]. In the United States, the disorder has changed from a symptomatic condition characterized by “stones, bones, and psychic moans” before the era of multichannel laboratory tests to a less symptomatic condition [2]. The recently published 5th international workshop on the evaluation and management of primary hyperparathyroidism [3] suggested classifying patients with PHPT in 3 groups (Table 1). Broadly these are symptomatic PHPT, asymptomatic PHPT, and normocalcemic PHPT (NPHPT).
Table 1.
Classification of primary hyperparathyroidism
|
Biochemically, PHPT is classically characterized by hypercalcemia with elevated or inappropriately normal PTH levels and normal to elevated urinary calcium. In some patients, only the ionized calcium is elevated, and in others the hypercalcemia is intermittent [3]. The diagnosis is important because mild PHPT may be associated with low bone mineral density (BMD), microarchitectural changes in bone, increased vertebral and other fracture risk, and an increased prevalence of kidney stones [4]. There is an association with neuropsychiatric symptoms and cardiovascular disease; however, causality is uncertain as benefits from parathyroidectomy that are evident on observational studies are less clear in randomized trials [4].
There are several physiologic considerations in the measurement of PTH. Specifically, whereas serum calcium is relatively stable throughout adult life, PTH secretion increases with age, and an estimated 20% of women ≥80 years will have an elevated PTH level, primarily related to age-related decreases in renal function [5]. Furthermore, PTH secretion is dynamic, with intermittent pulses superimposed on a background of basal secretion [6], and has a circadian rhythm [7, 8]. Additional factors to consider that can influence PTH and calcium measurement include hemoconcentration, albumin and protein levels, immobilization, and pH-dependent changes in protein-bound calcium.
NPHPT was described many years ago [9] but has been better characterized more recently [10-17]. The major diagnostic dilemma is differentiating NPHPT from secondary hyperparathyroidism (SHPT) (Table 2). Classical hypercalcemic PHPT (HPHPT) is characterized by hypercalcemia with elevated or normal (nonsuppressed) PTH levels and is caused by autonomous parathyroid function of 1 or more glands. The hypercalcemia may be intermittent or only elevated when the ionized calcium is measured [3]. NPHPT is characterized by elevated PTH levels with persistently normal albumin-corrected total calcium and ionized calcium levels over at least 3 to 6 months [3]. It is worth noting that some patients with apparent NPHPT will be redefined as hypercalcemic after the ionized calcium is measured [18]. Furthermore, untreated patients with NPHPT can transition to normal, whereas patients with milder PHPT often can transition to normal calcium with elevated PTH levels only mimicking NPHPT [19]. Secondary hyperparathyroidism is characterized by elevated PTH levels associated with persistently normal (or low) albumin-corrected total calcium and ionized calcium levels and is caused by a physiologic stimulus to PTH secretion rather than autonomous parathyroid function. From these definitions, it is clear that NPHPT and SHPT may sometimes be difficult to differentiate.
Table 2.
Definitions
|
There are many medical conditions and medications that can cause secondary hyperparathyroidism [16]. These are shown in Table 3.
Table 3.
Causes of secondary hyperparathyroidism
| Chronic kidney disease |
| Malabsorption Bariatric surgery Gastrectomy Inflammatory bowel disease Celiac disease Short gut syndrome |
| Idiopathic hypercalciuria |
| Vitamin D insufficiency/deficiency |
| Calcium deficiency |
| Paget's disease of bone |
| Obesity |
| Fibroblast growth factor-23-mediated disorders |
| Medications Bisphosphonates Denosumab Romosozumab Diuretics Sodium-glucose contransporter-2 inhibitors Lithium Anticonvulsants (phenytoin/phenobarbital) Tenofovir Calcitonin Estrogen replacement Calcium channel blockers Ferric carboxymaltose |
Pathophysiology of NPHPT
Potential mechanisms of NPHPT have recently been reviewed by Zavatta and Clarke [13]. One explanation is based on the observation that the intraindividual variation in serum calcium is much less than the population variation [20]. For example, the mean total serum calcium level in healthy outpatients without chronic kidney disease at Mayo Clinic Rochester and Arizona is approximately 9.2 mg/dL throughout adult life (personal communication with R.A.W., Nikola A. Baumann, PhD). Hence, a patient with average adult levels who now has calcium levels of 9.8 mg/dL may have developed autonomous parathyroid function. Other mechanisms include age-related decreased 1,25-dihydroxyvitamin D [1,25(OH)2D] production with decreased feedback on parathyroid glands [21]. With prolonged stimulation, autonomous parathyroid gland function could occur. Additional proposed mechanisms include partial PTH resistance [22], decreased free 25-hydroxyvitamin D levels despite normal total 25-hydroxyvitamin D levels [23], and abnormal sensing of calcium by the calcium sensing receptor on parathyroid glands [24]. The biochemical constellation that fits NPHPT likely has heterogenous causes [13].
Cases
Four cases are presented to illustrate relevant clinical scenarios.
Case 1: Idiopathic Hypercalciuria
A 38-year-old woman was evaluated for hypocalcemia 6 months after parathyroid surgery. She had a history of kidney stones since age 23. Calcium levels in the 6 years prior to parathyroid surgery were 9 to 10 mg/dL. The ionized calcium prior to surgery was 1.25 mmol/L (1.15-1.33) with intact PTH levels of 93, 88, and 67 pg/mL (normal, 14-72 pg/mL). The 25(OH)D level was 40 ng/mL, creatinine 0.68 mg/dL, phosphate 3.2 mg/dL, magnesium 2.1 mg/dL, and urinary calcium 748 mg/24 hours. Her family history (FH) was significant for a sister with a pituitary tumor and genetic testing for multiple endocrine neoplasia type 1 was negative. At surgery she had 3 parathyroid glands removed (right upper 88 mg, right lower 108 mg, left upper 147 mg). She also had a left thyroid lobectomy (benign). Post-operatively, she developed hypocalcemia. When evaluated 6 months post-operatively she was on calcitriol 0.25 mcg daily and calcium as carbonate 600 mg 3 times daily. At that time, laboratory data revealed calcium 8.3 mg/dL, magnesium 2.2 mg/dL, phosphate 3.7 mg/dL, creatinine 0.82 mg/dL, PTH 29 pg/mL, and urinary calcium 946 mg/24 hours. Thiazide therapy was advised.
Case discussion
Case 1 demonstrates idiopathic hypercalciuria likely causing SPHPT. In 1973, Coe et al described SHPT due to renal hypercalciuria [25]. Idiopathic hypercalciuria probably has complex mechanisms, and the frequency of elevated PTH levels is unclear [26]; however, marked hypercalciuria is considered an exclusion to the diagnosis of NPHPT [16]. In the patient described here, a trial of a thiazide diuretic to lower urinary calcium and possibly normalize PTH secretion would have been preferred prior to considering parathyroid surgery. In patients with hypercalciuria and elevated PTH levels, the development of hypercalcemia on the thiazide diuretic could provide evidence of HPHPT [27] as most patients with thiazide-associated hypercalcemia have an underlying disorder of calcium metabolism [28].
Case 2: Undiagnosed Malabsorption Due to Celiac Disease
A 74-year-old woman was seen for osteoporosis and elevated PTH levels. There was no fracture history. BMD by dual-energy x-ray absorptiometry (DXA) revealed T-scores of −2.3 to −3.3. She was treated with calcium 600 mg twice daily, vitamin D2 50 000 units weekly, and risedronate for the 4 years prior to evaluation. Her biochemical evaluation revealed a serum of calcium 9.0 mg/dL, albumin 4.4 g/dL, phosphate 3.6 mg/dL, creatinine 0.67 mg/dL, magnesium 2.2 mg/dL, PTH 101 pg/mL (normal, 15-72 pg/mL), 25OHD 29 mg/dL, and urinary calcium 14 mg/24H. Celiac serology was strongly positive, and duodenal biopsy revealed villous blunting, intraepithelial lymphocytosis, and crypt hyperplasia, consistent with celiac disease (CD). Risedronate was stopped and she was treated with a gluten-free diet, calcium, and vitamin D. Eight months later the PTH level and celiac serology had normalized. Despite adherence to a gluten-free diet, the BMD did not improve, and she was subsequently treated with a bisphosphonate.
Case discussion
Case 2 demonstrates undiagnosed malabsorption may cause SHPT. The most common disorder in this setting is CD. CD is relatively common with a prevalence of about 1% [29]. It is more common is patients with a FH of celiac disease as well as autoimmune conditions such as type 1 diabetes and autoimmune thyroid disease [29]. Clues to diagnosis of CD include gastrointestinal symptoms, FH, autoimmune conditions, low urinary calcium, and high vitamin D requirements. Many patients have no gastrointestinal symptoms [29]. Bone loss in celiac disease is in part related to SHPT [30]. The PTH level may be elevated even with normal 25(OH)D levels because abnormal duodenal and jejunal mucosa may malabsorb calcium and other nutrients. The diagnosis of CD is made by celiac serology such as tissue transglutaminase antibodies in addition to small bowel biopsy [29]. Immunoglobulin A (IgA) deficiency, which is 10 to 15 times more common in patients with CD than in the general population, may cause false-negative IgA tissue transglutaminase antibodies, and IgA deficiency should be screened for during serologic testing for CD [31]. A gluten-free diet remains the only treatment, although a transglutaminase 2 inhibitor is being studied [32]. Other disorders associated with malabsorption such as inflammatory bowel disease, gastrectomy, and short gut syndrome may be accompanied by SHPT.
