Abstract
A cross-sectional study was conducted to determine the seroprevalence of the Peste des Petits Ruminant (PPR) virus (PPRV) in sheep populations and to determine the potential epidemiological risk factors associated with this infection. Between October 2014 and March 2017, 2420 sheep serum samples were collected from ten selected PPR outbreak-prone districts in Bangladesh. The collected sera were analysed by competitive enzyme-linked immunosorbent assay (cELISA) test to detect antibodies against PPR. A previously designed disease report form was used to gather data on important epidemiological risk factors, and a risk analysis was performed to ascertain their association with PPRV infection. By cELISA, 44.3 % (95 % confidence interval:42.4–46.4 %) of sheep sera were positive for PPRV antibodies against PPR. In univariate analysis, the Bagerhat district had significantly higher seropositivity (54.1 %, 156/288) than other districts. Moreover, significantly higher (p < 0.05) seropositivity was found in the Jamuna River Basin (49.1 %, 217/442) compared to other ecological zones, in crossbreeds (60 %; 600/1000) related to native sheep, in males (69.8 %, 289/414) associated with females, in imported sheep (74.3 %, 223/300) compared to other sources, and in winter (57.2 %, 527/920) than in other seasons. In the multivariate logistic regression model, six possible risk factors were identified: study location, ecological zone, breed, sex, source, and season. The high seroprevalence of PPRV is significantly associated with several risk factors, suggesting that PPR is epizootic throughout the country.
Keywords: Sheep, PPR, Epidemiology, Risk factors, cELISA, Seroprevalence, Bangladesh
1. Introduction
Sheep are an important livestock species worldwide. Asia accounts for approximately 43.6 % of sheep worldwide (FAO, 2013). Sheep are the third most common ruminant species in Bangladesh, with a population of approximately 3.679 million and a density of 24.78 per square kilometre (DLS, 2021). In Bangladesh, the vast majority of sheep are indigenous and very few are crossbred (Bhuiyan, 2006). Sheep are typically used for meat production in Bangladesh. Sheep are found all around the country, although they are more concentrated in certain areas. Although these animals have been neglected, sheep farming can help with income production, poverty reduction, job creation, and the filling of protein gaps (Hassan and Talukder, 2012). Consequently, the government of Bangladesh has taken steps to strengthen sheep production systems and productivity. However, several factors, such as heredity, diet, and disease, impede the output and productivity of sheep. Peste des Petits Ruminants (PPR) is one of Bangladesh’s most frequent infectious diseases affecting small ruminants, including sheep (Rahman et al., 2021).
PPR is a contagious and infectious viral disease that affects both domestic and wild ruminants (Dou et al., 2020). Cattle and pigs are also susceptible to PPR; however, they do not develop any obvious clinical characteristics (Banyard et al., 2010). The highly contagious nature and rapid spread capacity of PPR make it one of the most important diseases. Morbidity and mortality rates associated with PPR can be as high as 100 % (Dou et al., 2020). As a result, it has a significant socioeconomic impact on the livestock business in nations whose economies are based on small ruminants, especially in countries with endemic poverty, including Bangladesh.
The etiological agent of PPR belongs to the Morbillivirus genus under the Paramyxoviridae family and Mononegavirales order and is associated with rinderpest, canine distemper, and measles (Murphy et al., 1999). As the virus primarily affects the digestive and respiratory tracts, it can be found in all body secretions, making direct contact between PPR-infected and vulnerable animals the most likely way for the PPR virus (PPRV) to spread. PPR causes fever, diarrhoea, erosive stomatitis, pneumonia, mouth sores, conjunctivitis, ocular and nasal discharges, and death in sheep and goats (Almeshay et al., 2017). The viral strain, local environmental factors, and immune status of the infected host influence disease severity.
The PPR was initially documented in West Africa and subsequently expanded to other African and Asian regions (Baazizi et al., 2017). Studies indicate that PPR has become epizootic in Bangladesh, Pakistan, India, Nepal, China, Afghanistan, and others (Shaila et al., 1989, Abdollahpour et al., 2006). In Bangladesh, PPR was first reported in 1993 and first described in 1995. In a short period, the disease has become epizootic throughout the country due to high morbidity and mortality (Rahman et al., 2018).
Although PPR has been prevalent in Bangladesh for over two decades, seroepidemiological studies on this disease are very limited in sheep. Additionally, PPR outbreaks in sheep have been reported in different areas of Bangladesh. Furthermore, the eradication of PPR is important to keep the country’s economy stable; however, the recurrent endemic cycles of the disease throughout the country provide a significant hurdle to its eradication. This necessitates a more in-depth examination of their epidemiological properties. Therefore, this study was carried out to determine the seroprevalence of PPR in sheep in several selected areas of Bangladesh and to analyse the epidemiological risk factors associated with PPRV infection.
2. Materials and methods
2.1. Ethical statement
Experienced veterinarians obtained blood samples from sheep while adhering to ethical guidelines and considering animal welfare. The farmers verbally consented to the collection of samples before they were taken.
