Highlights
-
•
Melanocortin-4 receptor functions as a vital pharmaceutical target to maintain controllable appetite and body weight for professional athletes.
-
•
Exploration of the peripheral actions of melanocortin-4 receptor in the enteroendocrine and cardiovascular system.
-
•
Direct central–local communications of hypothalamic melanocortin 4 receptor signaling with bone-derived Lipocalin-2.
-
•
Melanocortin-4 receptor signaling is required for proper limb regeneration in the Xenopus model system.
Keywords: Body weight, Energy homeostasis, GPCR, Melanocortin-4 receptor
Abstract
Melanocortin 4 receptor (MC4R), the most important monogenetic cause of human metabolic disorders, has been of great interest to many researchers in the field of energy homeostasis and public health. Because MC4R is a vital pharmaceutical target for maintaining controllable appetite and body weight for professional athletes, previous studies have mainly focused on the central, rather than the peripheral, roles of MC4R. Thus, the local expression of MC4R and its behavioral regulation remain unclear. In an attempt to shed light on different directions for future studies of MC4R signaling, we review a series of recent and important studies exploring the peripheral functions of MC4R and the direct physiological interaction between peripheral organs and central MC4R neurons in this article.
Graphical Abstract
1. Introduction
In the past 3 decades, increasing numbers of obese patients live in the shadow of diabetes,1, 2, 3 depression,1,4, 5, 6 cancer7,8 and cardiovascular disease.2,4 Melanocortin 4 receptor (MC4R), the most famous monogenetic cause of human obesity,9 contributes to more than 5% of all types of early-onset obesity reported in childhood patients.10 In the face of a severe obesity epidemic and obese patients with MC4R deficiency, it is urgent to understand the physiological challenges that occur throughout the whole body. Previous studies have found that MC4R mRNA is expressed in several areas within the central nervous system (CNS), including the brainstem, dorsal motor nucleus of the vagus, nucleus tractus solitarius, and parabrachial nucleus.11, 12, 13, 14 MC4R is co-expressed with the melanocortin receptor accessory protein 2 , a known accessory protein in vivo,15, 16, 17, 18 and is pharmacologically regulated by the unique endogenous agonist α-melanocyte-stimulating hormone (α-MSH)19 and other antagonists, such as agouti-related protein (AgRP),20,21 that control energy expenditure and food intake. Recently, with the aid of more advanced approaches, several new features of MC4R have been explored and elucidated. One study found that Kir7.1, a potassium channel, was specifically coupled to MC4R in the hypothalamus.22 Recently, Yu et al.23 resolved the 3-dimensional conformation of the cyclic peptide SHU9119-bound human MC4R complex (a potent MC4R antagonist) by crystallization, which revealed the functional participation of divalent calcium for the ligand binding and pharmacological modulation of MC4R signaling.
Due to the complicated nature of metabolic regulation and neuronal networks, it seems that a point of saturation has now been reached for the exploration of the functions of MC4R in the CNS. However, MC4R expression outside of the brain region has recently attracted much attention. Many encouraging discoveries have emerged showing that peripheral MC4R expression and communication greatly contribute to the energy balance of the whole body. The pharmacology and physiology of MC4R (e.g., its impact on the reproductive system) have been thoroughly discussed in another review article.24 Instead, in this paper we primarily focus on the crucial findings related to MC4R signaling outside the CNS and its direct central regulation by novel polypeptide ligands generated from peripheral organs.
