Abstract
The objective of this study was to compare the meat quality characteristics and collagen-related gene expression levels in the broiler pectoralis major (PM) muscle among the meat quality groups, including normal, pale, soft, and exudative (PSE), and white striping (WS) groups. The group was classified by their WS degree (moderate or severe striping) and quality traits including pH and lightness values at 24 h postmortem (normal group: pH24 h ≥5.7, 48≤L*≤53, without WS features; PSE group: pH24 h <5.7, L* >53, without WS features; WS group: pH24 h ≥5.7, 48≤L*≤53, with moderate or severe striping). The WS group revealed no differences in all measured meat quality traits compared to the normal group (P > 0.05). PM muscles exhibiting PSE conditions without WS indicated lower pH15 min and pH24 h values (P < 0.05). Whereas, lower lightness and cooking loss values were observed in the normal and WS groups compared to the PSE group (P < 0.05). No significant difference was observed in the level of type I collagen among the groups (P > 0.05), whereas a higher type III collagen level was observed in the WS group than in the other groups (P < 0.05). Additionally, the WS group showed a higher type IV collagen level compared to the normal group (P < 0.05) and a level not different from that of the PSE group (P > 0.05). In contrast, the expression levels of matrix metalloproteinase (MMP) 2, involved in type IV collagen degradation, and angiopoietin-like protein 7, associated with collagen accumulation, were higher in the WS group compared to the normal group (P < 0.05). However, no difference was detected in the MMP1 level among the all groups (P > 0.05). These results suggest that the occurrence of WS features in broiler PM muscle, unlike PSE and normal conditions, can be influenced by the expression levels of collagen-related genes associated with abnormalities in extracellular matrix components.
Key words: white striping, PSE, collagen-gene expression, meat quality, chicken breast
INTRODUCTION
Modern broilers have been rigorously selected for their higher growth rate and muscle mass, which can lead to histological and biochemical modifications of muscle tissues (Livingston et al., 2019). Consequently, broilers are experiencing an increasing incidence of muscular abnormalities, especially in the pectoralis major (PM) muscle, due to being increasingly susceptible to various acute and chronic stressors (Petracci et al., 2019). Critical problems pertaining to stress-induced muscular disorders include pale, soft, and exudative (PSE) conditions and white striping (WS) features (Petracci et al., 2013; Kuttappan et al., 2016). The existence of PSE and WS can potentially affect the consumer preference for the product owing to impaired visual appearance and technical quality, although these abnormalities do not affect the safety of meat as food (Kuttappan et al., 2013). Thus, the poultry industry has been striving to reduce the incidence of muscular abnormalities by identifying their etiology (Bordini et al., 2021). PSE development is primarily attributed to the rapid pH decline and high temperature owing to accelerated glycolysis and higher apoptotic cell death during the postmortem period, and is associated with the expression of genes involved in the calcium signaling and apoptosis pathway (Kuttappan et al., 2016; Kim et al., 2022; Lee and Choi, 2022; Malila et al., 2022; Mudalal and Zaazaa, 2022). Unlike PSE, the precise etiology of WS with the presence of parallel streaks in the same orientation of muscle fibers remains unknown; however, there are several plausible speculations regarding the histological lesions around WS (Kuttappan et al., 2016). Its occurrence can cause degeneration and necrosis of fibers according to chronic stress and the consequent damage to fibers, which can be related to accumulated collagen and fat within the skeletal muscle (Kuttappan et al., 2013).
