Abstract
Purpose
The most common cause of mortality for many cancer survivors is cardiovascular disease (CVD). This requires a shift in thinking where control of CVD risk factor-related comorbidity is paramount. Our objective was to provide an understanding of adherence to medications for the management of CVD risk factor-related comorbidities among cancer survivors.
Methods
We systematically searched for articles indexed in MEDLINE (via PubMed), Embase, Cochrane (Wiley), PsyclNFO, and Scopus (via Elsevier) for articles published from inception to October 31, 2019, and updated the search on June 7, 2021. English language, original research that assessed medication adherence to common CVD risk factor-related comorbidities among cancer survivors was included. We assessed risk of bias using the Mixed Methods Appraisal Tool.
Results
Of the 21 studies included, 57% focused on multiple cancer types. Seventy-one percent used pharmacy-based adherence measures. Two were prospective. Adherence was variable across cancer types and CVD risk factor-related comorbidities. Among the studies that examined changes in comorbid medication adherence, most noted a decline in adherence following cancer diagnosis and throughout cancer treatment. There was a focus on breast cancer populations.
Conclusions
CVD risk factor-related medication adherence is low among cancer survivors and declines over time. Given the risk for CVD-mortality among cancer survivors, testing of interventions aimed at improving adherence to non-cancer medications is critically needed.
Implications for Cancer Survivors
For many cancer survivors, regularly taking medications to manage CVD risk is important for longevity. Engaging with primary care throughout the cancer care trajectory may be important to support cardiovascular health.
Keywords: Medication adherence, Cancer survivors, Cardiovascular diseases, Heart disease risk factors
Introduction
There are approximately 17 million cancer survivors, defined as persons living from the time of diagnosis until the end of life, in the USA, and approximately 64% are at least aged 65 [1]. With aging comes an increased likelihood of chronic comorbid conditions, including hypertension, diabetes, and dyslipidemia [2, 3]. Certain cancer treatments may also increase the risk of developing or exacerbating existing chronic comorbid conditions [3–5]. Accordingly, the majority (70%) of cancer survivors have chronic conditions that require comprehensive care [6, 7]. While survivors must manage multiple health demands, the most common cause of mortality is not cancer, but cardiovascular disease (CVD) [8–10]. In fact, from the point of cancer diagnosis onward into survivorship, compared to the general US population, cancer patients with all types of cancer are at elevated risk of dying from CVD [11]. For patients with a high likelihood of survival, this requires a shift in thinking where control of CVD risk factors is paramount.
Managing CVD risk factors is complex, often involving lifestyle modification and prescription medications to reduce CVD risk. Much of the existing literature around reducing CVD risk among cancer survivors has focused on maintaining physical activity, reducing sedentary time, and eating a healthy, well-balanced diet [12–17]. A healthy lifestyle, particularly physical activity, is associated with decreased likelihood of cancer recurrence, prolonged survival, and decreased mortality in survivors [18–23]. While lifestyle modification is important, one of the most powerful tools to manage comorbidities is taking medications as prescribed.
Over the last decade, prescription medication use has steadily increased. Nearly 70% of Americans are prescribed at least one medication and more than half take two [24]. While medication use to control CVD risk factor-related comorbidities is ubiquitous, so too is non-adherence. Non-adherence to cardiovascular medications has been described as a pandemic; a European study identified that approximately three out of every four adults do not take their medications as prescribed [25]. Medication adherence is consistently suboptimal across multiple conditions and patient populations [26], even individuals that have experienced a major medical event. For example, among patients who previously had a myocardial infarction, nearly one-quarter did not fill their medications within 1 week of discharge, and over one-third discontinued at least one of their medications within 1 month of discharge [27, 28]. While evidence from other cardiac patient populations may be inferred, the literature about adherence to medication for CVD risk factor-related comorbidities among cancer survivors is limited. Our objective was to systematically search medical and public health literature to provide an evidence-based understanding of adherence to medications for the management of CVD risk factor-related comorbidities among cancer survivors.
Methods
We systematically searched MEDLINE (via PubMed), Embase, Cochrane (Wiley), PsycINFO, and Scopus (via Elsevier) to identify articles addressing adherence to medications prescribed to treat CVD risk factor-related conditions among cancer survivors. Our initial review was identified articles published from inception to October 31, 2019. We then updated the search on June 7, 2021, to identify any published work. We defined cancer survivors as persons living from the time of diagnosis until the end of life, including those in active treatment [1]. Bibliographic references and the review process were managed in Covidence, which automatically removed duplicate articles. To refine and clarify our eligibility guidance, two reviewers (LZ, CM) completed a mock title and abstract screening of 50 articles prior to the formal review. Next, the title and abstract of each article were reviewed for eligibility by two of five multidisciplinary reviewers (LZ, MS, RA, CW, CM) using a double-blind screening process. Finally, abstract and full-text conflicts, all conflicts were discussed and voted on by four reviewers (KO, LZ, RA, CW and LZ, RA, MS, and CW for abstract and full texts, respectively), and the remaining articles were triaged for full text review.
Eligibility criteria were developed in collaboration with medical librarians and were consistently applied across all review phases (Fig. 1). English language articles were included if they addressed medication adherence to common CVD risk factor-related comorbidities — hypertension, diabetes, and dyslipidemia — among cancer survivors. We included articles independent of study design (e.g., randomized trials, quasi-experimental, observational); however, articles were excluded if they reported on non-original research (e.g., reviews, case reports, editorials) or lacked full text (e.g., conference abstracts). We further excluded articles if they focused on adherence to cancer-related therapies (e.g., only described adherence to oral chemotherapy or endocrine therapies), non-cardiovascular outcomes (e.g., nausea, vomiting), CVD risk factor-related comorbidity management among non-cancer populations, lifestyle-only interventions to support comorbidity management among cancer survivors (e.g., improve diet without attention to medication adherence), or clinical outcomes (e.g., health service utilization) where medication adherence was an independent variable.
Fig. 1.
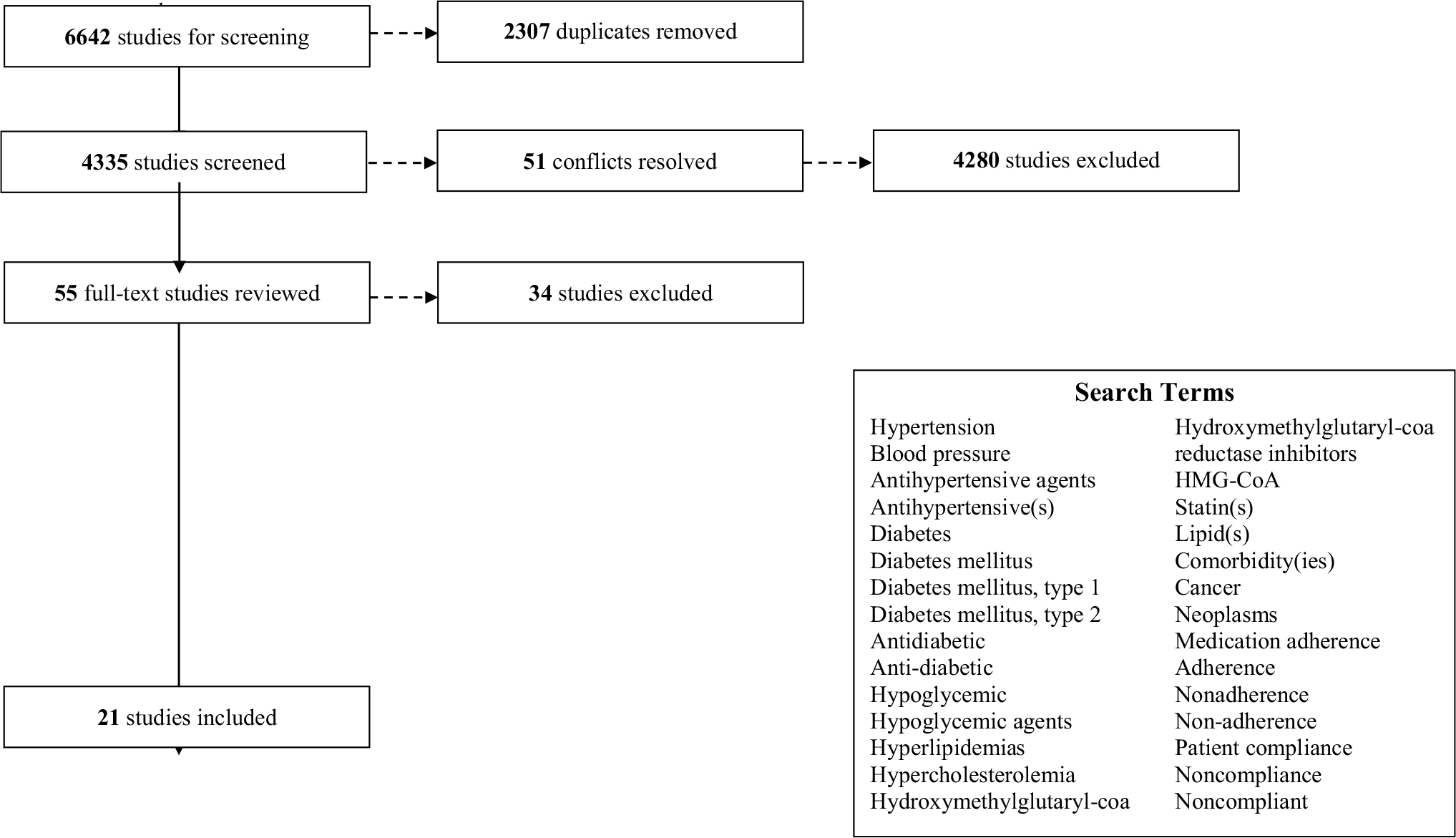
Article flow
Due to various designs and methodological approaches, we used the Mixed Methods Appraisal Tool (MMAT) to assess study quality [29]. Three reviewers (LZ, MS, CD) completed the risk assessment and calculated a quality score for each article by dividing satisfied criteria by the five MMAT criteria (e.g., 20% if 1 MMAT criteria was satisfied, 100% if all 5 criteria were satisfied). Our approach is consistent with reviews that have synthesized findings from studies of diverse research methods [30, 31].