2. Exploration of the peripheral roles of MC4R signaling
2.1. MC4R in the enteroendocrine system
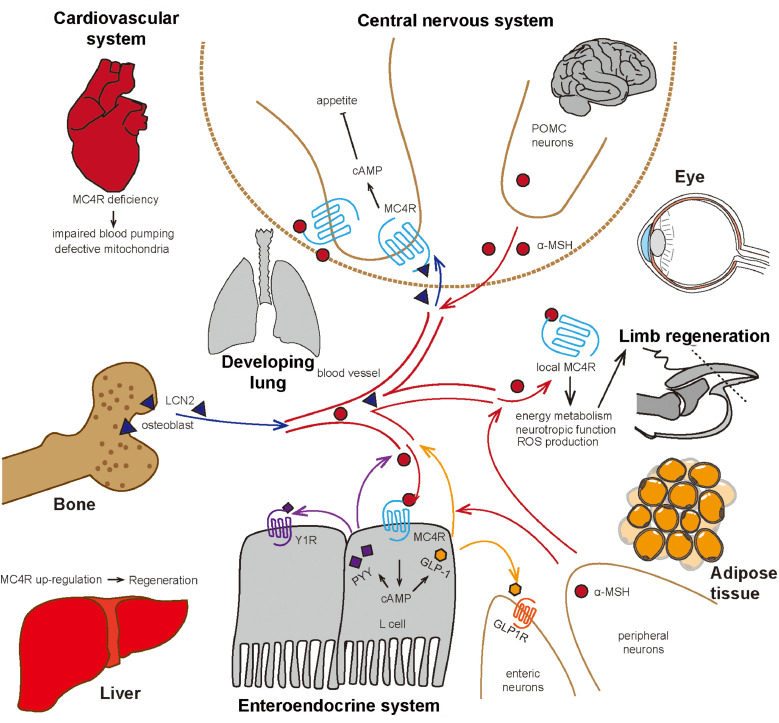
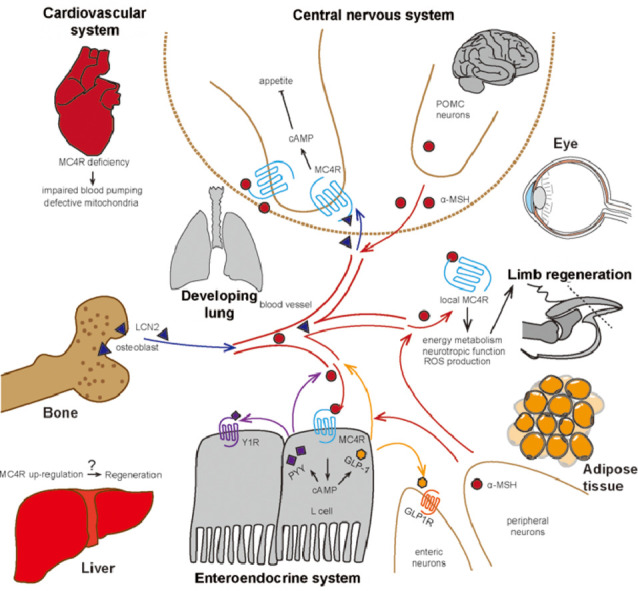
Previous studies on MC4R have mainly focused on the CNS and its effects on appetite and feeding behaviors. Recently, there has been evidence to suggest that MC4R is expressed in and regulates the gastrointestinal system. Using a localization experiment, researchers have revealed that MC4R expression is highest in the dorsal motor nucleus of vagus,11 where preganglionic parasympathetic vagal efferent nerves regulate gastrointestinal function. MC4R positive cells in the nodose ganglion of the nervi vagus also innervate the stomach and duodenum to regulate postprandial functions.25 Gastric contractions decrease when Melanotan II (MTII) and α-MSH are delivered into the dorsal motor nucleus of vagus or solitary tract.26 Gastric bypass surgery has verified that MC4R signaling is required in order for sustained effects on weight loss and physiological mechanisms of action to take place,27 yet it seems to not be required for a short-term response.28 Furthermore, MC4R has also been found to be highly expressed in gastric ghrelin-positive cells.29 Although they focus on the CNS, these findings confirm the role of MC4R in regulating the anabolic enteroendocrine system, suggesting that MC4R expression in local digestive organs may directly affect gastric cells.
In their pioneering work, Panaro et al.30 identified a previously unrecognized peripheral role for the MC4R cascade in these gastric cells. This expanded research into MC4R began a new era of inquiry that focused on the peripheral and localized functions of MC4R and the physiological interactions associated with peripheral-central MC4R. Using gene expression analysis29 and fluorescence-assisted cell sorting followed by real-time polymerase chain reaction, they found that MC4R mRNA was enriched in glucagon-like peptide 1 (GLP-1) positive enteroendocrine cells nearly 430-fold compared to no GLP-1 cells. 30 Using MC4R-Sapphire transgenic mice, Liu et al.31 found that MC4R was co-expressed with GLP-1 and peptide YY (PYY) in L cells of the colon. To further examine the potential function of MC4R in L cells, short-circuit current (SCC) changes, mostly induced by Cl– secretion, were measured in the intestinal mucosae of MC4R+/+ or MC4R−/− mice after stimulation by α-MSH on the apical or basolateral sides of L cells. The results showed that basolateral α-MSH administration could induce a sharp depolarization of SCC in MC4R+/+ mice only, suggesting that MC4R in the basolateral side of L cells regulated Cl– secretion. BIBO 3304 trifluoroacetate, a neuropeptide Y Y1 receptor antagonist, or the replacement of glucose with mannitol, blocked α-MSH's action on MC4R in L cells, indicating that MC4R function may be mediated by Neuropeptide Y Y1 receptor (Y1R) and glucose signaling. Finally, they found a concentration-dependent upregulation of PYY and GLP-1 only in the plasma of MC4R+/+ mice after intraperitoneal (i.p.) administration of LY2112688 and other MC4R agonists, which could be prevented by co-injection of SHU9119. Thus, a novel role of MC4R, independent from central melanocortin circuits, was uncovered in peripheral energy metabolism.30, 31–32
This work established a link among the gut microbiota, energy metabolism and the endocrine regulation of satiety. Additional studies on the Escherichia coli chaperone protein caseinolytic protease B have suggested that an increase in circulating GLP-1 and PYY can suppress food intake.33 Additionally, caseinolytic protease B may directly activate MC4R expression in gut cells because it has antigen mimetic properties related to α-MSH.34 However, because these studies have been confined to a separate function independent of the gut or brain, further analysis, such as tissue-specific knock-out of MC4R or its related peptides, should be performed in order to elucidate whether a causal relationship exists. Pharmacological intervention may also be an effective biomedical approach for modulating the gut metabolic function against obesity and diabetes via MC4R signaling in the enteroendocrine system (Fig. 1).
