Abstract
Adverse environmental factors severely affect crop productivity. Improving crop resistance to multiple stressors is an important breeding goal. Although CBFs/DREB1s extensively participate in plant resistance to abiotic stress, the common mechanism underlying CBFs/DREB1s that mediate resistance to multiple stressors remains unclear. Here, we show the common mechanism for MaDREB1F conferring cold and drought stress resistance in banana. MaDREB1F encodes a dehydration-responsive element binding protein (DREB) transcription factor with nuclear localization and transcriptional activity. MaDREB1F expression is significantly induced after cold, osmotic, and salt treatments. MaDREB1F overexpression increases banana resistance to cold and drought stress by common modulation of the protectant metabolite levels of soluble sugar and proline, activating the antioxidant system, and promoting jasmonate and ethylene syntheses. Transcriptomic analysis shows that MaDREB1F activates or alleviates the repression of jasmonate and ethylene biosynthetic genes under cold and drought conditions. Moreover, MaDREB1F directly activates the promoter activities of MaAOC4 and MaACO20 for jasmonate and ethylene syntheses, respectively, under cold and drought conditions. MaDREB1F also targets the MaERF11 promoter to activate MaACO20 expression for ethylene synthesis under drought stress. Together, our findings offer new insight into the common mechanism underlying CBF/DREB1-mediated cold and drought stress resistance, which has substantial implications for engineering cold- and drought-tolerant crops.
Introduction
Adverse environmental factors, such as low temperature, drought, and salt, severely limit crop growth, development, and productivity. To combat these harsh stressors, plants have developed various complicated mechanisms for their protection, including the perception and transduction of stress signaling, regulation of gene expression, and physiological and metabolic changes [1, 2]. Of these, transcription factors (TFs) are crucial for regulating abiotic stress signaling and gene expression. The TFs of the APETALA2/ethylene-responsive factor (AP2/ERF) superfamily are subclassified into three families, namely ERF, AP2, and Related to ABI3/VP1 (RAV) [3]. The ERF family, which contains the ERF and DREB (dehydration-responsive element-binding protein) subfamilies, has a single AP2 domain and fewer introns and plays a key role in abiotic stress response [2]. Evidence has suggested the induction of ERFs by abiotic stress, such as drought, cold, heat, salt, and hormones, including abscisic acid (ABA), ethylene, gibberellin, salicylic acid, jasmonate (JA), and auxin, in various plant species [4]. Further genetic studies support the positive or negative function of ERFs in plant resistances to drought, cold, heat, salt, and heavy metals [4].
DREB TFs act on the activation of stress-responsive genes by directly binding to the C-repeat/dehydration responsive element (CRT/DRE) with a core sequence of G/ACCGAC, constituting the major component of the ABA-independent pathway during the stress response [2]. In Arabidopsis, DREBs are classified into six subclades, with subclade A1 mainly including C-repeat binding factors (CBF1–4) [5]. Extensive overexpression and silence studies have suggested that CBF1 and CBF3 play central roles in cold acclimation [6, 7]. In contrast, the function of CBF2 is ambiguous; the T-DNA insertion mutation of CBF2 increased freezing resistance [8], while the cbf2 mutant was susceptible to freezing stress [9]. CBFs have been characterized in various plant species [10, 11] and overexpression of CBFs induces cold-responsive (COR) gene expression and increases cold resistance in some plant species, including rice, tomato, Zea mays, and Brassica napus [12–17]. Although this indicates the functional conservation of CBFs in cold resistance, other roles have been verified for diverse CBF members. CBF4 plays a role in drought resistance and CBF2 regulates dehydration and salt stress resistance in Arabidopsis [8, 18]. Overexpression of the orthologs (OsDREB1A, OsDREB1B, OsDREB1E, OsDREB1F, and OsDREB1G) of Arabidopsis CBFs increased rice resistance to drought and/or salt stress [19]. However, the common mechanism underlying CBF/DREB1-mediated multiple stress resistance remains unclear.
Accumulated evidence has shown that the crucial targets of CBFs/DREB1s are COR genes [6, 20, 21]. COR genes include COR/LEA, low-temperature induced (LTI), early dehydration-inducible (ERD), and responsive to desiccation (RD) genes [6, 20, 22–24]. The products of COR genes mainly include key enzymes involved in cell wall modifiers, osmolyte biosynthesis, lipid metabolism, carbohydrate metabolism, proteins for hormone responses, and molecular chaperones [6, 20, 21]. The promoters of COR genes harbor the CRT/DRE cis-element that is responsible for cold-, dehydration-, and salt-induced expression [20]. CBFs can regulate transcriptional changes in many genes associated with stress responses, TFs, kinases, hormonal signaling, carbohydrate metabolism, and cell wall modification [9, 25, 26]. However, in addition to COR genes, the target genes directly bound by CBF are still poorly understood.
Banana is one of the nutritional fruits and staple foods worldwide. It is widely distributed in tropical and subtropical developing countries, but these regions have high rates of abiotic stress and plant disease, severely decreasing the yield and quality of banana [27]. Moreover, banana plants are sensitive to cold, drought, and salt stress [28]. It is essential to study the mechanism underlying banana’s response to abiotic stress to enhance resistance to abiotic stress. Several genes, including MusaPIP2;6, MusaPIP1;2, MusaNAC042, MusaWRKY71, MusaSAP1, MusaSNAC1, MaPIP2-7, and MaSIP2-1, have been shown to have a positive effect on banana resistance to abiotic stress [28–35]. However, there is currently no evidence of the genetic function and molecular mechanism of the AP2/ERF subfamily in banana’s response to abiotic stress.
In this study, MaDREB1F overexpression increased banana resistance to cold and drought stress. Combining physiological and transcriptomic analyses, we discovered that MaDREB1F overexpression in common triggered the accumulation of soluble sugar and proline, activated antioxidant system, and promoted the synthesis of jasmonate and ethylene under cold and drought stress. The findings offer new insights into the common mechanism of MaDREB1F conferring cold and drought stress and will aid in breeding strategies to improve crop resistance to cold and drought stress.
Results
MaDREB1F encodes a DREB transcription factor
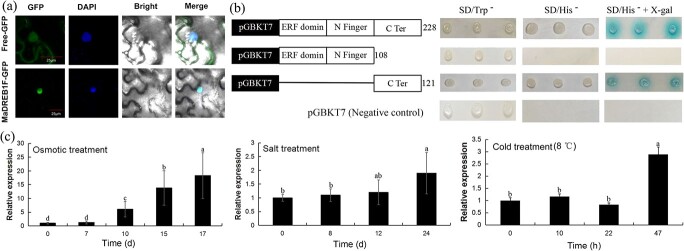
Previously, our transcriptomic data indicated that MaDREB1F is induced after osmotic, cold, and salt treatments [36]. In this study, to investigate its role, we cloned the open reading frame (ORF) of MaDREB1F, which is 687 bp in length and encodes 229 amino acids. The BLASTX analysis showed that MaDREB1F has high homology with EgDREB1F (60%) from Elaeis guineensis, PdDREB1F (60%) from Phoenix dactylifera, and AcDREB1F (58%) from Ananas comosus. The putative MaDREB1F protein had typical sequence characteristics of a conserved AP2 domain, a nuclear localization signal (NLS), and an activation domain (Supplementary Data Fig. S1). Phylogenetic analysis showed that OsDREB1F belongs to the A-1 group of the DREB subfamily and is close to OsDREB1F (Supplementary Data Fig. S2). Subcellular localization analysis suggested that the fluorescence of 35S::GFP was distributed throughout the cell, whereas fluorescence of the 35S::MaDREB1F-GFP chimera was localized in the cellular nucleus (Fig. 1a). Transcriptional activity analysis showed that yeast cells containing pGBKT7-MaDREB1F and pGBKT7-MaDREB1F-C grew well in SD/His− medium and turned blue in SD/His− medium with X-gal, indicating transcriptional activity of MaDREB1F protein and its C-terminal domain (Fig. 1b). These results suggest that MaDREB1F encodes a DREB TF in banana.