Case 3: Bariatric Surgery
A 60-year-old woman was seen for osteoporosis and elevated PTH levels. Nine months earlier, she fractured the right femur and right radius and ulna when she fell in the bathroom on a wet floor. She had surgery on both fractures, which healed. This prompted a BMD by DXA, which revealed an L1-L4 T score of −3.5. There was a history of Roux-en-Y gastric bypass (RYGB) for obesity approximately 9 years early. She was taking calcium 500 mg twice daily, vitamin D 2000IU daily, and a daily multivitamin. Laboratory data revealed calcium 9.4 mg/dL, albumin 4.5 g/dL, creatinine 0.71 mg/dL, phosphate 3.6 mg/dL, magnesium 2.1 mg/dL, 25(OH) vitamin D 40 ng/mL, intact PTH 101 pg/mL, and urinary calcium 122 mg/24H. She was treated with continued vitamin D and calcium as citrate about 600 mg elemental 3 times daily. Two months later the PTH level was high normal at 58 pg/mL with calcium 9.4 mg/dL, albumin 4.5 g/dL, creatinine 0.85 mg/dL, and ionized calcium 1.25 mmol/L. She was ultimately treated with romosozumab, and the PTH level has remained high normal to slightly elevated with normal albumin-corrected and ionized calcium levels.
Case discussion
Case 3 demonstrates SHPT due to bariatric surgery. Elevation of PTH levels in obesity was described many years ago by Bell et al [33]. PTH level elevations have subsequently been noted in many patients prior to bariatric surgery [34-37]. The mechanism of elevated PTH levels in obesity is probably in part related to lower vitamin D levels but is not completely understood [33]. RYBG and other malabsorptive bariatric procedures are well known to be associated with an Increased likelihood of SHPT [34-37], which may occur even with normal vitamin D nutrition [38]. Shafer et al [38] showed significantly decreased calcium absorption after bariatric surgery despite normal vitamin D status presumably because of bypass of calcium absorptive surface in duodenum and jejunum. PTH levels and bone turnover markers can stay elevated years after both sleeve gastrectomy and RYGB [39]. Treatment includes calcium and vitamin D. Calcium as citrate may be more easily absorbed in this setting [40]; however, it may be difficult to normalize PTH levels in these patients with calcium citrate or carbonate [41]. Interestingly, there have been reports of PHPT with hypercalcemia after RYBG [42]. These cases may represent classical PHPT in a patient with RYGB or tertiary hyperparathyroidism in patients with prolonged PTH stimulation somewhat akin to kidney disease [43].
Case 4: Medication-associated Hyperparathyroidism
A 66-year-old woman was referred for possible NPHPT. Ten months earlier she was diagnosed with osteoporosis on DXA scanning (lowest T-sore −2.8). There was no fracture history. At that time her calcium was 9.7 mg/dL, albumin 4.2 g/dL, and creatinine 0.78 mg/dL. Her provider started her on calcium, vitamin D, and denosumab 60 mg every 6 months. Six weeks after the second dose she changed providers and laboratory evaluation revealed calcium 9.4 mg/dL, albumin 4.1 g/dL, creatinine 0.81 mg/dL, phosphorus 3.0 mg/dL, magnesium 2.1 mg/dL, 25(OH)vitamin D 43 ng/mL, and intact PTH 87 pg/mL. She was referred to an endocrine surgeon who advised her to see endocrinology for further evaluation. At that time, she was on calcium 600 mg twice daily and vitamin D 2000 units daily. Laboratory data obtained about 5.5 months after the last denosumab dose revealed calcium 9.6 mg/dL, albumin 4.2 g/dL, ionized calcium 1.24 mmol/L, creatinine 0.79 mg/dL, phosphate 3.1 mg/dL, intact PTH 51 pg/mL, and urinary calcium 145 mg/24H.
Case discussion
Case 4 demonstrates SHPT caused by a medication (denosumab). Several medications can raise the PTH level (Table 2). Lithium interferes with the calcium sensing receptor and increases the set point for PTH secretion [44, 45]. Lithium may also cause HPHPT [44, 45]. Loop diuretics may increase urinary calcium excretion and cause secondary hyperparathyroidism [46, 47]. The effect of thiazide diuretics is less clear. A study in dogs [48] suggested thiazide diuretics increase PTH secretion. One study in humans [49] also suggested increased PTH levels despite lowering urinary calcium. Other studies in humans suggest PTH levels may be lower or unchanged in patients on thiazide diuretics [50-53]. Older anticonvulsants such as phenytoin and phenobarbital can interfere with vitamin D metabolism and cause secondary hyperparathyroidism [54]. Although results are mixed, some studies suggest sodium-glucose contransporter-2 inhibitors may increase PTH levels [55-58]. Proton pump inhibitors (PPIs) have complicated effects on the calcium-parathyroid hormone axis. A relative rare complication of PPIs is reversible hypomagnesemic hypoparathyroidism [59]. However, higher PTH levels have been reported in elderly patients on PPIs [60]. Notable among drugs that can increase PTH levels are the potent bone antiresorptive drugs such as bisphosphonates [61] and denosumab [62, 63]. PTH level elevation is more commonly seen with denosumab, especially in the first few weeks after administration, and can cause confusion in the patient being evaluated for secondary causes of osteoporosis. Romosozumab has both anabolic and antiresorptive effects and is associated with a modest decrease in serum calcium [64, 65], as well as elevations in PTH levels [66]. Other medications that may increase the PTH level include tenofovir [67], calcitonin [68], estrogen [69], calcium channel blockers [50], and ferric-carboxymaltose [70].
Other Causes of Elevated PTH Levels in Eucalcemic Patients
Chronic Kidney Disease
Chronic kidney disease (CKD) is well known to cause SHPT [71, 72]. This is due to decreased production of 1,25(OH)2D as well as phosphate retention. PTH levels may begin to increase when glomerular filtration rate (GFR) decreases below 60 mL/minute [71]. Patients found to have both PHPT and elevated PTH levels with normocalcemia are often being evaluated for osteoporosis [2]. This population is also at risk for age-related decline in GFR. Additionally, the serum creatinine may overestimate GFR in elderly patients with decreased muscle mass [73, 74]. In this setting, another measurement of renal function either by plasma cystatin C, which is less dependent on muscle mass than serum creatinine, or based on a 24-hour urine creatinine clearance is advisable to better assess renal function.
Vitamin D Deficiency
Vitamin D deficiency is well known to cause SHPT. The 25(OH)D level at which PTH levels go up is unclear, and the literature is complicated by different 25(OH)D assays [75]. That said, a reasonable approach is to obtain a 25(OH)D level above 30 ng/mL before considering the diagnosis of NPHPT [3]. It is also worth noting it may take several months for PTH levels to normalize after attaining vitamin D sufficiency. Vitamin D replacement can also reduce PTH levels in PHPT [76].
Low Calcium Intake
Low calcium intake may be associated with SHPT [77]. Recently, Shokry et al reported a calcium challenge test in 8 women and 1 man with eucalcemic hyperparathyroidism and low calcium intake. The patients were treated with calcium 600 mg twice daily, and PTH levels normalized in all patients [77]. They all remained eucalcemic. In addition, secretion of PTH in PHPT can be modified by calcium consumption [78, 79].
Fibroblast Growth Factor-23-mediated Hypophosphatemic Disorders
Fibroblast growth factor-23-mediated hypophosphatemic disorders such as X-linked hypophosphatemia, autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets, and tumor-induced osteomalacia may be associated with hyperparathyroidism due to decreased production of 1,25(OH)2D and phosphate supplementation [80]. Such patients may also develop PHPT with hypercalcemia and biochemistry suggestive of NPHPT [81]. Generally, these conditions can be differentiated from primary hyperparathyroidism based on clinical history and the marked degree of hypophosphatemia usually present.
Assay Issues
False elevation in PTH levels may be caused by heterophile antibodies and rheumatoid factor [82, 83]. Antimurine heterophile antibodies causing PTH level elevation have also been reported in a transplant patient treated with muromonab-CD3 immunosupression [84]. Additionally, a study from the UK found significant variation in the diagnosis of NPHPT using 3 different commercial assays [85].
Epidemiology of Normocalcemic PHPT
There have been several population-based studies to assess the prevalence of normal calcium and elevated PTH levels [86-95]. Most of these studies excluded patients with 25(OH)D levels <20 to 30 ng/mL and estimated GFR (eGFR) < 40 to 70 mL/minute. Some of these studies did baseline and follow-up measurements. Marques et al [87] studied 156 women being evaluated for osteoporosis in Brazil. They excluded patients with eGFR <40 mL/minute and 25(OH)D < 30 ng/mL as well as patients with known malabsorption or use of medications that can raise the PTH level. They found 8.9% with elevated PTH levels and normal albumin-corrected calcium. Garcia-Martin et al [88] analyzed data from 100 healthy postmenopausal women in Spain. After excluding GFR <70 and 25(OH)D < 30 ng/mL, 6% had elevated PTH levels with normal albumin-corrected calcium at baseline and at follow-up. Other studies, however, found elevated PTH levels with normal albumin-corrected calcium much less common with a prevalence of 0.2% to 3.3% at baseline [89-95] and 0% to 0.6% on follow-up measurement [89, 90, 93, 95]. Berger et al [91] studied 1872 men and women ages >35 years being evaluated for fracture risk (CaMOS). After excluding eGFR <60 mL/minute and 25(OH)D < 20 ng/mL, they found 3.3% had elevated PTH levels and normal albumin-corrected calcium. However, of the 53 participants suspected of having NPHPT, 28 were using bone antiresorptive agents and 16 were using diuretics.