Fig. 1.
Schematic illustration of the peripheral actions and direct central–local communications of melanocortin 4 receptor signaling. α-MSH = α-melanocyte-stimulating hormones; cAMP = cyclic adenosine monophosphate; GLP-1 = glucagon-like peptide1; GLP1R = glucagon-like peptide-1 receptor; LCN2 = Lipocalin 2; MC4R = melanocortin 4 receptor; POMC = pro-opiomelanocortin; PYY = peptide YY; Y1R = Neuropeptide Y Y1 receptor; ROS = reactive oxygen species.
2.2. Lipocalin 2, a peripheral-derived novel MC4R agonist
Bone is traditionally defined as a unique multifunctional organ essential to normal action triggered by muscle or to the protection of internal tissues against physical injury.35 Bone also plays an important role in controlling the homeostasis of several minerals, including calcium and phosphate.36 Using symmetric cellular analysis, it found that bone not only possessed hematopoietic properties but also secreted a variety of hormones, making bone a crucial endocrine organ. One of these hormones, osteocalcin, secreted by osteoblasts, is capable of improving glucose homeostasis and insulin secretion in both mice and humans.37,38 Another hormone, fibroblast growth factor 23, produced by osteoblasts and osteocytes, is able to maintain phosphorus metabolic homeostasis in the kidney. 39, 40, 41
Neutrophil gelatinase-associated lipocalin 2 (LCN2), a previously defined adipokine, is exclusively secreted by adipose tissue and has been associated with obesity and insulin resistance.42,43 Mosialou et al.44 screened osteoblast-secreted molecules downstream of forkhead box O1 (FOXO1). Surprisingly, they observed LCN2 as one of the most highly upregulated secreting factors in FOXO1osb−/− mice when compared to the wild-type control group. When LCN2 was knocked out in osteoblasts, the mouse model showed decreased glucose tolerance, lower insulin concentration in serum (nearly 50% less compared to normal) and increased fatty tissue and body weight. Using osteoblast or adipocyte-specific LCN2 knockdown mice, they found that the influence of osteoblast-derived LCN2 was the most essential. Interestingly, LCN2 was able to pass the blood–brain barrier and was observed in the brainstem, thalamus, and hypothalamus after i.p. administration. Intracerebroventricular (i.c.v.) injection of LCN2, without leakage to serum, inhibited appetite, suggesting that LCN2 was mainly functional in the CNS. LCN2 increased cyclic adenosine monophosphate cAMP signaling in vitro rather than the mitogen activated protein kinase (MAPK) signaling or the extracellular-signal-regulated kinase (ERK) 1/2 phosphorylation and tyrosine kinase activation. Additionally, LCN2 showed concentration responsive to cAMP activation in human embryonic kidneys 293T (HEK293T) cells transfected with MC4R. The binding of LCN2 to MC4R was further confirmed by competitive binding assay. Through electrophysiology analysis, the researchers found that LCN2 depolarized Sim1 neurons in paraventricular hypothalamus rather than pro-opiomelanocortin (POMC) or neuropeptide Y neurons. The most interesting finding was that LCN2 suppressed the appetite and body weight gain only in wild type mice and not in MC4R knockout mice. Even in 5 obese human patients with MC4R mutations, LCN2 levels were higher than in body mass index-matched control individuals.44
Until now, LCN2 has been verified as a novel potent polypeptide agonist for MC4R. LCN2 performs its function through activating MC4R in Neuropeptide Y Y1 receptor (Y1R) neurons of the hypothalamus, suggesting that osteoblast-derived LCN2 could pass the blood–brain barrier and directly bind and stimulate MC4R signaling in the brain. Leptin (ob) is the first identified circulating hormone predominantly made by adipose cells, helping to regulate energy balance by inhibiting hunger.45 Specifically, leptin acts on its receptor in AgRP/POMC neurons, inhibiting AgRP and stimulating α-MSH release, the 2 upstream endogenous ligands for MC4R. No direct bridge between peripheral organs and hypothalamic MC4R neurons was reported until Mosialou et al.’s findings,44 which represented a new conceptual breakthrough, in that MC4R neurons in the CNS could directly detect the signal from peripheral organs to control food intake, bypassing the traditional neuronal regulation mediated by upstream AgRP/α-MSH ligands. LCN2 functions as a messenger, linking bone and the CNS for metabolic homeostasis of the whole body, and may be a key player for MC4R-associated linear growth and final height reported in humans9,46 and mouse models.47 Interestingly, mature LCN2, which is more than 150 amino acids long, has been identified as a novel polypeptide agonist for both MC4R and MC3R. It remains unknown whether LCN2 possesses the ability to activate other melanocortin receptors or G protein-coupled receptor, highlighting the need for further research in this area.