Figure 1.
Subcellular localization, transactivation activity, and expression of MaDREB1F after drought, cold, and salt treatments. (a) Subcellular localization of MaDREB1F. 35S::MaDREB1F-GFP fusion and 35S::GFP were transiently transformed in tobacco leaves and visualized on the third day post-infiltration. (b) Transactivation activity of MaDREB1F in yeast. Fusion protein was transformed into the AH109 strain. Transactivation activity was examined on SD/Trp−, SD/His−, and SD/His− with X-gal media. (c) Expression of MaDREB1F after osmotic, cold, and salt treatments. Duncan’s range test was used for significance examination (n = 3; P < .05).
MaDREB1F expression is upregulated after osmotic, cold, and salt treatments
The expression of the MaDREB1F gene was induced by osmotic treatment and reached the highest level after 17 days of treatment. After salt treatment, the expression of MaDREB1F was slightly upregulated and reached the highest transcription level at 24 days. After cold treatment, MaDREB1F was induced at 47 hours of treatment (Fig. 1c). These results indicate the induction of MaDREB1F expression by osmotic, salt, and cold stress.
Generation of transgenic banana plants overexpressing MaDREB1F
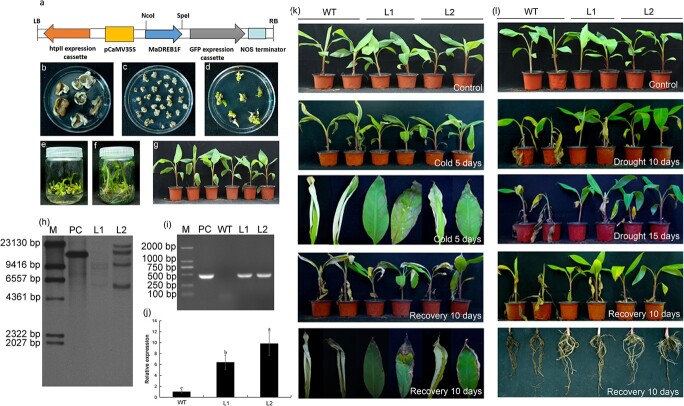
To investigate the role of MaDREB1F in planta, MaDREB1F was introduced into the pCAMBIA1302 vector (Fig. 2a). The floral apices of the Gongjiao (Musa corniculata L. AAA group) were cut into slices that were subsequently placed on a differentiation medium to induce callus growth (Fig. 2b). Two-millimeter slices were cut from the callus and infected with A. tumefaciens EHA105 harboring the binary vector (Fig. 2c). The infected callus slices were transferred to a differentiation medium to induce shoot growth (Fig 2d). After the shoot had been cultured to 3 cm in height (Fig. 2e), the explants were placed in a rooting medium (Fig. 2f). When the roots reached 8 cm in length, the plantlets were moved onto coconut coir medium for 100 days (Fig. 2g). Finally, we acquired 27 hygromycin-resistant lines, of which six transgenic lines could be verified by PCR amplifying the hpt-II gene from the binary vector. Then, the T2 generation was produced from these transgenic lines. To provide molecular evidence for these transgenic lines, we performed a molecular characterization in detail. Southern blot analysis indicated the integration of multiple copies of the MaDREB1F transgene in lines L1 and L2 (Fig. 2h). In addition, PCR amplification indicated the existence of the hpt-II gene from the vector in L1 and L2 (Fig. 2i). The expression levels of MaDREB1F were 6–10 times higher in L1 and L2 than in the WT (Fig 2j). These results suggest that MaDREB1F was successfully overexpressed in banana plants.
Figure 2.
MaDREB1F overexpression increases banana resistance to cold and drought stress. (a) T-DNA region of pCAMBIA1302 for generating transgenic plants. (b) Floral apex slices on differentiation medium. (c) Callus regenerated from floral apex slices. (d) Induction of shoot growth on shooting medium. (e) Shoot growth to 3 cm in height. (f) Rooting of explants on rooting medium. (g) Hardening of rooted transgenic plants. (h) Integration of MaDREB1F transgene in lines L1 and L2 from Southern blot analysis. M, marker; PC, positive control. (i) Amplification of hpt-II gene by PCR in transgenic lines. (j) MaDREB1F expression in transgenic lines using qRT–PCR. Duncan’s range test was used for significance examination (n = 3; P < .05). (k) 100-day-old banana seedlings were exposed to cold conditions (8°C) for 5 days and recovery for 10 days, then photographs were taken. (i) 100-day-old banana seedlings were treated by withholding water for 10 and 15 days and recovery for 10 days, then photographs were taken.
MaDREB1F overexpression enhances banana resistance to cold and drought stress
When banana seedlings were under cold and recovery conditions, WT plants displayed more severe leaf curling and chlorosis than transgenic lines (Fig. 2k). After 10 and 15 days of withholding water and 10 days of recovery, WT plants showed more severe growth inhibition, and leaf wilt and chlorosis compared with transgenic lines. Moreover, WT plants had shorter roots and more root damage than transgenic lines under drought and recovery conditions (Fig. 2l). MaDREB1F overexpression enhances banana’s resistance to cold and drought stress.
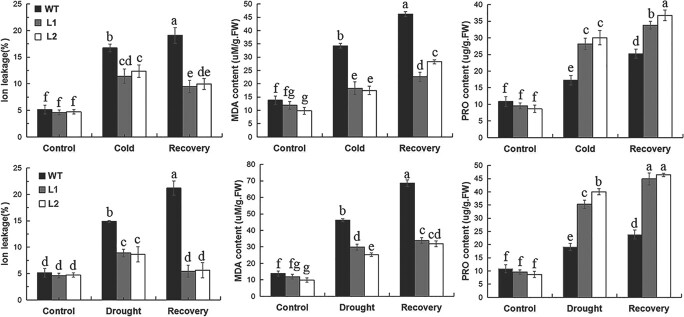
Under normal growth conditions, the content of MDA was lower in transgenic plants than in WT plants. Under drought, cold, and recovery conditions, transgenic plants exhibited lower levels of ion leakage and MDA, but higher proline content compared with WT plants (Fig. 3). Ion leakage and MDA are crucial indicators of reactive oxygen species (ROS)-mediated injury and membrane injury, respectively [37,38]. This further supports the positive role of MaDREB1F during drought and cold stress.
Figure 3.
Physiological differences between transgenic lines and WT under normal, cold, drought, and recovery conditions. 100-day-old banana seedlings were exposed to low temperature at 8°C for 5 days and recovery for 10 days or treated by withholding water for 15 days and recovery for 10 days; leaf samples were then collected to examine physiological indices. Duncan’s range test was used for significance examination (n = 3; P < .05).
Identification of cold- and drought-responsive genes affected by MaDREB1F overexpression
Comparative transcriptomic analysis was conducted between WT and MaDREB1F-overexpressing plants before and after drought and cold treatments. Because MaDREB1F-overexpressed plants were more tolerant than WT under drought and cold conditions, we focused on the differentially expressed genes (DEGs) that either specifically changed in MaDREB1F-overexpressing plants or changed ≥2-fold in MaDREB1F-overexpressing plants relative to the WT. Since these two types of DEGs are probably associated with stress resistance in banana conferred by MaDREB1F, we defined them as stress-responsive genes affected by MaDREB1F overexpression.