2.2. Study area sites
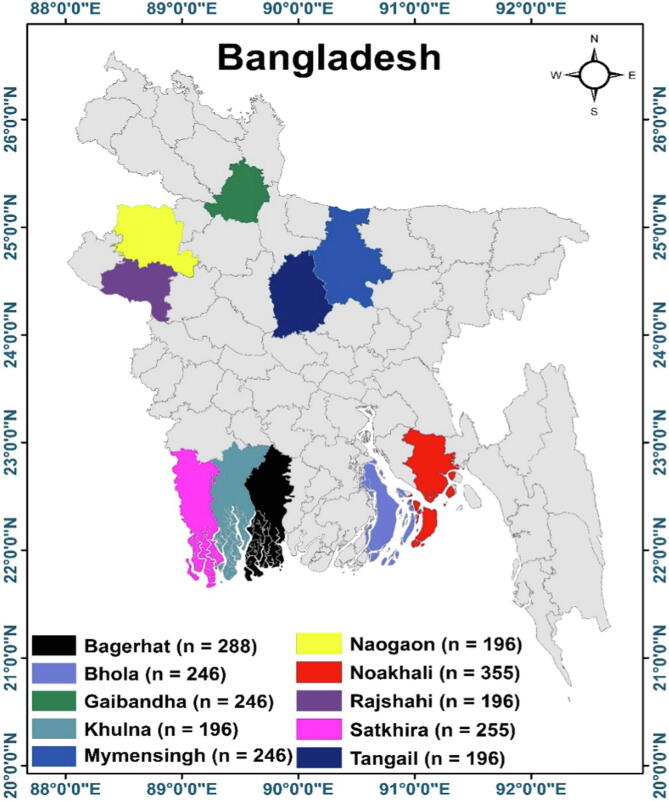
This study was conducted between October 2014 and March 2017. Four ecological zones i.e. coastal zone [Noakhali (22.8246° N, 91.1017° E), Bhola (22.6855° N, 90.6439° E), Bagerhat (22.6555° N, 89.7662° E), and Satkhira (22.3155° N, 89.1115° E), and Khulna (22.8456° N, 89.5403° E) districts], Barind [Rajshahi (24.3745° N, 88.6042° E) and Naogaon (24.7936° N, 88.9318° E) districts], Jamuna River Basin [Tangail (24.2513° N, 89.9167° E) and Gaibandha (25.3290° N, 89.5415° E) districts], and plane land [Mymensingh (24.7471° N, 90.4203° E) district] were selected in the present cross-sectional study (Fig. 1). The above-mentioned study areas were selected based on geographical location, ecology, the higher number of sheep population, husbandry practices, and increased animal movement through the areas.
Fig. 1.
Study area map of different districts of Bangladesh. Image was extracted from DIVA-GIS (https://www.diva-gis.org/) using the Geographical Information System (GIS) to develop the map with ArcMap software (version 10.7), n = the numbers represent the number of sheep sampled in each region.
2.3. Sample size calculation
The sample size was computed using a previous formula (Daniel, 1999), which was then supported by Naing et al. (2006), who recommended the use of a 50 % prevalence or ranges of 10 %-90 % prevalence if the precise prevalence is unclear. Therefore, the sample size was calculated in the following study with an expected prevalence ranging from 20 % to 50 %, an estimated desired precision from 5 % to 10 %, and a confidence interval (CI) of 95 % (Daniel, 1999):
Here, n = sample size; Z = standard score at the 95 % confidence level (Z = 1.96), p = expected prevalence (p = 0.2–0.5), q = 1-p, and d = precision limit of sampling error (d = 0.05 0.1).
Using this formula, the calculated sample sizes ranged from 196 to 355. Subsequently, 196–355 sheep blood samples were collected from each district, and 2420 samples were obtained from ten different districts of Bangladesh.
2.4. Epidemiological data collection
This study used a disease report form designed by the Regional Diagnostic Laboratory for PPR, Bangladesh Livestock Research Institute, Savar, Dhaka, which included general information such as the farmer's name and address, farm location, herd size, breed, sex, age, morbidity, mortality of affected sheep, and climatic conditions during outbreaks. The report forms were filled out during visits to the study locations. Nine variables were used as risk factors for PPRV infection in sheep: location, ecological zone, breed, age, sex, source of sheep, season, rearing system, and feeding system of the farms. The farmers were able to understand the substance of the questionnaire because it was translated into their native language. During the face-to-face interview data collection phase, two expert veterinarians, two expert microbiologists, and a trained enumerator were involved.
2.5. Sampling
Whole blood samples (5 mL) were aseptically taken from each sheep’s jugular vein into a sterile syringe, which was then kept at the slanting position and left at room temperature to clot. Following completion of the clotting process within the syringes, the collected blood samples were placed in an icebox until they could be transported in a cool chain to the Virology Laboratory at the Department of Microbiology and Hygiene at Bangladesh Agricultural University in Mymensingh. After transportation to the laboratory, the clotted blood was carefully removed using a sterile needle, and the sera were transferred to clean, sterile 1.5 mL Eppendorf tubes. After that, serum samples were purified by centrifugation (at 1,150 × g for 5–7 min), transferred to clean and sterile Eppendorf tubes, and stored at −20 °C for further use.
2.6. Serological analysis
Serological analysis in the present study was conducted at the Regional Diagnostic Laboratory for PPR, Bangladesh Livestock Research Institute (BLRI), Savar, Bangladesh. A competitive enzyme-linked immunosorbent assay (cELISA) kit (The Pirbright Institute, Surrey, UK) was used for PPRV detection during the serological study. cELISA was run according to the protocol laid out in the client manual provided with the kits (Anderson et al., 1991). The optical density (OD) values of the final plates were measured at 492 nm using an ELISA reader, and the obtained data were calculated using the following formula:
Here, PI = Percentage inhibition; OD = Optical density; Cm = Monoclonal antibody.
The results were interpreted (following the manufacturer’s instructions) by the PI value of each sample, as follows:
-
•
Samples with a ≥ 50 PI value were deemed positive
-
•
Samples with a < 50 PI value were considered negative.
