Abstract
Analgesic trials pose unique scientific, ethical, and practical challenges in pediatrics. Participants in a scientific workshop sponsored by the US Food and Drug Administration developed consensus on aspects of pediatric analgesic clinical trial design. The standard parallel-placebo analgesic trial design commonly used for adults has ethical and practical difficulties in pediatrics, due to the likelihood of subjects experiencing pain for extended periods of time. Immediate-rescue designs using opioid-sparing, rather than pain scores, as a primary outcome measure have been successfully used in pediatric analgesic efficacy trials. These designs maintain some of the scientific benefits of blinding, with some ethical and practical advantages over traditional designs. Preferred outcome measures were recommended for each age group. Acute pain trials are feasible for children undergoing surgery. Pharmacodynamic responses to opioids, local anesthetics, acetaminophen, and nonsteroidal antiinflammatory drugs appear substantially mature by age 2 years. There is currently no clear evidence for analgesic efficacy of acetaminophen or nonsteroidal antiinflammatory drugs in neonates or infants younger than 3 months of age. Small sample designs, including cross-over trials and N of 1 trials, for particular pediatric chronic pain conditions and for studies of pain and irritability in pediatric palliative care should be considered. Pediatric analgesic trials can be improved by using innovative study designs and outcome measures specific for children. Multicenter consortia will help to facilitate adequately powered pediatric analgesic trials.
Keywords: pain, pediatric, infant, child, neonate, analgesic, clinical trial, placebo
Special ethical and clinical considerations must be invoked when conducting drug research with infants and children. 1 In this article, we discuss unique scientific, ethical, and practical considerations for designing pediatric analgesic trials, followed by recommendations regarding outcome measures, appropriate patient groups for study, and study designs for classes of analgesic medications. The epidemiology and prevalence of acute and chronic pain in children are briefly reviewed. We also discuss available evidence regarding age-dependent actions of several analgesic classes and their relevance for extrapolating the efficacy and risks of these drugs from older children to younger ones.
Analgesic Trials Are Difficult
Clinical trials of analgesics pose challenges for patients of all ages. Pain perception and analgesic responsiveness show marked variability among individuals, even among those receiving similar experimental or clinical pain stimuli. Placebo and nocebo effects are common, variable in magnitude, and history-dependent. 2
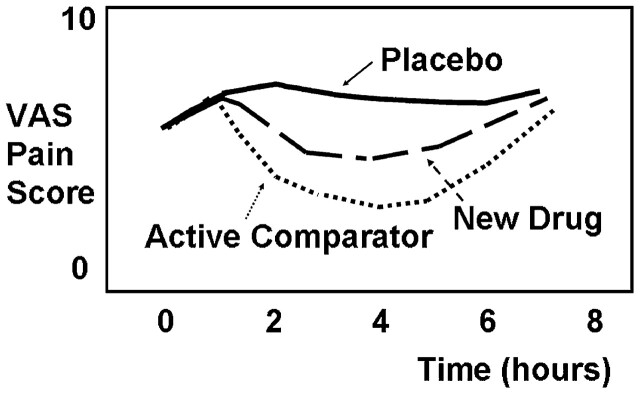
For regulatory agencies, such as the US Food and Drug Administration (FDA), there is an imperative to prevent marketing drugs that are unsafe or ineffective. Placebo-controlled trials are an ideal way to avoid the false conclusion that a drug is effective when it is not. Thus, large randomized parallel-group, placebo-controlled clinical trials are a preferred methodology for evaluating efficacy and side effects of analgesics. 3 A typical single-dose acute pain analgesic trial, idealized in Fig 1, including an experimental group, a placebo group, and an active comparator group (using a well-established drug), might require 100 to 200 patients for adequate statistical power. Comparison of a new drug against an existing effective drug is important for comparative effectiveness or pharmacoeconomics research, but without a placebo group, conclusions about the new drug’s efficacy may be erroneous. Because analgesic trials often show large variability and small effect sizes, there is no “transitivity principle” for efficacy: if drug A is significantly more effective than placebo and drug B is not significantly distinguishable from drug A, it does not follow that drug B is significantly more effective than placebo. 3
FIGURE 1.
Typical time course of pain scores for a double-blind, parallel-group, placebo-controlled, active-comparator analgesic trial. Note that in general, requirement for rescue analgesia results in termination of pain scoring for that subject. VAS, visual analog scale.
The FDA frequently requires new drug applications to include randomized placebo-controlled trials in children if the drug is indicated for this age group. Beliefs and expectations among clinicians, investigators, and parents can influence pain assessments, particularly in trials involving nonverbal subjects. Nevertheless, placebo controls with infants and children pose serious ethical and practical challenges, which have severely limited the number of new analgesics approved by the FDA for children.
The purpose of this article is to review these challenges and suggest alternative strategies for investigators and regulators for analgesic research in pediatrics.
Ethical Considerations in Pediatric Drug Trials
Children are vulnerable research participants who often cannot represent their own views and self-interests. Their participation in research with more than minimal risk must be justified by potential direct benefit to the individual child. 1 , 4 , 5 A placebo-controlled pediatric analgesic trial may be ethically problematic if exposure to more than minor pain occurs and there are effective treatments for the disorder or condition being studied. 1 Despite the availability of accepted drugs with known efficacy, competent adults can consent to trials including a placebo analgesic but children cannot.
Pediatric trials are complicated by 3 unique but interrelated issues: (1) lack of agreement about pediatric analgesic study designs and measures, (2) limitations in pain assessment by surrogates, 1 , 6 and (3) limits on extrapolation of efficacy and risks, due to developmental differences in metabolism, excretion, drug efficacy, receptor subtypes, receptor induction, signal transduction, and cellular regulatory pathways. 7 – 10 All these considerations are accentuated at earlier developmental stages, maximally so in extremely preterm infants. 11 These factors generate a tension between the demands of scientific rigor and the need for adequately controlled studies on the one hand and ethical considerations on the other.
The Development of Nociception and Pain
Peripheral and spinal nociceptive pathways are substantially developed in newborn animals and human newborns. Noxious stimuli evoke specific responses in the contralateral somatosensory cortex in preterm neonates as early as 25 weeks’ postconception. 12 – 14 Untreated pain from surgery or repeated noxious procedures may evoke hyperalgesia, stress responses, 15 and short- and long-term sequelae. 16 , 17 Although prevention of short- or long-term sequelae are desirable secondary goals of analgesic therapies, amelioration of the patient’s experience of pain and suffering are important in their own right.
