Abstract
Introduction:Craniosynostosis is a congenital anomaly defined as early ossification of the cranial sutures. It is a rare pathology worldwide, implicitly also in our country, with a prevalence of 1:2100-1:2500. However, it represents a condition with potentially severe complications in terms of patient functionality. At the same time, not much research has been done in this field. Thus, it was considered useful to conduct a study on the epidemiology of craniosynostosis in Bihor county.
Objectives: The present study had the following objectives: updating epidemiological data; analysis of the clinical data of the study group; identification of risk factors in the occurrence of the disease; evaluating the prospects for a genetic approach to the disease, including genetic testing and genetic counseling.
Materials and method: This is a retrospective cross-sectional study. Data from a cohort of 35 patients were collected using the database which were made available by the Bihor Regional Center for Medical Genetics. Only patients with imaging-confirmed craniosynostosis in the last three decades were included in the study.
Outcomes:Most patients were diagnosed in the age range of one month – one year, the mean being 197 days. The most frequently affected suture was the sagittal suture (60%) and the least affected one the metopic suture (5%). Combined lesions were present in three cases. The majority (75%) of cases were isolated craniosynostosis, with the remaining 25% being diagnosed in the context of a genetic syndrome (most frequently Apert syndrome). Throughout the three explored decades, a significant increase in the number of cases was observed.
Conclusion:The most commonly affected groups included male patients, those from rural areas, those born after year 2000, especially from 2011 to the present. Most cases were isolated craniosynostosis. Heredo-collateral antecedents were insignificant. Three risk factors were present, including male sex, maternal smoking during pregnancy and advanced parents’ ages. Complications of the disease were rare and a minority of patients benefited from surgical treatment. Genetic counseling is an important component of disease prevention and should be offered as soon as possible.
Keywords:craniosynostozis, congenital anomaly, retrospective study.
INTRODUCTION
Craniosynostosis is a congenital anomaly defined as early ossification of the cranial sutures before brain development is complete. The direct consequence of this ossification is the abnormal development of the shape of the skull, while the brain continues to develop normally.
Notions of anatomy
There are five main cranial sutures: metopic (located on the midline of the frontal bone), sagittal (between the parietal bones), coronal (between the parietal and frontal bones) and lambdoid (between the parietal and occipital bones). Other sutures include frontoethmoidal, occipitomastoid, petrosquamous, petroclival, sphenofrontal, sphenoparietal, sphenopetrosal, sphenosquamous, sphenoethmoidal, squamous, parietomastoid and parieto-temporal sutures (1).
It is a rare pathology worldwide, implicitly also in our country, with a prevalence of 1:2100-1:2500 (2). The risk factors associated with this pathology are not very clear, but in some studies significant correlations between sagittal craniosynostosis and maternal smoking during pregnancy have been identified (3) such as those between the occurrence of metopic craniosynostosis and male sex, multiple pregnancy, prematurity, low weight at birth and emergency caesarean section (4). Maternal thyroid disease and treatment with clomiphene citrate are also contributing factors according to the CDC (5).
Also, several studies identified a relationship between the occurrence of craniosynostosis and the advanced ages of both parents (6, 7). Maternal alcohol consumption as well as uterine defects have been blamed in some cases.
Embryological development of cranial sutures
Cranial sutures are made of fibrous tissue and are located between the cranial bones. They are flexible, which on the one hand facilitates birth through the vaginal canal by temporarily deforming the bones, and on the other hand, they allow the expansion of the cranial vault during brain development. The process of morphological development begins in the 20th week of gestation and is completed in adulthood.
The development of the cranial vault begins with a precondensation phase that results in the appearance of cells of two different origins: the paraaxial mesoderm and the neural crest (8). Neural crest cells are a group of multipotent specialized cells which possess the ability to migrate (9).
Condensation is the second phase of development. This involves the aggregation of undifferentiated mesenchymal cells around the sutures. Condensation of mesenchymal cells triggers the proliferation of chondrocytes and perichondrial cells. During the ossification process, several transcription factors are important such as RUNX2 and OSX in osteoblastic development, transforming growth factor beta (TGF-β) in inhibiting chondrocyte proliferation, and MSX2 in inhibiting calvarial osteoblast differentiation. The main regulatory pathway of the differentiation process is the fibroblast growth factor (FGF) signaling pathway. Overgrowth of bone tissue from unmineralized bone matrix is a likely cause of premature suture fusion.
During the embryonic period, multiple signaling pathways (FGF, TGF-β, Wnt) require proper functioning for calvarial development. In addition, the presence of osteoinhibitory and osteoinductive substances is also necessary for the formation of cranial sutures, including soluble heparin-binding factors, FGF2, and isoforms of TGF-β secreted by dura mater cells (10). These molecules regulate the growth and formation of the membranous skull bones.