Taken together, these studies suggest elevated PTH levels with normal serum calcium is uncommon. Further, in some of these studies all causes of SHPT were not rigorously excluded, ionized calcium levels were not usually measured, and subjects were not always proven to have this biochemical pattern persistently. Indeed, the prevalence of true NPHPT may be even lower than indicated in these studies.
Morbidity in NPHPT (Skeletal and Renal)
Most of the cohorts studied are in patients in whom a PTH level was measured because of bone disease or kidney stones resulting in selection bias [10, 18, 22, 87, 96-102]. These reports are summarized in Table 4. In summary, these studies found osteoporosis in 15% to 57% of patients with NPHPT and nephrolithiasis in 4% to 36% of patients with NPHPT. Some studies comparing HPHPT and NPHPT found similar skeletal and renal morbidity [100, 101], whereas the study by Palermo et al found that patients with NPHPT had an intermediate biochemical phenotype between HPHPT and controls with BMD and fracture risk in NPHPT similar to controls [102]. Charopoulos et al [103] used peripheral quantitative computed tomography (CT) (tibia) and found patients with NPHPT had evidence of catabolic actifons at both cortical and cancellous sites and that cortical geometric properties are adversely affected in NPHPT whereas trabecular properties are generally preserved.
Table 4.
Summary of skeletal and renal morbidity
| Reference | Number of patients | Skeletal | Kidney stones |
|---|---|---|---|
| Maurini et al, 2003 [22] | 34 NPHPT | 18% radiographic demineralization | 35% |
| Tordjman et al, 2004 [96] | 32 NPHPT | 36% osteoporosis | 9% |
| Lowe et al, 2006 [10] | 37 NPHPT | 57% osteoporosis 11% fragility fractures |
14% |
| Marques et al, 2011 [87] | 14 NPHPT | 21% fractures | 29% |
| Wade et al, 2012 [18] | 8 NPHPT | 25% osteoporosis | 25% |
| Amaral et al, 2012 [97] | 33 NPHPT 37 HPHPT |
15% fractures NPHPT 11% fractures HPHPT |
18% NPHPT 19% HPHPT |
| Cakir et al, 2012 [98] | 18 NPHPT | 47% osteoporosis | 11% |
| Tuna et al, 2015 [100] | 23 NPHPT 284 HPHPT |
53% osteoporosis NPHPT 44% osteoporosis HPHPT |
15% NPHPT 19% HPHPT |
| Siprova et al, 2016 [99] | 187 NPHPT | 42% decreased BMD | 4% |
| Pirreaux et al, 2018 [101] | 25 NPHPT 106 HPHPT |
25% osteoporosis NPHPT 32% osteoporosis HPHPT |
36% NPHPT 24% HPHPT |
| Palermo et al, 2020 [102] | 47 NHPT 41 PHPT |
47% osteoporosis in NPHPT BMD FN, LS similar in NPHPT and HPHPT TH lower in HPHPT than NPHPT Morphometric vertebral fractures 60% HPHPT 28% NPHPT 23% controls |
13% NPHPT 10% HPHPT |
Abbreviations: BMD, bone mineral density; FN, femoral neck; HPHPT, hypercalcemic primary hyperparathyroidism; LS, lumbar spine; NPHPT, normocalcemic primary hyperparathyroidism; TH, total hip.
It is not surprising that many of these series are enriched in patients with bone and renal stone disease as the PTH level was often measured during the evaluation of these conditions. Further, ionized calcium was not always measured, and it is quite possible some of the patients had HPHPT. Lastly, causes of SHPT were not always rigorously excluded, and SHPT could also have deleterious skeletal effects.
Nonclassical (Quality of Life and Cardiovascular/Metabolic Effects or Symptoms)
Patients with severe HPHPT clearly may have neuropsychiatric and neuromuscular symptoms [4]. In patients with mild HPHPT, the causality of these symptoms is less clear [4, 104]. In a small study (13 patients), Voss et al found decreased physical functioning and quality of life (QOL) by SF-36 in patients with NPHPT [105].
In asymptomatic HPHPT, observational studies suggest an increased prevalence of abnormalities in markers of cardiovascular outcomes; however, reversibility after parathyroid surgery has not been proven [4]. One study found no increase in arterial stiffness in patients with NPHPT [106]. There is conflicting data on coronary calcification in NPHPT [107, 108]. Several studies have examined insulin resistance in NPHPT. Cakir and colleagues [98] found no evidence of insulin resistance in NPHPT and at a 4-year follow-up [109]. Two additional studies observed normal insulin sensitivity in patients with NPHPT [110, 111]. Hagstrom et al [112] found patients with NPHPT had a more atherogenic lipid profile, higher body mass index, and higher glucose levels than age-matched controls. A study that compared patients with HPHPT, NPHPT, and controls observed that HPHPT and NPHPT were similar in metabolic profile [113]. In this study, NPHPT patients had a higher prevalence of glucose intolerance and hypertension than controls [113]. Chen et al also found a higher risk of hypertension in NPHPT than controls [114]. Karras et al found NPHPT was associated with higher fasting glucose than controls [115] and that glucose homeostasis may improve after parathyroidectomy [116].
In summary, the data on nonclassical symptoms and cardiovascular risks associated with NPHPT is inconclusive. Further, because many of the patients in these studies may not have met rigorous criteria for the diagnosis of NPHPT, interpretation of this data is difficult.
Biochemical Course in Apparent NPHPT
Tordjman et al [96] followed 20 patients with NPHPT and none became hypercalcemic during 4 years of follow-up. Lowe et al [10] found that 7/37(19%) became hypercalcemic over about 3 years of follow-up, and the individuals who became hypercalcemic had a higher baseline serum calcium (9.7 mg/dL), were older, and had higher baseline 24-hour urine calcium measures. Forty-one percent developed either hypercalcemia, hypercalciuria, fracture, and/or kidney stones during that time. In one study of 187 patients with NPHPT [99], 19% developed hypercalcemia during up to 6 years of follow-up; however, most of these patients became hypercalcemic in the first 2 years. In a different study of 71 patients with apparent NPHPT, none became hypercalcemic, with a median follow-up of 23.1 months [117]. It is also notable than some patients with elevated PTH levels and normal serum calcium levels have normalization of PTH levels over time [19, 89, 90, 91, 95]. In summary, some patients with NPHPT develop hypercalcemia over time, making the diagnosis more definitive. In addition, some patients will experience normalization of PTH levels over time.
Localization
Preoperative localization is now used routinely to guide the parathyroid surgeon [118]. Successful localization appears to be less likely in NPHPT compared to HPHPT. A study comparing imaging modalities in patients with NPHPT and HPHPT [119] found that ultrasound localized 22%, TC-99-Sestamibi scintigraphy localized 11.1%, and 4D parathyroid CT localized 55.6% of patients with NPHPT. In contrast, in HPHPT, ultrasound localized 58.3%, TC-99-Sestamibi scintigraphy localized 75%, and 4DCT localized 75% (95% CI, 50.5-99.5). In the study by Siprova et al [99], TC-99-Sestamibi scintigraphy was successful in 14% of normocalcemic patients compared to 73% of the patients who became hypercalcemic. A recent study using TC-99-Sestamibi SPECT/CT reported localization in 86.0% of patients with HPHPT and 59.4% of patients with NPHPT [120].
Surgical Findings
There is some evidence that multiple gland disease (MGD) is more common in NPHPT than in HPHPT. Kiriakopoulos and colleagues [121] observed no difference in MGD (16.8%), but adenomas were smaller in NPHPT compared to HPHPT. Lim et al [122] evaluated 573 patients who underwent parathyroidectomy for PHPT. They divided the patients into 3 groups: 405 with elevated PTH levels and calcium, 72 with hypercalcemia and normal PTH levels, and 96 with elevated PTH levels and normal calcium. NPHPT was associated with MGD in 43 (45%, P < .001). Ten percent of the patients with hypercalcemia and normal PTH levels and 9% of the patients with elevated PTH levels and calcium had MGD. On logistic regression, significant predictors for MGD were the normocalcemic subtype and positive FH. Twelve-month biochemical normalization rates after operative treatment were >98% in all 3 groups. Another study [117] observed a high incidence of MGD in their patients with NPHPT who underwent parathyroidectomy (53.5%).
Pandian et al [123] analyzed the Collaborative Endocrine Surgery Quality Improvement Program database (6836 with HPHPT and 733 with NPHPT). Single gland resection was more common in HPHPT than NPHPT (73.3%% vs 47.5%, P < .05). Patients with NPHPT had a higher rate of subtotal (3.5 gland) resection compared to patients with HPHPT (10.0% vs 4.7%, P < .05). Multigland hyperplasia was reported in 43.1% of patients with NPHPT vs 21.9% of patients with HPHPT. In the NPHPT cohort, 47 patients (6.4%) underwent remedial surgery compared with 307 patients (4.5%) with HPHPT.
