Highlights
-
•
Endurance exercise capacity may be influenced by gut microbiota.
-
•
Veillonella atypica and lactic acid bacteria may affect lactate metabolism.
-
•
Short-chain fatty acids may modulate substrate metabolism in skeletal muscle.
-
•
Prebiotics, probiotics, and postbiotics could have ergogenic potential.
-
•
More human research is needed to understand the gut microbiota–exercise connection.
Keywords: Exercise performance, Gut microbiota, Short-chain fatty acids
Abstract
The gut microbiota refers to the collection of trillions of intestinal microorganisms that modulate central aspects of health and disease through influential effects on host physiology. Recently, a connection has been made between the gut microbiota and exercise. Initial investigations demonstrated the beneficial effects of exercise on the gut microbiota, with cross-sectional studies revealing positive correlations between exercise-associated states, and healthy gut microbiota and exercise interventions showed post-intervention increases in the abundance of beneficial bacterial taxa. More recent investigations have focused on exploring the reverse relationship: the influence of the gut microbiota on exercise performance. Murine investigations have revealed that certain bacterial taxa may enhance endurance exercise performance by augmenting various aspects of lactate metabolism. Further, short-chain fatty acids—which modulate metabolism at various organ sites, including within skeletal muscle—have been shown to enhance endurance exercise capacity in mice. This review highlights what is currently known about the connection between the gut microbiota and exercise, with a particular focus on the ergogenic potential of the gut microbiota and how it may be leveraged to enhance endurance exercise performance.
GraphicAbstract
1. Introduction
Efforts to understand the key physiological determinants contributing to elite human endurance exercise performance has been a long-term pursuit of sports science research and continues to be a primary focus.1 Currently, most research is directed at understanding how cardiovascular and muscular adaptations to exercise training, such as cardiac output, hemoglobin concentration, and muscle capillarization, affect well-established evaluations of endurance exercise performance, including maximal oxygen consumption (V̇O2max) and the boundary of sustainable V̇O2.2 While central (i.e., cardiovascular) and peripheral (i.e., musculoskeletal) factors remain key determinants of exercise performance, recent research has attempted to reach beyond the roles of traditional physiological mechanisms to explore how exercise performance may be influenced by less well-established factors—in particular, the gut microbiota.
Exercise bouts lasting seconds to hours require a continuous supply of adenosine triphosphate (ATP) in order to maintain skeletal muscle contraction.3 The ATP-phosphocreatine, glycolytic, and aerobic energy systems provide ATP to exercising tissues via substrate-level phosphorylation and oxidative phosphorylation, with the relative contribution of each system dependent on exercise intensity and duration.3 The gut microbiota influences host energy metabolism through production of its own metabolites, which provide ∼10% of the daily caloric requirements of the host,4 and via its direct and/or indirect influence on appetite, fat storage, and glucose tolerance. Given its diverse influence on key metabolic processes, sports science research has begun focusing effort on better understanding how the gut microbiota may be implicated in exercise and exploring ways it may be leveraged to enhance exercise tolerance and capacity. Initial insights from animal and human models have demonstrated improvements in exercise capacity following interventions that target microbial optimization (i.e., probiotic, prebiotic, and short-chain fatty acid (SCFA) supplementation), revealing the ergogenic potential of the gut microbiota. These findings suggest that the gut microbiota may represent a previously unexplored determinant of exercise performance, and exogenous strategies targeting the gut microbiota may represent a novel mechanism for enhancing human exercise tolerance, capacity, and performance.
2. Gut microbiota
The gut microbiota—which represents a complex ecosystem of approximately 40 trillion bacteria, viruses, archaea, and fungi—resides predominantly in the large intestine and has a biomass of approximately 1.5 kg.5 Bacteria represent the largest microbial contribution to the gut microbiota, consisting of well over 1000 unique bacterial species belonging largely to 4 dominant phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria.6 The microbiota inhabits the entire gastrointestinal tract, with the lowest abundance of bacteria in the stomach (10 bacteria/gram of content) and highest abundance in the colon (1012 bacteria/gram of content).7 The increase in bacterial abundance along the gastrointestinal tract is met with an increase in biodiversity, with the colon harboring the most diverse bacterial organisms.8 In comparison to the human genome, which encodes approximately 23,000 genes, the gut microbiome—a term used when the genetic potential of the microbiota is referred to alongside the microbes themselves—contains approximately 3.3 million genes that modulate several aspects of host health and function, including energy harvest and metabolism,9 inflammation,10 vitamin synthesis,11 and immunity.12
All humans share similarities in the general composition and function of their gut microbiomes, and some evidence suggests there may even be a “core microbiome” that exists among all individuals.13 Nevertheless, variations in microbial abundance, composition, diversity, and gene function lead to immense interindividual differences in the gut microbiomes of humans.14 The diversity of this community, which is believed to be as unique as a fingerprint,15 is stark when compared to human genomic diversity. Of the 23,000 genes that make up our host genome, approximately 99.9% are identical among all humans,16 whereas the genes that make up our gut microbiome can differ substantially from one another.13 Given the immense variability of human gut microbiomes and the significant influence of the microbiome on host health, research in the medical sciences is now pivoting to begin considering the native microbiome in the development of personalized approaches to medicine and to enhance the effectiveness of disease treatments.17 In time, these efforts may be extended to approaches focused on enhancing exercise performance.
