Abstract
Introduction
The immunosuppressive tumor microenvironment (TME) is a major barrier to the efficacy of chimeric antigen receptor T cells (CAR-T cells) in glioblastoma (GBM). Transgenic expression of IL15 is one attractive strategy to modulate the TME. However, at present, it is unclear if IL15 could be used to directly target myeloid-derived suppressor cells (MDSCs), a major cellular component of the GBM TME. Here, we explored if MDSC express IL15Rα and the feasibility of exploiting its expression as an immunotherapeutic target.
Methods
RNA-seq, RT-qPCR, and flow cytometry were used to determine IL15Rα expression in paired peripheral and tumor-infiltrating immune cells of GBM patients and two syngeneic murine GBM models. We generated murine T cells expressing IL13Rα2-CARs and secretory IL15 (CAR.IL15s) or IL13Rα2-CARs in which IL15 was fused to the CAR to serve as an IL15Rα-targeting moiety (CAR.IL15f), and characterized their effector function in vitro and in syngeneic IL13Rα2+glioma models.
Results
IL15Rα was preferentially expressed in myeloid, B, and dendritic cells in patients’ and syngeneic GBMs. In vitro, CAR.IL15s and CAR.IL15f T cells depleted MDSC and decreased their secretion of immunosuppressive molecules with CAR.IL15f T cells being more efficacious. Similarly, CAR.IL15f T cells significantly improved the survival of mice in two GBM models. TME analysis showed that treatment with CAR.IL15f T cells resulted in higher frequencies of CD8+T cells, NK, and B cells, but a decrease in CD11b+cells in tumors compared with therapy with CAR T cells.
Conclusions
We demonstrate that MDSC of the glioma TME express IL15Ra and that these cells can be targeted with secretory IL15 or an IL15Rα-targeting moiety incorporated into the CAR. Thus, IL15-modified CAR T cells act as a dual targeting agent against tumor cells and MDSC in GBM, warranting their future evaluation in early-phase clinical studies.
Keywords: Immunotherapy, Adoptive; Cell Engineering; Myeloid-Derived Suppressor Cells; T-Lymphocytes; Brain Neoplasms
WHAT IS ALREADY KNOWN ON THIS TOPIC
Chimeric antigen receptor (CAR) T cells have been developed and studied for targeting tumor-associated antigens in preclinical and clinical studies for glioblastoma (GBM). Infiltration of myeloid-derived suppressor cells (MDSCs) are a hallmark of GBMs and contribute to the immunosuppressive microenvironment which promotes tumor progression and therapeutic resistance.
WHAT THIS STUDY ADDS
Here, we report that MDSCs of human and murine GBMs express IL15Rα. To simultaneously target MDSCs and malignant GBM cells, and further stimulate T cell effector function, we modified T cells to express an IL13Rα2-CAR and secretory IL15 or an IL13Rα2-CAR in which IL15 was directly fused to the CAR. We demonstrate that dual targeting CAR T-cells deplete MDSCs and decrease their secretion of immunosuppressive molecules, resulting in improved antitumor activity in murine glioma models.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study highlights the benefit of targeting not only glioma cells but also MDSCs and provides the impetus to develop MDSC/tumor cell-targeting CAR T cells for a broad range of malignancies.
Introduction
Glioblastoma (GBM) is the most common primary brain tumor in adults. Despite improvements in surgical and radiochemotherapy techniques, GBM remains a devastating diagnosis as current treatment regimens provide no cure and short survival.1 2 The success of T cells modified with chimeric antigen receptor (CAR) in the treatment of blood cancers3 4 inspired the investigation of CAR T cells in solid tumors,5 6 including GBM.7–9 However, multiple barriers exist in GBM that continue to hinder the efficacy of CAR-T cells, including (1) heterogeneity in the antigen expression or concerns of on target/off cancer toxicities,7 8 (2) the immunosuppressive tumor microenvironment (TME),10 11 and (3) inefficient trafficking to glioma sitescrossing12 13 all contribute to poor performance of CAR T cells in GBM.
We and other have demonstrated that interleukin-13 receptor alpha 2 (IL13Rα2) can serve as a CAR target in both xenografts and syngeneic models of GBM, and IL13Rα2-CAR T cells been evaluated in early phase clinical studies. However, their activity in clinical studies as well syngeneic GBM models was limited.14–17 Expressing cytokines, chimeric cytokine receptors, or constitutively active cytokine receptors improve the effector function of CAR T cells in preclinical models.18–22 For example, we and other investigators have demonstrated that cells enhances their antitumor activity, and early clinical studies are in progress.15 23 24
The majority of preclinical studies with IL15-expressing CAR T cells have been conducted in xenograft models, which do not allow studying the effects of IL15 on tumor-infiltrating immune cells. IL15 binds not only the IL2/15Rβ and γC chain expressed in T cells, but is also transpresented to T cells by binding to IL15 receptor alpha (IL15Rα) expressed on antigen presenting cells. At present, it was unknown if myeloid cells, a major component of the GBM TME,25 26 express IL15Rα and can be targeted with CAR T cells. To address this gap in our knowledge, we examined the expression of IL15Rα in primary human GBM samples and syngeneic mouse models and explored the feasibility of targeting IL15Rα-positive immune cells. We demonstrate that IL15Rα is expressed on myeloid-derived suppressor cell (MDSC) in human and murine GBMs, and that targeting these cells with secretory IL15 or an IL15Rα-targeting moiety incorporated into the CAR reverses immunosuppression in GBMs and improved antitumor activity in preclinical murine glioma models.
Materials methods
Detailed information on selected methods is provided in online supplemental methods.
jitc-2022-006239supp001.pdf (184.9KB, pdf)
Generation of retroviral constructs and CAR T cells
The IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ were cloned in the pRV2011(M) vector, as previously described.17 For the generation of the IL15 constructs, we (1) subcloned a cDNA encoding T2A and murine IL15 at the C-terminal part of the CAR construct to generate a retroviral vector that encoded the CAR, T2A, and secretory IL15 (IL15s), or (2) subcloned murine IL15 and (Gly4S)3 linker at the N-terminus part of CAR to generate an IL15-CAR fusion protein (IL15f). All CAR constructs were verified by Sanger’ sequencing (GenScript, Piscataway, New Jersey, USA).
Processing of patients’ blood and tumor samples
Prior the use in the study, the patient’s tumor and blood samples (peripheral blood mononuclear cells, PBMCs) were deidentified. Patient consents were obtained prior to biospecimen collection. Isolation of T cells from PBMC and tumor tissue was performed as previously described.17
Animal experiments
All animal experiments were performed according to the protocols approved by the Northwestern University Institutional Animal Care and Use Committee, protocol IS00009472. CD45.1, CD45.2 C57BL/6 and IL-15Ra knock-out (KO) mice were obtained from Jackson Laboratory. As previously described, male and female 6–8 weeks old mice were used for intracranial glioma implantation.17 In 7 days after tumor implantation, animals received an intratumoral (i.t.) injection of saline, non-transduced (NT) or 1×106 CAR T cells. All animals were then randomly assigned to housing cages, separated by gender, and monitored for survival.
