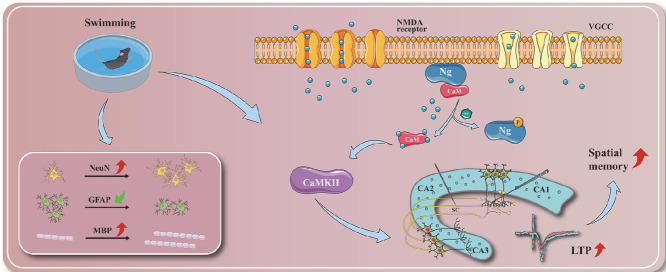
Highlights
-
•
Neurogranin conditional knockout (Ng cKO) mice exhibit significant spatial memory impairment and the memory disability in bilateral common carotid artery stenosis (BCAS) mice is progressively aggravated.
-
•
Swimming training improves the spatial memory dysfunction of BCAS mice by regulating the expression of Ng.
-
•
Neurogranin-Calmodulin-Calcium/calmodulin-dependent protein kinase II (Ng-CaM-CaMKII) cascade plays a crucial role in swimming training regulating synaptic plasticity to produce positive effect on the spatial memory.
-
•
Ng cKO hinders the beneficial role on swimming training in improving white matter injury, neuronal damage and inflammation in BCAS mice.
Keywords: Neurogranin, Swimming training, Synaptic plasticity, Vascular cognitive impairment
Abstract
Background
Vascular cognitive impairment caused by chronic cerebral hypoperfusion (CCH) has become a hot issue worldwide. Aerobic exercise positively contributes to the preservation or restoration of cognitive abilities; however, the specific mechanism has remained inconclusive. And recent studies found that neurogranin (Ng) is a potential biomarker for cognitive impairment. This study aims to investigate the underlying role of Ng in swimming training to improve cognitive impairment.
Methods
To test this hypothesis, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) system was utilized to construct a strain of Ng conditional knockout (Ng cKO) mice, and bilateral common carotid artery stenosis (BCAS) surgery was performed to prepare the model. In Experiment 1, 2-month-old male and female transgenic mice were divided into a control group (wild-type littermate, n = 9) and a Ng cKO group (n = 9). Then, 2-month-old male and female C57BL/6 mice were divided into a sham group (C57BL/6, n = 12) and a BCAS group (n = 12). In Experiment 2, 2-month-old male and female mice were divided into a sham group (wild-type littermate, n = 12), BCAS group (n = 12), swim group (n = 12), BCAS + Ng cKO group (n = 12), and swim + Ng cKO group (n = 12). Then, 7 days after BCAS, mice were given swimming training for 5 weeks (1 week for adaptation and 4 weeks for training, 5 days a week, 60 min a day). After intervention, laser speckle was used to detect cerebral blood perfusion in the mice, and the T maze and Morris water maze were adopted to test their spatial memory. Furthermore, electrophysiology and Western blotting were conducted to record long-term potential and observe the expressions of Ca2+ pathway-related proteins, respectively. Immunohistochemistry was applied to analyze the expression of relevant markers in neuronal damage, inflammation, and white matter injury.
Results
The figures showed that spatial memory impairment was detected in Ng cKO mice, and a sharp decline of cerebral blood flow and an impairment of progressive spatial memory were observed in BCAS mice. Regular swimming training improved the spatial memory impairment of BCAS mice. This was achieved by preventing long-term potential damage and reversing the decline of Ca2+ signal transduction pathway-related proteins. At the same time, the results suggested that swimming also led to improvements in neuronal death, inflammation, and white matter injury induced by CCH. Further study adopted the use of Ng cKO transgenic mice, and the results indicated that the positive effects of swimming training on cognitive impairments, synaptic plasticity, and related pathological changes caused by CCH could be abolished by the knockout of Ng.
Conclusion
Swimming training can mediate the expression of Ng to enhance hippocampal synaptic plasticity and improve related pathological changes induced by CCH, thereby ameliorating the spatial memory impairment of vascular cognitive impairment.
Graphical Abstract
1. Introduction
Chronic cerebral hypoperfusion (CCH) can cause an insufficient supply of cerebral blood and oxygen, resulting in damage to the structure and function of the white matter of the brain. This leads to induced vascular cognitive impairment (VCI), evident in the dysfunction of learning and memory, executive, and other domains.1,2 VCI may progress to vascular dementia in the late stage, which can seriously affect the quality of daily life of patients. Among the elderly population over 65 years old in China, the overall prevalence of mild cognitive impairment is 20.8%, of which 42.0% is caused by cerebrovascular diseases and vascular risk factors.3 Among all cases of dementia, vascular dementia accounts for about 20.0% and is the most common type of dementia other than Alzheimer's disease (AD).4
In recent years, the results of several meta-analyses have shown that exercise, as one of the methods of rehabilitation for cognitive dysfunction, can effectively rescue the cognitive dysfunction of patients after stroke.5,6 And randomized controlled experiments have shown that long-term regular aerobic exercise for subjects with mild subcortical ischemic VCI can significantly reduce the score of AD assessment scale–cognitive subscale and improve cognitive dysfunction.7 Further studies have found that aerobic exercise can enhance synaptic plasticity by up-regulating the expression of hippocampal synapse-related proteins, thereby ameliorating the performance of mice that were subjected to the bilateral common carotid artery stenosis (BCAS) model in behavioral tests and restoring the memory function.8,9
As we all know, the hippocampus is a crucial region of the brain for the formation of spatial learning and memory, and its synaptic plasticity is considered to be an essential neurophysiological foundation. However, CCH can induce neuronal death, inflammation, white matter injury, synaptic dysfunction, and abnormal calcium signal transduction in the hippocampus, all of which lead to spatial learning and memory impairment. In recent years, studies have found that neurogranin (Ng), a specific post-synaptic protein of the nervous system, was regarded as a novel biomarker for cognitive dysfunction.10, 11, 12 In fact, in a previous review, our team systematically examined the effects of Ng levels in cerebrospinal fluid and blood exosomes on the decline of cognitive and memory functions,13 which was expressed as an increase in the cerebrospinal fluid of patients with AD and mild cognitive impairment and a decrease in blood exosomes. However, in patients with cerebrovascular diseases, current studies have not yet yielded consistent results. One study suggested that the Ng level in the cerebrospinal fluid of VCI patients decreased significantly,14 and another study showed that the Ng level of VCI patients was similar to that of the cognitively normal control group.15 Ng is composed of 78 amino acids and mainly distributed in cerebral cortex and hippocampus. Studies showed that Ng is the binding protein of calmodulin (CaM) and the postsynaptic substrate of protein kinase C (PKC), which plays an important role in calcium signal transduction and synaptic plasticity, and can effectively promote the formation of learning and memory.16 Unfortunately, there is a current lack of studies looking at Ng levels in VCI patients as the primary outcome.
In summary, Ng is closely related to synaptic plasticity and is considered to be one of the biomarkers of cognitive dysfunction. However, to date, the evidence supporting the role of Ng in swimming to improve cognitive dysfunction after chronic cerebral ischemia has remained sparse and inconclusive. To test this hypothesis, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) system was utilized to conditionally knock out the Ng gene in the nervous system of subjects, thereby constructing a strain of transgenic mice. BCAS surgery was conducted to prepare the model for the attempt to clarify the biological mechanism that explains how swimming regulates hippocampal synaptic plasticity and improves the memory function in BCAS mice.
2. Methods
2.1. Animals
C57BL/6 mice and Ng conditional knockout (Ng cKO) mice were selected for animal experiments. The C57BL/6 mice were purchased from Shanghai Slack Laboratory Animal Co. (Shanghai, China), and the Ngflox/wt heterozygous (flox/wt indicates that one allele is flox and the other allele is wild type.) mice and Nestin-cre mice were purchased from Nanjing Institute of Biomedicine, Nanjing University, China (Permit number: SCXK(Su)-2015-0001). The genetic background of the transgenic mice is C57BL/6. The Ngflox/wt were crossed with Nestin-cre mice to generate the Ng cKO strain. All animals were raised and bred in the Experimental Animal Center of Fujian University of Traditional Chinese Medicine (Permit number: SYXK(Min)2019-0007), and all the mice were housed under a 12-h light/dark cycle in a temperature (22°C–26°C) and humidity (40%–50%) controlled room. Mice were housed in groups of 5 per cage and had free access to water and standard chow. All animal experiments have been approved by the Animal Experiment Ethics Committee of Fujian University of Traditional Chinese Medicine and comply with the National Laboratory Animal Health Guidance Principles.