The initial development of the gut microbiota occurs during the first 3 years of life,18 and early-life exposures, such as birth mode, infant diet, and antibiotic exposure, play a critical role in microbial colonization.19 Once established, the gut microbiota composition remains stable from 3 years of age until late adulthood;20 however, environmental factors including diet, stress, and exercise can alter the community composition towards a more eubiotic (balanced microbiota) or dysbiotic (unbalanced microbiota) state, which directly influences host functions.21 As a result, there is now intense interest in exploring how various intrinsic and extrinsic factors influence the gut microbiota (and thus host function) and how the microbiota can be optimized to support health status, or in the context of this review, exercise performance.
3. Prebiotics, probiotics, and postbiotics
Prebiotics, probiotics, and postbiotics comprise a family of substances that function to modulate the gut microbiota and can be referred to collectively as “biotics”.22 The use of prebiotics and probiotics to increase the abundance of beneficial bacterial species (probiotics) or fertilize existing commensal bacterial species (prebiotics) is an exogenous approach for improving the structure and function of the gut microbiota.23 Probiotics are “live microorganisms that confer a health benefit to the host when administered in adequate amounts”,24 and they are most often found in the form of single or consortium blends of Lactobacillus and Bifidobacterium strains. A prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit,” and it can be found in non-digestible oligosaccharides, fructans, and galactans.25 The concept of postbiotics has emerged more recently and is defined as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”.26 Postbiotics contain inactivated microbial cells or cell components, with or without metabolites such as SCFAs,27 although commercial availability of these supplements is currently low.
4. Gut microbiota fermentation and SCFAs
Following consumption, the constituents of non-digestible carbohydrates such as dietary fiber remain intact until they reach the large intestine, where they become available to the gut microbiota for fermentation and energy extraction.28 Specifically, commensal gut microbiota can ferment non-digestible substrates into 3 main SCFAs—acetate, propionate, and butyrate—which are important metabolites for maintaining colonic health and integrity.29,30 Production of acetate, propionate, and butyrate occurs at an approximate molar ratio of 60:20:20, respectively,31 although factors such as bacterial population (type and abundance), diet, and gut transit time can affect SCFA production.32 Concentrations of SCFA are highest in the proximal colon (70–140 mM) and fall to 20–70 mM in the distal colon.31 In the cecum and colon, approximately 95% of SCFA are rapidly absorbed by the colonocytes, with the remaining 5% being excreted in the feces (this being the fraction that is measured in fecal samples).32 Butyrate is the preferred fuel for colonocytes and is extensively oxidized by the intestinal epithelium to improve intestinal health through various local effects.33 Acetate and propionate are also utilized, but they are oxidized to a lesser extent than butyrate.34 SCFA not oxidized in colonocytes travel through the portal vein to the liver, where they can be used as substrates for Krebs cycle or enter systemic circulation and elicit beneficial effects in several cells and organs.35 Recent insights suggest one of these target organs is skeletal muscle containing G-protein-coupled receptors GPR41 and GPR43, which are SCFA-specific receptors believed to play a role in skeletal muscle metabolism.36 Given the fundamental role of energy metabolism during exercise, the influence that SCFA may have on metabolic processes within skeletal muscle is of particular interest. However, more research is needed to discern the underlying direct or indirect mechanisms and potential interaction that gut-derived SCFA may have on exercise performance.
5. Effects of exercise on the gut microbiota
Efforts to understand the relationship between exercise and the gut microbiota have emerged largely over the past decade. In 2014, Clarke and colleagues37 published a landmark study in exercise and gut microbiota research showing that professional rugby athletes had higher alpha diversity and a greater relative abundance of the health-associated genus Akkermansia compared to high- and low-body mass index sedentary controls. These findings prompted several subsequent investigations focused on better understanding the relationship between exercise and the gut microbiota. Several observational studies have revealed that exercise-associated states are correlated with higher alpha diversity,38,39 an enrichment of beneficial taxa,38,40, 41, 42 and a higher abundance of fecal SCFA.43 Alternatively, exercise training intervention studies in healthy and clinical populations have only seldomly revealed post-intervention increases in alpha diversity,44,45 although increases in the relative abundance of health-associated taxa have been consistently demonstrated.46, 47, 48, 49 While observational and intervention investigations have revealed the influential effects of exercise on the gut microbiota, less described is the reverse relationship: the influential effects of the gut microbiota on exercise. The remainder of this paper will focus on reviewing the current evidence demonstrating the influential effects of the gut microbiota and its metabolites on proxy measures of endurance exercise performance—mainly time to exhaustion (TTE) tasks—in human and murine models, and it will describe the potential physiological mechanisms underlying this unique relationship.