Animal studies (11) have shown that TGF-β2 had an osteoinductive role, while TGF-â3 had an osteoinhibitory role in terms of suture formation. The TGF-âs superfamily is a key factor in the process of suture fusion. It includes more than 30 members and is involved in the regulation of cell proliferation, cell differentiation, and various other embryological developmental processes (12). This is grouped into three classes: TGF-β1, which includes bone morphogenic protein (BMP), TGF-β2, and TGF-β3. There is a notable lack of published genetic studies identifying mutations in these genes associated with known craniosynostosis syndromes.
In general, skull bones undergo a process of intramembranous ossification, in which mesenchymal stem cells (MSCs) differentiate into osteoblasts to form bone tissues. Mesenchymal stem cells are produced in the hematogenous marrow, with their differentiation into osteogenic, chondrogenic, adipogenic or myogenic lineages being controlled by various signaling pathways. Of note, a 2016 study (13) identified a significant involvement of TGF-â, BMP, Wnt, Hedgehog, FGF, and Notch pathways in directing MSCs to bone developmental lineages. In addition, the most recent studies demonstrated the role of the TWIST protein in the regulation and differentiation of sutural MSCs (14), respectively of Axin2, whose expression was elevated in a certain population of MSCs in the sutures of the median line (15). In addition to the abovementioned data, the signaling pathways also play a major role in the actual processes of intramembranous and enchondral ossification, regulating cell proliferation, differentiation and migration during the intraembryonic period. The FGF family comprises of 23 members that bind to five different types of receptors (FGFR1-4 and FGFRL) and thus, induce cellular responses. Among these receptors, FGFR1 is involved in regulating osteogenic differentiation and FGFR2 orchestrates stem cell proliferation. FGFR2 mutations are the most common FGFR mutations that have been identified in syndromic craniosynostosis (16).
Inhibitors of the FGF pathway are also important in the process of skull development. The majority of identified FGFR mutations in craniosynostosis are gain-of-function, whereas TWIST1 mutations are loss-of-function. There is evidence that TWIST1 protein heterodimerizes with TCF12 in the cell nucleus and inhibits RUNX2 (runt-related transcription factor 2) activation. The RUNX2 gene encodes the RUNX2 protein, a transcription factor essential in bone and cartilage development. Thus, the RUNX2 gene is considered to have a considerable influence on osteoblasts in bone development (17).
Clinical aspects
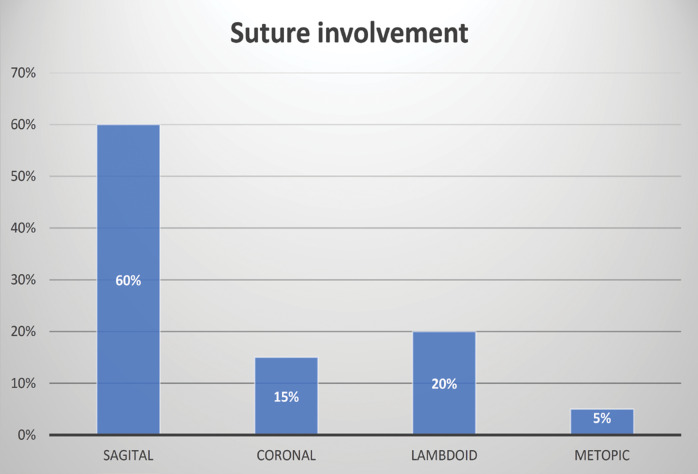
There are four types of craniosynostosis that correspond to the cranial sutures, including sagittal, coronal, lambdoid and metopic sutures. The sagittal suture is the most frequently affected one (18), having a frequency between 41% and 68% of all forms of craniosynostosis.