Cornish et al.48 reported improved cell proliferation in primary osteoblasts/chondrocytes and stimulated osteoclastogenesis in bone marrow upon treatment with α-MSH. Additionally, Dumont et al.49 found mRNA expression of MC4R in developing rat limb buds, teeth and skull bone, which suggests a potential function of MC4R in bone metabolism. Thus far, the connection between bone MC4R and its central/local ligands remains largely unclear. It would greatly increase our knowledge of MC4R function if researchers closely examined the dynamic regulation and expression of MC4R in bone development.
2.3. MC4R in the cardiovascular system
For a long time, MC4R was defined as a pivotal factor in appetite control. Tallam et al.50 found that MC4R−/− mice were hyperleptinemic and hyperinsulinemic, but not hypertensive. Greenfield et al.51 then showed that the prevalence of hypertension is lower in MC4R-haploinsufficient patients, who also presented with significantly lower blood pressure after antihypertensive medical treatment. Greenfield et al.51 found that heterozygous MC4R-deficient patients have stronger cardiovascular stability than other obese patients with matched body mass index, age and ancestry. However, the insulin-independent increase in blood pressure via MC4R agonism has limited the clinical potential of MC4R agonists for obesity treatment. Given the expression of MC4R in the cardiovascular system and other syndromes caused by obesity, the cardioprotective effects of MC4R deletion is challenged. Additional studies have shown that MC4R deficiency-induced obesity exhibits characteristics of several types of cardiovascular-associated diseases, such as hyperinsulinemia52 and hyperlipidemia.53
This feature was supported by recent creative work by Litt et al.,54 suggesting that MC4R could protect the heart from dilated cardiomyopathy via its own function rather than the pathology caused by obesity. Using echocardiography detection in MC4R−/−, MC4R+/−, and MC4R+/+ mice, a series of cardiomyopathy symptoms, such as increased left ventricular diameter and lower heart rate, was found in MC4R-deficient mice. Further investigation using weight-matched, diet-induced obese mice and MC4R−/− mice showed that these symptoms were independent of genetic background or obesity caused by MC4R deficiency and instead were caused by the loss of MC4R function. MC4R−/− mice exhibited slower heart rate, lower fractional shortening and ejection fraction and larger increased left ventricular diameter than the diet-induced obese control group. Transmission electron microscopy of the myocardium revealed mitochondrial dysfunction with mitochondrial pleomorphy and cardiomyocyte dropout. In seeking a potential cause of this dysfunction, study54 using a respirometry assay confirmed higher O2 consumption without any increased adenosine triphosphate content in cardiomyocytes after being treated with adenosine diphosphate, L-Octanoylacanrtine and succinate, all of which represented state III respiration.54 These phenotypes indicated a likely hypertrophic cardiomyopathy instead of dilated cardiomyopathy or right heart failure. Because reactive oxidative species (ROS) induced by increased O2 consumption may destroy mitochondria function, researchers tested ROS content in cardiomyocytes from MC4R−/− mice. ROS detected by 2′,7′-dichlorofluorescein diacetate oxidation assays and 4-hydroxynonenal protein adduct induced by ROS indicated an increasing level of the irreversible lipid-protein covalent bonds. Bioinformatic approaches, such as gene set enrichment and pathway ontology analysis, indicate that MC4R deficiency contributes to several pathways involved in cardiac tissue function and oxidative stress and may increase ROS accumulation and heart failure.
The study above provides some important clues on the peripheral roles of MC4R in the cardiovascular system and extends investigations of myocardial developmental function using heart-specific MC4R knockout mice. Since the last part of the mechanical study in this work is inadequate, more studies on ROS function with MC4R deficiency should be performed. Given that Mountjoy et al.55 indicated that MC4R was observed in early onset of heart development in rats, the developmental appearance of MC4R signaling may contribute to the clinical applications for future treatment of heart-associated disorders.