6. Effects of the gut microbiota on endurance exercise capacity: Human to mouse experiments
In 2019, Scheiman and colleagues50 were the first to demonstrate that endurance exercise capacity may be influenced by a microbially mediated mechanism. In this multi-part study, 16S rDNA analyses of daily stool samples provided by Boston marathoners approximately 5 days prior to and following the marathon revealed a significant increase in the relative abundance of the genus Veillonella in the athletes post-marathon. Veillonella is a gram-negative bacterium that utilizes lactate as its primary source of carbon energy.51 Given that marathon running is characterized by marked increases in lactate production (and removal) by skeletal muscle fibers, consequent of high rates of glycolysis to meet the ATP demands,3 researchers isolated a human strain of Veillonella atypica to explore the potential ergogenic effects of this bacterium during high-intensity endurance exercise. Inoculation experiments in mice revealed that compared to a control bacterium that does not catabolize lactate, Veillonella atypica increased treadmill run times during a TTE task, suggesting improvements due to a mechanism that augments lactate metabolism. Follow-up experiments revealed that systemic lactate can enter the gut, where it is catabolized by Veillonella atypica and subsequently metabolically converted to the SCFA propionate.51 Propionate can then leave the gut to be metabolized by hepatocytes52 or enter systemic circulation and elicit effects on peripheral tissues and organs expressing SCFA receptors, such as skeletal muscle.53 To explore the potential ergogenic effects of propionate, a final experiment completed by Scheiman and colleagues50 revealed that mice receiving a rectal infusion of propionate demonstrated longer treadmill run times during exhaustive running when compared to mice receiving saline.
While the precise mechanisms underlying the performance improvements resulting from Veillonella atypica colonization and propionate infusion remain unclear, it is possible that endurance exercise performance may be enhanced thorough microbiota targeted interventions that increase the abundance of lactate catabolizing bacterial species and/or increase the production and bioavailability of propionate.
Other studies exploring the ergogenic effects of microbial-based interventions using “human to murine” inoculation experiments have produced similar results. After isolating a strain of Bifidobacterium longum from the feces of a 2008 Olympic weightlifting gold medalist, Lee and colleagues54 introduced a once daily, 4-week oral gavage intervention into 4 groups of specific pathogen-free mice in the following human-equivalent dosages: 0 colony forming units (CFU)/kg (vehicle), 2.05 × 109 CFU/kg, 4.10 × 109 CFU/kg, and 1.03 × 1010 CFU/kg. After the 4-week intervention, significant dose-dependent effects were observed in several outcome measures, including endurance exercise capacity, fatigue-related biochemical variables, and histological analyses. Compared to control animals, mice receiving the Bifidobacterium longum intervention demonstrated improvements in exhaustive swim times and grip strength, reduced serum lactate levels immediately after and 20 min following an acute exercise challenge, and increased muscle and liver glycogen content. A follow-up investigation completed by the same group using a similar methodology but different bacterial strain (Lactobacillus salivarius) replicated these findings. Lee and colleagues55 demonstrated that in a dose-dependent manner, a once daily, 4-week Lactobacillus salivarius intervention improved endurance exercise capacity, increased muscle strength and glycogen storage in liver and muscle, and decreased serum lactate immediately after and 20 min following an acute exercise bout. The studies completed by Lee et al.54,55 provide evidence that markers related to physical performance, fatigue, and energy availability are improved in mice in a dose-dependent manner following a probiotic intervention.
6.1. Microbial effects on lactate metabolism
Results from the investigations completed by Scheiman et al. 50 and Lee et al. 54,55 provide promising evidence that the gut microbiome has the capacity to positively influence exercise performance. However, the mechanisms underlying the ergogenic effects of the gut microbiota and its metabolites remain unclear.50,54,55 Findings from the investigation done by Scheiman et al.50 suggest that some observed improvements in exercise capacity may result from a microbially mediated mechanism that augments lactate metabolism. Lactate is a metabolic by-product produced by muscle fibers during aerobic and anaerobic energy provision.56 Historically, lactate has been unfairly labeled as a fatigue-causing waste product; however, it is now more correctly recognized as a major fuel source and gluconeogenic precursor.57 While lactate is produced by nearly all cells in the body, skeletal muscle is both a major producer and utilizer of lactate, particularly during exercise.58 During exercise, the majority of lactate produced by skeletal muscle fibers is consumed by oxidizing tissues to produce ATP (mainly red oxidative skeletal muscle fibers), while the remainder is transported to the liver and converted to glucose via the Cori cycle.56,59 The newly formed glucose can then enter systemic circulation and becomes available as a glycolytic substrate for exercising skeletal muscle. Given the metabolic importance of lactate uptake and utilization by consumer tissues during exercise, a mechanism that enhances lactate disposal and clearance is likely to have beneficial effects on exercise capacity and performance. Therefore, the gut microbiota (in the presence of Veillonella atypica) could theoretically act as an additional consumer site for lactate, alongside the liver and skeletal muscle, which may optimize energy provision during exercise.
To explore whether Veillonella atypica colonization enhances lactate clearance, Scheiman and colleagues50 injected mice with a standardized dose of sodium lactate and measured capillary blood lactate concentrations at select time points during rested conditions using a lactate analyzer. No differences in lactate clearance were observed in the Veillonella atypica treated versus control mice. While lactate analyzers are practical, inexpensive, and widely used, they are unable to distinguish precise lactate kinetics and may lack a level of sensitivity in determining lactate flux.60,61 In addition, measuring blood lactate concentrations at various time points following exogenous lactate infusion during rested conditions may not be an appropriate proxy for characterizing endogenous skeletal muscle lactate production and removal as it would naturally occur during exercise.