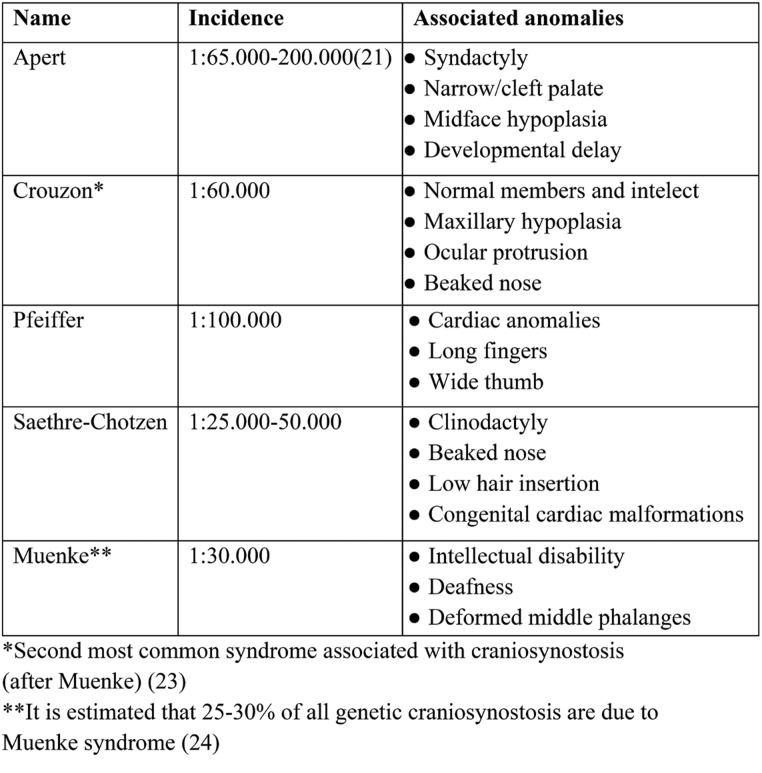
From a clinical point of view, craniosynostoses are classified as syndromatic [approximately 15% of cases (19), which involve the association with multiple morphological anomalies, respectively non-syndromatic [73-85% (16, 17)] (20). Although more than 200 syndromes associated with this malformation have been identified, five were the most commonnly: found, including Apert, Crouzon, Pfeiffer, Saethre-Chotzen and Muenke syndromes. All of these syndromes have fibroblast growth factor receptor (FGFR) mutations, except for Saethre-Chotzen syndrome, in which TWIST1 is the affected gene is TWIST1. A clinical summary of these conditions is presented in Table 1.
Diagnostic
Clinical diagnosis is based on inspection and palpation of the skull. Transfontanel ultrasound cannot always be performed due to the advanced ossification process. The certainty diagnosis is based on the computed tomography (CT) scan. Although magnetic resonance imaging (MRI) is a more desirable imaging investigation for children, it does not adequately highlight bone tissue, is slow, and has high costs.
Molecular diagnosis should be offered to patients with a known diagnosis of craniosynostosis within a genetic syndrome. Mutations within genes listed in Table 1 can be identified today. Johnson and Wilkie recommend that at least the FGFR (exon 7 p.Pro250Arg mutation) and FGFR2 (exons 7, 8) genes be tested in patients with multiple suture involvement or involvement of the coronary suture. If point mutations are not identified, Whole Genome or Exome Sequencing (WGS/WES) can be used. Some sources (25) directly recommend these techniques because of the significant heterogeneity in the number of mutations that can be identified.
Genetic counseling
It is necessary to differentiate isolated craniosynostosis from that within a genetic syndrome. Most syndromic forms are transmitted in an autosomal dominant manner, but most cases show de novo mutations. Somatic mosaicism is rare, but it is recommended that the patients' parents be tested for the presence of mutations, as the risk of recurrence in these families is higher than in the general population. In the rare cases of autosomal recessive transmission, the risk of recurrence is 25%.
If the family history is negative for craniosynostosis and no genetic changes have been identified, the risk of recurrence is approximately 5% for isolated cases affecting the metopic or sagittal suture, and 30-50% for cases involving multiple sutures. If the mutation occurs in only one gene (cannot be identified in both parents), the risk of recurrence is very low (<1%). A slightly higher risk was reported for TWIST1 mutations – 2%. (26).
Premise and objective of research
Craniosynostosis is a well-known congenital anomaly that creates a serious disability unless diagnosed and treated in time. Better knowledge of the clinical, epidemiological and, more recently, genetic mechanisms can prevent the onset of the disease and improve the prognosis and evolution.
The aim of this study is to have a more accurate knowledge of the clinical, genetic and epidemiological aspects of CS in an area circumscribed by Bihor county.
The objectives of the present study included updating epidemiological data; analysis of the clinical data of the study group; identification of risk factors in the occurrence of the disease; and evaluation of the prospects for a genetic approach to the disease, including genetic testing and genetic counseling.
MATERIALS AND METHODS
This is a retrospective cross-sectional study. Data from a cohort of 35 patients were collected using the database which was made available by the Bihor Regional Center for Medical Genetics and included demographic data, clinical data and treatments.
The following inclusion criteria were used: inclusion in the institutional database; craniosynostosis clinical diagnosis confirmed by imaging; survival until the start of the study (2016) of diagnosed patients; year of birth in the last three decades.
The following exclusion criteria were used: patients born before 1987; inclusion in the database after the start of the study; patients with an uncertain diagnosis of craniosynostosis; patients who did not survive to the start of the study.
In order to process the study data, softwares from the Office 365 package (Word, Excel) and R were used.