PTH Levels After Surgery
Caldwell et al reported that about one-third of patients with successful parathyroid surgery at a tertiary care center had elevated PTH levels after surgery [124]. In a literature review, de la Plaza Llamas et al found a mean prevalence of elevated PTH levels after parathyroid surgery for PHPT of 23.5%, ranging from 3% to 46% [125]. Surgical resolution of hypercalcemia presumably represents a cure; however, persistent elevation of PTH levels may be associated with a higher risk of recurrence [126]. Persistence of PTH level elevation after successful parathyroid surgery is probably multifactorial, including calcium uptake into the skeleton, vitamin D deficiency, inadequate calcium intake or absorption, reduced peripheral sensitivity to PTH, underlying CKD, and renal leak of calcium [127]. There is much less data about persistence of PTH levels elevation after surgery in NPHPT, although Sho et al found persistently elevated PTH levels in 46.5% of patients [117]. From a practical standpoint, persistent elevation of PTH levels after parathyroid surgery is harder for patients and clinicians to understand in the patient who has never been hypercalcemic than in the patient whose hypercalcemia was surgically cured.
Skeletal Response to Surgery
BMD increases after curative parathyroid surgery in HPHPT [128-133], and fracture risk may decrease after curative parathyroid surgery [134-137]. There is limited data in NPHPT. Koumakis et al [138] found similar increases in BMD 1 year after parathyroid surgery in patients with HPHPT compared to patients with NPHPT. Sho et al [117] reported a 5.6% increase in BMD more than 2 years postoperatively in the patients in whom PTH levels normalized. However, in the patients with persistently elevated PTH levels, there was no change in BMD (46.5% of the group).
Cardiovascular and Neuropsychiatric Changes After Surgery
The effects of parathyroid surgery on cardiovascular risks and neuropsychiatric symptoms in mild HPHPT are unclear [4]. In these patients, QOL improvement is greater in observational studies than in randomized trials, suggesting there may be a bias. There is less data available for NPHPT. Beysal et al [139] reported cardiovascular risk factors in patients undergoing parathyroid surgery for HPHPT and NPHPT. After parathyroidectomy, blood pressure, total cholesterol, and homeostatic model assessment of insulin resistance decreased in both PHPT groups. As noted, one study observed improved glucose homeostasis after parathyroidectomy in NPHPT [116]. A study of QOL in patients undergoing parathyroidectomy for PHPT suggested mild improvement in QOL in NPHPT [140]. The same limitations apply to this study as other surgical series of parathyroid surgery on QOL without a control group.
Medical Management
Like patients with mild HPHPT being medically managed, a normal total calcium intake from diet and supplements of about 1000 to 1200 mg daily is appropriate [3]. Normal doses of vitamin D to attain a 25(OH)D level of 30 to 50 ng/mL is reasonable [3]. Alendronate has been shown to improve bone mineral density in HPHPT [141-144]. Casereo et al studied 30 patients with NPHPT randomized to 2800 IU vitamin D weekly or 2800 IU vitamin D and alendronate 70 mg weekly [145]. At 12 months, BMD in the alendronate group increased significantly at the lumbar, femoral neck, and total femur. The largest increase (4.7%) was at the lumbar spine.
Cinacalcet is approved for management of significant hypercalcemia in patients with parathyroid cancer and HPHPT who cannot or will not have surgery. As the major effect is on serum calcium, cinacalcet would not typically be recommended in NPHPT. Brardi et al [146] studied 4 patients with HPHPT and 6 patients with NPHPT and kidney stones. Cinacalcet was given in a dose to normalize PTH levels. During a 10-month follow-up period there was a decrease in number and size of kidney stones. This study is limited by the mix of HPHPT and NPHPT as well as small sample size and short follow-up time.
Diagnosis of the Eucalcemic Patient With Elevated PTH Levels
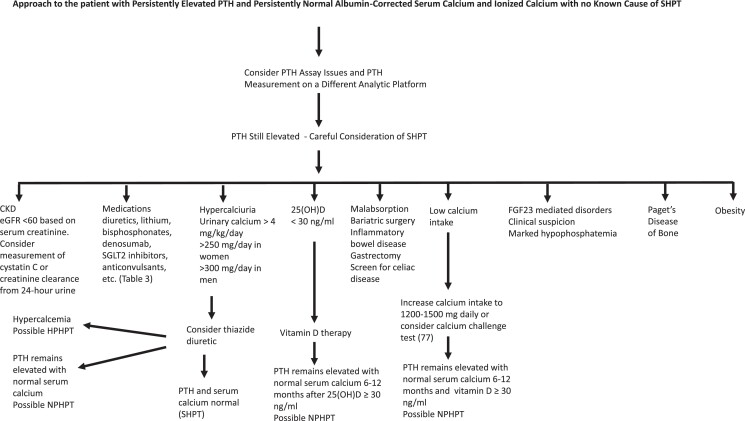
The diagnosis of NPHPT should only be made in the patient with persistently elevated PTH levels for at least 3 to 6 months with normal albumin corrected and ionized calcium after causes of secondary hyperparathyroidism have been rigorously excluded and medications that might raise the PTH level have been considered (Fig. 1). The presence of intermittent hypercalcemia or ionized hypercalcemia should result in reclassifying the patient as HPHPT. In addition, older women with elevated PTH levels without secondary factors and serum calcium levels at the higher end of normal compared to lower normal or with a serum calcium that has progressed from lower normal to higher normal may be more likely to develop HPHPT in the future [10]. Based on the findings of Kalairia et al [85], we also suggest considering measurement of PTH levels on at least 2 different analytic platforms. In addition, we recommend consideration of assessment of renal function by a second method (plasma cystatin C or creatinine clearance from a 24-hour urine) in elderly patients and patients with low muscle mass. Some have suggested the use of a calcium/phosphorus ratio [147] or a parathyroid function index (using calcium, phosphate, and PTH levels) [148] to discriminate NPHPT from SHPT. These studies are limited by the lack of ionized calcium measurement and retrospective design [13].
Figure 1.
Suggested approach to the eucalcemic patient with elevated parathyroid hormone levels.
Surgery in NPHPT
Because the pathophysiology is complicated, we suggest making the diagnosis of NPHPT with caution. Patients with apparent NPHPT are less likely to localize on imaging and more likely to have MGD and need bilateral exploration than patients with HPHPT. Lastly, persistent elevation of PTH levels is not uncommon after surgery in both HPHPT and NPHPT. If the primary reason for surgery is hypercalciuria/kidney stones, a trial of a thiazide diuretic seems appropriate. If the only reason for surgery is low BMD, a trial of an antiresorptive agent such as a bisphosphonate is a consideration.
Summary and Conclusions
Although NPHPT has been better characterized in recent years, the clinical problem of distinguishing NPHPT from SHPT remains. We believe the diagnosis should be made with caution only after a careful evaluation for other causes of PTH level elevation and observation over a period of time. The decision to proceed with surgery should be made with even greater caution. Although imaging is typically used only after surgery is planned to aid the surgeon, it would be reasonable to reserve surgery in NPHPT to those patients who have a clear surgical target [16]. We agree with Zavatta and Clarke that the patients with eucalcemic PTH level elevation represent a heterogeneous group and NPHPT may be frequently misdiagnosed [13].
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- BMD
bone mineral density
- CD
celiac disease
- CKD
chronic kidney disease
- CT
computed tomography
- DXA
dual-energy x-ray absorptiometry
- eGFR
estimated GFR
- FH
family history
- GFR
glomerular filtration rate
- HPHPT
hypercalcemic primary hyperparathyroidism
- IgA
immunoglobulin A
- MGD
multiple gland disease
- NPHPT
normocalcemic primary hyperparathyroidism
- PHPT
primary hyperparathyroidism
- PPIs
proton pump inhibitors
- PTH
parathyroid hormone
- QOL
quality of life
- RYGB
Roux-en-Y gastric bypass
- SHPT
secondary hyperparathyroidism
Contributor Information
Joseph L Shaker, Email: jshaker@mcw.edu, Department of Medicine and Division of Endocrinology and Molecular Medicine, Medical College of Wisconsin, Milwaukee, WI, USA.
Robert A Wermers, Department of Medicine and Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN, USA.