Epidemiology of Pain in Children
Painful conditions are often characterized by their time course as acute, recurrent/episodic, or chronic/persistent. Acute pain can arise from (1) medical procedures, (2) surgery, (3) injuries, and (4) acute exacerbations of disease-related pain. Common sources of recurrent/episodic pain in childhood include headache, chest pain, abdominal pain, and limb pain. These recurrent pain conditions occur in at least 5% to 10% of children and adolescents in school-based populations. 18 , 19 The frequency of recurrent pains generally increases with onset of adolescence, where they are more prevalent among girls than boys and may persist over many years. 20
Chronic pain, commonly defined as pain persisting for 3 months or more, or beyond the expected period of healing, involves different neural mechanisms from acute pain. 21 The epidemiology of chronic persistent pain differs considerably between children and adults. 22 Chronic daily pain 23 can occur in children with chronic daily headaches, neurodegenerative disorders, inflammatory disorders, 24 post-traumatic neuropathic pain conditions, 25 including complex regional pain syndromes, and in children with advanced cancer and other life-limiting diseases. Overall, however, chronic daily pain is much less prevalent in children than in adults. Infant animals 26 and humans 27 appear less likely than adults to develop chronic pain after similar types of nerve injury.
Pain Assessment and Analgesic Drug Efficacy in Children
Accurate pain assessment, especially for infants and children who are unable to perform self-report measures, remains a significant challenge in pediatric clinical trials for acute and chronic pain. 28 , 29 The Newborn Drug Development Initiative 30 and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) groups recently recommended multidimensional outcome domains and measures for pediatric acute and chronic pain clinical trials. 31
For acute pain, outcome measures may include pain intensity, frequency, duration and extent, satisfaction with treatment, associated symptoms and adverse events, physical recovery, emotional response, and economic factors. For chronic pain, the same list applies, with the addition of physical functioning, social functioning/peer relationships, mood, sleep, and quality of life. Table 1 lists measures for assessing each of these factors, as recommended primarily by the evidence and secondarily by expert opinion. Few scales measuring interference by pain with daily function were available at the time of the Pediatric IMMPACT consensus meeting; therefore, we recommend inclusion of the Patient Reported Outcomes Measurement Information System (PROMIS) Pediatric Pain Interference Scale to include patient-reported outcomes on daily activities impacted by chronic pain. 32 For all pain assessment, developmental, communication, and cognitive capacities must be considered. In general, patient self-report is deemed optimal whenever feasible. 28
TABLE 1.
| Acute | Chronic | |
|---|---|---|
| Pain Intensity | ||
| Self-report | ||
| 4-12 y | • Faces Pain Scale–Revised (FPS-R) | • Same self-report indices with possible addition of diaries |
| >8 y | • Numerical Rating Scale (NRS; known as Verbal Numerical Scale, VNS, when given verbally) | |
| • Visual Analog Scale (VAS) | ||
| Observation | ||
| >1 y | • Toddler-Preschooler Postoperative Pain Scale (TPPPS) | • No well-validated measures |
| • Face, Legs, Arms, Cry, Consolability Scale (FLACC) | ||
| • Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) | ||
| >5 y with neurological or communication impairment | • Non-Communicating Children's Pain Checklist (NCCPC-R; for post-operative pain use NCCPC-PV) | • Non-Communicating Children's Pain Checklist (NCCPC-R) |
| Global judgment of satisfaction with treatment | ||
| For parent or child > 7 years | • Same indices | |
| • Global open-ended question | ||
| • 0-10 numeric rating scale | ||
| Symptoms and adverse events | ||
| • Active capture of symptoms and adverse events; severity and importance of each event | • Same indices | |
| Physical recovery | ||
| • No well-validated measure | • Functional Disability Inventory | |
| • PedsQL | ||
| Emotional response | ||
| Self-report | • Adolescent Pediatric Pain Tool (APPT) | • Children's Depression Inventory (7-17 yrs) |
| • Facial Affective Scale (FAS) | ||
| • Revised Childhood Anxiety and Depression Scale | ||
| • PedsQL | ||
| Observation >1 y | • Procedure Behavior Check List (PBCL) | |
| • Procedure Behavioral Rating Scale Revised (PBRS-R) | ||
| Economic factors | ||
| • No well-validated measures | • No well-validated measures | |
| Role functioning | ||
| 6-18 y | • Not applicable | • School attendance |
| • PedMIDAS | ||
| • PedsQL | ||
| 8-17 y | • Not applicable | • PROMIS Pediatric Pain Interference Scale |
| Sleep | ||
| • Not applicable | • Polysomnography | |
| • Accelerometry | ||
| • Sleep Habits Questionnaire |
The PROMIS Pediatric Pain Interference Scale was not available at the time of the IMPACCT consensus process. We believe that it is a useful scale that should be considered particularly for analgesic studies for chronic pain. PedsQL, pediatric quality of life inventory; PedMIDAS, pediatric migraine disability assessment.
The Pediatric IMMPACT group addressed pain assessment in children age 3 years and older. 31 Many future analgesic trials will include preterm and term neonates, as well as older infants, for whom pain measurement poses particular challenges. 30
Neural maturation in both healthy and critically ill newborns affects the behavioral and autonomic responses to acute, repetitive, or persistent pain. Extremely low birth weight infants show inconsistent pain responses over time because responses are dampened and modified by contextual factors (eg, severity of illness, preceding painful stimuli, proximity to feeding, behavioral state, maternal contact, or use of anesthesia).
There are over 40 measures of infant pain; however, only a few have been specifically developed to address the unique responses of preterm, term, or older infants. In the Supplemental Information, item A1, we summarize infant pain measures that have been studied more extensively and outline clinical settings and intervention trials in which these measures have been used. 33 – 59 For example, the Premature Infant Pain Profile was designed for acute procedural pain, as opposed to disease-related or postoperative pain. There are few indices of ongoing continuous pain in newborns and there are conflicting data on the influence of previous painful procedures, cumulative pain experience, severity of illness or codiagnoses on subsequent pain sensitivity. 6 , 60
Behavioral measures of pain in older children may reflect fear or anxiety and they may underrate pain relative to self-report in the setting of persistent pain. 61 Nevertheless, behavioral measures 28 remain the most useful pain intensity measures for both clinical management and clinical research for infants, preverbal children, and nonverbal children.
Physiologic indicators of pain are widely used in critical care. They generally show poor specificity because many processes unrelated to pain can cause sympathetic stimulation or arousal. 62 Cerebral near infrared spectroscopy 12 , 14 , 63 and other neuroimaging techniques and various biomarkers, such as heart rate variability and cortisol, 64 , 65 are of current research interest but have not demonstrated reliability or validity for use in analgesic drug studies.