Given that the lactate catabolizing characteristics of Veillonella atypica make this bacterial species an intriguing potential player in lactate metabolism, it is possible the condition (i.e., rested) and methodology (i.e., capillary blood lactate measurements) were simply insufficient to observe whether lactate removal is enhanced in the presence of Veillonella atypica. Therefore, future investigations should (a) assess lactate kinetics during exercising states, and (b) employ isotope tracer techniques to identify specific characteristics of lactate metabolism, including the rate of lactate appearance, lactate disposal, and metabolic clearance rate,62 in order to better understand the relationship between the gut microbiota and lactate during exercise. Lastly, the prospect of the gut as a metabolic sink for lactate is an interesting possibility; however, despite the ability of lactate produced by skeletal muscle fibers during exercise to enter the gut,50 it is unknown how much circulating lactate is diverted to the large intestine during exercise states characterized by marked lactate production. Given that skeletal muscle accounts for the overwhelming majority of lactate removal and oxidation during exercise,59 it is likely that a large amount of lactate would have to be diverted to the gut and subsequently catabolized by Veillonella atypica in order to produce observable exercise improvements.
Similar to the Scheiman and colleagues' investigation,50 a limitation of both studies completed by Lee and colleagues is that blood lactate concentration was not evaluated during exercise tasks.54,55 However, researchers did measure and observe a decrease in blood lactate concentration immediately after and 20 min following an acute endurance exercise challenge in both intervention groups (i.e., Bifidobacterium and Lactobacillus groups), suggesting a possible probiotic-mediated influence on lactate removal and utilization. The researchers propose that probiotic inoculation may augment exercise performance through a physiological mechanism that exerts anti-fatigue effects and/or bolsters energy provision. Lactobacillus and Bifidobacterium are 2 main genera of the heterogenous group of lactic acid bacteria (LAB) that ferment non-digestible carbohydrates to produce lactate.63 The microbially produced lactate can then be consumed by lactate-utilizing bacteria such as Anaerostipes caccae and Eubacterium halli to produce acetyl CoA and eventually butyrate, with the concomitant production of ATP.64 Therefore, increasing the abundance of lactate producing bacteria in the gut may increase substrate availability for butyrate- (and thus ATP-) producing bacteria, thus increasing energy availability and delaying fatigue. While increasing lactate availability and thus ATP production could theoretically influence energy provision, it is more likely that the downstream ATP produced in this process would be utilized for local processes within the gut. Therefore, it remains unclear how this process could contribute to delayed fatigue within in the exercising skeletal muscle itself.
An alternative hypothesis for the ergogenic effects observed following probiotic supplementation is that increasing the amount of LAB in the gut may increase the production of microbial lactate (assuming sufficient dietary fiber consumption), which can then be utilized by consumer tissues such as skeletal muscle. Specifically, lactate produced by LAB in the gut can be transported from the large intestine into systemic circulation via sodium-coupled monocarboxylate transporters and then taken up and oxidized by skeletal muscle during exercise.65 Given the value of lactate as a fuel source, increasing the bioavailability of lactate via a Lactobacillus or Bifidobacterium intervention could theoretically optimize energy metabolism and augment endurance exercise capacity. However, it is currently unknown how much lactate leaves the gut during exercise and whether the quantity produced by LAB would be sufficient to influence skeletal muscle metabolism and improve endurance exercise capacity.
In summary, a gut microbiota-mediated mechanism that targets specific aspects of lactate metabolism may in part be responsible for the observed improvements in endurance exercise capacity following inoculation with Veillonella atypica or LAB. Specifically, the ergogenic effects of Veillonella atypica and LAB may result from the action of the bacteria itself on lactate produced by skeletal muscle fibers that then enters the gut or a mechanism that targets lactate produced by LAB and provides additional fuel to the host, respectively. Future studies should focus on distinguishing the microbially mediated influences on lactate metabolism during endurance exercise using more sensitive methodological approaches in the effort to better understand this relationship.
6.2. Gut microbiota, glycogen storage, and skeletal muscle metabolism
The investigations completed by Lee and colleagues54,55 also demonstrated enhancements in glycogen storage capacity following inoculation with a probiotic microorganism. Muscle glycogen depletion is one of the primary factors limiting exercise performance during prolonged strenuous exercise bouts. Accordingly, a considerable amount of research has focused on understanding best practices for maximizing glycogen storage and optimizing fuel availability during exercise. Perhaps the most popular method for maximizing glycogen storage is “carbohydrate loading”, which involves targeted exercise to deplete glycogen stores followed by the consumption of a high carbohydrate diet in an effort to achieve a super-compensatory response. While carbohydrate loading is a widely used technique among endurance athletes, high carbohydrate intakes can result in debilitating gastrointestinal distress,66 and some studies suggest carbohydrate loading may not be as effective in women.67,68 As such, interventions that increase glycogen storage capacity, replenish glycogen stores, and spare glycogen without reliance on carbohydrate-targeted approaches are attractive.