We used a narrative data synthesis approach, where a single reviewer (LZ, MS, RA, CW, CD) abstracted information about each study’s population (e.g., demographics, cancer type), key outcomes, and primary results (Table 1). Two reviewers then assessed extracted information for reporting consistency. We followed the PRISMA reporting guidelines [32].
Table 1.
Summary of study characteristics (n = 21)
| Author, year | Sample size | Population | Cancer type | Comorbidities | Outcomes | Study design |
|---|---|---|---|---|---|---|
|
| ||||||
| Multiple comorbidities | ||||||
| Birand, 2019 [33] | • 81 | • 65.4% female • Race NR • Mean age 59.1 (SD 11.34) • North Cyprus |
• Breast • GI • Genitourinary • Gynecology • Lung • Lymphoma • Others |
Condition: • Hyperlipidemia • Hypertension • Diabetes • Asthma • Hyperthyroid • Hypothyroidism • Others Medication: • Oral chemotherapies • Medicines used for chronic illnesses • All medicines used during chemotherapy cycles |
Primary: • Beliefs about medications (Beliefs about Medicines Questionnaire) Secondary: • Morisky Green Levine Test (MGLT) (aka Morisky Medication Adherence Scale) — score of 4 indicated adherence |
• Pro |
| Calip, 2017 [34] | • 2,308 | • 100% female • 87.4% white • 3.9% AA • 8.7% Others (Asian, American Indian, unknown) • Median age 67 (IQR 57–75) • 1% 18–39 yrs • 8.6% 40–49 yrs • 20.6% 50–59 yrs • 27.7% 60–69 yrs • 28.1% 70–79 yrs • 14% ≥ 80 yrs • USA |
• Breast |
Condition: • Hypertension • Diabetes • Hyperlipidemia Medication: • Antihypertensives (angiotensin converting-enzyme inhibitors, beta blockers, calcium channel blockers, diuretics) • Oral diabetes meds (metformin, sulfonylureas) • Statins |
Primary: • MPR during the 2nd year post-cancer diagnosis • Non-adherence defined as MPR <0.80 Secondary: • NR |
• Retro • Data linkage |
| Dashputre, 2019 [35] | • CML = 1,378 • CLL/SLL − 1,126 • MM = 3,527 |
• CML: • 43.9% female CLL/SLL: • 34.6% female MM: • 44% female • Race NR CML: • Mean age 54.7 (SD 14.8) CLL/SLL: • Mean age 67.2 (SD 11.4) MM: • Mean age 63.8 (SD 11.0) • USA |
• CML • CLL/SLL • MM |
Condition: • Diabetes • Hypertension • Hyperlipidemia **Pre-existing for all Medication: • Chronic conditions (anti-diabetic, antihypertensive, lipid-lowering agent) • Oral oncolytics |
Primary: • PDC ≥ 80% (changed to 85%, 90% in sensitivity analyses) Secondary: • NR |
• Retro |
| Drzayich Antol, 2018 [36] | • 1,847 • (524 w/ CRC, 623 w/ lung, 700 w/ breast) |
• 66.8% female • 84.9% white • 13.0% non-white • 0.3% unknown **Only reported for 1,624 Medicare pts • Mean age 69.2 (SD 9.2) • USA |
• Breast • Lung • CRC **All metastatic |
Condition: • Ischemia • CVD • Hypertension • Diabetes • Others (anemia, anxiety, depression, renal disease, pneumonia) Medication: • NR |
Primary: • 8-item Morisky Medication Adherence Scale (MMAS) Secondary: • Unhealthy days |
• Cross-sect • Retro |
| Santorelli, 2016 [37] | • 298 (BC + diabetes) and 1,192 comparators • 508 (B + lipid disorder) and 2,032 comparators • 1,062 (BC + HTN) and 4,282 comparators |
• 100% female BC + DM: • 73.2% white • 12.4% black • 14.4% others BC + lipid: • 87.8% white • 5.7% black • 6.5% other BC + HTN: • 83.7% white • 8.8% black • 7.5% others BC + DM: • 24.8% 66–69 yrs • 28.2% 70–74 yrs • 23.5% 75–79 yrs • 14.1% 80–84 yrs • 9.4% 85 + yrs BC + lipid: • 23.2% 66–69 yrs • 26.4% 70–74 yrs • 23% 75–79 yrs • 16.3% 80–84 yrs • 11% 85 + yrs BC + HTN: • 20% 66–69 yrs • 25.9% 70–74 yrs • 21.6% 75–79 yrs • 17.4% 80–84 yrs • 15.2% 85 + yrs • USA |
• Breast |
Condition: • Diabetes • Lipid disorders • Hypertension Medication: • All oral diabetes meds. (excluded those on insulin) • Any antihypertensive • Statins |
Primary: • PDC (non-adherent < 80%; changed to 70% and 90% in sensitivity analysis) • Persistence (largest # of consecutive days w/o coverage by med. class → 26 days for hypertension and diabetes; 62 days for lipids) Secondary: • NR |
• Retro |
| Yang, 2016 [38] | • 36,149 | • 100% female • Race NR • 13.6% 18–54 yrs • 34.1% 55–64 yrs • 52.4% ≥ 65 yrs • USA |
• Early stage breast cancer (ESBC) |
Condition: • Hypertension • Hyperlipidemia • Diabetes • Thyroid disease • GERD • Osteoporosis Medication: • Hypertension (diuretics, CA channel blocker, beta-blocker, ACE inhibitor, angiotensin-II receptor blocker, alpha blocker, alpha-2 receptor agonist, peripheral adrenergic inhibitor) • Hyperlipidemia (anti-hyperlipidemic agent, bile acid sequestrants, cholesterol absorption inhibitors, fibric acid derv., PCSK9 inhibitors, statins) • Diabetes (insulin, alpha-glucosidase inhibitors, amylin analogs, incretin mimetics, meglitinides, non/sulfonylurea, SGLT-2 inhibitors, thiazolidinediones) |
Primary: • MPR ≥ 80% • Compared change in adherence pre/post cancer treatment (20% decline considered sig. change) Secondary: • NR |
• Retro |
| Zhou, 2019 [39] | • 25,381 (23,349 chemo only [reference grp], 1382 autologous HCT, 650 allogeneic HCT) |
Chemo: • 47.1% female Auto HCT: • 39.4% female Allo HCT: • 42.5% female • Race NR Chemo: • Mean age 50.3 (SD 11.8) Auto HCT: • Mean age 54.2 (SD 8.4) Allo HCT: • Mean age 50 (SD 11.7) • USA |
• Blood cancer |
Condition: • Diabetes • Hypertension • Dyslipidemia Medication: • NR |
Primary: • Medication discontinuation (treatment gap ≥60 days) • Medication adherence (PDC ≥ 0.80) Secondary: • NR |
• Retro |
| Diabetes | ||||||
| Calip, 2015 [40] | • 509 (of 516 treated with oral diabetes medications) | • 100% female • 82% white • 5.0% AA • 13% others (Asian, American Indian/Alaska native, unknown) **Reported for 516 pts • Median age at BC diagnosis = 65 • Median age at BC diagnosis = 643 (IQR 11.4) **2nd measure is for 516 pts • USA |
• Breast |
Condition: • Diabetes Medication: • Oral diabetes medications |
Primary: • MPR; % adherent (MPR ≥ 0.80) • Discontinuation rates (gap ≥ 90 days btw. end of previous supply and subsequent medication) Secondary: • HbA1C |
• Retro |
| James, 2018 [41] | • 56 | • 52% female • 40% White • 27% Black • 32% Other (Asian, other races) • Mean age 62.2 (SD 9.2) • USA |
• Prostate • Breast • Lung • CRC |
Condition: • Diabetes Medication: • Oral hypoglycemic agents |
Primary: • Diabetes self-management behaviors, including self-reported medication adherence • 8-item Morisky Medication Adherence Scale (MMAS); non-adherence defined as a positive answer to > 2 questions on the MMAS Secondary: • Other DM self-mgt. behaviors (Summary of Diabetes Self-care Activities) |
• Pro |
| Letinier, 2018 [42] | • 13,943 (654 w/cancer; 13,289 w/o cancer) |
Cancer: • 34.9% female Non-cancer: • 49.6% female • Race NR Cancer: • Mean age 66.3 (SD 10.1) Non-cancer: • Mean age 59.3 (SD 14.2) • France |
Main analysis: • All Secondary analysis: • CRC • Pulmonary • Prostate • Breast • Others |
Condition: • Diabetes Medication: • Biguanides • Sulfonylureas • Sulfonamide • Alpha glucosidases inhibitors • Thiazolidinediones • Dipeptidyl peptidase 4 (DPP-4) inhibitors • Other diabetes medicines • Combinations of the above |
Primary: • Medication persistence — nonpersistent at occurrence of first OAD discontinuation (refill gaps of 90 days; 60 days in sensitivity analysis) • Sensitivity analysis where pts. taking insulin were excluded Secondary: • NR |
• Retro |
| Stuart, 2015 [43] | • 4,348 pre-exiting diabetes with cancer • 28,507 controls (diabetics without cancer) |
Cancer: • 61.9% female Non-cancer: • 66.9% female Cancer: • 78% white • 13.5% black • 8.4% others (including Hispanics) Non-cancer: • 74.7% white • 14.9% black • 10.4% others (including Hispanics) Cancer: • 13.3% <65 yrs • 17.7% 65–69 yrs • 20.4% 70–74 yrs • 21.1% 75–79 yrs • 15.8% 80–84 yrs • 11.6% > 84 yrs Non-cancer: • 24% < 65 yrs • 17.7% 65–69 yrs • 18.5% 70–74 yrs • 17.3% 75–79 yrs • 12.3% 80–84 yrs • 10.2% > 84 yrs • USA |
• New cancer diagnosis |
Condition: • Diabetes Medication: • Oral hypoglycemic agents (OHAs) • Renin–angiotensin–aldosterone system inhibitors (RAAS-I) • Statins |
Primary: • PDC (6 months pre/post diagnosis/index date) Secondary: • NR |
• Retro |
| Tan, 2016 [44] | • 1,918 (cancer and diabetes) • 1,918 matched non-cancer controls |
• 56.5% female • Race NR • Mean age 56.7 (SD NR) • 28.4% 18–54 years • 42.1% 55–60 yrs • 29.5% 61–64 yrs • USA |
• Breast • Prostate • Colon • Lung |
Condition: • Diabetes Medication: • Oral anti-diabetic (sulfonylureas, thiazolidinediones [TZD], metformin, dipeptidyl peptidase 4 [DPP-4] inhibitors |