2.7. Statistical analysis
Field and laboratory data were incorporated into a Microsoft Excel 2013 (Los Angeles, CA, USA) spreadsheet. The data were then thoroughly checked to evaluate errors and irregularities before sorting, coding, and testing for integrity. Finally, the data were exported to STATA (version 13; StataCorp LLC, Texas 77845, USA) for statistical analysis.
2.7.1. Descriptive analysis
Frequency and percentages were enumerated to determine demographic characteristics. The PPR seroprevalence of sheep blood samples obtained using cELISA was estimated for different epidemiological risk factors. In addition, PPR seroprevalence values are presented as percentages and 95 % CIs.
2.7.2. Risk factor analysis
2.7.2.1. Univariable analysis
For the univariable risk factor analysis, nine distinct epidemiological factors were first considered as follows: (1) study areas or ten important districts, (2) four important ecological zones, (3) whether sheep were native or crossed (crossed between native and Perendale breed, native and Dorper breed, and Chottanagpuri crossbred), (4) variation in the ages of sheep; (5) whether sheep were male or female; (6) whether sheep were born in the farm/house or imported or brought from markets; (7) seasonal variation; (8) whether sheep were reared either by farming or non-farming systems; and (9) whether sheep were fed either by grazing or non-grazing systems.
The relationship between the seroprevalence of PPRV and epidemiological risk factors was investigated using a univariate chi-square test (two-tailed). Statistical significance was set at a p-value ≤ 0.05, and a 95 % CI was determined for each variable.
2.7.2.2. Multivariable analysis
The initial consideration for inclusion in the multivariate analysis was given to factors with a tendency toward significance (p ≤ 0.1, chi-square test). The model was fitted using a backward stepwise logistic regression analysis. After running a full model, variables that had p ≤ 0.05 in a likelihood ratio test were kept. Interactions between biologically plausible epidemiological risk factors were also evaluated, and if significant (p ≤ 0.05), were retained in the final phase. In addition, Fisher's exact test was used to check for collinearity between variables. Two factors were termed collinear only if they had p < 0.05. Subsequently, the final model's sensitivity was evaluated for goodness-of-fit using the Hosmer-Lemeshow test (Dohoo et al., 2003), and the receiver operating characteristic (ROC) curve was employed to determine the post-estimate of predictive ability (Dohoo et al., 2003). For each adjusted predictor variable, the results were presented as odds ratios (OR), p-value, and 95 % CI.
3. Results
3.1. Sample description
The highest sheep blood samples were collected from the Noakhali district (14.7 %), coastal zone (55.4 %), native sheep (58.7 %), female sheep (82.9 %), 13–24 months of age sheep (40.3 %), sheep born in farm/house (54.5 %), winter season (38 %), sheep reared in the farming system (59.7 %), and sheep feed in grazing system (58.7 %) (Table 1).
Table 1.
Univariate association between cELISA results with different factors (N = 2420).
| Variable | Category (N*) | Positive, n (%) | 95 % CI | p-value |
|---|---|---|---|---|
| Districts | Noakhali (355) | 146 (41.1) | 36.1–46.3 | 0.00 |
| Bhola (246) | 131 (53.2) | 47.0–59.4 | ||
| Bagerhat (288) | 156 (54.1) | 48.4–59.8 | ||
| Khulna (196) | 91 (46.4) | 39.6–53.4 | ||
| Satkhira (255) | 112 (43.9) | 37.9–50.1 | ||
| Rajshahi (196) | 55 (28.0) | 22.2–34.7 | ||
| Naogaon (196) | 49 (25.0) | 19.5–31.5 | ||
| Gaibandha (246) | 115 (46.7) | 40.6–52.9 | ||
| Tangail (196) | 102 (52.0) | 45.1–58.9 | ||
| Mymensingh (246) | 117 (47.5) | 41.4–53.8 | ||
| Ecological zone | Coastal (1340) | 636 (47.4) | 44.8–50.1 | 0.00 |
| Barind (392) | 104 (26.5) | 22.4–31.1 | ||
| Jamuna river basin (442) | 217 (49.1) | 44.5–53.7 | ||
| Plane land (246) | 117 (47.5) | 41.4–3.8 | ||
| Breed | Crossbred (1000) | 600 (60) | 56.9–62.9 | 0.00 |
| Native (1420) | 474 (33.3) | 30.9–35.9 | ||
| Sex | Male (414) | 289 (69.8) | 65.2–74.0 | 0.00 |
| Female (2006) | 785 (39.1) | 37.0–41.3 | ||
| Age | 0–6 months (386) | 170 (44.0) | 39.2–49.0 | 0.93 |
| 7–12 months (404) | 175 (43.3) | 38.6–48.2 | ||
| 13–24 months (976) | 440 (45.0) | 41.9–48.2 | ||
| Above 24 months (654) | 289 (44.1) | 40.4–48.0 | ||
| Source | Animal market (800) | 450 (56.2) | 52.8–59.7 | 0.00 |
| Imported (300) | 223 (74.3) | 69.1–78.9 | ||
| Born in farm/house (1320) | 401 (30.3) | 27.9–32.9 | ||
| Season | Winter (Nov-Feb) (920) | 527 (57.2) | 54.1–60.4 | 0.00 |
| Rainy (Jul-Oct) (750) | 287 (38.2) | 34.9–41.8 | ||
| Summer (Mar-Jun) (750) | 260 (34.6) | 31.3–38.1 | ||
| Rearing system | Non farming (975) | 440 (45.1) | 42.0–48.3 | 0.54 |
| Farming (1445) | 634 (43.8) | 41.3–46.5 | ||
| Feeding system | Stall grazing (1000) | 429 (42.9) | 39.9–45.9 | 0.21 |
| Grazing (1420) | 645 (45.4) | 42.9–48.0 |
Here, a p ≤ 0.05 was deemed statistically significant, N* = Number of samples collected from each category, CI = Confidence interval, Nov = November, Feb = February, Jul = July, Oct = October, Mar = March, Jun = June.