After cold treatment, a total of 8902 and 8811 DEGs were identified from the transgenic line (TL)_cold/TL and WT_cold/WT, respectively (Supplementary Data Tables S2 and S3). Of these genes, 4812 were uniquely identified in TL_cold/TL, 4721 genes were exclusively found in WT_cold/WT, and 4090 genes were regulated in common in both WT_cold/WT and TL_cold/TL. Of the 4090 commonly regulated DEGs, 1336 genes changed more (fold change >2) in TL_cold/TL than in WT_cold/WT. In total, we identified 6148 genes as cold-responsive genes affected by MaDREB1F overexpression (Fig. 4a and Supplementary Data Table S4).
Figure 4.
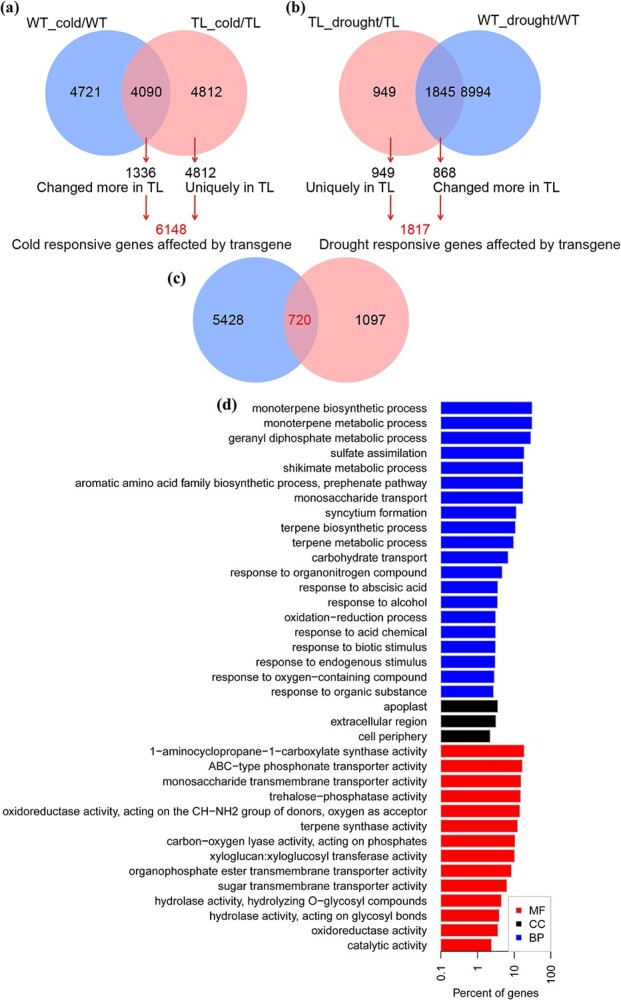
Identification of cold- and drought-responsive genes affected by MaDREB1F overexpression. (a) Venn diagram showing the cold-responsive genes in WT and the transgenic line. (b) Venn diagram showing the drought-responsive genes in WT and the transgenic line. (c) Venn diagram showing the genes regulated in common by MaDREB1F overexpression after cold and drought treatments. (d) GO enrichment of the genes regulated in common by MaDREB1F overexpression after cold and drought treatments.
After drought treatment, a total of 10 839 and 2794 DEGs were identified from WT_drought/WT and TL_drought/TL, respectively (Supplementary Data Tables S5 and S6). Of these, 8994 genes were exclusively found in WT_drought/WT, 949 genes were uniquely identified in TL_drought/TL, and 1845 genes were commonly regulated in both WT_drought/WT and TL_drought/TL. Of these 1845 commonly regulated genes, 868 genes changed more (fold change >2) in TL_drought /TL than in WT_drought/WT. In total, 1817 genes were identified as drought-responsive genes affected by MaDREB1F overexpression (Fig. 4b and Supplementary Data Table S7).
We further overlapped the cold- and drought-responsive genes affected by MaDREB1F overexpression and found 720 genes that were commonly regulated by MaDREB1F overexpression after cold and drought treatment (Fig. 4c and Supplementary Data Tables S8 and S9). These genes belonged to 37 categories from the Gene Ontology (GO) enrichment analysis (Fig. 4d and Supplementary Data Table S10). Notably, these GO terms included carbohydrate transport (GO_0008643), sugar transmembrane transporter activity (GO_0051119), response to abscisic acid (GO_0009737), oxidoreductase activity (GO_0016491), oxidoreductase activity, acting on the CH-NH2 group of donors (GO_0016641), oxidation–reduction process (GO_0055114), and response to oxygen-containing compound (GO_1901700), suggesting that carbohydrate metabolism, ABA response, and oxidation–reduction-associated processes were commonly affected by MaDREB1F overexpression after cold and drought treatments (Supplementary Data Fig. S3 and Supplementary Data Tables S11 and S12). These seven GO terms harbor 146 genes and their expression patterns were classified into four groups. Of these, more genes (73 genes) in these biological processes showed common induction, but fewer genes (18 genes) showed common repression after cold and drought treatments in MaDREB1F-overexpressing plants than in the WT (Supplementary Data Fig. S4 and Supplementary Data Tables S13–S16).
Further physiological analyses showed that the H2O2 content was lower but activities of CAT, POD, and SOD and content of soluble sugar were higher in transgenic plants than in WT plants under normal, cold, drought, and recovery conditions. This indicates that MaDREB1F overexpression improves antioxidative enzyme activity and sugar metabolism, supporting the above transcriptomic results (Fig. 5).
Figure 5.
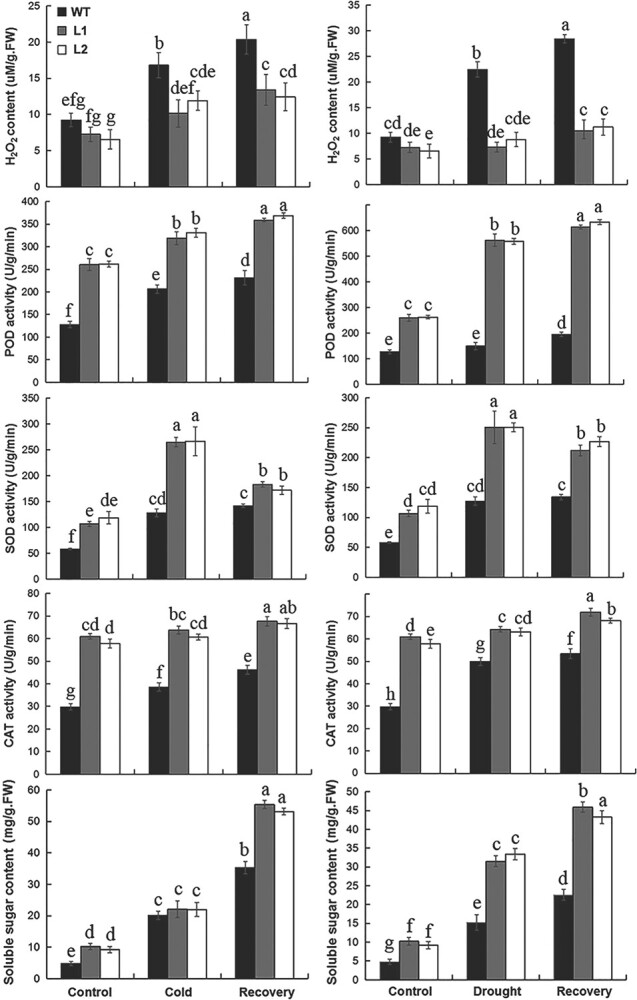
Measurement of H2O2 content, enzymatic activities of POD, SOD, and CAT, and soluble sugar content in WT and transgenic lines under normal, cold, drought, and recovery conditions. 100-day-old banana seedlings were exposed to low temperature at 8°C for 5 days and recovery for 10 days or treated by withholding water for 15 days and recovery for 10 days, then the leaf samples were collected to examine physiological indices. Duncan’s range test was used for significance examination (n = 3; P < .05).