Statistical consideration
All statistical analyses of data were performed with GraphPad V.9 Software (Prism, La Jolla, California, USA). As indicated, significance, defined as p<0.05 in all statistical tests, was calculated with an unpaired Mann-Whitney test or an unpaired two-tailed Student’s t-test. Multiple groups were analyzed with either a one-way or two-way analysis of variance, followed by a Tukey’s multiple comparisons test. Kaplan-Meier plots were generated using GraphPad V.8 Prism, and p values for curve comparisons were calculated using the log-rank method. Data are presented as mean±SEM.
ResultsMC
IL15Rα is expressed by cells of the TME at higher levels than in peripheral blood of GBM patients
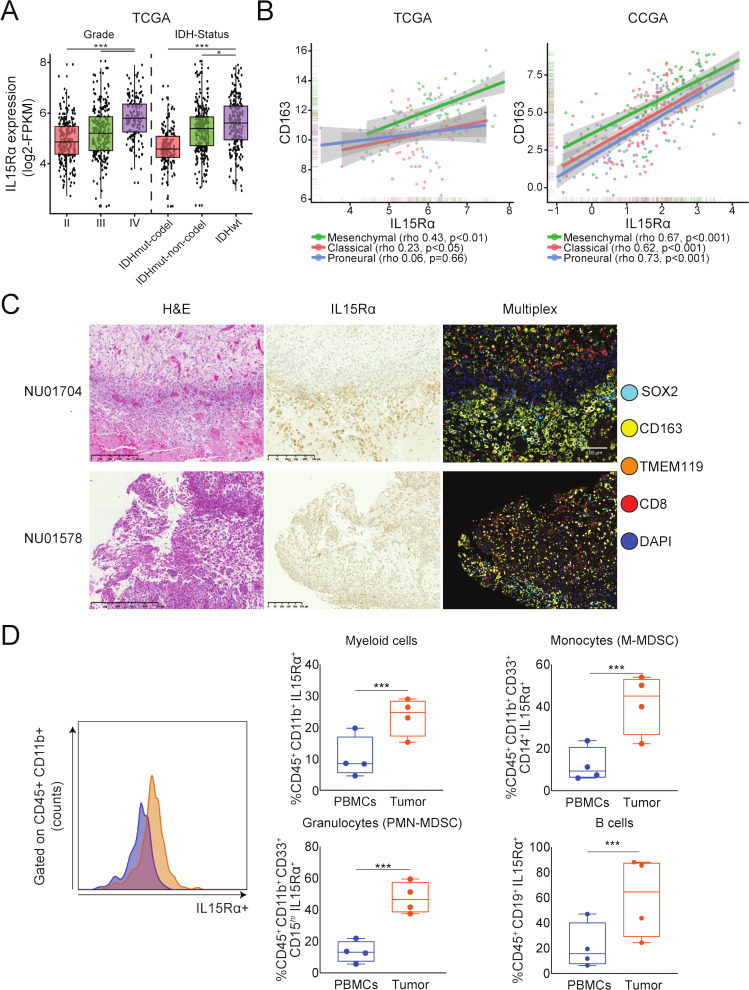
IL15 binds to βγ receptor complex expressed on T cells and IL15Rα expressed on antigen presenting cells.27 28 To investigate if myeloid cells express IL15Rα in the GBM TME, we first performed a transcriptomic analysis of GBMs from The Cancer Genome Atlas (TCGA). IL15Rα expression correlated with histological grade, with significantly higher expression seen in grade IV compared with lower grade brain tumors, and positively correlates with Isocitrate Dehydrogenase-wild type (IDHwt) status, with significantly higher expression seen in IDHwt compared with both codel and non-codel IDH mutant tumors (figure 1A). Further analysis revealed a positive correlation between IL15Rα and CD163, CD206, and TGFβ1, markers associated with M2 phenotype of macrophages,29–31 in both TCGA and Chinese Glioma Genome Atlas data sets (figure 1B, online supplemental figure 1B). Analysis of human GBM tissue in the Ivy Glioblastoma Atlas Project (Ivy GAP) revealed higher IL15Rα expression levels in areas of microvascular proliferation and hyperplastic blood vessels of GBM (online supplemental figure 1A). Immunostaining of tumor tissue sections from GBM patients displayed IL15Rα expression coinciding with macrophage-rich (CD163+) regions (figure 1C). Analysis of four paired peripheral blood and GBM tissue samples revealed a significantly higher expression surface IL15Rα expression in the myeloid compartment (CD45+CD11b+ cells) of GBM than in peripheral blood (figure 1D). Further stratification of CD45+ cell subsets showed higher IL15Rα expression in tumor derived monocytic MDSC (CD45+, CD1lb+, CD33+, and CD14+), granulocytic MDSCs (PMN-MDSC; CD45+, CD1lb+, CD33+, and CD15hi), and B Cells (CD45+ and CD19+) compared with peripheral blood (figure 1D, online supplemental figure 1C). Immunostaining of ten patient samples shows that IL15Rα is also expressed by glioma cells, although with great intra and intertumor variability (online supplemental figure 2B). RT-qPCR and flow cytometry analysis of three patient-derived and two murine glioma cell lines detected IL15Rα expression at both the mRNA and protein level, respectively (online supplemental figure 2B, C).
Figure 1.
Expression of IL15Rα in the immune infiltrates of tumor and peripheral blood of GBM patients. (A) The analysis of the TCGA data set for the RNA expression of IL15Rα shows that GBM (n=152) expresses IL15Rα at significantly higher levels than oligodendroglioma (n=191), oligoastrocytoma (n=130), and astrocytoma (n=194) tumors (***adj. p<0.001, *adj. p<0.05, Tukey’s honest significant difference test). (B) Spearman rank correlation analysis of GBMs from TCGA (n=156) and CGGA (n=225) of RNA-seq data sets shows that IL15Rα expression correlates with expression of CD163+, a marker of infiltrating macrophages. (C) Immunostaining of GBM patient tissue section for the expression of IL15Rα and immune cells. (D) Flow cytometry analysis of IL15Rα expression on the surface of immune cells harvested from GBM microenvironment and peripheral blood (peripheral blood mononuclear cells, PBMC) of patients (paired samples, n=4). Isotype control IgG served as a negative control to anti-IL15RA. Representative histogram shows IL15Rα detection in CD45+ CD11b+ cells. The data show a higher expression of IL15Rα in many CD45+ cell types, including subsets of monocytes, granulocytes, and B cells harvested from tumors, compared with cells purified from peripheral blood. Paired t-test. ***p<0.001. CGGA, Chinese Glioma Genome Atlas; GBM, glioblastoma; TCGA, The Cancer Genome Atlas.
