Abstract
Sialic-acid-binding immunoglobulin-like lectins are cell surface immune receptors known as Siglecs that play a paramount role as modulators of immunity. In recent years, research has underscored how the underlaying biology of this family of receptors influences the outcome of viral infections. While Siglecs are needed to promote effective antiviral immune responses, they can also pave the way to viral dissemination within tissues. Here, we review how recent preclinical findings focusing on the interplay between Siglecs and viruses may translate into promising broad-spectrum therapeutic interventions or key biomarkers to monitor the course of viral infections.
Keywords: Siglecs, HIV-1, Ebola virus, SARS-CoV-2, Antiviral
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- COVID-19
Coronavirus disease 2019
- DCs
dendritic cells
- DC-SIGN
Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin
- EBOV
Ebola virus
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- GM1
Ganglioside 1 (monosialotetrahexosylganglioside)
- GM3
Ganglioside 3 (monosialodihexosylganglioside)
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV-1
Human immunodeficiency virus
- HSV
Herpes simplex virus
- IFN
Interferon
- ITIM
Tyrosine-based inhibitory motives
- LSECtin
Liver sinusoidal endothelial cell lectin
- LPS
Lipopolysaccharide;
- MAG
Myelin-associated glycoprotein
- MLV
Murine leukemia virus
- NK
Natural killer cells
- NTD
N-terminal domain
- PRRSV
Porcine reproductive and respiratory syndrome virus
- RBD
Receptor Binding Domain
- RSV
Respiratory syncytial virus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- Siglecs
Sialic acid binding immunoglobulin-like lectins
- TLR
Toll-like receptor
- VCC
Virus-containing compartment
- VSV
Vesicular stomatitis virus
- VZV
Varicella-zoster virus
1. Introduction
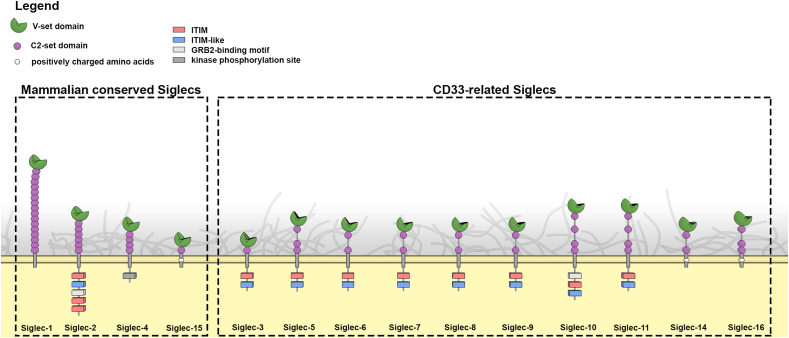
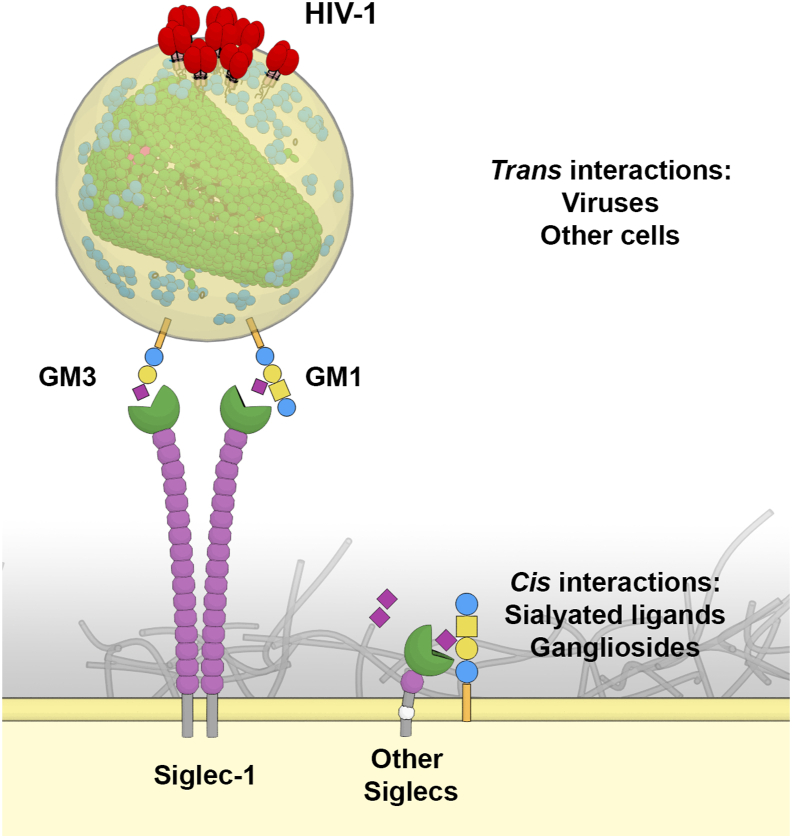
The sialic acid binding immunoglobulin-like lectins or Siglecs are immune membrane receptors that have the capacity to interact with sialylated glycans that decorate proteins or lipids and are exposed on the cellular membranes. The recognition between Siglecs and their counterpart ligands facilitates the modulation of immune responses, triggering key cellular contacts that influence the outcome of adaptative immune responses. Siglec-1 together with Siglec-2, -4 and -15 belongs to a sub-family of Siglecs structurally conserved among vertebrates (Fig. 1). The rest of Siglecs, including −3, 5–12, −14 and −16 are referred as CD33-related, since they share similar extracellular domains that vary in number between species (Pillai et al., 2012). The structural properties of each Siglec receptor determine their functional ability to interact with particular sialylated ligands through their terminal V-set domain (Fig. 2). These interactions can be established either with molecules located in the same cell where they are expressed (in cis), or with distant counterpart ligands expressed on the surface of other cells (in trans).
Fig. 1.
Structural overview of Siglec proteins. Conserved and CD33-related Siglecs contain a V-set domain interacting with syalilated ligands and a variable number of C2-set domains that project the receptors extracellularly. Transmembrane and intracellular moieties are also indicated. Siglec-12 is not included given that it does not recognize sialic acids in humans (Angata et al., 2001). ITIM: immunoreceptor tyrosine-based inhibitory motif, GRB2: growth-factor-receptor-bound protein 2.
Fig. 2.
Trans and cis ligand recognition of Siglec proteins. All Siglecs recognize sialylated ligands. Siglec-1 is the only Siglec who's V-set domain is projected to recognize sialylated molecules on the surface of other cells or viruses, such as HIV-1. High avidity interactions are established via clustering of Siglec-1 molecules that recognize thousands of gangliosides in the viral membrane. Other Siglec members interact mostly with sialylated molecules exposed on the surface of the same cell.
Recognition of sialylated ligands exposed on the surface of different pathogens is also a key function of Siglecs. Detected pathogens includes a wide range of microorganisms such as enveloped viruses, whose sensing by Siglecs can either promote or subvert immune responses. In recent years, numerous studies have addressed the role of Siglecs during viral infections, allowing us to understand their capacity to initiate innate immunity or modulate adaptative immune responses against infection (Crocker et al., 2007; Macauley et al., 2014). Yet, these studies have also underscored the capacity of very different viruses, including HIV-1, SARS-CoV-2 and Ebola virus (EBOV), to escape these immune defenses and exploit Siglec biology to promote viral dissemination (Perez-Zsolt et al., 2019b, 2021; Rempel et al., 2008). While the therapeutic potential of Siglec receptors is currently being explored in the fields of cancer and autoimmune diseases (Murugesan et al., 2021), we still lack any Siglec-related therapy to treat viral infections. Moreover, currently there is no registered clinical trial to address the diagnostic or therapeutic value of these lectins in infectious diseases (ClinicalTrial.gov, accession on May 28, 2022). Despite this, promising preclinical studies have been carried out in recent years that may facilitate future clinical development of these molecules for infectious diseases. Here, we review the effects of a double-edged sword interaction between Siglecs and viruses, and highlight how this interplay can impact the course of viral infections to counteract or even promote pathogenesis, but also offer novel therapeutic solutions.
2. The first and the largest I-type lectin receptor: Siglec-1
Siglec-1 (CD169 or Sialoadhesin) was the first member of the family identified by Paul Crocker and colleagues in the early 90s (Crocker et al., 1991, 1994). It is the Siglec member with the longest extracellular part composed of 17 immunoglobulin-like domains and contains a terminal V-set domain (Fig. 1). The numerous Ig-like domains that separate the ligand-binding site from the trans-membrane domain favor the interaction with external ligands in trans and avoids binding to cell-surface molecules in cis, as it happens with shorter members of the Siglec family (Fig. 2) (Crocker et al., 2007). Its cytoplasmic domain lacks a tyrosine containing motif and therefore does not trigger an intracellular signaling cascade (Crocker et al., 2007).
Siglec-1 is expressed in immune cells such as dendritic cells (DCs), macrophages and monocytes, and it binds to multiple target cells including granulocytes, natural killer cells (NK) (Crocker et al., 2007; Hartnell et al., 2001), B-cells and erythrocytes and T-cells. Thus, this receptor participates in cell-to-cell signaling via recognition of sialylated counterparts to modulate cellular immune responses (Macauley et al., 2014; Pillai et al., 2012). Siglec-1 is an interferon (IFN)-inducible receptor (Rempel et al., 2008), and, consequently, immune activating signals present throughout the course of viral infections increase the expression of Siglec-1 in myeloid cells and contribute to the modulatory capacity of this receptor (Pulliam et al., 2004; Rempel et al., 2008).
Siglec-1 on myeloid cells also captures extracellular vesicles via recognition of sialylated lipids exposed on the membrane of these particles (Izquierdo-Useros et al., 2009, 2012b). These extracellular vesicles are released by different cellular types and mediate antigen transfer between immune cells such as DCs, promoting the initiation of adaptive immunity against invading viruses (Théry et al., 2002, 2009). Yet, enveloped viruses can also interact with Siglec-1 via recognition of viral glycolipids such as gangliosides containing specific sialylated moieties (Izquierdo-Useros et al., 2012a; Puryear et al., 2012). These gangliosides are ubiquitous lipids present in the biological membranes where viral budding occurs (Bavari et al., 2002; Nguyen and Hildreth, 2000), and as it happens with extracellular vesicles, the incorporation of such ligands in the viral membrane facilitates an efficient viral capture via Siglec-1 that could help to control viral replication.