Microbially mediated influences on glycogen storage capacity and skeletal muscle metabolism have been demonstrated in murine models and appear to result from the metabolic actions of SCFA. Strains within the Lactobacillus and Bifidobacterium genera are well-established probiotics that ferment non-digestible carbohydrates to produce SCFA.69 Current research in animal models and cell lines demonstrates that SCFA may enhance glycogen storage capacity by increasing the expression of glucose transporter type 4 (GLUT4) in skeletal muscle, thus increasing glucose uptake and glycogen repletion.70,71 In addition, SCFA have been shown to influence lipid and carbohydrate metabolism in the liver and skeletal muscle.72 After being produced in the gut, SCFA can leave the gut via the portal vein and be taken up in varying amounts by the liver, where they can be used as substrates for lipid or carbohydrate synthesis.72 Accordingly, SCFA—particularly propionate, which can be used as a precursor for gluconeogenesis—can support energy demands during exercise by providing an additional substrate for exercising skeletal muscle. Interestingly, SCFA have been shown to modulate skeletal muscle metabolism toward a phenotype similar to that induced by chronic aerobic exercise training. Specifically, in animal models and cell lines, SCFA have been shown to enhance lipid uptake70 and oxidation,73 increase glucose uptake,74 and increase the rate of glycogen synthesis.75
Given that both Scheiman et al.50 and Lee et al.54,55 employed interventions capable of directly or indirectly enhancing SCFA production and/or bioavailability, it is possible these interventions increased SCFA availability, resulting in one or more of the following effects: enhanced glycogen storage, increased glucose availability, greater fatty acid oxidation, and endogenous glucose sparing. Accordingly, any one or a combination of these factors could have augmented endurance exercise capacity. Unfortunately, colonic SCFA abundance was not measured in either investigation done by Lee et al.,54,55 and Scheiman and colleagues50 failed to detect labeled propionate in the colons or ceca of Veilonella atypica-inoculated mice following lactate infusion (although the researchers suggested this may have been because the time period between inoculation and sacrifice was too short for Veilonella atypica to convert lactate to propionate). Therefore, it is possible that propionate would have been detected in these tissues if longer time frames were used.
In summary, the available evidence suggests that SCFA may have performance-enhancing effects through their influence on glycogen storage and skeletal muscle metabolism; however, the precise mechanisms underlying their ergogenic potential remain unclear. To complement gut microbiome analyses, future studies should also include metabolomic analyses (host and microbial metabolites) to better understand the relationship between bacterial interventions, SCFA, and exercise performance.
6.3. Improved intestinal barrier integrity
Lastly, it is possible that endurance exercise capacity was indirectly improved in the Lee et al. investigations54,55 due to the beneficial effects of probiotics on the intestinal barrier. Through a variety of mechanisms—including enhanced capacity for butyrate production via cross-feeding—Lactobacillus and Bifidobacterium have been shown repeatedly to enhance intestinal barrier function.76,77 This reduces the translocation of bacteria and endotoxins from the intestinal lumen into systemic circulation, thus reducing inflammation and improving immunity.78 As a result, it is possible the Lactobacillus and Bifidobacterium interventions indirectly improved endurance exercise capacity by enhancing intestinal barrier integrity and improving aspects of host health that have been repeatedly implicated in various disease states.79 Ultimately, evidence demonstrating the ergogenic effects of probiotics is lacking, and more research is needed to substantiate the indirect effects of probiotics on exercise performance.
7. Microbial perturbations and endurance exercise capacity: Mice
Perturbations in the gut microbial community have been shown to have significant consequences on skeletal muscle function and exercise capacity. For example, germ-free mice (i.e., those lacking a gut microbiota) display impairments in mitochondrial function and reduced skeletal muscle mass and strength,80 and mice receiving broad spectrum antibiotics demonstrate significantly shorter treadmill run times during TTE trials.81 However, restorative interventions that rescue the gut microbiota have been shown to reverse these impairments and normalize endurance exercise capacity in microbiota-perturbed mice.
7.1. Dietary interventions
Food-based interventions targeting the gut microbiota have been shown to have a significant influence on exercise capacity. In a recent study completed by Okamoto et al.,82 mice were administered a high microbiome-accessible carbohydrate diet or low microbiome- accessible carbohydrate diet for 6 weeks. Following the dietary intervention, mice receiving the diet containing a non-fermentable, microbiota-inaccessible fiber source displayed significantly shorter treadmill run times during a TTE trial and lower overall muscle mass compared to mice receiving the diet with microbiota-accessible fiber sources. Interestingly, impairments in exercise capacity in the low microbiome- accessible carbohydrate diet group were reversed following administration of an oral prebiotic and fecal microbiota transplantation from the high microbiome-accessible carbohydrate diet group, with the 2 groups displaying no differences in TTE following the intervention. While diet has a well-established influence on the gut microbiota, these findings highlight the downstream effects of this relationship and the magnitude by which dietary components may influence exercise performance by way of the gut microbiota.
7.2. SCFA interventions: Acetate
To further highlight the influence of the gut microbiota on exercise performance, researchers subcutaneously infused acetate into microbiota-depleted mice for 1 week, demonstrating that acetate but not saline normalized treadmill run times in a TTE trial.82 These findings suggest that acetate has restorative and possibly ergogenic effects and may be a key substrate for skeletal muscle metabolism. The metabolic importance of acetate has also been highlighted by Sakakibara et al.,83 who observed that mice lacking Acetyl-CoA synthetase 2—the sole enzyme responsible for converting acetate to acetyl-CoA—display low endurance exercise tolerance under fasting conditions. This finding confirms that acetate generated by the gut microbiota is an important substrate for skeletal muscle metabolism and possibly influences exercise performance. Of all SCFA produced through microbial fermentation, acetate is produced in the largest molar quantities and is the least oxidized by colonic cells.34 The combination of these factors makes acetate the most detectable SCFA in peripheral blood and, therefore, most available to tissues expressing SCFA receptors. Given the expression of GPR41 and GPR43 in skeletal muscle, interventions that directly (by administering acetate) or indirectly (by increasing metabolite production) increase the bioavailability of acetate are likely to improve exercise tolerance, although it is unclear whether this is specific to certain pre-exercise feeding conditions.