Primary: • Adherence to oral antidiabetic medications among different regimens and cancer types and cancers cases and matched cancer-free controls • Assessed using MPR (for each patient & each drug class) • 0.8 ≤ MPR ≤ 1 is adherent Secondary: • All-cause hospitalization and ER visits • Total medical costs (inpatient, outpatient, emergency room) |
• Retro • Data linkage |
| Zanders, 2015 [45] | • 3,281 cancer patients • 12,891 controls w/o cancer |
Cancer: • 44% female Control: • 44% female • Race NR Cancer: • mean age 67.7 (SD 9.8) Control: • mean age 67.5 (SD 9.7) **Age at first diabetes drug dispensing • Netherlands |
• Any cancer (except nonmelanoma skin) 6 most frequent: • Colorectal • Other GI (esophageal, stomach, pancreas, liver) • Prostate • Breast • Pulmonary • Urinary |
Condition: • Diabetes Medication: • Anti-diabetic/glucose lowering drug (metformin monotherapy, sulfonylurea monotherapy, any insulin and others) |
Primary: • Change in MPR associated with cancer diagnosis (calculated every month) Secondary: • NR |
• Retro • Data linkage |
| Hyperlipidemia | ||||||
| Banegas, 2018 [46] | • 10,177 | • 39.1% female • 63.8% White, non-Hispanic • 9.2% AA, non-Hispanic • 21% others (Hispanic, non-Hispanic Asian, Native American, multirace, unknown) • 5.8% < 55 yrs • 26.4% 55–64 yrs • 42% 65–74 yrs • 25.8% 75 + yrs • USA |
• Breast • CRC • Prostate |
Condition: • NR Medication: • Statins (prevalent users) |
Primary: • PDC during 4 periods (12–24 months before cancer dx, 0–11 months before cancer dx, month of cancer to 11 months after cancer dx, and 12–24 months after cancer dx) • PDC ≥ 0.80 → adherent; 0.20–0.79 → partially adherent; <0.20 → non-adherent Secondary: • PDC during the same periods by race and ethnicity |
• Retro |
| Calip, 2013 [47] | • 1,393 (with ≥ 1 statin medication prior to cancer dx) | • 100% female • 86.8% white • 3.8% AA • 9.8% others (Asian, American Indian, unknown) • Mean age 62.4 (SD 11.0) • USA |
• Breast |
Condition: • Hyperlipidemia Medication: • Statins (prevalent users) |
Primary: • MPR ≥ 80% (adherent) • Discontinuation rates (gap ≥ 90 days btw, supplies) Secondary: • LDL, HDL levels |
• Retro |
| Feng, 2021 [48] | • 20,046 (breast cancer) • 11,719 (colorectal cancer) • 6,430 (melanoma) |
• 100% female9 Breast cancer: • Mean age of 70.1 Colorectal cancer: • Mean age of 73.6 Melanoma: • Mean age of 71.9 • Australia • Race NR |
• Breast • CRC • Melanoma |
Condition: • Hyperlipidemia Medication: • Lipid lowering medications (primarily statins) |
Primary: • Breast cancer mortality • CRC mortality • Melanoma mortality • Any cancer mortality • Non-cancer mortality • All-cause mortality Secondary: • NR |
• Retro • Data linkage |
| Hypertension | ||||||
| Jeong, 2015 [49] | • 56 breast cancer survivors • 280 non-cancer controls |
• 100% female **Both cohorts • Race NR Cancer: • Mean age 65.7 (SD 1.7) Cancer: • Mean age 65.7 (SD 1.7) **After PSM • Korea |
• Breast |
Condition: • Hypertension Medication: • Antihypertensive |
Primary: • Antihypertensive medication use was measured by monthly frequency • Good adherence defined as daily medication Secondary: • Health behaviors (alcohol consumption, smoking, diet control, physical activity) |
• Retro • Cross-sect |
| Jo, 2015 [50] | • 480 in final analysis (80 cancer; 400 non-cancer) • **After PSM |
Cancer: • 38.4% female Non-cancer: • 38% female • Race NR Cancer: • Mean age 69.2 (SD 1.26) Non-cancer: • Mean age 69.0 (SD 1.46) • Korea |
• Gastric |
Condition: • Hypertension Medication: Antihypertensive |
Primary: • Antihypertensive medication was measured by frequency (per month) • Good adherence was daily medication; levels below this were not sufficient adherence Secondary: • Health behaviors (alcohol consumption, smoking, diet control, physical activity) |
• Retro |
| Shin, 2010 [51] | • 2,455,193 (include 12,636 cancer survivors) | • Cancer: • 55.1% female General pop: • 57.8% female • Race NR Cancer: • 0.7% 20–39 yrs • 6.7% 40–49 yrs • 20.8% 50–59 yrs • 36.1% 60–69 yrs. 4 • 35.7% >/ = 70 yrs General Pop: • 2.9% 20–39 yrs • 13.7% 40–49 yrs • 26.6% 50–59 yrs • 31.2% 60–69 yrs • 25.6% >/ = 70 yrs • Korea |
• Oral cavity and pharynx • Esophagus • Stomach • CRC • Liver • Gallbladder • Pancreas • Larynx • Lung • Melanoma • Breast • Cervix uteri • Corpus uteri • Ovary • Prostate • Testis • Kidney • Bladder • Brain and CNS • Thyroid • Hodgkin’s lymphoma • NHL • MM • Leukemia |
Condition: • Hypertension Medication: • Antihypertensive |
Primary: • CMA scale (% of days patient has pills available — calculated by dividing total number of days of medication dispensed (excluding the final prescription) by total number of days between the 1 st and last prescriptions) • CMA reported as continuous (median and IQR and proportion) appropriate adherence = cumulative medication adherence 80 + % Secondary: • NR |
• Retro • Data linkage |
| Shin, 2012 [52] | • 1,956 cancer patients (include 385 w/ HTN) • 1,124 comparison group w/HTN & w/o cancer) |
Cancer: • 48.8% female General pop: • 56.1% female • Race NR Cancer: • Mean age 65.2 (SD 8.9) General pop: • Mean age 59.9 (SD 12.2) • Korea |
• Stomach • Lung • CRC • Breast • Others (includes all other types of cancer) |
Condition: • Hypertension Medication: Antihypertensive |
Primary: • Questionnaires in relation to anti-hypertensive medication adherence Secondary: • Questionnaires in relation to frequency BP monitoring and perceived BP control |
• Cross-sect |
| Other CVD-related risk factors | ||||||
| Cheung, 2013 [53] | • 1,119 cancer survivors (CS) • 7,886 propensity score-matched non-cancer patients (NCP) |
Cancer: • 65% female Non-cancer: • 67% female Cancer: • 92% white Non-cancer: • 91% white Cancer: • Mean 80.6 (SD 6.9) Non-cancer: • Mean 80.5 (SD 7.2) • USA |
• Cancer survivors |
Condition: • Secondary prevention of myocardial infarction (MI) Medication: • Statins • Beta-blockers • Angiotensin-converting enzyme inhibitors (ACEIs) • Angiotensin-II receptor blockers (ARBs) |
Primary: • PDC Secondary: • Receipt of revascularization procedures |
• Retro • Data linkage |
Abbreviations:aOR adjusted odds ratio; CI confidence interval; CLL chronic lymphocytic leukemia; CML chronic myeloid leukemia; CNS central nervous system; CRC colorectal cancer; CVD cardiovascular disease; D-in-D difference in difference; GERD gastroesophageal reflux disease; GI gastrointestinal; OR odds ratio; MM multiple myeloma; MPR medication possession ratio; NHL non-Hodgkin lymphoma; NR not reported; PDC proportion of days covered; Pro prospective; PSM propensity score matching; Retro retrospective; SLL small lymphocytic leukemia
Results
Our search yielded 6,642 articles. After removing duplicates, there were 4,335 articles remaining. Following the initial two-reviewer title and abstract reviews, 53 articles with different decisions were discussed with the full review team. This equates to a nearly 99% agreement rate between reviewers. After resolving conflicts, we identified 30 articles for full text review. During the full text review process, 9 additional articles were excluded. The majority were excluded because adherence to CVD risk factor-related medications was not the primary outcome or the full text was unavailable. This resulted in a total of 21 articles for data extraction and synthesis (Fig. 1).
Study characteristics
Included studies had diverse characteristics. Over half (n = 13, 62%) of the studies originated in the USA [34–41, 43, 44, 46, 47, 53]. Several studies are originated in Korea (n = 4, 19%) [49–52] and Europe (n = 3, 14%) [33, 42, 45]. The majority of studies (n = 18, 86%) used retrospective study designs [34–40, 42–51, 53, 54], while only two studies (1%) were prospective [33, 41, 52]. Studies were also varied with respect to the population of cancer survivors. Over half of studies included survivors of multiple cancer types (n = 12, 57%) [33, 35, 36, 41–46, 51–53]. One-third of studies were conducted among patients with breast cancer (n = 7, 33%) [37, 38, 40, 47, 49, 54]. Survivors of cancer hematologic malignancy [35, 39] and gastric cancer [50] were also addressed in single studies. The CVD risk factor-related comorbidities assessed were similarly heterogeneous. Thirty-three percent (n = 7) of studies addressed multiple CVD risk factor-related comorbidities [33–39]. Diabetes (n = 6, 29%) [40–45], hypertension (n = 4, 19%) [49–52], and dyslipidemia (n = 2, 10%) [46, 47] were specifically addressed in a few studies. One study addressed secondary prevention of myocardial infarction among cancer survivors [53].