Common regulation of jasmonate and ethylene biosynthesis by MaDREB1F overexpression under cold and drought conditions
In the GO terms ‘response to oxygen-containing compound’ (GO_1901700) and ‘oxidation–reduction process’ (GO_0055114) from the commonly regulated gene set by MaDREB1F overexpression after cold and drought treatments, there are an AOC and two ACOs encoding key enzymes for biosynthesis of JA and ethylene, respectively (Supplementary Data Table S10). This guided us to investigate the effects of MaDREB1F on the JA and ethylene biosynthesis pathways under cold and drought conditions.
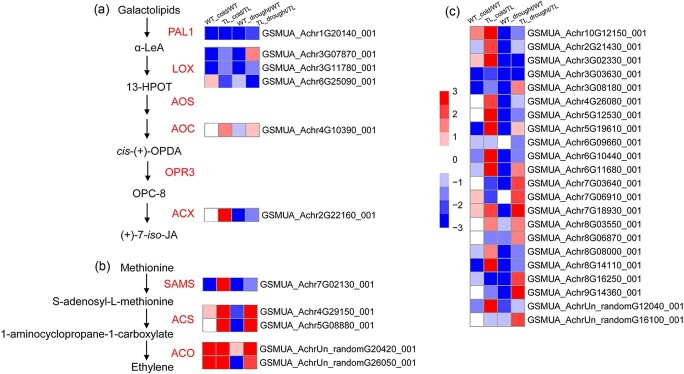
We found that the expression of six genes encoding key enzymes in JA biosynthesis was significantly affected by MaDREB1F overexpression after cold and drought treatments (Fig. 6a and Supplementary Data Table S17). Under cold treatment, phospholipase A1 (PAL1) and two lipoxygenases (LOXs) (GSMUA_Achr3G07870_001 and GSMUA_Achr3G11780_001) were repressed in WT and transgenic plants, but the degree of repression in transgenic plants was alleviated. Moreover, allene oxide cyclase (AOC) and acyl-CoA oxidase (ACX) did not show significant changes in the WT, but were significantly induced in transgenic plants under cold treatment. Under drought treatment, PAL1, LOX (GSMUA_Achr3G11780_001), and ACX were repressed in the WT, but the degree of repression was alleviated in MaDREB1F-overexpressing plants. In addition, another LOX (GSMUA_Achr3G07870_001) and AOC were repressed in WT plants, but were significantly induced in transgenic plants after drought treatment. These results indicated that MaDREB1F activated or alleviated the repression of genes in the JA biosynthesis pathway under cold and drought conditions.
Figure 6.
Common regulation of JA and ethylene biosynthesis by MaDREB1F overexpression under cold and drought conditions. (a) Expression profile of six genes commonly regulated by MaDREB1F overexpression after cold and drought treatments in the JA synthesis pathway. (b) Expression profile of five genes in the ethylene synthesis pathway commonly regulated by MaDREB1F overexpression after cold and drought treatments. (c) Expression profile of 22 ERF genes commonly regulated by MaDREB1F overexpression after cold and drought treatments.
The transcripts of five genes encoding ethylene biosynthesis-associated enzymes were commonly affected by MaDREB1F overexpression after cold and drought treatments (Fig. 6b and Supplementary Data Table S18). Ethylene biosynthesis includes three core enzymatic steps: S-adenosyl-l-methionine synthase (SAMS), ACS, and ACO. Under cold treatment, SAMS was downregulated and one ACS (GSMUA_Achr5G08880_001) did not show significant changes in WT, but they were significantly upregulated in MaDREB1F-overexpressing plants. One ACS (GSMUA_Achr4G29150_001) and two ACOs were upregulated in WT and transgenic plants, but the degree of induction was significantly improved in the transgenic plants. Under drought treatment, SAMS was repressed in WT and transgenic plants, but the degree of repression was alleviated in transgenic plants. Two ACS and one ACO (GSMUA_AchrUn_randomG26050_001) were repressed in WT plants, but were significantly induced in transgenic plants. The expression of one ACO (GSMUA_AchrUn_randomG26050_001) was upregulated in the WT and transgenic plants, but the degree of induction was significantly enhanced in the transgenic plants. These results indicate that MaDREB1F activated or alleviated the repression of genes in the ethylene biosynthesis pathway under cold and drought conditions.
Notably, we observed that expressions of 22 ERFs were commonly affected by MaDREB1F overexpression after cold and drought treatments. Under cold treatment, 15 ERFs showed more induction or less repression in transgenic plants than in WT. Similarly, 21 ERFs showed more induction or less repression in transgenic plants than in WT under drought treatment (Fig. 6c and Supplementary Data Table S19).
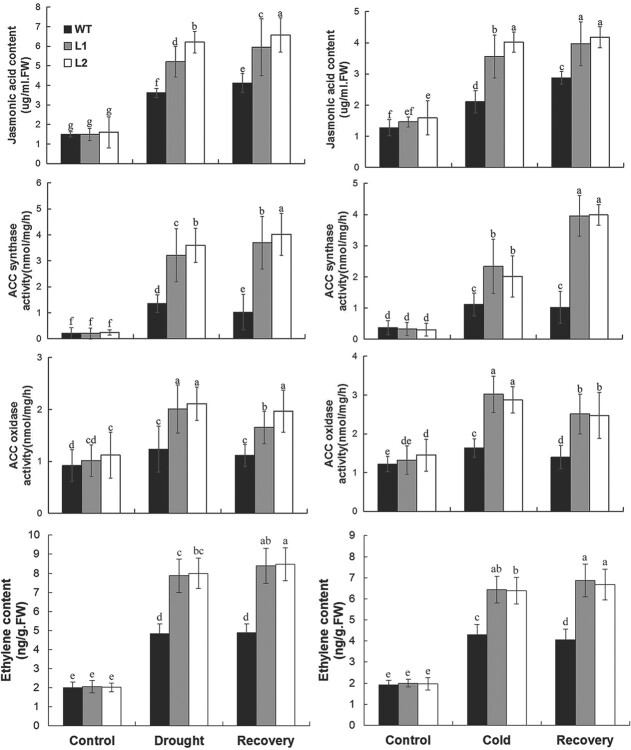
Physiological analyses showed that JA content, ACS and ACO activities, and ethylene content were significantly higher in transgenic lines than in WT under cold, drought, and recovery conditions, further supporting the activation or alleviation of repression of JA and ethylene biosynthetic genes in transgenic plants under cold and drought conditions (Fig. 7).
Figure 7.
Measurement of JA content, ACS and ACO activities, and ethylene content in WT and transgenic lines cold, drought, and recovery conditions. 100-day-old banana seedlings were exposed to cold conditions (8°C) for 5 days and recovery for 10 days or treated by withholding water for 15 days and recovery for 10 days. The leaves were collected to examine JA content and activities of ACS and ACO. Whole seedlings were enclosed in an airtight container to collect and measure ethylene content. Duncan’s range test was used for significance examination (n = 3; P < .05).