3.2. Seroprevalence
The overall seroprevalence of PPR in sheep samples based on cELISA was 44.3 % (1074/2420; 95 % CI:42.4–46.4 %), among them, the highest seroprevalence was recorded in the Bagerhat district (54.1 %; 156/288; 95 % CI:48.4–59.8 %); in the coastal ecological zone (47.4 %; 636/1340; 95 % CI:44.8–50.1 %); in cross breed sheep (60 %; 600/1000; 95 % CI:56.9–62.9 %); in male sheep (69.8 %; 289/414; 95 % CI:65.2–74.0 %); in sheep of 13–24 months of ages (45 %, 440/976; 95 % CI:41.9–48.2 %); in imported sheep (74.3 %; 223/300; 95 % CI:69.1–78.9 %); in the winter season (57.2 %; 527/920; 95 % CI:54.1–60.4 %); in non-farming rearing system (45.1 %; 440/975; 95 % CI:42.0–48.3 %); and grazing feeding system (45.4 %; 645/1420; 95 % CI:42.9–48.0 %) as shown in Table 1.
3.3. Univariate association analysis for the risk factors
The results of univariable analysis between cELISA and different epidemiological risk factors for PPR in Table 1 show that six risk factors included a strong significant distribution with the seroprevalence, including district/area (p < 0.001), ecological zone (p < 0.001), breed (p < 0.001), age (p < 0.001), source (p < 0.001), and season (p < 0.001). There was no significant variation in the age of the sheep (p = 0.93), rearing system (p = 0.54), or feeding system (p = 0.21).
3.4. Multivariable regression
Multivariable logistic regression analysis revealed that variables such as study location, ecological zone, breed, age, source, and season were possible risk factors for the occurrence of PPRV infection in sheep in Bangladesh. The results showed that the significant effect of location on the seroprevalence of PPRV was 1.8 times in Bhola (OR = 1.8, 95 % CI = 1.2–2.5, p < 0.001), 1.9 times in Bagerhat (OR = 1.9, 95 % CI = 1.3–2.6, p < 0.001), 0.5 times in Rajshahi (OR = 0.5, 95 % CI = 0.3–0.7, p < 0.001), 0.4 times in Naogaon (OR = 0.4, 95 % CI = 0.2–0.6, p < 0.001), and 1.7 times in Tangail (OR = 1.7, 95 % CI = 1.1–2.4, p = 0.01) compared to Noakhali district. Similarly, the ecological zone Barind (OR = 0.3, 95 % CI = 0.3–0.5, p < 0.001) was a significant association (0.3 times) with the occurrence of PPRV antibodies against PPR in the coastal zone. Similarly, native sheep had 0.4 times the odds of having seroprevalence of PPRV than crossbreds (OR = 0.4, 95 % CI = 0.2–0.7, p < 0.001); female sheep had 0.3 times than male (OR = 0.3, 95 % CI = 0.2–0.4, p < 0.001); imported sheep had 2.8 times (OR = 2.8, 95 % CI = 1.8–4.4, p < 0.001) and sheep born in the farm/house had 0.2 times (OR = 0.2, 95 % CI = 0.1–0.4, p < 0.001) than in sheep which were brought from the market; and finally, the winter season had 6.3 times (OR = 6.3, 95 % CI:3.2–12.3, p < 0.001), and the rainy season had 3.6 times (OR = 3.6, 95 % CI:1.9–6.8, p < 0.001) the odds of seropositivity for PPRV than in the summer season (Table 2).
Table 2.
Multivariable logistic regression model between the binary response variable and the selected factors (N = 2420).
| Possible risk factors | Category | OR | 95 % CI | p-value |
|---|---|---|---|---|
| Districts | Noakhali | Ref | ||
| Bhola | 1.8 | 1.2–2.5 | 0.00 | |
| Bagerhat | 1.9 | 1.3–2.6 | 0.00 | |
| Khulna | 1.4 | 0.9–2.1 | 0.22 | |
| Satkhira | 1.2 | 0.8–1.7 | 0.49 | |
| Rajshahi | 0.5 | 0.3–0.7 | 0.00 | |
| Naogaon | 0.4 | 0.2–0.6 | 0.00 | |
| Gaibandha | 1.3 | 0.9–1.9 | 0.17 | |
| Tangail | 1.7 | 1.1–2.4 | 0.01 | |
| Mymensingh | 1.4 | 0.9–1.9 | 0.11 | |
| Ecological zone | Costal | Ref | ||
| Barind | 0.3 | 0.3–0.5 | 0.00 | |
| Jamuna river basin | 1.1 | 0.8–1.3 | 0.55 | |
| Plane land | 1.0 | 0.7–1.3 | 0.97 | |
| Breed | Crossbreds | Ref | ||
| Native | 0.4 | 0.2–0.7 | 0.00 | |
| Sex | Male | Ref | ||
| Female | 0.3 | 0.2–0.4 | 0.00 | |
| Source | Animal market | Ref | ||
| Imported | 2.8 | 1.8–4.4 | 0.00 | |
| Born in farm/house | 0.2 | 0.1–0.4 | 0.00 | |
| Season | Summer (Mar-Jun) | Ref | ||
| Rainy (Jul-Oct) | 3.6 | 1.9–6.8 | 0.00 | |
| Winter (Nov-Feb) | 6.3 | 3.2–12.3 | 0.00 |
Here, a p ≤ 0.05 was deemed as statistically significant, OR = Odds ratio, CI = Confidence interval, Nov = November, Feb = February, Jul = July, Oct = October, Mar = March, Jun = June.
