Abstract
Rationale & Objective
Variability in estimated glomerular filtration rate (eGFR) over time is often observed, but it is unknown whether this variation is clinically important. We investigated the association between eGFR variability and survival free of dementia or persistent physical disability (disability-free survival) and cardiovascular disease (CVD) events (myocardial infarction, stroke, hospitalization for heart failure, or CVD death).
Study Design
Post hoc analysis.
Setting & Participants
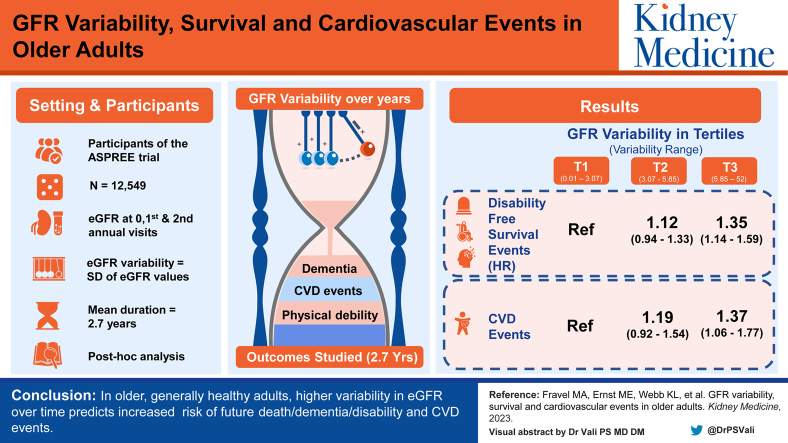
12,549 participants of the ASPirin in Reducing Events in the Elderly trial. Participants were without documented dementia, major physical disability, previous CVD, and major life-limiting illness at enrollment.
Predictors
eGFR variability.
Outcomes
Disability-free survival and CVD events.
Analytical Approach
eGFR variability was estimated using the standard deviation of eGFR measurements obtained from participants’ baseline, first, and second annual visits. Associations between tertiles of eGFR variability with disability-free survival and CVD events occurring after the eGFR variability estimation period were examined.
Results
During median follow-up of 2.7 years after the second annual visit, 838 participants died, developed dementia, or acquired a persistent physical disability; 379 had a CVD event. The highest tertile of eGFR variability had an increased risk of death/dementia/disability (HR, 1.35; 95% CI, 1.14-1.59) and CVD events (HR, 1.37; 95% CI, 1.06-1.77) compared with the lowest tertile after covariate adjustment. These associations were present in patients with and without chronic kidney disease at baseline.
Limitations
Limited representation of diverse demographics.
Conclusions
In older, generally healthy adults, higher variability in eGFR over time predicts increased risk of future death/dementia/disability and CVD events.
Index Words: Aging, cardiovascular disease, disability-free survival, eGFR variability, long-term follow-up
Graphical abstract
Plain-Language Summary.
Using isolated estimated glomerular filtration rate (eGFR) measures to establish the prognosis in older adults is problematic given that eGFR declines even in healthy aging. In patients with multiple comorbid conditions, studies have linked eGFR variability with an increased risk of poor health outcomes. The prognostic significance of eGFR variability in healthy, older adults is unclear. In a cohort of generally healthy older adults, we found that high eGFR variability was associated with a 35% increased risk of a composite of death, dementia, or physical disability and a 37% increased risk of cardiovascular disease events. Increased risk with high eGFR variability was observed regardless of the chronic kidney disease status at baseline. Incorporating assessment of visit-to-visit eGFR variability into routine laboratory reporting should be further investigated as a novel biomarker of health risk in older adults.
Editorial, 100599
Persistently low estimated glomerular filtration rate (eGFR), the hallmark of chronic kidney disease (CKD), is associated with multiple adverse health outcomes, including anemia, bone and mineral disorders, cardiovascular disease (CVD), and death.1 In contrast, the clinical significance of fluctuations in eGFR commonly observed across serial health care encounters is less clear, particularly in adults without established CKD. In a small number of studies, eGFR variability was associated with increased risk of hospitalization, CVD events, CKD progression, and overall mortality2, 3, 4, 5, 6, 7, 8; however, these studies were conducted in populations with a high level of comorbid conditions at baseline, including established CKD, CVD, or high risk of CVD. Thus, the significance of eGFR variability in a healthy, older population needs further investigation.
Although older adults with multiple comorbid conditions usually have lower eGFR than their healthy counterparts, healthy older adults have lower eGFR than healthy younger individuals because of the expected decline in eGFR of 1 mL/min/1.73 m2 per year.9, 10, 11 This suggests that healthy aging can occur in spite of declining eGFR, and calls into question the use of making clinical prognoses based on isolated eGFR measurements in this population. Moreover, an upward trajectory in eGFR in specific older adult populations has, paradoxically, been associated with poorer, as opposed to improved, health outcomes, possibly due to multimorbidity or malnourishment.12 Such observations further highlight the complexity involved in evaluating kidney function in older adults and the challenges of applying universal eGFR thresholds to diagnose CKD and establish long-term prognoses. Examining variability across serial eGFR measures, similar to methods assessing visit-to-visit blood pressure variability, may yield prognostic information not otherwise ascertained through individual measures or the mean of serial measures.13
We hypothesized that higher long-term variability in kidney function among generally healthy older adults would be associated with an increased risk of future adverse health outcomes. To test this hypothesis, we examined the association between visit-to-visit variability in eGFR and disability-free survival and CVD events in participants of the ASPirin in Reducing Events in the Elderly (ASPREE) trial.14 ASPREE is an optimal cohort in which to examine the prognostic capability of long-term eGFR variability with key advantages, including its large sample size, comprehensive phenotyping, including serial measures of eGFR collected as part of the study protocol, significant length of follow-up, stringent adjudication of health events, and use of disability-free survival as a novel composite end point encapsulating important health outcomes, such as dementia and physical disability that are relevant to older adults.15
Methods
Study Design and Participants
We conducted a post hoc analysis using data from participants enrolled in ASPREE, a multicenter, randomized, placebo-controlled trial of 100 mg daily aspirin versus placebo conducted in Australia and the United States from 2010-2017 and currently continuing follow-up in an observational phase. Detailed reports of the ASPREE study and the main findings of the intervention phase have been previously published.16, 17, 18 In brief, ASPREE included 19,114 community-dwelling adults aged 70 years and older (65 years for US minorities) who were without CVD, dementia, major physical disability, known high risk of bleeding, contraindication to aspirin, systolic blood pressure ≥180 mm Hg or diastolic ≥105 mm Hg, and chronic illness expected to limit survival to less than 5 years. All participants provided written informed consent to participate, and the trial was conducted in accordance with the principles of the Declaration of Helsinki and approved by local institutional review boards at each site. The trial was registered with ClinicalTrials.gov (NCT01038583) and ISRCTN83772183.