Transcriptional regulation of MaAOC4, MaACO20, and MaERF11 by MaDREB1F
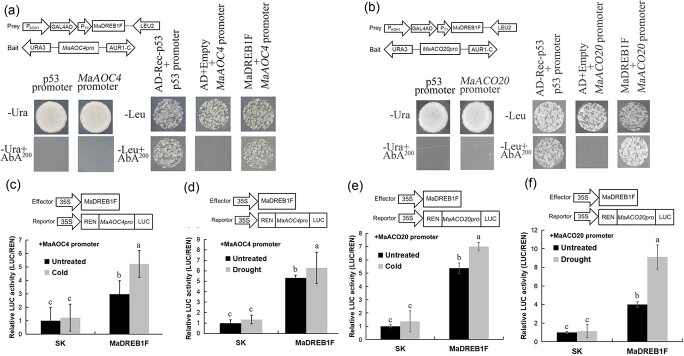
MaAOC4 [GSMUA_Achr4G10390_001, belonging to response to oxygen-containing compound (GO_1901700)] and MaACO20 [GSMUA_AchrUn_randomG20420_001, belonging to oxidation–reduction process (GO_0055114)] encode an allene oxide cyclase and a 1-aminocyclopropane-1-carboxylate oxidase, respectively, which are key enzymes for JA and ethylene biosynthesis. Because the promoter regions of these two genes contain the DRE/CRT cis-element (G/ACCGAC) (Supplementary Data Figs S5 and S6), the interactions of these promoters with MaDREB1F were detected using the Y1H assay. The results showed that yeast cells containing MaDREB1F-MaAOC4Pro and MaDREB1F-MaACO20Pro survived on the selective medium, suggesting interactions between MaDREB1F and the MaAOC4 promoter and between MaDREB1F and the MaACO20 promoter (Fig. 8a and b). To assess the effects of MaDREB1F on MaAOC4/MaACO20 promoters, dual-luciferase assays were performed. Tobacco leaves that harbored MaDREB1F and luciferase (LUC) driven by MaAOC4Pro or MaACO20Pro showed significantly higher LUC activity than those that harbored SK vector and LUC driven by MaAOC4Pro or MaACO20Pro under normal, cold, or drought conditions (Fig. 8c–f). This indicates that MaDREB1F has an effect on activating MaAOC4 and MaACO20 promoters after cold and drought treatments.
Figure 8.
Direct activation of MaAOC4 and MaACO20 by MaDREB1F. (a, b) Physical interactions between MaDREB1F and MaAOC4/MaACO20 promoters using a Y1H assay. Autoactivation of the promoters was examined on SD/−Ura + AbA medium, while the interactions between MaDREB1F and MaAOC4/MaACO20 promoters were tested on SD/−Leu + AbA medium. (c–f) Analysis of relative LUC activity showed that MaDREB1F activates MaAOC4/MaACO20 promoters under normal, cold, and drought conditions. Duncan’s range test was used for significance examination (n = 3; P < .05).
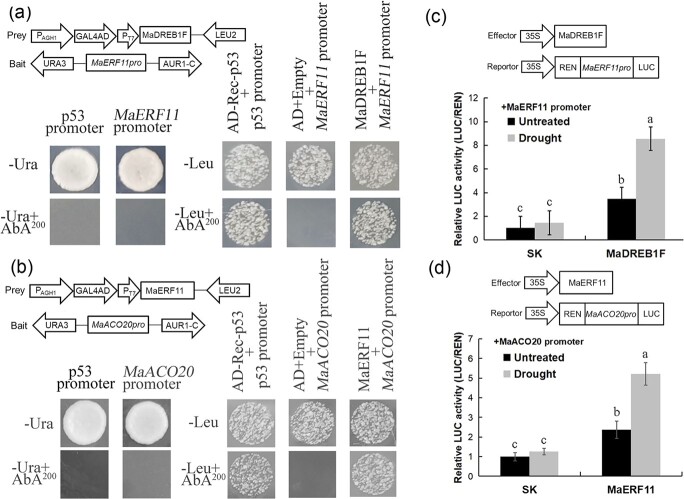
Interestingly, an ethylene-responsive factor (MaERF11) exists in the gene set commonly regulated by MaDREB1F and the promoter region of MaERF11 harbors the DRE/CRT cis-element. We thus investigated the interaction between MaERF11 promoter and MaDREB1F using a Y1H assay and found that yeast cells containing MaDREB1F-MaERF11Pro survived on the selective medium, suggesting interaction between MaDREB1F and the MaERF11 promoter (Fig. 9a). Moreover, the Y1H assay also indicated interaction between MaERF11 and the MaACO20 promoter (Fig. 9b). Further dual-luciferase assays showed that MaDREB1F had an effect on activating the MaERF11 promoter and that MaERF11 also activated the MaACO20 promoter after drought treatment (Fig. 9c and d).
Figure 9.
Direct activation of MaERF11 by MaDREB1F and MaACO20 by MaERF11. (a, b) Physical interactions between MaDREB1F and MaERF11 promoter and between MaERF11 and MaACO20 promoter using a Y1H assay. Autoactivation of the promoters was examined on SD/−Ura + AbA medium, while interactions between MaDREB1F and the MaERF11 promoter and between MaERF11 and the MaACO20 promoter were tested on SD/−Leu + AbA medium. (c, d) Relative LUC activity analysis showing that MaDREB1F activates the MaERF11 promoter and MaERF11 activates the MaACO20 promoter under normal and drought conditions. Duncan’s range test was used for significance examination (n = 3; P < .05).
Discussion
Improving crop resistance to multiple stresses is an important breeding goal. It is important to use the core functional genes for improving overall resistance of crops in molecular breeding [36]. Extensive studies have revealed that DREB/CBFs are critical for cold acclimation as well as resistance to drought and salt stress in higher plants [6, 19]. Currently, the common mechanism underlying CBF/DREB1-mediated multiple stress response remains unclear.
In this study we observed the transcriptional induction of MaDREB1F after cold, drought, and salt treatment, and MaDREB1F overexpression significantly enhanced banana resistance to cold and drought stress, in agreement with previous reports showing the positive effect of CBFs in the cold and drought responses of various plant species [12–19]. MaDREB1F has the typical characteristics of TFs supported by its nuclear localization and transcriptional activity. Due to the crucial function of TFs in regulating gene transcripts [1], further integrated transcriptomic analyses identified 720 genes that are commonly regulated by MaDREB1F overexpression after cold and drought treatments. Of these genes, those involved in carbohydrate metabolism, ABA response, and oxidation-reduction associated processes were affected in common by MaDREB1F overexpression after cold and drought treatments. Moreover, more genes (73 genes) in these biological processes showed common induction, but fewer genes (18 genes) showed common repression after cold and drought treatments in MaDREB1F-overexpressing plants than in WT, implying the possibly integrative activation of these biological processes by MaDREB1F overexpression after cold and drought treatments. Further physiological analyses support this hypothesis, by which H2O2 content decreased, but activities of CAT, POD, and SOD as well as the content of soluble sugar increased in transgenic plants under normal, cold, drought, and recovery conditions. This indicates that MaDREB1F overexpression improves antioxidant system and carbohydrate metabolism, and is thus beneficial for protecting transgenic plants against overproduced ROS and osmotic imbalance caused by cold and drought stress [39].
MaDREB1F has an effect on activating or alleviating repression of the genes in the JA and ethylene biosynthesis pathways under cold and drought conditions. This is further supported by physiological analyses showing the high JA contents and the activities of ACS and ACO in transgenic lines after cold and drought treatment. JA and ethylene are widely involved in stress responses in plants. There is evidence showing that exogenous JA and ethylene increased plant resistance to cold or drought stress [36,40–44]. Endogenous JA and ethylene levels increased under cold or drought stress [36,42,45,46]. Accordingly, JA and ethylene biosynthetic genes showed induction upon cold or drought stress [43,47–50]. Thus, coincident with the possible positive roles of JA and ethylene in cold and drought resistance, MaDREB1F confers cold and drought stress resistance through commonly activating or alleviating repression of JA and ethylene biosynthetic genes.