The structural and mechanical integrity of skeletal muscles can be maintained by the extracellular matrix (ECM), which mainly comprises collagen, especially types I, III, and IV (Stern et al., 2009). Regulation of ECM components plays a critical role that affects various physiological and pathological conditions in tissues (Eckes et al., 2000). Abnormalities in the ECM organization result in histological lesions, such as fibrosis (Ricard-Blum et al., 2018). The ECM remodeling pathway, including the degradation and accumulation of collagens, is linked to the expression of matrix metalloproteinases (MMPs) and angiopoietin-like proteins (ANGPTLs) (Ricard-Blum et al., 2018; Santos et al., 2022). MMPs, as a family of extracellular zinc- and calcium-dependent endoproteinases, are involved in the degradation of selected ECM components, and MMP1 and MMP2 can cleave fibrillary collagen types I and III and nonfibrillar collagen type IV, respectively (Ricard-Blum et al., 2018). In contrast, ANGPTL7, an ANGPTL family member, is a critical regulator of the accumulation and deposition of specific collagen types, particularly types I and IV (Tanigawa et al., 2020; Santos et al., 2022). Thus, the expression levels of these molecules that affect the balance between collagen synthesis and breakdown in the ECM can coordinate the occurrence of fibrotic abnormalities (Picard et al., 2006). Despite fibrosis being a prominent finding in the WS muscle, the association between WS features and collagen-related molecules has been poorly elucidated. Unlike the WS muscle (Kuttappan et al., 2016), there is no direct correlation between fibrotic lesions caused by ECM remodeling and PSE development, but the degradation of ECM by MMP expressions might be associated with the PSE characteristics, especially soft textured phenotype. Therefore, to determine the association between collagen-related molecules and muscular abnormalities, the aim of this study was to compare meat quality and collagen-related gene levels (collagen type I, III, IV, MMP1, MMP2, and ANGPTL7) among the normal, PSE, and WS conditions of broiler PM muscles.
MATERIALS AND METHODS
Animals and Sample Procedure
A total of 84 PM muscles from Ross 308 broilers (male; 4 wk old; average weight, 1,515 ± 127 g) were used in this study. The birds were slaughtered in 5 batches (16–17 birds per batch) according to the standard slaughtering process of the Korea Institute for Animal Products Quality Evaluation (KAPE, 2020). At 15 min postmortem, the left and right PM muscles from all birds were excised and then the muscle pH values (pH15 min) were measured by penetrating the left PM muscle using a pH meter (Testo 206-pH2, Test Inc., Lenzkirch, Germany). Simultaneously, approximately 20 g of muscle from the left samples was obtained and frozen using liquid nitrogen and stored at –80°C for quantitative real-time polymerase chain reaction (qPCR) analysis. The remaining left and entire right fillets were immediately cooled with an ice-water slurry and stored in a refrigerator (4°C) until further analysis. At 24 h postmortem, the remaining left breast tissue was used for meat quality measurements, including muscle pH and color. All right samples were used for classification according to their WS degree (Kuttappan et al., 2013) and analysis of water-holding capacity (WHC), including drip loss and cooking loss.
Meat Quality Characteristics
The muscle pH values (pH15 min and pH24 h) were determined using Testo 206-pH2 (Test Inc., Lenzkirch, Germany), with the probe penetrating the left fillets. At 24 h postmortem, the upper surface of the left PM muscle was used to assess the meat color, including lightness (L*), redness (a*), and yellowness (b*), using a Minolta CR-400 chromameter (diffuse D65; illumination C; viewing angle, 0˚; port/viewing area, 8 mm; Minolta Camera Co., Osaka, Japan) according to the recommendations of the Commission Internationale de l'Eclairage (CIE, 1978). After measuring pH and meat color values, meat quality classes were classified by their WS degree, pH24 h, and L* values, as described previously (Lee et al., 2021; Lee and Choi, 2022). The normal group (N = 45) had a pH24 h value of ≥5.7 and L* value ranging from 48 to 53 without striping, and the PSE group (N = 15) had a pH24 h value of under 5.7 and L* value of ≥53.0 without striping. The WS group (N = 24) exhibited pH24 h and L* values similar to those of the normal group and had moderate or severe striping. However, as only 2 chickens presented both PSE and WS features, these chickens were excluded from the statistical analysis. Moreover, moderate or severe wooden breast myopathy, which is often observed in modern poultry due to rapid growth rate (Kauffman et al., 1986; Bordini et al., 2021), was not detected in this study. For WHC, analysis of drip loss and cooking loss were analyzed as described by Honikel (1998) and Kauffman et al. (1986). Muscle samples (2.5 cm3; approximately 12.0 g) were used for drip loss measurement using a meat extract collector tube (Sarstedt Inc., Newton, NC) and stored for 48 h at 4°C. The drip loss percentage was calculated as the difference in sample weight before and after 48 h, and the average value was obtained from triplicate measurements. To determine cooking loss, fillets were weighed and sealed in a polyethylene bag and then heated in a water bath (80°C) until the core temperature reached 71°C. The cooked samples were re-weighed, and cooking loss was calculated as the difference in sample weight before and after cooking.