OUTCOMES
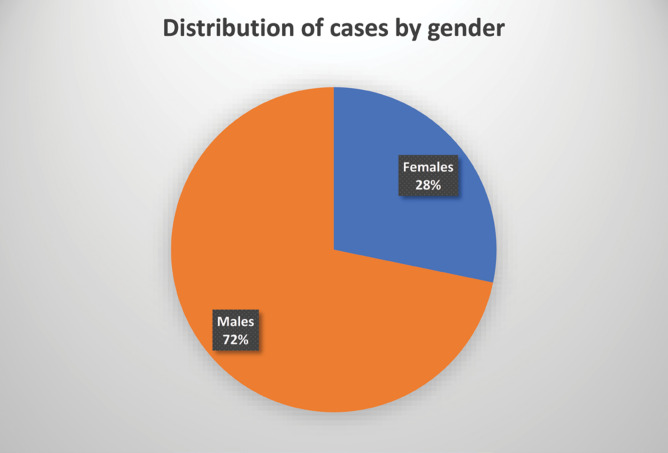
The study included 35 patients, of which 25 were males and 10 females (Figure 1).
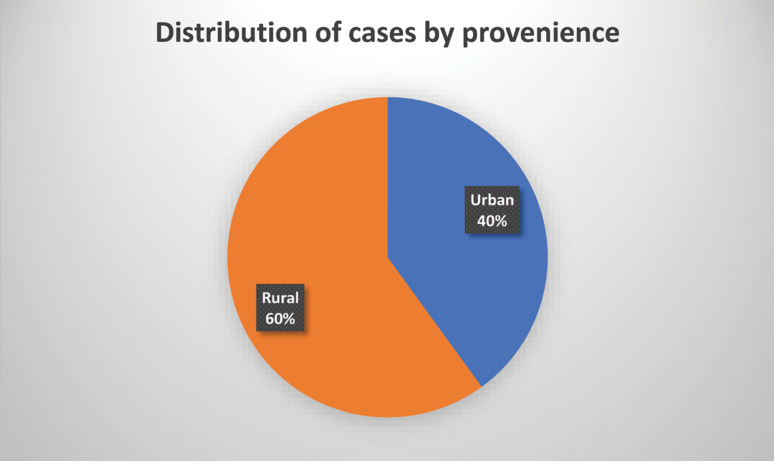
Most of the patients came from a rural environment (21), while 14 were from an urban environment (Figure 2).
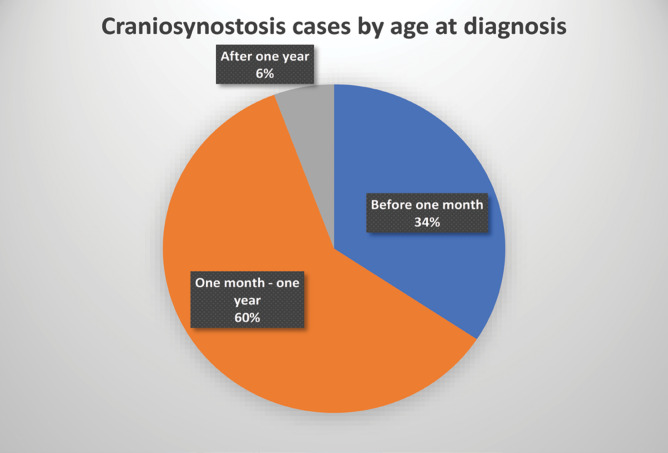
Regarding the age at diagnosis, there were three categories: at birth or before one month, less than one year, and after one year. Most cases (21) belonged to the second category and the fewest to the third (2). The mean age at diagnosis was 197 days, with extreme limits of one day and 152 days, respectively (Figure 3). There were no statistically significant differences between the mean age in girls compared to boys (p > 0.05).
The sagittal suture was most frequently affected (in 26 cases), followed by the lambdoid (seven cases), coronal (five cases) and metopic (two cases) suture (Figure 4). Combined lesions were recorded in three cases: one case presented an association between the sagittal suture and the lambdoid, and two cases, the coronal suture associated with the metopic.
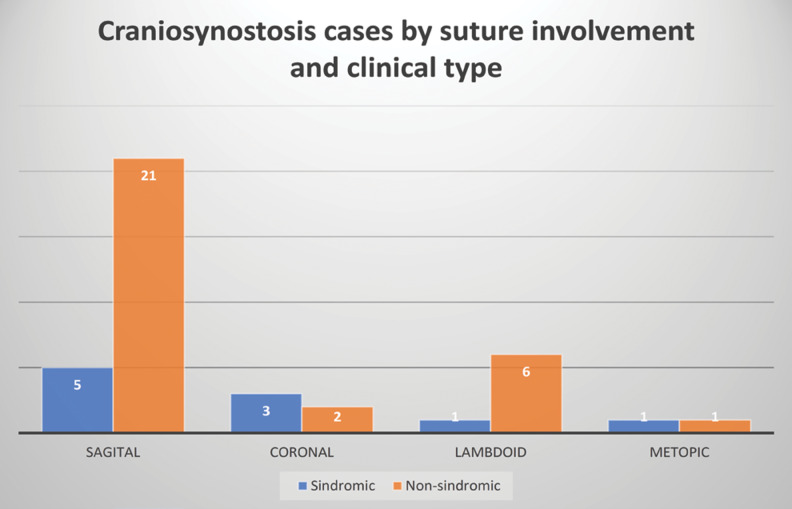
Ten (25%) of all cases were in the context of a syndrome (five cases of Apert syndrome, three cases of Muenke syndrome and two cases of Crouzon syndrome). Within this category, the sagittal suture was the most frequently affected, followed by the lambdoid suture, and the coronal and metopic sutures were relatively equal in frequency (Figure 5).