AOC, as a key enzyme in JA biosynthesis, participates extensively in plant resistance to various stress [51,52]. We found that MaDREB1F can directly bind to and activate the promoter of MaAOC4 under cold and drought treatment, resulting in increased JA content in transgenic plants. The ICE-CBF transcriptional regulatory pathway plays a crucial role in plant response to cold stress. Upon cold stress, ICE1 and ICE2 are activated and directly bind to CANNTG cis-elements in the CBF promoters, leading to the induction of COR genes and increased cold resistance [36]. JA was reported to positively modulate the ICE-CBF pathway to improve cold resistance in Arabidopsis [53]. Moreover, the JA-induced ICE-CBF pathway also has a positive effect in resistance to cold stress of banana fruit [48]. Our results indicate that MaDREB1F can positively regulate the synthesis of JA through MaAOC4 under cold stress. Thus, there may be a positive feedback loop, thereby improving plant resistance to cold stress.
ACO is a rate-limiting enzyme for ethylene biosynthesis [54]. Accompanied by an increase in ethylene production under cold and drought conditions, expression of ACOs significantly increased in various plant species [47,55–59]. We found that MaDREB1F can directly bind to and activate the promoter of MaACO20 under cold and drought treatment. Additionally, MaDREB1F can also directly activate MaERF11 expression and MaERF11 can directly activate MaACO20 expression after drought treatment. This resulted in increased ACO activity and ethylene content in transgenic plants after cold and drought treatment. Although previous studies widely demonstrated the transcriptional response of the AP2/ERF superfamily (including CBFs) to ethylene upon abiotic stress [6,60], there is evidence showing that MaERF9 directly bound to and activated MaACO1 promoter activity in banana fruit ripening [61]. Together, these findings reveal a multilayered regulatory cascade that promotes MaACO20 transcription in the banana response to cold and drought stress.
Based on our present findings, we propose a possible working model for MaDREB1F conferring cold and drought stress resistance (Fig. 10). Upon cold and drought stress, MaDREB1F commonly affects carbohydrate metabolism- and antioxidant enzyme-associated genes, resulting in accumulation of soluble sugar and scavenging of ROS. Besides, MaDREB1F commonly activates or alleviates repression of MaPAL1, MaLOXs, MaACX, and MaAOC4 for JA synthesis and of MaERF11, MaSAMS, MaACOs, and MaACSs for ethylene synthesis under cold and drought conditions. MaDREB1F directly activates promoter activities of MaAOC4 and MaACO20 for JA and ethylene synthesis, respectively, under cold and drought stress. Moreover, MaDREB1F can also target the MaERF11 promoter to activate MaACO20 expression for ethylene synthesis under drought stress. Our findings reveal a common mechanism underlying CBFs/DREB1-mediated resistance to cold and drought stress in plants and identify several new targets (MaAOC4, MaACO20, and MaERF11) of CBFs/DREB1s, beneficial for designing breeding strategies to improve crop resistance to cold and drought stress.
Figure 10.
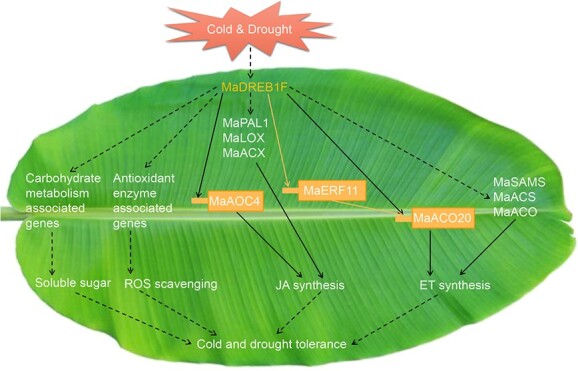
Proposed model for MaDREB1F conferring cold and drought stress resistance by commonly regulating hormone synthesis and protectant metabolite contents in banana. Solid black lines indicate a direct role, black dashed lines indicate an indirect role, and yellow lines indicate specific interactions for drought response.
Materials and methods
Plant growth and stress treatment
Plants of the banana cultivar ‘Brazil’ (Musa acuminata L. AAA group) were used in this study. Seedlings were grown in a greenhouse (28°C, 16 hours light/8 hours dark cycle, 70% relative humidity). The stress treatments were applied on banana plants at the five-leaf stage. For cold treatment, the plants were placed in an incubator at 8°C for 47 hours with 70% relative humidity. For osmotic treatment, the plants were treated with 200 mM mannitol for 17 days at 28°C with 70% relative humidity. For NaCl treatment, plants were treated with 300 mM NaCl for 24 days at 28°C with 70% relative humidity.
qRT–PCR
Banana leaves with or without cold, drought, and salt treatments were collected to examine MaDREB1F expression using qRT–PCR on a Stratagene Mx3000P instrument. The optimal primer and template concentrations were determined using a series of dilutions before the quantification experiments. Agarose gel electrophoresis and the melting curve were used to measure the specificity of the primers (Supplementary Data Table S1). Primer efficiency was examined in the range of 0.9–1.1. The expression of target genes was normalized using the comparative 2–ΔΔCt method [62]. Expression of target genes was normalized with internal controls MaUBQ2 (HQ853254) and MaRPS2 (HQ853246).
Cloning and subcellular localization of MaDREB1F
The coding sequence of MaDREB1F was amplified from the M. acuminata L. AAA group using RT–PCR (Supplementary Data TableS1). The PCR product was inserted into the pMD19-T vector and sequenced. The full-length cDNA sequence, which removed the stop codon, was inserted into the pCAMBIA1302 vector to produce an MaDREB1F:GFP fusion (Supplementary Data Table S1). Agrobacterium tumefaciens strain (GV3101, pSoup+p19) harboring the recombinant vector was independently infiltrated into the leaves of Nicotiana benthamiana by injection. After 3 days of culture, fluorescence was measured using a confocal laser-scanning microscope.
Transcription activation activity of the MaDREB1F protein
The complete coding sequence and N- and C-terminals of MaDREB1F were cloned into the pGBKT7 vector (Supplementary Data Table S1). The constructs were transferred into yeast strain AH109, which was subsequently plated on plates with SD/Trp− or SD/His−. Yeast cells were grown on medium containing X-gal to assess transcription activation activity.
Generation of transgenic plants
Green fluorescent protein (GFP) as a gene expression reporter and vital marker has been widely used for studying plant development, gene expression regulation, and protein folding, and is not cytotoxic to plants [63–65]. The pCAMBIA1302-MaDREB1F-GFP construct was transferred into Agrobacterium strain GV3101. Overexpression of MaDREB1F in banana plants (Musa corniculata L. AAA group) was conducted using the Agrobacterium-mediated method [27]. Hygromycin-resistant transgenic plants were examined by amplifying the hpt-II gene from pCAMBIA1302. Then, the T2 generation was produced. Southern blot analysis was employed to further verify the integration of MaDREB1F in Line 1 (L1) and L2 genomes.
Southern blotting assay
Total genomic DNA was extracted and digested with the EcoRI enzyme, followed by transfer to nylon membranes. The cDNA probe of hpt-II in pCAMBIA1302 was amplified with primer set 5′-GCTCCTACAAATGCCATCATTGC-3′ and 5′-GATAGTGGGATTGTGCGTCATCCC-3′ and labeled with a random primer labeling system. Hybridizations were conducted following the manufacturer’s instructions (Roche 11745832910, USA).