Study Measurements
Following a 4-week placebo run-in period to finalize eligibility, randomized participants subsequently underwent annual study visits for collection of standardized, comprehensive assessments of physical and cognitive function by trained study staff following standard operating procedures. Serum creatinine and urinary albumin-creatinine ratio were scheduled to be obtained at baseline, and again at annual visits in years 3 and 5, and closeout (which could be year 3 or any year thereafter, depending on the participant’s year of enrollment). However, based on sites’ study visit workflow, all laboratory assessments could be obtained at each visit. Although uncommon, measurements obtained through regular clinical care could be substituted in place of study visit collections if they occurred within a 6-month window of the study visit (<5% of measures were obtained this way). For this study, eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation at baseline, annual visit 1, and annual visit 2 when serum creatinine measurements were available.19
Assessment of eGFR Variability
eGFR variability was estimated by calculating the standard deviation (SD) of 3 eGFR measurements obtained for each participant during the eGFR variability estimation period, which we defined as baseline, annual visit year 1, and annual visit year 2. Participants who were missing any of these 3 measures were excluded from analyses. eGFR variability was first treated as a continuous variable and then participants were divided into tertiles with the lowest tertile used as a reference in the analysis.
Disability-Free Survival and CVD Composite End Point
The main end point for this analysis was the ASPREE primary adjudicated end point of disability-free survival, measured as a composite of participants’ first event of death or incident dementia or persistent physical disability (referred to here as death/dementia/disability). A second end point in this analysis was incident CVD events, which was also a pre-specified secondary end point of the main ASPREE trial, defined as a composite of fatal coronary heart disease (death from myocardial infarction, sudden cardiac death, cardiac failure death, or any other death in which the underlying cause was considered to be coronary heart disease), nonfatal myocardial infarction, fatal or nonfatal stroke (hemorrhagic or ischemic), noncoronary cardiac or vascular death, or hospitalization for heart failure. All CVD events were adjudicated by a panel of experts blinded to treatment group assignment. To minimize the potential for immortal time bias, only those outcomes occurring after the estimation period for eGFR variability, ie, from the second annual visit onward, were included in this analysis.
Statistical Analysis
Cox proportional hazards regression models were used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) to explore the association of kidney function variability (as a continuous variable and by tertiles of SD) with disability-free survival and with time to the first occurrence of CVD events. Participants were followed from the second annual visit until they experienced an end point or were censored because of death or loss to follow-up. A directed acyclic graph was developed to identify variables to include in the adjusted models (Fig S1). An initial unadjusted model (model 1) was followed by adjustment for age, sex, race/ethnicity (model 2) and randomized treatment arm, body mass index, mean eGFR and mean albumin-creatinine ratio (over the eGFR variability estimation period), diabetes, baseline frailty, smoking status, total cholesterol, low-density lipoprotein, mean systolic blood pressure (over the eGFR variability estimation period), and use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and diuretics (over the eGFR estimation period) (model 3). The addition of mean diastolic blood pressure (over the eGFR variability estimation period) to the model did not impact the results. Proportional hazards assumptions were tested using scaled Schoenfeld residuals; all P values were found to be >0.1, indicating satisfaction of the assumption for all end points. Statistical analyses were performed using the R version 4.0.2 (R Core Team, 2020).
We conducted sensitivity analyses by repeating the main analyses examining eGFR variability and the disability-free survival and CVD end points but stratifying by CKD status during the eGFR variability estimation period. Participants who had eGFR <60 mL/min/1.73 m2 or albumin-creatinine ratio ≥3 mg/mmol at the second annual visit and at their baseline and/or first annual visit were considered to have CKD. In an additional sensitivity analysis, each of the individual components of disability-free survival (first event of death or dementia or persistent physical disability) was explored individually with eGFR variability. Finally, given that 30% of participants were excluded from the main analysis because of missing eGFR data, a sensitivity analysis was completed, including participants with 2 or more baseline eGFR measures.
We also undertook analyses using alternate methods to calculate kidney function variability using all participants with 3 measurements of creatinine at baseline, year 1 and year 2. First, we used the coefficient of variation (CV) for both eGFR and serum creatinine levels noting that for eGFR, its symmetric distribution ensures a lack of correlation between mean and SD, but this is not the case for serum creatinine level, which has a skewed distribution and for which CV may be more appropriate. Second, given that clinicians often use the serum creatinine (rather than eGFR) in actual practice, we repeated analyses using the SD of serum creatinine level. There was a high correlation between all measures of eGFR variability (Table S1). Mean eGFR had a weak correlation with eGFR variability (SD) but a strong correlation existed between mean eGFR and eGFR variability (CV) indicating that SD was the preferable measure to describe differences between participants in their eGFR variability. Each of these alternative estimates of kidney function variability were examined one by one in analyses corresponding to models 1-3 as continuous variables and divided into tertiles.