Hence, Siglec-1 also regulates immune responses against viral infections. Macrophages expressing Siglec-1 are involved in cross-presenting vaccinia virus antigens to a specific DC subset, which induce effector and memory CD8+ and CD4+ T cells mediated-immunity (van Dinther et al., 2018). In a murine model of retroviral infection, Siglec-1 expressing macrophages triggered cytotoxic CD8+ T cell responses and prevented Friend virus complex spreading (Uchil et al., 2019). For the respiratory syncytial virus (RSV), the major cause of respiratory viral infections in infants, Siglec-1+ alveolar macrophages trigger early innate protection and recruitment of effector CD8+ T-cells to the lung after RSV infection (Oh et al., 2017). Vesicular stomatitis virus (VSV) also infects macrophages expressing Siglec-1, initiating type I IFN responses and limiting virus propagation to the central nervous system (Iannacone et al., 2010; Junt et al., 2007). The absence of Siglec-1 expressing macrophages hampers antigen uptake, leading to a decrease in the CD8+ T-cell responses against VSV (Camara et al., 2022). Similar observations have been done for the lymphocytic choriomeningitis virus (LCMV) infection, where Siglec-1 expressing macrophages produce type I IFN that regulates PD-L1 expression, avoiding viral propagation and preventing acute infection (Shaabani et al., 2016). However, viral capture mediated by Siglec-1 does not always lead to an effective immune response, and can also favor viral evasion or dissemination within tissues. In the following sections, we will review how different enveloped viruses that constitute a major threat to human health incorporate sialylated gangliosides on their membranes and directly interact with Siglec-1, offering a getaway for viral evasion and dissemination of infectivity within tissues.
2.1. Siglec-1 and HIV-1 trans-infection
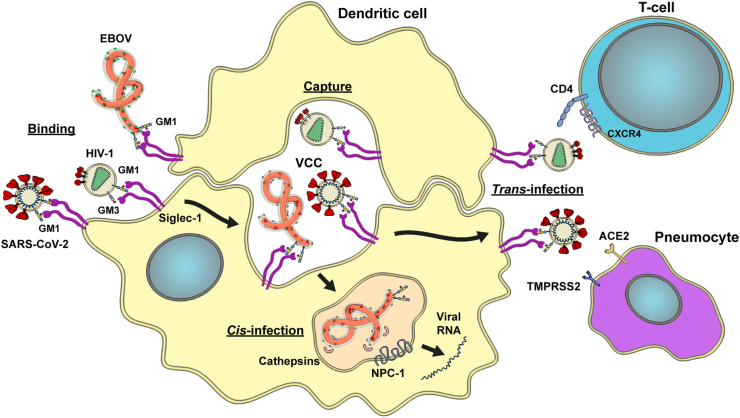
The first evidence of how Siglec-1 promotes viral infection of target cells came from the field of HIV-1 cell-to-cell transmission. This retrovirus is responsible for the AIDS pandemic and preferentially infects CD4+ T cell lymphocytes expressing CCR5 or CXCR4 co-receptors. Although Siglec-1 expressing myeloid cells are also susceptible to HIV-1 infection (Perot et al., 2020; Ruffin et al., 2019), several restriction factors limit productive viral replication (Hrecka et al., 2011; Laguette et al., 2011). However, via Siglec-1, these cells can trap HIV-1 (Izquierdo-Useros et al., 2012b; Puryear et al., 2013), which display specific gangliosides in their enveloped membranes. Gangliosides, such as GM3 or GM1, expose the sialyllactose moiety recognized by Siglec-1 (Izquierdo-Useros et al., 2012a; Puryear et al., 2012). These virions are then trafficked by Siglec-1 to a virus-containing compartment (VCC) (Izquierdo-Useros et al., 2011; Yu et al., 2008). In primary cervical tissues from HIV-1-infected individuals, cell-associated viral accumulations have been identified in Siglec-1-positive compartments, confirming the formation of VCCs in vivo (Perez-Zsolt et al., 2019a). Virions accumulated are effectively transferred to interacting CD4+ T-cells through the formation of an infectious synapse (McDonald et al., 2003). This mechanism, known as trans-infection (Fig. 3), was initially observed in vitro by the laboratory of Ralph Steinman (Cameron et al., 1992), who won the Nobel prize in medicine in 2011 for the discovery of DCs and their role in adaptive immunity. Yet, it took two additional decades to identify Siglec-1 as the key molecule mediating this process (Izquierdo-Useros et al., 2012b; Puryear et al., 2013). Moreover, this extents to other retroviruses, including HIV-2 and murine leukemia virus (MLV) (Erikson et al., 2015; Kijewski et al., 2016).
Fig. 3.
Siglec-1 trans- and cis-infection pathway. SARS-CoV-2 and Ebola virus (EBOV) bind to nanoclusters of Siglec-1 molecules via GM1, whereas HIV-1 binds via both GM1 and GM3. Viral binding is followed by accumulation in a virus containing compartment (VCC) within dendritic cells. HIV-1 and SARS-CoV-2 are subsequently transferred to target cells expressing their respective receptors ie. CD4/CXCR4/CCR5 for HIV-1 (CD4+ T lymphocyte) and ACE2/TMPRSS2 for SARS-CoV-2 (pneumocytes) via trans-infection, whereas EBOV is directed to the late endosome, where cathepsins cleave the viral glycoprotein and via Nieman Pick Receptor 1 (NPC-1), releases viral RNA into the cytoplasm leading to the production of new EBOV via cis-infection in dendritic cells.
During the course of HIV-1 infection, several immune activating signals are up-regulated in the plasma of infected individuals, increasing the expression levels of Siglec-1 receptor on myeloid cells (Pino et al., 2015; Rempel et al., 2008). That is the case of LPS, a component of bacterial cell wall that translocate due to the loss of integrity of the gut barrier caused by HIV-1 (Brenchley et al., 2006). In addition, type I IFN released by immune cells that sense HIV-1 replication, such as plasmacytoid DCs (Beignon et al., 2005), also increases Siglec-1 expression (Rempel et al., 2008). Indeed, Siglec-1 is transcribed from an IFN-inducible gene whose activity in circulating monocytes correlates with viral load of HIV-1 infected individuals (Pino et al., 2015; Rempel et al., 2008). Thus, Siglec-1 is up-regulated upon infection via type I IFNs, and attenuates the antiviral effects of these molecules (Akiyama et al., 2017) even at mucosal sites where HIV-1 transmission occurs (Perez-Zsolt et al., 2019a), promoting viral infection and dissemination to distant tissues. This has been confirmed in vivo, using a murine model infected with another retrovirus, the MLV, whose robust infection in lymph nodes and spleen required Siglec-1 and trans-infection for viral spread (Sewald et al., 2015).
2.2. Trans-infection of SARS-CoV-2 via Siglec-1
Siglec-1 also facilitates viral infection of respiratory viruses which invade the upper respiratory tract and their subsequent dissemination to distant pulmonary tissues. The newest member of the coronavirus family, the β-coronavirus SARS-CoV-2 that causes COVID-19, incorporates sialylated gangliosides on the membrane and can be efficiently trans-infected via Siglec-1-expressing DCs (Fig. 3) (Perez-Zsolt et al., 2021). Sialylated gangliosides from SARS-CoV-2 membranes, such as GM1, interact with Siglec-1, facilitating the capture of viral particles in VCCs (Perez-Zsolt et al., 2021), as reported for HIV-1. SARS-CoV-2 trans-infection takes place from DCs to ACE2-and TMPRSS2-expressing cells (Lempp et al., 2021; Perez-Zsolt et al., 2021). As transmission of SARS-CoV-2 via Siglec-1 is not dependent on the viral spike and relies on the interaction with viral gangliosides, it occurs similarly for different variants of concern (Perez-Zsolt et al., 2021). Thus, on DCs, Siglec-1 acts as an attachment receptor rather than an entry receptor, facilitating the infection process of bystander target cells by the canonical ACE2 entry pathway. Yet, HIV-1 or SARS-CoV-2 are poorly transmitted by Siglec-1-expressing macrophages (Perez-Zsolt et al., 2021; Pino et al., 2015), indicating that capture of viral particles does not always lead to trans-infection. In the particular case of macrophages, Siglec-1 facilitates SARS-CoV-2 fusion, where restrictive non-productive infection leads to the induction of proinflammatory responses (Jalloh et al., 2022).
As previously discussed for chronic HIV-1 infection, Siglec-1 is also overexpressed on monocytes during the acute phase of SARS-CoV-2 infection (Bedin et al., 2021; Ortillon et al., 2021), where its expression peaks within 3 days after onset of symptoms, and decreases within 3–4 weeks, when infection is usually resolved. This correlates with the amount of viral load, which may trigger immune activating factors such as type I IFNs that induce Siglec-1 expression. Within pulmonary myeloid cells that express Siglec-1, high amounts of SARS-CoV-2 RNA were detected, but these cells showed very low levels of ACE2, TMPRSS2, or classical SARS-CoV-2 entry factors (Lempp et al., 2021; Perez-Zsolt et al., 2021). In a non-pathogenic macaque model, increased IFN responses along with the accumulation of Siglec-1 macrophages that harbor SARS-CoV-2 correlated with decline in viremia and recovery (Singh et al., 2022). Yet, since severe COVID-19 is driven by persistent lung inflammation, cytokine production, viral replication and sustained IFN responses, Siglec-1 may also fuel pathology within this context. Recently it has been proposed that the restricted SARS-CoV-2 infection of macrophages is opposed by inflammasome activation. This cytosolic multiprotein innate immune protein complex senses viral entry, and to prevent viral replication, induces inflammatory cytokine secretion and suicide by pyroptosis (Sefik et al., 2022). As blockade of inflammasome leads to the release of infectious virus by infected macrophages (Sefik et al., 2022), strategies aimed at targeting Siglec-1 could decrease COVID-19 pathology exacerbated by infected macrophages.
2.3. Productive infection mediated by Siglec-1: filoviruses and beyond
Other enveloped viruses, such the highly pathogenic EBOV, bind to Siglec-1, but in this particular case the interaction leads to the viral fusion of Siglec-1 expressing cells (Perez-Zsolt et al., 2019b), which are the ones productively infected (Geisbert et al., 2003). EBOV sporadic outbreaks are associated with high mortality rates, and viral transmission occurs through contact with blood and fluids from infected individuals (Baseler et al., 2017). This results in the infection of a broad cell repertoire including hepatocytes, fibroblasts and myeloid cells like DCs, macrophages and monocytes (Geisbert et al., 2003). Given their patrolling function at the viral entry sites, DCs are among the first cells to encounter EBOV, becoming productively infected early during EBOV disease (Bosio et al., 2003; Geisbert et al., 2003). Following their migration to lymphoid tissues, DCs contribute to EBOV systemic dissemination (Geisbert et al., 2003) playing a similar role to that described for HIV-1 infection.
In addition to promoting EBOV dissemination, infected myeloid cells display a dysregulated phenotype that impairs their capacity to mount innate and adaptive immune responses (Bosio et al., 2003; Lubaki et al., 2016). Moreover, infection of myeloid cells leads to a potent activation status characterized by the secretion of inflammatory mediators (Escudero-Pérez et al., 2014; Gupta et al., 2001), a ‘cytokine storm’ that results in organ damage and contributes to the severity of EBOV disease (Colavita et al., 2019; Reynard et al., 2019). EBOV entry into myeloid cells begins with viral attachment to the cell surface, which involves different viral and host factors (Davey et al., 2017). C-type lectins such as DC-SIGN and LSECtin mediate EBOV binding through recognition of the EBOV glycoprotein (Dominguez-Soto et al., 2007; Simmons et al., 2003), while the TIM/TAM receptors act as attachment factor through the recognition of phosphatidylserine on the viral membrane (Jemielity et al., 2013).