Analyses of physiological indices
Ion leakage was tested following the method reported by Jiang and Zhang [66]. The content of malondialdehyde (MDA) was examined based on the thiobarbituric acid colorimetric method [67]. Proline content was examined following Bates et al. [68]. The content of H2O2 was measured with a detection kit (W-100, G-CLONE, Beijing, China). The activities of perox idase (POD), catalase (CAT) , and superoxide dismutase (SOD) were spectrophotometrically tested with detection kits (A007-1-1, A084-3-1, and A001-1-1; Jiancheng, Nanjing, China).. The soluble sugar content was examined using a detection kit (A145-1-1, Jiancheng, Nanjing, China). The content of jasmonate and the activities of 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) were measured with detection kits (ml062281, ml062340-2, ml062341-2, Meilian, Shanghai, China). Banana seedlings were enclosed in an airtight container for 8 hours to collect ethylene. Ethylene production was examined by gas chromatography (GC-2010 Plus) following the manufacturer’s instructions.
Transcriptomic analyses
Total RNA was extracted from the leaves of transgenic line L1 and wild type (WT) under normal, cold (5 days of cold treatment), or drought (10 days of drought treatment) conditions with an RNA extraction kit (EX1882, G-CLONE, Beijing, China). cDNA was converted from 3 μg RNA for each sample using the RevertAid First-Strand cDNA Synthesis Kit (Promega, Madison, WI, USA). Eighteen cDNA libraries were constructed and sequenced using Illumina GAII. After removing adapter sequences with the FASTX toolkit, FastQC was used to evaluate clean read quality. Assembly of the transcriptome was conducted using cufflinks with alignment files. Gene expression levels were represented as fragments per kilobase of transcript per million mapped reads (FPKM). DEGseq was employed to identify differentially expressed genes (DEGs) based on the threshold of 2-fold expression change and P-value ≤.05 of three replicate reads per gene.
Yeast one-hybrid assay
In order to characterize the interaction between MaDREB1F and target promoters, each promoter sequence was constructed into pAbAi as a bait vector. The 2000-bp DNA sequences upstream of the transcription start site of the target genes were obtained from the banana A genome website (http://banana-genome.cirad.fr/). The 2000-bp fragment of the target promoter was used to construct the bait vector. The coding sequence of MaDREB1F was constructed into the pGADT7 as a prey vector (Supplementary Data Table S1). The suitable aureobasidin A (AbA) concentration for each bait vector is 200 mg/l. The prey and bait constructs were co-transformed into Y1H (yeast one-hybrid) yeast strains, followed by culture on SD/−Leu + AbA 200 selective medium at 30°C for 3 days.
Dual-luciferase activity analysis
A dual-luciferase assay was conducted with the method by Niu et al. [69]. The promoter and coding sequence of the gene were cloned into pGreenII 0800-LUC and pGreenII 62-SK vectors, respectively, and were then transferred into A. tumefaciens GV3101 (Supplementary Data Table S1). Four-week-old tobacco leaves were infiltrated with the transformed A. tumefaciens using injection. Firefly luciferase (LUC) and Renilla luciferase (REN) activities were detected by a dual-luciferase reporter assay system. Three biological replicates were carried out for each assay.
Acknowledgements
We thank the National Key Research and Development Program of China (2019YFD1000204), the 2020 Research Program of Sanya Yazhou Bay Science and Technology City (SKJC-2020-02-002), the National Natural Science Foundation of China (32260736), and the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630052020006) for funding resources.
Author contributions
W.H., B.X., and Z.J. conceived the study. Y.X. and W.H. wrote the manuscript. Other authors carried out experiments and analysis.
Data availability
The data that support the findings are available in the paper and the supplementary materials published online.
Conflict of interest
The authors declare no competing interests.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Yi Xu, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China.
Wei Hu, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Shun Song, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China.
Xiaoxue Ye, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Zehong Ding, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Juhua Liu, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Zhuo Wang, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Jingyang Li, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Xiaowan Hou, Key Laboratory of Hainan Province for Postharvest Physiology and Technology of Tropical Horticultural Products, South Subtropical Crops Research Institute, Chinese Academy of Tropical Agricultural Sciences, Guangdong, China.
Biyu Xu, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
Zhiqiang Jin, Haikou Experimental Station, Key Laboratory of Genetic Improvement of Bananas, Sanya Research Institute, State Key Laboratory of Biological Breeding for Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Key Laboratory for Protection and Utilization of Tropical Bioresources, Hainan Institute for Tropical Agricultural Resources, Chinese Academy of Tropical Agricultural Sciences, Hainan, China; Hainan Yazhou Bay Seed Laboratory, Hainan, China; Hainan Key Laboratory for Biosafety Monitoring and Molecular Breeding in Off-Season Reproduction Regions, Key Laboratory of Biology and Genetic Resources of Tropical Crops, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Hainan, China.
References
- 1. Hu W, Huang C, Deng Xet al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013;36:1449–64. [DOI] [PubMed] [Google Scholar]
- 2. Fan W, Hai M, Guo Yet al. The ERF transcription factor family in cassava: genome-wide characterization and expression analyses against drought stress. Sci Rep. 2016;6:37379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakhwani D, Pandey A, Dhar YVet al. Genome-wide analysis of the AP2/ERF family in Musa species reveals divergence and neofunctionalisation during evolution. Sci Rep. 2016;6:18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muller M, Munne-Bosch S. Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol. 2015;169:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang X, Song X, Chen Ret al. Genome-wide analysis of the DREB subfamily in Saccharum spontaneum reveals their functional divergence during cold and drought stresses. Front Genet. 2019;10:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi Y, Ding Y, Yang S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018;23:623–37. [DOI] [PubMed] [Google Scholar]
- 7. Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novillo F, Alonso JM, Ecker JRet al. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:3985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao C, Zhang Z, Xie Set al. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016;171:2744–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agarwal PK, Agarwal P, Reddy MKet al. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006;25:1263–74. [DOI] [PubMed] [Google Scholar]
- 11. Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819:86–96. [DOI] [PubMed] [Google Scholar]
- 12. Qin F, Sakuma Y, Li Jet al. Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol. 2004;45:1042–52. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Fowler SG, Cheng Het al. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004;39:905–19. [DOI] [PubMed] [Google Scholar]
- 14. Savitch LVAA, Allard G, Seki Met al. The effect of overexpression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant Cell Physiol. 2005;46:1525–39. [DOI] [PubMed] [Google Scholar]
- 15. Ito YJIR, Katsura K, Maruyama Ket al. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006;47:141–53. [DOI] [PubMed] [Google Scholar]
- 16. Wang Q, Guan Y, Wu Yet al. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol. 2008;67:589–602. [DOI] [PubMed] [Google Scholar]
- 17. Dubouzet JG, Sakuma Y, Ito Yet al. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003;33:751–63. [DOI] [PubMed] [Google Scholar]
- 18. Haake V, Cook D, Riechmann JLet al. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 2002;130:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niu X, Luo T, Zhao Het al. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses. Gene. 2020;740:144514. [DOI] [PubMed] [Google Scholar]