Our approach to estimating within-person kidney function variability, based on similar commonly used methods for blood pressure variability, is simple and transparent but known to potentially lead to bias in estimating associations with outcomes.20 Hence, in additional 2-stage analyses, eGFR SD was estimated using 2 different linear mixed effects models based on borrowing information across individuals, with the first model ignoring any correlation between the SD and mean eGFR and the second model, including a parameter for this correlation. Model parameters were estimated using Bayesian Markov chain Monte Carlo implemented using the JAGS version 4.3.0 and the R2Jags package in R, with the posterior mean of the residual SD used as the measure of renal variability.20
Results
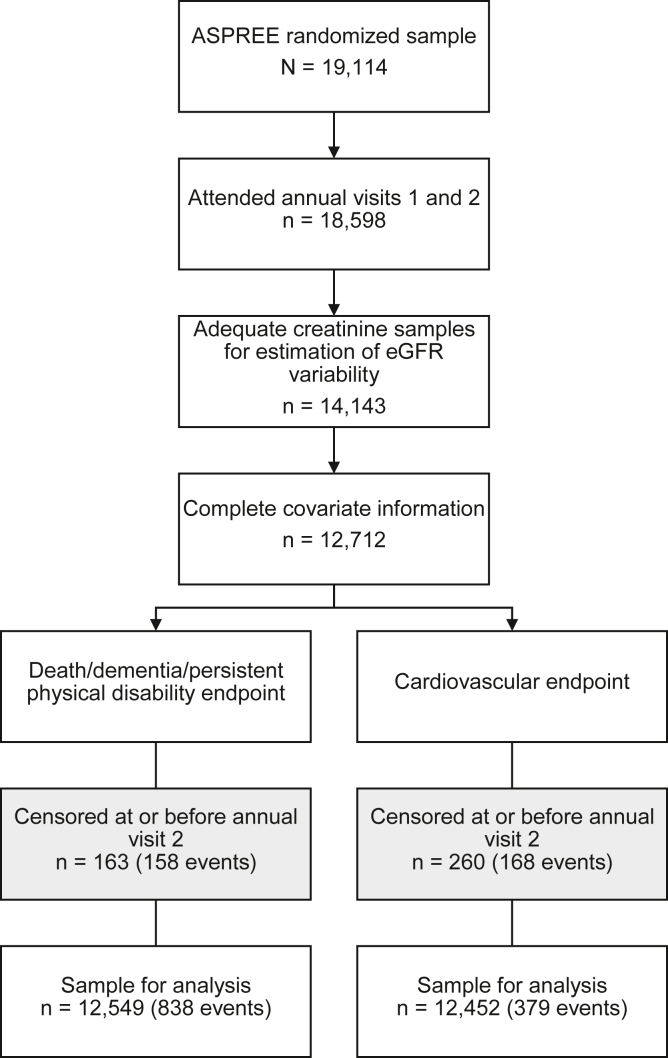
Of the 19,114 ASPREE participants, 12,712 remained eligible for analysis after screening for the availability of kidney function measurements and complete covariate information during the eGFR variability estimation period. Death, dementia, or persistent physical disability occurred as the first event in 162 participants during the eGFR variability estimation period, leaving 12,549 available for the primary disability-free survival analysis. In the CVD analysis, 260 experienced a CVD event during the eGFR variability estimation period, leaving 12,452 available for this analysis (Fig 1).
Figure 1.
Derivation of participants in each analysis. Abbreviations: ASPREE, ASPirin in Reducing Events in the Elderly; eGFR, estimated glomerular filtration rate.
Among the 12,549 participants in the disability-free survival analysis, the mean age was 75.1 years (ranging from 65-98 years); 54.6% of participants were women and 96.0% were White. Baseline mean systolic blood pressure was 138.2 mm Hg and baseline eGFR was 71.9 mL/min/1.73 m2. Table 1 shows the baseline characteristics of the participants in the disability-free survival analysis overall and by eGFR variability according to tertile. The eGFR variability range was 0.01-3.07 mL/min/1.73 m2 (tertile 1), 3.07-5.85 mL/min/1.73 m2 (tertile 2), and 5.85-52.00 mL/min/1.73 m2 (tertile 3). Baseline characteristics of participants in the analysis examining CVD events were similar to those in the disability-free survival analysis (Table S2). In both analyses, participants in the highest tertile of eGFR variability were more likely to have lower baseline eGFR, have a body mass index >30 kg/m2, have diabetes, be frail, and be using an angiotensin-converting enzyme inhibitor/angiotension receptor blocker and/or diuretic compared with those in the lowest eGFR variability tertile.
Table 1.