2.2. Polymerase chain reaction (PCR)
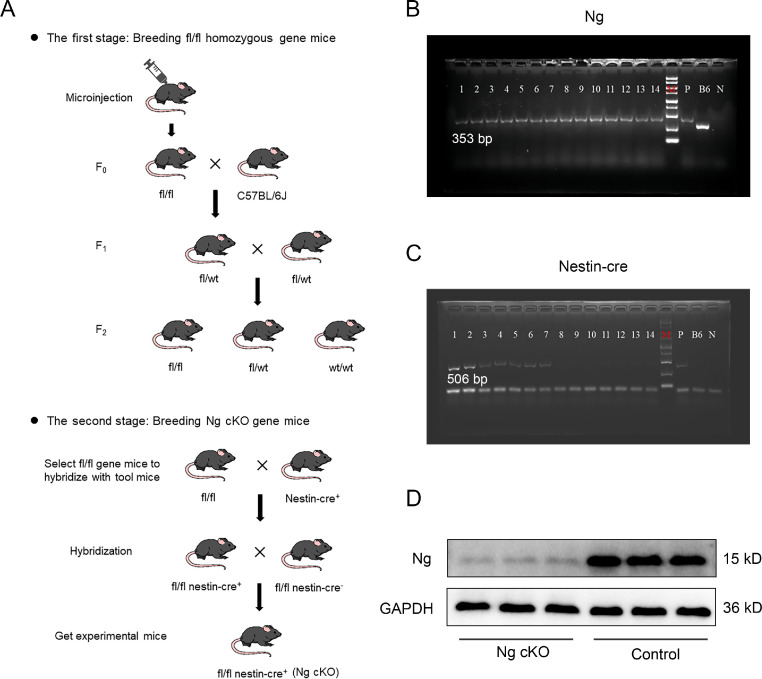
The CRISPR-Cas9 system was utilized to generate novel conditional knockout mice by inactivating Ng in the nervous system; the hybridization process was shown in Fig. 1A. Then, in order to identify the mice with the target gene, PCR was used to detect the genotype of all mice. Tail tips of mice were cut and placed into Eppendorf tubes. Samples were heated (at 56°C for 15 min) and then centrifuged (14,000 r, 2 min). The DNA was isolated according to the instructions of the DNA extraction kit (Hangzhou Bioer Technology Co., Hangzhou, China), and then the extracted DNA samples were subjected to an amplification reaction (100 V, 60 min). Finally, the amplified DNA samples were prepared for electrophoresis and gel imaging. The primer sequences, amplification reaction system, and amplification reaction procedure were as shown in Supplementary Tables 1–3.
Fig. 1.
Cultivation and identification of Ng cKO mice. (A) Schematic diagram of breeding of Ng cKO mice; (B) Electrophoretic bands of Ng gene with PCR. The Ngflox/flox homozygous gene band is 353 bp, the Ngflox/wt heterozygous gene bands are 353 bp and 260 bp; and the Ngwt/wt wild-type gene band is 260 bp; (C) Electrophoretic bands of Nestin-cre gene with PCR. The positive band of Nestin-cre is 506 bp; and (D) Western blotting of Ng in the hippocampus of Ng cKO mice. B6 = negative control; F0 = chimera obtained by microinjection; F1 = first-generation gene knockout mice obtained by crossing chimeras with wild-type; fl = flox; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; kD = kilodalton; N = blank control; Ng = neurogranin; Ng cKO = neurogranin conditional knockout; P = positive control; PCR = polymerase chain reaction; wt = wild type.
2.3. Experimental design
2.3.1. Experiment 1
The first experiment sought to determine whether conditional knockout of Ng will induce spatial memory dysfunction in transgenic mice and to observe the time course in the change of spatial memory and cerebral blood perfusion (CBF) in BCAS mice. Eighteen 2-month-old male and female transgenic mice were divided into a control group (n = 9) and a Ng conditional knockout (Ng cKO) group (n = 9) according to the PCR results. Experiments were performed with sex-matched and age-matched littermates of the indicated genotypes. The T maze and Morris water maze (MWM) were used to test spatial memory ability. Then, 24 C57BL/6 male and female mice were divided into a sham operation (sham) group (n = 12) and a BCAS group (n = 12). The C57BL/6 mice were used as the sham group. BCAS surgery was conducted on the BCAS group, whereas the bilateral common carotid arteries in the sham group were only separated. Laser speckle was used to monitor CBF, and T maze was used to detect spatial memory.
2.3.2. Experiment 2
The second experiment sought to observe the therapeutic effect of swimming training and to investigate how Ng functions in swimming training to improve the spatial memory dysfunction of BCAS mice. Forty-two 2-month-old male and female mice were divided into a sham group (n = 12) and an operation group (n = 30). Wild-type (wt) littermate controls were used as the sham group. BCAS surgery was conducted on the operation group, whereas the bilateral common carotid arteries in the sham group were only separated. Then, 24 mice that were successfully modeled and qualified in the operation group were randomly divided into a BCAS group (n = 12) and a swimming training (swim) group (n = 12). In addition, thirty-two 2-month-old male and female Ng cKO mice were subjected to BCAS surgery, and the same methods were used to identify the model after surgery. Finally, 24 Ng cKO mice that were successfully modeled and qualified were randomly divided into a BCAS + Ng cKO group (n = 12) and a swim + Ng cKO group (n = 12).
2.4. BCAS
BCAS surgery was conducted to prepare the CCH model.17 After general anesthesia with 2% isoflurane, the mouse was fixed on the operating table. The neck of the mouse was disinfected with 75% alcohol, and a small incision was made in the middle of the neck (1.0–1.5 cm). The common carotid artery and vagus nerve on the right were fully exposed, 2 medical sutures were placed under the distal and proximal parts of the right common carotid artery, and then the common carotid artery was lifted and clamped with a miniature spring coil 0.18 mm in diameter (Zhangchiyoudu Hardware Products Factory, Shenzhen, China). After continuous anesthesia for 30 min, the left common carotid artery was clamped in the same way to make it narrow. During the operation, a heating pad was used to maintain body temperature and electrocardiogram monitoring was used to monitor respiratory rate. After the operation, the wound was cleaned, the skin was sutured, penicillin was used to prevent infection, manage pain and reduce mortality, and the mouse was put back into the cage with soft bedding for normal feeding. In the sham operation group, the bilateral common carotid artery and vagus nerve were only separated without clamping.
2.5. Laser speckle
Laser speckle imaging technology (Perimed AB, Stockholm, Sweden) was used to detect postoperative blood flow distribution and changes in blood perfusion of cerebral vessels in order to evaluate the success of CCH in model mice. After general anesthesia with 2% isoflurane, the mouse was placed on the operating table, and the skin was cut longitudinally to obtain a small incision (1.0–1.5 cm) to expose the skull. The exposed area was irradiated with a 780 nm laser, then the average blood perfusion in the area of interest was monitored and recorded.
2.6. Intervention
Swimming training was adopted in the swim group and swim + Ng cKO group. The specific plan was as follows:18 The mice were trained in a water tank (50 cm in diameter, 40 cm in depth, and 20 cm in water depth) to swim without weight, and the water temperature was maintained at 32°C ± 1°C. Swimming training was divided into an adaptation period and a training period. The adaptation period was 1 week. For the first 5 days, the mice swam for 10 min, 15 min, 30 min, 45 min, and 60 min, respectively, and then they rested for 2 days. The training period, which followed, lasted for 4 weeks; the mice swam 5 days a week for 60 min a day. After swimming, the mice were dried with a towel and put back into the cage. During swimming training, changes in the body weight of the mice were recorded every day. The mice in the sham group, model group, and model + Ng cKO group were picked up and placed in the same box as the swimming training group, but without water.
2.7. T maze
The T maze was performed to test the spatial working memory of the mice based on their autonomous alternative selection behavior.19 It consisted of 2 target arms (50 cm × 10 cm × 20 cm) and a starting arm (60 cm × 10 cm × 20 cm). The test included 2 parts, and the specific steps were as follows: During the first forced test, one of the arms was identified as the target arm and was closed. The mouse was placed in the starting arm and forced to choose the target arm with the gate open. Then, the mouse was expected to return to the starting arm. During the free choosing test, after the mouse returned to the starting arm, the gate of the starting arm was closed, and 15 s later, the gates of all arms were opened. The mouse was allowed to freely choose to enter any target arm, and once it entered 1 target arm, the other target arms would be closed. Then the mouse was expected to return to the starting arm, and the starting arm gate was closed for 15 s. The above steps would be repeated 14 times. The continuous entry of the mouse into different target arms was considered to be a correct autonomous alternation.
The autonomous alternation rate is the number of autonomous alternation times/total number of choices × 100%.
2.8. MWM
The MWM was used to test the learning and memory abilities of the mice. The MWM was a round pool (120 cm in diameter and 50 cm in depth). The escape platform was a transparent circular platform with a diameter of 6 cm. The water maze was divided into 4 quadrants, and there were different markers on the pool wall in each quadrant. The MWM included 2 experiments, and the specific experimental schemes were as follows:20 For the orientation navigation experiment, mice were trained for 4 days. After training, researchers recorded the time it took (within 90 s) for a mouse to find the hidden platform; this was called escape latency. If the mouse could not find the platform within 90 s, it would be guided to the platform to learn for 15 s before the next navigation experiment. This experiment was repeated 4 times a day. Then, on the fifth day, a space exploration experiment was conducted. The escape platform was removed, the mice were put into the water facing the pool wall from the opposite quadrant of the original escape platform, and the number of crossing the corresponding position of the original platform within 90 s was recorded.