Pain measurement and pain management pose unique challenges for children with neurodevelopmental impairments. 66 – 69 Although self report is not available in this setting, an emerging set of tools are now available that offer indices of pain and irritability to guide clinical and research questions. 67 , 70 , 71 Standardized assessment tools may not capture pain responses accurately in this population. Clinical trials and outcome studies for patients with neurodevelopmental impairments should examine efficacy and emphasize impact on daily function, as well as risks and side effects that may be especially prevalent among these children. 72
Developmental Pharmacology of Analgesics
Important aims of pediatric analgesic clinical trials are to provide information on safe and effective drug dosing, based on dose-scaling with body size and organ development. 73 Pediatric textbooks and interprofessional in-house manuals often list single weight-scaled drug doses, but this approach sometimes leads to significant over- or underdosing. 74 For example, extrapolation of adult doses based strictly on a “mg/kg” approach leads to overdosing of morphine in neonates, but underdosing of bupivacaine in infant spinal anesthesia. 75 Age-related trends 10 in pharmacokinetics (PKs) 76 are summarized in Table 2. Some pharmacodynamic trends relevant for analgesic clinical trials are summarized in the Supplemental Information, item A2. 74 , 76 – 78
TABLE 2.
| Action | Trend | Implications or Clinical Examples |
|---|---|---|
| Hepatic CYP-dependent metabolism | Immature at birth, maturation over 6–12 mo | 1. Prolonged action of fentanyl in neonates and young infants 2. Ineffectiveness of CYP-dependent prodrugs, such as codeine, in infants 3. Increased risk of accumulation and systemic toxicity with amino-amide local anesthetics |
| Hepatic glucuronidation | Immature at birth, maturation over 6–12 mo | Prolonged action of morphine in neonates and young infants |
| Plasma esterases | Lower levels at birth, maturation over 3 mo | Despite lower esterase levels in neonates and young infants, clearance of chloroprocaine and remifentanil appears rapid by 2 mo of age |
| Renal clearance | Immature at birth, maturation over 6 mo | Accumulation of metabolites that may contribute to analgesia (morphine -6G) or to seizures (MEGX, normeperidine, morphine-3G) in infants |
| Protein binding | Reduced plasma concentrations of albumin and α-1 acid glycoprotein in neonates and young infants | Increased unbound concentrations of lipophilic drugs (eg, bupivacaine) may potentiate systemic toxicities |
| Composition of body compartments | Greater total body water and larger blood vol per kg in infants compared with older subjects | Larger volumes of distribution for hydrophilic drugs |
Pharmacogenomic variability can dramatically influence drug efficacy and toxicity. An emphasis on pharmacogenomic studies should be especially strong for drugs that act partially (oxycodone, tramadol) or almost entirely (codeine) as prodrugs. For codeine, developmental immaturity and/or a slowly metabolizing form of cytochrome p450 type 2D6 can lead to very poor analgesia, 79 whereas a rapidly metabolizing form of the enzyme can lead to overdose. 80 Interactions between developmental, pharmacogenetic, and pharmacogenomic factors on analgesic drug efficacy, safety, and tolerance 81 remain unexplored.
An Alternative Trial Design: Immediate-Rescue With Nurse-Controlled Analgesia or Patient-Controlled Analgesia
Ethical and practical considerations pose substantial obstacles for pediatric analgesic efficacy trials. Medical ethicists and institutional review boards commonly oppose enrolling children in trials similar to Fig 1 above. We propose a different pediatric analgesic trial design integrating immediate rescue with patient-controlled analgesia (PCA) or nurse-controlled analgesia (NCA), using analgesic sparing as a surrogate primary efficacy endpoint, while still incorporating the scientific and regulatory advantages of placebo-controlled trials. A typical trial would proceed as summarized in Fig 2. Additional details are provided in the Supplemental Information, item A3.
FIGURE 2.
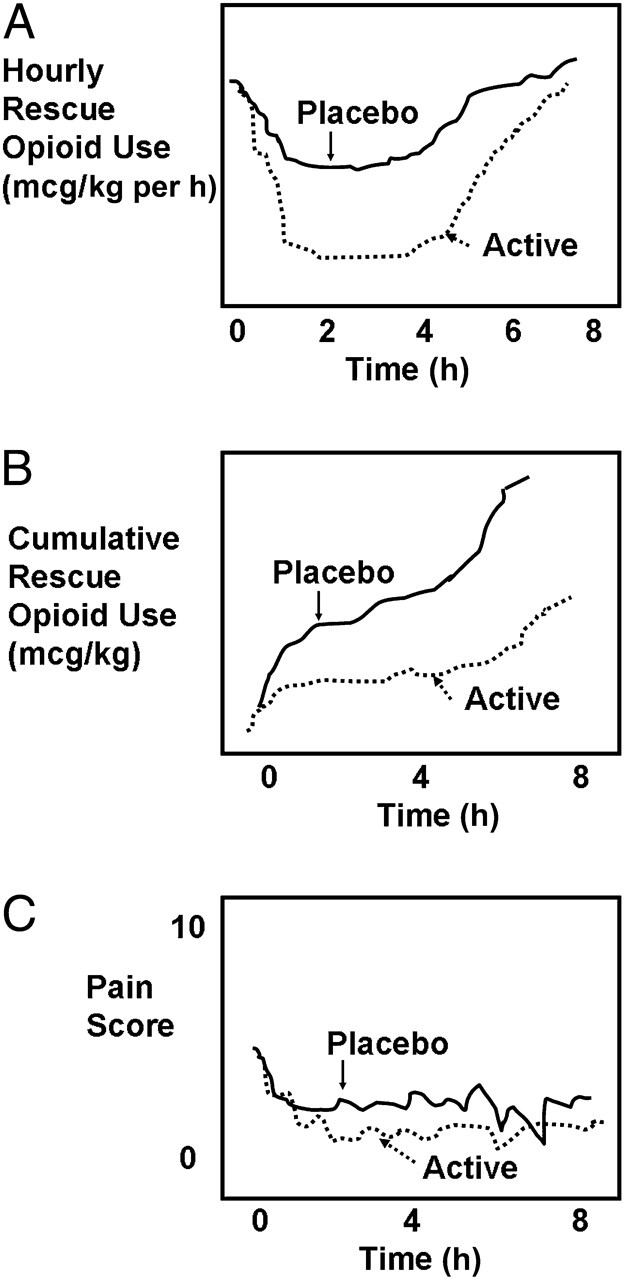
Idealized immediate rescue analgesic trial for a single-dose analgesic study. A, The time course of hourly rescue dosing of a short-acting opioid. B, The cumulative rescue opioid dosing over time. C, The time course of pain scores. Note that, depending on the dosing schedule for rescue analgesics, in some trials of this design, pain scores remain lower in the active drug group than for the placebo group.