Baseline Characteristics of Study Participants for Analysis of Death/Dementia/Persistent Physical Disability by eGFR Variability, With Variability According to Tertiles of Standard Deviation
|
Variable |
Standard Deviation of eGFR (eGFR Variability) |
Overall | ||
|---|---|---|---|---|
| T1 (0.01-3.07) | T2 (3.07-5.85) | T3 (>5.85) | ||
| Number of participants | 4,183 | 4,183 | 4,183 | 12,549 |
| Age (y), mean (SD) | 75.1 (4.2) | 75.2 (4.3) | 75.0 (4.3) | 75.1 (4.3) |
| Female, n (%) | 2,199 (52.6%) | 2,212 (52.9%) | 2,446 (58.5%) | 6,857 (54.6%) |
| Race/ethnicity, n (%) | ||||
| White | 4,050 (96.8%) | 4,044 (96.7%) | 3,947 (94.4%) | 12,041 (96.0%) |
| Black | 35 (0.8%) | 36 (0.9%) | 135 (3.2%) | 206 (1.6%) |
| Hispanic | 44 (1.1%) | 39 (0.9%) | 43 (1.0%) | 126 (1.0%) |
| Asian | 29 (0.7%) | 48 (1.1%) | 33 (0.8%) | 110 (0.9%) |
| Other | 25 (0.6%) | 16 (0.4%) | 25 (0.6%) | 66 (0.5%) |
| Aspirin treatment assignment, n (%) | 2,063 (49.3%) | 2,061 (49.3%) | 2,112 (50.5%) | 6,236 (49.7%) |
| Kidney function (BL-AV2) | ||||
| eGFR (ml/min/1.73 m2), mean (SD) | 74.4 (14.6) | 70.9 (12.9) | 70.3 (10.8) | 71.9 (13.0) |
| ACR (mg/g), median (IQR) | 8.0 (5.0-14.2) | 8.0 (5.0-14.2) | 8.3 (5.3-15.3) | 8.0 (5.0-14.5) |
| Serum creatinine level (mg/dL), mean (SD) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) |
| Mean (BL-AV2) SBP, mm Hg, mean (SD) | 138.4 (13.3) | 138.3 (13.6) | 137.9 (13.8) | 138.2 (13.6) |
| Mean (BL-AV2) DBP, mm Hg, mean (SD) | 76.2 (7.9) | 75.9 (8.1) | 75.4 (8.2) | 75.8 (8.1) |
| BMI, n (%) | ||||
| <18.5 kg/m2 | 20 (0.5%) | 23 (0.5%) | 18 (0.4%) | 61 (0.5%) |
| 18.5-25 kg/m2 | 1,117 (26.7%) | 1,035 (24.7%) | 1,025 (24.5%) | 3,177 (25.3%) |
| 25-30 kg/m2 | 1,910 (45.7%) | 1,918 (45.9%) | 1,869 (44.7%) | 5,697 (45.4%) |
| ≥30 kg/m2 | 1,136 (27.2%) | 1,207 (28.9%) | 1,271 (30.4%) | 3,614 (28.8%) |
| Total cholesterol (mg/dL), mean (SD) | 203.2 (37.4) | 202.9 (37.4) | 203.3 (38.5) | 203.2 (37.7) |
| LDL (mg/dL), mean (SD) | 118.9 (33.3) | 118.4 (33.7) | 118.2 (34.4) | 118.5 (33.8) |
| Diabetes, n (%) | 379 (9.1%) | 409 (9.8%) | 470 (11.2%) | 1,258 (10.0%) |
| Baseline frailty, n (%)a | ||||
| Not frail | 2,636 (63.0%) | 2,648 (63.3%) | 2,540 (60.7%) | 7,824 (62.3%) |
| Prefrail | 1,480 (35.4%) | 1,477 (35.3%) | 1,563 (37.4%) | 4,520 (36.0%) |
| Frail | 67 (1.6%) | 58 (1.4%) | 80 (1.9%) | 205 (1.6%) |
| Antihypertension drug use | ||||
| ACEi/ARBs, n (%) | ||||
| No use at BL or AV1 | 2,329 (55.7%) | 2,255 (53.9%) | 2,041 (48.8%) | 6,625 (52.8%) |
| BL or AV1 | 110 (2.6%) | 141 (3.4%) | 157 (3.8%) | 408 (3.3%) |
| Both BL and AV1 | 1,744 (41.7%) | 1,787 (42.7%) | 1,985 (47.5%) | 5,516 (44.0%) |
| Diuretics, n (%) | ||||
| No use at BL or AV1 | 3,390 (81.0%) | 3,365 (80.4%) | 3,201 (76.5%) | 9,956 (79.3%) |
| BL or AV1 | 70 (1.7%) | 77 (1.8%) | 103 (2.5%) | 250 (2.0%) |
| Both BL and AV1 | 723 (17.3%) | 741 (17.7%) | 879 (21.0%) | 2343 (18.7%) |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-creatinine ratio; ARB, angiotensin receptor blocker; AV1, first annual visit; AV2, second annual visit; BL, baseline; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
Frailty/prefrailty: participants were classified as frail if they met at least 3 of the following criteria and prefrail if they met 1 or 2 of the criteria: (1) BMI < 20 kg/m2; (2) lowest 20% of grip strength taking into account sex and BMI; (3) the participant endorsed “I felt that everything I did was an effort” and/or “I could not get going” for 3 or more days during the last week, according to the Center for Epidemiological Studies Depression 10 scale; (4) time to walk 3 m (10 ft) was in the lowest 20% taking into account sex and height; and (5) no walking outside the home in the last 2 weeks, or the longest amount of time walking outside without sitting down to rest was less than 10 min, according to Lifestyle Interventions and Independence for Elders study Disability questionnaire responses.
