Allogeneic hematopoietic stem cell transplantation (allo-HSCT), the pioneer and mainstream form of cellular therapy, represents the only curative or preferred treatment for various malignant and nonmalignant disorders or facilitates organ transplantation by reconstituting the new blood and immune system of a patient with donor’s regenerative HSCs, similar to the comics character “Groot,” who has an incredible regeneration ability. The annual cases of allo-HSCT in mainland China increased from 10042 in 2020 to 12744 in 2021, suggesting that the negative impact of the severe acute respiratory syndrome coronavirus 2 pandemic might be eliminated, while Europe and North American registries reported 18796 and 8326 cases in 2020, respectively.1 In addition, cutting-edge progress in other cellular therapies, including chimeric antigen receptor T (CAR-T) cells, mesenchymal stromal cells, and specific cytotoxic T lymphocyte (CTL) cells, promotes the combination of allo-HSCT with these therapies to compose the new chapter “Strong Together, Guardians of Life” to save more patients worldwide.
Harmony of engrafted “seeds” and environment “soil”
Human leukocyte antigen (HLA) is critical to the immune system, which might prevent the HSC “seeds” from “homing” in the new bone marrow “soil” of the recipient or may contribute to the conflict between grown “seeds” and “soil” known as graft-versus-host diseases (GvHDs). Therefore, HLA-matched donors have previously been indispensable for allo-HSCT. Thanks to the innovative regimens “Beijing Protocol” and “Baltimore Protocol,” which bypass these barriers and fulfill allo-HSCT with haploidentical or even more than 5/10 mismatched donors, the overall survival with alternative donors has dramatically increased to 60%–70%. The haploidentical donor has been the largest source in China since 2013, accounting for 60% of allo-HSCT cases reported by the Chinese registry and for 20% in North America and Europe.1
The new era of “everyone has a donor” brings in-depth investigations of bridging the gap of the HLA barrier. Granulocyte colony-stimulating factor (G-CSF) is involved in 80%–90% of worldwide allo-HSCT activity, while the mechanism of facilitating engraftment and preventing GvHDs remains to be elucidated. Multiomics analysis of the chromatin structure and transcriptome of immune cells suggested that suppressor of cytokine signaling 1 (SOCS1) inhibits T cell activation, while loss of SOCS1 exacerbates GvHDs. In addition, G-CSF upregulates glucocorticoid receptors and SOCS1 expression in natural killer (NK) cells.2 Moreover, single-cell RNA sequencing before and after G-CSF administration suggested significantly changed progenitors and trajectory inference for hematopoiesis, which might promote the development of the G-CSF-based platform.
To better understand how donor “seeds” renew their lives in new “soil,” research on the bone marrow (BM) microenvironment has been an important part of optimizing allo-HSCT practice. M2 macrophages, rather than M1 macrophages, upregulate PI3K–AKT activity and support megakaryopoiesis, which provides a potential therapeutic strategy to reduce thrombocytopenia post-HSCT. In addition, reduced and dysfunctional BM endothelial cells could induce the exhaustion of successfully engrafted donor “seeds” and lead to poor graft function. By in vitro and clinical studies targeting reactive oxygen species with the classic antioxidant N-acetylcysteine, a potential strategy was developed to promote “seeds” to regenerate in new recipient “soils.” The latest phase III randomized trial indicated that N-acetylcysteine could reduce the +60-day incidence of prolonged isolated thrombocytopenia or poor graft function (22.5% versus 7.5%, p = 0.021), reduce the ROC level, and improve BM endothelial cells and CD34+ HSCs. This series of studies exerts a perfect example of “how to solve the clinical dilemma by revisiting classics in a new pathway,” which provides an opportunity to consider this type of trial design to identify new regimens for harmonious coexistence of engrafted “seeds” and environment “soil.”
Orchestrate the balance between graft and host
Although the progression of prophylaxis has reduced the risk of GvHDs, steroid-refractory (SR) acute GvHD (aGvHD) and chronic GvHD (cGvHD) remain unavoidable “growing pains,” especially considering the need to maximize the power of the graft-versus-leukemia effect to reduce relapse of various hematological malignancies. Therefore, it is critical to refine the treatment of GvHDs by orchestrating the balance between the graft and host post-HSCT.