Quantitative RT-PCR
Total RNA isolation and complementary DNA synthesis were followed in a previous study (Kim et al., 2021) using muscle samples at 15 min postmortem. The total RNA quantity was determined using an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA), and RNA quality was assessed by gel electrophoresis and normalized accordingly. Complementary DNA was synthesized from 1 ng of total RNA and was used to assess the expression of collagen type 1 alpha 1 chain (COL1A1), COL3A1, COL4A1, MMP1, MMP2, ANGPTL7, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using qPCR. The sequences of the forward and reverse primers for the respective genes were as follows: 5′-ACC CTA AGA CAA AGA GCC CC-3′ and 5′-GTT CGG GTT TCC ACA CAT CC-3′; 5′-AGG CTG AAG GAA ACA GCA AA-3′ and 5′-TGC GCG TTC TGT ATT CAA AG-3′; 5′-GGA CCA TCA GGA TTT CCA GGA-3′ and 5′-GCG GCG GCT TCG ACT TCA G-3′; 5′-TGG AGC AGT GTG ACA CCA TTG AC-3′ and 5′-CAT GAG CCA GCA GCC CAT TAG G-3′; 5′-TCG CAA TGA TGG CAA GCT GTG G-3′ and 5′-GGC GGA AGT TCT TGG TGT AGG TG-3′; 5′-AGC TAC CGT CTG TTT GTG GG-3′ and 5′-TGG AGC AGT GTG ACA CCA TTG AC-3′; 5′-CGT CCT CTC TGG CAA AGT CC-3′ and 5′-AAG ATA GTG ATG GCG TGC CC-3′. qPCR was performed using SYBR green dye (A2574, Applied Biosystems) and an StepOnePlus qPCR instrument (Applied Biosystems). The condition was 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, and 60°C for 1 min. Relative gene expression was calculated using the comparative 2−ΔΔCt method for relative quantification (Livak and Schmittgen, 2001). GAPDH, a housekeeping gene, was used to normalize the qPCR calculation.
Statistical Analysis
Meat quality traits and levels of collagen-related factors in the meat quality classes were analyzed using a general linear mixed model (SAS Institute, Cary, NC). Meat quality classes were treated as fixed effects, and sample batches were treated as random effects in the model. Significant differences in the least-squares means (LSM) for the investigated parameters among the groups were evaluated using the probability difference by setting the significance level at 5%. All data are presented as LSM with standard errors.