Conflict of Interest
The authors report no conflicts of interest relevant to this subject matter or materials included in this work.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122–1129. doi: 10.1210/jc.2012-4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griebeler ML, Kearns AE, Ryu E, Hathcock MA, Melton LJ III, Wermers RA. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1–7. doi: 10.1016/j.bone.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilezikian JP, Khan AA, Silverberg SJ, et al. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the fifth international workshop. J Bone Mineral Res. 2022;37(11):2293–2314. doi: 10.1002/jbmr.4677 [DOI] [PubMed] [Google Scholar]
- 4. El-Hajj Fuleihan G, Chakhtoura M, Cipriani C, et al. Classical and nonclassical manifestations of primary hyperparathyroidism. J Bone Mineral Res. 2022;37(11):2330–2350. doi: 10.1002/jbmr.4679 [DOI] [PubMed] [Google Scholar]
- 5. Buchebner D, Malmgren L, Christensson A, et al. Longitudinal assessment of PTH in community-dwelling older women—elevations are not associated with mortality. J Endocr Soc. 2017;1(6):615–624. doi: 10.1210/js.2017-00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samuels MH, Veldhuis J, Cawley C, et al. Pulsatile secretion of parathyroid hormone in normal young subjects: assessment by deconvolution analysis. J Clin Endocrinol Metab. 1993;77(2):399–403. doi: 10.1210/jcem.77.2.8345044 [DOI] [PubMed] [Google Scholar]
- 7. Ledger GA, Burritt MF, Kao PC, O’Fallon WM, Riggs BL, Khosla S. Role of parathyroid hormone in mediating nocturnal and age-related increases in bone resorption. J Clin Endocrinol Metab. 1995;80(11):3304–3310. doi: 10.1210/jcem.80.11.7593443 [DOI] [PubMed] [Google Scholar]
- 8. Calvo MS, Eastell R, Offord KP, Bergstralh EJ, Burritt MF. Circadian variation in ionized calcium and intact parathyroid hormone: evidence for sex differences in calcium homeostasis. J Clin Endocrinol Metab. 1991;72(1):69–76. doi: 10.1210/jcem-72-1-69 [DOI] [PubMed] [Google Scholar]
- 9. Wills MR, Pak CYC, Hammond WG, Bartter FC. Normocalcemic primary hyperparathyroidism. Am J Med. 1969;47(3):384–391. doi: 10.1016/0002-9343(69)90222-8 [DOI] [PubMed] [Google Scholar]
- 10. Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007;92(8):3001–3005. doi: 10.1210/jc.2006-2802 [DOI] [PubMed] [Google Scholar]
- 11. Cusano NE, Silverberg SJ, Bilezikian JP. Normocalcemic primary hyperparathyroidism. J Clin Densitom. 2013;16(1):33–39. doi: 10.1016/j.jocd.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cusano NE, Cipriani C, Bilezikian JP. Management of normocalcemic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(6):837–845. doi: 10.1016/j.beem.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 13. Zavatta G, Clarke BL. Normocalcemic hyperparathyroidism: a heterogeneous disorder often misdiagnosed? JBMR Plus. 2020;4(8):e10391. doi: 10.1002/jbm4.10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawood NB, Yan KL, Shieh A, Livhits MJ, Yeh MW, Leung AM. Normocalcaemic primary hyperparathyroidism: an update on diagnostic and management challenges. Clin Endocrinol. 2020;93(5):519–527. doi: 10.1111/cen.14315 [DOI] [PubMed] [Google Scholar]
- 15. Cusano NE. Evaluation and management of elevated parathyroid hormone levels in normocalcemic patients. Med Clin North Am. 2021;105(6):1135–1150. doi: 10.1016/j.mcna.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 16. Bollerslev J, Rejnmark L, Zahn A, et al. European expert consensus on practical management of specific aspects of parathyroid disorders in adults and in pregnancy: recommendations of the ESE educational program of parathyroid disorders (PARAT 2021). Eur J Endocrinol. 2022;186(2):R33–R63. doi: 10.1530/eje-21-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88(11):5348–5352. doi: 10.1210/jc.2003-031014 [DOI] [PubMed] [Google Scholar]
- 18. Wade TJ, Yen TWF, Amin AL, Wang TS. Surgical management of normocalcemic primary hyperparathyroidism. World J Surg. 2012;36(4):761–766. doi: 10.1007/s00268-012-1438-y [DOI] [PubMed] [Google Scholar]
- 19. Yeh MW, Zhou H, Kuo EJ, Adams AL, Li N, Haigh PI. Biochemical dynamics of untreated primary hyperparathyroidism: an observational study. Endocr Pract. 2019;25(5):470–476. doi: 10.4158/ep-2018-0489 [DOI] [PubMed] [Google Scholar]
- 20. Farquharson RF, Dorothy M, Tibbetts DM. Studies of calcium and phosphorus metabolism: XVIII. On temporary fluctuations in the level of calcium and inorganic phosphorus in blood serum of normal individuals. J Clin Invest. 1930;10(2):271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ledger GA, Burritt MF, Kao PC, O’Fallon WM, Riggs BL, Khosla S. Abnormalities of parathyroid hormone secretion in elderly women that are reversible by short term therapy with 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1994;79(1):211–216. doi: 10.1210/jcem.79.1.8027229 [DOI] [PubMed] [Google Scholar]
- 22. Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88(10):4641–4648. doi: 10.1210/jc.2002-021404 [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Meng L, Su C, Shapses SA. Low free (but not total) 25-hydroxyvitamin D levels in subjects with normocalcemic hyperparathyroidism. Endocr Pract. 2020;26(2):174–178. doi: 10.4158/ep-2019-0325 [DOI] [PubMed] [Google Scholar]
- 24. Díaz-Soto G, Romero E, Castrillón J, Jauregui O, de Luis Román D. Clinical expression of calcium sensing receptor polymorphism (A986S) in normocalcemic and asymptomatic hyperparathyroidism. Horm Metab Res. 2015;48(3):163–168. doi: 10.1055/s-0035-1559723 [DOI] [PubMed] [Google Scholar]
- 25. Coe FL, Canterbury JM, Firpo JJ, Reiss E. Evidence for secondary hyperparathyroidism in idiopathic hypercalciuria. J Clin Invest. 1973;52(1):134–142. doi: 10.1172/jci107156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28(2):120–132. doi: 10.1016/j.semnephrol.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisner BH, Ahn J, Stoller ML. Differentiating primary from secondary hyperparathyroidism in stone patients: the “thiazide challenge.” J Endourol. 2009;23(2):191–192. doi: 10.1089/end.2008.0567 [DOI] [PubMed] [Google Scholar]
- 28. Griebeler ML, Kearns AE, Ryu E, et al. Thiazide-associated hypercalcemia: incidence and association with primary hyperparathyroidism over two decades. J Clin Endocrinol Metab. 2016;101(3):1166–1173. doi: 10.1210/jc.2015-3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17(1):142. doi: 10.1186/s12916-019-1380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Selby PL, Davies M, Adams JE, Mawer EB. Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res. 1999;14(4):652–657. doi: 10.1359/jbmr.1999.14.4.652 [DOI] [PubMed] [Google Scholar]
- 31. Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin A deficiency: how effective are the serological methods of diagnosis? Clin Vaccine Immunol. 2002;9(6):1295–1300. doi: 10.1128/cdli.9.6.1295-1300.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schuppan D, Mäki M, Lundin KEA, et al. A randomized trial of a transglutaminase 2 inhibitor for celiac disease. N Engl J Med. 2021;385(1):35–45. doi: 10.1056/nejmoa2032441 [DOI] [PubMed] [Google Scholar]
- 33. Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–373. doi: 10.1172/jci111971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei JH, Lee WJ, Chong K, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg. 2017;28(3):798–804. doi: 10.1007/s11695-017-2932-y [DOI] [PubMed] [Google Scholar]
- 35. Ministrini S, Ricci MA, Daviddi G, et al. Determinants of high parathyroid hormone levels in patients with severe obesity and their relationship with the cardiometabolic risk factors, before and after a laparoscopic sleeve gastrectomy intervention. Obes Surg. 2020;30(6):2225–2232. doi: 10.1007/s11695-020-04453-z [DOI] [PubMed] [Google Scholar]
- 36. Grethen E, McClintock R, Gupta CE, et al. Vitamin D and hyperparathyroidism in obesity. J Clin Endocrinol Metab. 2011;96(5):1320–1326. doi: 10.1210/jc.2010-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carlin AM, Rao DS, Yager KM, Genaw JA, Parikh NJ, Szymanski W. Effect of gastric bypass surgery on vitamin D nutritional status. Surg Obes Relat Dis. 2006;2(6):638–642. doi: 10.1016/j.soard.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 38. Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30(8):1377–1385. doi: 10.1002/jbmr.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crawford MR, Pham N, Khan L, Bena JF, Schauer PR, Kashyap SR. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr Pract. 2018;24(3):256–264. doi: 10.4158/ep-2017-0072 [DOI] [PubMed] [Google Scholar]