Rescue-analgesic designs have been used previously in pediatric analgesic studies, including those evaluating nonsteroidal antiinflammatory drugs (NSAIDs), 82 acetaminophen, 83 regional anesthesia, 84 oral opioids, and analgesic combinations. 85 Patients expected to experience moderate to severe pain, such as pain after surgery, are enrolled and randomly assigned to placebo or active drug groups in a double-blind manner. All patients have immediate access to either PCA (based on age, motor capacity, and developmental readiness), or NCA, if they are unable to use PCA effectively. PCA and NCA appear ideal for this design, because they permit rapid “catch-up” in the event of unrelieved pain, and because they allow small incremental doses, increasing assay sensitivity. In contrast to a PCA or NCA paradigm, large intermittent rescue doses or continuous opioid infusions seem less desirable because of the greater likelihood of washing out differences between randomized groups. 86 Basal infusions are omitted with PCA or NCA so that if subjects had very little pain, whether from specific analgesic effects, placebo effects, or resolution of pain, they would not receive additional analgesia. Subjects in both treatment and placebo arms who have inadequate analgesia would receive prompt rescue analgesia. Differences in cumulative rescue dosing between drug and placebo groups are regarded as primary surrogate measures of drug efficacy (Fig 2B); pain scores become secondary outcome measures (Fig 2C).
PCA dosing by nurses or family members (PCA by proxy) is widely accepted in pediatric settings, with excellent safety of NCA in acute pain management, and broad acceptance of dosing by both nurses and family members in palliative care. 87 – 89 Immediate-rescue paradigms have several attractive features, as well as significant limitations, as detailed in the Supplemental Information, item A4.
The time course of opioid analgesic effects is not represented solely, or even primarily, by its elimination half-life. Rather, it reflects other processes, including distribution and binding to the central nervous system and other tissues, as well as the analgesic actions of active metabolites. The concepts of “effect site concentration” and “context-sensitive half-time” are well described elsewhere. 90 The authors of many previous studies used long-acting opioids such as morphine as the rescue analgesic 84 , 86 despite its comparatively long duration of action and active metabolites. For single-dose studies over a short time period, it is plausible that there will be better discrimination between test drug and placebo by using rescue opioids with more rapid onset and offset of action and context-sensitive half-lives that are less dependent on previous dosing compared with morphine, such as alfentanil, 91 fentanyl, or sufentanil. With short-acting agents using an NCA paradigm, availability of study nurses must be sufficient to ensure prompt repeat dosing at frequent intervals. The ultrashort action of remifentanil might be impractical for these designs, particularly if a study extends overnight. Although rapid intravenous bolus administration of these opioids can produce chest wall and respiratory muscle rigidity, this risk appears very low for small incremental dosing used with PCA pumps. 92
What Pediatric Patient Populations Should Participate in Analgesic Studies? When Can Efficacy Be Extrapolated to Children?
In an ideal world, efficacy of each new drug would be established in every age group and for a broad range of clinical circumstances. To require demonstration of efficacy in every circumstance would unacceptably delay availability and pediatric licensing because of the impracticality of completing efficacy studies in a timely fashion.
Furthermore, for drug classes with well-understood mechanisms of action (eg, μ-opioid agonist interaction with μ-receptors in the central nervous system, or local anesthetic sodium channel blockade), there is little biological reason to postulate that the drug would be less effective (at similar effect site concentrations) in children older than 2 years of age compared with adults. What is most needed to guide safe prescribing are studies that generate valid data on drug metabolism, dose response, and toxicity. For drugs with less well-understood mechanisms of action or with less well-established efficacy in adults, pediatric efficacy, PK, and safety studies should be required as long as there is a potential clinical role for these drugs in infants and younger children.
Provisional recommendations about selection of patient populations for different drug classes and clinical indications are listed in Table 3. These recommendations are based on expert opinion, enrollment experience in previous pediatric analgesic trials, and on the pediatric pharmacoepidemiology of analgesic classes.
TABLE 3.
Recommended Pediatric Studies for Different Analgesic Classes
| Drug Class | Recommended Studies: Acute Use (<1 wk) | Recommended Studies: Subacute Use (1–3 wk) | Recommended Studies: Chronic Use (≥1 mo) |
|---|---|---|---|
| Acetaminophen | Efficacy (extrapolation to age 2 y; special emphasis on <3 mo of age) PK (all ages) Dose response (all ages) Safety (hepatic toxicity) | PK (all ages) Dose response (all ages) Safety (hepatic toxicity) | Rarely indicated for chronic pain in pediatrics Not recommended for chronic headache in pediatrics |
| Antiinflammatory Agents: Nonsteroidal antiinflammatory drugs Coxibs | Efficacy (extrapolation to age 2 y; special emphasis on <3 mo of age) PK (all ages) Dose response (all ages) Safety (gastric, renal toxicity) | Efficacy (extrapolation to age 2 y) PK (ages ≥2) Dose response Safety (gastric, renal toxicity) | Efficacy for selected antiinflammatory indications, age >2 y PK (ages ≥2) Dose response Safety (gastric, renal, hepatic toxicity) |
| μ Receptor Opioids | Efficacy (extrapolation to age 2 y) PK (all ages) Dose response (all ages) Safety (CNS toxicity, respiratory sensitivity) | Efficacy (extrapolation to age 2 y) PK (all ages) Dose response (all ages) Safety (CNS toxicity, immune response, withdrawal) | Small, heterogeneous populations for study; consider small-sample designs Safety (CNS toxicity, immune response, withdrawal) |
| Mixed Receptor Opioids | Efficacy (all ages) PK (all ages) Dose response Safety (CNS toxicity, respiratory sensitivity) | Efficacy (all ages) PK (all ages) Dose response (all ages) Safety (CNS toxicity, immune response, withdrawal) | Small, heterogeneous populations for study; consider small-sample designs Safety (CNS toxicity, immune response, withdrawal) |
| Local anesthetics | PK (all ages) Dose response (all ages) Safety (surrogate measures of CNS toxicity, cardiotoxicity) Postoperative infusion studies over 2–4 d | Rarely indicated for use beyond 1 wk | Rarely indicated for use beyond 1 wk |
| Antiepileptic drugs | Not applicable (used predominantly for chronic pain rather than acute pain) | Not applicable (used predominantly for chronic pain rather than acute or subacute pain) | Efficacy: Migraine prophylaxis and functional abdominal disorders in adolescents Consider small-sample designs for neuropathic pain and fibromyalgia PK Dose response Safety (CNS, psychiatric toxicity) |
| Antidepressants | Not applicable (used predominantly for chronic pain rather than acute pain) | Not applicable (used predominantly for chronic pain rather than acute or subacute pain) | Efficacy: Migraine prophylaxis and functional abdominal disorders in adolescents Consider small-sample designs for neuropathic pain and fibromyalgia PK Dose response Safety (CNS, psychiatric toxicity) |
CNS, central nervous system.