RESULTS AND DISCUSSION
The meat quality characteristics of the quality classes are presented in Table 1. The normal and WS groups did not differ in muscle pH, meat color, and WHC (P > 0.05), and were significantly different from that of the PSE group (P < 0.05). Lower muscle pH15 min (6.41 vs. 6.67 and 6.63, P < 0.01) and pH24 h (5.61 vs. 6.03 and 6.01, P < 0.001) values were observed in the PSE group compared to the other groups. Broiler breast muscles with striping exhibited lower lightness (48.8 vs. 56.6, P < 0.001) and yellowness (6.59 vs. 9.01, P < 0.001) values and higher redness values (1.80 vs. 0.65, P < 0.001) compared to muscles showing PSE condition. Regarding the WHC, drip loss was similar among the normal, WS, and PSE groups (P > 0.05), whereas a higher cooking loss was observed in the PSE group than in the other groups (13.6 vs. 11.5 and 10.6, P < 0.001, respectively). PSE and WS features are well-known rapid growth-related abnormalities in poultry (Petracci et al., 2013), and these muscular abnormalities can manifest as myofibrillar protein degeneration caused by exceeding the physiological muscle growth rate and consequent muscle damage (Kuttappan et al., 2013). The development of PSE conditions relies on the structural and metabolic changes within the muscle during postmortem by various stressors before and after slaughter (Kuttappan et al., 2016). In contrast, WS myopathy occurs primarily in heavier chickens with increased breast yield (Petracci et al., 2013), and is thus more associated with hypertrophic muscle growth than PSE abnormalities (Lee and Choi, 2021). Typically, consumer panelists and trained panelists can visually distinguish fresh samples from the PSE and WS conditions compared to samples from the normal condition, thereby avoiding the purchase of compromised meat (Kuttappan et al., 2012; Lee et al., 2021). Marked differences in the meat quality characteristics were exhibited between PSE and normal condition muscles, whereas the occurrence of WS features in chicken breasts was not closely associated with impairing meat quality and cooked texture (Lee and Choi, 2021). In the present study, similar to a previous study (Lee and Choi, 2021), clear differences were observed between the PSE and other quality groups in all measured quality attributes, particularly lightness and muscle pH24 h used as quality classification criteria. The paler surface color and decreased WHC observed in PSE breasts may be associated with increased protein degradation and conversion of oxymyoglobin to metmyoglobin via rapid glycolysis (Carvalho et al., 2014). On the other hand, there were no significant differences in the meat quality characteristics between WS and normal condition breasts classified as reddish, firm, and non-exudative (RFN) (P > 0.05). Thus, these muscular abnormalities exert different effects on meat quality characteristics.
Table 1.
Comparison of meat quality characteristics among the meat quality classes in broiler pectoralis major muscle.
| Meat quality class |
||||
|---|---|---|---|---|
| Normal (N = 45) | PSE (N = 15) | White striping (N = 24) | Significance level | |
| Muscle pH | ||||
| pH15 min | 6.67a (0.04)1 | 6.41b (0.06) | 6.63a (0.05) | ** |
| pH24 h | 6.03a (0.02) | 5.61b (0.04) | 6.01a (0.03) | *** |
| Meat color | ||||
| Lightness (L*) | 49.7b (0.34) | 56.6a (0.59) | 48.8b (0.47) | *** |
| Redness (a*) | 1.38a (0.13) | 0.65b (0.24) | 1.80a (0.18) | *** |
| Yellowness (b*) | 7.13b (0.17) | 9.01a (0.28) | 6.59b (0.22) | *** |
| Water-holding capacity | ||||
| Drip loss (%) | 2.28 (0.20) | 2.75 (0.33) | 2.09 (0.27) | NS |
| Cooking loss (%) | 11.5b (0.34) | 13.6a (0.52) | 10.6b (0.44) | *** |
Abbreviation: PSE, pale, soft, and exudative.
Level of significance: NS, no significant; ** P < 0.01; *** P < 0.001.
Different superscript letters in the same row represent significant differences (P < 0.05).
Standard error of least-square means.