2.4. MC4R in limb regeneration
Recently, Zhang et al.56 performed a series of experiments on limb regeneration regulated by the MC4R pathway, primarily in the Xenopus model system. To investigate the mechanism through which energy metabolism regulates limb regeneration, one side of the hypothalamus of stage-53 tadpoles were electronically destroyed. Amputated limbs failed to form blastema and regenerate following this treatment. In-situ hybridization and real-time polymerase chain reaction found that the expression of agrp, pomc, and melanocortin receptors were upregulated 3 days post-amputation. This interesting study suggest the potential physiological roles of MC4R signaling in the CNS and local organs, yet the regulating networks of MC4R-dependent regeneration still remain unknown. Zhang et al.56 next employed MC4R-morpholino to inhibit endogenous MC4R translation and induce whole-body overexpression of AgRP, driven by a heat-shock promoter. Inhibition of MC4R signaling resulted in enhanced appetite, faster growth rate, inhibited limb regeneration and failure of blastema formation upon injury. All of these defects could be rescued by local transfection of POMC DNA. POMC overexpression in Xenopus promoted blastema formation and better limb regeneration, even though the regenerated limbs were not morphologically perfect. Immunofluorescent results showed increased Proliferating Cell Nuclear Antigen (PCNA) expression and ERK 1/2 phosphorylation, indicating improved cellular proliferation and stimulated MC4R cascades. The same phenomenon was also found in the digit tip regeneration of the MC4R-deficient mouse models, which partly proved that this mechanism was conserved in vertebrates. In the Xenopus and murine model, regional MC4R and α-MSH were mostly expressed in β3-tubulin-marked neurons, suggesting MC4R-mediated regeneration might be neuron dependent. Further pathway analysis confirmed cAMP-mediated signaling through the upregulation of cAMP response element modulator and c-fos and down-regulation of protein kinase inhibitor (PKI). Seahorse eahorse Extracellular Flux (XF) glycolysis and cell Mito stress tests uncovered an advanced glycolytic response; mitochondrial adenosine triphosphate accumulation and massive mitochondrial stress in MC4R-Morpholino (MO)- or AgRP-overexpressed Xenopus tissues resulted. Based on these findings, MC4R-associated ROS production was revealed as an essential mechanism for body regeneration in multiple model systems. Oxiselect ROS/RNS assays found that ROS content decreased in MC4R-MO- and AgRP-overexpressed Xenopus limbs, which could be rescued by the administration of POMC DNA. Upregulation of ROS by Cyba DNA also improved limb regeneration progress.56 Similar to Xu et al.’s57 results in studies involving rat livers, Zhang et al.56 found that local MC4R and POMC expression in the limb dramatically increased upon injury. These results suggest that local expression of MC4R and its agonist POMC58 may play a vital role in Xenopus and mouse limb regeneration and shed light on the clinical applications of MC4R agonism in treatments involving wound-associated rehabilitation. However, before commencing any clinical trials, the roles of MC4R signaling in the regeneration of limbs and other organs needs to be further explored in other vertebrate model systems.
2.5. MC4R in other organs
In early 2003, Mountjoy et al.55 reported the expression of MC4R mRNA in the cardiorespiratory, musculoskeletal, and integumentary systems of rats. These researchers also showed that MC4R mRNA expression was time dependent in the lungs of E16-E20 fetuses, in the respiratory muscles of E14 fetuses and in the tongue and occipital muscles of E15-E20 fetuses. The eyes were also found to express MC4R.55 Fifteen years later, Cai et al.59 showed that MC4R expression in retinal microvessel endothelial cells could protect the early diabetic retina upon stimulation with α-MSH. In 2007, Tanabe et al.60 observed MC4R expression in nerve-injured motor and sensory neurons, suggesting that MC4R could be a key player in peripheral nerve regeneration. Kobayashi et al.61 reported that food deficiency in barfin flounder increased MC4R expression levels in the liver. Later, Xu et al.57 suggested the potential role of MC4R signaling in liver regeneration due to locally upregulated expression of MC4R mRNA during rat liver regeneration. In addition, MC4R has also been found in pancreatic tissues. For example, in a murine model, intrapancreatic ganglia neurons received projection fibers from MC4R-expressing neurons in the dorsal motor nucleus of the vagus nerves, modulating serum insulin levels independent of leptin receptors.62,63 Researchers have also discovered that leptin secretion is mediated via MC4R signaling in adipose tissue because the administration of α-MSH could spur leptin release in rat adipocytes, revealing a feedback loop in leptin-MSH synergetic energy homeostasis.64 Furthermore, LY2112688, a MC4R-selective agonist, may stimulate lipolysis in intact white adipose tissue.65 MC4R is also expressed in the periosteum of mouse bone49 and in mesencephalic periaqueductal gray innervating renal tissue.66 However, additional studies need to be carried out to elucidate the physiological role of MC4R expression in the above areas.