2.9. Western blotting
Mice were anesthetized with 3% isoflurane and euthanized by cervical dislocation. Mouse hippocampi were extracted and lysed in radioimmunoprecipitation assay with protease and phosphatase inhibitors. The protein lysates were quantified according to the bicinchoninic acid kit (Beyotime, Shanghai, China). Then, different gel concentrations were chosen according to the size of the protein molecules. Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under 20 V for 10 min, 60 V for 30 min, and 100 V for 60 min before being transferred to the polyvinylidene fluoride membrane. The membrane transfer voltage and time was selected according to the molecular weight of the protein. After washing in triethanolamine-buffered saline with Tween 20 and blocking in 5% milk, the membrane was incubated with the primary antibody, including rabbit anti-calcium/CaM-dependent protein kinase II (CaMKII) (1:1000; Abcam, Cambridge, UK), rabbit anti-CaM (1:10000; Abcam), rabbit anti-Ng (1:1000; Abcam), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:8000; Proteintech, Wuhan, China) 4°C overnight. The next day, the membrane was washed and incubated for 1 h at room temperature in secondary antibodies, including goat anti-rabbit (1:5000; Proteintech) and goat anti-mouse (1:5000; Proteintech). Finally, the membrane was put into the chromogenic solution for development using an imaging system (Bio-Rad, Hercules, CA, USA), and the gray value of the protein band was analyzed quantitatively.
2.10. Electrophysiology
Mice were quickly anesthetized with 3% isoflurane and euthanized by cervical dislocation. The brains were quickly removed and put into the artificial cerebrospinal fluid filled with 95% oxygen and 5% carbon dioxide. The brain tissue was cut into 400 μm with a vibration microtome. The brain slices were transferred to the incubation tank, where they were incubated at 37°C for 30 min and at room temperature for 1 h. Then, the brain slices were transferred to the recording tank. The recording electrode was filled with artificial cerebrospinal fluid, and the resistance was maintained at 3–6 MΩ. The stimulating electrode was placed on the fiber in the area of Cornu Ammonis 3 (CA3) to CA1, and the recording electrode was placed on the stratum radiatum in the CA1. The test stimulus intensity was defined as the stimulus intensity that could induce 30% of the maximum response amplitude. The brain slice was stimulated by a single pulse with test stimulus intensity for 20 min and recorded as the baseline. Then, the stimulating electrode was given a high-frequency stimulation of 100 Hz to induce long-term potential (LTP), and the field excitatory postsynaptic potential (fEPSP) was observed and recorded for 60 min.21
2.11. Immunohistochemistry
Mice were anesthetized with 2% pentobarbital sodium, transcardially perfused with 0.9% saline, and fixed with 4% paraformaldehyde. Then, the brain tissues were fixed with 4% paraformaldehyde for 24 h and embedded in paraffin. For immunohistochemical detection of neuronal nuclei (NeuN), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP) expression, brain tissue is prepared into 5-μm coronal sections. First, sections were deparaffinized by xylene and gradient alcohol, and after heat-mediated antigen retrieval with citrate buffer or ethylene diamine tetraacetic acid, sections were stained according to the steps of the immunohistochemistry kit (MX Biotechnologies Co., Ltd, Fuzhou, China). Brain sections were then rinsed briefly in phosphate-buffered saline, treated with 1% hydrogen peroxide for 10 min, and blocked for 10 min. Next, the sections were incubated with a mouse anti-NeuN antibody (1:1000; Abcam), mouse anti-GFAP antibody (1:1000; Proteintech), and rabbit anti-MBP (1:200; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. They were incubated with secondary antibody and streptomyces antibiotic protein-peroxidase on the second day. Diaminobenzidine chromogenic agent was used to develop color. And ImageJ Version 1.8.0 (NIH, New York, NY, USA) was used to analyze the average optical density values for quantification.
2.12. Statistical analysis
In this study, SPSS software Version 25.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis of all experimental data. The data were in accordance with normal distribution and were expressed as mean ± SD. The escape latency of the MWM was analyzed by two-way repeated measure analysis of variance, and the rest of the experimental data was analyzed by one-way analysis of variance. According to whether or not the variance conformed to the homogeneous distribution, least significant difference and Dunnett's T3 were performed for post hoc comparison of data. In all cases, significance levels were established at a level of p < 0.05.
3. Results
3.1. Identification of gene and protein expression in Ng cKO mice
For detecting the target genes of Ngflox/flox and Nestin-cre, the mice tail tips were cut for gene identification. The genes showed in Fig. 1B and 1C were Ng and Nestin-cre, respectively. The Ngwt/wt wt gene band was 260 bp, the Ngflox/wt heterozygous gene band was 353 bp and 260 bp, and the Ngflox/flox homozygous gene band was 353 bp. The Nestin-cre positive gene band was 506 bp. The PCR results showed that mice numbered 1–7 were all homozygous for Ng and the expressions of Nestin-cre were positive, indicating that the target gene was successfully knocked out; mice numbered 8–14 were all homozygous for Ng, but Nestin-cre expressions were negative, indicating that the target gene had not been knocked out. In order to detect the protein level of Ng in the Ng cKO group, mouse brain hippocampi were extracted for WB. The band showed that the Ng protein level in Ng cKO mice was significantly lower than that of control mice, suggesting that the Ng gene was successfully knocked out conditionally in the nervous system (Fig. 1D). C57BL/6 mice were used as the sham group in Experiment 1. The wt littermate controls were used as the control group in Experiment 1, and the sham group in Experiment 2.
3.2. Changes of spatial memory and CBF in Ng cKO mice and BCAS mice
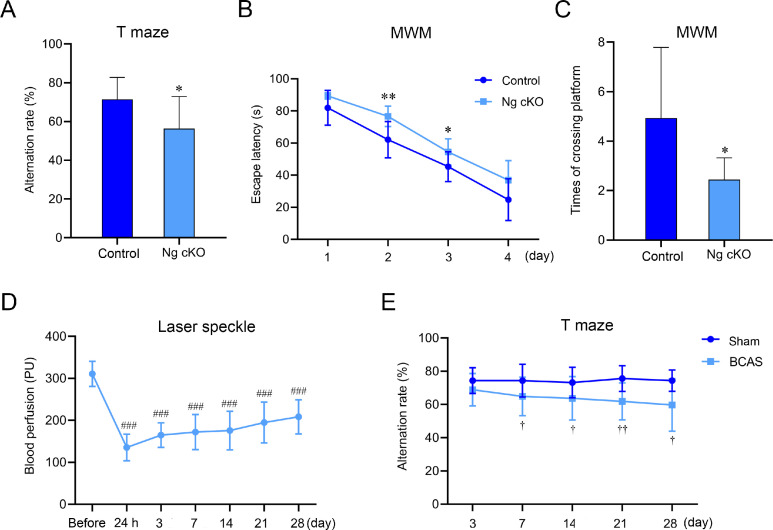
In Experiment 1, to explore the behavioral characteristics of the Ng cKO mice, the T maze and MWM were used to detect the spatial memory of 2-month-old mice. The results showed that compared with the control group, the autonomous alternation rate of the T maze in the Ng cKO group was significantly reduced (p < 0.05, Fig. 2A), the escape latency of the water maze was significantly prolonged (p < 0.05, p < 0.01, Fig. 2B), and the number of platform crossings was significantly reduced (p < 0.05, Fig. 2C). There was no significant difference in swimming speed during the orientation navigation trial between groups (p > 0.05, Supplementary Fig. 1A). These data proved that the knockout of Ng impaired spatial memory ability.
Fig. 2.
Changes of spatial memory and cerebral blood perfusion in Ng cKO mice and BCAS mice. (A) The autonomous alternating rate of T maze in control and Ng cKO mice (n = 9 per group); (B) The escape latency of Morris water maze in control group and Ng cKO mice (n = 9 per group); (C) The number of platform crossings of MWM in control and Ng cKO mice (n = 9 per group); (D) Changes of cerebral blood perfusion in BCAS mice before operation to 28 days after operation (n = 12 per group); and (E) Changes of autonomous alternating rate of T maze in sham group and BCAS mice from 3 days to 28 days after operation (n = 12 per group). * p < 0.05, ** p < 0.01, compared with control group; ###p < 0.001, compared with cerebral blood perfusion before operation; †p < 0.05, ††p < 0.01, compared with sham group. BCAS = bilateral common carotid artery stenosis; MWM = Morris water maze; Ng cKO = neurogranin conditional knockout; PU = perfusion unit.