Collagen plays a crucial role in the development and maintenance of skeletal muscle tissue by regulating the interaction between cells and ECM (Ahmad et al., 2020). The proportions of collagen types classified by their supramolecular structure in matrices differ to complement the unique functions and structures of each organ (Kisling et al., 2019). Among the interstitial matrices, the epimysium and perimysium are mainly composed of type I collagen, and the endomysium primarily comprises types I and III (Mahdy, 2019). Type IV collagen is the main collagen in the basement membrane surrounding the muscle fibers (Mahdy, 2019). However, biochemical changes in chronically diseased tissues cause ECM remodeling by greater expression of collagen type
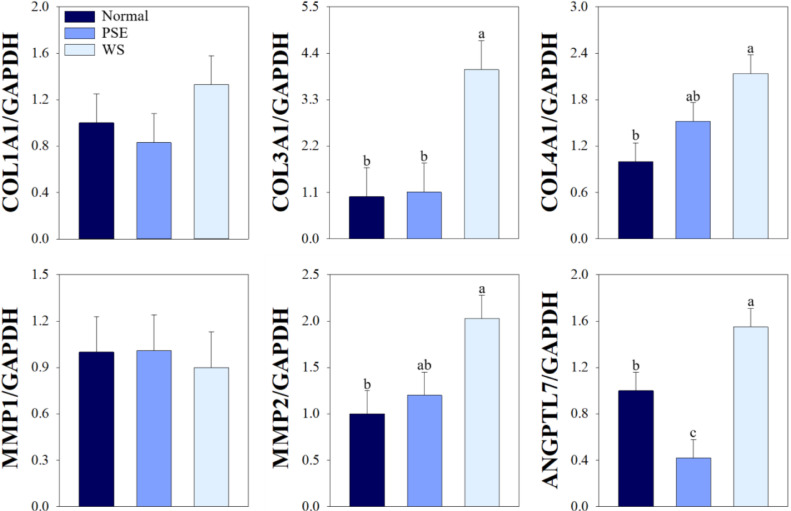
ECM remodeling, which indicates connective tissue degradation with increased deposition of fibrous collagens and ECM, leading to the development of myopathic conditions observed in chicken breast muscle (Soglia et al., 2021). Giannandrea and Parks (2014) reported that excessive deposition of fibrillary collagen types I and III could contribute to the structural and functional disruption of skeletal muscles. Additionally, high levels of nonfibrillar collagen type IV gene (COL4A1) triggered the onset of muscular disorders, including WS condition (Bordini et al., 2021). These results indicate that collagen level influences muscular dystrophy, which supports our findings. In this study, a higher COL3A1 level was observed in white-striped breast fillets compared to normal and PSE breast fillets (4.04 vs. 1.00 and 1.11, P < 0.01; Figure 1). However, there was no difference in COL1A1 levels among the groups (P > 0.05). The normal group exhibited lower expression levels of COL4A1 compared to the WS group (1.00 vs. 2.14, P < 0.01), whereas the levels in the PSE group did not differ from the other groups (P > 0.05). Therefore, paler streaks on the muscle surface are associated with increased expression of fibrillar and nonfibrillar collagens.
Figure 1.
Quantitative RT-PCR analysis of COL1A1, COL3A1, COL4A1, MMP1, MMP2, and ANGPTL7 expression in broiler pectoralis major muscle at 15 min postmortem. The expression of each gene was normalized against that of GAPDH, a housekeeping gene. Bars indicate the standard error of the mean. a-c Different letters denote significant differences (P < 0.05). Abbreviations: ANGPTL7, angiopoietin-like 7; COL1A1, collagen type 1 alpha 1 chain; COL3A1, collagen type 3 alpha 1 chain; COL4A1, collagen type 4 alpha 1 chain; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP1, matrix metalloproteinase 1; MMP2, matrix metalloproteinase 2; PSE, pale, soft, and exudative; WS, white striping.