jitc-2022-006239supp002.pdf (10.4MB, pdf)
Murine syngeneic models of glioma recapitulate the expression IL15Rα pattern in human GBM
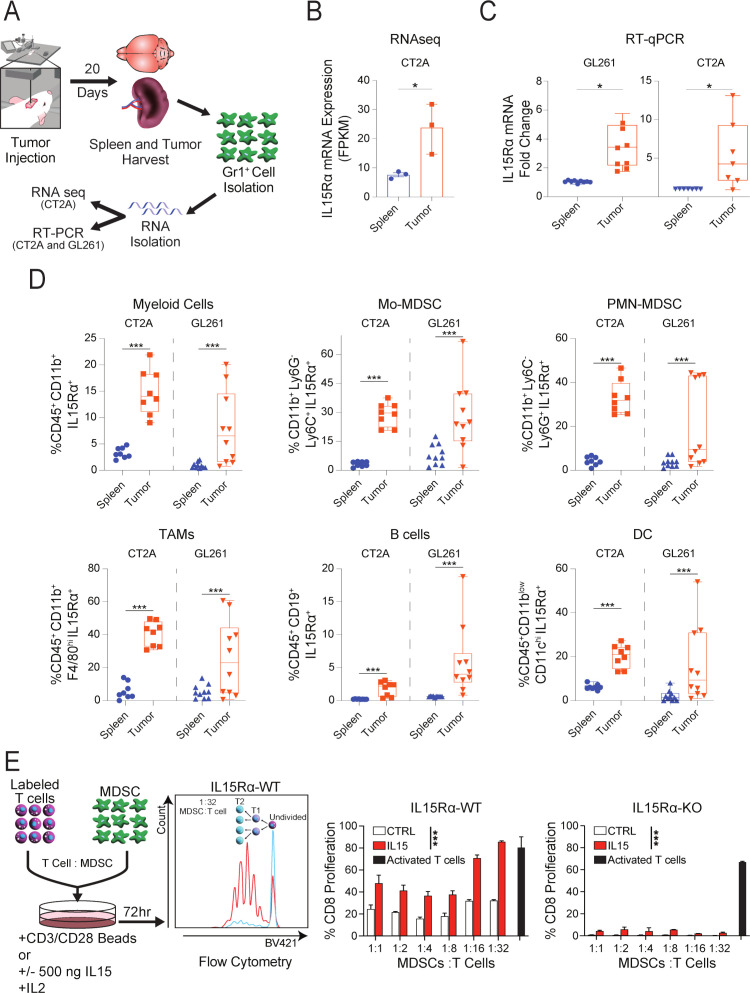
We next sought to determine if the preferential expression of IL15Rα by myeloid cells is recapitulated in murine glioma models. To do so, we isolated Gr1+ cells from the tumors and spleens of CT2A and GL261 bearing mice, extracted the mRNA from these cells, and, in separate experiments, performed either bulk RNAseq (PRJNA909275)32 or RT-qPCR (figure 2A). RNA-seq analysis of CT2A bearing mice showed 3.7-fold higher IL15Rα expression in tumor-associated Gr1+ cells (log 2-fold change 1.9, n=3, adj. p=1.19E-10) compared with splenic Gr1+ cells (figure 2B). Similar results were obtained from RT-qPCR analysis, with 3.5-fold and 4.2-fold higher IL15Rα expression in tumor-derived cells compared with splenocytes from GL261 and CT2A bearing models, respectively (figure 2C). To determine any expression differences in individual cell types, we used flow cytometry to further stratify the immune subsets of CT2A and GL261 bearing animals and compared IL15Rα expression of tumor-derived cells to that of the spleen. The overall tumor-derived myeloid compartment (CD45+CD11b+) displayed significantly higher IL15Rα expression than myeloid cells from the spleen (figure 2D) in both GL261 and CT2A models. Similar results were seen following stratification, with Mo-MDSC (CD45+, CD11b+, Ly6G-, Ly6C+), PMN-MDSC (CD45+, CD11b+, Ly6C-, Ly6G+), and TAMs (CD45+, CD11b+, F4/80hi), all displaying significantly higher IL15Rα expression differences compared with splenic cells (figure 2D). In both CT2A and GL261-bearing animals, the same comparison of B cells (CD45+CD19b+) and dendritic cells (CD11b-CD11chi) showed significantly higher expression of IL15Rα in the tumor compartment (figure 2D; representative flow cytometry plots are shown in online supplemental figure 2D). Taken together, the expression of IL15Rα in these cell types suggests the myeloid compartment may be able to influence T cell activity through IL15 transpresentation.
Figure 2.
Expression of IL15Rα in brain tumor tissue of murine models of GBM. (A) The diagram shows the experimental set up used for generation of Gr1+ cells for downstream analysis of IL15Rα expression. (B) RNAseq analysis of Gr1+ cells harvested from the brain and spleens (paired samples) of mice bearing established CT2A tumors Gr1+ cells were harvested from 10 mice in three independent experiments, n=3. Paired t-test. *p<0.05. (C) RT-qPCR analysis of IL15Rα mRNA expression in Gr1+ cells harvested from the brains and spleens of mice bearing CT2A and GL261, n=8. Paired t-test. *p<0.05. (D) Flow cytometry analysis of IL15Rα expression on the surface of total population of myeloid cells (CD45+ CD11b+ cells) and different subsets of myeloid cells, as well monocytic MDSCs (CD11c-CD11B+ Ly6G- Ly6C+), polymorphonuclear MDSCs (CD11c-CD11B+ Ly6G+ Ly6C-), TAMs (CD45+ CD11B+ F4/80hi Ly6C-), B cells (CD45+ CD19+), and dendritic cells (CD45+ CD11blow CD11chi) harvested from brains and splenocytes of mice bearing intracranial CT2A and GL261 murine glioma tumors, n=4. Paired t-test. ***p<0.001. (E) CD8+T cells proliferate in the presence of recombinant murine IL15 in coculture with wild type MDSC, but not with IL15Rα KO MDSCs or absence of IL15 (negative control). A representative flow plot showing proliferation of Cell Trace Violet labeled CD8+T cells at 1:32 ratio of MDSCs to T cells (red) or negative control (blue; unstimulated). Quantitative analysis of T cells proliferation in cocultures are shown. CD8+T cells activated with activating beads served as a positive control in the proliferation assay. GBM, glioblastoma; KO, knock-out; MDSCs, myeloid-derived suppressor cell.
To test this, we labeled CD8+ T cells with CTV proliferation dye, prepared bone-marrow-derived MDSCs from either wild-type (WT) or IL15Rα KO mice, and cocultured them for 72 hours at various ratios with or without recombinant murine IL15 (rIL15), and determined proliferation of T cells via flow cytometry (figure 2E).32 CD3/CD28/CD2-bead activated T cells served as the control for this experiment. In the absence of rIL15, no proliferation of T cells was seen in either condition after 72 hours of coculture (figure 2E). Following the addition of recombinant rIL15 to cultures, proliferation of CD8+ T cells was observed in IL15Rα WT-MDSCs setting, but not IL15Rα KO-MDSC (figure 2E).
In summary, murine glioma models follow a similar pattern of IL15Rα expression seen in GBM patients. Furthermore, as shown by the dependency of IL15Rα for IL15 mediated T cell proliferation, we confirmed the ability of IL15Rα expressing MDSCs to transpresent IL15 to CD8+ T cells and modulate their activity.