Medication adherence was assessed using a variety of methods. Pharmacy-based measures were the most commonly used approach (n = 15, 71%) [35, 37–40, 42–48, 51, 53]. Among these studies, there was an almost even divide between use of medication possession ratio (MPR) (n = 6, 29%) [34, 38, 40, 44, 45, 47] and proportion of days covered (PDC) (n = 7, 33%) [35, 37, 39, 43, 46, 48, 53]. One study used cumulative medication adherence [51]. Among studies that used a pharmacy-based adherence measure, most defined adherence dichotomously and many used an 80% threshold to define medication adherence (n = 8, 38%) [34, 35, 37–40, 44, 47]. A few studies also examined multiple adherence thresholds [35, 37, 46] (often in sensitivity analyses) or changes in adherence over time [38, 45, 46]. Studies less commonly used self-reported measures of adherence (n = 7, 33%) [33, 36, 41, 49–52]. Among the studies using self-reported measures, the 4-item [33] and 8-item Morisky [36, 41] measures [55–58] were the most common. Three of the studies of Korean origin used self-report but did not specify a particular measure [49, 50, 52]. None of the studies combined the use of pharmacy-based and self-reported measures. A minority (n = 5, 24%) of studies named a phase of medication adherence. Studies addressed persistence [37], discontinuation [39, 40, 47], or persistence and discontinuation jointly [42],
Many studies were conducted entirely in cancer survivor populations [33–36, 38–41, 46–48]. Of those studies that had a non-cancer comparison group [37, 42–45, 49–53], findings about adherence comparing cancer survivors to the general population were mixed [37,42,43,45,49–53], One study reported that cancer survivors were more adherent to CVD-related medications [52]; a few identified worse adherence among cancer survivors compared with the general population [49, 51],
Adherence to CVD risk factor-related medications
Rates of adherence were variable across cancer types and specific CVD risk factor-related comorbidities. In the context of breast cancer survivors, at 2-year post-diagnosis, Calip et al. reported adherence to medications for the management of diabetes, statins, and antihypertensives at 75%, 39%, and 37%, respectively [40]. Conversely, Santorelli and colleagues found adherence to be approximately 74% for medications for diabetes management, 71% for lipid control, and 86% for blood pressure management among breast cancer survivors [37].
Of the studies that examined change in medication adherence over time, the majority noted a decline after diagnosis and over the course of the cancer experience [35, 36, 38, 43, 46]. For example, among a cohort of over 36,000 with breast cancer survivors, Yang et al. reported that average adherence before breast cancer treatment was over 90% but decreased to approximately 78% following cancer treatment [38]. Similarly, among breast cancer survivors, Calip and colleagues reported that mean MPR for metformin/sulfonylureas declined during breast cancer treatment. While adherence rose slightly during the first 3 years after diagnosis, adherence was never fully restored to the pre-cancer diagnosis adherence level [40].
In instances when survivors had multiple CVD risk factor-related comorbidities, a few studies examined which CVD risk factor-related comorbidities had the most severe declines in medication adherence. In these cases, there was a lack of consensus about which CVD risk factor-related comorbidities were most impacted. One study identified adherence for diabetes-related medications was the worst, while [38] another reported no difference in adherence to diabetes-related medications and identified that adherence to lipids suffered the most [35].
Factors associated with CVD risk factor-related medication adherence
Findings about factors associated with medication non-adherence were diverse. Regarding sociodemographic characteristics, both younger age [39, 44] and older age [38, 45] were associated with increased likelihood of non-adherence. Type of insurance was associated with non-adherence; one study found that for most medications, patients with health maintanance organization (HMO), preferred provider organization (PPO), and other insurance types were less likely to experience a decrease in adherence compared to patients with comprehensive insurance [38]. Use of a mail pharmacy, as opposed to a retail pharmacy, was protective against medication non-adherence [44]. In terms of patient beliefs, negative beliefs about the necessity of taking medications [33] and avoidant thoughts about cancer [49] resulted in increased non-adherence.
There were also mixed findings related to clinical factors that impacted medication non-adherence. Higher cancer stage [45] was associated with non-adherence to glucose lowering therapies, and non-adherence with anticancer medications [35] was associated with worse adherence to lipid-lowering therapies and antihypertensives. Receipt of certain cancer treatments was associated with greater likelihood of non-adherence, as was less frequent engagement with primary care [40]. More specifically, among women with breast cancer, those who were treated with adjuvant chemotherapy and endocrine therapy were more likely to be non-adherent. Only receipt of adjuvant chemotherapy was a significant association [40]. Interestingly, higher comorbidity burden was both positively [38, 40] and negatively associated with non-adherence [36]. Taking more medications daily, which may be related to increased comorbidity burden, was associated with worse non-adherence [38, 44].
Prospective studies
We identified two prospective studies [33, 41]. Both studies were non-randomized, single arm designs that included patients with multiple cancer types and small sample sizes (n < 100). Both studies replied on a repeated measures approach and patient self-report for adherence assessment. One study was interventional [33], and one was observational [41]. We describe these two studies in detail below.
Birand and colleagues conducted an interventional, prospective, single-arm study in a tertiary hospital in Northern Cyprus [33]. Patients of all cancer types were eligible for participation. The most frequent comorbid CVD-related risk factors were hypertension and type II diabetes mellitus. Before and after participating in the intervention, patients reported information about their beliefs about medications [59] and their medication adherence [57]. The intervention included a face-to-face educational intervention delivered by an oncology pharmacist and was tailored to each patient’s chemotherapy protocol. The intervention was delivered by the oncology pharmacists when patients visited the oncology clinic for chemotherapy administration. Patients were taught about the importance of medication adherence and rational drug use. Data collection was completed in Turkish. Eighty-one patients were included in analysis. Birand and colleagues found that pharmacist education significantly enhanced the mean patient necessity-concern by two-fold with the greatest gains experienced by patients receiving the educational intervention for the first time. While a direct impact of the intervention on adherence was not assessed, the authors also reported that patients who had a negative balance between their beliefs about the necessity of the medication and their concerns were less likely to adhere to medications [33].
James and colleagues examined the impact of cancer-related post-traumatic stress symptoms on self-management behaviors for people with diabetes mellitus, including medication adherence [41]. Patients with existing diabetes mellitus and a new early-stage cancer diagnosis (diagnosis of prostate, breast, lung, or colorectal cancer within the previous 6 months; also had diabetes mellitus for at least 1 year) were recruited from two medical centers in New York City. There were 56 patients included in analysis. Elevated cancer-related post-traumatic stress symptoms were associated with poor diet, but not with medication adherence behaviors.
Risk of bias
While most studies were retrospective and quantitative in nature, there were differences in study design that prohibited a common method for evaluating risk of bias. Most of the included studies had low or no perceived risk of bias (Table 2). Of those articles that had some risk of bias, in nearly all cases, the risk of bias was attributed to data analysis techniques. As an example, a common limitation was the use of unadjusted models that did not account for potential measured or observed confounders.
Table 2.