Disability-free Survival and CVD Events
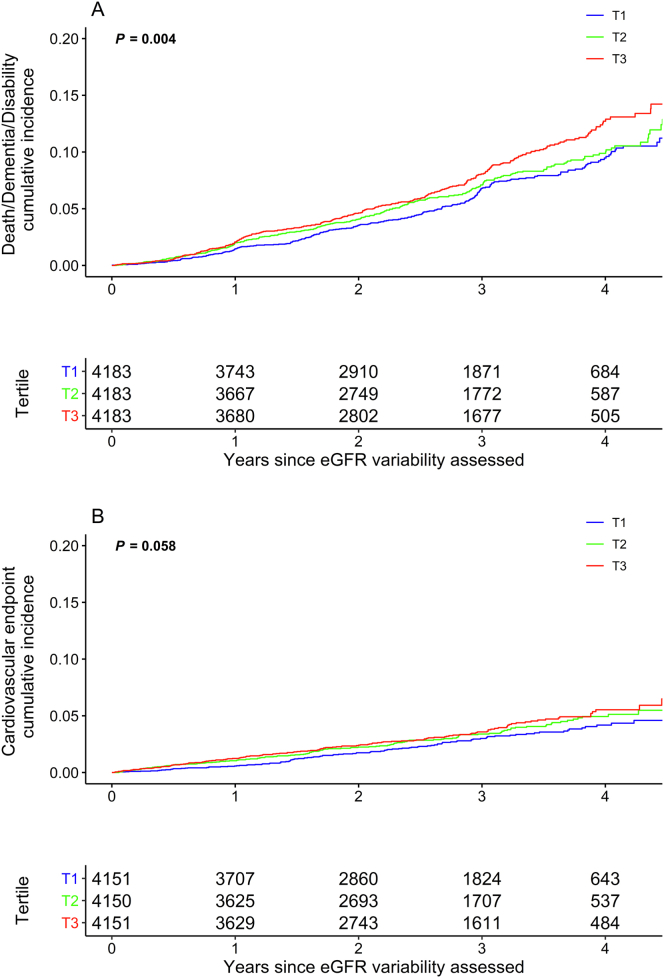
During a mean follow-up of 2.7 years after the eGFR variability estimation period, 838 participants died or developed dementia or persistent physical disability (381 deaths, 282 dementia, and 175 disability) and 379 experienced a CVD event (38 coronary heart disease death, 125 nonfatal myocardial infarction, 151 fatal and nonfatal stroke, and 65 hospitalizations for heart failure). There were 448 deaths in total (217 [48.45%] cancer-related deaths, 90 [20.1%] cardiovascular deaths, 25 [5.6%] major hemorrhage deaths, and 116 [25.9%] other or insufficient information). Figure 2 shows the cumulative incidence for death, dementia, or persistent physical disability and the CVD events. The rate of death, dementia, or physical disability increased across tertiles of eGFR variability, from 22.3 events per 1,000 person-years in the lowest (reference) tertile, to 24.9 events per 1,000 person-years in the second tertile, and 22.8 events per 1,000 person-years in the third tertile. In the fully adjusted model, individuals in the highest tertile of eGFR variability had increased risk of death/dementia/disability compared with the lowest (HR, 1.35; 95% CI, 1.14-1.59) (Table 2). When examined as a continuous variable, a difference of 3 mL/min/1.73 m2 in eGFR variability was associated with an 11% increased risk of the composite of death/dementia/disability (HR, 1.11; 95% CI, 1.05-1.17). Higher eGFR variability was also associated with increased risk of CVD events, with the highest tertile of eGFR variability having a higher risk of CVD events compared with the lowest (HR, 1.37; 95% CI, 1.06-1.77) (Fig 2, Table 2). Point estimates of the HRs for both outcomes in the second tertile were intermediate between the point estimates of the first and third tertiles. CVD event rates ranged from 9.8 of 1,000 person-years in the lowest tertile of eGFR variability to 13.1 of 1,000 person-years in the highest tertile.
Figure 2.
Cumulative incidence of (A) death/dementia/persistent physical disability and (B) CVD events by eGFR variability, with variability according to tertiles (T) of standard deviation. Abbreviations: CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate. ∗P value for log-rank test.
Table 2.
Hazard Ratio (95% CI) for Association Between eGFR Variability and (A) Death/Dementia/Persistent Physical Disability (DFS) and (B) CVD Events, With Variability as Continuous Variable and According to Tertiles (T) of Standard Deviation
| Variability Measure | Variability (Continuous, Per 3 mL/min/1.73 m2 Difference in SDa) | Variability (Tertiles) |
||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| A. DFS, N | 12,549 | 4,183 | 4,183 | 4,183 |
| Variability range | -- | 0.01-3.07 | 3.07-5.85 | 5.85-52.00 |
| Number of events | 838 | 254 | 272 | 312 |
| Event rate per 1,000 person-y | 25.3 | 22.3 | 24.9 | 28.8 |
| HR (95% CI) | ||||
| Model 1 | 1.09 (1.04-1.16) | REF | 1.13 (0.95-1.34) | 1.32 (1.12-1.55) |
| Model 2 | 1.12 (1.05-1.18) | REF | 1.10 (0.93-1.31) | 1.36 (1.15-1.60) |
| Model 3 | 1.11 (1.05-1.17) | REF | 1.12 (0.94-1.33) | 1.35 (1.14-1.59) |
| B. CVD, N | 12,452 | 4,151 | 4,150 | 4,151 |
| Variability range | -- | 0.01-3.07 | 3.07-5.85 | 5.85-52.00 |
| Number of events | 379 | 110 | 130 | 139 |
| Event rate per 1,000 person-y | 11.7 | 9.8 | 12.1 | 13.1 |
| HR (95% CI) | ||||
| Model 1 | 1.08 (1.00-1.17) | REF | 1.24 (0.96-1.60) | 1.35 (1.05-1.73) |
| Model 2 | 1.11 (1.02-1.20) | REF | 1.22 (0.95-1.58) | 1.42 (1.11-1.83) |
| Model 3 | 1.09 (1.01-1.19) | REF | 1.19 (0.92-1.54) | 1.37 (1.06-1.77) |
Note: Model 1: unadjusted. Model 2: adjusted for age, sex, and race/ethnicity. Model 3: includes model 2 adjustments and randomized arm (aspirin vs placebo), BMI, mean eGFR (BL-AV2), mean ACR (BL-AV2), diabetes, baseline frailty, smoking status, total cholesterol, LDL, mean SBP (BL-AV2), ACEi/ARB use, and diuretic use.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-creatinine ratio; ARB, angiotensin receptor blocker; BL-AV2, baseline to annual visit 2; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; DFS, disability-free survival; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LDL, low-density lipoprotein; REF, reference; SD, standard deviation.
A difference of 3 mL/min/1.73 m2 is approximately the difference between tertile cut-offs and allows for easier comparison between hazard ratios.