In order to monitor the temporal characteristics of CBF in BCAS mice, laser speckle was used to detect the change of CBF in 12 BCAS mice from before operation to 28 days after operation. The figure illustrated that compared with CBF before operation, it was significantly reduced from 24 h to 28 days after operation (p < 0.001, Fig. 2D). As time went by, the cerebral blood flow was found to have a trend of recovery, but the CBF was still lower 28 days after operation than it was before operation.
To investigate the temporal characteristics of memory impairment in BCAS mice, the T maze was used to test the memory function from 3 to 28 days after operation. The results demonstrated that compared with the sham group, the autonomous alternation rate of the BCAS group was decreased significantly from the 7th day and was aggravated until 28 days after the operation (p < 0.05, p < 0.01, Fig. 2E). Therefore, swimming training would be carried out on the 7th day after surgery.
3.3. Swimming training can improve spatial memory dysfunction by regulating Ng in BCAS mice
In Experiment 2, Fig. 3A presented the schematic diagram of the chronological order of BCAS mice undergoing swimming training, cognitive testing, and synaptic function detecting. Laser speckle was used to detect the changes in CBF after operation in each group of mice to determine whether the model establishment was successful. The data illustrated that compared with the sham group, the CBF of the BCAS group, swim group, BCAS + Ng cKO group, and swim + Ng cKO group were all decreased by about 55% (p < 0.001, Fig. 3B and 3C), and there was no difference among the 4 groups (p > 0.05, Fig. 3B and 3C). This suggested that the CBF of the BCAS mice in each group was significantly reduced after the operation. The T maze was used to test the spatial memory ability. The results demonstrated that compared with the sham group, the autonomous alternation rates of the BCAS group, swim group, BCAS + Ng cKO group, and swim + Ng cKO group were all decreased (p < 0.05, p < 0.01, Fig. 3D), and there was no statistical difference among the 4 groups (p > 0.05, Fig. 3D). The above results indicated that CCH would lead to spatial memory impairment in BCAS mice and that the baseline of spatial memory impairment in the 4 groups was similar.
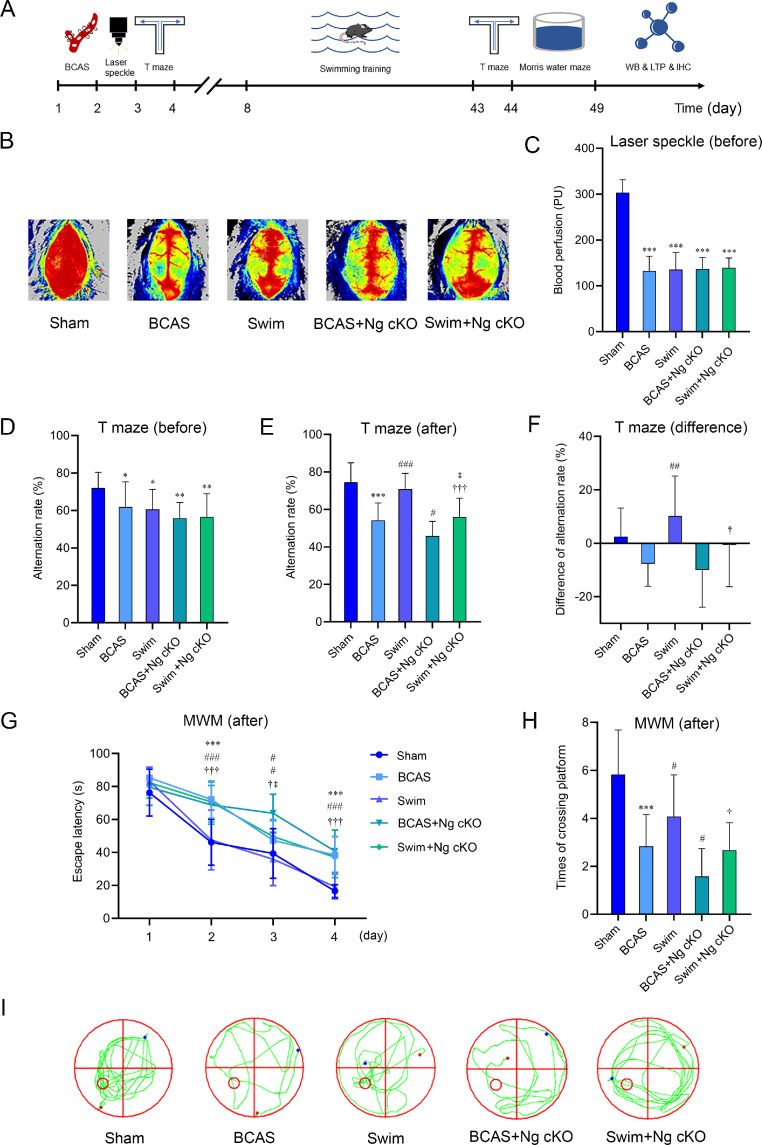
Fig. 3.
The role of Ng in swimming training to improve spatial memory impairment in BCAS mice. (A) Schematic diagram of the chronological order of BCAS mice undergoing swimming training, cognitive testing, and synaptic function detecting; (B) Typical schematic of CBF perfusion in 5 groups before swimming training; (C) The quantification of CBF in 5 groups before swimming training (n = 12 per group); (D) The quantification of autonomous alternating rate of T maze in 5 groups before swimming training (n = 12 per group); (E) The quantification of autonomous alternating rate of T maze in 5 groups after swimming training (n = 12 per group); (F) The difference of autonomous alternating rate before and after swimming training in 5 groups (n = 12 per group); (G) The escape latency of Morris water maze after swimming training in 5 groups (n = 12 per group); (H) The number of platform crossings of MWM after swimming training in 5 groups (n = 12 per group); and (I) Typical swimming traces after swimming training in 5 groups. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with sham group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with BCAS group; †p < 0.05, †††p < 0.001, compared with swim group; ‡p < 0.05, compared with BCAS + Ng cKO group. BCAS = bilateral common carotid artery stenosis; CBF = cerebral blood flow; IHC = immunohistochemistry; LTP = long-term potential; MWM = Morris water maze; Ng cKO = neurogranin conditional knockout; PU = perfusion unit; WB = Western blotting.
After swimming, the T maze and MWM were used to evaluate the effects of swimming training on the spatial memory function of mice with CCH. The T maze was adopted to detect spatial working memory. The results showed that compared with the sham group, a significant decrease in the autonomous alternation rate was reported in the BCAS group (p < 0.001, Fig. 3E). After the intervention, the autonomous alternation rate of the swim group was higher than that of the BCAS group (p < 0.001, Fig. 3E), while the autonomous alternation rate of the BCAS + Ng cKO group was lower than that of the BCAS group (p < 0.05, Fig. 3E). Compared with the swim group, a significantly lower voluntary alternation rate was observed in the swim + Ng cKO group (p < 0.001, Fig. 3E), and compared with the BCAS + Ng cKO group, the autonomous alternation rate of the swim + Ng cKO group was significantly increased (p < 0.05, Fig. 3E). Then, further analysis was conducted, and the difference before and after the intervention was calculated to detect the change in characteristics during the behavioral test. Compared with the BCAS group, a significant increase in the autonomous alternation rate was found in the swim group (p < 0.01, Fig. 3F), while the autonomous alternation rate of the swim + Ng cKO group was significantly higher than that of the swimming group (p < 0.05, Fig. 3F). These findings suggested that swimming can improve the spatial working memory of mice with CCH, while the knockout of Ng weakened the beneficial effects of swimming.
The MWM was adopted to detect spatial learning and memory, and it included 2 experimental stages. The first 4 days were the orientation navigation trial, and the 5th day was the space probe trial. The data indicated that compared with the sham group, the escape latency of the BCAS group was markedly increased and the number of platform crossings was significantly reduced (p < 0.001, Fig. 3G–3I). After swimming training, the escape latency of the swim group was significantly lower than that of the BCAS group, and the number of platform crossings was markedly higher (p < 0.05, p < 0.001, Fig. 3G–3I), while the escape latency of the BCAS + Ng cKO group was significantly higher than that of the BCAS group, and the number of platform crossings was significantly reduced (p < 0.05, p < 0.001, Fig. 3G–3I). Compared with the swim group, the swim + Ng cKO group spent more time searching for the platform, and the number of platform crossings was significantly decreased (p < 0.05, p < 0.001, Fig. 3G–3I). Compared with the BCAS + Ng cKO group, the escape latency of the swim + Ng cKO group was significantly lower (p < 0.05, Fig. 3G), and the number of platform crossings showed an upward trend, but there was no statistical significance (Fig. 3H–3I). In line with the results of the T maze, these findings suggested that swimming can also reduce the escape latency and increase the number of platform crossings of BCAS mice, thereby rescuing spatial learning and memory functions. However, conditionally knocking out Ng in the nervous system relatively offset the therapeutic effects of swimming training. In addition, there was no significant difference in swimming speed during the orientation navigation trial between groups (p > 0.05, Supplementary Fig. 1B), suggesting that performance in the MWM was not affected by swimming training.