- 40. Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg. 2009;19(9):1256–1261. doi: 10.1007/s11695-009-9850-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ring Madsen L, Espersen R, Rejnmark L, Richelsen B. Effect of calcium citrate vs calcium carbonate on elevated parathyroid hormone after Roux-en-Y gastric bypass. A double-blinded, randomized trial. Clin Endocrinol. 2018;89(6):734–741. doi: 10.1111/cen.13836 [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Lubitz CC, Shikora SA, et al. Primary hyperparathyroidism after Roux-en-Y gastric bypass. Obes Surg. 2014;25(4):700–704. doi: 10.1007/s11695-014-1444-2 [DOI] [PubMed] [Google Scholar]
- 43. Keskin M, Öztürk D, Or Koca A, Ertuğrul DT, Bulus H. Does bariatric surgery increase the formation of parathyroid adenoma? Bariatr Surg Pract Patient Care. 2020;15(3):155–159. doi: 10.1089/bari.2019.0043 [DOI] [Google Scholar]
- 44. Haden ST, Stoll AL, McCormick S, Scott J, El-Hajj Fuleihan G. Alterations in parathyroid dynamics in lithium-treated subjects. J Clin Endocrinol Metab. 1997;82(9):2844–2848. doi: 10.1210/jcem.82.9.4218 [DOI] [PubMed] [Google Scholar]
- 45. Albert U, De Cori D, Aguglia A, et al. Effects of maintenance lithium treatment on serum parathyroid hormone and calcium levels: a retrospective longitudinal naturalistic study. Neuropsychiatr Dis Treat. 2015;11:1785–1791. doi: 10.2147/ndt.s86103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Corapi KM, McMahon GM, Wenger JB, Seifter JL, Bhan I. Association of loop diuretic use with higher parathyroid hormone levels in patients with normal renal function. JAMA Intern Med. 2015;175(1):137. doi: 10.1001/jamainternmed.2014.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Effects of long-term treatment with loop diuretics on bone mineral density, calcitropic hormones and bone turnover. J Intern Med. 2005;257(2):176–184. doi: 10.1111/j.1365-2796.2004.01434.x [DOI] [PubMed] [Google Scholar]
- 48. Pickleman JR, Straus FH 2nd, Forland M, Paloyan E. Thiazide-induced parathyroid. Metab Clin Exp. 1969;18(10):867–873. doi: 10.1016/0026-0495(69)90062-6 [DOI] [PubMed] [Google Scholar]
- 49. Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Effects of thiazide- and loop-diuretics, alone or in combination, on calcitropic hormones and biochemical bone markers: a randomized controlled study. J Intern Med. 2001;250(2):144–153. doi: 10.1046/j.1365-2796.2001.00868.x [DOI] [PubMed] [Google Scholar]
- 50. Zaheer S, de Boer I, Allison M, et al. Parathyroid hormone and the use of diuretics and calcium-channel blockers: the multi-ethnic study of atherosclerosis. J Bone Miner Res. 2016;31(6):1137–1145. doi: 10.1002/jbmr.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Den Berg CJ, Tucker RM, Dousa TP. Idiopathic hypercalciuria: hydrochlorothiazide decreases urinary calcium without altered renal response to parathyroid hormone. J Clin Endocrinol Metab. 1982;55(1):23–26. doi: 10.1210/jcem-55-1-23 [DOI] [PubMed] [Google Scholar]
- 52. Hvarfner A, Bergström R, Lithell H, Mörlin C, Wide L, Ljunghall S. Changes in calcium metabolic indices during long-term treatment of patients with essential hypertension. Clin Sci. 1988;75(5):543–549. doi: 10.1042/cs0750543 [DOI] [PubMed] [Google Scholar]
- 53. Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics alter the diurnal rhythm of endogenous parathyroid hormone secretion. A randomized-controlled study on the effects of loop- and thiazide-diuretics on the diurnal rhythms of calcitropic hormones and biochemical bone markers in postmenopausal. Eur J Clin Invest. 2001;31(9):764–772. doi: 10.1046/j.1365-2362.2001.00883.x [DOI] [PubMed] [Google Scholar]
- 54. Verrotti A, Coppola G, Parisi P, Mohn A, Chiarelli F. Bone and calcium metabolism and antiepileptic drugs. Clin Neurol Neurosurg. 2010;112(1):1–10. doi: 10.1016/j.clineuro.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 55. Vinke JSJ, Heerspink HJL, de Borst MH. Effects of sodium glucose cotransporter 2 inhibitors on mineral metabolism in type 2 diabetes mellitus. Curr Opin Nephrol Hypertens. 2019;28(4):321–327. doi: 10.1097/mnh.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ye Y, Zhao C, Liang J, Yang Y, Yu M, Qu X. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2019;9:1517. doi: 10.3389/fphar.2018.01517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Jong MA, Petrykiv SI, Laverman GD, et al. Effects of dapagliflozin on circulating markers of phosphate homeostasis. Clin J Am Soc Nephrol. 2018;14(1):66–73. doi: 10.2215/cjn.04530418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blau JE, Bauman V, Conway EM, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3(8):e99123. doi: 10.1172/jci.insight.99123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sivakumar J. Proton pump inhibitor-induced hypomagnesaemia and hypocalcaemia: case review. Int J Physiol Pathophysiol Pharmacol. 2016;8(4):169–174. [PMC free article] [PubMed] [Google Scholar]
- 60. Hinson AM, Wilkerson BM, Rothman-Fitts I, Riggs AT, Stack BC Jr, Bodenner DL. Hyperparathyroidism associated with long-term proton pump inhibitors independent of concurrent bisphosphonate therapy in elderly adults. J Am Geriatr Soc. 2015;63(10):2070–2073. doi: 10.1111/jgs.13661 [DOI] [PubMed] [Google Scholar]
- 61. Vasikaran SD. Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem. 2001;38(6):608–623. doi: 10.1258/0004563011901037 [DOI] [PubMed] [Google Scholar]
- 62. Makras P, Polyzos SA, Papatheodorou A, Kokkoris P, Chatzifotiadis D, Anastasilakis AD. Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin Endocrinol. 2013;79(4):499–503. doi: 10.1111/cen.12188 [DOI] [PubMed] [Google Scholar]
- 63. Emin T, Sevnaz Ş, Sumru S, Saraç ZF, Akçiçek SF. A case of denosumab-associated hyperparathyroidism: a differential diagnostic challenge. Int J Immunother Cancer Res. 2021;7(1):024–025. doi: 10.17352/2455-8591.000033 [DOI] [Google Scholar]
- 64. McClung MR, Brown JP, Diez-Perez A, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33(8):1397–1406. doi: 10.1002/jbmr.3452 [DOI] [PubMed] [Google Scholar]
- 65. Kobayakawa T, Suzuki T, Nakano M, et al. Real-world effects and adverse events of romosozumab in Japanese osteoporotic patients: a prospective cohort study. Bone Rep. 2021;14:101068. doi: 10.1016/j.bonr.2021.101068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. doi: 10.1056/nejmoa1305224 [DOI] [PubMed] [Google Scholar]
- 67. Mingione A, Maruca K, Chiappori F, et al. High parathyroid hormone concentration in tenofovir-treated patients are due to inhibition of calcium-sensing receptor activity. Biomed Pharmacother. 2018;97:969–974. doi: 10.1016/j.biopha.2017.11.037 [DOI] [PubMed] [Google Scholar]
- 68. Burckhardt PM, Singer FR, Potts JT Jr. Parathyroid function in patients with Paget's disease treated with salmon calcitonin. Clin Endocrinol. 1973;2(1):15–22. doi: 10.1111/j.1365-2265.1973.tb03480.x [DOI] [PubMed] [Google Scholar]
- 69. Prince RL, Schiff I, Neer RM. Effects of transdermal estrogen replacement on parathyroid hormone secretion. J Clin Endocrinol Metab. 1990;71(5):1284–1287. doi: 10.1210/jcem-71-5-1284 [DOI] [PubMed] [Google Scholar]
- 70. Frazier R, Hodakowski A, Cai X, et al. Effects of ferric carboxymaltose on markers of mineral and bone metabolism: a single-center prospective observational study of women with iron deficiency. Bone. 2020;141:115559. doi: 10.1016/j.bone.2020.11555910.1155/2019/5496710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kritmetapak K, Pongchaiyakul C. Parathyroid hormone measurement in chronic kidney disease: from basics to clinical implications. Int J Nephrol. 2019;2019:1–9. doi: 10.1155/2019/5496710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Isakova T, Nickolas TL, Denburg M, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751. doi: 10.1053/j.ajkd.2017.07.01910.1371/journal.pone.0226509 [DOI] [PubMed] [Google Scholar]
- 73. Willey JZ, Moon YP, Husain SA, et al. Creatinine versus cystatin C for renal function-based mortality prediction in an elderly cohort: the Northern Manhattan Study. PLoS One. 2020;15(1):e0226509. doi: 10.1371/journal.pone.0226509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013;33(9):912–921. doi: 10.1002/phar.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd international conference on controversies in vitamin D. Rev Endocr Metab Disord. 2020;21(1):89–116. doi: 10.1007/s11154-019-09532-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90(4):2122–2126. doi: 10.1210/jc.2004-1772 [DOI] [PubMed] [Google Scholar]
- 77. Shokry G, Morkos M. Calcium challenge to confirm secondary hyperparathyroidism caused by decreased calcium intake. Endocr Pract. 2022;28(10):1069–1071. doi: 10.1016/j.eprac.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 78. Tohme JF, Bilezikian JP, Clemens TL, Silverberg SJ, Shane E, Lindsay R. Suppression of parathyroid hormone secretion with oral calcium in normal subjects and patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1990;70(4):951–956. doi: 10.1210/jcem-70-4-951 [DOI] [PubMed] [Google Scholar]