New analgesic drugs are commonly evaluated in phase 1 studies in healthy adult volunteers, and then in phase 2 and 3 studies in patients with the indicated condition. Phase 1 volunteer studies are rarely appropriate in pediatric analgesic research because they incur some risk and no opportunity for direct benefits to the child.
To define the risks and benefits of a drug, it is necessary to conduct studies in patient populations likely to use the drug. If a drug is indicated for pediatric pain, it is highly desirable to require relevant studies of PKs and safety to provide a rational basis for dose calculation and patient selection. Because needlesticks evoke pain and distress in infants and children, PK studies should be performed whenever possible in patients with indwelling venous or arterial lines or those who require venipuncture for clinical indications.
Other Alternative Study Designs
The challenges noted above in applying traditional parallel-placebo designs for pediatric acute pain studies become even more burdensome for repeated dose studies for treatment of chronic persistent or recurrent pain. The PCA/NCA paradigm can be adapted for relatively short-term repeated dose studies, particularly for pain due to major surgery, sickle cell vaso-occlusive crises, mucositis, or advanced cancer. For studies lasting several days, short half-life opioids are not suitable for rescue therapy, and intermediate half-life opioids may be more appropriate. In principle, an oral opioid analgesic rescue paradigm can be used for longer-term or outpatient studies. However, comparatively few children receive round-the-clock opioids for severe pain for time periods more than 4 weeks. In addition, these children are heterogeneous and their analgesic requirements often escalate markedly over time. 93 This trend may reflect important age-related biological differences in the development of opioid tolerance or induced hyperalgesia. 81 , 94 , 95 Previous efforts to conduct efficacy studies of chronically administered opioids in pediatrics have been extremely challenging. Feasibility remains a severe challenge for opioid analgesic efficacy studies lasting more than 2 weeks in children.
In the Supplemental Information, item A5, numbers from national cancer statistics 96 and from a recent study from 2 pediatric cancer centers 97 are extrapolated to estimate the total population of children younger than 15 years of age with advanced cancer receiving chronic daily round-the-clock opioid dosing for more than 1 month or 3 months as roughly 386 and 232 patients, respectively. These estimates do not include children during active cancer treatment, after hematopoietic stem cell transplantation, or with severe graft-versus-host disease who may require prolonged opioid dosing. Not surprisingly, multicenter clinical trials of long-term opioids for children with cancer 98 have yielded very low enrollment rates (eg, 0.1 subjects per site per month).
Small sample designs are recommended for situations that necessarily involve small patient samples (eg, astronauts 99 ), marked individual variability, or ethical limitations with placebo controls when traditional parallel-group randomized trials (including immediate-rescue designs) are not feasible. Small sample designs have significant limitations that require sophistication in statistical design and analysis. Nevertheless, they may be the best practical option for some situations involving pediatric chronic pain and cancer pain. For children in palliative care, the N-of-1 design has potential to benefit the child by providing a clinically definite answer for that individual subject in real-time. 100 , 101 Enriched enrollment and randomized withdrawal designs have been studied extensively in adult analgesic trials, with some theoretical advantages but with mixed results in actual practice. 102 Open-label extension studies can provide important descriptive information about adverse events, drug interactions, and late toxicities of analgesics in children. However, open-label extension studies can provide only provisional information about efficacy, clinical effectiveness, or side effects due to inability to control for placebo and nocebo effects, natural history, regression to the mean, and other threats to internal validity. Conclusions about causation versus association from open-label extension studies should be extremely circumspect.
Conclusions
Analgesic trials in pediatrics are challenging and require a delicate balance between scientific, ethical, and practical concerns. There are biological, empirical, and experiential bases to justify extrapolation of efficacy from studies in adults to children aged 2 years for μ-opioids, local anesthetics, NSAIDs, and acetaminophen. Although complete efficacy trials may not be required for these pharmaceutical classes, drug trials should define PKs, dose response, and safety in all pediatric age groups for which a new drug is indicated. Pediatric analgesic efficacy data should be demonstrated and not extrapolated from adult studies for any age group for pharmaceuticals in other classes or with novel mechanisms (eg, non-μ or multiple-receptor opioids). Immediate-rescue paradigms using analgesic sparing as a primary surrogate outcome measure may be a reasonable compromise for studies of efficacy and dose response in the acute pain setting.
In our view, the pediatric indications for chronic (>3 month) daily administration of opioids for chronic pain are primarily life-limiting conditions; relatively few pediatric patients with chronic pain due to nonlife-limiting conditions should receive long-term opioids. In this population, efficacy of μ-opioids, local anesthetics, NSAIDs, and acetaminophen may also be extrapolated, but PK, dose-ranging, and safety studies must be performed to define prescribing parameters for practitioners. PK and dose-ranging studies require relatively smaller patient samples compared with traditional efficacy studies. Safety data may be collected both during the performance of PK and dose-ranging studies and used during the drug approval process, but safety data collection must be continued in the form of postmarketing surveillance. It is our hope that with greater collaboration across sites, such as through established clinical trials consortia, some limitations related to adequate sampling and control of contextual factors will be reduced and that more meaningful safety and efficacy data will emerge for the use of analgesic medications in infants and children.
Supplementary Material
Acknowledgments
We thank Ms Elizabeth Carpino and Ms Katherine Kurgansky for outstanding editorial assistance.
Glossary
- FDA
Food and Drug Administration
- IMPACCT
Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials
- NCA
nurse-controlled analgesia
- NSAIDs
nonsteroidal antiinflammatory drugs
- PCA
patient-controlled analgesia
- PK
pharmacokinetic
Footnotes
All authors participated in the FDA scientific workshop in December 2009. Participants were assigned writing of specific sections of the article and/or roles in editing and revision. Authors reviewed the final manuscript prior to submission.
The authors participated in a scientific workshop on pediatric analgesic trials sponsored by the US Food and Drug Administration in December 2009. The views expressed in this article are those of the authors. No official endorsement by the US Food and Drug Administration or the US National Institutes of Health should be inferred.