3. Summary
Since the first discovery of MC4R, researchers have identified the hypothalamus, which is part of the CNS, as its highest expression site,19 and from then on, MC4R signaling and its endogenous ligands have been closely studied as the combination of the central command for appetite and metabolic homeostasis. This focus has gradually expanded in recent years. For example, researchers have explored the physiological role of MC4R in enteroendocrine L cells,30 the novel endogenous agonists generated from bone marrow,44 the association between MC4R and cardiovascular disorders54 and tissue regeneration under the control of local MC4R expression.56 Additionally, recently published work has shown that LCN2 contributes to skeletal muscle regeneration by regulating the extracellular matrix, implying an unknown connection between LCN2 and the regeneration process in a MC4R-dependent manner.67 Each of these studies has opened a new window that elucidates additional roles that MC4R plays and that establishes multiple exciting hypotheses that other scientists could investigate. With new studies highlighting the role of MC4R in heart growth54 and limb regeneration,56 many puzzles remain regarding MC4R's spatial and temporal expression, area-specific distribution and regulating networks.
Moreover, researchers have shown that athletes are at a high risk of eating disorders due to strict energy control and psychological stress.68,69 Some professional athletes, especially those involved in weightlifting, boxing, judo, and artistic gymnastics, require extra attention to manage their body weight. As an ideal pharmaceutical G protein-coupled receptor (GPCR) target to control appetite and food intake, modulation of MC4R activities may benefit the treatment of obesity,9,47 cachexia,70 senescence-induced weight loss,71 pediatric failure to thrive,72 anhedonia73,74 and obsessive-compulsive disorders.75 However, small-molecule compounds that directly (agonist or antagonist) or indirectly (allosteric modulator) act against MC4R signaling have not yet been successfully developed for clinical applications. A previous peptide drug development failed at clinical trial during Phase 2 due to side effects such as hypertension.9 In June 2019, the US Food and Drug Administration approved bremelanotide (Brand name: Vyleesi®), a potent MC4R agonist, to treat hypoactive sexual desire disorder in premenopausal women. Setmelanotide, also known as RM493, a selective MC4R-specific agonist developed by Rhythm Pharmaceuticals, is now in a Phase 3 clinical trial.76 In August 2019, Rhythm Pharmaceuticals announced positive topline results from pivotal Phase 3 clinical trials evaluating Setmelanotide in POMC- and leptin receptor-deficiency obesities. Both studies met primary and all key secondary endpoints, with statistically significant and clinically meaningful results in reductions of weight and hunger in these patients. Setmelanotide may become the first drug approved by the US Food and Drug Administration that is safe for use in weight management through central MC4R signaling. Moreover, with increased combinatorial understanding of MC4R's structure, physiology and pharmacology, drugs and therapies to treat bulimia nervosa and anorexia nervosa by repressing MC4R are likely to be developed in the near future.
Acknowledgments
Acknowledgments
Fundings supported by grants from the National Key Research and Development Program of China (Grant No. 2017YFA0103902, 2018YFA0800300, 2019YFA0801900, 2019YFA0111400), National Natural Science Foundation of China (Grant No. 31771283, 91749104, 31971074), the Fundamental Research Funds for the Central Universities of Tongji University (No. 22120190210), Innovative Research Team of High-Level Local Universities in Shanghai (No. SSMU-ZDCX20180700), Key Laboratory Program of the Education Commission of Shanghai Municipality (No. DSYS14005), and the Science and Technology Innovation Action Plan of Shanghai Science and Technology Committee (No. 18140901300).
Authors’ contributions
LL, TL and ChZ designed the study and contributed to the manuscript writing; JL, and CZ participated in the data collection and interpretation of the results. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.02.001.
Contributor Information
Tiemin Liu, Email: tiemin_liu@fudan.edu.cn.
Chao Zhang, Email: zhangchao@tongji.edu.cn.