While commercial availability of SCFA-based supplements is low, inulin and oligofructose, which are naturally occurring prebiotic fibers, have been shown to significantly increase SCFA production and may be an effective alternative approach for increasing the bioavailability of SCFA. Specifically, a study assessing SCFA production after various dietary fiber interventions found that replacing wheat starch with 10% inulin increased SCFA production by 85% in the rat cecum.84 In human studies, a 12-week, 10 g/day oligofructose-enriched inulin supplement increased fecal SCFA concentrations by 31%, with the most significant increase observed in acetate production.85 These findings suggest that supplementing the diet with inulin-type fructans is an effective method for stimulating SCFA production and may be an appropriate method for increasing SCFA bioavailability without the use of SCFA supplements. Therefore, increasing the systemic availability of SCFA may represent a novel strategy for supporting exercise capacity by providing an additional substrate to exercising tissues.
8. Conclusion
The evidence presented in this review highlights the influence of the gut microbiota on endurance exercise capacity and tolerance. While more research is needed to further substantiate its ergogenic potential, the gut microbiota and its metabolites may enhance exercise capacity through mechanisms that augment lactate metabolism, increase glycogen storage capacity, or influence substrate metabolism in skeletal muscle (Fig. 1). The possibility of leveraging a previously unsuspecting “organ” to enhance performance is intriguing, but ultimately the gut microbiota/exercise research area is still in its infancy, and more rigorous scientific studies are needed to better understand the gut microbiota–exercise connection and determine whether there is a causal link between the gut microbiota and exercise performance. Future investigations should employ more sensitive methodological approaches, such as tracer technologies and metabolomics, to better understand the precise mechanisms underlying the proposed ergogenic effects of the gut microbiota. Further, there currently exists a general lack of gut microbiota and exercise research in humans, and more “biotic” interventions in humans are needed to better understand their effectiveness on exercise performance. Lastly, it is important this research area expands beyond interventions limited to laboratory settings and aims, in time, to explore the effectiveness of “biotics” and other microbiota-modulating therapies in real-life competition scenarios. By seeking to further understand and explore how the microbiota and its associated metabolites can influence and optimize performance, researchers may initiate an exciting new frontier in exercise science.
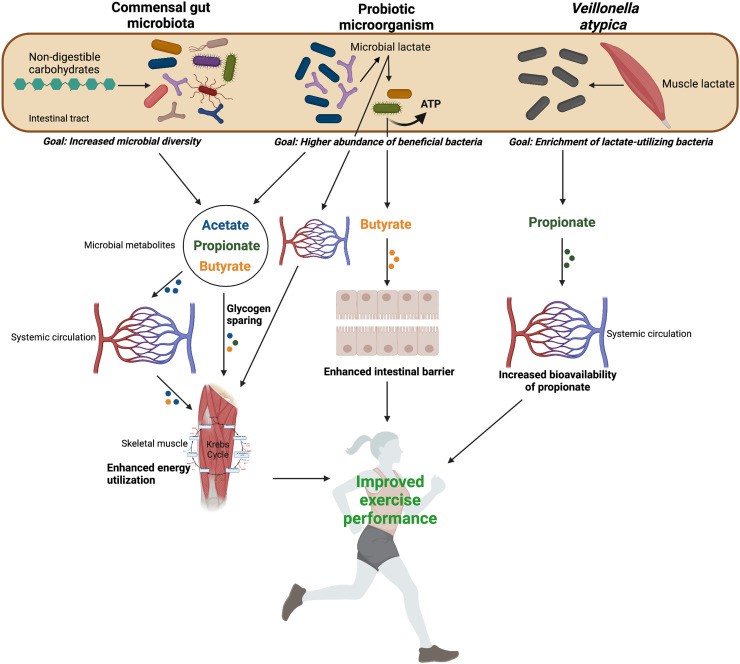
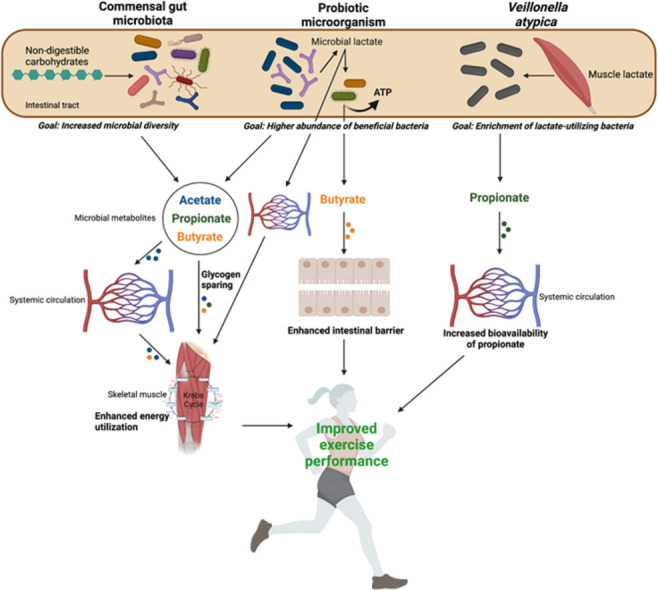
Fig. 1.