CAR T cells armored with IL15 fusion are more potent than CAR T cells secreting IL15 in killing IL13Rα2-expressing glioma cells
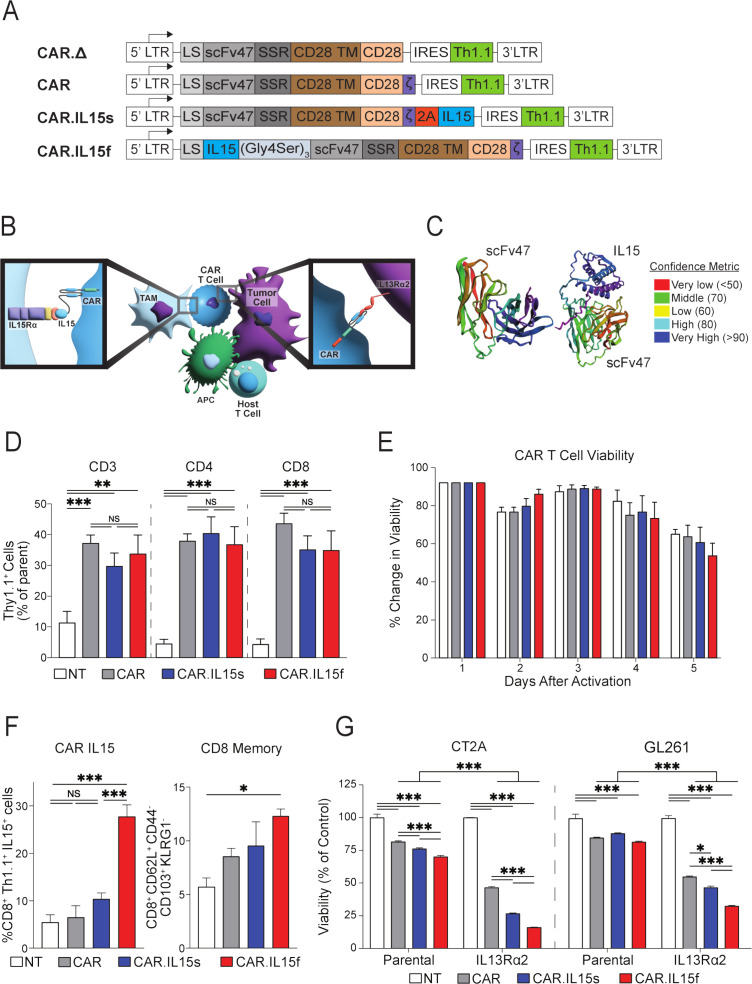
Having confirmed IL15Rα expression in various cells within both human and murine GBM, and demonstrating the capacity of these cells to enhance T cell proliferation, we hypothesize that the addition of IL15 modalities to our IL13Rα2-CAR.CD28.ζ T Cells (CAR T cells)17 would further potentiate their therapeutic efficacy. Additionally, given that (1) IL15 action requires transpresentation through IL15Rα expressed predominately on myeloid cells, (2) the low bioactivity of the single-chain IL15 in biological systems,33 34 and (3) our multiplex staining of tissue sections from GBM patients (N=25) showing that~20% (mean=19.39, 95% CI: 12.39 to 26.39) of glioma cells (SOX2+) are in direct contact with macrophages (CD163+) (online supplemental figure 3A, B), we hypothesized that fusing IL15 to the scFv portion of CAR may allow for better bioavailability to direct interactions with both IL13Rα2-glioma cells and IL15Rα-expressing MDSCs (figure 3B). To test both hypotheses, we generated the following retroviral vectors for subsequent T cell transduction as previously described17: (1) a control vector encoding a non-functional CAR (CAR.Δ), (2) a functional CAR, (3) a CAR encoding cDNA with a secretable IL15 (CAR.IL15s), and (4) a CAR cDNA, where IL15 fused to the N-terminal part of the heavy chain of the scFv IL13Rα2 (clone 47) via (Gly4S)3 flexible linker (CAR.IL15f) (figure 3A). The AlphaFold2 modeling of scFv47, an antigen-binding portion of CAR T cells, versus IL15 fusion with the scFv47 antibody, shows that the IL15-scFv fusion could be modeled with a high confidence (figure 3C).
Figure 3.
CAR T cells modified to express IL15 successfully kill glioma cells in vitro. (A) CAR.Δ, CAR, CAR.IL15s and CAR.IL15f murine constructs were designed as described in the Materials and Methods section. (B) Schematic diagram shows potential interaction of CAR.IL15s and CAR.IL15f with glioma and cells of the tumor microenvironment. (C) Modeling of antigen-binding part of CAR protein, a scFvIL13Rα2 (clone 47) alone and as a fusion with IL15. (D) Flow cytometry analysis to quantify CAR T cell transduction efficiency using a fluorochrome congjugated antibody against Thy1.1 (n=3, **p<0.01, ***p<0.001). (E) Trypan blue exclusion assay was perfromed to determine viability of CAR, CAR.IL15s, and CAR.IL15f T cells. Values presented as % change in viability over 5 days postactivation. (F) Flow cytometry analysis of IL15 expression on the surface of CD8+Thy1.1+ CAR T cells (n=3, ***p<0.001), and analysis of the CAR T cells for their memory phenotype after vital transduction following 2 days of rest after retroviral transduction in vitro (n=3, *p<0.05). (G) Analysis of the viability of parental and IL13Rα2-expressing GL261 and CT2A cells in coculture with CAR T cells. Non-transduced (NT) T cells were used as a negative control (n=3, ***p<0.001). Viability values in each group were normalized to NT cells. The experiments was repeated two times. CAR, chimeric antigen receptor.
The transduction efficiency of CD4+ and CD8+ T cells was similar across of all constructs (figure 3D), and there was no difference in viability and CAR T cell expansion (figure 3E, online supplemental figure 3C). Following transduction, flow cytometry analysis showed no difference in surface expression of IL15 between NT, conventional CAR (CAR), or CAR.IL15s T cells, but a significant increase in surface IL15 expression was seen in CAR.IL15f T cells (figure 3F). Expression of T cell memory markers was also significantly higher in CAR.IL15f T cells compared with NT, CAR, and CAR.IL15s T cells (figure 3F). To test the functionality of all constructs, we cocultured NT, CAR, CAR.IL15s, and CAR.IL15f T cells with either parental or IL13RA2 expressing CT2A and GL261 glioma cells. All three constructs displayed cytotoxic capacity against glioma cells in an antigen-dependent fashion, with the highest activity seen in CAR.IL15f T cells (figure 3G).
CAR.IL15f T cells mediate the killing of MDSC cells and modulate their immunosuppressive phenotype
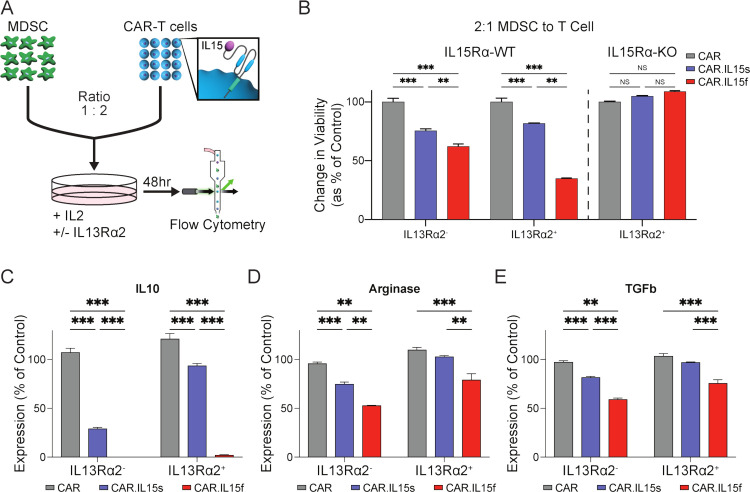
To directly compare the functional activity of CAR.IL15s and CAR.IL15f against MDSCs, CAR T cells were cocultured at a 2:1 ratio of CAR T cells to MDSCs for 48 hours (figure 4A). Flow cytometry analysis showed a greater loss in the viability of MDSCs cocultured with CAR.IL15f in comparison to CAR.IL15s and conventional CAR T cells. This effect was fully abrogated in cocultures with MDSCs derived from IL15Rα KO mice (figure 4B). Additionally, decreased viability of MDSC was more pronounced when CAR T cells are stimulated with IL13Rα2. The analysis of MDSC for the production of IL10, Arginase 1 (Arg1), and TGF-β revealed significant decreased levels of these immunosuppressive molecules in coculture with both CAR.IL15s and CAR.IL15f (figure 4C–E). In a similar fashion, this effect was also more pronounced when CAR T cells were activated with IL13Rα2. Thus, our data show that IL15-modified CAR T cells exert a direct cytotoxic effect on MDSCs and modulate the immunosuppressive function of MDSCs mediated by IL15/IL15Rα interaction with CAR.IL15f is being superior to IL13Rα2-CAR.IL15s in these actions.