References
- 1. Shaddy RE , Denne SC Committee on Drugs and Committee on Pediatric Research . Clinical report—guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics. 2010;125(4):850–860 [DOI] [PubMed] [Google Scholar]
- 2. Weisman SJ , Bernstein B , Schechter NL . Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med. 1998;152(2):147–149 [DOI] [PubMed] [Google Scholar]
- 3. Tramèr MR , Reynolds DJ , Moore RA , McQuay HJ . When placebo controlled trials are essential and equivalence trials are inadequate. BMJ. 1998;317(7162):875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.21 CFR. Section 50.53April, 2010
- 5. E6 R. Guideline for Good Clinical Practice . In: I.C.H., ed, 1996. Available at: www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf. Accessed January 9, 2012 [Google Scholar]
- 6. Anand KJ . Pain assessment in preterm neonates. Pediatrics. 2007;119(3):605–607 [DOI] [PubMed] [Google Scholar]
- 7. Baba H , Doubell TP , Moore KA , Woolf CJ . Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. J Neurophysiol. 2000;83(2):955–962 [DOI] [PubMed] [Google Scholar]
- 8. Rahman W , Dashwood MR , Fitzgerald M , Aynsley-Green A , Dickenson AH . Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108(1-2):239–254 [DOI] [PubMed] [Google Scholar]
- 9. Durrmeyer X , Vutskits L , Anand KJS , Rimensberger PC . Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2010;67(2):117–127 [DOI] [PubMed] [Google Scholar]
- 10. Kearns GL , Abdel-Rahman SM , Alander SW , Blowey DL , Leeder JS , Kauffman RE . Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167 [DOI] [PubMed] [Google Scholar]
- 11. Anand KJ , Aranda JV , Berde CB , et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117(3 pt 2):S9–S22 [DOI] [PubMed] [Google Scholar]
- 12. Slater R , Boyd S , Meek J , Fitzgerald M . Cortical pain responses in the infant brain. Pain. 2006;123(3):332 , author reply 332–334 [DOI] [PubMed] [Google Scholar]
- 13. Slater R , Fitzgerald M , Meek J . Can cortical responses following noxious stimulation inform us about pain processing in neonates? Semin Perinatol. 2007;31(5):298–302 [DOI] [PubMed] [Google Scholar]
- 14. Bartocci M , Bergqvist LL , Lagercrantz H , Anand KJ . Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1-2):109–117 [DOI] [PubMed] [Google Scholar]
- 15. Anand KS . Relationships between stress responses and clinical outcome in newborns, infants, and children. Crit Care Med. 1993;21(s uppl 9):S358–S359 [DOI] [PubMed] [Google Scholar]
- 16. Taddio A , Shah V , Atenafu E , Katz J . Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain. 2009;144(1-2):43–48 [DOI] [PubMed] [Google Scholar]
- 17. Taddio A , Shah V , Gilbert-MacLeod C , Katz J . Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA. 2002;288(7):857–861 [DOI] [PubMed] [Google Scholar]
- 18. Apley J , Naish N . Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958;33(168):165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perquin CW , Hazebroek-Kampschreur AA , Hunfeld JA , et al. Pain in children and adolescents: a common experience. Pain. 2000;87(1):51–58 [DOI] [PubMed] [Google Scholar]
- 20. El-Metwally A , Salminen JJ , Auvinen A , Kautiainen H , Mikkelsson M . Prognosis of non-specific musculoskeletal pain in preadolescents: a prospective 4-year follow-up study till adolescence. Pain. 2004;110(3):550–559 [DOI] [PubMed] [Google Scholar]
- 21. Costigan M , Scholz J , Woolf CJ . Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones GT , Macfarlane GJ . Predicting persistent low back pain in schoolchildren: a prospective cohort study. Arthritis Rheum. 2009;61(10):1359–1366 [DOI] [PubMed] [Google Scholar]
- 23. Huguet A , Miró J . The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9(3):226–236 [DOI] [PubMed] [Google Scholar]
- 24. Schanberg LE , Anthony KK , Gil KM , Maurin EC . Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum. 2003;48(5):1390–1397 [DOI] [PubMed] [Google Scholar]
- 25. Krane EJ , Heller LB . The prevalence of phantom sensation and pain in pediatric amputees. J Pain Symptom Manage. 1995;10(1):21–29 [DOI] [PubMed] [Google Scholar]
- 26. Howard RF , Walker SM , Mota PM , Fitzgerald M . The ontogeny of neuropathic pain: postnatal onset of mechanical allodynia in rat spared nerve injury (SNI) and chronic constriction injury (CCI) models. Pain. 2005;115(3):382–389 [DOI] [PubMed] [Google Scholar]
- 27. McCann ME , Waters P , Goumnerova LC , Berde C . Self-mutilation in young children following brachial plexus birth injury. Pain. 2004;110(1-2):123–129 [DOI] [PubMed] [Google Scholar]
- 28. von Baeyer CL , Spagrud LJ . Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127(1-2):140–150 [DOI] [PubMed] [Google Scholar]
- 29. Hicks CL , von Baeyer CL , Spafford PA , van Korlaar I , Goodenough B . The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183 [DOI] [PubMed] [Google Scholar]
- 30. Anand KJ , Aranda JV , Berde CB , et al. Analgesia and anesthesia for neonates: study design and ethical issues. Clin Ther. 2005;27(6):814–843 [DOI] [PubMed] [Google Scholar]
- 31. McGrath PJ , Walco GA , Turk DC , et al. PedIMMPACT . Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–783 [DOI] [PubMed] [Google Scholar]
- 32. Varni JW , Stucky BD , Thissen D , et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carbajal R , Paupe A , Hoenn E , Lenclen R , Olivier-Martin M . APN: evaluation behavioral scale of acute pain in newborn infants [in French]. Arch Pediatr. 1997;4(7):623–628 [DOI] [PubMed] [Google Scholar]
- 34. Debillon T , Zupan V , Ravault N , Magny JF , Dehan M . Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F36–F41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duhn LJ , Medves JM . A systematic integrative review of infant pain assessment tools. Adv Neonatal Care. 2004;4(3):126–140 [DOI] [PubMed] [Google Scholar]
- 36. Grunau RVE , Craig KD . Pain expression in neonates: facial action and cry. Pain. 1987;28(3):395–410 [DOI] [PubMed] [Google Scholar]
- 37. Holsti L , Grunau RE . Initial validation of the Behavioral Indicators of Infant Pain (BIIP). Pain. 2007;132(3):264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hummel P , Puchalski M , Creech SD , Weiss MG . Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28(1):55–60 [DOI] [PubMed] [Google Scholar]
- 39. Stevens B . Pain assessment and management in infants with cancer. Pediatr Blood Cancer. 2007;49(s uppl 7):1097–1101 [DOI] [PubMed] [Google Scholar]