Sensitivity Analyses
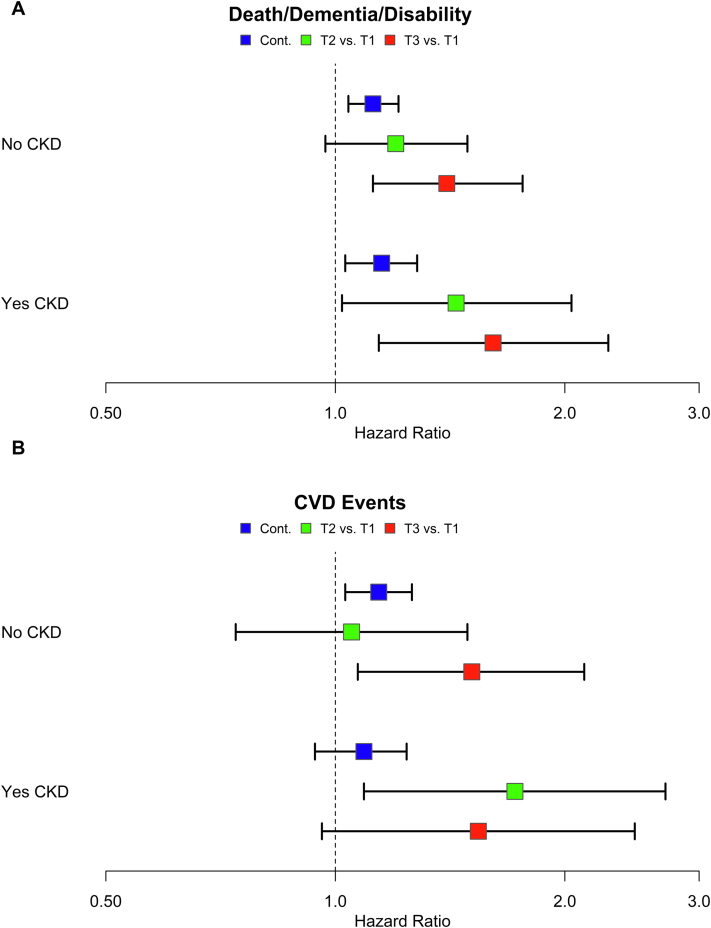
Subgroup analysis for the death/dementia/disability and CVD end points stratified by baseline CKD status are shown in Fig 3. In this analysis, the relationship between higher eGFR variability and increased risk of death/dementia/disability persisted for those with and without CKD at baseline, with the highest tertile of eGFR variability associated with higher risk than the lowest for both CKD and non-CKD participants (baseline CKD: HR, 1.61; 95% CI, 1.14-2.28; no baseline CKD: HR, 1.40; 95% CI, 1.12-1.76) (Table S3). The relationship between higher eGFR variability and risk of CVD events in the subgroup analysis was also observed (baseline CKD: HR, 1.54; 95% CI, 0.96-2.47; no baseline CKD: HR, 1.51; 95% CI, 1.07-2.12) (Table S3).
Figure 3.
Hazard ratio (95% CI) for association between eGFR variability and (A) death/dementia/persistent physical disability and (B) CVD events stratified by baseline CKD status with variability as a continuous variable (per 3 ml/min/1.73 m2 difference) and according to tertiles (T) of standard deviation. Adjusted for age, sex, race/ethnicity, randomized arm (aspirin vs placebo), BMI, mean eGFR (BL-AV2), mean ACR (BL-AV2), diabetes, baseline frailty, smoking status, total cholesterol, LDL, mean SBP (BL-AV2), ACEi/ARB use, and diuretic use. Baseline CKD status determined by eGFR < 60 mL/min/1.73 m2 or ACR ≥ 3 mg/mmol at year 2 visit and baseline or year 1 visit. Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BL-AV2, baseline to annual visit 2; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; SBP, systolic blood pressure.
When exploring the individual components of the death/dementia/disability end point, eGFR variability was associated with death, which appeared to drive the overall composite finding (Table S4, Fig S2). However, death was the most common outcome overall in the cohort, and the point estimates for the associations with physical disability and dementia also suggested increased risk with higher eGFR variability. In contrast, the composite CVD outcome appeared to be driven by nonfatal myocardial infarction and hospitalization for heart failure (Table S4).
Findings for both death/dementia/disability and CVD events remained similar when patients with at least 2 baseline eGFR measures who were originally excluded for missing data were included (Table S5) and in other sensitivity analyses when eGFR variability was calculated using CV and when both CV and SD were used to estimate serum creatinine variability (Tables S6 and S7). Additionally, results were similar when eGFR SD was re-estimated using 2 different linear mixed effects models to allow for information to be used across individuals (Table S8).
Discussion
In the present analysis, we found that generally healthy older adults with higher variability in eGFR were less likely to survive free of dementia or persistent physical disability and more likely to experience a CVD event during follow-up than those with lower variability in eGFR. These associations were found to be independent of baseline kidney function and were consistent across different analytic approaches. The risk of death or dementia, or physical disability increased by 11% and risk of cardiovascular events increased by 9% with each 3 mL/min/1.73 m2 difference in SD of eGFR. Individuals in the cohort who were in the highest tertile of eGFR variability had a 35% increased risk of death or dementia or physical disability and a 37% increased risk of a CVD event compared with those in the lowest tertile. Notably, these increased risks were present in individuals irrespective of whether they had CKD at baseline.
Visit-to-visit variability in routine health measurements, such as blood pressure, once viewed as measurement artifacts, are now known to be independently predictive of health outcomes, such as CVD events and cognitive decline.21, 22, 23 This variability presumably signals disruptions in underlying hemodynamic and pathological vascular pathways that isolated measurements, or even mean values across measures, may fail to reveal. Given the close relationship between blood pressure and kidney function, evaluating variability in kidney function may identify individuals at higher risk of adverse health outcomes that might have otherwise escaped notice when focused only on infrequent measures viewed in isolation. As such, including a variety of measures of kidney function, including eGFR variability, as part of risk prediction strategies8 may aid in better prediction of future outcomes.
To date, there have been few studies examining the prognostic significance of variability in eGFR, and the majority of these studies were conducted in participants who already had CKD or other significant comorbidities.2, 3, 4, 5, 6, 7, 8 In a large cohort of older adults with reduced eGFR, patients with the greatest eGFR variability had a greater than 30% increased risk of death compared with those with the least variability.2 In the Systolic Blood Pressure Intervention Trial, which included adults with and without CKD and who were at high risk of CVD, eGFR variability, regardless of baseline eGFR, was associated with increased risk of all-cause mortality (a nearly 30% increase in HR for each SD increase in variability). However, an association was not found between increased eGFR variability and CVD. Our study is distinguished by the fact that to our knowledge, (1) it is the first to examine the clinical significance of eGFR variability in a large cohort of older adults who were relatively healthy at baseline, (2) it was not conducted exclusively in those with established CKD, CVD, or at high risk of CVD as a criterion of enrollment, and (3) it did not involve an intervention on a health parameter that is directly related to kidney function.