Figure 4.
CAR T cells modified to express IL15 kill MDSC and modulate their immunosuppressive phenotype. (A) Experimental setup of cocultures of bone-marrow-derived MDSC and CAR T cells in vitro. (B) Comparison of cytotoxic activity of CAR T cells towards MDSC (n=3, *p<0.05, ***p<0.001). (C–E) Flow cytometry analysis of MDSC from cocultures with CAR T cells for production of IL10, arginase, and TGFβ (n=3, **p<0.01, ***p<0.001). The experiments was repeated two times. CAR, chimeric antigen receptor; MDSC, myeloid-derived suppressor cell.
The ability of CAR T cells modified with IL15 to modulate the TME was further confirmed by bulk RNAseq analysis of immune cells (CD45+) harvested from GL261 tumors of mice treated CAR T cells (PRJNA908873; online supplemental figure 4A). Violin plot shows the top differentially expressed genes (DEG) between CAR.IL15s compared with conventional CAR T cells and PBS (online supplemental figure 4B). Gene ontology (GO) analysis of DEGs revealed the most significant changes are related to chemokine-mediated signaling pathways, response to chemokines, and leukocytes’ migration (online supplemental figure 4C). Consistent with in vitro data, the downregulation of genes associated with an immunosuppressive function of tumor-associated macrophages was observed in tumors from mice treated with CAR.IL15s (online supplemental figure 4D). Collectively, these data show that IL15-armored CAR T cells have the ability to either modulate the immunosuppressive function of MDSCs or deplete them from the TME.
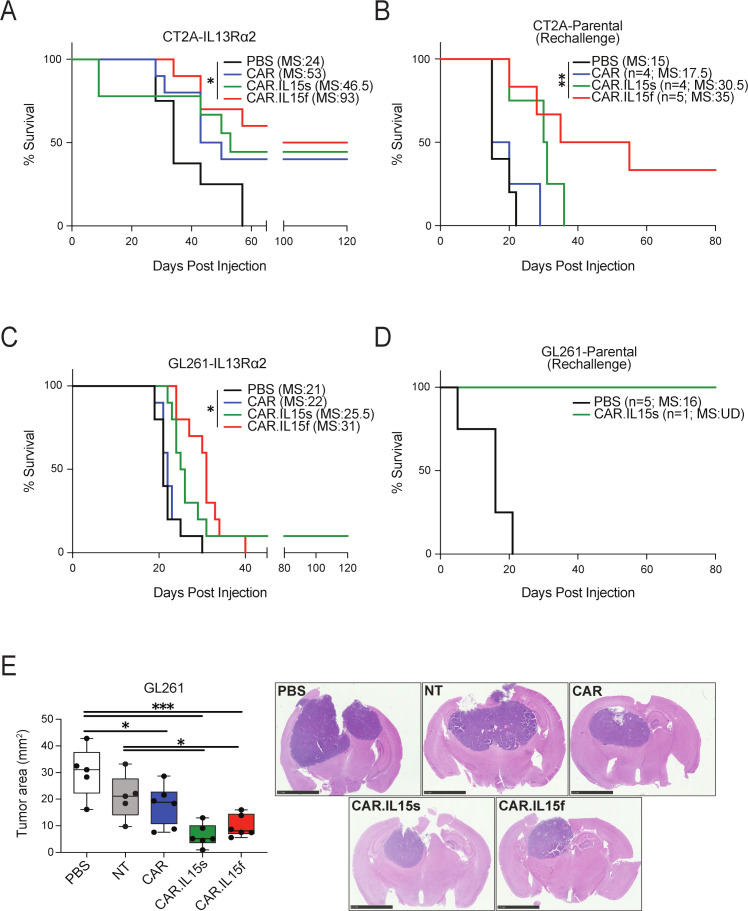
IL15-modified CAR T cells improve the survival of mice in two syngeneic murine models of GBM
We next investigated the therapeutic properties of IL15-modified CAR T cells in comparison to conventional CAR T cells in vivo using syngeneic murine models of GBM. Mice bearing CT2A or GL261 orthotopic tumors expressing IL13Rα2 were treated via direct i.t. injection with 1×106 CAR T cells. In the CT2A model, mice treated with CAR.IL15f had a superior median survival of 93 days in comparison to 46.5 days in CAR.IL15s, 53 days in CAR, and 34 days in PBS group (n=10, *p<0.05) (figure 5A). Long-term surviving mice from each group were rechallenged with parental CT2A cells; age-matched mice served as the control. On the rechallenge, the mice from CAR.IL15f group showed superior survival over mice treated with CAR.IL15s T cells or unmodified CAR T cells groups (figure 5B). Histological analysis of brain tissue confirmed a complete tumor regression, with CD8+ T cells present at the site of injection and in other areas of the brain (online supplemental figure 5A). In GL261 models, the treatment of IL15-modified CAR T cells resulted in improved although modest survival in GL261 model, with better survival seen in mice treated with CAR.IL15f compared with all other groups (figure 5C). The long-term surviving animal was also resistant to rechallenge with parental GL261 line (figure 5D). Histological analysis of tissue showed no tumor in the brain of the rechallenged animal (online supplemental figure 5B). To better understand the survival analysis, we measured the area of tumors in all experimental groups of GL261 model (figure 5E, F). Mice treated with IL15-modified CAR T cells had 2–3 times smaller tumors than tumors in CAR or non-treated control groups. Collectively, these experiments show the IL15-modified CAR T cells provide survival benefit for tumor-bearing animals and further confirm that IL15-modification enhances the functionality and therapeutic efficacy of CAR T cells. Histopathological examination of the tissues showed no evidence of focal or diffuse meningoencephalitis, demyelination, neuronal dropout, infarction, or vasculitis.
Figure 5.
CAR-IL15f T cells are superior to CAR.IL15s and conventional CAR T in mediating survival of mice in syngeneic models of glioma. (A) Survival analysis of mice bearing CT2A-IL13Rα2 glioma and treated with 1×106 of CAR T cells (n=10, *p<0.05). (B) Mice surviving long-term were rechallenged with parental CT2A line (**p<0.01). (C) Survival analysis of mice bearing GL261-IL13Rα2 glioma and treated with 1×106 of CAR T cells (n=10, *p<0.05). (D) Mice surviving long-term were rechallenged with parental Gl261 line. (E) Morphometric analysis of tumor area in the brain of mice bearing GL261-IL13Rα2 glioma tumors 10 days after the treatments of CAR T cells (n=5/group, *p<0.05, *** p<0.001), and representative images of H&E stain of the brain of mice after CAR T cell treatment. CAR, myeloid-derived suppressor cell; NT, non-transduced.