Basiliximab is a novel monoclonal antibody that binds to the interleukin-2 receptor (IL-2R) and inhibits activated T lymphocytes in allo-HSCT, but high-quality evidence remains to be obtained. In the largest real-world study of basiliximab in patients with SR aGvHD (ie, BRIGHT study), the day-28 overall response rate (ORR) was 79.4% after basiliximab treatment. Machine learning helps to define the optimal protocol for basiliximab at the fourth dose to balance the risk of infection and therapeutic response in severe SR aGvHD.3 The phase III randomized trial (ClinicalTrials.gov: NCT02241018) also confirmed that adding mesenchymal stromal cells to basiliximab and calcineurin inhibitor resulted in better day-28 ORR than the control group in SR aGvHD (82.8% versus 70.7%; p = 0.043), extended the median failure-free survival (11.3 versus 6 months, p = 0.024), and reduced the 2-year cGvHD (39.5% versus 62.7%, p = 0.005) without increasing relapse risk.
Ruxolitinib, a Janus kinase inhibitor, replicated its success in SR cGvHD as aGvHD. In the REACH3 phase III randomized trial, compared with the best available treatment, the ruxolitinib group demonstrated a greater week-24 ORR (49.7% versus 25.6%; p < 0.001), longer failure-free survival (median >18.6 versus 5.7 months; p < 0.001), and higher incidence of relieved symptoms (24.2% versus 11%; p = 0.001). The latest meta-analysis also confirmed the efficacy and safety of ruxolitinib in SR GvHD, which were comparable between children and adults. The complete response rate and ORR were 0.49 and 0.77 for aGvHD and 0.15 and 0.78 for cGvHD, respectively. Another selective Janus kinase 1 inhibitor, itacitinib, might improve the day-28 ORR of aGvHD in combination with steroids (74% versus 53%, p = 0 · 078) and requires further studies.
Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil–containing protein kinase 2, which could enhance regulatory T cells and prevent proinflammatory cytokines IL-17 and IL-21. In the randomized study evaluating belumosudil in patients with SR cGvHD who had received two to five lines of therapy, the ORR was 74%–77%, and the median duration of response was 54 weeks, which promoted its approval by the United States in 2022.
Growing evidence suggests a significant reduction in microbial diversity peri-GvHD, and the intestinal microbiome can serve as a target.4 Pooled allo-microbiotherapy MaaT013 resulted in a 56% ORR in severe gastrointestinal aGvHD, while bacteremia was lower than the historical control (14% versus 31%–74%). As the United States approved the first fecal microbiota product for the prevention of Clostridioides difficile infection in 2022, it is anticipated that the microbiota will play an important role in controlling GvHDs.
In addition, as more novel agents, such as RGI-2001, baricitinib, etc., might become available in the near future, we would be more confident in helping patients eliminate “growing pains,” which might broaden allo-HSCT application in the near future.
Strong together: Boost HSCT by combining cellular therapy
The ultimate purpose of allo-HSCT is to restore a healthy blood and immune system, which would help patients fight against malignancies or eliminate BM failure, immune deficiency, inherited diseases, etc. Combining other cellular therapies pre- and post-HSCT, the regenerated naive system could be “boosted” to refine its ability against tumors or infections.
As patients transplanted in relapse/refractory diseases have inferior survival compared with those in complete remission (CR), it has been one of the standard options to bridge relapse/refractory acute B cell lymphoblastic leukemia to allo-HSCT by pre-HSCT CAR-T treatment, considering the high remission possibility (67%–93% by CD19) but low long-term survival (20%–40%) by CAR-T alone. In 2022, CAR-T targeting CD7 resulted in encouraging CR rates of 85%–95.8%.5 By bridging to consolidation allo-HSCT within 3 months after CD7 CAR-T, approximately 76%–90% of patients achieved long-term survival. It is anticipated that more novel targets will emerge in the next few years, especially in acute myelogenous leukemia or myelodysplastic syndromes.
For patients with measurable residual disease post-HSCT, preemptive CAR-T therapy could eliminate leukemia cells more rapidly (14 versus 43 days, p < 0.001) and achieve negative measurable residual disease (100% versus 66.7%, p = 0.032) than traditional donor lymphocyte infusion. By post-HSCT infusion of donor-derived multiple leukemia antigen-specific T cells targeting frequently expressed biomarkers (PRAME, WT1, and surviving) in ALL, patients could strengthen their tumor-reactive T cells against tumor antigens by in vivo amplification and prevent relapse.
