Chlormethine (also known as mechlorethamine) gel is a skin-directed chemotherapy specifically developed for treatment of patients with mycosis fungoides (MF), the most common form of cutaneous T-cell lymphoma (1). In the pivotal study known also as the 201 trial (NCT00168064), treatment with chlormethine gel was compared with a chlormethine ointment formulation (2). The gel formulation resulted in a high response rate (58.5%) and observed adverse events were skin related and manageable. In addition, chlormethine gel was non-inferior in terms of response to chlormethine ointment (2). A post hoc analysis showed significantly higher response rates with chlormethine gel (79.8%) than with chlormethine ointment (49.2%) for patients with stage IA disease (p = 0.0014). Moreover, time to response was shorter and overall response trends were better when patients were treated with chlormethine gel compared with ointment (3).
Treatment guidelines recommend chlormethine gel as a first-line option for early-stage MF (4–6); however, in real-world clinical practice, patients may have received other therapies for MF prior to chlormethine gel, with bexarotene and phototherapy being among the most common. Currently, no data are available about the possible influence of prior therapies on the efficacy of chlormethine gel. Herein, we present results from a second post hoc analysis of the 201 trial, evaluating whether prior bexarotene and/or phototherapy affects the response to chlormethine gel in patients with MF.
MATERIALS AND METHODS
Data from the chlormethine gel arm of the randomized controlled 201 trial were used for this post hoc analysis. The detailed study design was published previously (2). Briefly, patients received daily chlormethine gel monotherapy for ≥ 12 months and efficacy was assessed every 1–2 months using Composite Assessment of Index Lesion Severity (CAILS). Per inclusion criteria, all patients were required to have received at least 1 prior therapy for MF. Prior therapies included corticosteroids, phototherapy, bexarotene (or other retinoids), topical chlormethine (>2 years prior to the study), interferon therapy, methotrexate, and radiation. In the current analysis, complete response (CR) was defined as 100% skin clearance (CAILS score 0), very good partial response (VGPR) as 75–<100% reduction from the baseline score, and partial response (PR) as 50–<75% reduction from baseline.
Time-to-response and time-trend analyses were performed as published previously (3). The intent-to-treat (ITT) population was used for the analysis, as well as a modified ITT population that excluded all patients who received prior bexarotene, to obtain a more homogeneous data set on the effect of prior phototherapy.
Time-to-response was estimated using Kaplan–Meier curves with an event defined as the first occurrence of a CAILS response, and served as surrogate of time-point estimates. Separate curves were produced with response defined either as CR only, at least VGPR, or at least PR.
Time-trend analysis was performed using generalized estimating equation models with baseline scores, group (with/without prior bexarotene or phototherapy), visit, and group-by-visit interaction applied as model fixed effects. For this analysis, response was defined as at least PR.
RESULTS
Patients
In total, 118 patients were included in the analysis. Of these, 21 had received oral or topical bexarotene prior to chlormethine gel and 97 had received other prior therapies. In the ITT population, 46 patients had received prior phototherapy and 72 had received other prior therapies; the modified ITT population included 30 patients with prior phototherapy and 67 with other prior therapies.
Time-to-response analyses
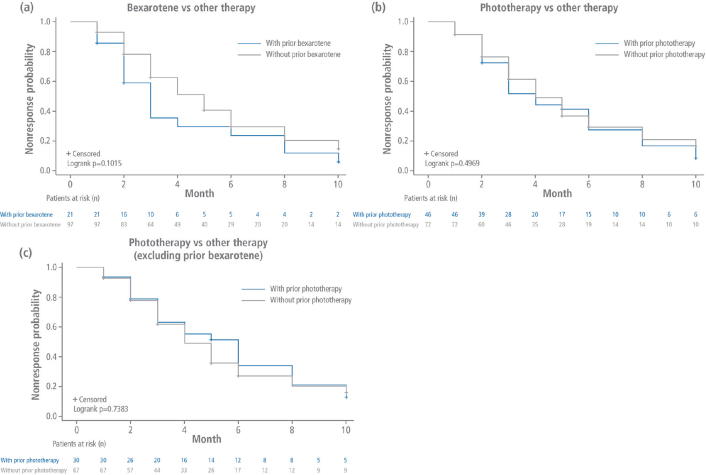
There was no statistically significant difference in time to first CAILS response for patients who received prior bexarotene compared with those who received other prior therapies when response was defined as CR only (p = 0.3149), at least VGPR (p = 0.6182), or at least PR (p = 0.1015, Fig. 1a). Similarly, time to first response was not significantly different for patients in the ITT population who had previously received phototherapy than for those who had received other prior therapies when response was defined as CR only (p = 0.5937), at least VGPR (p = 0.6458), or at least PR (p = 0.4969, Fig. 1b).
Fig. 1.
Time to response analysis. Kaplan–Meier curves for time to first Composite Assessment of Index Lesion Severity response (at least partial response) for patients who received (a) prior bexarotene or (b) prior phototherapy in the intent-to-treat (ITT) population, or (c) prior phototherapy in the modified ITT population vs any other prior therapies.
When patients who received prior bexarotene were excluded in the modified ITT population, time to first response was also not significantly different for patients who received prior phototherapy compared with those who received any other prior therapies when response was defined as CR only (p = 0.9834), at least VGPR (p = 0.4358), or at least PR (p = 0.7383, Fig. 1c). Individual point estimates were also similar for the 3 comparisons.