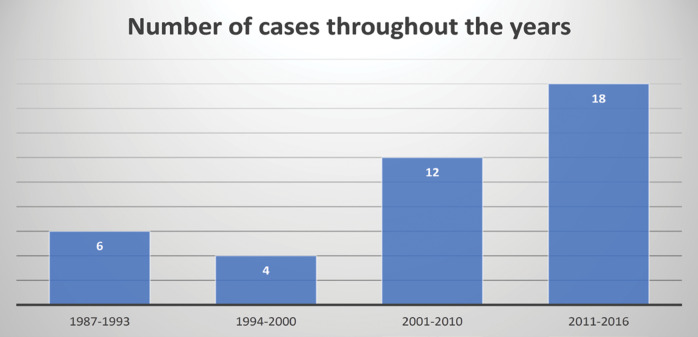
The date of diagnosis in the Genetic Service was divided into three periods: 1987-2000, 2001-2010 and 2011-2016. Their distribution is shown in Figure 7. An increasing trend was found over the years, most (75%) of cases being diagnosed after the year 2000. In the last period (2011-2016), the number of cases increased three times compared to the first period (1987-1993), which represented an increase of 300%. This increase was statistically significant (p < 0.05).
Only two of the 35 cases (5.7%) had a family history of craniosynostosis.
Risk factors (other than male sex) have not been identified except for maternal smoking during pregnancy (11 mothers, representing 31.42%). Advanced ages of both parents have also been noticed but with low frequencies (two couples with both parents over 35 years old, three couples wherein the mother was under 30 but the father over 35 years old and one couple wherein the mother was over 30 and the father over 35 years old).
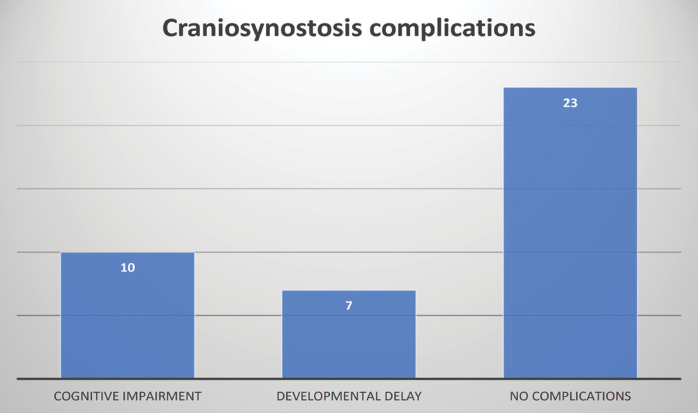
With regards to the consequent damage to the brain due to craniosynostosis, the frequency of the occurrence of cognitive impairment and developmental delay was analyzed. Most patients did not present these complications (65.71%). Their distribution can be seen in Figure 7.
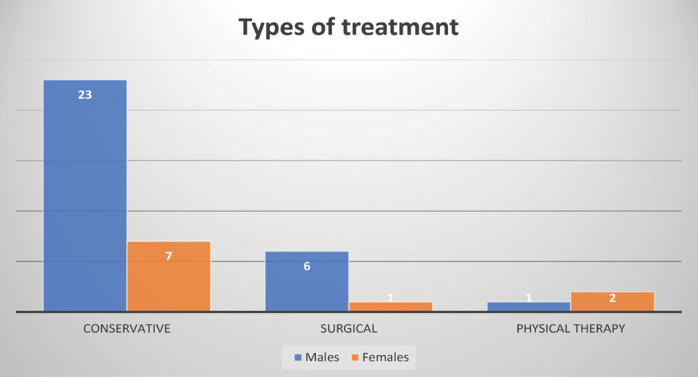
Surgery was not performed in most cases due to less severe deformities and parents’ refusal due to concerns regarding surgical risks, which is why most patients benefited from conservative treatment (77.14%). Some patients underwent physical or occupational therapy (7.5%) for associated hand anomalies (such as those with Apert syndrome).
From the group of those undergoing surgery, the majority were males.
The distribution of treatments is shown in Figure 9.
All patients benefited from genetic counselling. Unfortunately, no genetic testing was performed as molecular testing has not always been available in Romania and the financial aspect was and continues to be an obstacle for all families.