- 79. Insogna KL, Mitnick ME, Stewart AF, Burtis WJ, Mallette LE, Broadus AE. Sensitivity of the parathyroid hormone–1, 25-dihydroxyvitamin D axis to variations in calcium intake in patients with primary hyperparathyroidism. N Engl J Med. 1985;313(18):1126–1130. doi: 10.1056/nejm198510313131805 [DOI] [PubMed] [Google Scholar]
- 80. Lecoq A, Chaumet-Riffaud P, Blanchard A, et al. Hyperparathyroidism in patients with X-linked hypophosphatemia. J Bone Miner Res. 2020;35(7):1263–1273. doi: 10.1002/jbmr.3992 [DOI] [PubMed] [Google Scholar]
- 81. Kotwal A, Ferrer A, Kumar R, et al. Clinical and biochemical phenotypes in a family with ENPP1 mutations. J Bone Miner Res. 2020;35(4):662–670. doi: 10.1002/jbmr.3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cavalier E, Carlisi A, Chapelle JP, Delanaye P. False positive PTH results: an easy strategy to test and detect analytical interferences in routine practice. Clinica Chimica Acta. 2008;387(1-2):150–152. doi: 10.1016/j.cca.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 83. Zanchetta MB, Giacoia E, Jerkovich F, Fradinger E. Asymptomatic elevated parathyroid hormone level due to immunoassay interference. Osteoporos Int. 2021;32(10):2111–2114. doi: 10.1007/s00198-021-05950-2 [DOI] [PubMed] [Google Scholar]
- 84. Levin O, Morris LF, Wah DT, Butch AW, Yeh MW. Falsely elevated plasma parathyroid hormone level mimicking tertiary hyperparathyroidism. Endocr Pract. 2011;17(2):e8–e11. doi: 10.4158/ep10235.cr [DOI] [PubMed] [Google Scholar]
- 85. Kalaria T, Fenn J, Sanders A, et al. The diagnosis of normocalcaemic hyperparathyroidism is strikingly dissimilar using different commercial laboratory assays. Horm Metab Res. 2022;54(7):429–434. doi: 10.1055/a-1856-4900 [DOI] [PubMed] [Google Scholar]
- 86. Lundgren E, Rastad J, Thurfjell E, Åkerström G, Ljunghall S. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121(3):287–294. doi: 10.1016/s0039-6060(97)90357-3 [DOI] [PubMed] [Google Scholar]
- 87. Marques TF, Vasconcelos R, Diniz E, Rêgo D, Griz L, Bandeira F. Normocalcemic primary hyperparathyroidism in clinical practice: an indolent condition or a silent threat? Arq Bras Endocrinol Metab. 2011;55(5):314–317. doi: 10.1590/s0004-27302011000500003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. García-Martín A, Reyes-García R, Muñoz-Torres M. Normocalcemic primary hyperparathyroidism: one-year follow-up in one hundred postmenopausal women. Endocrine. 2012;42(3):764–766. doi: 10.1007/s12020-012-9694-z [DOI] [PubMed] [Google Scholar]
- 89. Cusano NE, Maalouf NM, Wang PY, et al. Normocalcemic hyperparathyroidism and hypoparathyroidism in two community-based nonreferral populations. J Clin Endocrinol Metab. 2013;98(7):2734–2741. doi: 10.1210/jc.2013-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kontogeorgos G, Trimpou P, Laine CM, Oleröd G, Lindahl A, Landin-Wilhelmsen K. Normocalcaemic, vitamin D-sufficient hyperparathyroidism—high prevalence and low morbidity in the general population: a long-term follow-up study, the WHO MONICA project, Gothenburg, Sweden. Clin Endocrinol. 2015;83(2):277–284. doi: 10.1111/cen.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Berger C, Almohareb O, Langsetmo L, et al. Characteristics of hyperparathyroid states in the Canadian Multicentre Osteoporosis Study (CaMos) and relationship to skeletal markers. Clin Endocrinol. 2014;82(3):359–368. doi: 10.1111/cen.12569 [DOI] [PubMed] [Google Scholar]
- 92. Vignali E, Cetani F, Chiavistelli S, et al. Normocalcemic primary hyperparathyroidism: a survey in a small village of southern Italy. Endocr Connect. 2015;4(3):172–178. doi: 10.1530/ec-15-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Palermo A, Jacques R, Gossiel F, et al. Normocalcaemic hypoparathyroidism: prevalence and effect on bone status in older women. The OPUS Study. Clin Endocrinol. 2015;82(6):816–823. doi: 10.1111/cen.12732 [DOI] [PubMed] [Google Scholar]
- 94. Rosário P, Calsolari M. Normocalcemic primary hyperparathyroidism in adults without a history of nephrolithiasis or fractures: a prospective study. Horm Metab Res. 2019;51(4):243–247. doi: 10.1055/a-0859-1020 [DOI] [PubMed] [Google Scholar]
- 95. Schini M, Jacques RM, Oakes E, Peel NFA, Walsh JS, Eastell R. Normocalcemic hyperparathyroidism: study of its prevalence and natural history. J Clin Endocrinol Metab. 2020;105(4):e1171–e1186. doi: 10.1210/clinem/dgaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tordjman KM, Greenman Y, Osher E, Shenkerman G, Stern N. Characterization of normocalcemic primary hyperparathyroidism. Am J Med. 2004;117(11):861–863. doi: 10.1016/j.amjmed.2004.06.037 [DOI] [PubMed] [Google Scholar]
- 97. Amaral LM, Queiroz DC, Marques TF, Mendes M, Bandeira F. Normocalcemic versus hypercalcemic primary hyperparathyroidism: more stone than bone? J Osteoporos. 2012;2012:1–4. doi: 10.1155/2012/128352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cakir I, Unluhizarci K, Tanriverdi F, Elbuken G, Karaca Z, Kelestimur F. Investigation of insulin resistance in patients with normocalcemic primary hyperparathyroidism. Endocrine. 2012;42(2):419–422. doi: 10.1007/s12020-012-9627-x [DOI] [PubMed] [Google Scholar]
- 99. Šiprová H, Fryšák Z, Souček M. Primary hyperparathyroidism, with a focus on management of the normocalcemic form: to treat or not to treat? Endocr Pract. 2016;22(3):294–301. doi: 10.4158/ep15704.or [DOI] [PubMed] [Google Scholar]
- 100. Tuna MM, Çalışkan M, Ünal M, et al. Normocalcemic hyperparathyroidism is associated with complications similar to those of hypercalcemic hyperparathyroidism. J Bone Miner Metab. 2015;34(3):331–335. doi: 10.1007/s00774-015-0673-3 [DOI] [PubMed] [Google Scholar]
- 101. Pierreux J, Bravenboer B, Velkeniers B, Unuane D, Andreescu C, Vanhoeij M. Correction: normocalcemic primary hyperparathyroidism: a comparison with the hypercalcemic form in a tertiary referral population. Horm Metab Res. 2018;50(11):e7. doi: 10.1055/a-0799-5387 [DOI] [PubMed] [Google Scholar]
- 102. Palermo A, Naciu AM, Tabacco G, et al. Clinical, biochemical, and radiological profile of normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab. 2020;105(7):e2609–e2616. doi: 10.1210/clinem/dgaa174 [DOI] [PubMed] [Google Scholar]
- 103. Charopoulos I, Tournis S, Trovas G, et al. Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab. 2006;91(5):1748–1753. doi: 10.1210/jc.2005-2102 [DOI] [PubMed] [Google Scholar]
- 104. Pretorius M, Lundstam K, Heck A, et al. Mortality and morbidity in mild primary hyperparathyroidism: results from a 10-year prospective randomized controlled trial of parathyroidectomy versus observation. Ann Intern Med. 2022;175(6):812–819. doi: 10.7326/m21-4416 [DOI] [PubMed] [Google Scholar]
- 105. Voss L, Nóbrega M, Bandeira L, Griz L, Rocha-Filho PAS, Bandeira F. Impaired physical function and evaluation of quality of life in normocalcemic and hypercalcemic primary hyperparathyroidism. Bone. 2020;141:115583. doi: 10.1016/j.bone.2020.115583 [DOI] [PubMed] [Google Scholar]
- 106. Tordjman KM, Yaron M, Izkhakov E, et al. Cardiovascular risk factors and arterial rigidity are similar in asymptomatic normocalcemic and hypercalcemic primary hyperparathyroidism. Eur J Endocrinol. 2010;162(5):925–933. doi: 10.1530/eje-09-1067 [DOI] [PubMed] [Google Scholar]
- 107. Mesquita PN, Dornelas Leão Leite AP, Chagas Crisóstomo SD, Veras Filho E, da Cunha Xavier L, Bandeira F. Evaluation of coronary calcium score in patients with normocalcemic primary hyperparathyroidism. Vasc Health Risk Manag. 2017;13:225–229. doi: 10.2147/vhrm.s128084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Koubaity O, Mandry D, Nguyen-Thi PL, et al. Coronary artery disease is more severe in patients with primary hyperparathyroidism. Surgery. 2020;167(1):149–154. doi: 10.1016/j.surg.2019.05.094 [DOI] [PubMed] [Google Scholar]
- 109. Diri H, Unluhizarci K, Kelestimur F. Investigation of glucose intolerance in patients with normocalcemic primary hyperparathyroidism: 4-year follow-up. Endocrine. 2014;47(3):971–972. doi: 10.1007/s12020-014-0263-5 [DOI] [PubMed] [Google Scholar]
- 110. Temizkan S, Kocak O, Aydin K, et al. Normocalcemic hyperparathyroidism and insulin resistance. Endocr Pract. 2015;21(1):23–29. doi: 10.4158/ep14195.or [DOI] [PubMed] [Google Scholar]
- 111. Tassone F, Maccario M, Gianotti L, et al. Insulin sensitivity in normocalcaemic primary hyperparathyroidism. Endocrine. 2013;44(3):812–814. doi: 10.1007/s12020-013-0059-z [DOI] [PubMed] [Google Scholar]
- 112. Hagström E, Lundgren E, Rastad J, Hellman P. Metabolic abnormalities in patients with normocalcemic hyperparathyroidism detected at a population-based screening. Eur J Endocrinol. 2006;155(1):33–39. doi: 10.1530/eje.1.02173 [DOI] [PubMed] [Google Scholar]