Siglec-1 is also an EBOV attachment factor that contributes to EBOV cytoplasmic viral entry into DCs through the recognition of sialylated gangliosides (Perez-Zsolt et al., 2019b) (Fig. 3). Of note, Siglec-1-inducing factors such as LPS and type I IFNs are present during the course of EBOV infection, especially in fatality cases of the disease (Kreuels et al., 2014; Villinger et al., 2008). This, together with the fact that Siglec-1 allows viral entry into key target cells for EBOV, suggests a role for this receptor in the progression EBOV infection. EBOV captured via Siglec-1 is also directed towards a VCC that is associated with viral trans-infection (Perez-Zsolt et al., 2019b) as previously demonstrated for HIV-1 (Izquierdo-Useros et al., 2011; Yu et al., 2008) and more recently for SARS-CoV-2 (Perez-Zsolt et al., 2021). Whether or not this VCC also favors EBOV trans-infection remains to be elucidated.
Other highly pathogenic enveloped viruses such as Marburg virus, and Nipah and Hendra paramyxoviruses are also efficiently captured via Siglec-1 (Akiyama et al., 2014; Perez-Zsolt et al., 2019b). Overall, this conserved mechanism of viral evasion and systemic viral dissemination is common to retrovirus, coronavirus, filovirus and henipavirus, and offers the unique opportunity to design pan antiviral solutions targeting Siglec-1 recognition of different enveloped viruses.
2.4. Therapeutic opportunities of Siglec-1 biology
Knowledge accumulated in the last decade has laid the grounds for the development of broad-spectrum antivirals targeting Siglec-1 viral recognition. An advantage of targeting host factors like Siglec-1 is that these agents are not affected by the high mutation rates experienced by RNA viruses, especially retroviruses, which can result in the appearance and selection of resistant variants insensitive to treatments targeting viral proteins (Fischer et al., 2021; Kugelman et al., 2015).
Cells infected by some viruses including EBOV and SARS-CoV-2 can secrete soluble forms of the glycoproteins required for viral infection, which might act as decoy factors reducing the efficacy of anti-glycoprotein agents, including neutralizing antibodies (Letarov et al., 2021; Mohan et al., 2012). Targeting Siglec-1 would not only tackle a broad range of enveloped viruses, but also bypass the drawbacks associated to glycoprotein-directed strategies. Although tackling host factors could increase the risk of potential toxicity, the identification of Siglec-1 null individuals which naturally lack this receptor but display common phenotypes (Martinez-Picado et al., 2016), suggest that blocking this receptor may not cause serious side effects.
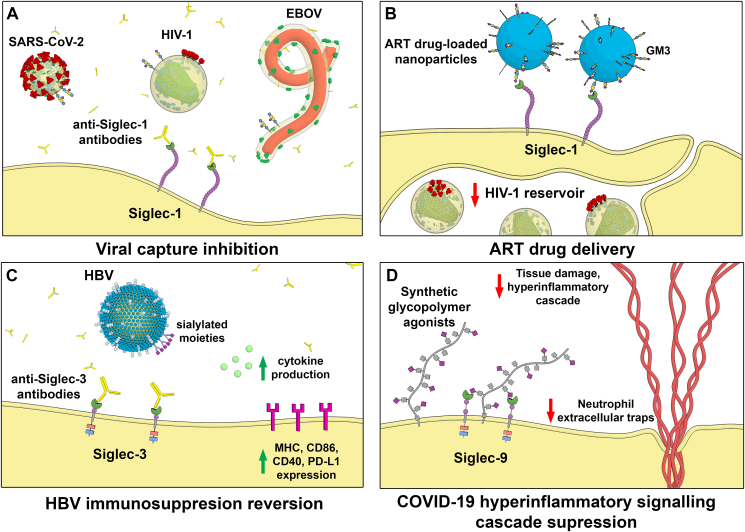
An interesting approach to target Siglec-1 is the development of anti-Siglec-1 antibodies, which reduced HIV-1 and SARS-CoV-2 trans-infection in vitro (Izquierdo-Useros et al., 2012b; Lempp et al., 2021; Perez-Zsolt et al., 2021) and also reduced EBOV cis-infection (Perez-Zsolt et al., 2019b) (Fig. 4a). However, to achieve the full potential of an anti-Siglec-1 based strategy, it should be combined with other antivirals. In the context of HIV-1 acquisition, a prophylactic strategy targeting Siglec-1 to reduce HIV-1 dissemination by cervical DCs (Perez-Zsolt et al., 2019a) could be integrated with an anti-retroviral therapy to block cell-free virus infection of target cells. Upon settlement of HIV-1 infection, nanoparticles loaded with antiretrovirals targeting Siglec-1 could be helpful to eradicate viral reservoirs of macrophages expressing this receptor (Fig. 4b), which compromise cure strategies (Chen et al., 2012; Eshaghi et al., 2022). In the case of SARS-CoV-2, the combination of an anti-Siglec-1 with antibodies against the N-terminal domain (NTD) or the conserved site of the spike Receptor Binding Domain (RBD), which effectively block lectin-facilitated infection (Lempp et al., 2021), could increase the potency of each treatment. Efficacy of a Siglec-1-directed therapy could be also maximized including an antiviral such as remdesivir in those cases in which SARS-CoV-2 dissemination has already occurred (Grein et al., 2020), a strategy that could also apply to EBOV treatment (Mulangu et al., 2019). Hence, Siglec-1-directed therapies in combination with antivirals may be useful against a broad spectrum of enveloped viruses and aid to control viral dissemination.
Fig. 4.
Examples of Siglec-based therapeutic antiviral strategies. A) Blocking with Siglec-1 antibodies reduces EBOV fusion and both HIV-1 and SARS-CoV-2 trans-infection by DCs. B) Nanoparticles loaded with ART may be useful to eradicate HIV-1 reservoirs of macrophages. C) Blocking Siglec-3 antibodies activate myeloid cells and may reactivate host immunity to HBV. D) Synthetic glycopolymers agonists can activate Siglec-9 and suppress neutrophil extracellular traps formation and hyperinflammation in the context of COVID-19.
Siglec-1 may also prove valuable as a biomarker of type I IFN responses induced by viral infections (Bourgoin et al., 2020). As already discussed, Siglec-1 is upregulated on monocytes of HIV-1-infected individuals, correlating with the presence of high viral loads (Pino et al., 2015; Rempel et al., 2008). However, its expression is reduced after successful antiretroviral treatment (Pino et al., 2015). Similarly, Siglec-1 is upregulated on monocytes of COVID-19 patients, but is reduced after viral load clearance (Bedin et al., 2021; Ortillon et al., 2021). This is also the case during acute infection with Dengue and Zika virus (Fenutria et al., 2021; Michlmayr et al., 2020). Future strategies aimed at using Siglec-1 as a viral infection biomarker, along with specific treatments to counteract viral transmission mediated by this lectin, will aid to implement novel interventions that need now to move to pre-clinical and clinical trials to test their safety and efficacy. Comparable diagnostic and therapeutic applications could also arise by focusing on the biology of other members of the Siglec family, with yet unknown roles during viral infections, but with great potential to offer new clinical solutions or antivirals.
3. The role on infection of other conserved Siglecs
Several members of the Siglec family are structurally conserved between different vertebrates. These include Siglec-1, along with Siglec-2, Siglec-4 and Siglec-15. The structure of Siglec −2, −4 and −15 is much shorter compared to Siglec-1 (Fig. 1), what reduces their capacity to directly interact with sialylated viruses, as these molecules remain mostly bound in cis (Crocker et al., 2007). Yet, as we will now discuss, some of these Siglecs may directly favor viral infection.
3.1. Siglec-2: a B-cell regulator still unexplored in viral infections
Siglec-2 (CD22) is readily available to interact in cis with sialylated ligands. This lectin is expressed on B-cells and to a lesser extent on DCs and mast cells (Crocker et al., 2007; Ravetch and Lanier, 2000). In the cytoplasmatic tail, this receptor displays three tyrosine-based inhibitory motives (ITIM), an ITIM-like domain and a growth-factor-receptor-bound protein binding motif. Upon antigen interaction on B-cells, Siglec-2 might recognize endogenous sialylated structures and block activation of B-cell receptor through the inhibitory effect of ITIM motives, protecting the organism from over-aggressive B-cells and autoimmunity (Jin et al., 2002; Meyer et al., 2021). Murine deficiency of Siglec-2 leads to a higher susceptibility to rotavirus and West Nile Virus infection (Ballet et al., 2021; Ma et al., 2013). However, Siglec-2 deficient mice infected with LCMV and VSV had no differences in survival compared to wild-type mice (Ballet et al., 2021; Ma et al., 2013). Currently, compounds targeting Siglec-2 are being evaluated as a putative treatment for tumors and autoimmune disorders (Drgona et al., 2018; Shah et al., 2019), but none is still directed to treat viral infections.
3.2. Siglec-4: a neurotropic viral target
Siglec-4 is shorter than Siglec-2 (Fig. 1). It was discovered as a myelin-associated glycoprotein (MAG), and in contrast to other Siglecs, it is exclusively expressed in the nervous system by myelinating cells, oligodendrocytes and Schwann cells (Quarles, 2007). Siglec-4 is implicated in the maintenance of myelin integrity and the regulation of neuronal contacts (Quarles, 2007), being associated with several viruses causing neuropathy. For example, in patients infected with HCV, an increased prevalence of demyelinating neuropathy due to anti-Sigle-4 IgM antibodies has been shown (Mariotto et al., 2015). In addition, Siglec-4 mediates varicella-zoster virus (VZV) and herpes simplex virus (HSV) infection, via sialic acid recognition of the B glycoprotein from the viral envelopes (Suenaga et al., 2010, 2015). Thus, targeting Siglec-4 interaction with viral glycoproteins might help to prevent certain neural damage of neurotropic viruses.
3.3. Siglec-15 and its unknow antiviral function
Siglec-15 (CD33L3) is the shortest family member, and although its cytoplasmic tail lacks any signaling moieties, it associates with the activating adaptor proteins DAP12 and DAP10 via a lysine residue in the transmembrane region (Fig. 1). This allows Siglec-15 to function as an activating signaling molecule (Angata et al., 2007). Siglec-15 is expressed on macrophages and/or DCs of human spleen and lymph nodes (Angata et al., 2007). Siglec-15 plays a role in bone homeostasis by regulating osteoclast development and function (Angata, 2020), and it has recently been associated with bacterial diseases (Bhattacharyya et al., 2019; Jaeger et al., 2019), but not directly to viral infections yet.