Supplementary materials
References
- 1.Sevilla-Gonzalez MDR, Quintana-Mendoza BM, Aguilar-Salinas CA. Interaction between depression, obesity, and type 2 diabetes: A complex picture. Arch Med Res. 2017;48:582–591. doi: 10.1016/j.arcmed.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Medina-Remon A, Kirwan R, Lamuela-Raventos RM, Estruch R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2018;58:262–296. doi: 10.1080/10408398.2016.1158690. [DOI] [PubMed] [Google Scholar]
- 3.Chukir T, Shukla AP, Saunders KH, Aronne LJ. Pharmacotherapy for obesity in individuals with type 2 diabetes. Expert Opin Pharmacother. 2018;19:223–231. doi: 10.1080/14656566.2018.1428558. [DOI] [PubMed] [Google Scholar]
- 4.Gross AC, Kaizer AM, Ryder JR, et al. Relationships of anxiety and depression with cardiovascular health in youth with normal weight to severe obesity. J Pediatr. 2018;199:85–91. doi: 10.1016/j.jpeds.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosio G, Kaufmann FN, Manosso L, et al. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology. 2018;91:132–141. doi: 10.1016/j.psyneuen.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Schachter J, Martel J, Lin CS, et al. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Peterson LL. Obesity and cancer: Evidence, impact, and future directions. Clin Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 8.Reggiani F, Bertolini F. Roles of obesity in the development and progression of breast cancer. Discov Med. 2017;24:183–190. [PubMed] [Google Scholar]
- 9.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 10.Stutzmann F, Tan K, Vatin V, et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes. 2008;57:2511–2518. doi: 10.2337/db08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 12.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 13.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Forlano PM, Cone RD. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012;15:256–264. doi: 10.1016/j.cmet.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J, Li L, Jin X, et al. Pharmacological effect of human melanocortin-2 receptor accessory protein 2 variants on hypothalamic melanocortin receptors. Endocrine. 2018;61:94–104. doi: 10.1007/s12020-018-1596-2. [DOI] [PubMed] [Google Scholar]
- 17.Chan LF, Webb TR, Chung TT, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci U S A. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Elmquist JK, Williams KW. Mrap2: An accessory protein linked to obesity. Cell Metab. 2013;18:309–311. doi: 10.1016/j.cmet.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantz I, Miwa H, Konda Y, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 20.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 21.Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 22.Ghamari-Langroudi M, Digby GJ, Sebag JA, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94–98. doi: 10.1038/nature14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Gimenez LE, Hernandez CC, et al. Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science. 2020;368:428–433. doi: 10.1126/science.aaz8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson J, Cruz MT, Majumdar U, et al. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci. 2013;33:13286–13299. doi: 10.1523/JNEUROSCI.0780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatoum IJ, Stylopoulos N, Vanhoose AM, et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocr Metab. 2012;97:E1023–E1031. doi: 10.1210/jc.2011-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jelin EB, Daggag H, Speer AL, et al. Melanocortin-4 receptor signaling is not required for short-term weight loss after sleeve gastrectomy in pediatric patients. Int J Obesity. 2016;40:550–553. doi: 10.1038/ijo.2015.230. [DOI] [PubMed] [Google Scholar]
- 29.Engelstoft MS, Park WM, Sakata I, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panaro BL, Tough IR, Engelstoft MS, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 In vivo. Cell Metab. 2014;20:1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Kishi T, Roseberry AG, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tough IR, Forbes S, Tolhurst R, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tennoune N, Chan P, Breton J, et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide alpha-MSH, at the origin of eating disorders. Transl Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breton J, Tennoune N, Lucas N, et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 2016;23:324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Brotto M, Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone. 2015;80:109–114. doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murshed M. Mechanism of bone mineralization. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 39.Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Jiang Y, Xia W. FGF23 and phosphate wasting disorders. Bone Res. 2013;1:120–132. doi: 10.4248/BR201302002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 43.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 44.Mosialou I, Shikhel S, Liu JM, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brennan AM, Mantzoros CS. Drug Insight: The role of leptin in human physiology and pathophysiology–emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 46.Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–E188. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 47.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 48.Cornish J, Callon KE, Mountjoy KG, et al. alpha -melanocyte-stimulating hormone is a novel regulator of bone. Am J Physiol Endocrinol Metab. 2003;284:E1181–E1190. doi: 10.1152/ajpendo.00412.2002. [DOI] [PubMed] [Google Scholar]