- 40. Stevens B , Johnston C , Petryshen P , Taddio A . Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12(1):13–22 [DOI] [PubMed] [Google Scholar]
- 41.Stevens B. Pilai Riddell RR, Oberlander T, Gibbins S. Assessment of pain in neonates and infants. In: Anand KJS, Stevens BJ, McGrath PJ, eds. Pain in Neonates and Infants. 3rd ed. Amsterdam: Elsevier; 2007:67–90 [Google Scholar]
- 42. Stevens B , Yamada J , Ohlsson A . Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2010;(1):CD001069 [DOI] [PubMed] [Google Scholar]
- 43. O’Sullivan A , O’Connor M , Brosnahan D , McCreery K , Dempsey EM . Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F419–F422 [DOI] [PubMed] [Google Scholar]
- 44. Overgaard C , Knudsen A . Pain-relieving effect of sucrose in newborns during heel prick. Biol Neonate. 1999;75(5):279–284 [DOI] [PubMed] [Google Scholar]
- 45. Carbajal R , Lenclen R , Jugie M , Paupe A , Barton BA , Anand KJ . Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics. 2005;115(6):1494–1500 [DOI] [PubMed] [Google Scholar]
- 46. Carbajal R , Veerapen S , Couderc S , Jugie M , Ville Y . Analgesic effect of breast feeding in term neonates: randomised controlled trial. BMJ. 2003;326(7379):13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carbajal R , Lenclen R , Gajdos V , Jugie M , Paupe A . Crossover trial of analgesic efficacy of glucose and pacifier in very preterm neonates during subcutaneous injections. Pediatrics. 2002;110(2 pt 1):389–393 [DOI] [PubMed] [Google Scholar]
- 48. Guinsburg R , Kopelman BI , Anand KJ , de Almeida MF , Peres CA , Miyoshi MH . Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr. 1998;132(6):954–959 [DOI] [PubMed] [Google Scholar]
- 49. Lehr VT , Taddio A . Topical anesthesia in neonates: clinical practices and practical considerations. Semin Perinatol. 2007;31(5):323–329 [DOI] [PubMed] [Google Scholar]
- 50. Long CP , McCafferty DF , Sittlington NM , Halliday HL , Woolfson AD , Jones DS . Randomized trial of novel tetracaine patch to provide local anaesthesia in neonates undergoing venepuncture. Br J Anaesth. 2003;91(4):514–518 [DOI] [PubMed] [Google Scholar]
- 51. Mucignat V , Ducrocq S , Lebas F , Mochel F , Baudon JJ , Gold F . Analgesic effects of Emla cream and saccharose solution for subcutaneous injections in preterm newborns: a prospective study of 265 injections [in French]. Arch Pediatr. 2004;11(8):921–925 [DOI] [PubMed] [Google Scholar]
- 52. Oberlander TF , Grunau RE , Fitzgerald C , Papsdorf M , Rurak D , Riggs W . Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115(2):411–425 [DOI] [PubMed] [Google Scholar]
- 53. e Silva YP , Gomez RS , Marcatto JO , Maximo TA , Barbosa RF , e Silva AC . Early awakening and extubation with remifentanil in ventilated premature neonates. Paediatr Anaesth. 2008;18(2):176–183 [DOI] [PubMed] [Google Scholar]
- 54. Bouwmeester NJ , Anand KJ , van Dijk M , Hop WC , Boomsma F , Tibboel D . Hormonal and metabolic stress responses after major surgery in children aged 0-3 years: a double-blind, randomized trial comparing the effects of continuous versus intermittent morphine. Br J Anaesth. 2001;87(3):390–399 [DOI] [PubMed] [Google Scholar]
- 55. Bouwmeester NJ , van den Anker JN , Hop WC , Anand KJ , Tibboel D . Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth. 2003;90(5):642–652 [DOI] [PubMed] [Google Scholar]
- 56. Bouwmeester NJ , Hop WC , van Dijk M , Anand KJ , van den Anker JN , Tibboel D . Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003;29(11):2009–2015 [DOI] [PubMed] [Google Scholar]
- 57. Shah V , Taddio A , Rieder MJ HELPinKIDS Team . Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta-analyses. Clin Ther. 2009;31(suppl 2):S104–S151 [DOI] [PubMed] [Google Scholar]
- 58. Taddio A , Appleton M , Bortolussi R , et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline. CMAJ. 2010;182(18):E843–E855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taddio A , Hogan ME , Moyer P , et al. Evaluation of the reliability, validity and practicality of 3 measures of acute pain in infants undergoing immunization injections. Vaccine. 2011;29(7):1390–1394 [DOI] [PubMed] [Google Scholar]
- 60. Hermann C , Hohmeister J , Demirakça S , Zohsel K , Flor H . Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125(3):278–285 [DOI] [PubMed] [Google Scholar]
- 61. Beyer JE , McGrath PJ , Berde CB . Discordance between self-report and behavioral pain measures in children aged 3-7 years after surgery. J Pain Symptom Manage. 1990;5(6)350–356 [DOI] [PubMed] [Google Scholar]
- 62. Berde C , McGrath P . Pain measurement and Beecher’s challenge: 50 years later. Anesthesiology. 2009;111(3):473–474 [DOI] [PubMed] [Google Scholar]
- 63. Slater R , Cantarella A , Gallella S , et al. Cortical pain responses in human infants. J Neurosci. 2006;26(14):3662–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grunau RE , Haley DW , Whitfield MF , Weinberg J , Yu W , Thiessen P . Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Grunau RE , Holsti L , Haley DW , et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Breau L . The science of pain measurement and the frustration of clinical pain assessment: does an individualized numerical rating scale bridge the gap for children with intellectual disabilities? Pain. 2010;150(2):213–214 [DOI] [PubMed] [Google Scholar]
- 67. Breau LM , Burkitt C . Assessing pain in children with intellectual disabilities. Pain Res Manag. 2009;14(2):116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Breau LM , Camfield C , McGrath PJ , Rosmus C , Finley GA . Measuring pain accurately in children with cognitive impairments: refinement of a caregiver scale. J Pediatr. 2001;138(5):721–727 [DOI] [PubMed] [Google Scholar]
- 69. Solodiuk JC , Scott-Sutherland J , Meyers M , et al. Validation of the Individualized Numeric Rating Scale (INRS): a pain assessment tool for nonverbal children with intellectual disability. Pain. 2010;150(2):231–236 [DOI] [PubMed] [Google Scholar]
- 70. Regnard C , Reynolds J , Watson B , Matthews D , Gibson L , Clarke C . Understanding distress in people with severe communication difficulties: developing and assessing the Disability Distress Assessment Tool (DisDAT). J Intellect Disabil Res. 2007;51(pt 4):277–292 [DOI] [PubMed] [Google Scholar]