DISCUSSION
Strictly respecting the imposed criteria, our study includes a cohort of 35 cases, representative of the geographical reference region. Similar studies (27) from our country refer to the surgical treatment of craniosynostosis of the sagittal suture, from the Bucharest area (Bagdasar-Arseni University Emergency Clinic Hospital) on groups of 231 patients. The respective cases were treated over a period of 15 years (1997-2012), 120 (52%) being sagittal suture cases. A study carried out in Vietnam between 2015 and 2019(28) included 76 patients, mostly males, with a male:female ratio of 3.3:1, close to our group, which recorded a ratio of 2.5:1. Similarly to the cited study, the sagittal suture was most commonly affected (50% vs. 60% in our study). A similar study (29) from Saxony-Anhalt (Germany), that collected data from 2000–2017, included 91 cases of craniosynostosis, with a rate of 4.8 cases per 10,000 live births; 75 patients were males, with a male:female ratio of 4.7:1. From a clinical point of view, 51 isolated craniosynostoses were identified (56.04% versus 71.42% in our study), of which five presented multisutural involvement (5.49% versus 8.57% in our study), 29 craniosynostoses associated with other congenital malformations and 11 within genetic syndromes compared to 28.57% in our study. The mean age at diagnosis was 5.5 months versus 197 days in our study. Our study highlights a close distribution of these clinical features. Significant differences were found regarding the trend of the appearance of new cases: if Neusel et al (29) did not identify increases in prevalence in recent years, in our study we identified a significant increase. This evolutionary trend was noted by many other studies in the literature, for example Tonne et al (17), where the incidence was 5.5 per 10,000 live newborns (1/1800) and increased significantly in the last five years (data being collected from 2003-2017). Notably, this increase occurred almost exclusively in the isolated craniosynostosis group. Among the 328 patients, the majority were men in this case as well (2:1) and 9.5% were familial cases, a much higher frequency than in our study (5.7%). Another study from 2019 (30) found a higher rate of cases with a positive family history (14.7%). In this study, most patients were males as well, except for complex isolated craniosynostosis (equal ratios between the two sexes). An interesting aspect was the damage to the metopic suture most frequently, a contradictory result of our research and less common in the literature. On the other hand, in a study conducted in the Netherlands between 2008-2013 Cornelissen et al (31) found a much higher number of craniosynostosis, 759, with the frequency being 7.2 per 10,000 live newborns. Cases of secondary craniosynostosis, for example, ventriculoperitoneal shunts or microcephaly, were excluded from this study. Also, patients born in other countries but treated in the Netherlands, as well as cases with mild trigonocephaly without an indication for surgical intervention, were also not included in the study. The sagittal suture was the most frequently affected (44%). Poisson regression revealed a statistically significant increase in prevalence from 1997 to 2013. The large differences between this study and ours could be explained by the different genetic predisposition of the two populations as well as the regions included (national versus regional study).
A meta-analysis by Shlobin et al (32) that included 24 studies identified a prevalence of craniosynostosis of 5.9 per 10,000 live newborns (95% confidence interval [CI]: 3.9–8.4; I2 = 100%). Isolated craniosynostoses had a lower prevalence of 5.2 per 10,000 live births (95% CI: 3.4–7.3; I2 = 98%); it was estimated that, in 2019, 84,665 children were born with craniosynostosis globally (95% CI: 55,965–120,540), and of these, 72,857 (86%) were isolated cases.
The high rate of conservative management in our study was in part due to refusal but at the same time, many cases were mild isolated craniosynostosis cases. A recent Canadian study (32) compared the quality of life in patients who underwent surgical treatment to those who did not across two surgical centers. One of them had a rate of 22% conservative treatments, while the other one 37%. Most parents who opted for surgical treatment did so because of severe appearance while most parents of children treated conservatively did so because of mild appearance and concerns regarding surgical risks. The study could not identify an independent association between quality of life and treatment type (surgical vs conservative); however, it concluded that families in Alberta had a high number of craniosynostosis cases treated with conservative management.
In recent years, various molecules potentially effective in the non-surgical treatment of craniosynostosis have been studied. Considering the importance of FGFR2 and 1 mutations in the occurrence of this pathology, most studies have tested FGFR-tyrosine kinase inhibitor therapy in vitro, for example Eswarakumar et al (34) who used PLX052 with positive results – prevention of early coronary suture fusion. On the other hand, the study by Perlyn et al (35) with the inhibitor PD173074 revealed a lack of response of coronary craniosynostosis to treatment. Another therapeutic target is the TGF-â pathway; this was used by Mooney et al (36), respectively Frazier et al (37), testing a TGF-â2 neutralizing antibody in an animal model (rabbits) with surgically repaired bilateral craniosynostosis. The antibody prevented postoperative resinostosis and improved intracranial volume and cranial vault growth. Finally, in vivo studies focusing on the NOGGIN pathway (38, 39) had the effect of inhibiting the premature fusion of sutures, respectively decreasing postoperatory recurrences, suggesting an adjunct role to surgical treatment (39).