4. CD33-related Siglecs and viral infections
The rest of the members of this family are known as CD33-related Siglecs (Fig. 1), sharing a similar short extracellular domain but varying in number between species (Pillai et al., 2012). Despite their short structure, displaying 1 to 5 extracellular domains, these Siglecs have been suggested to interact with sialylated viral ligands. This contrast to what is known for Siglec-1 engineered constructs containing less than six Ig-like C2-type domains, which do not recognize sialylated ligands unless they are treated with sialidases to deplete cell-surface sialic acids and reduce cis interactions (Munday et al., 1999). While Siglec-3, -7 and -9 interact with HIV-1 envelope via sialylated gp120 recognition to promote infection of macrophages (Varchetta et al., 2013; Zou et al., 2011), these interactions are less favored than those mediated by Siglec-1 binding to sialylated gangliosides in the lipid membrane. It has been estimated that there are an average of 14 ± 7 envelope trimers per HIV-1 particle (Zhu et al., 2003), but more than 12,000 sialylated gangliosides per virion (Brügger et al., 2006). Siglec-1 nanoclustering (Fig. 2) enhances the receptor avidity for reduced concentrations of gangliosides (Gutiérrez-Martínez et al.,2022). Thus, Siglec-1 supports high -avidity interactions by binding thousands of sialylated gangliosides despite the relatively poor affinity of each individual interaction, whereas the shorter CD33-related Siglecs bind with the reduced number of envelope glycoproteins suggesting a less prominent role during viral recognition.
4.1. Siglec-3 and Hepatitis B virus
Siglec-3 (CD33) is expressed in myeloid progenitors, macrophages, monocytes, microglia and granulocytes (Macauley et al., 2014). This inhibitory receptor containing ITIM and an ITIM-like intracellular domains can recruit SHP-1/2 phosphatases, negatively regulating cell activation on myeloid cells, or SOCS3, which downregulate the expression of this Siglec (Orr et al., 2007a, 2007b). Accordingly, the reduction of Siglec-3 expression increases secretion of IL-1β, IL-8, and TNF-α (Lajaunias et al., 2005). In the context of Hepatitis B virus (HBV) infection, Siglec-3 binding to sialylated viral moieties triggers the recruitment of SHP-1 and -2 to reduce TLR ligand–induced cytokine secretion (Tsai et al., 2021). This immunosuppression can be reverted with an anti-Siglec-3 monoclonal antibody, that enhances cytokine production and HBV antigen expression in response to a TLR-7 agonist (Tsai et al., 2021). Thus, blockade of Siglec-3 is a promising strategy to reactivate host immunity against HBV (Fig. 4c).
4.2. Siglec-6 and HIV-1 infection
Siglec-6 (CD327) was discovered in parallel by two groups: one isolated this protein from a human placental library (Takei et al., 1997) and other as a leptin-binding protein (Patel et al., 1999). Siglec-6 does not require the glycerol side chain of sialic acid for its recognition, being the only Siglec member that recognizes the sialyl-Tn glycans epitope (Brinkman-VDL and Varki, 2000; Patel et al., 1999). Siglec-6 is expressed on placental trophoblasts, B-cells and also on circulating T-cells. Siglec-6 expression is increased in B-cells of HIV-1 infected individuals, and its downregulation leads to higher levels of HIV-specific antibody-secreting cells and to B-cell associated chemokines and cytokines (Kardava et al., 2011). Therefore Siglec-6 is a promising target for regulating B cell activation during HIV-1 infection.
4.3. Siglec-7 and viremic status
Siglec-7 (CD328) has a similar structure to Siglec-6 (Fig. 1), and is predominantly expressed in NK cells (Falco et al., 1999; Nicoll et al., 1999), where it is associated with a better killer cell function, higher levels of activating receptors and lower levels of inhibitory receptors (Shao et al., 2016). In fact, Siglec-7 acts as an important regulator of NK activation, as the association of its ITIM domain with SHP-1 transduces inhibitory signals (Falco et al., 1999). Siglec-7 is also expressed in a small subset of CD8+ T cells, monocytes and granulocytes (Nicoll et al., 1999), DCs (Lock et al., 2004), eosinophils (Munitz, 2006), macrophages (Miyazaki et al., 2012), mast cells and basophils (Mizrahi et al., 2014), platelets of healthy donors (Nguyen et al., 2014), and in β-cells of human pancreatic islets (Dharmadhikari et al., 2017).
During HIV-1 infection, a decreased expression of Siglec-7 is an early marker of dysfunctional NK-cell subsets that is linked with high levels of viremia (Brunetta et al., 2009). This impaired response of NK cells is due to an expansion of anergic NK-cell subsets in HIV-1–infected patients (Brunetta et al., 2009). Higher serum levels of soluble Siglec-7 (sSiglec-7) are found in HIV-1-infected patients concomitant to the expansion of Siglec-7 negative NK cells, suggesting that the continuous exposure to ongoing viral replication induces the shedding of Siglec-7 from NK cell surface (Brunetta et al., 2009; Varchetta et al., 2013). Similarly, in vitro stimulation of NK cells with HCV induces both a decrease of Siglec-7 expression and a significant release of sSiglec-7, and identifies a dysfunctional NK subset associated with chronic HCV infection (Varchetta et al., 2016). Recently it was found that Siglec-7 binds to VZV envelope glycoprotein B in a sialic acid-dependent manner (Suenaga et al., 2022). Thus, sSiglec-7 monitoring could inform about the viremic status of distinct infections.
4.4. Siglec-8 and inflammation mediated by respiratory viruses
Siglec-8 has a similar structure to Siglec- 6 and -7 (Fig. 1), and is expressed in human mast cells, eosinophils and basophils, where it plays a role as an inhibitory receptor (Youngblood et al., 2020). Another study showed that mast cells derived proteases and eosinophils mediators are increased in COVID-19 patient sera and lungs, suggesting that these cells may play a role in COVID-19 inflammation (Gebremeskel et al., 2021). In this context, Siglec-8 mAb treatment suppressed TLR-driven inflammation in response to viral RNA, and also decreased disease severity and airway inflammation in a RSV infection model (Gebremeskel et al., 2021; Youngblood et al., 2019). Therefore, anti-Siglec-8 antibodies such as lirentelimab could be used to limit mast cells and eosinophil infiltration and activation, reducing the excessive inflammation in SARS-CoV-2 patients (Gebremeskel et al., 2021).
4.5. Siglec-9 as a therapeutic antiviral target
Siglec-9 (CD329) is an inhibitory receptor with a structure like Siglec-6, -7 and -8 (Zhang et al., 2000) (Fig. 1). Siglec-9 is expressed quite broadly among human blood leukocytes, including monocytes, neutrophils, B cells, NK cells, and minor subsets of T cells (Zhang et al., 2000). The binding of Siglec-9 to sialic acid on target cells induces an inhibitory signal transduction cascade by recruiting SHP-1, which counteracts the phosphorylation-mediated activation of other signaling molecules (Avril et al., 2004), and restrains NK cytotoxicity (Crocker et al., 2007).
During viral infections, Siglec-9 levels on NK cells decrease and negatively correlated with virus replication in HBV-infected patients (Zhao et al., 2018). Moreover, Siglec-9+ NK cells showed a more activated phenotype in HBV-infected patients and exhibited higher cytotoxicity capacity against HIV-infected cells compared to Siglec-9- NK cells (Adeniji et al., 2021). A recent study revealed a specific binding of the SARS-CoV-2 spike protein to Siglec-9 by using an ELISA (Chiodo et al., 2020).
From a therapeutic point of view, neutralization of Siglec-9 by a blocking antibody on NK cells of HBV-infected patients restores their cytokine synthesis and cytotoxic capability (Zhao et al., 2018). Besides, a novel proof-of-concept immunotherapy has been tested to selectively disrupt Siglec-9/sialoglycan interactions between NK cells and HIV--infected cells (Adeniji et al., 2021). A sialidase conjugated to HIV-1 broadly neutralizing antibodies selectively de-sialylated HIV-infected cells and enhanced NK capacity to kill these cells. Additionally, synthetic glycopolymers that activate Siglec-9 in neutrophils have shown to suppress neutrophil extracellular traps (Fig. 4d) and the hyperinflammatory signaling cascade that occurs in COVID-19 patients (Delaveris et al., 2021). Thus, Siglec-9 targeting is a promising therapeutic strategy to both restore NK cell function in HBV and HIV infections, and curb neutrophilic hyperinflammation in COVID-19.
4.6. Siglec-10 as a viral recognition receptor
Siglec-10 was discovered in 2001 and has five extracellular Ig-like domains (Fig. 1) (Whitney et al., 2001). Siglec-10 is expressed in B cells, eosinophils, monocytes, DCs and a minor population of NK cells (Li et al., 2001; Munday et al., 2001; Whitney et al., 2001). The binding of Siglec-10 to CD24 induces an inhibitory cascade that blocks TLR-mediated inflammation, and leads to immunosuppression (Chen et al., 2009). The interaction of CD24 expressed in the placenta with Siglec-10 suggest a possible role in mediating immune tolerance (Sammar et al., 2017).
Siglec-10 mediates viral entry of porcine reproductive and respiratory syndrome virus (PRRSV) along with Siglec-1, and this is dependent of the sialic-acid binding activity of these lectins (Xie et al., 2017, 2018). Siglec-10 also recognizes glycan epitopes on the RBD of the Spike of SARS-CoV-2 (Lenza et al., 2020), a finding confirmed using immunoassays, as also shown for Siglec-9 and -3 (Chiodo et al., 2020). The murine ortholog of human Siglec-10 is Siglec-G, which inhibits the innate immune response induced by RNA viruses such as VSV or Sendai, by promoting RIG-I degradation, but not for DNA viruses or bacteria (Chen et al., 2013). Further work is needed to understand the outcome of Siglec-10 interactions with human viruses on immune responses, and how this interplay could be therapeutically targeted.
5. Siglec-5 & Siglec-14: an antiviral role yet to decipher
In humans there are two pairs of Siglecs that share nearly identical ligand binding extracellular regions, but display divergent transmembrane and cytoplasmic regions containing inhibitory ITIM domains or positively charged amino acid residues that associate to the activating DAP12 (Crocker et al., 2007). There is a long-standing hypothesis suggesting that activating counterparts of these paired receptors evolved as a response to pathogen molecular mimicry of host ligands for inhibitory receptors (Schwarz et al., 2017). Two examples of this paired Siglecs are known: the repressing Siglec-11 coupled to the activating Siglec-16, but here we focused on Siglec-5 and Siglec-14, whose role in viral infections is better studied.
Siglec-5 (CD170) is expressed on monocytes, B cells and neutrophils (Cornish et al., 1998) and it contains ITIM and ITIM-like motifs (Jaroenpool et al., 2007; Macauley et al., 2014) (Fig. 1). During HIV-1 infection, Siglec-5 may be protective, since the transduction of human T cells with Siglec-5 had a higher percentage of survival after HIV-1 infection and a decreased expression of apoptosis-related factors, such as the active form of caspase-3 and TRAILR2/DR5 (Soto et al., 2013). Siglec-14 is expressed on neutrophils, macrophages, granulocytes and monocytes, it has three Ig domains and no tyrosine motifs, and it associates with the adaptor DAP12, via positively charged amino acids in its transmembrane region (Macauley et al., 2014) (Fig. 1). This interaction activates receptors by recruiting spleen tyrosine kinases.