- 113. Yener Ozturk F, Erol S, Canat MM, et al. Patients with normocalcemic primary hyperparathyroidism may have similar metabolic profile as hypercalcemic patients. Endocr J. 2016;63(2):111–118. doi: 10.1507/endocrj.ej15-0392 [DOI] [PubMed] [Google Scholar]
- 114. Chen G, Xue Y, Zhang Q, et al. Is normocalcemic primary hyperparathyroidism harmful or harmless? J Clin Endocrinol Metab. 2015;100(6):2420–2424. doi: 10.1210/jc.2014-4432 [DOI] [PubMed] [Google Scholar]
- 115. Karras SN, Koufakis T, Tsekmekidou X, Antonopoulou V, Zebekakis P, Kotsa K. Increased parathyroid hormone is associated with higher fasting glucose in individuals with normocalcemic primary hyperparathyroidism and prediabetes: a pilot study. Diabetes Res Clin Pract. 2020;160:107985. doi: 10.1016/j.diabres.2019.107985 [DOI] [PubMed] [Google Scholar]
- 116. Karras S, Annweiler C, Kiortsis D, Koutelidakis I, Kotsa K. Improving glucose homeostasis after parathyroidectomy for normocalcemic primary hyperparathyroidism with co-existing prediabetes. Nutrients. 2020;12(11):3522. doi: 10.3390/nu12113522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sho S, Kuo EJ, Chen AC, Li N, Yeh MW, Livhits MJ. Biochemical and skeletal outcomes of parathyroidectomy for normocalcemic (incipient) primary hyperparathyroidism. Ann Surg Oncol. 2018;26(2):539–546. doi: 10.1245/s10434-018-6998-0 [DOI] [PubMed] [Google Scholar]
- 118. Perrier N, Lang BH, Farias LCB, et al. Surgical aspects of primary hyperparathyroidism. J Bone Mineral Res. 2022;37(11):2373–2390. doi: 10.1002/jbmr.4689 [DOI] [PubMed] [Google Scholar]
- 119. Cunha-Bezerra P, Vieira R, Amaral F, et al. Better performance of four-dimension computed tomography as a localization procedure in normocalcemic primary hyperparathyroidism. J Med Imaging Radiat Oncol. 2018;62(4):493–498. doi: 10.1111/1754-9485.12728 [DOI] [PubMed] [Google Scholar]
- 120. Musumeci M, Pereira LV, San Miguel L, et al. Normocalcemic primary hyperparathyroidism: 99mTc SestaMibi SPECT/CT results compare with hypercalcemic hyperparathyroidism. Clin Endocrinol (Oxf). 2022;96(6):831–836. doi: 10.1111/cen.14667 [DOI] [PubMed] [Google Scholar]
- 121. Kiriakopoulos A, Petralias A, Linos D. Classic primary hyperparathyroidism versus normocalcemic and normohormonal variants: do they really differ? World J Surg. 2018;42(4):992–997. doi: 10.1007/s00268-018-4512-2 [DOI] [PubMed] [Google Scholar]
- 122. Lim JY, Herman MC, Bubis L, et al. Differences in single gland and multigland disease are seen in low biochemical profile primary hyperparathyroidism. Surgery. 2017;161(1):70–77. doi: 10.1016/j.surg.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 123. Pandian TK, Lubitz CC, Bird SH, Kuo LE, Stephen AE. Normocalcemic hyperparathyroidism: a collaborative endocrine surgery quality improvement program analysis. Surgery. 2020;167(1):168–172. doi: 10.1016/j.surg.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 124. Caldwell M, Laux J, Clark M, Kim L, Rubin J. Persistently elevated PTH after parathyroidectomy at one year: experience in a tertiary referral center. J Clin Endocrinol Metab. 2019;104(10):4473–4480. doi: 10.1210/jc.2019-00705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. de la Plaza Llamas R, Ramia Ángel JM, Arteaga Peralta V, et al. Elevated parathyroid hormone levels after successful parathyroidectomy for primary hyperparathyroidism: a clinical review. Eur Arch Otorhinolaryngol. 2017;275(3):659–669. doi: 10.1007/s00405-017-4836-9 [DOI] [PubMed] [Google Scholar]
- 126. Ryder CY, Jarocki A, McNeely MM, et al. Early biochemical response to parathyroidectomy for primary hyperparathyroidism and its predictive value for recurrent hypercalcemia and recurrent primary hyperparathyroidism. Surgery. 2021;169(1):120–125. doi: 10.1016/j.surg.2020.05.049 [DOI] [PubMed] [Google Scholar]
- 127. Oltmann SC, Maalouf NM, Holt S. Significance of elevated parathyroid hormone after parathyroidectomy for primary hyperparathyroidism. Endocr Pract. 2011;17 (Supplement 1):57–62. doi: 10.4158/ep10324.ra [DOI] [PubMed] [Google Scholar]
- 128. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341(17):1249–1255. doi: 10.1056/nejm199910213411701 [DOI] [PubMed] [Google Scholar]
- 129. Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocr Metab. 2008;93(9):3462–3470. doi: 10.1210/jc.2007-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rao DS, Phillips ER, Divine GW, Talpos GB. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2004;89(11):5415–5422. doi: 10.1210/jc.2004-0028 [DOI] [PubMed] [Google Scholar]
- 131. Ambrogini E, Cetani F, Cianferotti L, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective randomized clinical trial. J Clin Endocrinol Metab. 2007;92(8):3114–3121. doi.org/10.1210/jc.2007-0219 [DOI] [PubMed] [Google Scholar]
- 132. Bollerslev J, Jansson S, Mollerup CL, et al. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J Clin Endocrinol Metab. 2007;92(5):1687–1692. doi.org/10.1210/jc.2006-1836 [DOI] [PubMed] [Google Scholar]
- 133. Lundstam K, Heck A, Godang K, et al. Effect of surgery versus observation: skeletal 5-year outcomes in a randomized trial of patients with primary HPT (the SIPH study). J Bone Miner Res. 2017;32(9):1907–1914. doi: 10.1002/jbmr.3177 [DOI] [PubMed] [Google Scholar]
- 134. Lundstam K, Heck A, Mollerup C, et al. Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100(4):1359–1367. doi: 10.1210/jc.2014-3441 [DOI] [PubMed] [Google Scholar]
- 135. VanderWalde LH. The effect of parathyroidectomy on bone fracture risk in patients with primary hyperparathyroidism. Arch Surg. 2006;141(9):885. doi: 10.1001/archsurg.141.9.885 [DOI] [PubMed] [Google Scholar]
- 136. Vestergaard P. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321(7261):598–602. doi: 10.1136/bmj.321.7261.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nilsson M, Ståhl E, Åkesson KE, et al. Reduced fracture incidence in patients having surgery for primary hyperparathyroidism. Clin Endocrinol (Oxf). 2022;97(3):276–283. doi: 10.1111/cen.14703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Koumakis E, Souberbielle JC, Sarfati E, et al. Bone mineral density evolution after successful parathyroidectomy in patients with normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab. 2013;98(8):3213–3220. doi: 10.1210/jc.2013-1518 [DOI] [PubMed] [Google Scholar]
- 139. Beysel S, Caliskan M, Kizilgul M, et al. Parathyroidectomy improves cardiovascular risk factors in normocalcemic and hypercalcemic primary hyperparathyroidism. BMC Cardiovasc Disord. 2019;19(1):106. doi: 10.1186/s12872-019-1093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bannani S, Christou N, Guérin C, et al. Effect of parathyroidectomy on quality of life and non-specific symptoms in normocalcaemic primary hyperparathyroidism. Br J Surg. 2018;105(3):223–229. doi: 10.1002/bjs.10739 [DOI] [PubMed] [Google Scholar]
- 141. Khan AA, Bilezikian JP, Kung AWC, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(7):3319–3325. doi: 10.1210/jc.2003-030908 [DOI] [PubMed] [Google Scholar]
- 142. Khan AA, Bilezikian JP, Kung A, Dubois SJ, Standish TI, Syed ZA. Alendronate therapy in men with primary hyperparathyroidism. Endocr Pract. 2009;15(7):705–713. doi: 10.4158/ep08178.orr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rossini M, Gatti D, Isaia G, Sartori L, Braga V, Adami S. Effects of oral alendronate in elderly patients with osteoporosis and mild primary hyperparathyroidism. J Bone Miner Res. 2001;16 (1):113–119. doi.org/10.1359/jbmr.2001.16.1.113 [DOI] [PubMed] [Google Scholar]
- 144. Chow CC, Chan WB, Li JKY, et al. Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003;88(2):581–587. doi: 10.1210/jc.2002-020890 [DOI] [PubMed] [Google Scholar]
- 145. Cesareo R, Di Stasio E, Vescini F, et al. Effects of alendronate and vitamin D in patients with normocalcemic primary hyperparathyroidism. Osteoporos Int. 2014;26(4):1295–1302. doi: 10.1007/s00198-014-3000-2 [DOI] [PubMed] [Google Scholar]
- 146. Brardi S, Cevenini G, Verdacchi T, Romano G, Ponchietti R. Use of cinacalcet in nephrolithiasis associated with normocalcemic or hypercalcemic primary hyperparathyroidism: results of a prospective randomized pilot study. Arch Ital Urol Androl. 2015;87(1):66. doi: 10.4081/aiua.2015.1.66 [DOI] [PubMed] [Google Scholar]
- 147. Madeo B, De Vincentis S, Repaci A, et al. The calcium-to-phosphorous (Ca/P) ratio in the diagnosis of primary hyperparathyroidism and hypoparathyroidism: a multicentric study. Endocrine. 2020;68(3):679–687. doi: 10.1007/s12020-020-02276-7 [DOI] [PubMed] [Google Scholar]
- 148. Guo Y, Wang Q, Lu C, et al. New parathyroid function index for the differentiation of primary and secondary hyperparathyroidism: a case-control study. BMC Endocr Disord. 2020;20(1):5. doi: 10.1186/s12902-019-0487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.