3.4. Swimming training can enhance synaptic plasticity by regulating Ng in BCAS mice
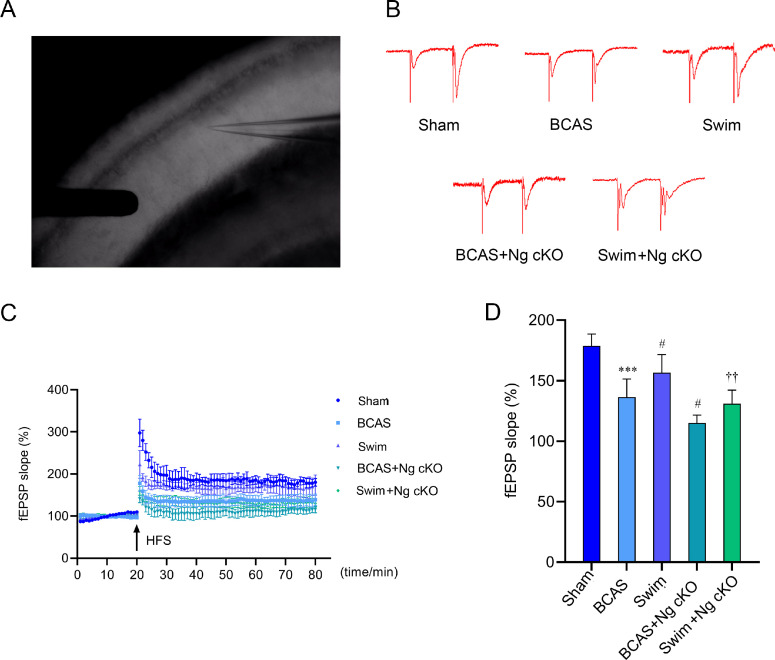
LTP in hippocampal CA3–CA1 region was recorded to evaluate synaptic plasticity, 100 Hz high-frequency stimulation was given to induce LTP response, and the slope of excitatory postsynaptic potential (fEPSP) was recorded as the index of LTP. Fig. 4A was the practical diagram of electrophysiological recording, and Fig. 4B showed that the postsynaptic potential of mice in each group obviously changed before and after high-frequency stimulation, which proved that LTP had been successfully induced. Electrophysiological results showed that the slope of fEPSP in the BCAS group was significantly lower than that in the sham group (p < 0.001, Fig. 4C and 4D). Compared with the BCAS group, the increase of fEPSP was reported in the swim group (p < 0.05, Fig. 4C and 4D), while it decreased significantly in the BCAS + Ng cKO group (p < 0.05, Fig. 4C and 4D). In addition, the slope of fEPSP in the swim + Ng cKO group was significantly lower than that in the swim group (p < 0.01, Fig. 4C and 4D). Compared with the BCAS + Ng cKO group, the slope of fEPSP in the swim + Ng cKO group showed an upward trend, but there was no statistical significance (Fig. 4C and 4D). These findings suggested that swimming training can enhance the synaptic plasticity of BCAS mice, while the knockout of Ng may hinder the positive effects by aggravating synaptic damage.
Fig. 4.
The role of Ng in swimming training to enhance synaptic plasticity in BCAS mice. (A) Practical diagram of electrophysiological recording; (B) Typical schematic of high-frequency stimulation-induced LTP and its corresponding baseline in the CA3–CA1 region after swimming training in 5 groups; (C) Time course of the fEPSP after swimming training in 5 groups before and after HFS; and (D) The quantification of fEPSP slopes after swimming training in 5 groups (n = 4 per group). *** p < 0.001, compared with sham group; #p < 0.05, compared with BCAS group; ††p < 0.01, compared with swim group. BCAS = bilateral common carotid artery stenosis; CA = Cornu Ammonis; fEPSP = field excitatory postsynaptic potential; HFS = high frequency stimulation; LTP = long-term potential; Ng = neurogranin; Ng cKO = neurogranin conditional knockout.
3.5. Swimming training can up-regulate Ca2+ signal transduction pathway-related proteins by regulating Ng in BCAS mice
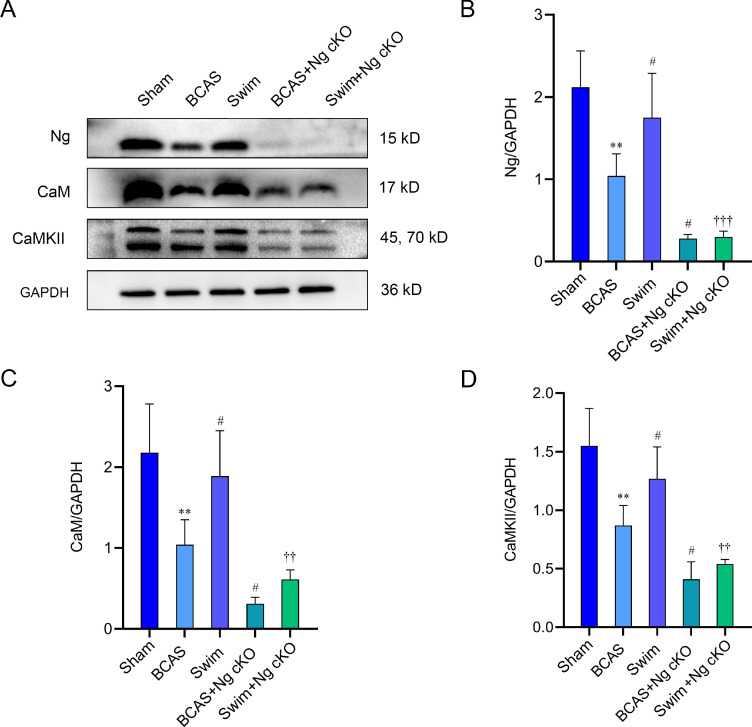
Western blotting was used for detecting the abundance of Ca2+ signal transduction pathway-related proteins. The results showed that levels of Ng, CaM, and CaMKII in the BCAS group were significantly lower than those in the sham group (p < 0.01, Fig. 5A–5D). Compared with the BCAS group, the expression levels of Ng, CaM, and CaMKII in the swim group were significantly increased (p < 0.05, Fig. 5A–5D), while their levels in the BCAS + Ng cKO group were significantly decreased (p < 0.05, Fig. 5A–5D). Besides, the levels of Ng, CaM, and CaMKII in the swim + Ng cKO group were significantly lower than those in the swim group (p < 0.01, p < 0.001, Fig. 5A–5D). Compared with the swim + Ng cKO group, the Ng expression level of the BCAS + Ng cKO group was not statistically different (p > 0.05, Fig. 5A–5D), and a rising trend was observed in the levels of CaM and CaMKII, but there was no significant difference (p > 0.05, Fig. 5A–5D). These results indicated that swimming training can up-regulate the abundance of calcium signal transduction pathway-related proteins, while Ng knockout will reduce the concentration of these proteins and impair the flow of Ca2+.
Fig. 5.
The role of Ng in swimming training to up-regulate Ca2+ signal transduction pathway-related proteins in BCAS mice. (A) Western blotting of Ng, CaM, and CaMKII in hippocampus from 5 groups after swimming training; (B) The quantification of Ng in hippocampus from 5 groups after swimming training (n = 3 per group); (C) The quantification of CaM in hippocampus from 5 groups after swimming training (n = 3 per group); and (D) The quantification of CaMKII in hippocampus from 5 groups after swimming training (n = 3 per group). ** p < 0.01, compared with sham group; #p < 0.05, compared with BCAS group; ††p < 0.01, †††p < 0.001, compared with swim group. BCAS = bilateral common carotid artery stenosis; CaM = calmodulin; CaMKII = calcium/calmodulin-dependent protein kinase II; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; Ng = neurogranin; Ng cKO = neurogranin conditional knockout.