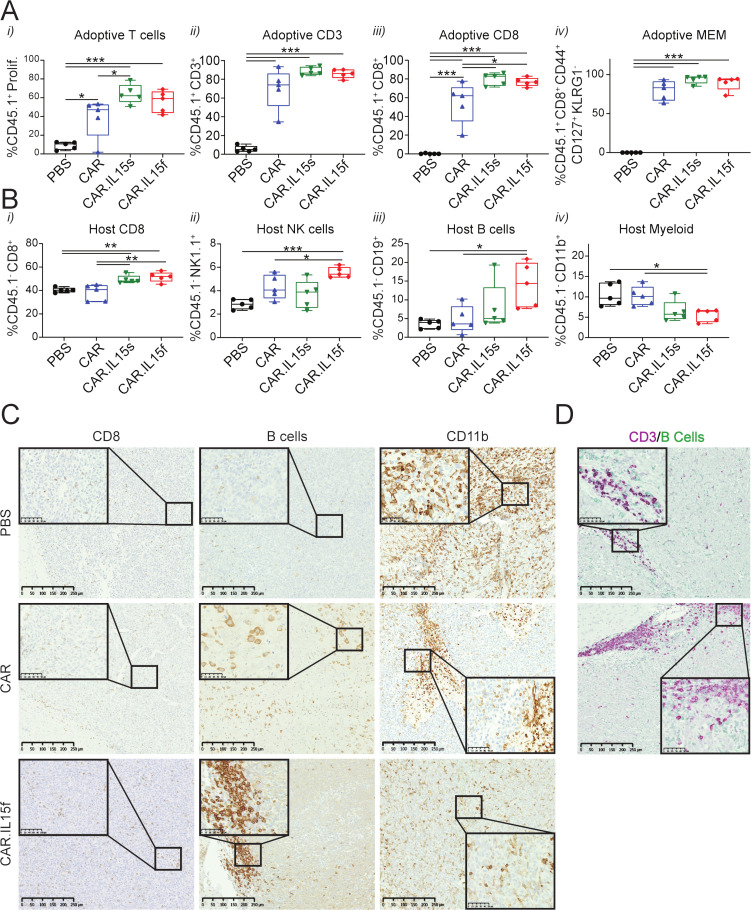
IL15-modified CAR T cells modulate the TME by diminishing the myeloid compartment and increasing frequencies of the host’s CD8+, NK, and B cells
To gain further insights into the therapeutic properties of IL15-modified CAR T cells, we treated GL261-bearing mice with CAR T cells, IL15-modified CAR T cells, or PBS, and analyzed the TME of animals 7 days post-treatment. To distinguish between host and adoptive T cells, we used CD45.1 congenital mice to generate CAR T cells and adoptively transferred them into CD45.2 mice. The data showed (1) increased adoptive T cell proliferation, and higher frequency of (2) CD3+, (3) CD8+ in the TME of mice treated with CAR.IL15s and CAR.IL15f T cells compared with CAR T cell and PBS groups (figure 6A). No difference in (4) memory phenotype of adoptively transferred cells was detected between groups (figure 6A). Analyzing the host cells of the TME, we observed increased (1) CD8+ T cells, (2) NK, and (3) B cells 7 days post-CAR T cells treatments compared with PBS, with CAR.IL15f T cell group being superior to the other CAR T cell group (figure 6B). We also observed a twofold decrease in the frequency of (4) myeloid cells (CD45+ and CD11b+) when treated with CAR.IL15s and CAR.IL15f (figure 6B). Histological analysis of tissues revealed CD8+ T cells were distributed throughout the tumor, and an increased presence of B cells within tumors treated with CAR.IL15f (figure 6C). Moreover, dual staining of tumors showed colocalization of B and CD3+T cells within the brains of CAR.Il15f-treated mice (figure 6D). Staining for CD11b showed lower intensity within tumors treated with CAR.IL15f compared with all other groups (figure 6C). To assess the distribution in extracranial sites, we assessed the immune compartment in spleens of mice treated in all groups. Small changes in the frequency of CD8+ T cells, NK, B, and CD11b+ cells were observed in the spleen (online supplemental figure 6), suggesting that the local treatment with CAR T cells, to a lesser degree, may also influence peripheral immune compartments.
Figure 6.
IL15-modified CAR T cells alter the tumor microenvironment in murine glioma. (A) (i) Frequency of adoptively transferred proliferating (CTV-labeled) CAR T cells in the brain of mice-bearing GL261-IL13Rα2 tumors 7 days after CAR T cells treatment, (ii–iii) CD3+, CD8+ T cells, and (iv) CD8+ T cells expressing markers of adoptive memory. (B) Frequency of host (i) CD45+CD8+ T cells, (ii) NK cells, (iii) B cells, and (iv) CD11b+ TAMs in the brain of mice bearing GL261-IL13Rα2 tumors 7 days after CAR T cells treatment. * p <0.05, ** p<0.01, *** p<0.001. (C) Immunostaining of tumor tissue sections for CD8+ T cells, B cells, and CD11b+ cells in PBS, CAR, and CAR.IL15f groups. (D) Double stain for B cells and CD3+ T cells in the area of B cells’ accumulation on the tumor/brain border. CAR, chimeric antigen receptor; PBS, phosphate buffered saline.
Discussion
Here, we describe that MDSC in the TME of human and murine GBMs express IL15Rα. To target glioma cells and MDSC, we generated murine T cells expressing IL13Rα2-CARs and secretory IL15 (CAR.IL15s) or IL13Rα2-CARs in which IL15 was fused to the CAR to serve as an IL15Rα-targeting moiety (CAR.IL15f). We demonstrate that CAR.IL15s and CAR.IL15f T cells (1) deplete MDSCs and (2) decrease their secretion of immunosuppressive molecules in vitro with CAR.IL15f T cells being more efficacious. In vivo, CAR.IL15f T cells reversed the immunosuppressive TME to a greater extend than CAR or CAR.IL15s T cells, resulting in improved survival in two glioma models.
We and other investigators have demonstrated that transgenic expression of IL15 improves the effector function of CAR T cells against solid tumors, including glioma.15 24 35 However, it was unclear if IL15Rα is expressed on myeloid cells, which comprise up to 30%–50% of the GBM tumor mass,36 and can be exploited as an immunotherapeutic target. Taking advantage of the TCGA data set we demonstrated that IL15Rα is expressed in glioma and that expression correlates with histological grade. IL15Rα expression colocalized with CD163 expression, a marker of macrophages, as judged by IHC analyses. Further, analyses of paired peripheral blood and tumor-infiltrating cells of GBM patient samples revealed significantly higher IL15Rα expression in immune cells of brain tumors compared with those of peripheral blood. Since the myeloid compartment of GBMs consists predominantely of MDSCs, these data suggested that IL15Rα is expressed by MDSCs. IL15Rα expression by MDSCs was confirmed in two (GL261, CT2A) murine glioma models. In addition, higher IL15Rα expression in myeloid cells of the TME compared with the peripheral compartment was also observed in murine models, mirrowing our findings in humans. To our knowledge, this is the first study that demonstrates preferential expression of IL15Rα in myeloid cells or the GBM TME, providing the impetus to conduct mechanistic studies in the future.