The inhibitory receptor Siglec-5 is paired with Siglec-14, suggesting that these two proteins may work in a cooperative manner, balancing activating and inhibitory signaling through sialic-acid recognition (Angata et al., 2006). In fact, interaction of bacterial proteins with both Siglec-5 and Siglec-14 allow the activation of signaling pathways that counteract the pathogen-induced host immune suppression (Ali et al., 2014). This example of a paired receptor system in the Siglec family has multiple implications for regulation of host immunity and may also apply to viral infections.
6. Future perspectives
The unprecedent coronavirus pandemic started in 2019, but still poses a major toll on global health worldwide. This highlights the need to understand biology behind infection to design effective therapeutic counter measurements against viruses. Immunotherapies and host directed pan-antiviral solutions are promising preclinical tools, and could become the next arsenal to combat future viral outbreaks. Insights gained into the biology of Siglec-1, which promotes viral recognition and clearance in particular contexts, but can be subverted by certain enveloped viruses, offers new avenues to implement broad antiviral therapies. Yet, to fully achieve clinical applicability, potential toxicities, challenges with drug delivery and efficacy will need to be carefully evaluated. Fine tune balancing of the immunomodulatory role of other members of the Siglec family could also benefit the clinical outcome of infected individuals, and bring novel therapeutic solutions. Finally, soluble and membrane-bound Siglecs could aid to better predict clinical courses based on the immune profile of these lectins. Here, we have summarized what is known about Siglecs and their interaction with particular viruses (Table 1), focusing on immune responses and the putative role of these lectins during infection (Table 2). Underscoring the underlaying biology of Siglecs, with capacity to immunomodulate and interact with lethal enveloped viruses, may offer novel antiviral solutions and preparedness for future outbreaks.
Table 1.
Siglec binding to different viruses.
| Siglec | Viruses | Reference |
|---|---|---|
| 1 | HIV-1 and HIV-2 | Izquierdo-Useros et al., 2012b,Puryear et al., 2013,Kijewski et al., 2016 |
| Murine Leukemia Virus | Erikson et al., 2015,Sewald et al., 2015 | |
| Ebola and Marburg viruses | Perez-Zsolt et al., 2019b | |
| SARS-CoV-2 | Perez-Zsolt et al., 2021,Lempp et al., 2021 | |
| Nipah and Hendra viruses | Akiyama et al., 2014 | |
| 3 | Hepatitis B virus | Tsai et al., 2021 |
| SARS-CoV-2 | Chiodo et al., 2020 | |
| 4 | Herpes virus | Suenaga et al., 2010,Suenaga et al., 2015 |
| 7 | Varicella-zoster virus | Suenaga et al., 2022 |
| 9 | SARS-CoV-2 | Chiodo et al., 2020 |
| 10 | Porcine reproductive and respiratory virus | Xie et al., 2017,Xie et al., 2018 |
| SARS-CoV-2 | Lenza et al., 2020,Chiodo et al., 2020 |
Table 2.
Impact of each Siglec on the antiviral immune response and putative role upon infection.
| Siglec | Virus | Outcome | Reference |
|---|---|---|---|
| 1 | HIV-1 | Increased Siglec-1 expression in myeloid cells | Pulliam et al., 2004;Pino et al., 2015;Rempel et al., 2008 |
| Vaccinia virus | Induce effector and memory CD8+ and CD4+ T cells | van Dinther et al., 2018 | |
| Retrovirus Friend virus complex | Promote cytotoxic CD8 T cell responses in murine models | Uchil et al., 2019 | |
| Respiratory syncytial virus | Recruitment of effector CD8+ T-cells to the lung | Oh et al., 2017 | |
| SARS-CoV-2 | Increased Siglec-1 expression in myeloid cells | Bedin et al., 2021;Ortillon et al., 2021 | |
| 2 | Rotavirus and West Nile virus | Murine deficiency of Siglec-2 leads to higher susceptibility of infection | Ballet et al., 2021;Ma et al., 2013 |
| 4 | Hepatitis C virus | Increased demyelinating neuropathy due to anti-Sigle-4 IgM antibodies | Mariotto et al., 2015 |
| 6 | HIV-1 | Increased Siglec-6 expression in B-cells of infected individuals | Kardava et al., 2011 |
| 7 | HIV-1 | Decreased Siglec-7 expression, increased sSiglec-7 and dysfunctional NK cells | Brunetta et al., 2009,Varchetta et al., 2013 |
| HCV | Decreased Siglec-7 expression, increased sSiglec-7 and dysfunctional NK cells | Varchetta et al., 2016 | |
| 8 | Respiratory syncytial Virus | Anti-Siglec-8 mAb decreased inflammation in murine models | Gebremeskel et al., 2021 |
| 9 | Hepatitis B virus | Decreased Siglec-9 expression on NK cells | Zhao et al., 2018 |
| HIV-1 | Siglec-9+ NK showed higher cytotoxicity capacity against HIV-infected cells | Adeniji et al., 2021 |
Funding
NI-U is supported by the Spanish Ministry of Science and Innovation (grant PID2020-117145RB-I00, Spain), EU HORIZON-HLTH-2021-CORONA-01 (grant 101046118, European Union) and by institutional funding of Grifols, Pharma Mar, HIPRA, Amassence and Palobiofarma (Spain). Research in the JM-P lab is supported by the Spanish Ministry of Science and Innovation (grants PID2019-109870RB-I00 and CB21/13/00063, Spain), NIH/NIAID (1 UM1 AI164561-01, United States of America), EU HORIZON-HLTH-2021-DISEASE-04-07 (grant 101057100, European Union), Research Centers of Catalonia (CERCA, grant 2017 SGR 252, Spain), Fundació La Marató de TV3 (grant 202130-30-31-32, Spain), Generalitat de València (grant PROMETEO/2021/036, Spain), and Grifols (Spain). XM-T is supported by the Spanish Ministry of Science, Innovation and Universities and the European Regional Development Fund under agreement No. BES-2017-082900 (Spain). FL-N is supported by the Spanish Ministry of Science and Innovation and the European Social Fund under agreement No. PRE-2020-095004 (Spain).
Declaration of competing interest
The authors are inventors of distinct patent applications based on Siglec-1 interactions with enveloped viruses. This review was written in the absence of any other competing interest.
References
- Adeniji O.S., Kuri-Cervantes L., Yu C., Xu Z., Ho M., Chew G.M., Shikuma C., Tomescu C., George A.F., Roan N.R., Ndhlovu L.C., Liu Q., Muthumani K., Weiner D.B., Betts M.R., Xiao H., Abdel-Mohsen M. Siglec-9 defines and restrains a natural killer subpopulation highly cytotoxic to HIV-infected cells. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Miller C., Patel H.V., Hatch S.C., Archer J., Ramirez N.-G.P., Gummuluru S. Virus particle release from glycosphingolipid-enriched microdomains is essential for dendritic cell-mediated capture and transfer of HIV-1 and henipavirus. J. Virol. 2014;88:8813–8825. doi: 10.1128/jvi.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H., Ramirez N.-G.P., Gibson G., Kline C., Watkins S., Ambrose Z., Gummuluru S. Interferon-inducible CD169/Siglec1 attenuates anti-HIV-1 effects of alpha interferon. J. Virol. 2017;91(21) doi: 10.1128/jvi.00972-17. e00972-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.R., Fong J.J., Carlin A.F., Busch T.D., Linden R., Angata T., Areschoug T., Parast M., Varki N., Murray J., Nizet V., Varki A. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 2014;211:1231–1242. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T., Varki N.M., Varki A. A second uniquely human mutation affecting sialic acid biology. J. Biol. Chem. 2001;276(43):40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- Angata T. Siglec-15: a potential regulator of osteoporosis, cancer, and infectious diseases. J. Biomed. Sci. 2020;27:10. doi: 10.1186/s12929-019-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T., Hayakawa T., Yamanaka M., Varki A., Nakamura M. Discovery of Siglec‐14, a novel sialic acid receptor undergoing concerted evolution with Siglec‐5 in primates. Faseb. J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- Angata T., Tabuchi Y., Nakamura K., Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- Avril T., Floyd H., Lopez F., Vivier E., Crocker P.R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by siglecs-7 and -9, CD33-related siglecs expressed on human monocytes and NK cells. J. Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- Ballet R., Brennan M., Brandl C., Feng N., Berri J., Cheng J., Ocón B., Alborzian Deh Sheikh A., Marki A., Bi Y., Abram C.L., Lowell C.A., Tsubata T., Greenberg H.B., Macauley M.S., Ley K., Nitschke L., Butcher E.C. A CD22–Shp1 phosphatase axis controls integrin $β$7 display and B cell function in mucosal immunity. Nat. Immunol. 2021;22:381–390. doi: 10.1038/s41590-021-00862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler L., Chertow D.S., Johnson K.M., Feldmann H., Morens D.M. The pathogenesis of Ebola virus disease. Annual Rev. Pathol. 2017;12:387–418. doi: 10.1146/annurev-pathol-052016-100506. [DOI] [PubMed] [Google Scholar]
- Bavari S., Bosio C.M., Wiegand E., Ruthel G., Will A.B., Geisbert T.W., Hevey M., Schmaljohn C., Schmaljohn A., Aman M.J. Lipid raft microdomains A gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedin A.-S., Makinson A., Picot M.-C., Mennechet F., Malergue F., Pisoni A., Nyiramigisha E., Montagnier L., Bollore K., Debiesse S., Morquin D., Bourgoin P., Veyrenche N., Renault C., Foulongne V., Bret C., Bourdin A., Moing V., de Perre Le, Van P., Tuaillon E. Monocyte CD169 expression as a biomarker in the early diagnosis of COVID-19. J. Infect. Dis. 2021;223(4):562–567. doi: 10.1093/infdis/jiaa724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beignon A.S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D.G., Larsson M., Gorelick R.J., Lifson J.D., Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya C., Majumder P.P., Pandit B. An exome wide association study of pulmonary tuberculosis patients and their asymptomatic household contacts. Infect. Genet. Evol. 2019;71:76–81. doi: 10.1016/j.meegid.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Bosio C., Aman M., Grogan C., Hogan R., Ruthel G., Negley D., Mohamadzadeh M., Bavari S., Schmaljohn A. Ebola and Marburg viruses replicate in monocyte‐derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 2003;188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- Bourgoin P., Biéchelé G., Ait Belkacem I., Morange P.E., Malergue F. Role of the interferons in CD64 and CD169 expressions in whole blood: relevance in the balance between viral- or bacterial-oriented immune responses. Immunity Inflamm. Dis. 2020;8:106–123. doi: 10.1002/iid3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B.R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J.N., Hecht F.M., Picker L.J., Lederman M.M., Deeks S.G., Douek D.C. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brinkman-VDL E.C., Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. J. Biol. Chem. 2000;275:8625–8632. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F.T., Kräusslich H.-G.G. The {HIV} lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetta E., Fogli M., Varchetta S., Bozzo L., Hudspeth K.L., Marcenaro E., Moretta A., Mavilio D. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer–cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114:3822–3830. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara A., Lavanant A.C., Abe J., Desforges H.L., Alexandre Y.O., Girardi E., Igamberdieva Z., Asano K., Tanaka M., Hehlgans T., Pfeffer K., Pfeffer S., Mueller S.N., Stein J.V., Mueller C.G. CD169+ macrophages in lymph node and spleen critically depend on dual RANK and LTbetaR signaling. Proc. Natl. Acad. Sci. USA. 2022;119(3) doi: 10.1073/pnas.2108540119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P.U., Freudenthal P.S., Barker J.M., Gezelter S., Inaba K., Steinman R.M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Chen G.-Y., Tang J., Zheng P., Liu Y. CD24 and siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Han C., Xie B., Hu X., Yu Q., Shi L., Wang Q., Li D., Wang J., Zheng P., Liu Y., Cao X. Induction of siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Chen W.C., Kawasaki N., Nycholat C.M., Han S., Pilotte J., Crocker P.R., Paulson J.C. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo F., Bruijns S.C.M., Rodriguez E., Li R.J.E., Molinaro A., Silipo A., Di Lorenzo F., Garcia-Rivera D., Valdes-Balbin Y., Verez-Bencomo V., van Kooyk Y. 2020. Novel ACE2-independent Carbohydrate-Binding of SARS-CoV-2 Spike Protein to Host Lectins and Lung Microbiota. BioRxiv [Preprint]. May 14, 2020 [cited 2022 July 20]. Available from: [DOI] [Google Scholar]
- Colavita F., Biava M., Castilletti C., Lanini S., Miccio R., Portella G., Vairo F., Ippolito G., Capobianchi M.R., Di Caro A., Lalle E. Inflammatory and humoral immune response during Ebola virus infection in survivor and fatal cases occurred in Sierra Leone during the 2014–2016 outbreak in west Africa. Viruses. 2019;11:373. doi: 10.3390/v11040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish A.L., Freeman S., Forbes G., Ni J., Zhang M., Cepeda M., Gentz R., Augustus M., Carter K.C., Crocker P.R. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. doi: 10.1182/blood.v92.6.2123. [DOI] [PubMed] [Google Scholar]
- Crocker P.R., Kelm S., Dubois C., Martin B., McWilliam A.S., Shotton D.M., Paulson J.C., Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991;10:1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P.R., Mucklow S., Bouckson V., McWilliam A., Willis A.C., Gordon S., Milon G., Kelm S., Bradfield P. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994;13:4490–4503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P.R., Paulson J.C., Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Davey R.A., Shtanko O., Anantpadma M., Sakurai Y., Chandran K., Maury W. Mechanisms of filovirus entry. Curr. Top. Microbiol. Immunol. 2017;411:323–352. doi: 10.1007/82_2017_14. [DOI] [PubMed] [Google Scholar]
- Delaveris C.S., Wilk A.J., Riley N.M., Stark J.C., Yang S.S., Rogers A.J., Ranganath T., Nadeau K.C., Blish C.A., Bertozzi C.R. Synthetic siglec-9 agonists inhibit neutrophil activation associated with COVID-19. ACS Cent. Sci. 2021;7:650–657. doi: 10.1021/acscentsci.0c01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmadhikari G., Stolz K., Hauke M., Morgan N.G., Varki A., de Koning E., Kelm S., Maedler K. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci. Rep. 2017;7 doi: 10.1038/srep45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Soto A., Aragoneses-Fenoll L., Martin-Gayo E., Martinez-Prats L., Colmenares M., Naranjo-Gomez M., Borras F.E., Munoz P., Zubiaur M., Toribio M.L., Delgado R., Corbi A.L. The DC-SIGN–related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109:5337–5345. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- Drgona L., Gudiol C., Lanini S., Salzberger B., Ippolito G., Mikulska M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33. Clin. Microbiol. Infect. 2018;24:S83–S94. doi: 10.1016/j.cmi.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Erikson E., Wratil P.R., Frank M., Ambiel I., Pahnke K., Claveria M.P., Azadi P., Izquierdo-Useros N., Martinez-Picado J., Meier C., Schnaar R.L., Crocker P.R., Reutter W., Keppler O.T. Mouse {Siglec}-1 mediates trans-infection of surface-bound murine leukemia virus in a sialic acid {N}-acyl side chain-dependent manner. J. Biol. Chem. 2015;290:27345–27359. doi: 10.1074/jbc.M115.681338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero-Pérez B., Volchkova V.A., Dolnik O., Lawrence P., Volchkov V.E. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog. 2014;10(11) doi: 10.1371/journal.ppat.1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaghi B., Fofana J., Nodder S.B., Gummuluru S., Reinhard B.M. Virus-mimicking polymer nanoparticles targeting CD169+ macrophages as long-acting nanocarriers for combination antiretrovirals. ACS Appl. Mater. Interfaces. 2022;14:2488–2500. doi: 10.1021/acsami.1c17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco M., Biassoni R., Bottino C., Vitale M., Sivori S., Augugliaro R., Moretta L., Moretta A. Identification and molecular cloning of P75/airm1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 1999;190:793–802. doi: 10.1084/jem.190.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenutria R., Maringer K., Potla U., Bernal-Rubio D., Evans M.J., Harris E., Rahman A.H., Fernandez-Sesma A., Ramos I. CyTOF profiling of Zika and Dengue virus-infected human peripheral blood mononuclear cells identifies phenotypic signatures of monotype subsets and upregulation of the interferon-inducible protein CD169. mSphere. 2021;6:1–16. doi: 10.1128/mSphere.00505-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Giorgi E.E., Chakraborty S., Nguyen K., Bhattacharya T., Theiler J., Goloboff P.A., Yoon H., Abfalterer W., Foley B.T., Tegally H., San J.E. 1093–1110. 2021. (Review HIV-1 and SARS-CoV-2 : Patterns in the Evolution of Two Pandemic Pathogens 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremeskel S., Schanin J., Coyle K.M., Butuci M., Luu T., Brock E.C., Xu A., Wong A., Leung J., Korver W., Morin R.D., Schleimer R.P., Bochner B.S., Youngblood B.A. Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: implications for an anti-siglec-8 antibody. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Hensley L.E., Larsen T., Young H.A., Reed D.S., Geisbert J.B., Scott D.P., Kagan E., Jahrling P.B., Davis K.J. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Mahanty S., Ahmed R., Rollin P.E. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with Ebola virus secrete MIP-1α and TNF-α and inhibit poly-IC-induced IFN-α in vitro. Virology. 2001;284:20–25. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Martínez, Benet S., Mateos N., Erkizia I., Nieto-Garai, Ander J., Lorizate M., Manzo C., Campelo F., Izquierdo-Useros N., Martinez-Pica J., Garcia-Parajo M.F. Actin-regulated Siglec-1 nanoclustering influences HIV-1 capture and virus-containing compartment formation in dendritic cells. BioRxiv [Preprint] April 30, 2022 doi: 10.1101/2022.04.28.489919. [cited 2022 July 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnell A., Steel J., Turley H., Jones M., Jackson D.G., Crocker P.R. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.V97.1.288. [DOI] [PubMed] [Google Scholar]
- Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M.P., Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone M., Moseman E.A., Tonti E., Bosurgi L., Junt T., Henrickson S.E., Whelan S.P., Guidotti L.G., Von Andrian U.H. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N., Esteban O., Rodriguez-Plata M.T., Erkizia I., Prado J.G., Blanco J., García-Parajo M.F., Martinez-Picado J. Dynamic imaging of cell-free and cell-associated viral capture in mature dendritic cells. Traffic. 2011;12:1702–1713. doi: 10.1111/j.1600-0854.2011.01281.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Useros N., Lorizate M., Contreras F.-X.-.X., Rodriguez-Plata M.T., Glass B., Erkizia I., Prado J.G., Casas J., Fabriàs G., Kräusslich H.-G.-.G., Martinez-Picado J. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N., Lorizate M., Puertas M.C., Rodriguez-Plata M.T., Zangger N., Erikson E., Pino M., Erkizia I., Glass B., Clotet B., Keppler O.T., Telenti A., Kräusslich H.-G.-.G., Martinez-Picado J. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N., Naranjo-Gómez M., Archer J., Hatch S.C., Erkizia I., Blanco J., Borràs F.E., Puertas M.C., Connor J.H., Fernández-Figueras M.T., Moore L., Clotet B., Gummuluru S., Martinez-Picado J. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger M., Pinelli M., Borghi M., Constantini C., Dindo M., van Emst L., Puccetti M., Pariano M., Ricaño-Ponce I., Büll C., Gresnigt M.S., Wang X., Gutierrez Achury J., Jacobs C.W.M., Xu N., Oosting M., Arts P., Joosten L.A.B., van de Veerdonk F.L., Veltman J.A., ten Oever J., Kullberg B.J., Feng M., Adema G.J., Wijmenga C., Kumar V., Sobel J., Gilissen C., Romani L., Netea M.G. A systems genomics approach identifies SIGLEC15 as a susceptibility factor in recurrent vulvovaginal candidiasis. Sci. Transl. Med. 2019;11(496):eaar3558. doi: 10.1126/scitranslmed.aar3558. [DOI] [PubMed] [Google Scholar]
- Jalloh S., Olejnik J., Berrigan J., Nisa A., Suder E.L., Akiyama H., Lei M., Tyagi S., Bushkin Y., Mühlberger E., Gummuluru S. CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses. BioRxiv [Preprint] 2022 doi: 10.1101/2022.03.29.486190. March 30:2022.03.29.486190 [cited 2022 July 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroenpool J., Rogers K.A., Pattanapanyasat K., Villinger F., Onlamoon N., Crocker P.R., Ansari A.A. Differences in the constitutive and SIV infection induced expression of Siglecs by hematopoietic cells from non-human primates. Cell. Immunol. 2007;250:91–104. doi: 10.1016/j.cellimm.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielity S., Wang J.J., Chan Y.K., Ahmed A.A., Li W., Monahan S., Bu X., Farzan M., Freeman G.J., Umetsu D.T., DeKruyff R.H., Choe H. TIM-Family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., McLean P.A., Neel B.G., Wortis H.H. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J. Exp. Med. 2002;195:1199–1205. doi: 10.1084/jem.20011796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M., Fink K., Henrickson S.E., Shayakhmetov D.M., Di Paolo N.C., van Rooijen N., Mempel T.R., Whelan S.P., von Andrian U.H. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral {B} cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kardava L., Moir S., Wang W., Ho J., Buckner C.M., Posada J.G., O'Shea M.A., Roby G., Chen J., Sohn H.W., Chun T.W., Pierce S.K., Fauci A.S. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J. Clin. Invest. 2011;121:2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijewski S.D.G., Akiyama H., Feizpour A., Miller C.M., Ramirez N.G.P., Reinhard B.M., Gummuluru S. Access of HIV-2 to CD169-dependent dendritic cell-mediated trans infection pathway is attenuated. Virology. 2016;497:328–336. doi: 10.1016/j.virol.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuels B., Wichmann D., Emmerich P., Schmidt-Chanasit J., de Heer G., Kluge S., Sow A., Renné T., Günther S., Lohse A.W., Addo M.M., Schmiedel S. A case of severe Ebola virus infection complicated by gram-negative septicemia. N. Engl. J. Med. 2014;371:2394–2401. doi: 10.1056/nejmoa1411677. [DOI] [PubMed] [Google Scholar]