3.6. Swimming training can ameliorate white matter injury, neuronal damage, and inflammation by regulating Ng in BCAS mice
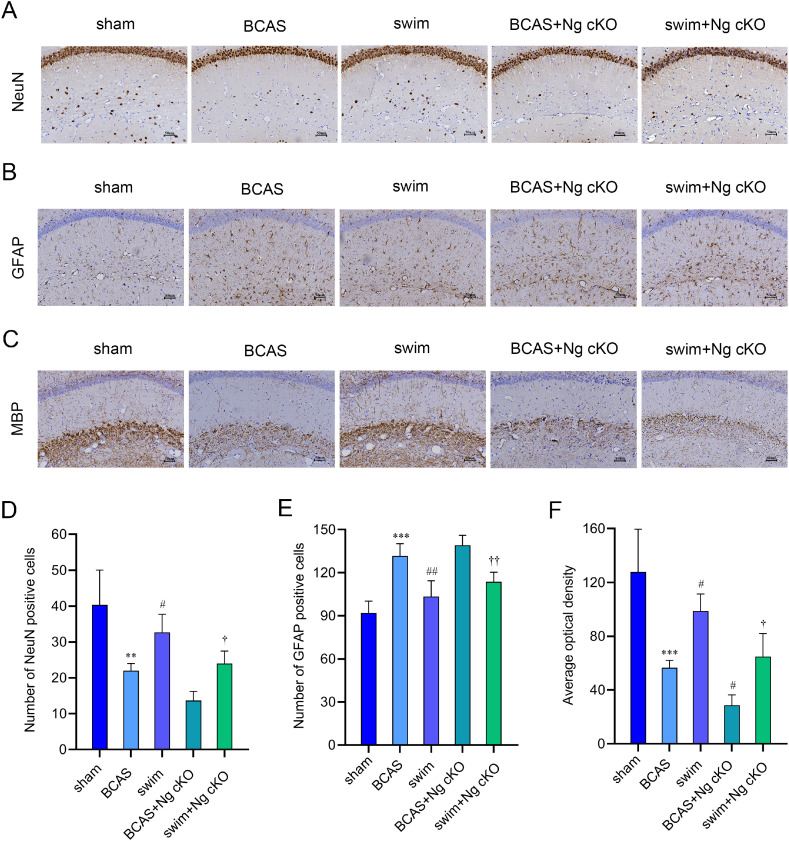
Immunohistochemistry staining was conducted to observe the expression levels of related markers involving white matter injury, neuronal damage and inflammation. It is believed that number of neurons can reflect the severity of neuronal death, that overactivation of astrocytes is involved in the inflammation induced by CCH, and that MBP is a critical membrane protein in the white matter of the brain. The results of the trial demonstrated that the expressions of NeuN, GFAP, and MBP in the hippocampus were lower in the BCAS group than in the sham group, and the expression of GFAP was higher (p < 0.01, p < 0.001, Fig. 6A–6F). The expressions of NeuN, GFAP, and MBP in the swim group were higher than those in the BCAS group, and the expression of GFAP was lower (p < 0.05, p < 0.01, Fig. 6A–6F). After the Ng gene was conditionally knocked out, the expression of MBP decreased significantly in the BCAS + Ng cKO group (p < 0.05, Fig. 6C and 6F), while a decreasing trend was observed in the level of NeuN, and an increasing trend was detected in the level of GFAP (p > 0.05, Fig. 6A–6B and 6D–6E). Furthermore, the expressions of NeuN and MBP were markedly increased in the swim + Ng cKO group, and the expression of GFAP was increased (p < 0.05, p < 0.01, Fig. 6A–6F). These data supported that swimming training improved and alleviated white matter injury, neuronal death, and inflammation induced by CCH by regulating Ng.
Fig. 6.
The role of Ng in swimming training to rescue neuronal damage, inflammation, and white matter injury in BCAS mice. (A) The expression level of NeuN in hippocampus from 5 groups after swimming training; (B) The expression level of GFAP in hippocampus from 5 groups after swimming training; (C) The expression level of MBP in hippocampus from 5 groups after swimming training; (D) The quantification of the number of NeuN positive cells (n = 3 per group); (E) The quantification of the number of GFAP positive cells (n = 3 per group); and (F) The quantification of the number of MBP positive cells (n = 3 per group). ** p < 0.01, *** p < 0.001, compared with sham group; #p < 0.05, ##p < 0.01, compared with BCAS group; †p < 0.05, ††p < 0.01, compared with swim group. BCAS = bilateral common carotid artery stenosis; GFAP = glial fibrillary acidic protein; MBP = myelin basic protein; NeuN = neuronal nuclear; Ng = neurogranin; Ng cKO = neurogranin conditional knockout.
4. Discussion
Cumulatively, our data showed that swimming training effectively up-regulated the expression of synapse-related proteins, enhanced synaptic plasticity, and thereby improved the spatial memory deficits induced by CCH. Further evidence proved that conditional knockout of Ng attenuated the beneficial effects of swimming training on spatial memory impairment in BCAS mice, which may be achieved by (A) down-regulating the contents of CaM and CaMKII, (B) affecting Ca2+ transduction, and (C) weakening synaptic plasticity. Moreover, the results showed swimming training improved and alleviated neuronal death, inflammation, and white matter injury by regulating Ng. These findings explained the potential mechanism by which swimming training may mediate Ng to regulate synaptic plasticity and related brain injury induced by CCH, thereby improving spatial memory impairment.
VCI is a type of cognitive impairment syndrome caused by continuous insufficient blood supply to the brain due to a variety of vascular factors. Atherosclerosis and cardioembolic diseases are the most common causes of vascular brain injury or infarction, and small vessel disease characterized by arteriosclerosis and lacunar infarction can also lead to cortical and subcortical microinfarction, which seems to be the most serious basis for cognitive impairment.22 Long-term hypoperfusion cerebral ischemia can cause white matter lesions and neuronal death, induce neuro-inflammatory response, and impair synaptic function, aggravating cognitive dysfunction.23 In this study, the CBF decreased most severely 2 h after the operation. With the development of pathology, the CBF showed a gradual upward trend, and it was decreased by about 30% at 28 days after the operation, which was consistent with the results of previous studies.24 In the T maze, there was no difference in the autonomous alternation rate of the BCAS mice at 3 days after the operation, and it had decreased significantly 7–28 days after the operation, suggesting that the memory impairment in BCAS mice occurred at the 7th day after surgery and then gradually worsened. Therefore, swimming training was given to BCAS mice at this point in time.
In recent years, meta-analyses have shown that exercise training can effectively improve the cognitive dysfunction of patients with cerebral ischemic diseases.5,6 Aerobic exercise is widely accepted and regarded as a potential treatment for cognition because it is cost-effective, convenient, and has no side effects. As one of the classic types of aerobic exercise, swimming has been proven to play a beneficial role with respect to cognitive impairment. Studies found that swimming for 4 weeks can effectively improve the cognitive dysfunction of rats with bilateral common carotid artery ligation and cerebral infarction. This finding was manifested by the shortened escape latency of the water maze, increased platform crossing times, and up-regulated levels of synaptic associated protein and neurotrophic factor.25 These results further strengthened the evidence that aerobic exercise can improve cognitive dysfunction in cerebral ischemic diseases, but the specific mechanism remains controversial.
Cumulative evidence supports the assumption that the hippocampus is an important part of the limbic system, and it is recognized as one of the critical brain regions in spatial memory coding and storage.26, 27, 28 The hippocampal classic three-synaptic circuit, which is composed of entorhinal cortex, dentate gyrus, CA3, and CA1, is an important neural connection for learning and memory, and spatial information is encoded and stored through synaptic transmission of neurons.29 However, the hippocampus is sensitive to ischemic injury and susceptible to neuronal death and inflammation induced by ischemia and hypoxia, which lead to cognitive dysfunction.29 The formation and encoding of memory are determined by the strength of synaptic connections between neurons, and the change of synaptic strength depends on synaptic plasticity.30 Generally, the electrophysiological activities of neurons are considered to reflect changes in the plasticity of synaptic functions, and long-term potentiation and long-term depression are the main manifestations; these are also considered to be important neurophysiological bases for learning and memory activities.31 In addition, the calcium signal transduction pathway is an important molecular regulation mechanism for synaptic plasticity. As a second messenger, Ca2+ can participate in multiple signal pathways and play a critical role in the induction and maintenance of LTP.32,33 There were studies showing that in rats subjected to bilateral common carotid artery ligation, the length and number of dendritic spines were significantly reduced, LTP was impaired, and synaptic transmission efficiency was weakened.34,35 Therefore, synaptic plasticity is significant for spatial memory function, and the recovery of spatial memory impairment depends on the improvement of synaptic plasticity.
Our results pointed out that ischemic injury in BCAS mice inhibited hippocampal LTP, and swimming was able to rescue the impairment and enhance synaptic transmission. However, interestingly, the beneficial effects of swimming on synaptic dysfunction and spatial memory impairment were limited in the BCAS + Ng cKO group. Studies have shown that Ng knockout mice exhibited memory dysfunction in hippocampal-dependent spatial tasks, which was related to the inhibition of hippocampal excitatory postsynaptic field potentials. Ng gene defect will lead to the decline of synaptic transmission efficiency and the weakening of hippocampal neuron activity, which in turn affects the encoding and storage of spatial memory.36 Further studies have found that enriched environment caused a significant increase in hippocampal Ng levels, both in Ng+/+ and Ng+/− mice, which seemed to contribute to their improved LTP and behavioral performances; however, the same intervention conditions cannot ameliorate the LTP deficiency and spatial memory impairment in Ng−/− mice.37
Similarly, many studies in recent years have shown that Ng is also expressed abnormally in a variety of diseases and can seriously affect cognitive function. Our team's previous meta-analysis found that the level of Ng significantly increased in the cerebrospinal fluid of patients with mild cognitive impairment and AD. Further analysis found that the Ng level in cerebrospinal fluid increases with cognitive decline and is negatively associated with Mini-mental State Examination scores.13 In addition, the concentration of Ng in the blood plasma exosomes of patients with AD and mild cognitive impairment decreased.13 At the same time, in patients with VCI, it was also found that the expression level of Ng in cerebrospinal fluid decreased and was associated with cognitive dysfunction.14,38 However, other articles demonstrated that the Ng level in patients with VCI, as measured by 3 assays targeting different epitopes, was not significantly different from that in the cognitively normal control group,15 and negative correlations were found between Ng and neuropsychiatric symptoms.39 In patients with acute ischemic stroke, the Ng level of cerebrospinal fluid in patients with an infarct volume >5 mL was significantly higher than in patients with smaller infarct volumes.40 Furthermore, an animal experiment found that brain contusions showed loss of Ng immunoreactivity at the site of the lesion, and acute cerebral ischemia resulted in an extensive loss of Ng staining in the ipsilateral hemisphere along with a decrease in the number of neuronal dendrites.41 Although there are few relevant studies at present, the existing results still suggested that the abnormal changes of Ng in cerebrospinal fluid are closely related to the cognitive dysfunction of patients with vascular diseases.