Reasons for variability in eGFR are varied and include influences from comorbid conditions, instances of acute kidney injury, medications, fluctuation in fluid status, and age-related changes in creatinine levels, among others. In older adults, the predominant pattern is a progressively declining eGFR with increasing age, and small transient changes in either direction are not usually viewed with concern. It has been suggested, however, that these fluctuations may signal diminished renal reserve and reflect a limited ability to compensate in the setting of otherwise routine events that require the kidney to autoregulate.2,3,24 Additionally, it has been proposed that increased eGFR variability may represent not only a deficit in intrinsic kidney function but also the function of other organ systems involved in maintaining renal stability in the presence of external renal insults.2 This theory attributes eGFR variability as a representation of overall organ system resilience and adaptability and may offer a potential explanation why more eGFR variability was associated with a reduction in the global composite of disability-free survival in our trial. In this sense, increased eGFR variability could be viewed as a measure of accelerated aging. Although the pathophysiologic mechanisms underlying eGFR variability and its strong association with future adverse health events are uncertain, the strength and consistency of our observed associations suggests it merits further consideration as a useful biomarker of kidney function in healthy older adults.
Strengths of our study include the large sample size, significant length of follow-up, expert adjudication of end points, and use of the unique composite end point representing disability-free survival, a highly relevant outcome for studies in healthy aging. We used more than 1 analysis technique for estimating eGFR variability to address the potential bias in commonly used approaches.20 Additionally, the fact that serum creatinine level was measured at pre-specified time points according to the ASPREE protocol minimizes the potential for measurement bias, which can occur when individuals in poorer health are disproportionately monitored more frequently, thus increasing the likelihood of observing higher variability in the measurements of those individuals.
Our study results need to be acknowledged in the context of important limitations. First, a number of patients had missing eGFR data leading to exclusions of approximately 30% of the overall enrolled ASPREE cohort. Although this still allowed for analysis of more than 12,000 patients, a sensitivity analysis was conducted to include participants with fewer eGFR measurements, which added an additional 3,000 participants. This analysis demonstrated findings consistent with the original analyses. Second, our study was primarily limited to White participants with limited racial/ethnic diversity, limiting the generalizability to other more ethnically diverse cohorts. Third, the presence of unmeasured confounders may have also affected our findings, although we were able to adjust for a large number of relevant covariates. Although lower serum creatinine levels, leading to higher eGFR estimates, may serve as a surrogate for low muscle mass and poor outcomes in acutely ill patients,25 our model controlled for body mass index and baseline frailty, helping to mitigate these concerns. Nevertheless, replication studies assessing variability in GFR estimated with other markers of kidney function, such as serum cystatin C, may provide additional insight into the applicability of eGFR variability and health outcomes in this population. Finally, future investigations incorporating eGFR trajectory with variability may provide additional insight into the association between eGFR change and adverse clinical outcomes.
In summary, we observed that in older adults without dementia, physical disability, or prior CVD enrolled in ASPREE, higher long-term variability in eGFR, independent of initial eGFR level, is associated with increased risk of future death/dementia/disability and CVD events. Our findings suggest it may be important to monitor variation in eGFR over time in healthy older adults, as a novel predictor of the risk of future poor health outcomes.
Article Information
Authors’ Full Names and Academic Degrees
Michelle A. Fravel, PharmD, Michael E. Ernst, PharmD, Katherine L. Webb, MA, James B. Wetmore, MD, MS, Rory Wolfe, PhD, Robyn L. Woods, PhD, Christopher M. Reid, PhD, Enayet Chowdhury, MBBS, MPH, PhD, Anne M. Murray, MD, MSc, and Kevan R. Polkinghorne, MBChB, PhD; on behalf of the ASPirin in Reducing Events in the Elderly Investigator Group
Authors’ Contributions
Study concept and design: MAF, MEE, KLW, JBW, RW, RLW, CMR, EC, AMM, KRP; acquisition of data: MEE, RLW, CMR, AMM; analysis and interpretation of data: MAF, MEE, KLW, JBW, RW, RLW, CMR, EC, AMM, KRP; statistical analysis: KLW, RW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by grants (U01AG029824 and U19AG062682) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (334047 and 1127060) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received April 4, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form October 26, 2022.
Footnotes
Complete author and article information provided before references.
ASPirin in Reducing Events in the Elderly Investigator Group Members: A list of investigators is available at https://aspree.org/usa/about-us.
Figure S1: Directed acyclic graph.
Figure S2: Hazard ratio (95% CI) for association between eGFR variability and the individual components of disability-free survival, with variability as a continuous variable (per 3 mL/min/1.73 m2 difference) and according to tertiles (T) of standard deviation.
Table S1: Pearson’s Correlation Coefficients Summarizing Associations Between Standard Deviation of eGFR and Other Kidney Function Variability Estimates.
Table S2: Baseline Characteristics of Study Participants for Analysis of CVD Events by eGFR Variability, With Variability According to Tertiles of Standard Deviation.
Table S3: Hazard Ratio (95% CI) for Association Between eGFR Variability and (A, B) Death/Dementia/Persistent Physical Disability (DFS) and (C, D) CVD, Stratified by Baseline CKD Status With Variability as a Continuous Variable and According to Tertiles (T) of Standard Deviation.
Table S4: Hazard Ratio (95% CI) for Association Between eGFR Variability and (A, B, C) the Individual Components of Disability-Free Survival and (D, E, F, G) the Individual Components of CVD With Variability as a Continuous Variable and According to Tertiles (T) of Standard Deviation.