- Kugelman J.R., Kugelman-Tonos J., Ladner J.T., Pettit J., Keeton C.M., Nagle E.R., Garcia K.Y., Froude J.W., Kuehne A.I., Kuhn J.H., Bavari S., Zeitlin L., Dye J.M., Olinger G.G., Sanchez-Lockhart M., Palacios G.F. Emergence of Ebola virus escape variants in infected nonhuman primates treated with the MB-003 antibody cocktail. Cell Rep. 2015;12:2111–2120. doi: 10.1016/j.celrep.2015.08.038. [DOI] [PubMed] [Google Scholar]
- Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Ségéral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajaunias F., Dayer J.-M., Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 2005;35:243–251. doi: 10.1002/eji.200425273. [DOI] [PubMed] [Google Scholar]
- Lempp, Soriaga L.B., Montiel-Ruiz M., Benigni F., Noack J., Park Y.-J., Bianchi S., Walls A.C., Bowen J.E., Zhou J., Kaiser H., Joshi A., Agostini M., Meury M., Dellota E., Jaconi S., Cameroni E., Martinez-Picado J., Vergara-Alert J., Izquierdo-Useros N., Virgin H.W., Lanzavecchia A., Veesler D., Purcell L.A., Telenti A., Corti D. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature. 2021;598:342–347. doi: 10.1038/s41586-021-03925-1. [DOI] [PubMed] [Google Scholar]
- Lenza M.P., Oyenarte I., Diercks T., Quintana J.I., Gimeno A., Coelho H., Diniz A., Peccati F., Delgado S., Bosch A., Valle M., Millet O., Abrescia N.G.A., Palazón A., Marcelo F., Jiménez‐Osés G., Jiménez‐Barbero J., Ardá A., Ereño‐Orbea J. Structural of N‐linked glycans in the receptor binding domain of the SARS‐CoV‐2 spike protein and their interactions with human lectins. Angew. Chem. Int. Ed. 2020;59:23763–23771. doi: 10.1002/anie.202011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarov A.V., Babenko V.V., Kulikov E.E. Free SARS-CoV-2 spike protein S1 particles may play a role in the pathogenesis of COVID-19 infection. Biochemistry (Moscow) 2021;86:257–261. doi: 10.1134/S0006297921030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhang W., Wan T., Zhang J., Chen T., Yu Y., Wang J., Cao X. Cloning and characterization of siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. J. Biol. Chem. 2001;276:28106–28112. doi: 10.1074/jbc.M100467200. [DOI] [PubMed] [Google Scholar]
- Lock K., Zhang J., Lu J., Lee S.H., Crocker P.R. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lubaki N.M., Younan P., Santos R.I., Meyer M., Iampietro M., Koup R.A., Bukreyev A. The Ebola interferon inhibiting domains attenuate and dysregulate cell-mediated immune responses. PLoS Pathog. 2016;12(12) doi: 10.1371/journal.ppat.1006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D.Y., Suthar M.S., Kasahara S., Gale M., Clark E.A. CD22 is required for protection against West Nile virus infection. J. Virol. 2013;87:3361–3375. doi: 10.1128/jvi.02368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M.S., Crocker P.R., Paulson J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotto S., Ferrari S., Monaco S. HCV-related central and peripheral nervous system demyelinating disorders. Inflamm. Allergy - Drug Targets. 2015;13:299–304. doi: 10.2174/1871528113666140908113841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Picado J., McLaren P.J., Erkizia I., Martin M.P., Benet S., Rotger M., Dalmau J., Ouchi D., Wolinsky S.M., Penugonda S., Günthard H.F., Fellay J., Carrington M., Izquierdo-Useros N., Telenti A. Identification of siglec-1 null individuals infected with HIV-1. Nat. Commun. 2016;7 doi: 10.1038/ncomms12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D., Wu L., Bohks S.M., KewalRamani V.N., Unutmaz D., Hope T.J. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Meyer S.J., Steffensen M., Acs A., Weisenburger T., Wadewitz C., Winkler T.H., Nitschke L. CD22 controls germinal center B cell receptor signaling, which influences plasma cell and memory B cell output. J. Immunol. 2021;207:1018–1032. doi: 10.4049/jimmunol.2100132. [DOI] [PubMed] [Google Scholar]
- Michlmayr D., Kim E.-Y.Y., Rahman A.H., Raghunathan R., Kim-Schulze S., Che Y., Kalayci S., Gümüş Z.H., Kuan G., Balmaseda A., Kasarskis A., Wolinsky S.M., Suaréz-Fariñas M., Harris E. Comprehensive immunoprofiling of pediatric Zika reveals key role for monocytes in the acute phase and No effect of prior Dengue virus infection. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Sakuma K., Kawamura Y.I., Izawa M., Ohmori K., Mitsuki M., Yamaji T., Hashimoto Y., Suzuki A., Saito Y., Dohi T., Kannagi R. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J. Immunol. 2012;188:4690–4700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- Mizrahi S., Gibbs B.F., Karra L., Ben-Zimra M., Levi-Schaffer F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J. Allergy Clin. Immunol. 2014;134:230–233. doi: 10.1016/j.jaci.2014.03.031. e3. [DOI] [PubMed] [Google Scholar]
- Mohan G.S., Li W., Ye L., Compans R.W., Yang C. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.-J. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday J., Floyd H., Crocker P.R. Sialic acid binding receptors (siglecs) expressed by macrophages. J. Leukoc. Biol. 1999;66:705–711. doi: 10.1002/jlb.66.5.705. [DOI] [PubMed] [Google Scholar]
- Munday J., Kerr S., Ni J., Cornish A.L., Zhang J.Q., Nicoll G., Floyd H., Mattei M.-G., Moore P., Liu D., Crocker P.R. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem. J. 2001;355(Pt 2):489–497. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz A. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107:1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- Murugesan G., Weigle B., Crocker P.R. ScienceDirect Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 2021;62:34–42. doi: 10.1016/j.cbpa.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Nguyen D.H., Hildreth J.E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.A., Hamzeh-Cognasse H., Palle S., Anselme-Bertrand I., Arthaud C.-A., Chavarin P., Pozzetto B., Garraud O., Cognasse F. Role of siglec-7 in apoptosis in human platelets. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll G., Ni J., Liu D., Klenerman P., Munday J., Dubock S., Mattei M.-G., Crocker P.R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- Oh D.S., Oh J.E., Jung H.E., Lee H.K. Transient depletion of CD169+ cells contributes to impaired early protection and effector CD8+ T cell recruitment against mucosal respiratory syncytial virus infection. Front. Immunol. 2017;8:819. doi: 10.3389/fimmu.2017.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr S.J., Morgan N.M., Buick R.J., Boyd C.R., Elliott J., Burrows J.F., Jefferies C.A., Crocker P.R., Johnston J.A. SOCS3 targets siglec 7 for proteasomal degradation and blocks siglec 7-mediated responses. J. Biol. Chem. 2007;282:3418–3422. doi: 10.1074/jbc.C600216200. [DOI] [PubMed] [Google Scholar]
- Orr S.J., Morgan N.M., Elliott J., Burrows J.F., Scott C.J., McVicar D.W., Johnston J.A. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–1068. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- Ortillon M., Coudereau R., Cour M., Rimmelé T., Godignon M., Gossez M., Yonis H., Argaud L., Lukaszewicz A.C., Venet F., Monneret G. Monocyte CD169 expression in COVID-19 patients upon intensive care unit admission. Cytometry A. 2021;99:466–471. doi: 10.1002/cyto.a.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Brinkman-Van Der Linden E.C.M., Altmann S.W., Gish K., Balasubramanian S., Timans J.C., Peterson D., Bell M.P., Bazan J.F., Varki A., Kastelein R.A. OB-BP1/Siglec-6. A leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J. Biol. Chem. 1999;274:22729–22738. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- Perez-Zsolt D., Cantero-Pérez J., Erkizia I., Benet S., Pino M., Serra-Peinado C., Hernández-Gallego A., Castellví J., Tapia G., Arnau-Saz V., Garrido J., Tarrats A., Buzón M.J., Martinez-Picado J., Izquierdo-Useros N., Genescà M. Dendritic cells from the cervical mucosa capture and transfer HIV-1 via siglec-1. Front. Immunol. 2019;10:825. doi: 10.3389/fimmu.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Zsolt D., Erkizia I., Pino M., García-Gallo M., Martin M.T., Benet S., Chojnacki J., Fernández-Figueras M.T., Guerrero D., Urrea V., Muñiz-Trabudua X., Kremer L., Martinez-Picado J., Izquierdo-Useros N. Anti-Siglec-1 antibodies block Ebola viral uptake and decrease cytoplasmic viral entry. Nat. Microbiol. 2019;4:1558–1570. doi: 10.1038/s41564-019-0453-2. [DOI] [PubMed] [Google Scholar]