4. Discussion
Bangladesh has a large population of sheep, which have a significant impact on the country’s GDP by providing income sources to farmers and being a significant source of meat and wool for the country. However, PPR has hampered the development of the sheep sector by causing morbidity and mortality and increasing treatment costs. Therefore, this study was designed to determine the seroepidemiology of PPR in sheep using cELISA in several specific areas of Bangladesh.
In this study, 44.3 % (1074/2420) of sheep blood samples collected from ten districts were found to be seropositive for PPRV antibodies, and the Bagerhat district had significantly higher seropositivity (p < 0.05) compared to other districts. The seroprevalence of PPR in sheep in this study was higher than that reported by Banik et al. (2008) (27 %); however, comparable to that reported by Rahman et al. (2004) (36 %). Although PPR has long been epizootic in Bangladesh, the degree of seropositivity in the field in the absence of vaccination is still unknown. The findings of the present study show that PPR has been circulating in parts of Bangladesh without being reported to the veterinary authorities. As a result, national disease monitoring systems for controlling PPR were found to be ineffective. Previously, the similar, higher, and lower seroprevalence of PPRV antibodies in this study were reported in the neighbouring countries of Bangladesh, for example, in India, 44.7 % by Hota et al. (2018), and 2.98 % by Krishna et al. (2001); and in Pakistan, 51.34 % by Khan et al. (2007), 53 % by Abubakar et al. (2009), and 37.2 % by Nizamani et al. (2015). Globally, similar seroprevalence was recorded, for example, 46.68 % by Gari et al. (2017) in Ethiopia and 46.7 % by Almeshay et al. (2017) in Libya; still higher seroprevalence was reported by Saeed et al. (2018) as 68.1 % and lower was reported by Mebrahtu et al. (2018) (16.2 %). The disparity in seroprevalence rates could be attributed to a variety of factors, including immunity levels such as vaccination status or previous PPR exposure, differences in sheep farming systems across regions, allocation of sheep populations, animal trading, nutritional components, genetic makeup, animal trading, farmers' socioeconomic status, and diagnostic procedures.
Bangladesh is classified into 30 agro-ecological zones, of which only four are included in this study. Among them, the Jamuna River Basin zone had the highest seroprevalence of PPR (49.1 %) and the lowest was in the Barind zone (26.5 %). PPR seroprevalence varied significantly (p < 0.05) among the ecological zone zones, which could be attributable to changes in agro-ecology, native sheep population variance, sheep flock movement for the market, and small ruminant management systems. The high PPR seroprevalence in the study locations could be linked to the occurrence of an active PPR epizootic during the sample collection period. Fentie et al., 2018, Hota et al., 2018 found that PPR seroprevalence varies significantly across different ecological zones. The causes of the variance in seroprevalence among ecological zones could be linked to various climatic factors, such as temperature, humidity, rainfall, and soil type (Chauhan et al., 2012).
In this study, PPR infection in sheep was more common in crossbreeds than in indigenous breeds. Seroprevalence was significantly higher (p < 0.05) in crossbreeds (60 %) than in native sheep (33.3 %). Due to unrestrained animal migration across borders with neighbouring nations, the number of imported animals in Bangladesh has increased dramatically in recent years. This may be an attributable cause of the increased PPR seroprevalence in crossbreeds. In addition, inbreeding or unethical breeding of crossed sheep for profit may make them more susceptible to infectious diseases, including PPR. In comparison to native or indigenous sheep, crossbred sheep may have lower resistance to disease because of a possible lack of immunity.
Based on the sex of sheep, the seroprevalence of PPRV was higher in males (69.8 %) than in females (39.1 %), and the effect of sex on PPR seropositivity in sheep was statistically significant. In addition, the OR of PPR infection in sheep revealed that male sheep were more prone to PPR than female sheep. Mahajan et al. (2012) also reported similar outcomes and revealed that the seroprevalence of PPRV was significantly (p < 0.05) higher in male sheep (38.88 %) than in female sheep (19.44 %). A similar pattern was reported by Rahman et al., 2004, Tajpara et al., 2022. An increased ratio of male sheep to female sheep in the vulnerable age range of 4–24 months could explain why PPR is more common in male sheep. When compared with the proportion of females in the same age range, the proportion of male sheep between the ages of 4 and 24 months was significantly greater. After the age of 24 months, male small ruminants (including sheep) in Bangladesh are typically not reared but sold at local markets for slaughter (Rony et al., 2017). However, the exact host and pathogen rationale of variation remains unknown, it is a fact that the high need for male animals for meat purpose have forced them to the market, where they are more likely to be infected than females, which are typically kept at home for breeding purposes (Tajpara et al., 2022). Moreover, another possible explanation for the increased seroprevalence and OR in males is that during the mating season, males frequently interact with females from various flocks, increasing their chances of becoming infected (Mahajan et al., 2012).