The contributions of this study include carrying out the only epidemiological-clinical analysis of this condition in Bihor county, and one of the few in Romania.
The limitations of this study were related to the difficulty in obtaining data on clinical evolution over a longer period of time, mainly due to the non-cooperative attitude of the majority of patients. Moreover, the absence of genetic analyses due to lack of funding and limited accessibility in the area was an important aspect. Unfortunately newer technologies are not available or routinely performed in our country so that many pediatric patients remain without a clear diagnosis (40).
CONCLUSIONS
This study identified 35 cases of craniosynostosis diagnosed in Bihor county over a period of 29 years. The most frequently affected groups comprised male patients, rural patients and those born after 2000, especially from 2011 to the present. Clinically, most cases of craniosynostosis were isolated congenital anomalies. Positive heredo-collateral antecedents of craniosynostosis were insignificant. The only identified risk factors were male sex, maternal smoking during pregnancy and advanced parents’ ages. The sagittal suture was most frequently involved, both in syndromic and isolated cases, and the metopic suture was least frequently involved in both cases. Complications due to craniosynostosis were rare, as was surgical treatment. Genetic testing could not be perfomed on our patients but can be an essential component in some cases and should be more accessible. Genetic counseling is an important component of disease prevention and should be offered to affected patients as soon as possible.
Conflict of interests: none declared.
Financial support: none declared.
TABLE 1.
Genetic syndromes associated with craniosynostosis
FIGURE 1.
Distribution of patients by gender
FIGURE 2.
Distribution of patients according to the environment of origin
FIGURE 3.
Distribution of patients according to age at diagnosis
FIGURE 4.
Distribution of patients according to age at diagnosis
FIGURE 5.
Distribution of cases by clinical type of craniosynostosis and suture involvement
FIGURE 6.
Multiannual distribution of newly registered cases
FIGURE 7.
Distribution of craniosynostosis complications
FIGURE 8.
Distribution of treatments performed according to gender
Contributor Information
Claudia-Victoria SOMOLAN, ”Prof. Dr. Ioan Puscas” City Hospital, Simleu Silvaniei, Romania.
Codruta Diana PETCHESI, University of Oradea, Faculty of Medicine and Pharmacy, Oradea, Romania.
Claudia JURCA, University of Oradea, Faculty of Medicine and Pharmacy, Oradea, Romania.
Marius BEMBEA, University of Oradea, Faculty of Medicine and Pharmacy, Oradea, Romania.
References
- 2.van de Lande LS, Greig AVH, Dunaway DJ. Craniosynostosis. Plastic Surgery – Principles and Practice, 2021.
- 4.Lee HQ, Hutson JM, Wray AC, et al. Changing epidemiology of nonsyndromic craniosynostosis and revisiting the risk. Journal of Craniofacial Surgery, 2012. [DOI] [PubMed]
- 6.Gill SK, Broussard C, Devine O, et al. Association between Maternal Age and Birth Defects of Unknown Etiology - United States, 1997–2007. Birth Defects Res A Clin Mol Teratol. [DOI] [PMC free article] [PubMed]
- 9.Jin SW, Sim KB, Kim SD. Development and Growth of the Normal Cranial Vault : An Embryologic Review. J Korean Neurosurg Soc, 2016. [DOI] [PMC free article] [PubMed]
- 10.Levi B, Wan DC, Wong VW, et al. Cranial suture biology: From pathways to patient care. Journal of Craniofacial Surgery 2012. [DOI] [PubMed]
- 11.Opperman LA, Adab K, Gakunga PT. Transforming Growth Factor-2 and TGF-3 Regulate Fetal Rat Cranial Suture Morphogenesis by Regulating Rates of Cell Proliferation and Apoptosis. Dev Dyn. 2000;219:237–247. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1044>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Roth DA, Longaker MT, Mccarthy JG, et al. Studies in Cranial Suture Biology: Part I. Increased Immunoreactivity for TGF-β Isoforms (β1, β2, and β3) During Rat Cranial Suture Fusion. Journal of Bone and Mineral Research, [Internet]. 1997 Mar 1 [cited 2022 Apr 20] [DOI] [PubMed]
- 13.Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death & Differentiation. 2016;23:7. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsianou MA, Adamopoulos C, Vastardis H, Basdra EK. Signaling mechanisms implicated in cranial sutures pathophysiology: Craniosynostosis. BBA Clin, 2016. [DOI] [PMC free article] [PubMed]