- Perez-Zsolt D., Muñoz-Basagoiti J., Rodon J., Elosua-Bayes M., Raïch-Regué D., Risco C., Sachse M., Pino M., Gumber S., Paiardini M., Chojnacki J., Erkizia I., Muñiz-Trabudua X., Ballana E., Riveira-Muñoz E., Noguera-Julian M., Paredes R., Trinité B., Tarrés-Freixas F., Blanco I., Guallar V., Carrillo J., Blanco J., Telenti A., Heyn H., Segalés J., Clotet B., Martinez-Picado J., Vergara-Alert J., Izquierdo-Useros N. SARS-CoV-2 interaction with Siglec-1 mediates trans-infection by dendritic cells. Cell. Mol. Immunol. 2021;18:2676–2678. doi: 10.1038/s41423-021-00794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perot B.P., García-Paredes V., Luka M., Ménager M.M. Dendritic cell maturation regulates TSPAN7 function in HIV-1 transfer to CD4+ T lymphocytes. Front. Cell. Infect. Microbiol. 2020;10(70):1–17. doi: 10.3389/fcimb.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Netravali I.A., Cariappa A., Mattoo H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino M., Erkizia I., Benet S., Erikson E., Fernández-Figueras M.T., Guerrero D., Dalmau J., Ouchi D., Rausell A., Ciuffi A., Keppler O.T., Telenti A., Kräusslich H.-G.G., Martinez-Picado J., Izquierdo-Useros N. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology. 2015;12:37. doi: 10.1186/s12977-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L., Sun B., Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Puryear W.B., Akiyama H., Geer S.D., Ramirez N.P., Yu X., Reinhard B.M., Gummuluru S. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on siglec-1/CD169. PLoS Pathog. 2013;9(4) doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear W.B., Yu X., Ramirez N.P., Reinhard B.M., Gummuluru S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:7475–7480. doi: 10.1073/pnas.1201104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R.H. Myelin-associated glycoprotein (MAG): past, present and beyond. J. Neurochem. 2007;100(6):1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Lanier L.L. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Rempel H., Calosing C., Sun B., Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard S., Journeaux A., Gloaguen E., Schaeffer J., Varet H., Pietrosemoli N., Mateo M., Baillet N., Laouenan C., Raoul H., Mullaert J., Baize S. Immune parameters and outcomes during Ebola virus disease. JCI Insight. 2019;4(1) doi: 10.1172/jci.insight.125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffin N., Gea-Mallorquí E., Brouiller F., Jouve M., Silvin A., See P., Dutertre C.A., Ginhoux F., Benaroch P. Constitutive Siglec-1 expression confers susceptibility to HIV-1 infection of human dendritic cell precursors. Proc. Natl. Acad. Sci. U.S.A. 2019;116:21685–21693. doi: 10.1073/pnas.1911007116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammar M., Siwetz M., Meiri H., Fleming V., Altevogt P., Huppertz B. Expression of CD24 and Siglec-10 in first trimester placenta: implications for immune tolerance at the fetal–maternal interface. Histochem. Cell Biol. 2017;147:565–574. doi: 10.1007/s00418-016-1531-7. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Landig C.S., Siddiqui S., Secundino I., Olson J., Varki N., Nizet V., Varki A. Paired Siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J. 2017;36:751–760. doi: 10.15252/embj.201695581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E., Qu R., Junqueira C., Kaffe E., Mirza H., Zhao J., Brewer J.R., Han A., Steach H.R., Israelow B., Blackburn H.N., Velazquez S., Chen Y.G., Halene S., Iwasaki A., Meffre E., Nussenzweig M., Lieberman J., Wilen C.B., Kluger Y., Flavell R.A. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606(7914):585–593. doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewald X., Ladinsky M.S., Uchil P.D., Beloor J., Pi R., Herrmann C., Motamedi N., Murooka T.T., Brehm M.A., Greiner D.L., Shultz L.D., Mempel T.R., Bjorkman P.J., Kumar P., Mothes W. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science. 2015;350:563–567. doi: 10.1126/science.aab2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaabani N., Duhan V., Khairnar V., Gassa A., Ferrer-Tur R., Häussinger D., Recher M., Zelinskyy G., Liu J., Dittmer U., Trilling M., Scheu S., Hardt C., Lang P.A., Honke N., Lang K.S. CD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.350. e2446--e2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.N., Qin H., Yates B., Su L., Shalabi H., Raffeld M., Ahlman M.A., Stetler-Stevenson M., Yuan C., Guo S., Liu S., Hughes S.H., Fry T.J., Wu X. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3:2317–2322. doi: 10.1182/bloodadvances.2019000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J.-Y., Yin W.-W., Zhang Q.-F., Liu Q., Peng M.-L., Hu H.-D., Hu P., Ren H., Zhang D.-Z. Siglec-7 defines a highly functional natural killer cell subset and inhibits cell-mediated activities. Scand. J. Immunol. 2016;84:182–190. doi: 10.1111/sji.12455. [DOI] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Grogan C.C., Vandenberghe L.H., Baribaud F., Whitbeck J.C., Burke E., Buchmeier M.J., Soilleux E.J., Riley J.L., Doms R.W., Bates P., Pöhlmann S. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Singh D.K., Aladyeva E., Das S., Singh B., Esaulova E., Swain A., Ahmed M., Cole J., Moodley C., Mehra S., Schlesinger L.S., Artyomov M.N., Khader S.A., Kaushal D. Myeloid cell interferon responses correlate with clearance of {SARS}-{CoV}-2. Nat. Commun. 2022;13:679. doi: 10.1038/s41467-022-28315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto P.C., Karris M.Y., Spina C.A., Richman D.D., Varki A. Cell-intrinsic mechanism involving Siglec-5 associated with divergent outcomes of HIV-1 infection in human and chimpanzee CD4 T cells. J. Mol. Med. 2013;91:261–270. doi: 10.1007/s00109-012-0951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T., Matsumoto M., Arisawa F., Kohyama M., Hirayasu K., Mori Y., Arase H. Sialic acids on varicella-zoster virus glycoprotein B are required for cell-cell fusion. J. Biol. Chem. 2015;290:19833–19843. doi: 10.1074/jbc.M114.635508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T., Mori Y., Suzutani T., Arase H. Siglec-7 mediates varicella-zoster virus infection by associating with glycoprotein B. Biochem. Biophys. Res. Commun. 2022;607:67–72. doi: 10.1016/j.bbrc.2022.03.060. [DOI] [PubMed] [Google Scholar]
- Suenaga T., Satoh T., Somboonthum P., Kawaguchi Y., Mori Y., Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U.S.A. 2010;107:866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y., Sasaki S., Fujiwara T., Takahashi E., Muto T., Nakamura Y. Molecular cloning of a novel gene similar to myeloid antigen CD33 and its specific expression in placenta. Cytogenet. Genome Res. 1997;78:295–300. doi: 10.1159/000134676. [DOI] [PubMed] [Google Scholar]
- Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Tsai T.Y., Huang M.T., Sung P.S., Peng C.Y., Tao M.H., Yang H.I., Chang W.C., Yang A.S., Yu C.M., Lin Y.P., Bau C.Y., Huang C.J., Pan M.H., Wu C.Y., Hsiao C.D., Yeh Y.H., Duan S., Paulson J.C., Hsieh S.L. SIGLEC-3 (CD33) serves as an immune checkpoint receptor for HBV infection. J. Clin. Invest. 2021;131(11) doi: 10.1172/JCI141965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil P.D., Pi R., Haugh K.A., Ladinsky M.S., Ventura J.D., Barrett B.S., Santiago M.L., Bjorkman P.J., Kassiotis G., Sewald X., Mothes W. A protective role for the lectin CD169/siglec-1 against a pathogenic murine retrovirus. Cell Host Microbe. 2019;25:87–100. doi: 10.1016/j.chom.2018.11.011. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther D., Veninga H., Iborra S., Borg E.G.F., Hoogterp L., Olesek K., Beijer M.R., Schetters S.T.T., Kalay H., Garcia-Vallejo J.J., Franken K.L., Cham L.B., Lang K.S., van Kooyk Y., Sancho D., Crocker P.R., den Haan J.M.M. Functional CD169 on macrophages mediates interaction with dendritic cells for CD8+ T cell cross-priming. Cell Rep. 2018;22:1484–1495. doi: 10.1016/j.celrep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Varchetta S., Lusso P., Hudspeth K., Mikulak J., Mele D., Paolucci S., Cimbro R., Malnati M., Riva A., Maserati R., Mondelli M.U., Mavilio D. Sialic acid-binding Ig-like lectin-7 interacts with HIV-1 gp120 and facilitates infection of CD4posT cells and macrophages. Retrovirology. 2013;10:154. doi: 10.1186/1742-4690-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varchetta S., Mele D., Lombardi A., Oliviero B., Mantovani S., Tinelli C., Spreafico M., Prati D., Ludovisi S., Ferraioli G., Filice C., Aghemo A., Lampertico P., Facchetti F., Bernuzzi F., Invernizzi P., Mondelli M.U. Lack of Siglec-7 expression identifies a dysfunctional natural killer cell subset associated with liver inflammation and fibrosis in chronic HCV infection. Gut. 2016;65:1998–2006. doi: 10.1136/gutjnl-2015-310327. [DOI] [PubMed] [Google Scholar]
- Villinger F., Rollin P.E., Brar S.S., Chikkala N.F., Winter J., Sundstrom J.B., Zaki S.R., Swanepoel R., Ansari A.A., Peters C.J. Markedly elevated levels of interferon (IFN)‐σ, IFN‐α, Interleukin (IL)‐2, IL‐10, and tumor necrosis factor‐α associated with fatal Ebola virus infection. J. Infect. Dis. 2008;179:S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- Whitney G., Wang S., Chang H., Cheng K.-Y., Lu P., Zhou X.D., Yang W.-P., McKinnon M., Longphre M. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33: immune-restricted siglec-10. Eur. J. Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- Xie J., Christiaens I., Yang B., Breedam W. Van, Cui T., Nauwynck H.J. Molecular cloning of porcine Siglec-3, Siglec-5 and Siglec-10, and identification of Siglec-10 as an alternative receptor for porcine reproductive and respiratory syndrome virus (PRRSV) J. Gen. Virol. 2017;98:2030–2042. doi: 10.1099/jgv.0.000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Christiaens I., Yang B., Trus I., Devriendt B., Cui T., Wei R., Nauwynck H.J. Preferential use of Siglec-1 or Siglec-10 by type 1 and type 2 PRRSV strains to infect PK15S1–CD163 and PK15S10–CD163 cells. Vet. Res. 2018;49:67. doi: 10.1186/s13567-018-0569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B.A., Brock E.C., Leung J., Falahati R., Bryce P.J., Bright J., Williams J., Shultz L.D., Greiner D.L., Brehm M.A., Bebbington C., Tomasevic N. AK002, a humanized sialic acid-binding immunoglobulin-like lectin-8 antibody that induces antibody-dependent cell-mediated cytotoxicity against human eosinophils and inhibits mast cell-mediated anaphylaxis in mice. Int. Arch. Allergy Immunol. 2019;180:91–102. doi: 10.1159/000501637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B.A., Leung J., Falahati R., Williams J., Schanin J., Brock E.C., Singh B., Chang A.T., O'Sullivan J.A., Schleimer R.P., Tomasevic N., Bebbington C.R., Bochner B.S. Discovery, function, and therapeutic targeting of siglec-8. Cells. 2020;10:19. doi: 10.3390/cells10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.J., Reuter M.A., McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4(8) doi: 10.1371/journal.ppat.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Q., Nicoll G., Jones C., Crocker P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- Zhao D., Jiang X., Xu Y., Yang H., Gao D., Li X., Gao L., Ma C., Liang X. Decreased siglec-9 expression on natural killer cell subset associated with persistent HBV replication. Front. Immunol. 2018;9:1124. doi: 10.3389/fimmu.2018.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., Chertova E., Bess J., Lifson J.D., Arthur L.O., Liu J., Taylor K.A., Roux K.H. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15812–15817. doi: 10.1073/pnas.2634931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., Chastain A., Moir S., Ford J., Trandem K., Martinelli E., Cicala C., Crocker P., Arthos J., Sun P.D. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024559. [DOI] [PMC free article] [PubMed] [Google Scholar]