Summary of the proposed microbially mediated mechanisms contributing to improvements in endurance exercise performance. Non-digestible carbohydrates are fermented by the commensal gut microbiota (including probiotic microorganisms) to produce SCFA. SCFA influence skeletal muscle substrate metabolism and promote glycogen sparing effects, leading to enhanced energy utilization. Probiotic microorganisms produce lactate as a by-product of fermentation. Microbially produced lactate can be transported into systemic circulation to become available to oxidizing skeletal muscle fibers, or it can be consumed by lactate-utilizing bacteria that concomitantly produce butyrate and ATP. Butyrate fuels colonocytes, which enhances the intestinal barrier. Lactate produced by muscle fibers during exercise enters the gut (an additional removal site for lactate) and is metabolically converted by Veillonella atypica into propionate, which enters systemic circulation and is available to SCFA receptor expressing tissues, including skeletal muscle. (Created with BioRender.com). ATP = adenosine triphosphate; SCFA = short-chain fatty acid.
Acknowledgments
RAR is supported by a Canadian Institutes of Health Research Grant (PJT-159626). The funding source had no role in the preparation or submission of the article.
Authors’ contributions
KMS wrote the main manuscript text; KMS and RAR prepared the figure and contributed to the editing of the manuscript text. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Both authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.09.002.
Supplementary materials
References
- 1.Joyner MJ, Coyle EF. Endurance exercise performance: The physiology of champions. J Physiol. 2008;586:35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2:817–828. doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 4.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 5.Stephens RW, Arhire L, Covasa M. Gut microbiota: From microorganisms to metabolic organ influencing obesity. Obesity (Silver Spring) 2018;26:801–809. doi: 10.1002/oby.22179. [DOI] [PubMed] [Google Scholar]
- 6.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 8.Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017;32:300–313. doi: 10.1264/jsme2.ME17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 10.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6(Suppl. 1):S43–S45. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Mohr AE, Jager R, Carpenter KC, et al. The athletic gut microbiota. J Int Soc Sports Nutr. 2020;17:24. doi: 10.1186/s12970-020-00353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de La Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21:167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Franzosa EA, Huang K, Meadow JF, et al. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci U S A. 2015;112:E2930–E2938. doi: 10.1073/pnas.1423854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler DA, Srinivasan M, Egholm M, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- 17.Behrouzi A, Nafari AH, Siadat SD. The significance of microbiome in personalized medicine. Clin Transl Med. 2019;8:16. doi: 10.1186/s40169-019-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaser MJ, Dominguez-Bello MG. The human microbiome before birth. Cell Host Microbe. 2016;20:558–560. doi: 10.1016/j.chom.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 20.D'Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Iebba V, Totino V, Gagliardi A, et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016;39:1–12. [PubMed] [Google Scholar]
- 22.Kocot AM, Jarocka-Cyrta E, Drabińska N. Overview of the importance of biotics in gut barrier integrity. Int J Mol Sci. 2022;23:2896. doi: 10.3390/ijms23052896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzapfel WH, Schillinger U. Introduction to pre- and probiotics. Food Res Int. 2002;35:109–116. [Google Scholar]
- 24.Hill C, Guarner F, Reid G, et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 25.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 26.Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar-Toalá J, Garcia-Varela R, Garcia H, et al. Postbiotics: An evolving term within the functional foods field. Trends Food Sci Tech. 2018;75:105–114. [Google Scholar]
- 28.Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiology spectrum. 2017;5 doi: 10.1128/microbiolspec.BAD-0019-2017. [DOI] [PubMed] [Google Scholar]
- 29.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 31.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 33.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer R.J. Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen J, Clausen M, Mortensen P. Oxidation of short and medium chain c2-c8 fatty acids in Sprague-Dawley rat colonocytes. Gut. 1997;40:400–405. doi: 10.1136/gut.40.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan g protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 37.Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 38.Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mörkl S, Lackner S, Müller W, et al. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord. 2017;50:1421–1431. doi: 10.1002/eat.22801. [DOI] [PubMed] [Google Scholar]
- 40.Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J Int Soc Sports Nutr. 2019;16:21. doi: 10.1186/s12970-019-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bressa C, Bailén-Andrino M, Pérez-Santiago J, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PloS One. 2017;12 doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donovan CM, Madigan SM, Garcia-Perez I, Rankin A, O'Sullivan O, Cotter PD. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J Sci Med Sport. 2020;23:63–68. doi: 10.1016/j.jsams.2019.08.290. [DOI] [PubMed] [Google Scholar]
- 43.Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 44.Cronin O, Barton W, Skuse P, et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. 2018;3:e00044–18. doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kern T, Blond MB, Hansen TH, et al. Structured exercise alters the gut microbiota in humans with overweight and obesity—A randomized controlled trial. Int J Obes (Lond) 2020;44:125–135. doi: 10.1038/s41366-019-0440-y. [DOI] [PubMed] [Google Scholar]