Summary of study results and quality assessment (n = 21)
| Author, year | Results | MMAT Score |
|---|---|---|
|
| ||
| Multiple comorbidities | ||
| Birand, 2019 [33] | • Pts. who had a negative balance between their beliefs about the necessity of the medication and their concerns were less likely to adhere to the medication (OR 0.138, 95% CI 0.025–0.772) • Among all participants, 91.4% were identified as adherent post intervention (patient education on adherence and rational drug use) and 8.6% were non-adherent (P < 0.0001) • Adherent pts. showed sig. higher specific necessity belief scores compared to non-adherent pts, and non-adherent pts. showed sig. higher general overuse and harm belief scores than adherent pts. (P < 0.05) |
• 100% |
| Calip, 2017 [34] | • 37% were non-adherent to antihypertensives (out of 1,1779 users) • 75% were non-adherent to diabetes medications (out of 499 users) • 39% were non-adherent to statins (out of 1,072 users) • In adjusted models, younger age was associated with non-adherence to all three therapeutic classes • Certain cancer treatments were modestly associated with non-adherence to antihypertensives (radiation: OR 1.21, 95% CI 1.01–1.47; endocrine therapy: OR 1.27, 95% CI 1.03–1.52) and diabetes medications (chemotherapy: OR 1.69, 95% CI 1.17–2.21) • Less frequent primary care provider visits were associated with higher odds of non-adherence to antihypertensives (OR 1.70, 95% CI 1.19–2.22) • Trends showed higher Charlson comorbidity scores associated with greater adherence to diabetes medications (P < 0.001) and statins (P = 0.032) |
• 100% |
| Dashputre, 2019 [35] | • Across all 3 cancers, patients prescribed lipid-lowering agents experienced most sig. declines in adherence post-oncologic initiation (MM: – 15.6%, P < 0.0001; CML: – 13.1%, P < 0.0001; CLL/SLL: – 10.7%, P = 0.01) • D-in-D models revealed consistent and sig. differences in changes of adherence to antihypertensives (CML: 5.1% vs. 11.3%, P = 0.03; CLL/SLL: 5.2% vs. 13.9%, P = 0.007; MM: 10.4% vs. 13.1%, P = 0.09); sensitivity analyses yielded similar results • No sig. differences for those taking antidiabetic agents • Amongst those adherent to comorbid therapy in baseline, non-adherence to oral oncolytics was associated with higher odds of non-adherence to comorbid therapy in follow-up, with sig. higher odds of non-adherence to antihypertensives in follow-up across all 3 cancers (CML: OR 1.90, 95% CI 1.06–3.42; CLL/SLL: 2.17,1.23–3.82; MM: 1.53, 1.18–1.99) and higher odds of non-adherence to lipid-lowering agents for CLL/SLL (2.51,1.18–5.32) and MM (1.52, 1.04–2.22) |
• 80%; unadjusted D-in-D models |
| Drzayich Antol, 2018 [36] | • Low adherence to comorbidity medication was reported by 29.5% of pts • Pts. with a higher comorbidity level were more frequently moderately or highly adherent to their comorbidity medication regimens • Pts. w/low comorbidity medication adherence reported a median of 15 total unhealthy days compared to 10 total unhealthy days for patients w/moderate or high comorbidity medication adherence (P < 0.05) • Pts. w/low comorbidity medication adherence reported more frequent mental and physical unhealthy days than pts. w/ moderate/high comorbidity medication adherence (30.3% vs 20.2%, P < 0.001; 38.7% vs 33.6%, P = 0.049) • Pts. reporting low comorbidity medication adherence had 23.4% more unhealthy days than pts. w/ moderate/high comorbidity medication adherence (P = 0.007) • A higher comorbidity index was the only factor observed as protective against the odds of low comorbidity medication adherence |
• 100% |
| Santorelli, 2016 [37] | • During the post-baseline period, the percentage of BC pts. who were non-adherent were 26.2% for diabetes medication, 28.9% for lipid-lowering medication, and 14.2% for hypertension medication • BC pts. experienced increased odds (44%) of diabetes medication non-adherence relative to women without cancer (P = 0.02) (effect was similar in sensitivity analyses) • Odds of hypertension medication non-adherence was 24% lower for pts. with BC than those w/o cancer (P < 0.01; when PDC < 90%) • No difference between BC and comparison women in the odds of non-adherence to lipid-lowering medication • Findings of non-persistence were similar to non-adherence → probability of non-persistence with diabetes medication was higher for women w/ BC compared to women w/o cancer, while the probability of non-persistence with HTM medication was lower for women w/BC relative to comparators • Women w/BC experienced a 31% increase in the hazard for diabetes medication non-persistence relative to women w/o cancer (P = 0.02), while women w/BC experienced a 27% decrease in hazard for hypertension medication non-persistence relative to comparators (P < 0.01) • The odds of non-adherence was higher in the post-baseline period relative to the prebaseline period for both the BC and comparison women in all three cohorts |
• 100% |
| Yang, 2016 [38] | • Average adherence to non-cancer medications before cancer diagnosis was 91.4%; after ESBC treatment, average adherence decreased to 77.9% (P < 0.001) • The largest decline in adherence was observed for osteoporosis medications (83.5 to 45.5%; P < 0.001), followed by diabetes medications (79.9 to 53.1%; P < 0.001) and hyperlipidemia medications (83.2 to 57.1%; P < 0.001) • Looking at drugs for each condition, non-adherence ranged from 15.6 to 38% (P < 0.001) • Older age, insurance type, number of medications, and comorbid conditions were associated with non-adherence |
• 100% |
| Zhou, 2019 [39] | • During follow-up period, 74.1% of the allogeneic HCT recipients and 68.3% of the autologous HCT recipients experienced treatment gaps for oral diabetes meds. compared to 54.3% in chemo-only group at 12 months after transplantation • A total of 67.1% of the allo HCT recipients and 60.0% of the auto HCT recipients had treatment gaps of oral antihypertensive meds. at 12 months compared to 47.8% in chemo-only group • For pts. with dyslipidemias, 79.0% of the allo HCT recipients and 63.1% of the auto HCT recipients experienced treatment gaps or oral lipid-lowering meds. at 12 months compared to 53.2% in chemo-only group • Sig. differences in medication adherence observed in allo HCT cohort (P < 0.001) and auto HCT cohort (P = 0.017) for lipid-lowering meds, compared with chemo-only cohort • Allo HCT pts. had greater risk of discontinuation of oral diabetes meds (HR = 1.93, 95% CI 1.10–3.39), hypertension (HR = 1.75, 95% CI 1.21–2.53), and dyslipidemias (HR = 2.02, 95% CI 1.39–2.93) compared to matched chemo-only cohort • Allo HCT recipients were less likely to be adherent to antihypertensive meds. (OR = 0.58, 95% CI 0.38–0.89) and lipid-lowering meds. (OR = 0.38, 95% CI 0.22–0.65); auto HCT recipients were less likely to be adherent to lipid-lowering meds. (OR = 0.73, 95% CI 0.53–0.995) • Younger age was associated with suboptimal adherence to diabetes and hypertensive meds |
• 100% |
| Diabetes | ||
| Calip, 2015 [40] | • Compared to year – 1, mean MPR for metformin/sulfonylureas (0.86 versus 0.49, P < 0.001) and %-adherent (75.3% versus 24.6%, P < 0.001) declined during breast cancer treatment • MPR and %-adherent rose slightly during years 1–3 post diagnosis but never returned to baseline • Discontinuation rate increased from treatment to year + 1 (59.3% versus 75.6%, P < 0.001) and remained elevated during subsequent observation periods |
• 80%; unadjusted models |
| James, 2018 [41] | • No sig. difference in self-management behaviors at enrollment compared with 3-month follow-up (57% vs. 65% adherent to their oral diabetes medication) • No differences in self-management behaviors by age, race, income, cancer type, or presence of anxiety • Compared to pts. w/o avoidant thoughts, pts. w/ avoidant thoughts were less likely to adhere to diabetes medications (77% vs. 30%, P = 0.02) • Adjusted regression did not show an independent association between elevated total PTSD symptoms and adherence to medications • Pts. w/avoidant thoughts about their cancer were less likely to be adherent to their diabetes medications (OR: 0.17, 95% CI: 0.03–1.00) • No sig. associations with pts. w/ intrusive thoughts about cancer and diabetes self-mgt |
• 80%; potential for differential factors in responders and non-responders |
| Letinier, 2018 [42] | • For cancer subjects, avg. duration of oral diabetes treatment before cancer was 29.6 months • One quarter of non-cancer and half of cancer subjects definitively stopped oral diabetes medication after a first discontinuation of ≥ 90 days • Estimated cumulative non-persistence incidence probabilities were 0.39 over the year after oral diabetes treatment initiation and 0.51 over the following 3 years; a higher risk of non-persistence was observed over the first year of f/up • A cancer occurrence sig. increased the cause-specific HR of non-persistence by 93% (similar results in sensitivity analyses) • A cancer occurrence sig. increased the specific HR of non-persistence for CRC, pulmonary, and other cancer groups; association btw. breast or prostate cancer was close to the sig. threshold of 0.05 |
• 100% |
| Stuart, 2015 [43] | Overall, PDC declined after index date for OHAs (3.7%), RAAS-Is (5.3%), and statins (3.2%) for individuals with cancer compared with controls (all P < 0.001) • Overall, individuals diagnosed with cancer had larger pre-post declines than controls for OHAs (− 8.5%; P < 0.01), RAAS-I (− 10.7%; P < 0.001), and statins (− 9.3%; P < 0.01) • Declines in PDC were larger for individuals with short life expectancy relative to those with longer-term survival for RAAS-I (− 6.1%; P < 0.05) and statins (− 6.7%; P < 0.05), whereas the effect was smaller and not significant for OHAs • The PDC values for all three drug classes declined in both the LIS and non-LIS subgroups for cancer survivors relative to controls |
• 100% |
| Tan, 2016 [44] | • Average MPR among all pts. w/ cancer was 0.60 • 62.4% diabetic cancer v. 64.2% diabetic non-cancer non-adherent (no sig. difference, P = 0.24) • 37.6% diabetic, cancer pts.newly initiated on oral diabetic medication adherent to oral diabetic medications; similar adherence proportion in non-cancer control population (35.8%, P = 0.24) • Breast and prostate higher adherence trend than colon and lung cancers (no sig. difference) • More sulfonylureas and TZD (mono/combination therapy) non-adherent than metformin & DPP-4 inhibitors (P = 0.02 and P = 0.01, respectively) • Monotherapy associated w/better adherence than combination therapy (P < 0.001) • Adherence sig. associated w/ demographics and medication-related factors — not clinical status • Younger (18–54) less adherent than older (61–64) (OR = 0.70, P = 0.006) • Mail order 33% more likely adherent than retail (P = 0.02) |
• 100% |
| Zanders, 2015 [45] | • MPR increased 0.10% per month before cancer diagnosis (95% CI: 0.10, 0.10) • Sig. MPR drop at time of cancer diagnosis (− 6.3%; 95% CI: – 6.5, – 6.0) and ongoing, yet lower monthly MPR decline after cancer diagnosis (− 0.20%, 95% CI: – 0.21, – 0.20) → indicating a clear decline in medication adherence because of cancer • No sig. MPR decline at time of breast/prostate cancer diagnosis, but large MPR decline at time of other GI and pulmonary cancers (~ 0.5% each month) • Higher TNM stage associated w/greater observed medication adherence decline • Chemotherapy or radiotherapy administration no effect modification • Pts. who did not receive surgery had more pronounced drop in MPR at cancer diagnosis (− 10.8%; 95% CI: – 11.2, – 10.4) than those who did receive surgery (− 2.8%; 95% CI: – 3.1, – 2.4) • Impact of cancer on adherence was sig. for all age grps., but larger decreases in MPR observed w/increasing age • The impact of cancer diagnosis on MPR most apparent w/ sulfonylurea + metformin and insulin monotherapy or combination therapy at cancer diagnosis |