Regarding the age group, the seroprevalence of PPR was higher in the 13–24 month age group and lower in the 0–6 month age group, but there was no significant difference (p > 0.05). The lower prevalence of PPR in young stock could be attributed to passive protection by colostral antibodies up to six months of age which was provided by exposed or vaccinated female sheep. Subsequently, a child’s susceptibility to PPRV infection rises in tandem with the natural drop in maternal antibodies. Obi et al., 1983, Tahir et al., 2000, Agrawal et al., 2006, Abubakar et al., 2009, and Nizamani et al. (2015) also reported an increase in PPR seropositivity with age.
According to the source, imported sheep (74.3 %) had a significantly higher (p < 0.05) seroprevalence of PPRV compared to other sources. In addition, imported sheep had significantly higher odds of PPR than sheep from other sources. The intensity of illegally imported sheep could explain the disparity in prevalence. The present study revealed that one of the main routes of PPRV infection and transmission was the introduction of new sheep to farms, which were purchased from different live-animal markets. This finding agrees with those of Abubakar et al., 2009, Almeshay et al., 2017. Most sheep in Bangladesh are native; however, some farmers prefer to raise exotic varieties. Farmers obtain them from other regions of the country, from a neighbouring country, or introduce fresh sheep to their farms without quarantine. PPR may be linked to the introduction of these freshly imported diseased animals from animal live markets to the farm or close contact of sheep with sick animals that were taken to the market for selling but remained unsold, and back again to the farms. In addition, illegally purchased sheep are commonly exploited for breeding purposes, which may influence the higher seroprevalence of PPRV in Bangladesh.
Although the disease exists epizootic in Bangladesh, seroepidemiological data show that abrupt weather changes, such as a quick rise or drop in temperature, extended droughts, and continuous rain, have an adverse influence on the disease. In this study, the seroprevalence of PPRV was significantly higher (p < 0.05) in the winter season (57.2 %) than in other seasons. Moreover, the odds of PPR were significantly higher in the winter and rainy seasons. Epizootics have been linked to weather changes, such as the start of the rainy season or differences in dry and cold time frames (OIE, 2008). The air is dry and frigid during the winter. Increased PPR incidence during the winter and rainy seasons may have their roots in the fact that both are stressful times of the year for small ruminants in Bangladesh (Rony et al., 2017). The extremely contagious PPR virus is believed to spread more easily during these times of the year since herding and, by extension, close contact is favoured. Previous studies have reported a significantly higher risk of PPR in the winter season in Bangladesh (Sarker and Islam, 2011, Chowdhury et al., 2014, Rony et al., 2017, Rahman et al., 2021). A scientifically sound policy for disease prevention can be formulated using data on the seasonal variation in PPR. This could involve, for example, scheduling vaccination programs before the start of the winter and rainy seasons.
Rearing and feeding systems are important factors for disease incidence. In this study, the seropositivity of PPRV was higher in non-farming rearing systems than in farming rearing and grazing feeding systems than in stall feeding systems. However, there were no significant variations in the rearing and feeding systems. In Congo, Bwihangane et al. (2016) found that animals kept in open grazing systems and free-range or non-farming rearing systems had greater seropositivity than those housed in stall grazing farming systems. During the dry season, the open grazing system around water sources in rural areas may play a significant role in the spread of the disease. In addition, infected sheep fed in an open grazing system may spread the PPRV infection to other healthy animals or water sources.
5. Conclusion
The high seroprevalence of PPRV in this study suggests that PPR is nearly epizootic and that the virus is widespread among sheep populations throughout Bangladesh. This study sheds light on the epizootic nature of PPR across Bangladesh, demonstrating potential epidemiological risk factors such as geographical locations, ecological zones, breed, sex, sources, and seasons. If preventive measures are not implemented promptly, it may harm national small ruminant production practices. Therefore, controlling and managing animal trading, as well as surveilling suspected cases, is critical for preventing disease transmission. In addition, a well-designed strategic mass vaccination program across the country and regular vaccination of young sheep would be viable approaches for preventing PPRV infection in the sheep population. Additional efforts should be made to enhance awareness among sheep farmers regarding this contagious disease. Government organisations such as the Department of Livestock Services (DLS) can help the Central Disease Investigation Laboratory (CDIL) and the Field Disease Investigation Laboratory (FDIL) improve their diagnostic capabilities for faster disease diagnosis. More extensive nationwide investigations to identify the seroprevalence and titre levels of PPRV antibodies, as well as virus isolation with molecular epidemiology, are strongly recommended, as they are an important aspect of developing PPR control and eradication measures.
Funding
This study was supported by the “Conservation and Improvement of Native Sheep through the Community Farming and Commercial Farming (Phase II) project, which was managed by the Asian Development Bank (ADB) from July 2012 to June 2019.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their gratitude to the National Institute of Biotechnology (NIB), Savar, Dhaka, Bangladesh Livestock Research Institute (BLRI), Savar, Dhaka, and the Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, for their support during the research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad Mojibur Rahman, Email: mojibur.rahman@dls.gov.bd.
Abdullah Al Momen Sabuj, Email: sabuj47041@bau.edu.bd.
Md. Saiful Islam, Email: dvm41257@bau.edu.bd.
Md. Alimul Islam, Email: alimul.vmh@bau.edu.bd.
Jahangir Alam, Email: jalam@nib.gov.bd.
Md. Ershaduzzaman, Email: ershaduzzaman@kgf.org.bd.
Sukumar Saha, Email: sukumar.saha@bau.edu.bd.