Time-trend analyses
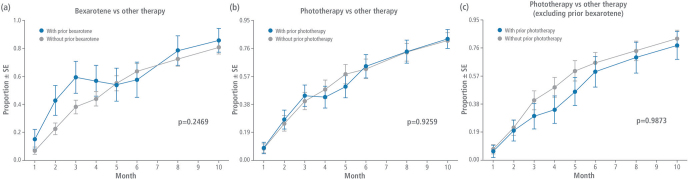
When evaluating the response trends at each visit, patients previously treated with bexarotene did not have a significantly different response to chlormethine gel treatment than those who received other prior therapies (p = 0.2469). Response trend analysis also showed no significant difference at each visit for prior phototherapy vs other prior therapies when including (ITT, p = 0.9259) or excluding (modified ITT, p = 0.9873) patients who received prior bexarotene (Fig. 2).
Fig. 2.
Time-response trend analysis. Response trends (at least partial response) for patients who received (a) prior bexarotene or (b) prior phototherapy in the intent-to-treat (ITT) population, or (c) prior phototherapy in the modified ITT population vs any other prior therapies. SE: standard error.
DISCUSSION
This post hoc analysis showed that the response to chlormethine gel in patients with MF was similar from a clinical viewpoint, regardless of prior treatment with bexarotene and/or phototherapy vs other therapies. These results support the assertion that chlormethine gel is a valid treatment option, not only as first-line therapy, but also for patients who received prior therapies.
In real-world clinical practice, patients with MF often receive prior treatments for MF before initiating chlormethine gel. In a retrospective evaluation of 18 patients, only 1 was treatment-naïve when initiating chlormethine gel, with 7 patients having received 2–4 prior lines of therapy and 8 patients having had more than 5 prior therapies (7). Another retrospective study included 58 patients who all received prior therapies, including prior phototherapy or bexarotene, and had good responses to chlormethine gel treatment (8).
In the real-world PROVe study, phototherapy and bexarotene were 2 of the most common treatments used in combination with chlormethine gel (9). Chlormethine gel was effective and had good tolerability when co-administered with other therapies (9). An expert consensus on the optimal use of chlormethine gel noted that concomitant therapy with chlormethine gel occurs in clinical practice, but sufficient evidence on the topic is currently lacking to provide recommendations (10).
In conclusion, this post hoc analysis demonstrates that chlormethine gel fits well in the current treatment algorithm and can be considered as treatment for patients with MF regardless of prior therapy.
ACKNOWLEDGEMENTS
The authors acknowledge and thank the volunteers, investigators, and the study teams at the centres participating in these studies, and thank Erminio Bonizzoni from the University of Milan, Italy, for performing the statistical analyses. Editorial and medical writing assistance was provided by Judith Land, PhD, from Aptitude Health, The Hague, the Netherlands, funded by Helsinn Healthcare SA.
Institutional Review Board approval of the 201 trial was obtained at all study sites and all patients provided written informed consent prior to enrolment.
Conflicts of interest
CA: Advisory board: 4SC, Takeda, Helsinn, Innate Pharma, Recordati Rare Diseases, Kyowa, Stemline Therapeutics. CQ: Steering Committee/Advisory Board: Helsinn, Kyowa Kirin; Investigator: Helsinn, Celgene (Bristol Myers Squibb), Kyowa Kirin, Eisai, Bioniz, miRagen, Trillium Therapeutics; Speakers’ Bureau: Helsinn; Funds/Research Grants: Celgene, Helsinn. MS, MT: Employees of Helsinn Healthcare SA. JJS: Consultancy: Takeda, Helsinn, Recordati, 4SC, Kyowa, Mallinckrodt, miRagen; Research grant: Kyowa.
REFERENCES
- 1.Valchlor (mechlorethamine gel) [prescribing information]. Iselin, NJ: Helsinn Therapeutics (U.S.), Inc. [Accessed 28 September 2022]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202317s009lbl.pdf. [Google Scholar]
- 2.Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol 2013; 149: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Querfeld C, Scarisbrick JJ, Assaf C, Guenova E, Bagot M, Ortiz-Romero PL, et al. Post hoc analysis of a randomized, controlled, phase 2 study to assess response rates with chlormethine/mechlorethamine gel in patients with stage IA–IIA mycosis fungoides. Dermatology 2022; 238: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M, ESMO Guidelines Committee . Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv30–40. [DOI] [PubMed] [Google Scholar]
- 5.Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – update 2017. Eur J Cancer 2017; 77: 57–74. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Primary cutaneous lymphoma. Version 2.2022. 2022. [Accessed 28 September 2022]. Available from: https://www.nccn.org/professionals/physician_gls/default_nojava.aspx.
- 7.Wehkamp U, Jost M, Gosmann J, Grote U, Bernard M, Stadler R. Management of chlormethine gel treatment in mycosis fungoides patients in two German skin lymphoma centers. J Dtsch Dermatol Ges 2021; 19: 1057–1059. [DOI] [PubMed] [Google Scholar]
- 8.Papadavid E, Koumourtzis M, Nikolaou V, Lampadaki K, Marinos L, Patsatsi A, et al. Chlormethine gel is effective for the treatment of skin lesions in patients with early- and late-stage mycosis fungoides in clinical practice. J Eur Acad Dermatol Venereol 2022; 36: 1751–1757. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Guitart J, Querfeld C, Girardi M, Musiek A, Akilov OE, et al. The PROVe study: US real-world experience with chlormethine/mechlorethamine gel in combination with other therapies for patients with mycosis fungoides cutaneous T-cell lymphoma. Am J Clin Dermatol 2021; 22: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assaf C, Booken N, Dippel E, Guenova E, Jonak C, Klemke CD, et al. The optimal use of chlormethine gel for mycosis fungoides: an expert consensus from Germany, Austria and Switzerland (DACH region). J Dtsch Dermatol Ges 2022; 20: 579–586. [DOI] [PubMed] [Google Scholar]