- 20. Wang D, Jin YN, Ding XHet al. Gene regulation and signal transduction in the ICE–CBF–COR signaling pathway during cold stress in plants. Biochemistry (Mosc). 2017;82:1103–17. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Dang P, Liu Let al. Cold acclimation by the CBF–COR pathway in a changing climate: lessons from Arabidopsis thaliana. Plant Cell Rep. 2019;38:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobayashi F, Takumi S, Nakata Met al. Comparative study of the expression profiles of the Cor/Lea gene family in two wheat cultivars with contrasting levels of freezing tolerance. Physiol Plant. 2004;120:585–94. [DOI] [PubMed] [Google Scholar]
- 23. Tang K, Zhao L, Ren Yet al. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J Integr Plant Biol. 2020;62:258–63. [DOI] [PubMed] [Google Scholar]
- 24. Li H, Ye K, Shi Yet al. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant. 2017;10:545–59. [DOI] [PubMed] [Google Scholar]
- 25. Shi Y, Huang J, Sun Tet al. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J Integr Plant Biol. 2017;59:118–33. [DOI] [PubMed] [Google Scholar]
- 26. Jia Y, Ding Y, Shi Yet al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–53. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Gao P, Sun Xet al. Efficient regeneration and genetic transformation platform applicable to five Musa varieties. Electron J Biotechnol. 2017;25:33–8. [Google Scholar]
- 28. Xu Y, Hu W, Liu Jet al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol Biochem. 2020;147:66–76. [DOI] [PubMed] [Google Scholar]
- 29. Sreedharan S, Shekhawat UK, Ganapathi TR. MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol Biol. 2012;80:503–17. [DOI] [PubMed] [Google Scholar]
- 30. Sreedharan S, Shekhawat UK, Ganapathi TR. Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotechnol J. 2013;11:942–52. [DOI] [PubMed] [Google Scholar]
- 31. Sreedharan S, Shekhawat UK, Ganapathi TR. Constitutive and stress-inducible overexpression of a native aquaporin gene (MusaPIP2;6) in transgenic banana plants signals its pivotal role in salt tolerance. Plant Mol Biol. 2015;88:41–52. [DOI] [PubMed] [Google Scholar]
- 32. Shekhawat UK, Ganapathi TR. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. 2013;8:e75506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tak H, Negi S, Ganapathi TR. Banana NAC transcription factor MusaNAC042 is positively associated with drought and salinity tolerance. Protoplasma. 2017;254:803–16. [DOI] [PubMed] [Google Scholar]
- 34. Negi S, Tak H, Ganapathi TR. A banana NAC transcription factor (MusaSNAC1) impart drought tolerance by modulating stomatal closure and H2O2 content. Plant Mol Biol. 2018;96:457–71. [DOI] [PubMed] [Google Scholar]
- 35. Xu Y, Li J, Song Set al. A novel aquaporin gene MaSIP2-1 confers tolerance to drought and cold stresses in transgenic banana. Mol Breed. 2020;40:1380–3743. [Google Scholar]
- 36. Hu Y, Jiang Y, Han Xet al. Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot. 2017;68:1361–9. [DOI] [PubMed] [Google Scholar]
- 37. Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic Res. 1998;28:659–71. [DOI] [PubMed] [Google Scholar]
- 38. Xu Y, Hu W, Liu Jet al. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biol. 2014;14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang C, Deng P, Chen Let al. A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS One. 2013;8:e65120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu T, Zou L, Li Yet al. Mitochondrial alternative oxidase-dependent autophagy involved in ethylene-mediated drought tolerance in Solanum lycopersicum. Plant Biotechnol J. 2018;16:2063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ullah A, Manghwar H, Shaban Met al. Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ Sci Pollut Res Int. 2018;25:33103–18. [DOI] [PubMed] [Google Scholar]
- 42. Sun X, Zhao T, Gan Set al. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ETHYLENE RESPONSE FACTOR 057. Sci Rep. 2016;6:24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu W, Wang H, Chen Yet al. Cold stress improves the production of artemisinin depending on the increase in endogenous jasmonate. Biotechnol Appl Biochem. 2017;64:305–14. [DOI] [PubMed] [Google Scholar]
- 44. Ruan J, Zhou Y, Zhou Met al. Jasmonic acid signaling pathway in plants. Int J Mol Sci. 2019;20:2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aharoni N. Relationship between leaf water status and endogenous ethylene in detached leaves. Plant Physiol. 1978;61:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu J, Jiang M, Guo C. Crop pollen development under drought: from the phenotype to the mechanism. Int J Mol Sci. 2019;20:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arraes FB, Beneventi MA, Lisei de Sa MEet al. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015;15:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao ML, Wang JN, Shan Wet al. Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ. 2013;36:30–51. [DOI] [PubMed] [Google Scholar]
- 49. Huang B, Li X, Liu Pet al. Transcriptomic analysis of Eruca vesicaria subs. sativa lines with contrasting tolerance to polyethylene glycol-simulated drought stress. BMC Plant Biol. 2019;19:419–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao R, Xie H, Lv Set al. LeMAPK4 participated in cold-induced ethylene production in tomato fruit. J Sci Food Agric. 2013;93:1003–9. [DOI] [PubMed] [Google Scholar]
- 51. Sun T, Cen G, You Cet al. ScAOC1, an allene oxide cyclase gene, confers defense response to biotic and abiotic stresses in sugarcane. Plant Cell Rep. 2020;39:1785–801. [DOI] [PubMed] [Google Scholar]
- 52. Zhao Y, Dong W, Zhang Net al. A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014;164:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu Y, Jiang L, Wang Fet al. Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Houben M, Van de Poel B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): the enzyme that makes the plant hormone ethylene. Front Plant Sci. 2019;10:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lelièvre JM, Tichit L, Dao Pet al. Effects of chilling on the expression of ethylene biosynthetic genes in Passe-Crassane pear (Pyrus communis L.) fruits. Plant Mol Biol. 1997;33:847–55. [DOI] [PubMed] [Google Scholar]
- 56. Nieuwenhuizen NJ, Chen X, Pellan Met al. Regulation of wound ethylene biosynthesis by NAC transcription factors in kiwifruit. BMC Plant Biol. 2021;21:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luo W, Gong Y, Tang Yet al. Glutathione and ethylene biosynthesis reveal that the glume and lemma have better tolerance to water deficit in wheat. Plant Physiol Biochem. 2021;160:120–9. [DOI] [PubMed] [Google Scholar]
- 58. Dalal M, Sahu S, Tiwari Set al. Transcriptome analysis reveals interplay between hormones, ROS metabolism and cell wall biosynthesis for drought-induced root growth in wheat. Plant Physiol Biochem. 2018;130:482–92. [DOI] [PubMed] [Google Scholar]
- 59. Wei H, Xue Y, Chen Pet al. Genome-wide identification and functional investigation of 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) genes in cotton. Plants (Basel). 2021;10:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xie Z, Nolan TM, Jiang Het al. AP2/ERF transcription factor regulatory networks in hormone and abiotic stress responses in Arabidopsis. Front Plant Sci. 2019;10:228–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiao Y, Chen JY, Kuang JFet al. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot. 2013;64:2499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 63. Stewart CN Jr. The utility of green fluorescent protein in transgenic plants. Plant Cell Rep. 2001;20:376–82. [DOI] [PubMed] [Google Scholar]
- 64. Waldo GS, Standish BM, Berendzen Jet al. Rapid protein-folding assay using green fluorescent protein. Nat Biotechnol. 1999;17:691–5. [DOI] [PubMed] [Google Scholar]
- 65. Bottin A, Larche L, Villalba Fet al. Green fluorescent protein (GFP) as gene expression reporter and vital marker for studying development and microbe-plant interaction in the tobacco pathogen Phytophthora parasitica var. nicotianae. FEMS Microbiol Lett. 1999;176:51–6. [DOI] [PubMed] [Google Scholar]
- 66. Jiang MHKB, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42:1265–73. [DOI] [PubMed] [Google Scholar]
- 67. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98. [DOI] [PubMed] [Google Scholar]
- 68. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7. [Google Scholar]
- 69. Niu Q, Li J, Cai Det al. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J Exp Bot. 2016;67:239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings are available in the paper and the supplementary materials published online.