- 15.Maruyama T, Jeong J, Sheu T, Hsu W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat Commun. 2016;1:10526. doi: 10.1038/ncomms10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoury SC, Reddy S, Shukla V, Deng CX. Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation Related Syndromic Craniosynostosis. Int J Biol Sci. [DOI] [PMC free article] [PubMed]
- 17.Cuellar A, Bala K, di Pietro L, et al. Gain-of-function variants and overexpression of RUNX2 in patients with nonsyndromic midline craniosynostosis. Bone. [DOI] [PMC free article] [PubMed]
- 18.Gonzalez SR, Light JG, Golinko MS. Assessment of Epidemiological Trends in Craniosynostosis: Limitations of the Current Classification System. Plast Reconstr Surg Glob Open. [DOI] [PMC free article] [PubMed]
- 19.Timberlake AT, Persing JA. Genetics of nonsyndromic craniosynostosis. Plast Reconstr Surg. [DOI] [PubMed]
- 20.Tønne E, Due-Tønnessen BJ, Wiig U, Stadheim BF, et al. Epidemiology of craniosynostosis in Norway. J Neurosurg Pediatr. [DOI] [PubMed]
- 21.Fearon JA. Treatment of the hands and feet in Apert syndrome: an evolution in management. Plast Reconstr Surg. [DOI] [PubMed]
- 22.den Ottelander BK, van Veelen MLC, de Goederen R, et al. Saethre–Chotzen syndrome: long-term outcome of a syndrome-specific management protocol. Dev Med Child Neurol. [DOI] [PMC free article] [PubMed]
- 23.Rannan-Eliya SV, Taylor IB, de Heer IM, et al. Paternal origin of FGFR3 mutations in Muenke-type craniosynostosis. Hum Genet. [DOI] [PubMed]
- 24.Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. [DOI] [PMC free article] [PubMed]
- 25.Miller KA, Twigg SRF, McGowan SJ, et al. Diagnostic value of exome and whole genome sequencing in craniosynostosis. J Med Genet. [DOI] [PMC free article] [PubMed]
- 26.Kutkowska-Kaźmierczak A, Gos M, Obersztyn E. Craniosynostosis as a clinical and diagnostic problem: molecular pathology and genetic counseling. Journal of Applied Genetics. 2018;59:2. doi: 10.1007/s13353-017-0423-4. [DOI] [PubMed] [Google Scholar]
- 27.Nica D, Mohan A, Ciurea AM, et al. Tratamentul modern al scafocefaliei. Experiența pe 120 de cazuri Modern Treatment of Scaphocephaly. Experience based on 120 cases. Revista de Neurologie şi Psihiatrie a Copilului şi Adolescentului din România.
- 28.Can DDT, Lepard JR, Anh NM, et al. Nonsyndromic craniosynostosis in Vietnam: initial surgical outcomes of subspecialty mentorship. J Neurosurg Pediatr. [DOI] [PubMed]
- 29.Neusel C, Class D, Eckert AW, et al. Multicentre approach to epidemiological aspects of craniosynostosis in Germany. Br J Oral Maxillofac Surg. 2018;56:881–886. doi: 10.1016/j.bjoms.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Hormozi H, Abbaszadeh-Kasbi A, Sharifi G, et al. Incidence of Familial Craniosynostosis Among Patients With Nonsyndromic Craniosynostosis. Journal of Craniofacial Surgery. [DOI] [PubMed]
- 31.Cornelissen M, Ottelander B den, Rizopoulos D, et al. Increase of prevalence of craniosynostosis. J Craniomaxillofac Surg. 2016;44:1273–1279. doi: 10.1016/j.jcms.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Shlobin NA, Baticulon RE, Ortega CA, et al. Global Epidemiology of Craniosynostosis: A Systematic Review and Meta-Analysis. World Neurosurg. 2022;164:413–423. doi: 10.1016/j.wneu.2022.05.093. [DOI] [PubMed] [Google Scholar]
- 33.Sader N, Mehta V, Hart S, Bliss L, et al. Quality of life and satisfaction in surgical versus conservative treatment of nonsyndromic children with craniosynostosis. J Neurosurg Pediatr. [DOI] [PubMed]
- 34.Eswarakumar VP, Özcan F, Lew ED, Bae JH, et al. Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed]
- 35.Perlyn CA, Morriss-Kay G, Darvann T, et al. A model for the pharmacological treatment of Crouzon syndrome. Neurosurgery. [DOI] [PMC free article] [PubMed]
- 37.Frazier K, Thomas R, Scicchitano M, et al. Inhibition of ALK5 signaling induces physeal dysplasia in rats. Toxicol Pathol. [DOI] [PubMed]
- 38.Shen K, Krakora SM, Cunningham M, et al. Medical treatment of craniosynostosis: Recombinant Noggin inhibits coronal suture closure in the rat craniosynostosis model. Orthod Craniofac Res. [DOI] [PMC free article] [PubMed]
- 39.Huard J, Cooper GM, Usas A, et al. Ex vivo noggin gene therapy inhibits bone formation in a mouse model of postoperative resynostosis. Plast Reconstr Surg. [DOI] [PubMed]
- 40.Duicu C. Genetic testing in pediatrics-a narrative essay of challenges and possibilities in Romania. Rev Rom Med Lab. 2019;27 [Google Scholar]