• 100% |
| Hyperlipidemia | ||
| Banegas, 2018 [46] | • Statin adherence decreased from pre- to post-cancer diagnosis (aOR: 0.91, 95% CI 0.88–0.94, P < 0001) • Statin adherence decreased from pre- to post-cancer diagnosis among breast (aOR: 0.94, 95% CI: 0.90–0.99, P = 0.011) and colorectal (aOR: 0.79, 95% CI: 0.74–0.85, P < 0.001) cancer patients • There was no difference in adherence observed among prostate cancer patients (aOR: 1.01, 95% CI: 0.97–1.05, P = 0.657) |
• 100% |
| Calip, 2013 [47] | • Mean MPR for statin use (0.78 vs. 0.68; P = 0.001) and proportion adherent (67.0 vs. 51.9%; P = 0.001) declined from baseline to cancer treatment period • Lowest MPR observed in year 2 (of treatment years) (0.63 and % adherent 35.9%) • Proportion of users that did not experience a discontinuation episode at baseline was 50.5% and greatest in the treatment period (57.7%; P < 0.001) • During treatment, non-adherent statin users had the highest mean LDL (160.4 mg/dL) and proportion not at goal LDL (91.8%) overall • Adherence did not return to baseline in subsequent years following treatment although LDL levels did • Mean HDL and proportion not at HDL goal did not differ by periods of interest or adherence levels • Adherence to statins in this population was poor, particularly in the treatment period, and lagged in returning to baseline |
• 80%; unadjusted models |
| Feng, 2021 [48] | • Each 10% increase in 1-year adherence to LLMs was associated with an 8% reduction in breast cancer mortality (fully adjusted hazard ratio (HR) = 0.92, 95% CI 0.91–0.93) • The HR for non-cancer mortality was also statistically significantly reduced (0.93, 95% CI 0.92–0.94). Findings were similar for use of any statins and lipophilic statins but were less marked for hydrophilic statins (breast cancer mortality, fully-adjusted HR = 0.96, 95% CI 0.94–0.99; non-cancer mortality, fully-adjusted HR = 0.97, 95% CI 0.94–0.99 • Similar 8% reductions between each 10% increase in 1-year adherence to LLMs, any statins, or any lipophilic statins and colorectal cancer mortality and non-cancer mortality • Reduction in colorectal cancer mortality was slightly less apparent for patients who increased 10% of 1-year adherence to hydrophilic statins (fully adjusted HR = 0.96, 95% CI 0.93–0.98) • Each 10% increase in 1-year adherence to LLMs reduced melanoma mortality and non-cancer mortality after adjustment for age (HR = 0.96, 95% CI 0.93–0.99 and HR = 0.94, 95% CI 0.92–0.96, respectively). After further adjustment for multivariables, the corresponding inverse associations were slightly attenuated for melanoma mortality (HR = 0.97, 95% CI 0.94–1.00) but slightly improved for non-cancer mortality (HR = 0.93, 95% CI 0.92–0.95). A weak reduction in melanoma mortality was observed in lipophilic statin users (fully adjusted HR = 0.99, 95% CI 0.95–1.02) • No statistically significant association in melanoma mortality among hydrophilic statin users |
• 100% |
| Hypertension | ||
| Jeong, 2015 [49] | • No significant differences in smoking, physical activity, antihypertensive medication adherence (aOR: 0.54, 95% CI: 0.19–1.54, P = 0.251), or self-reported diet control • Good medication adherence for BC survivors and non-cancer controls was 81.3% vs. 88.8%; poor medication adherence was 18.7% and 11.2% for cancer and non-cancer pts., respectively |
• 100% |
| Jo, 2015 [50] | • No significant difference in antihypertensive medication adherence among gastric cancer survivors and non-cancer controls (aOR = 1.66; 95% CI: 0.77–3.57; P = 0.195) | • 80%; approach for assessing adherence was not reported |
| Shin, 2010 [51] | • Cancer survivors less likely have appropriate medication adherence than general population (lower CMA) (54.4% vs. 57.5%; aOR, 0.85; 95% CI, 0.82–0.88) • Cancer population greater medication adherence variation based on greater interquartile range • Significant variation based on cancer type: thyroid more adherent and CRC/breast/prostate similarly adherent as general population • Factors affecting adherence: younger age (< 50), low income, rural, and treatment duration |
• 100% |
| Shin, 2012 [52] | • 92.7% of cancer survivors reported taking mediation regularly at all times; 73% of non-cancer comparison group reported taking medication regularly at all times • Cancer survivors were significantly more likely to report full adherence (aOR = 3.45; 95% CI: 2.08–5.73) • Subgroup analyses by cancer stage showed patients w/in situ or local tumors tended to report full adherence & very good perceived BP control (but no statistical significance by trend analysis) |
• 100% |
| Other CVD-related comorbidities | ||
| Cheung, 2013 [53] | • Among those that received a statin after discharge, 42 and 39% of CS and NCP, respectively, were adherent to therapy in 1997–1998 in contrast to 55 and 54%, respectively, in 2007–2008 (P < 0.001) • Of pts. prescribed a beta-blocker and all 3 medications, only NCP became increasingly adherent to therapy between 1997 and 2004 • Adherence to ACEIs/ARBs remained unchanged throughout the study period • Compared to NCP, more survivors received statins (38 vs. 31%) and b-blockers (67 vs. 59%), but fewer underwent bypass surgery (1.5 vs. 2.8%) after MI • Comorbidities (e.g., depression. lung disease) and demographics (advanced age, female) were associated w/ underuse of preventive care among survivors when compared to NCP |
• 100% |
Abbreviations:aOR adjusted odds ratio; CI confidence interval; CLL chronic lymphocytic leukemia; CML chronic myeloid leukemia; CNS central nervous system; CRC colorectal cancer; CVD cardiovascular disease; D-in-D difference in difference; GERD gastroesophageal reflux disease; GI gastrointestinal; OR odds ratio; MM multiple myeloma; MPR medication possession ratio; NHL non-Hodgkin lymphoma; NR not reported; PDC proportion of days covered; Pro prospective; PSM propensity score matching; Retro retrospective; SLL small lymphocytic leukemia
Discussion
We identified limited literature regarding adherence to CVD-related medications among cancer survivors, particularly in the area of prospective interventions to improve adherence. When we updated our search approximately 18 months following the initial literature pull, we only identified one additional article [48]. Cancer survivors may have pre-existing CVD-related comorbidities or new-onset or exacerbated CVD-related comorbidities because of cancer and its treatment [2–5]. Cancer survivors may be less likely to be prescribed medications for comorbidity control [3] and, relative to their contemporaries without a history of cancer, may struggle more with medication adherence [37, 43, 49, 51, 53]. This is a significant public health problem because many cancer survivors have a higher relative risk of mortality from CVD-related problems [60–63] than cancer and CVD medication adherence is critical for reducing morbid and mortality [64].
Importantly, we identified that adherence to medications for CVD-related comorbidities is often worse among cancer survivors than the general population [37, 43, 49, 51–53]. Moreover, for many cancer survivors, medication adherence declines following a cancer diagnosis and during active cancer treatment and may never fully recover to baseline levels [40, 47]. Thus, while cancer may be cured or controlled, the survivor may die of a preventable cause. This is an area ripe for intervention in two ways. First, self-management interventions could potentially be adapted from other patient populations for use among cancer survivors. A critical step is to identify which subgroups of survivors are most likely to struggle with medication adherence; however, a key gap in the literature is how to pinpoint this population. We identified mixed findings about which specific populations of cancer survivors are most at risk for non-adherence. Different studies identified various patient-level characteristics that were associated with medication adherence (e.g., both older age and younger age were associated with non-adherence depending on the study population and study design) [38, 39, 44, 51, 53, 54]. Second, there is an opportunity to develop guidelines for supporting chronic disease management for cancer survivors, both during and after cancer therapy. For example, is a shift away from chronic disease management during cancer treatment always warranted? The current state of the literature may hamper the ability to identify patients who would benefit most from chronic disease management support, both broadly and in relation to the timing of their cancer treatment. It is difficult for predictive analytic approaches to identify the most at risk for adherence problems. Similarly, all cancer survivors should not be treated the same way with regard to chronic disease management or medication adherence. Therefore, the best approach may be to screen individual cancer survivors and provide tailored medication adherence support where needed.
We also identified that less frequent primary care visits were associated with greater medication adherence challenges [34]. This is particularly relevant in survivorship, where many patients are primarily managed by their oncology care team during active cancer treatment. Indeed, this is especially problematic in the management of survivors with chronic cancer, such as chronic lymphocytic cancer. For example, patients managed on ibrutinib are at risk of atrial fibrillation; blood pressure management is essential and is often suboptimal and survivors may be at risk for hypertension-related health events without consistent interaction with primary care. While experts in the field have recommended engagement of primary care providers throughout the cancer care trajectory, this seldom occurs in practice [35, 65–67]. There are known issues with communication and coordination between cancer care and primary care teams [66–70]. Evidence supporting cancer survivorship care plans to facilitate this process is weak to moderate [69––72], and many cancer survivors are lost in transition [73]. This makes adherence to medications for CVD-related medications particularly challenging for cancer survivors, as many may not have an existing primary care team, are poorly engaged with them, or are in a process of regaining familiarity and trust [74].
In addition to these two key findings, we also identified several noteworthy methodological findings. Adherence has been defined as a process with three quantifiable phases: (1) initiation, when a patient takes the first dose of prescribed medication; (2) implementation, the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen; and (3) persistence, length of time between initiation and when the patient stops taking medication for any reason (discontinuation) [75]. Only five studies cited the phase of adherence that was assessed [37, 39, 40, 42, 47]. Similarly, the EMERGE (ESPACOMP Medication Adherence Reporting) Guidelines recommend standard reporting approaches based on the three adherence phases [76]. The EMERGE guidelines are comprised of 21 items organized into minimum reporting criteria that specify reporting each phase of medication adherence studied; an operational definition of each examined phase; the methods of adherence measurement used for each phase, including information on measure performance (validity, reliability, and potential bias); and the results of the analysis relevant to each phase [76]. Although these guidelines were published in 2018 (preceding the publication of many of the identified studies), it is worth noting that none of the included studies referenced the reporting guidelines. This may underscore the need for standardization of definitions across authors in multiple countries.