References
- Abdollahpour G., Raoofi A., Najafi J., Sasani F., Sakhaie E. Clinical and para-clinical findings of a recent outbreaks of peste des petits ruminants in Iran. J. Vet. Med. B. 2006;53:14–16. doi: 10.1111/j.1439-0450.2006.01013.x. [DOI] [Google Scholar]
- Abubakar M., Jamal S.M., Arshed M.J., Hussain M., Ali Q. Peste des petits ruminants virus (PPRV) infection; its association with species, seasonal variations and geography. Trop. Anim. Health Prod. 2009;41:1197–1202. doi: 10.1007/s11250-008-9300-9. [DOI] [PubMed] [Google Scholar]
- Agrawal R., Kumar M., Singh R.P. Epidemiological investigations of PPR in goats in some part of Uttaranchal. Indian Vet. J. 2006;83:790–791. [Google Scholar]
- Almeshay M.D., Gusbi A., Eldaghayes I., Mansouri R., Bengoumi M., Dayhum A.S. Peste des petits ruminants in Tripoli Region. Lybia. Vet. Ital. 2017;53:235–242. doi: 10.12834/vetit.964.5025.3. [DOI] [PubMed] [Google Scholar]
- Anderson J., McKay J.A., Butcher R.N. International Atomic Energy Agency; Vienna, Austria: 1991. The use of monoclonal antibodies in competition ELISA for detection of antibodies to rinderpest and peste des petits ruminants viruses. The sero-monitoring of rinderpest throughout Africa: phase I; pp. 43–53. [Google Scholar]
- Baazizi R., Mahapatra M., Clarke B.D., Ait-Oudhia K., Khelef D., Parida S. Peste des petits ruminants (PPR): A neglected tropical disease in Maghreb region of North Africa and its threat to Europe. PloS One. 2017;12:e0175461. doi: 10.1371/journal.pone.0175461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik S.C., Podder S.C., Samad M.A., Islam M.T. Sero-surveillance and immunization in sheep and goats against peste des petits ruminants in Bangladesh. Bangladesh J. Vet. Med. 2008;6:185–190. doi: 10.3329/bjvm.v6i2.2334. [DOI] [Google Scholar]
- Banyard A.C., Parida S., Batten C., Oura C., Kwiatek O., Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J. Gen. Virol. 2010;91:2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- Bhuiyan, A.K.F.H., 2006. Livestock genetic resources in Bangladesh: Preservation and Management. In International conference on livestock services, Chinese Academy of Agricultural Science (CAAS), Beijing, China, vol. 6, pp. 16-20
- Bwihangane, B.A., Misinzo, G., Sviteck, N., Bebora, L.C. and George, C.G., 2016. Sero-epidemiology of peste des petits ruminants infection and the associated risk factors in South Kivu, DR. Congo. In Fifth African Higher Education Week and RUFORUM Biennial Conference 2016,“ Linking agricultural universities with civil society, the private sector, governments and other stakeholders in support of agricultural development in Africa”, Cape Town, South Africa, 17-21 October 2016 (pp. 737-746).
- Chauhan H.C., Dadawala A.I., Chandel B.S., Kalyani I.H., Patel S.S., Kher H.N. Seroprevalence of Peste des petits ruminants in small ruminants under different managemental conditions. Indian J. Field Vets. 2012;7:37–39. [Google Scholar]
- Chowdhury E.H., Bhuiyan A.R., Rahman M.M., Siddique M.S.A., Islam M.R. Natural peste des petits ruminants virus infection in Black Bengal goats: virological, pathological and immunohistochemical investigation. BMC Vet. Res. 2014;10:1–10. doi: 10.1186/s12917-014-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel W.W., editor. Biostatistics: a Foundation for Analysis in the Health Sciences. 7th ed. Wiley; New York: 1999. pp. 141–142. [Google Scholar]
- DLS, 2021. Livestock economy at a glance, DLS. Department of Livestock Services. Available online: http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/files/a7c2c046_864e_41c5_adcc_c9858ebc7887/2021-08-18-05-41-28ed36a96d0db342b627f416f5d1f97a.pdf (Accessed on April 15, 2022).
- Dohoo I., Martin W., Stryhn H. Canada; AVC Inc, Charlottetown, PE: 2003. Veterinary Epidemiologic Research. [Google Scholar]
- Dou Y., Liang Z., Prajapati M., Zhang R., Li Y., Zhang Z. Expanding diversity of susceptible hosts in Peste Des Petits ruminants virus infection and its potential mechanism beyond. Front. Vet. Sci. 2020;7:66. doi: 10.3389/fvets.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2013. Supporting livelihoods and building resilience through Peste des Petits Ruminants (PPR) and small ruminant diseases control. Animal Production and Health Position Paper, Rome. Available online: http://www.fao.org/3/a-aq236e.pdf (Accessed on April 15, 2022).