- 49.Dumont LM, Wu CS, Tatnell MA, Cornish J, Mountjoy KG. Evidence for direct actions of melanocortin peptides on bone metabolism. Peptides. 2005;26:1929–1935. doi: 10.1016/j.peptides.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 51.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 52.do Carmo JM, da Silva AA, Dubinion J, et al. Control of metabolic and cardiovascular function by the leptin-brain melanocortin pathway. IUBMB Life. 2013;65:692–698. doi: 10.1002/iub.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Himmerich H, Minkwitz J, Kirkby KC. Weight gain and metabolic changes during treatment with antipsychotics and antidepressants. Endocr Metab Immune Disord Drug Targets. 2015;15:252–260. doi: 10.2174/1871530315666150623092031. [DOI] [PubMed] [Google Scholar]
- 54.Litt MJ, Okoye GD, Lark D, et al. Loss of the melanocortin-4 receptor in mice causes dilated cardiomyopathy. Elife. 2017;6:e28118. doi: 10.7554/eLife.28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology. 2003;144:5488–5496. doi: 10.1210/en.2003-0570. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Chen Y, Xu H, et al. Melanocortin receptor 4 signaling regulates vertebrate limb regeneration. Dev Cell. 2018;46:397–409. doi: 10.1016/j.devcel.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu M, Alwahsh SM, Ramadori G, Kollmar O, Slotta JE. Upregulation of hepatic melanocortin 4 receptor during rat liver regeneration. J Surg Res. 2016;203:222–230. doi: 10.1016/j.jss.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Harno E, Gali Ramamoorthy T, Coll AP, White A. POMC: The physiological power of hormone processing. Physiol Rev. 2018;98:2381–2430. doi: 10.1152/physrev.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai S, Yang Q, Hou M, et al. Alpha-melanocyte-stimulating hormone protects early diabetic retina from blood-retinal barrier breakdown and vascular leakage via MC4R. Cell Physiol Biochem. 2018;45:505–522. doi: 10.1159/000487029. [DOI] [PubMed] [Google Scholar]
- 60.Tanabe K, Gamo K, Aoki S, Wada K, Kiyama H. Melanocortin receptor 4 is induced in nerve-injured motor and sensory neurons of mouse. J Neurochem. 2007;101:1145–1152. doi: 10.1111/j.1471-4159.2006.04432.x. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi Y, Tsuchiya K, Yamanome T, Schioth HB, Kawauchi H, Takahashi A. Food deprivation increases the expression of melanocortin-4 receptor in the liver of barfin flounder, Verasper moseri. Gen Comp Endocrinol. 2008;155:280–287. doi: 10.1016/j.ygcen.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Mansour M, White D, Wernette C, et al. Pancreatic neuronal melanocortin-4 receptor modulates serum insulin levels independent of leptin receptor. Endocrine. 2010;37:220–230. doi: 10.1007/s12020-009-9289-5. [DOI] [PubMed] [Google Scholar]
- 63.Tsumori T, Oka T, Yokota S, Niu JG, Yasui Y. Intrapancreatic ganglia neurons receive projection fibers from melanocortin-4 receptor-expressing neurons in the dorsal motor nucleus of the vagus nerve of the mouse. Brain Res. 2013;1537:132–142. doi: 10.1016/j.brainres.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Hoggard N, Hunter L, Duncan JS, Rayner DV. Regulation of adipose tissue leptin secretion by alpha-melanocyte-stimulating hormone and agouti-related protein: Further evidence of an interaction between leptin and the melanocortin signalling system. J Mol Endocrinol. 2004;32:145–153. doi: 10.1677/jme.0.0320145. [DOI] [PubMed] [Google Scholar]
- 65.Moller CL, Pedersen SB, Richelsen B, et al. Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res Notes. 2015;8:559. doi: 10.1186/s13104-015-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ke QF, Wang LX. Neuroanatomical evidence of the melanocortin-4 receptor expression in the mesencephalic periaqueductal gray innervating renal tissues. Int J Clin Exp Med. 2015;8:6119–6123. [PMC free article] [PubMed] [Google Scholar]
- 67.Rebalka IA, Monaco CMF, Varah NE, et al. Loss of the adipokine lipocalin-2 impairs satellite cell activation and skeletal muscle regeneration. Am J Physiol Cell Physiol. 2018;315:C714–CC21. doi: 10.1152/ajpcell.00195.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milano W, Milano L, Capasso A. Eating disorders in athletes: From risk management to therapy. Endocr Metab Immune Disord Drug Targets. 2020;20:2–14. doi: 10.2174/1871530319666190418121446. [DOI] [PubMed] [Google Scholar]
- 69.Eichstadt M, Luzier J, Cho D, Weisenmuller C. Eating disorders in male athletes. Sports Health. 2020;12:327–333. doi: 10.1177/1941738120928991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodfellow VS, Saunders J. The melanocortin system and its role in obesity and cachexia. Curr Top Med Chem. 2003;3:855–883. doi: 10.2174/1568026033452212. [DOI] [PubMed] [Google Scholar]
- 71.Wysokiński A, Sobów T, Kłoszewska I, Kostka T. Mechanisms of the anorexia of aging-a review. Age (Dordrecht, Netherlands) 2015;37:9821. doi: 10.1007/s11357-015-9821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elks CE, Loos RJF, Sharp SJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goudarzi M, Nahavandi A, Mehrabi S, Eslami M, Shahbazi A, Barati M. Valproic acid administration exerts protective effects against stress-related anhedonia in rats. J Chem Neuroanat. 2020;105 doi: 10.1016/j.jchemneu.2020.101768. [DOI] [PubMed] [Google Scholar]
- 75.Xu P, Grueter BA, Britt JK, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc Natl Acad Sci U S A. 2013;110:10759–10764. doi: 10.1073/pnas.1308195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.