- 46.Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11:868. doi: 10.3390/nu11040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motiani KK, Collado MC, Eskelinen JJ, et al. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc. 2020;52:94–104. doi: 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Wang Y, Ni Y, et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31:77–91. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104–1109. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egland PG, Palmer RJ, Jr, Kolenbrander PE. Interspecies communication in streptococcus gordonii-veillonella atypica biofilms: Signaling in flow conditions requires juxtaposition. Proc Natl Acad Sci U S A. 2004;101:16917–16922. doi: 10.1073/pnas.0407457101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 53.Bloemen JG, Venema K, van de Poll MC, Damink SWO, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28:657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Lee MC, Hsu YJ, Chuang HL, et al. In vivo ergogenic properties of the bifidobacterium longum OLP-01 isolated from a weightlifting gold medalist. Nutrients. 2019;11:2003. doi: 10.3390/nu11092003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee MC, Hsu YJ, Ho HH, et al. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms. 2020;8:545. doi: 10.3390/microorganisms8040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118:691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 58.Lund J, Aas V, Tingstad RH, Van Hees A, Nikolić N. Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism. Sci Rep. 2018;8:9814. doi: 10.1038/s41598-018-28249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messonnier LA, Emhoff C-AW, Fattor JA, Horning MA, Carlson TJ, Brooks GA. Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol (1985) 2013;114:1593–1602. doi: 10.1152/japplphysiol.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonaventura JM, Sharpe K, Knight E, Fuller KL, Tanner RK, Gore CJ. Reliability and accuracy of six hand-held blood lactate analysers. J Sports Sci Med. 2015;14:203–214. [PMC free article] [PubMed] [Google Scholar]
- 61.Tanner RK, Fuller KL, Ross ML. Evaluation of three portable blood lactate analysers: Lactate pro, lactate scout and lactate plus. Eur J Appl Physiol. 2010;109:551–559. doi: 10.1007/s00421-010-1379-9. [DOI] [PubMed] [Google Scholar]
- 62.Bergman BC, Wolfel EE, Butterfield GE, et al. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol (1985) 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 63.Axelsson L. Marcel Dekker, Inc./CRC Press; New York, NY: 2004. Lactic acid bacteria: Classification and physiology. [Google Scholar]
- 64.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teramae H, Yoshikawa T, Inoue R, et al. The cellular expression of smct2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res. 2010;31:239–249. doi: 10.2220/biomedres.31.239. [DOI] [PubMed] [Google Scholar]
- 66.Rehrer NJ, van Kemenade M, Meester W, Brouns F, Saris WH. Gastrointestinal complaints in relation to dietary intake in triathletes. Int J Sport Nutr. 1992;2:48–59. doi: 10.1123/ijsn.2.1.48. [DOI] [PubMed] [Google Scholar]
- 67.Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD. Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol (1985) 1995;78:1360–1368. doi: 10.1152/jappl.1995.78.4.1360. [DOI] [PubMed] [Google Scholar]
- 68.Walker JL, Heigenhauser GJ, Hultman E, Spriet LL. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol (1985) 2000;88:2151–2158. doi: 10.1152/jappl.2000.88.6.2151. [DOI] [PubMed] [Google Scholar]
- 69.Markowiak-Kopeć P, Ślizewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maruta H, Yoshimura Y, Araki A, Kimoto M, Takahashi Y, Yamashita H. Activation of amp-activated protein kinase and stimulation of energy metabolism by acetic acid in l6 myotube cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamashita H, Maruta H, Jozuka M, et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima fatty (OLETF) rats. Biosci Biotechnol Biochem. 2009;73:570–576. doi: 10.1271/bbb.80634. [DOI] [PubMed] [Google Scholar]
- 72.Frampton J, Murphy KG, Frost G, Chambers ES. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab. 2020;2:840–848. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- 73.Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J-H, Kim I-S, Jung S-H, Lee S-G, Son H-Y, Myung C-S. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41. PloS One. 2014;9:e95268. doi: 10.1371/journal.pone.0095268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fushimi T, Tayama K, Fukaya M, et al. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J Nutr. 2001;131:1973–1977. doi: 10.1093/jn/131.7.1973. [DOI] [PubMed] [Google Scholar]
- 76.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 78.Bron PA, Kleerebezem M, Brummer R-J, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lahiri S, Kim H, Garcia-Perez I, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11:eaan5662. doi: 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nay K, Jollet M, Goustard B, et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019;317:E158–E171. doi: 10.1152/ajpendo.00521.2018. [DOI] [PubMed] [Google Scholar]
- 82.Okamoto T, Morino K, Ugi S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab. 2019;316:E956–E966. doi: 10.1152/ajpendo.00510.2018. [DOI] [PubMed] [Google Scholar]
- 83.Sakakibara I, Fujino T, Ishii M, et al. Fasting-induced hypothermia and reduced energy production in mice lacking acetyl-CoA synthetase 2. Cell Metab. 2009;9:191–202. doi: 10.1016/j.cmet.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Levrat MA, Rémésy C, Demigné C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J Nutr. 1991;121:1730–1737. doi: 10.1093/jn/121.11.1730. [DOI] [PubMed] [Google Scholar]
- 85.Drabińska N, Jarocka-Cyrta E, Markiewicz LH, Krupa-Kozak U. The effect of oligofructose-enriched inulin on faecal bacterial counts and microbiota-associated characteristics in celiac disease children following a gluten-free diet: Results of a randomized, placebo-controlled trial. Nutrients. 2018;10:201. doi: 10.3390/nu10020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.