ECM remodeling-induced myopathies can also be caused by the altered expression levels of MMP and ANGPTL genes involved in maintaining the collagen composition (Mahdy, 2019). Increased collagen degradation by the overexpression of MMPs is associated with the unregulated overgrowth of fibroblasts, myofibroblasts, and inflammatory cells, leading to muscle dysfunction (Polyakova et al., 2011); however, MMPs also play a pivotal role in skeletal muscle regeneration after tissue injury (Mahdy, 2019). In this study, we observed no difference in the expression levels of MMP1, which degrades type I and III collagen, among the groups (P > 0.05). However, similar to COL4A1 expression, PM muscles with pale streaks exhibited a higher level of MMP2 than those under normal conditions (2.03 vs. 1.00, P < 0.05), whereas the normal and PSE groups did not differ (1.20, P > 0.05). These results can be attributed to the relationship between the myopathic muscles and MMP2 expression. Upregulation of this gene was accompanied by increased degradation of type IV collagen, a major component of basal- and reticular-lamina, and both the excessive degradation and accumulation of this component are associated with myopathic PM in broilers (Soglia et al., 2021). Additionally, ANGPTL7 overexpression was found to alter the expression of ECM proteins, including collagen types I and IV and MMP1, indicating a role for this gene in ECM remodeling by collagen accumulation (Tanigawa et al., 2020). In this study, a higher level of ANGPTL7 was observed in the WS group compared to the normal and PSE groups (P < 0.001), although level of MMP1, which depends on ANGPTL7 expression, did not differ among the groups (P > 0.05). In contrast, the lowest ANGPTL7 level in the PSE condition in this study supports the previous finding that ANGPTL7 downregulation increases apoptotic cell death by suppressing the expression of antiapoptotic proteins, such as B-cell lymphoma 2 (Zhao et al., 2019). Moreover, Lee and Choi (2021) reported that apoptotic potential is associated with the occurrence of PSE condition. Collectively, our results suggested that the development of PSE condition is associated with rapid apoptosis caused by acute stress before and after slaughter compared to the RFN condition, resulting in impaired meat quality, but is not related to the accumulation and degradation of collagen. Unlike the PSE condition, the WS features did not link to deterioration of meat quality, such as decreased pH values, pale color, and poor WHC. Additionally, the development of WS myopathy can be associated with the overexpression of collagen-related molecules, especially collagen type III, collagen type IV, MMP2, and ANGPTL7, that affect the accumulation and degradation of ECM organization. On the other hand, the mechanisms of occurrence of 2 abnormalities may be different, as only two samples exhibiting both PSE and WS conditions were observed in this study.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF-2020R1A2C1010756).
DISCLOSURES
The authors did not provide a conflict of interest statement.
REFERENCES
- Ahmad K., Shaikh S., Ahmad S.S., Lee E.J., Choi I. Cross-talk between extracellular matrix and skeletal muscle: implications for myopathies. Front. Pharmacol. 2020;11:142. doi: 10.3389/fphar.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini M., Zappaterra M., Solgila F., Petracci M., Davoli R. Weighted gene co-expression network analysis identifies molecular pathways and hub genes involved in broiler White Striping and Wooden Breast myopathies. Sci. Rep. 2021;11:1776. doi: 10.1038/s41598-021-81303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho H., Soares A.L., Honorato D.C.B., Guarnieri P.D., Pedrao M.R., Paiao F.G., Oba A., Ida E.I., Shimokomaki M. The incidence of pale, soft, and exudative (PSE) turkey meat at a Brazilian commercial plant and the functional properties in its meat product. LWT. 2014;59:883–888. [Google Scholar]
- Commission Internationale de l'Eclairage . Bureau Central del la CIE; Paris, France: 1978. Recommendations on Uniform Color Spaces, Color Differences Equations, Psychometric Colour Terms. CIE Publication (15 (E-1.3.3) 1971/(TO-1.3) (Suppl. 15) [Google Scholar]
- Eckes B., Zigrino P., Kessler D., Holtkotter O., Shephard P., Mauch C., Krieg T. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol. 2000;19:325–332. doi: 10.1016/s0945-053x(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Giannandrea M., Parks W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Kauffman R.G., Eikelenboom G., Van der Wal P.G., Merku G., Zaar M. The use of filter paper to estimate drip loss of porcine musculature. Meat Sci. 1986;18:191–200. doi: 10.1016/0309-1740(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Choi Y.M., Suh Y., Shin S., Lee J., Hwang S., Lee S.S., Lee K. Research Note: Increased myostatin expression and decreased expression of myogenic regulatory factors in embryonic ages in a quail line with muscle hypoplasia. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.K., Yong H.I., Cha J.Y., Kim Y.J., Jung S., Choi Y.S. Effects of protein functionality on myofibril protein-saccharide graft reaction. Food Sci. Anim. Resour. 2022;42:849–860. doi: 10.5851/kosfa.2022.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisling A., Lust R.M., Katwa L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–34. doi: 10.1016/j.lfs.2019.04.042. [DOI] [PubMed] [Google Scholar]
- Korea Institute of Animal Products Quality Evaluation (KAPE). The beef carcass grading. http://www.ekape.or.kr/index.do (accessed June 2020).