In the field of medication adherence, there has been a great deal of attention to medication adherence measurement best practices. The general consensus is that there is no single “best” approach and that it is important to consider contextual factors [77]. Thus, our finding of diverse approaches to measuring adherence is not surprising. We identified several studies that relied on patient self-report to assess adherence [33, 36, 41, 51]. While self-report often overestimates medication adherence, it could also be a valuable tool in understanding potential adherence barriers [78], which in turn is essential in developing and implementing interventions to improve adherence. We also identified several studies that used pharmacy-based measures of adherence [35, 37–40, 43, 45–47, 51, 53, 54]. Compared to patient self-report, pharmacy-based measures are generally viewed as more objective; however, pharmacy-based measures have their own limitations. Notably, coding of pharmacy data could be incorrect or incomplete [79, 80]. In addition, pharmacy-based measures typically only measure prescription refills and are not well-suited to provide insight into whether a patient actually ingested the prescribed medication or the reasons why the patient did not initiate or discontinued therapy [79, 81–83].
The majority of identified studies that used pharmacy-based measures [35, 37–40, 42–47, 51, 53] primarily used MPR or PDC [84–86]. Both MPR and PDC are generally reported as a percentage of time when a patient has a medication available; however, there are important differences in these measures. MPR is the sum of the days’ supply for all fills of a given drug in a particular time period, divided by the number of days in the time period [81]. Interpretation of MPR may be challenging in some contexts because the calculation exceeds 100% (e.g., patient gets a refill before it is due and has extra medication supply on hand) [87]. PDC is a newer, more conservative measure of refill record-based adherence. The formula is similar to MPR, but instead of simply adding the days’ supplied in a given period, the PDC considers the days that are “covered” [81]. PDC is often capped at 100%. It is important to note that while MPR is the more commonly used measure, it may overestimate adherence [87, 88]. Moreover, PDC has been recommended by the Pharmacy Quality Alliance and incorporated into the Centers for Medicare and Medicaid Services which has plan ratings [89]. Thus, future work evaluating adherence to medications for CVD-related comorbidities among cancer survivors should consider using PDC. This recommendation may be particularly salient for US-based researchers as PDC is endorsed by the Centers for Medicare and Medicaid Services (CMS). Combining self-report and pharmacy-based assessments of medication adherence may present an interesting opportunity to triangulate true adherence behaviors while also understanding potential challenges to adherence. Interestingly, no studies used combined assessments in analysis.
Our findings have important clinical implications. First, medication adherence is a pervasive and multifactorial problem. While clinicians may not be able to address all barriers that an individual survivor faces, it is important to inform survivors of the importance of taking their medications as prescribed and, when possible, to provide resources to support survivors in achieving this goal. It may also be important to consider integrating assessment of adherence into clinical practice. It is critical to understand that there is no “one-size-fits-all” approach to improve medication adherence [77]. Different survivors will have different needs, thus requiring different tools, and these needs will vary over time. Improving CVD-related medication adherence among cancer survivors is critical to reduce morbidity and mortality among this population.
Acknowledgements
We would like to acknowledge Brandi Tuttle, MLS, AHIP, Research & Education Librarian at the Duke University Medical Center Library & Archives for her guidance with developing the literature search strategy. We also thank Shelley Jazowski, PhD, for her support in the conduct of the literature screening process. We thank the Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) at the Durham Veterans Affairs Health Care System for their support. The content is solely the responsibility of the authors and does not necessarily reflect the position or policy of Duke University, the US Department of Veterans Affairs, or the US government.
Funding
This work was supported by a grant from the National Cancer Institute (CA249568; MPI Oeffinger KC, Zullig LL). This study was also supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K12HL138030 (CD).
Footnotes
Competing interests Dr. Zullig reports research funding from Proteus Digital Health and the PhRMA Foundation, as well as consulting from Novartis — none related to the current work. All other authors have no relevant financial or non-financial interests to disclose.
Ethics approval This is a systematic literature review. Ethics approval was not required.
Consent to participate Not applicable.
Consent for publication Not applicable.
Data availability
No datasets were generated during this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019, (in eng). CA Cancer J Clin. 2019;69(1):7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(1):106–17. 10.1200/jco.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 3.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment, (in eng). CA Cancer J Clin. 2016;66(4):337–50. 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 4.Carver JR, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(25):3991–4008. 10.1200/jco.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 5.Haugnes HS, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer, (in eng). Ann Oncol Off J Eur Soc Med Oncol. 2007;18(2):241–8. 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 6.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases, (in eng). Cancer. 2000;88(3):653–63. . [DOI] [PubMed] [Google Scholar]
- 7.Sunga AY, Eberl MM, Oeffinger KC, Hudson MM, Mahoney MC. Care of cancer survivors, (in eng). Am Fam Physician. 2005;71(4):699–706. [PubMed] [Google Scholar]
- 8.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors, (in eng). Epidemiol (Cambridge, Mass). 2016;27(1):6–13. 10.1097/ede.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer, (in eng). J Am Coll Cardiol. 2012;60(24):2504–12. 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 10.Hanrahan EO, et al. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(31):4952–60. 10.1200/jco.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 11.Sturgeon KM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell KL, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable, (in eng). Med Sci Sports Exerc. 2019;51(11):2375–90. 10.1249/mss.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlebowski RT, et al. Dietary modification and breast cancer mortality: long-term follow-up of the women’s health initiative randomized trial, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(13):1419–28. 10.1200/jco.19.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Aragaki AK, Prentice RL. Dietary moderation and deaths from breast cancer, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2020; Jco2001218. 10.1200/jco.20.01218. [DOI] [PubMed] [Google Scholar]
- 15.Jones LW, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(23):2496–502. 10.1200/jco.2013.53.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones LW, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(32):3643–50. 10.1200/jco.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutschler NS, et al. Prognostic impact of weight change during adjuvant chemotherapy in patients with high-risk early breast cancer: results from the ADEBAR study, (in eng). Clin Breast Cancer. 2018;18(2):175–83. 10.1016/jxlbc.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Arem H, Pfeiffer RM, Moore SC, Brinton LA, Matthews CE. Body mass index, physical activity, and television time in relation to mortality risk among endometrial cancer survivors in the NIH-AARP diet and health study cohort, (in eng). Cancer Causes Control CCC. 2016;27(11):1403–9. 10.1007/s10552-016-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors, (in eng). Cancer Epidemiol Biomarkers Prev. 2013;22(5):792–802. 10.1158/1055-9965.Epi-13-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karavasiloglou N, Pestoni G, Wanner M, Faeh D, Rohrmann S. Healthy lifestyle is inversely associated with mortality in cancer survivors: results from the third national health and nutrition examination survey (NHANES III), (in eng). PLoS One. 2019;14(6):e0218048. 10.1371/journal.pone.0218048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(22):3535–41. 10.1200/jco.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 22.Nelson SH, et al. Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors, (in eng). Breast Cancer Res Treat. 2016;155(3):551–7. 10.1007/s10549-016-3694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Blarigan EL, et al. Association of survival with adherence to the american cancer society nutrition and physical activity guidelines for cancer survivors after colon cancer diagnosis: the CALGB 89803/alliance trial, (in eng ). JAMA Oncol. 2018;4(6):783–90. 10.1001/jamaoncol.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong W, et al. Age and sex patterns of drug prescribing in a defined American population, (in eng). Mayo Clin Proc. 2013;88(7):697–707. 10.1016/jkmayocp.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolandaivelu K, Leiden BB, O’Gara PT, Bhatt DL. Non-adherence to cardiovascular medications, (in eng). Eur Heart J. 2014;35(46):3267–76. 10.1093/eurheartj/ehu364. [DOI] [PubMed] [Google Scholar]
- 26.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients, (in eng). Am J Med. 2012;125(9):882–7.e1. 10.1016/amjmed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Ho PM, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction, (in eng). Arch Intern Med. 2006;166(17):1842–7. 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 28.Brown MT, Bussell JK. Medication adherence: WHO cares?, (in eng). Mayo Clin Proc. 2011;86(4):304–14. 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong QN, et al. Improving the content validity of the mixed methods appraisal tool: a modified e-Delphi study. J Clin Epidemiol. 2019;111:49–59. [DOI] [PubMed] [Google Scholar]
- 30.Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52(5):546–53. [DOI] [PubMed] [Google Scholar]
- 31.Maspero C, Abate A, Cavagnetto D, El Morsi M, Fama A, Farronato M. Available technologies, applications and benefits of teleorthodontics. a literature review and possible applications during the COVID-19 pandemic. J Clin Med. 2020;9(6):1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birand N, Bosnak AS, Diker O, Abdikarim A, Basgut B. The role of the pharmacist in improving medication beliefs and adherence in cancer patients. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract. 2019;25(8):1916–26. 10.1177/1078155219831377. [DOI] [PubMed] [Google Scholar]
- 34.Calip GS, Elmore JG, Boudreau DM. Characteristics associated with nonadherence to medications for hypertension, diabetes, and dyslipidemia among breast cancer survivors. Breast Cancer Res Treat. 2017;161(1):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dashputre AA, Gatwood KS, Schmidt J, Gatwood J. Impact of oral oncolytic initiation on medication adherence for pre-existing comorbid chronic conditions. J Oncol Pharm Pract. 2019; 1078155219875206. 10.1177/1078155219875206. [DOI] [PubMed] [Google Scholar]