Ng is a brain-specific postsynaptic protein highly expressed in the cerebral cortex, hippocampus, striatum, and amygdala that plays an important regulatory role in learning and memory abilities.42 It is a binding protein of Ca2+-sensitive CaM and substrate of PKC, the CaM-binding affinity of which is modulated by phosphorylation and oxidation. This protein can participate in downstream core protein activation and synaptic activities through calcium signaling pathways, thereby affecting cognitive function.43 The interaction between Ng and CaM is related to the regulation of synaptic response. Specifically, Ng can be phosphorylated at its serine 36 residue within the isoleucine and glutamine–motif by PKC, rendering CaM incapable of rebinding to phosphorylated Ng. Then, free CaM is released, which further promotes the combination of free Ca2+ and CaM to form an activated complex.44 This complex can activate downstream CaMKII, which is considered to be a synaptic protein closely related to learning and memory and is called a molecular switch of memory, and the activation and autophosphorylation of CaMKII can increase the sensitivity of NMDA and AMPA receptors and promote the flow of Ca2+ to induce and maintain LTP, thereby enhancing synaptic plasticity.45
Additionally, Ng knockout mice showed spatial memory impairment and impaired LTP as well as significantly decreased activities of CaMKII and PKC in hippocampus as compared with controls.36 Under novel context exposure, the Ng messenger RNA was recruited to the actively translating messenger RNA pool, and the expression level of protein was significantly increased in the hippocampus.46 Long-term blockade of N-Methyl-D-Aspartate receptors substantially decreased Ng and CaM expression in hippocampal neurons and impaired synaptic activity.43 These findings revealed that Ng was closely related to hippocampal synaptic activity, and both were involved in the encoding and formation of spatial memory. Furthermore, rehabilitation exercise can up-regulate the expression levels of synaptophysin and Ng in the hippocampus, promoting the recovery of cognitive function after cerebral ischemia.47 Similarly, this study showed that the impairment of spatial memory and LTP was observed and the concentrations of Ng, CaM and CaMKII were decreased in the hippocampus of BCAS mice. These results confirmed that the abnormal expression of Ng is an important pathological feature of VCI and that swimming training can reverse this situation.
In addition to the damage of synaptic function, neuronal death, neuroinflammation, and white matter injury were the other main pathological mechanisms of BCAS mice, and studies have shown that regular aerobic exercise can effectively promote neurogenesis,8 reduce neuronal death,48 alleviate the damage caused by oxidative stress,49 increase the number of oligodendrocytes, and improve white matter injury.50 In this study, the same experimental results were observed, but whether Ng knockdown exacerbated white matter injury and inflammation in the brain was inconclusive. Immunohistochemical analysis suggested that the knockout of Ng aggravated neuronal death and white matter injury. This may be because Ng is a neuron-specific protein that is located in dendrites and is closely related to neurogenesis and synaptic plasticity.43,51 However, despite the change trend, the effect of Ng knockout on the number of GFAP was not statistically significant. A clinical study showed that markers of neuronal damage, such as tau and Ng, were highly correlated with a specific set of inflammatory markers.52 The cerebrospinal fluid level of these proteins can be used as a readout of the inflammatory response in the neurodegeneration stage of AD. Further studies have found that CXC chemokine 12 and CXC chemokine receptor 4 released by excessive activation of microglia were related to the level of Ng expression, suggesting a close connection between postsynaptic injury and microglia activation in AD,53 which also provides a direction for future research.
Based on the results above, exercise can induce the enhancement of learning and memory by regulating the expression of Ng. Specifically, the results showed that exercise up-regulated the expression level of hippocampal Ng and promoted the phosphorylation of Ng, leading to the release of CaM, which combined with Ca2+ to activate CaMKII. The augmentation and enhancement of Ca2+ signaling further strengthened LTP and improved synaptic plasticity. At the same time, exercise-mediated Ng expression and activity level improved neuronal death, neuroinflammation, and white matter damage induced by cerebral ischemia. All of these changes have contributed to the improvement of cognition and explain possible underlying mechanisms.
This study utilized a chronic cerebral ischemia model with neurodegenerative changes. However, the expression level of total Ng was only detected at 1 time point, and neither a time-course evaluation of Ng in the brain nor a functional study of phosphorylated Ng has been carried out. In addition, this study only detected the expression level of full-length Ng, and studies have found that the Ng protein may exist in the form of peptides of different lengths in different regions, such as in cerebrospinal fluid, blood, and brain tissue. The role of different peptides of the Ng protein in diseases, and their beneficial role with respect to the way aerobic exercise improves cognition, may become a significant research direction in the field of cognitive neurology in the future. This study only adopted young animals, and the swimming therapy was initiated at 7 days after surgery. This time point may not be fully applicable to clinical treatment because patients currently clinically diagnosed with VCI are usually older, and may be accompanied by the neurological and motor deficits. Furthermore, another study demonstrates that a good pre-clinical model (inducible p25 mouse model) presented similar biomarker signs of synaptic alterations as those seen in patients with AD, proving that Ng is a translatable biomarker of value for future pre-clinical and clinical studies.54 However, our current research only detected the level of Ng in the brain tissue of this animal model induced by CCH. In the future, studies on CSF and blood levels of Ng and their correlation with cognitive changes in CCH models, together with the longitudinal clinical trials of patients with vascular diseases, will certainly promote the clinical translational application of Ng as a biomarker and shed light on the relationship between Ng and cognitive parameters.
5. Conclusion
To conclude, results demonstrated that swimming training elicited an enhancement of hippocampal synaptic plasticity and attenuation of cognitive deficits induced by CCH, and the beneficial effect was achieved by up-regulating the levels of Ng and promoting the transduction of Ca2+ signal. Moreover, swimming training reduced the number of neuronal death, relieved neuroinflammation, and improved white matter injury. This study strengthened the evidence that swimming training was regarded as a potentially promising therapy for improving cognitive impairment in VCI patients, and a prominent role is emerging for Ng and its downstream signaling cascades in this treatment.
Acknowledgments
Acknowledgment
Supported by the Youth Top Talent Project of Fujian Province, China “Young Eagle Project” (No. 2901-750102003).
Authors’ contributions
WLL contributed to the experimental design and the manuscript revision; HWL and JYZ contributed to performing the experiments, analyzing data, making figures, and writing manuscripts; YLD, HHL, XJH, and LWC played a role in performing the experiments and collecting the data; JT and CHL revised manuscripts. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.01.008.