In addition to strengthening the antitumor ability post-HSCT, it is also a critical step to refine antiviral immunity, especially in patients before good immune reconstitution. Adoptive therapy with cytomegalovirus-specific CTLs (CMV-CTLs) may help the recovery of endogenous immunity and “boost” patients post-HSCT to clear reactivated CMV. The first CMV-CTL infusion resulted in 80%–90% CR in 6 weeks, while third-party CMV-CTLs had comparable efficacy, toxicity, and endogenous immunity recovery compared with donor origin.
Other cellular treatments developed in the preclinical phase also suggest the potential to promote endogenous immunity by “boost” allo-HSCT, such as dual-CAR NK cells that incorporate both activating CAR against the tumor and an NK self-recognizing inhibitory CAR, the combination of OX40-specific CTLs and CAR-T cells, and Orca-Q with enriched CD34+ cells combined with specific T cell subsets, which deserve further in-depth investigation in the near future.
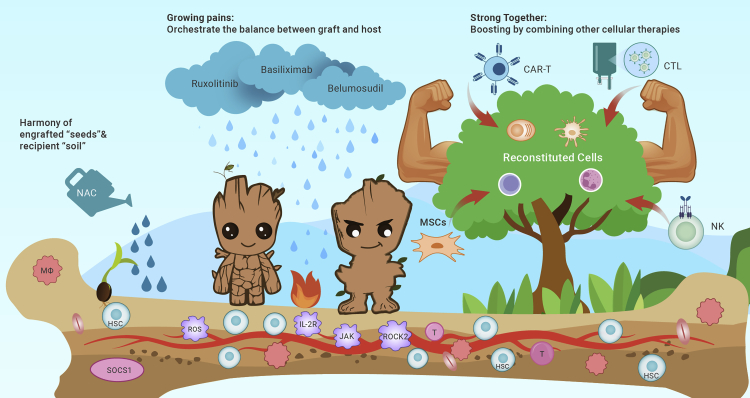
In summary, allo-HSCT would continue to be the major cellular therapy platform, which would regenerate “Groot” by the optimized “seed–soil” relationship, “boost” by other cellular treatments, and “heal” the infinite lives of the world (Figure 1).
Figure 1.
The figure illustrates three key steps for donor hematopoietic stem cells (HSCs) to reconstitute in the recipient’s environment and cure patients
(1) Promote harmony of engrafted HSCs “seeds” and recipient environment“soil". (2) Orchestrate the“adolescent” plant modes and cure the “growing pain”—refractory GvHD. (3) The reconstituted "Big Tree-Groot" refines its ability to cure patients by“boost” from CAR-T cells, MSCs, NK cells and CTL cells.
Acknowledgments
This work was supported by the National Key Research and Development Plan of China (2021YFA1100902 and 2022YFC2502606); National Natural Science Foundation of China (82070182, 82170208); Beijing Nova Program of Science and Technology (Z191100001119120); the CAMS Innovation Fund for Medical Sciences (CIFMS) (2022-I2M-C&T-B-121 and 2019-I2M-5-034); and Fund of China Scholarship Council (202106015007).
Declaration of interests
The authors declare no competing interests.
Published Online: January 2, 2023
References
- 1.Chang Y.,J., Pei X.Y., Huang X.J. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet. Haematol. 2022;9:e919–e929. doi: 10.1016/S2352-3026(22)00293-9. [DOI] [PubMed] [Google Scholar]
- 2.Guo H., Li R., Wang M., et al. Multiomics analysis identifies SOCS1 as restraining T cell activation and preventing Graft-Versus-Host Disease. Adv. Sci. 2022;9:e2200978. doi: 10.1002/advs.202200978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo X.D., Hong S.D., Zhao Y.L., et al. Basiliximab for steroid-refractory acute graft-versus-host disease: a real-world analysis. Am. J. Hematol. 2022;97:458–469. doi: 10.1002/ajh.26475. [DOI] [PubMed] [Google Scholar]
- 4.Burgos da Silva M., Ponce D.M., Dai A., et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood. 2022;140:2385–2397. doi: 10.1182/blood.2021015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu P., Liu Y., Yang J., et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood. 2022;140:321–334. doi: 10.1182/blood.2021014498. [DOI] [PubMed] [Google Scholar]