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentines B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscle. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F.C., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Lee B., Choi Y.M. Research note: comparison of histochemical characteristics, chicken meat quality, and heat shock protein expressions between PSE-like condition and white-stripping features of pectoralis major muscle. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Choi Y.M. Research note: PSE condition is associated with increased apoptotic potential in broiler pectoralis major muscle. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Park C.H., Kong C., Kim Y.S., Choi Y.M. Muscle fiber and fresh meat characteristics of white-striping chicken breasts, and its effects on palatability of sous-vide cooked meat. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livack K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Ferket P.R., Brake J., Livinston K.A. Dietary amino acids under hypoxic conditions exacerbates muscle myopathies including wooden breast and white striping. Poult. Sci. 2019;98:1517–1527. doi: 10.3382/ps/pey463. [DOI] [PubMed] [Google Scholar]
- Mahdy M.A.A. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375:575–588. doi: 10.1007/s00441-018-2955-2. [DOI] [PubMed] [Google Scholar]
- Malila Y., Thanatsang K.V., Sanpinit P., Arayamethakorn S., Soliga F., Zappaterra M., Bordini M., Sirri F., Rungrassamee W., Davoli R., Petracci M. Differential expression patterns of genes associated with metabolisms, muscle growth and repair in Pectoralis major muscles of fast- and medium-growing chickens. Plos One. 2022;17 doi: 10.1371/journal.pone.0275160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudalal S., Zaazaa A. Influence of slaughter age on the occurrence and quality characteristics of white striping and wooden muscle abnormalities. Food Sci. Anim. Resour. 2022;42:455–466. doi: 10.5851/kosfa.2022.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estevez M. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Saf. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Bonfiglio A., Cavani C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013;92:1670–1675. doi: 10.3382/ps.2012-03001. [DOI] [PubMed] [Google Scholar]
- Picard F., Brehm M., Fassbach M., Pelzer B., Scheuring S., Kury P., Strauer B.E., Schwartzkopff B. Increased cardiac mRNA expression of matrix metalloproteinase-1 (MMP-1) and its inhibitor (TIMP-1) in DCM patients. Clin. Res. Cardiol. 2006;95:261–269. doi: 10.1007/s00392-006-0373-z. [DOI] [PubMed] [Google Scholar]
- Polyakova V., Loeffler I., Hein S., Miyagawa S., Piotrowska I., Dammer S., Risteli J., Schaper J., Kostin S. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int. J. Cardiol. 2011;151:18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S., Baffet G., Theret N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018;68–69:122–149. doi: 10.1016/j.matbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Santos C.E., Peixoto J.D.O., Fernandes L.T., Marcelino D.E.P., Kawski V.L., Neis F.T., Ledur M.C., Ibelli A.M.G. Upregulated genes in articular cartilage may help to counteract femoral head separation in broilers with 21 days of age. Re. Vet. Sci. 2022;147:92–95. doi: 10.1016/j.rvsc.2022.04.006. [DOI] [PubMed] [Google Scholar]
- Soglia F., Petracci M., Davoli R., Zappaterra M. A critical review of the mechanisms involved in the occurrence of growth-related abnormalities affecting broiler chicken breast muscles. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.M., Myers R.L., Hammam N., Stern K.A., Eberli D., Krichevsky S.B., Soker S., Dyke M.V. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–2399. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa Y., Wainberg M., Karjalainen J., Kiiskinen T., Venkataraman G., Lemmela S., Turunen J.A., Grahma R.R., Havulinna A.S., Perola M., Palotie A., FinnGen M.J.Daly, Rivas M.A. Rare protein-altering variants in ANGPTL7 lower intraocular pressure and protect against glaucoma. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Liu K., Yin D., Lin Z. Angiopoietin-like 7 contributes to angiotensin II-induced proliferation, inflammation and apoptosis in vascular smooth muscle cells. Pharmacology. 2019;104:226–234. doi: 10.1159/000501296. [DOI] [PubMed] [Google Scholar]