Table S5: Hazard Ratio (95% CI) for Association Between eGFR Variability and (A) Death/Dementia/Persistent Physical Disability (DFS) and (B) CVD Events, With Variability as Continuous Variable and According to Tertiles (T) of Standard Deviation With Inclusion of Participants With 2 or More Baseline eGFR Values.
Table S6: Hazard Ratio (95% CI) for the Association Between eGFR Variability and (A) Death/Dementia/Persistent Physical Disability (DFS) and (B) CVD Events, With Variability as a Continuous Variable and According to Tertiles (T) of Coefficient of Variation.
Table S7: Hazard Ratio (95% CI) for the Association Between Serum Creatinine Variability (Standard Deviation (A, B) and Coefficient of Variation (C, D)) and Death/Dementia/Persistent Physical Disability (DFS) and CVD Events, With Variability as a Continuous Variable and According to Tertiles (T).
Table S8: Hazard Ratio (95% CI) for Association Between eGFR Variability (Mixed Model 2-Stage Approach) and (A, C) Death/Dementia/Persistent Physical Disability (DFS) and (C,D) CVD Events, With Variability as a Continuous Variable and According to Tertiles (T).
Supplementary Material
Figure S1-S2; Table S1-S8.
References
- 1.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Aly Z., Balasubramanian S., McDonald J.R., Scherrer J.F., O’Hare A.M. Greater variability in kidney function is associated with an increased risk of death. Kidney Int. 2012;82(11):1208–1214. doi: 10.1038/ki.2012.276. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra R., Katz R., Jotwani V., et al. Estimated GFR variability and risk of cardiovascular events and mortality in SPRINT (Systolic Blood Pressure Intervention Trial) Am J Kidney Dis. 2021;78(1):48–56. doi: 10.1053/j.ajkd.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins R.M., Tang X., Bengier A.C., Kirchner H.L., Bucaloiu I.D. Variability in estimated glomerular filtration rate is an independent risk factor for death among patients with stage 3 chronic kidney disease. Kidney Int. 2012;82(12):1332–1338. doi: 10.1038/ki.2012.281. [DOI] [PubMed] [Google Scholar]
- 5.Segar M.W., Patel R.B., Patel K.V., et al. Association of visit-to-visit variability in kidney function and serum electrolyte indexes with risk of adverse clinical outcomes among patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(1):68–77. doi: 10.1001/jamacardio.2020.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki A., Obi Y., Hayashi T., et al. Visit-to-visit variability in estimated glomerular filtration rate predicts hospitalization and death due to cardiovascular events. Clin Exp Nephrol. 2019;23(5):661–668. doi: 10.1007/s10157-019-01695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng C.L., Lafrance J.P., Lu S.E., et al. Variability in estimated glomerular filtration rate values is a risk factor in chronic kidney disease progression among patients with diabetes. BMC Nephrol. 2015;16:34. doi: 10.1186/s12882-015-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai C.W., Huang H.C., Chiang H.Y., et al. First-year estimated glomerular filtration rate variability after pre-end-stage renal disease program enrollment and adverse outcomes of chronic kidney disease. Nephrol Dial Transplant. 2019;34(12):2066–2078. doi: 10.1093/ndt/gfy200. [DOI] [PubMed] [Google Scholar]
- 9.Eriksen B.O., Palsson R., Ebert N., et al. GFR in healthy aging: an individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J Am Soc Nephrol. 2020;31(7):1602–1615. doi: 10.1681/ASN.2020020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glassock R.J., Rule A.D. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–29. doi: 10.1159/000445450. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt R., Melk A. Molecular mechanisms of renal aging. Kidney Int. 2017;92(3):569–579. doi: 10.1016/j.kint.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Weng S.C., Chen C.M., Chen Y.C., Wu M.J., Tarng D.C. Trajectory of estimated glomerular filtration rate and malnourishment predict mortality and kidney failure in older adults with chronic kidney disease. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.760391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst M.E., Fravel M.A., Webb K.L., et al. Long-term blood pressure variability and kidney function in participants of the ASPREE trial. Am J Hypertens. 2022;35(2):173–181. doi: 10.1093/ajh/hpab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil J.J., Woods R.L., Nelson M.R., et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci. 2017;72(11):1586–1593. doi: 10.1093/gerona/glw342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeil J.J. Rationale for ASPREE disability-free survival primary outcome and overview of primary outcome results. Innov Aging. 2019;3(suppl 1):S633. [Google Scholar]
- 16.McNeil J.J., Nelson M.R., Woods R.L., et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeil J.J., Wolfe R., Woods R.L., et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil J.J., Woods R.L., Nelson M.R., et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379(16):1499–1508. doi: 10.1056/NEJMoa1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett J.K., Huille R., Parker R., Yano Y., Griswold M. Estimating the association between blood pressure variability and cardiovascular disease: an application using the ARIC study. Stat Med. 2019;38(10):1855–1868. doi: 10.1002/sim.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens S.L., Wood S., Koshiaris C., et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo J.E., Shin D.W., Han K., et al. Blood pressure variability and the risk of dementia: a nationwide cohort study. Hypertension. 2020;75(4):982–990. doi: 10.1161/HYPERTENSIONAHA.119.14033. [DOI] [PubMed] [Google Scholar]
- 23.Parati G., Stergiou G.S., Dolan E., Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich) 2018;20(7):1133–1137. doi: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jufar A.H., Lankadeva Y.R., May C.N., Cochrane A.D., Bellomo R., Evans R.G. Renal functional reserve: from physiological phenomenon to clinical biomarker and beyond. Am J Physiol Regul Integr Comp Physiol. 2020;319(6):R690–R702. doi: 10.1152/ajpregu.00237.2020. [DOI] [PubMed] [Google Scholar]
- 25.Park J., Mehrotra R., Rhee C.M., et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol Dial Transplant. 2013;28(8):2146–2155. doi: 10.1093/ndt/gft213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S2; Table S1-S8.