To explore if IL15Rα expression could be leveraged therapeutically to enhance the antiglioma activity of IL13Rα2-CAR T cells we generated two CAR T cell effector population. One expressing secretory IL15 (CAR.IL15s) and one in which IL15 was directly fused to the IL13Rα2-CAR (CAR.IL15f T cells). Of interest, we did not observe fractercide of CAR.IL15f T cells. Studies have indicated that spacer length between the ligand and cell membrane modulates fractercids, with longer spacers being protective.37 Since in our CAR.IL15f design, the scFv of the CAR is between the IL15 molecule and the cell membrane, creating a significant distance, which likely protects from fratricide.
We compared the ability of CAR, CAR.IL15s, and CAR.IL15f T cells to reverse the immunosuppressive effects of MDSCs in coculture assays in vitro. While both IL15-modified CAR T cell populations were able to decrease MDSC-mediated immunosuppression as judged by downregulating the expression of IL10, Arginase-1, and TGF-β, CAR.IL15f T cells were more effective in comparison to CAR.IL15s T cells. Indeed, CAR.IL15f T cells also reduced MDSC viability, most likely through direct cytoxitic effects. In vivo, both IL15-modified CAR T cell populations extended the survival of animals bearing GL261 and CT2A gliomas, again with CAR.IL15f T cells being more efficacious. Neither CAR.IL15s or CAR.IL15f T cells induced toxicities post i.t. injection in our glioma models.
To better understand the differences in survival benefits between our CAR T cell populations, we characterized the immune compartment within the TME post therapy. In agreement with our in vitro studies, we observed a reduction of MDSC frequency in mice treated with IL15-modified CAR T cells, with CAR.IL15f T cells having slightly greater anti-MDSC activity, further confirming the dual targeting ability of our designed CAR T cells. Bayik et al have recently demonstrated that different subset of MDSC can drive immunosuppression in glioma tumors in a sex dependent manner.38 Whether response of different subsets of MDSC to CAR T cells therapy depends on animal’s sex will have to be determined in a future studies. B and NK cells, are normally found at low frequencies within the GBM TME.39 The presence of tertiary-like lymphoid structures and activated B cells within tumors has previously been established as positive predictors of patient survival in several types of cancer,40–42 and recruitment of NK cells has been reported to correlate with a better prognosis in gliomas.43 44 Although we did not fully characterize tertiary-like lymphoid structures in the brains of mice, we observed a colocalization of B and CD3+ T cells, and an increase in B and NK cells frequency following IL15-modified CAR T cell therapy. Given the increased survival post rechallenge with parental glioma cell lines that do not express IL13Rα2, it is possible that IL15-modified CAR T cells aid in the induction of endogenous immune responses to promote antigen spreading. Other investigators have explored if targeting TAMs with CAR T cells redirected to CD123 or FRβ improves CAR T cell efficacy in preclincial lymphoma or ovarian cancer models.45 46 Only one of these studies was conducted in an immune-competent animal model; timing was critical, and FRβ-CAR T cells only improved the antitumor activity of tumor-specific CAR T cells if there were given prior to their infusion. In our approach, we targeted tumor cells and MDSCs simultaneously, and based on the aforementioned study, exploring a multiple-dosing regimen might be advisable in the future.
To our knowledge, our study is the first to analyze the effects of IL15-modified CAR T cells in immunocompetent glioma models. Our detailed TME analysis highlights the benefit of these models over xenograft models that lack a functional immune system. While IL15-modified CAR T cells had superior antitumor activity, tumor free long-term survival was limited in particular in the GL261 model. This suggests that giving multiple doses of CAR T cells like in clinical studies,8 47 48 or additional genetic modification will be necessary to improve antitumor activity, including targeting multiple antigens expressed on glioma cells49–52 or deleting negative regulators in T cells.53–56
In conclusion, our study shows that modifying CAR T cells with IL15 improves their therapeutic properties. Furthermore, we show that the fusion of IL15 to the antibody part of CAR T cells generates a dual targeting system that not only diminishes the frequency MDSC and glioma cells, but also modulates their immunosuppressive phenotype. Most importantly, our studies open the opportunity for investigating other targeting moieties on the surface of MDSCs, specifically those enriched in cells of GBM TME, and applying these modifications to CAR T cells for their direct dual functions against glioma cells and immunosuppressive MDSC.
jitc-2022-006239supp003.pdf (1.5MB, pdf)
Footnotes
Twitter: @CatyLeechang, @jasonmiska1
Contributors: MZ, JTD, IVB designed the experiments. MZ, JTD, RNL, MS, QL, CJC, CMH, CL-C, JM, AP, VAA, generated data, developed protocols, and performed experiments. MZ and JTD performed statistical analyses. JTD and IVB wrote the manuscript. AMS, ML, SG, JM, CL-C contributed to the discussion of the data and revisions. IVB acts as guarantor of the study. All authors reviewed, edited, and commented on the manuscript.
Funding: The study is supported by the National Institute of Neurological Disorders and Stroke R01NS106379 (IVB) and R01NS122395 (IVB) grants, and the Translational Bridge Award funded by the Robert H. Lurie Comprehensive Cancer Center (IVB). The authors thank the Flow Cytometry Core Facility, Mouse Histology and Phenotyping, and Pathology Core supported by National Cancer Institute grant P30-CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. The authors are also grateful to patients donating their tissues for the data analysis through the Nervous System Tumor Bank, the Department of Neurosurgery, Northwestern University.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests: IVB, ML and SG are coinventors of a patent application for IL13Ralpha2-CAR T cells. IVB is an inventor on patent apllication pertaining to modification of IL13Ralpha2-CAR T cells with IL15. SG is a coinventor on patent applications in the fields of cell or gene therapy for cancer; he is a consultant of TESSA Therapeutics, a member of the Data and Safety Monitoring Board (DSMB) of Immatics, and has received honoraria from Tidal, Catamaran Bio, Sanofi, and Novartis within the last 2 years.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as online supplemental information. Raw Sequencing files deposited into the NIH Sequencing Read Archive under accession numbers PRJNA908873 and PRJNA909275.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Patient samples were collected under the auspices of a Northwestern University IRB-approved protocol # STU00095863. Participants gave informed consent to participate in the study before taking part.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021;384:705–16. 10.1056/NEJMoa2024850 [DOI] [PubMed] [Google Scholar]
- 5.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ErbB2. Mol Ther 2010;18:843–51. 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magee MS, Abraham TS, Baybutt TR, et al. Human GUCY2C-targeted chimeric antigen receptor (CAR) -expressing T cells eliminate colorectal cancer metastases. Cancer Immunol Res 2018;6:509–16. 10.1158/2326-6066.CIR-16-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused egfrviii-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9:eaaa0984. 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016;375:2561–9. 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Brawley V, Hegde M, et al. Her2-Specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 2017;3:1094–101. 10.1001/jamaoncol.2017.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016;19:20–7. 10.1038/nn.4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi VM, Neidert N, Will P, et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat Commun 2022;13:925. 10.1038/s41467-022-28523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res 2011;17:4296–308. 10.1158/1078-0432.CCR-10-2557 [DOI] [PubMed] [Google Scholar]