- Fentie T., Teshome Y., Ayele B., Molla W., Fenta N., Nigatu S., Assefa A., Leta S. Sero-epidemiological study of Peste des petits ruminants in small ruminants in Amahara region, Ethiopia. Comp. Clin. Path. 2018;27:1029–1036. doi: 10.1007/s00580-018-2697-2. [DOI] [Google Scholar]
- Gari G., Serda B., Negesa D., Lemma F., Asgedom H. Serological investigation of peste des petits ruminants in east Shewa and Arsi Zones, Oromia Region, Ethiopia. Vet. Med. Int. 2017;2017:9769071. doi: 10.1155/2017/9769071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.R., Talukder M.A.I. Comparative performance of different regional native sheep in Bangladesh. Bangladesh Vet. 2012;28:85–95. doi: 10.3329/bvet.v28i2.10692. [DOI] [Google Scholar]
- Hota A., Biswal S., Sahoo N., Rout M., Chaudhary D., Pandey A., Muthuchelvan D. Seroprevalence of PPR among sheep and goats of different agroclimatic zones of Odisha. Int. J. Livest. Res. 2018;8:2277–11964. doi: 10.5455/ijlr.20171028023420. [DOI] [Google Scholar]
- Khan H.A., Siddique M., Arshad M.J., Khan Q.M., Rehman S.U. Sero-prevalence of peste des petits ruminants (PPR) virus in sheep and goats in Punjab province of Pakistan. Pak. Vet. J. 2007;27:109–112. [Google Scholar]
- Krishna V., Rao M.S., Shaila M.S. Neutralizing antibodies to Peste-des-petits ruminants virus in small ruminants in Andhra Pradesh-a serological survey. Indian J. Anim. Sci. 2001;71:228–230. [Google Scholar]
- Mahajan S., Agrawal R., Kumar M., Mohan A., Pande N. Risk of seroconversion to peste des petits ruminants (PPR) and its association with species, sex, age and migration. Small Rumin. Res. 2012;104:195–200. doi: 10.1016/j.smallrumres.2011.10.009. [DOI] [Google Scholar]
- Mebrahtu K., Getachew S., Tesfaye T., Sahlu E., Aragaw K. Sero-epidemiological study of peste des petits ruminants (PPR) in sheep and goats under different production systems in South Omo, southern Ethiopia. Small Rumin. Res. 2018;169:90–93. doi: 10.1016/j.smallrumres.2018.06.017. [DOI] [Google Scholar]
- Murphy F.A., Gibbs E.P.J., Horzinek M.C., Studdert M.J. 3rd edition. Veterinary Virology; Academic Press, London, UK: 1999. Paramyxoviridae. [Google Scholar]
- Naing L., Winn T.B.N.R., Rusli B.N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006;1:9–14. [Google Scholar]
- Nizamani A.R., Nizamani Z.A., Umrani A.P., Dewani P., Vandiar M.A., Gandahi J.A., Soomro N.M. Prevalence of Peste des petits ruminants virus antibodies in small Ruminantsin Sindh, Pakistan. J. Anim. Plant Sci. 2015;25:1515–1529. [Google Scholar]
- Obi T.U., Ojo M.O., Taylor W.P., Rowe L.W. Studies on the epidemiology of peste des petits ruminants in Southern Nigeria. Trop. Vet. 1983;1:209–217. [Google Scholar]
- OIE, 2008. Peste des petits ruminants (PPR) in Morocco, OIE Alert Message. MAR 23-07-08. Available online: https://www.fao.org/3/aj120e/aj120e.pdf (Accessed on 25 April 2021).
- Rahman M.B., Razzaque M.A., Kafi M.A., Islam M.R., Khan M.F.R., Nazir K.H.M.N.H. Application of C-ELISA for detection of PPRV-specific antibodies in domestic ruminants in different areas of Mymensingh, Bangladesh. Mol. Biol. Biotechnol. J. 2004;2:40–43. [Google Scholar]
- Rahman M.Z., Haider N., Gurley E.S., Ahmed S., Osmani M.G., Hossain M.B., Islam A., Khan S.A., Hossain M.E., Epstein J.H., Zeidner N. Epidemiology and genetic characterization of Peste des petits ruminants virus in Bangladesh. Vet. Med. Sci. 2018;4:161–171. doi: 10.1002/vms3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A.K.M., Islam S.S., Sufian M., Talukder M., Ward M.P., Martínez-López B. Peste des Petits Ruminants Risk Factors and Space-Time Clusters in Bangladesh. Front. Vet. Sci. 2021;7:1190. doi: 10.3389/fvets.2020.572432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rony M.S., Rahman A.K.M.A., Alam M.M., Dhand N., Ward M.P. Peste des Petits Ruminants risk factors and space–time clusters in Mymensingh. Bangladesh. Transbound. Emerg. Dis. 2017;64:2042–2048. doi: 10.1111/tbed.12615. [DOI] [PubMed] [Google Scholar]
- Saeed F.A., Abdel-Aziz S.A., Gumaa M.M. Seroprevalence and associated risk factors of Peste des petits ruminants among sheep and goats in Kassala state, Sudan. Open J. Anim. Sci. 2018;8:381–395. doi: 10.4236/ojas.2018.84029. [DOI] [Google Scholar]
- Sarker S., Islam M.H. Prevalence and risk factor assessment of Peste des petits ruminants in goats in Rajshahi, Bangladesh. Vet. World. 2011;4:546–549. [Google Scholar]
- Shaila M.S., Purushothaman V., Bhavasar D., Venugopal K., Venkatesan R.A. Peste des petits ruminants of sheep in India. Vet. Rec. 1989;125:602. [PubMed] [Google Scholar]
- Tahir M.T., Rehan A., Iftikhar H., Ashfaque M. Serological study of peste-des-petits ruminants (PPR) using counter immunoelectrophoresis in Faisalabad. Pak. Vet. J. 2000;20:53–54. [Google Scholar]
- Tajpara M.M., Kathiriya J.B., Savsani H.H., Shah N.M., Gohil P.V., Patel D.R., Javia B.B., Kanani A.N. Seroprevalence of PPR Virus in Pre-and Post-Vaccinated Sheep and Goats of Saurashtra Region of Gujarat. Int. J. Curr. Microbiol. App. Sci. 2022;11:275–283. doi: 10.20546/ijcmas.2022.1102.031. [DOI] [Google Scholar]