Supplementary materials
References
- 1.Du SQ, Wang XR, Xiao LY, et al. Molecular mechanisms of vascular dementia: What can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017;54:3670–3682. doi: 10.1007/s12035-016-9915-1. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health. 2020;5:e661–e671. doi: 10.1016/S2468-2667(20)30185-7. [DOI] [PubMed] [Google Scholar]
- 4.Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10:1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Oberlin LE, Waiwood AM, Cumming TB, et al. Effects of physical activity on poststroke cognitive function: A meta-analysis of randomized controlled trials. Stroke. 2017;48:3093–3100. doi: 10.1161/STROKEAHA.117.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shu Y, He Q, Xie Y, Zhang W, Zhai S, Wu T. Cognitive gains of aerobic exercise in patients with ischemic cerebrovascular disorder: A systematic review and meta-analysis. Front Cell Dev Biol. 2020;8:582380. doi: 10.3389/fcell.2020.582380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu-Ambrose T, Best JR, Davis JC, et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology. 2016;87:2082–2090. doi: 10.1212/WNL.0000000000003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DH, Lee KH, Lee J. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep. 2016;13:2981–2990. doi: 10.3892/mmr.2016.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JM, Kim CJ, Park JM, Song MK, Kim YJ. Effect of treadmill exercise on spatial navigation impairment associated with cerebellar Purkinje cell loss following chronic cerebral hypoperfusion. Mol Med Rep. 2018;17:8121–8128. doi: 10.3892/mmr.2018.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L, Zhu M, Kong C, et al. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimers Dement. 2021;17:49–60. doi: 10.1002/alz.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headley A, De Leon-Benedetti A, Dong C, et al. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology. 2018;90:e887–e895. doi: 10.1212/WNL.0000000000005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvartsberg H, Duits FH, Ingelsson M, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimers Dement. 2015;11:1180–1190. doi: 10.1016/j.jalz.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Lin H, He X, et al. Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer's disease and mild cognitive impairment. Transl Psychiatry. 2020;10:125. doi: 10.1038/s41398-020-0801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janelidze S, Hertze J, Zetterberg H, et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer's disease. Ann Clin Transl Neurol. 2016;3:12–20. doi: 10.1002/acn3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willemse EAJ, De Vos A, Herries EM, et al. Neurogranin as cerebrospinal fluid biomarker for Alzheimer disease: An assay comparison study. Clin Chem. 2018;64:927–937. doi: 10.1373/clinchem.2017.283028. [DOI] [PubMed] [Google Scholar]
- 16.Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89:1782–1788. doi: 10.1212/WNL.0000000000004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Xue X, Xia J, Liu J, Qi Z. Swimming exercise reverses CUMS-induced changes in depression-like behaviors and hippocampal plasticity-related proteins. J Affect Disord. 2018;227:126–135. doi: 10.1016/j.jad.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Spowart-Manning L, van der Staay FJ. The T-maze continuous alternation task for assessing the effects of putative cognition enhancers in the mouse. Behav Brain Res. 2004;151:37–46. doi: 10.1016/j.bbr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Farkas E, Luiten PGM, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Lin PY, Kavalali ET, Monteggia LM. Genetic dissection of presynaptic and postsynaptic BDNF-TrkB signaling in synaptic efficacy of CA3-CA1 synapses. Cell Rep. 2018;24:1550–1561. doi: 10.1016/j.celrep.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol. 2016;131:659–685. doi: 10.1007/s00401-016-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishio K, Ihara M, Yamasaki N, et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41:1278–1284. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- 25.Song MK, Kim EJ, Kim JK, Park HK, Lee SG. Effect of regular swimming exercise to duration-intensity on neurocognitive function in cerebral infarction rat model. Neurol Res. 2019;41:37–44. doi: 10.1080/01616412.2018.1524087. [DOI] [PubMed] [Google Scholar]
- 26.Moser MB, Rowland DC, Moser EI. Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol. 2015;7:a021808. doi: 10.1101/cshperspect.a021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun C, Fukushi Y, Wang Y, Yamamoto S. Astrocytes protect neurons in the hippocampal CA3 against ischemia by suppressing the intracellular Ca2+ overload. Front Cell Neurosci. 2018;12:280. doi: 10.3389/fncel.2018.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patten AR, Yau SY, Fontaine CJ, Meconi A, Wortman RC, Christie BR. The benefits of exercise on structural and functional plasticity in the rodent hippocampus of different disease models. Brain Plast. 2015;1:97–127. doi: 10.3233/BPL-150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocchio M, Nabavi S, Capogna M. Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron. 2017;94:731–743. doi: 10.1016/j.neuron.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Lisman J, Cooper K, Sehgal M, Silva AJ. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci. 2018;21:309–314. doi: 10.1038/s41593-018-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D, Bacaj T, Morishita W, et al. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature. 2017;544:316–321. doi: 10.1038/nature21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster WJ, Taylor HBC, Padamsey Z, Jeans AF, Galione A, Emptage NJ. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling. Sci Signal. 2018;11:eaat9093. doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Yao Y, Wang L, et al. Gastrin-releasing peptide facilitates glutamatergic transmission in the hippocampus and effectively prevents vascular dementia induced cognitive and synaptic plasticity deficits. Exp Neurol. 2017;287:75–83. doi: 10.1016/j.expneurol.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Bhuvanendran S, Bakar SNS, Kumari Y, Othman I, Shaikh MF, Hassan Z. Embelin Improves the spatial memory and hippocampal long-term potentiation in a rat model of chronic cerebral hypoperfusion. Sci Rep. 2019;9:14507. doi: 10.1038/s41598-019-50954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pak JH, Huang FL, Li J, et al. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: A study with knockout mice. Proc Natl Acad Sci U S A. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang FL, Huang KP, Wu J, Boucheron C. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26:6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manniche C, Simonsen A, Hasselbalch SG, et al. Cerebrospinal fluid biomarkers to differentiate idiopathic normal pressure hydrocephalus from subcortical ischemic vascular disease. J Alzheimers Dis. 2020;75:937–947. doi: 10.3233/JAD-200036. [DOI] [PubMed] [Google Scholar]
- 39.Bloniecki VB, Zetterberg H, Aarsland D, et al. Are neuropsychiatric symptoms in dementia linked to CSF biomarkers of synaptic and axonal degeneration? Alzheimers Res Ther. 2020;12:153. doi: 10.1186/s13195-020-00718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vos A, Bjerke M, Brouns R, et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017;17:170. doi: 10.1186/s12883-017-0945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li GL, Farooque M, Lewen A, et al. MAP2 and neurogranin as markers for dendritic lesions in CNS injury. An immunohistochemical study in the rat. APMIS. 2000;108:98–106. doi: 10.1034/j.1600-0463.2000.d01-32.x. [DOI] [PubMed] [Google Scholar]
- 42.Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990;10:3782–3792. doi: 10.1523/JNEUROSCI.10-12-03782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido-García A, de Andrés R, Jiménez-Pompa A, et al. Neurogranin expression is regulated by synaptic activity and promotes synaptogenesis in cultured hippocampal neurons. Mol Neurobiol. 2019;56:7321–7337. doi: 10.1007/s12035-019-1593-3. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Huang KP, Huang FL. Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+- and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J Neurochem. 2003;86:1524–1533. doi: 10.1046/j.1471-4159.2003.01963.x. [DOI] [PubMed] [Google Scholar]
- 45.Ataei N, Sabzghabaee AM, Movahedian A. Calcium/calmodulin-dependent protein kinase II is a ubiquitous molecule in human long-term memory synaptic plasticity: A systematic review. Int J Prev Med. 2015;6:88. doi: 10.4103/2008-7802.164831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones KJ, Templet S, Zemoura K, et al. Rapid, experience-dependent translation of neurogranin enables memory encoding. Proc Natl Acad Sci U S A. 2018;115:E5805–E5814. doi: 10.1073/pnas.1716750115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wu Y, Cheng M, et al. Effects of rehabilitation on cognition, synaptophysin and neurogranin in rats with cerebral infarction. Chin J Rehabil Theory Pract. 2012;18:15–18. [in Chinese] [Google Scholar]
- 48.Lin Y, Xu Y, Feng H, et al. Involuntary, forced or voluntary exercise can ameliorate the cognitive deficits by enhancing levels of hippocampal NMDAR1, pAMPAR1 and pCaMKII in a model of vascular dementia. Neurol Res. 2021;43:349–357. doi: 10.1080/01616412.2020.1866351. [DOI] [PubMed] [Google Scholar]
- 49.Luca M, Luca A. Oxidative stress-related endothelial damage in vascular depression and vascular cognitive impairment: Beneficial effects of aerobic physical exercise. Oxid Med Cell Longev. 2019;2019:8067045. doi: 10.1155/2019/8067045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trigiani LJ, Lacalle-Aurioles M, Bourourou M, et al. Benefits of physical exercise on cognition and glial white matter pathology in a mouse model of vascular cognitive impairment and dementia. GLIA. 2020;68:1925–1940. doi: 10.1002/glia.23815. [DOI] [PubMed] [Google Scholar]
- 51.Huang KP, Huang FL, Shetty PK. Stimulation-mediated translocation of calmodulin and neurogranin from soma to dendrites of mouse hippocampal CA1 pyramidal neurons. Neuroscience. 2011;178:1–12. doi: 10.1016/j.neuroscience.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brosseron F, Kleemann K, Kolbe CC, et al. Interrelations of Alzheimer´s disease candidate biomarkers neurogranin, fatty acid-binding protein 3 and ferritin to neurodegeneration and neuroinflammation. J Neurochem. 2021;157:2210–2224. doi: 10.1111/jnc.15175. [DOI] [PubMed] [Google Scholar]
- 53.Sanfilippo C, Castrogiovanni P, Imbesi R, Nunnari G, Di Rosa M. Postsynaptic damage and microglial activation in AD patients could be linked CXCR4/CXCL12 expression levels. Brain Res. 2020;1749:147127. doi: 10.1016/j.brainres.2020.147127. [DOI] [PubMed] [Google Scholar]
- 54.Höglund K, Schussler N, Kvartsberg H, et al. Cerebrospinal fluid neurogranin in an inducible mouse model of neurodegeneration: A translatable marker of synaptic degeneration. Neurobiol Dis. 2020;134:104645. doi: 10.1016/j.nbd.2019.104645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.