- 36.Drzayich Antol D, et al. The relationship between comorbidity medication adherence and health related quality of life among patients with cancer. J Patient-Rep Outcomes. 2018;2:29. 10.1186/s41687-018-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santorelli ML, et al. Effects of breast cancer on chronic disease medication adherence among older women. Pharmacoepidemiol Drug Saf. 2016;25(8):898–907. 10.1002/pds.3971. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Neugut AI, Wright JD, Accordino M, Hershman DL. Non-adherence to oral medications for chronic conditions in breast cancer survivors. J Oncol Pract. 2016;12(8):e800–9. 10.1200/jop.2016.011742. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Han J, Nutescu EA, Patel PR, Sweiss K, Calip GS. Discontinuation and nonadherence to medications for chronic conditions after hematopoietic cell transplantation: a 6-year propensity score-matched cohort study, (in eng). Pharmacotherapy. 2019;39(1):55–66. 10.1002/phar.2197. [DOI] [PubMed] [Google Scholar]
- 40.Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM. Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol Drug Saf. 2015;24(1):75–85. 10.1002/pds.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James J, Harris YT, Kronish IM, Wisnivesky JP, Lin JJ. Exploratory study of impact of cancer-related posttraumatic stress symptoms on diabetes self-management among cancer survivors. Psychooncology. 2018;27(2):648–53. 10.1002/pon.4568. [DOI] [PubMed] [Google Scholar]
- 42.Letinier L, Mansiaux Y, Pariente A, Fourrier-Reglat A. Impact of cancer diagnosis on persistence of oral antidiabetic drugs. Diabetes Res Clin Pract. 2018;139:323–30. 10.1016/.diabres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Stuart BC, Davidoff AJ, Erten MZ. Changes in medication management after a diagnosis of cancer among medicare beneficiaries with diabetes. J Oncol Pract. 2015;11(6):429–34. 10.1200/jop.2014.003046. [DOI] [PubMed] [Google Scholar]
- 44.Tan X, Feng X, Chang J, Higa G, Wang L, Leslie D. Oral antidiabetic drug use and associated health outcomes in cancer patients. J Clin Pharm Ther. 2016;41(5):524–31. 10.1111/jcpt.12430. [DOI] [PubMed] [Google Scholar]
- 45.Zanders MM, Haak HR, van Herk-Sukel MP, van de Poll-Franse LV, Johnson JA. Impact of cancer on adherence to glucose-lowering drug treatment in individuals with diabetes. Diabetologia. 2015;58(5):951–60. 10.1007/s00125-015-3497-8. [DOI] [PubMed] [Google Scholar]
- 46.Banegas MP, et al. Patterns of medication adherence in a multi-ethnic cohort of prevalent statin users diagnosed with breast, prostate, or colorectal cancer. J Cancer Surviv Res Pract. 2018;12(6):794–802. 10.1007/s11764-018-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calip GS, Boudreau DM, Loggers ET. Changes in adherence to statins and subsequent lipid profiles during and following breast cancer treatment. Breast Cancer Res Treat. 2013;138(1):225–33. 10.1007/s10549-013-2424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng JL, Qin X. Does adherence to lipid-lowering medications improve cancer survival? A nationwide study of breast and colorectal cancer, and melanoma, (in English). Br J Clin Pharmacol. 2021;87(4):1847–58. 10.1111/bcp.14573. [DOI] [PubMed] [Google Scholar]
- 49.Jeong JR, Kim S, Jo SR, Joh JY, Kim YP. Health behaviors of breast cancer survivors with hypertension: a propensity analysis of KNHANES III-V (2005–2012). PLoS One. 2015;10(5):e0127346. 10.1371/journal.pone.0127346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jo SR, Joh JY, Jeong JR, Kim S, Kim YP. Health behaviors of Korean gastric cancer survivors with hypertension: a propensity analysis of KNHANES III-V (2005–2012). PLoS One. 2015;10(5):e0126927. 10.1371/journal.pone.0126927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin DW, Park JH, Park EC, Kim SY, Kim SG, Choi JY. Anti-hypertensive medication adherence in cancer survivors and its affecting factors: results of a Korean population-based study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2010;19(2):211–20. 10.1007/s00520-009-0802-4. [DOI] [PubMed] [Google Scholar]
- 52.Wook Shin D, et al. Comparison of hypertension management between cancer survivors and the general public. Hypertens Res Off J Jpn Soc Hypertens. 2012;35(9):935–9. 10.1038/hr.2012.54. [DOI] [PubMed] [Google Scholar]
- 53.Cheung WY, Levin R, Setoguchi S. Appropriateness of cardiovascular care in elderly adult cancer survivors. Med Oncol (Northwood, London, England). 2013;30(2):561. 10.1007/s12032-013-0561-4. [DOI] [PubMed] [Google Scholar]
- 54.Calip GS, Xing S, Jun DH, Lee WJ, Hoskins KF, Ko NY. Polypharmacy and adherence to adjuvant endocrine therapy for breast cancer. J Oncol Pract. 2017;13(5):e451–62. 10.1200/jop.2016.018317. [DOI] [PubMed] [Google Scholar]
- 55.Koschack J, Marx G, Schnakenberg J, Kochen MM, Himmel W. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties, (in eng). J Clin Epidemiol. 2010;63(3):299–306. 10.1016/j.jclinepi.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting, (in eng). J Clin Hypertens (Greenwich, Conn). 2008;10(5):348–54. 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence, (in eng). Med Care. 1986;24(1):67–74. 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Unni EJ, Farris KB. Development of a new scale to measure self-reported medication nonadherence, (in eng). Res Soc Adm Pharm RSAP 2015;11(3):e133–43. 10.1016/.sapharm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. [Google Scholar]
- 60.Armstrong GT, et al. Reduction in late mortality among 5-year survivors of childhood cancer, (in eng). N Engl J Med. 2016;374(9):833–42. 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gernaat SAM, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review, (in eng). Breast Cancer Res Treat. 2017;164(3):537–55. 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matasar MJ, Ford JS, Riedel ER, Salz T, Oeffinger KC, Straus DJ. Late morbidity and mortality in patients with Hodgkin’s lymphoma treated during adulthood, (in eng). J Natl Cancer Inst. 2015; 107(4). 10.1093/jnci/djv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Nimwegen FA, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk, (in eng). JAMA Intern Med. 2015;175(6):1007–17. 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 64.Leslie KH, McCowan C, Pell JP. Adherence to cardiovascular medication: a review of systematic reviews, (in eng). J Public Health (Oxf). 2019;41(1):e84–94. 10.1093/pubmed/fdy088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snyder CF, et al. Comorbid condition care quality in cancer survivors: role of primary care and specialty providers and care coordination, (in eng). J Cancer Surviv. 2015;9(4):641–9. 10.1007/s11764-015-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sussman J, Bainbridge D, Evans WK. Towards integrating primary care with cancer care: a regional study of current gaps and opportunities in Canada, (in eng). Healthc Policy Politiques de sante. 2017; 12(3):50–65. Vers une intégration des soins de santé primaires et des soins contre le cancer: étude régionale des lacunes et des occasions actuelles au Canada. [PMC free article] [PubMed] [Google Scholar]
- 67.Sussman J, Baldwin LM. The interface of primary and oncology specialty care: from diagnosis through primary treatment, (in eng), Journal of the National Cancer Institute. Monographs. 2010;2010(40):18–24. 10.1093/jncimonographs/lgq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Easley J, et al. Coordination of cancer care between family physicians and cancer specialists: importance of communication, (in eng). Can Fam Physician Medecin de famille canadien. 2016;62(10):e608–15. [PMC free article] [PubMed] [Google Scholar]
- 69.Salz T, Oeffinger KC, McCabe MS, Layne TM, Bach PB. Survivorship care plans in research and practice, (in eng). CA Cancer J Clin. 2012;62(2):101–17. 10.3322/caac.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver SJ, Jacobsen PB. Cancer care coordination: opportunities for healthcare delivery research, (in eng). Transl Behav Med. 2018;8(3):503–8. 10.1093/tbm/ibx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use, (in eng), Journal of cancer education : the official journal of the American Association for Cancer. Education. 2013;28(2):290–6. 10.1007/s13187-013-0469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forsythe LP, et al. Use of survivorship care plans in the United States: associations with survivorship care, (in eng). J Natl Cancer Inst. 2013;105(20):1579–87. 10.1093/jnci/djt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care, (in eng). Ann Intern Med. 2004;141(7):533–6. 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 74.Lawrence RA, McLoone JK, Wakefield CE, Cohn RJ. Primary care physicians’ perspectives of their role in cancer care: a systematic review, (in eng). J Gen Intern Med. 2016;31(10):1222–36. 10.1007/s11606-016-3746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, Vrijens B. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30–5. 10.7326/M18-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zullig LL, Mendys P, Bosworth HB. Medication adherence: a practical measurement selection guide using case studies, (in eng). Patient Educ Couns. 2017;100(7):1410–4. 10.1016/j.pec.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stirratt MJ, et al. Self-report measures of medication adherence behavior: recommendations on optimal use, (in eng). Transl Behav Med. 2015;5(4):470–82. 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crystal S, Akincigil A, Bilder S, Walkup JT. Studying prescription drug use and outcomes with medicaid claims data: strengths, limitations, and strategies, (in eng). Med Care. 2007;45(10 Supl 2):S58–65. 10.1097/MLR.0b013e31805371bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polinski JM, Schneeweiss S, Levin R, Shrank WH. Completeness of retail pharmacy claims data: implications for pharmacoepidemiologic studies and pharmacy practice in elderly patients, (in eng). Clin Ther. 2009;31(9):2048–59. 10.1016/j.clinthera.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures, (in eng). Ann Pharmacother. 2006;40(7–8):1280–8. 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 82.Winn AN, Keating NL, Dusetzina SB. Factors associated with tyrosine kinase inhibitor initiation and adherence among medicare beneficiaries with chronic myeloid leukemia, (in eng). J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(36):4323–8. 10.1200/jco.2016.67.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Virnig B ResDAC Research Data Assistance Center. https://www.resdac.org/articles/strengths-and-limitations-cms-administrative-data-research. Accessed 18 June 18.
- 84.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients, (in eng). Jama. 2002;288(4):455–61. 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 85.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases, (in eng). Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2007;10(1):3–12. 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 86.Nau D Proportion of days covered (PDC) as a preferred method of measuring medication adherence. http://www.pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf. Accessed 18 June 2020.
- 87.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases, (in eng). Med Care. 2013;51(8 Suppl 3):S11–21. 10.1097/MLR.0b013e31829b1d2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crowe M Do you know the difference between these adherence measures. https://www.pharmacytimes.com/view/do-you-know-the-difference-between-these-adherence-measures. Accessed 18 June 2020.
- 89.Centers for Medicare and Medicaid Services. Medicare 2018 part C & D star ratings technical notes. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated during this study.