- 71. Stallard P , Williams L , Velleman R , Lenton S , McGrath PJ , Taylor G . The development and evaluation of the pain indicator for communicatively impaired children (PICIC). Pain. 2002;98(1-2):145–149 [DOI] [PubMed] [Google Scholar]
- 72.Oberlander TF, Symons FJ, Johnson C. Assessing pain in children with developmental disabilities. In: Manjamer AM, ed. Clinical and Research Measures for Children With Developmental Disability: Framed by the ICF-CY. London, United Kingdom: MacKeith Press; 2010 (In Press) [Google Scholar]
- 73. Anderson BJ , Holford NH . Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36 [DOI] [PubMed] [Google Scholar]
- 74. Anderson BJ , Holford NH . Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332 [DOI] [PubMed] [Google Scholar]
- 75. Frawley G , Smith KR , Ingelmo P . Relative potencies of bupivacaine, levobupivacaine, and ropivacaine for neonatal spinal anaesthesia. Br J Anaesth. 2009;103(5):731–738 [DOI] [PubMed] [Google Scholar]
- 76. Anderson BJ , Allegaert K , Holford NH . Population clinical pharmacology of children: general principles. Eur J Pediatr. 2006;165(11):741–746 [DOI] [PubMed] [Google Scholar]
- 77. Morton NS , Errera A . APA national audit of pediatric opioid infusions. Paediatr Anaesth. 2010;20(2):119–125 [DOI] [PubMed] [Google Scholar]
- 78. Lesko SM , Mitchell AA . An assessment of the safety of pediatric ibuprofen. A practitioner-based randomized clinical trial. JAMA. 1995;273(12):929–933 [PubMed] [Google Scholar]
- 79. Williams DG , Patel A , Howard RF . Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br J Anaesth. 2002;89(6):839–845 [DOI] [PubMed] [Google Scholar]
- 80. Ciszkowski C , Madadi P , Phillips MS , Lauwers AE , Koren G . Codeine, ultrarapid-metabolism genotype, and postoperative death. N Engl J Med. 2009;361(8):827–828 [DOI] [PubMed] [Google Scholar]
- 81. Anand KJS , Willson DF , Berger J , et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network . Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5). Available at: www.pediatrics.org/cgi/content/full/125/5/e1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vetter TR , Heiner EJ . Intravenous ketorolac as an adjuvant to pediatric patient-controlled analgesia with morphine. J Clin Anesth. 1994;6(2):110–113 [DOI] [PubMed] [Google Scholar]
- 83. Korpela R , Korvenoja P , Meretoja OA . Morphine-sparing effect of acetaminophen in pediatric day-case surgery. Anesthesiology. 1999;91(2):442–447 [DOI] [PubMed] [Google Scholar]
- 84. Carney J , Finnerty O , Rauf J , Curley G , McDonnell JG , Laffey JG . Ipsilateral transversus abdominis plane block provides effective analgesia after appendectomy in children: a randomized controlled trial. Anesth Analg. 2010;111(4):998–1003 [DOI] [PubMed] [Google Scholar]
- 85. Hiller A , Meretoja OA , Korpela R , Piiparinen S , Taivainen T . The analgesic efficacy of acetaminophen, ketoprofen, or their combination for pediatric surgical patients having soft tissue or orthopedic procedures. Anesth Analg. 2006;102(5):1365–1371 [DOI] [PubMed] [Google Scholar]
- 86. van der Marel CD , Peters JW , Bouwmeester NJ , Jacqz-Aigrain E , van den Anker JN , Tibboel D . Rectal acetaminophen does not reduce morphine consumption after major surgery in young infants. Br J Anaesth. 2007;98(3):372–379 [DOI] [PubMed] [Google Scholar]
- 87. Czarnecki ML , Salamon KS , Jastrowski Mano KE , Ferrise AS , Sharp M , Weisman SJ . A preliminary report of parent/nurse-controlled analgesia (PNCA) in infants and preschoolers. Clin J Pain. 2011;27(2):102–107 [DOI] [PubMed] [Google Scholar]
- 88. Howard RF , Lloyd-Thomas A , Thomas M , et al. Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children’s hospital. Paediatr Anaesth. 2010;20(2):126–134 [DOI] [PubMed] [Google Scholar]
- 89. Monitto CL , Greenberg RS , Kost-Byerly S , et al. The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth Analg. 2000;91(3):573–579 [DOI] [PubMed] [Google Scholar]
- 90. Shafer SL , Varvel JR . Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology. 1991;74(1):53–63 [DOI] [PubMed] [Google Scholar]
- 91. Fiset P , Mathers L , Engstrom R , et al. Pharmacokinetics of computer-controlled alfentanil administration in children undergoing cardiac surgery. Anesthesiology. 1995;83(5):944–955 [DOI] [PubMed] [Google Scholar]
- 92. Douma MR , Verwey RA , Kam-Endtz CE , van der Linden PD , Stienstra R . Obstetric analgesia: a comparison of patient-controlled meperidine, remifentanil, and fentanyl in labour. Br J Anaesth. 2010;104(2):209–215 [DOI] [PubMed] [Google Scholar]
- 93. Collins JJ , Grier HE , Kinney HC , Berde CB . Control of severe pain in children with terminal malignancy. J Pediatr. 1995;126(4):653–657 [DOI] [PubMed] [Google Scholar]
- 94. Wang Y , Mitchell J , Moriyama K , et al. Age-dependent morphine tolerance development in the rat. Anesth Analg. 2005;100(6):1733–1739 [DOI] [PubMed] [Google Scholar]
- 95. Buntin-Mushock C , Phillip L , Moriyama K , Palmer PP . Age-dependent opioid escalation in chronic pain patients. Anesth Analg. 2005;100(6):1740–1745 [DOI] [PubMed] [Google Scholar]
- 96.National Cancer Institute. Childhood cancers. Available at: www.cancer.gov/cancertopics/factsheet/Sites-Types/childhood. Accessed October 31, 2011
- 97. Ullrich CK , Dussel V , Hilden JM , et al. Fatigue in children with cancer at the end of life. J Pain Symptom Manage. 2010;40(4):483–494 [DOI] [PubMed] [Google Scholar]
- 98. Finkel JC , Finley A , Greco C , Weisman SJ , Zeltzer L . Transdermal fentanyl in the management of children with chronic severe pain: results from an international study. Cancer. 2005;104(12):2847–2857 [DOI] [PubMed] [Google Scholar]
- 99. Evans C , Ildstad S . Small Clinical Trials: Issues and Challenges. Washington, DC: National Academy of Sciences Press; 2001. [PubMed] [Google Scholar]
- 100. Guyatt GH , Heyting A , Jaeschke R , Keller J , Adachi JD , Roberts RS . N of 1 randomized trials for investigating new drugs. Control Clin Trials. 1990;11(2):88–100 [DOI] [PubMed] [Google Scholar]
- 101. Guyatt GH , Keller JL , Jaeschke R , Rosenbloom D , Adachi JD , Newhouse MT . The n-of-1 randomized controlled trial: clinical usefulness. Our three-year experience. Ann Intern Med. 1990;112(4):293–299 [DOI] [PubMed] [Google Scholar]
- 102. Straube S , Derry S , McQuay HJ , Moore RA . Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. Br J Clin Pharmacol. 2008;66(2):266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.