- 13.Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020;577:689–94. 10.1038/s41586-019-1912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krenciute G, Krebs S, Torres D, et al. Characterization and functional analysis of scfv-based chimeric antigen receptors to redirect T cells to il13rα2-positive glioma. Mol Ther 2016;24:354–63. 10.1038/mt.2015.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krenciute G, Prinzing BL, Yi Z, et al. Transgenic expression of IL15 improves antiglioma activity of il13rα2-CAR T cells but results in antigen loss variants. Cancer Immunol Res 2017;5:571–81. 10.1158/2326-6066.CIR-16-0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CE, Aguilar B, Starr R, et al. Optimization of il13rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther 2018;26:31–44. 10.1016/j.ymthe.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pituch KC, Miska J, Krenciute G, et al. Adoptive transfer of il13rα2-specific chimeric antigen receptor T cells creates a pro-inflammatory environment in glioblastoma. Mol Ther 2018;26:986–95. 10.1016/j.ymthe.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmielewski M, Abken H. Car T cells releasing IL-18 convert to T-bethigh foxo1low effectors that exhibit augmented activity against advanced solid tumors. Cell Reports 2017;21:3205–19. 10.1016/j.celrep.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 19.Chmielewski M, Kopecky C, Hombach AA, et al. Il-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–706. 10.1158/0008-5472.CAN-11-0103 [DOI] [PubMed] [Google Scholar]
- 20.Shum T, Omer B, Tashiro H, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov 2017;7:1238–47. 10.1158/2159-8290.CD-17-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z, Li Y, Liu W, et al. Engineered IL-7 receptor enhances the therapeutic effect of AXL-CAR-T cells on triple-negative breast cancer. Biomed Res Int 2020;2020:4795171. 10.1155/2020/4795171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagoya Y, Tanaka S, Guo T, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med 2018;24:352–9. 10.1038/nm.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanitis E, Rota G, Kosti P, et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J Exp Med 2021;218:e20192203. 10.1084/jem.20192203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurton LV, Singh H, Najjar AM, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A 2016;113:E7788–97. 10.1073/pnas.1610544113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Xu S, Xin Y, et al. Tumor-Associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol 2012;189:444–53. 10.4049/jimmunol.1103248 [DOI] [PubMed] [Google Scholar]
- 26.Pinton L, Masetto E, Vettore M, et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J Immunother Cancer 2019;7:58. 10.1186/s40425-019-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirifu M, Hayashi C, Nakamura T, et al. Crystal structure of the IL-15-IL-15ralpha complex, a cytokine-receptor unit presented in trans. Nat Immunol 2007;8:1001–7. 10.1038/ni1492 [DOI] [PubMed] [Google Scholar]
- 28.Dubois S, Mariner J, Waldmann TA, et al. Il-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity 2002;17:537–47. 10.1016/s1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- 29.Lisi L, Ciotti GMP, Braun D, et al. Expression of inos, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett 2017;645:106–12. 10.1016/j.neulet.2017.02.076 [DOI] [PubMed] [Google Scholar]
- 30.Friebel E, Kapolou K, Unger S, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 2020;181:1626–42. 10.1016/j.cell.2020.04.055 [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–86. 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 32.Miska J, Rashidi A, Lee-Chang C, et al. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv 2021;7:eabc8929. 10.1126/sciadv.abc8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergamaschi C, Bear J, Rosati M, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15rα in human and mouse serum. Blood 2012;120:e1–8. 10.1182/blood-2011-10-384362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chertova E, Bergamaschi C, Chertov O, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer. J Biol Chem 2013;288:18093–103. 10.1074/jbc.M113.461756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batra SA, Rathi P, Guo L, et al. Glypican-3-specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res 2020;8:309–20. 10.1158/2326-6066.CIR-19-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Leo A, Ugolini A, Veglia F. Myeloid cells in glioblastoma microenvironment. Cells 2020;10:18. 10.3390/cells10010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer T, Parikh K, Sharma S, et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood 2021;138:318–30. 10.1182/blood.2020008221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayik D, Zhou Y, Park C, et al. Myeloid-Derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov 2020;10:1210–25. 10.1158/2159-8290.CD-19-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee-Chang C, Rashidi A, Miska J, et al. Myeloid-Derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res 2019;7:1928–43. 10.1158/2326-6066.CIR-19-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edin S, Kaprio T, Hagström J, et al. The prognostic importance of CD20+ B lymphocytes in colorectal cancer and the relation to other immune cell subsets. Sci Rep 2019;9:19997. 10.1038/s41598-019-56441-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Yin X, Wang Q, et al. A novel gene expression signature-based on B-cell proportion to predict prognosis of patients with lung adenocarcinoma. BMC Cancer 2021;21:1098. 10.1186/s12885-021-08805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012;18:3281–92. 10.1158/1078-0432.CCR-12-0234 [DOI] [PubMed] [Google Scholar]
- 43.Ren F, Zhao Q, Huang L, et al. The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunol Cell Biol 2019;97:457–69. 10.1111/imcb.12225 [DOI] [PubMed] [Google Scholar]
- 44.Vauléon E, Tony A, Hamlat A, et al. Immune genes are associated with human glioblastoma pathology and patient survival. BMC Med Genomics 2012;5:41. 10.1186/1755-8794-5-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Garcia A, Lynn RC, Poussin M, et al. Car-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun 2021;12:877. 10.1038/s41467-021-20893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruella M, Klichinsky M, Kenderian SS, et al. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov 2017;7:1154–67. 10.1158/2159-8290.CD-16-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of il13rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res 2015;21:4062–72. 10.1158/1078-0432.CCR-15-0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitanza NA, Johnson AJ, Wilson AL, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med 2021;27:1544–52. 10.1038/s41591-021-01404-8 [DOI] [PubMed] [Google Scholar]
- 49.Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and il13rα2 mitigate tumor antigen escape. J Clin Invest 2016;126:3036–52. 10.1172/JCI83416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol 2018;20:506–18. 10.1093/neuonc/nox182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi BD, Yu X, Castano AP, et al. Car-T cells secreting bites circumvent antigen escape without detectable toxicity. Nat Biotechnol 2019;37:1049–58. 10.1038/s41587-019-0192-1 [DOI] [PubMed] [Google Scholar]
- 52.Yin Y, Rodriguez JL, Li N, et al. Locally secreted bites complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther 2022;30:2537–53. 10.1016/j.ymthe.2022.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu B, Zou Y, Zhang L, et al. Nucleofection with plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in CD133-specific CAR T cells. Hum Gene Ther 2019;30:446–58. 10.1089/hum.2017.234 [DOI] [PubMed] [Google Scholar]
- 54.Prinzing B, Zebley CC, Petersen CT, et al. Deleting Dnmt3a in car T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med 2021;13:eabh0272. 10.1126/scitranslmed.abh0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Zhang X, Cheng C, et al. Crispr-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med 2017;11:554–62. 10.1007/s11684-017-0543-6 [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Shi L, Zhao Z, et al. Disruption of CTLA-4 expression on peripheral blood CD8 + T cell enhances anti-tumor efficacy in bladder cancer. Cancer Chemother Pharmacol 2019;83:911–20. 10.1007/s00280-019-03800-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006239supp001.pdf (184.9KB, pdf)
jitc-2022-006239supp002.pdf (10.4MB, pdf)
jitc-2022-006239supp003.pdf (1.5MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as online supplemental information. Raw Sequencing files deposited into the NIH Sequencing Read Archive under accession numbers PRJNA908873 and PRJNA909275.