Abstract
BACKGROUND AND OBJECTIVE:
Clinical practice guidelines (CPGs) assist clinicians in making appropriate diagnostic and treatment decisions based on available evidence. The objective of this study was to describe the availability and content of institutional community-acquired pneumonia (CAP) CPGs, and to evaluate the association between institutional CPGs and care utilization, antibiotic administration, and outcomes among children hospitalized with CAP.
METHODS:
This multicenter retrospective cohort study included children aged 1 to 18 years hospitalized with CAP from July 1, 2009, to June 30, 2011. CPGs from each institution were reviewed to abstract information regarding diagnostic testing and antimicrobial selection. We compared overall and specific utilization patterns, antimicrobial use, and hospital length of stay (LOS) for children with CAP between hospitals with and without CPGs.
RESULTS:
Thirteen (31.7%) of 41 hospitals had an institutional CPG for nonsevere CAP. There was marked heterogeneity among CPGs. Among the 19 710 children hospitalized with CAP, cost of care, hospital LOS, and 14-day readmission rate were not associated with the presence of a CPG. CPGs did not influence ordering patterns for most diagnostic tests, including blood culture and chest radiographs. Penicillin or aminopenicillins were prescribed to 46.3% of children at institutions where a CPG recommended the use of these antibiotics as first-line agents compared with 23.9% of children at institutions without a CPG (odds ratio = 2.7; 95% confidence interval = 1.4–5.5).
CONCLUSIONS:
The availability of a CAP CPG had minimal impact on resource utilization and was not associated with cost or hospital LOS. Institutional CPGs, however, did influence patterns of antimicrobial use.
Keywords: pneumonia, clinical practice guidelines, diagnostic testing
What’s Known on This Subject:
There are limited data on current testing and treatment patterns for children hospitalized with pneumonia, and on whether institutional guidelines affect care.
What This Study Adds:
The use of institutional clinical practice guidelines was not associated with changes in diagnostic testing, hospital length of stay, or costs for children hospitalized with pneumonia, but was associated with increased use of narrow-spectrum antibiotics.
Variability exists in a number of aspects of community-acquired pneumonia (CAP) management, including initial laboratory testing 1 and antibiotic prescribing. 2 , 3 Care variation contributes to increased costs and disparate outcomes. 4 – 6 Clinical practice guidelines (CPGs) can favorably influence utilization and clinical outcomes by developing evidence-based, peer-reviewed recommendations for patient care. 4 , 7 , 8 In a study of children hospitalized with urinary tract infection, hospitals with a CPG for this condition had a 20% shorter length of stay (LOS) and total charges that were $1060 less per admission than hospitals without a similar CPG. 4 The introduction of an institutional CPG for bronchiolitis was also associated with reduction of resource utilization, including chest physiotherapy and respiratory syncytial virus testing. 8 Application of nationally published guidelines for acute gastroenteritis lowered charges in emergency departments and observation units by 50% without any adverse outcomes. 5 Recently, implementation of an institutional CPG for CAP supported by an antibiotic stewardship program was associated with an increase in narrow-spectrum antibiotic use without increases in adverse outcomes. 9
Consensus national guidelines for the care of pediatric CAP were published in 2011 by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA). 10 These guidelines may serve as a resource for institutional CPG development. The objectives of this study were to describe characteristics of institutional pneumonia CPGs before publication of the Consensus National Guidelines, and to determine whether the presence or absence of an institutional CPG was associated with differences in clinical management of patients across institutions.
Methods
Data Source
Data for this multicenter retrospective cohort study were obtained from the Pediatric Health Information System, which contains resource utilization data from 43 freestanding tertiary care children’s hospitals with emergency departments. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia and accounted for 15% of all pediatric hospitalizations in the United States in 2009 (677 291 of 4 508 323 admissions). 11 , 12 These hospitals provide discharge data, including patient demographics, diagnoses, and procedures. Billing data detail all drugs, radiologic imaging studies, laboratory tests, and supplies charged to each patient. Data are de-identified before inclusion in the database; however, encrypted medical record numbers allow for tracking individual patients across admissions. The Children’s Hospital Association (Overland Park, KS) and participating hospitals jointly ensure data quality as described previously. 13 , 14 In accordance with the Common Rule (45 CFR 46.102[f]) and the policies of the Cincinnati Children’s Hospital Medical Center Institutional Review Board, this research, using a de-identified data set, was not considered human subjects research.
Questionnaire
A survey was sent to the quality officer at each participating hospital to determine whether the institution had a CPG addressing the treatment of children with CAP. Hospitals that responded to the questionnaire stating that they did have a CPG were asked to share them. All institutions reporting the use of a CAP CPG provided the actual CPG for review.
Patients
Children, 1 to 18 years of age, hospitalized for CAP were eligible for this study if they were discharged from participating hospitals between July 1, 2009, and June 30, 2011. Subjects were included if they satisfied one of the following International Classification of Diseases, Ninth Revision, discharge diagnosis code criteria: (1) primary diagnosis of pneumonia (481–483.8, 485–486); (2) primary diagnosis of a pneumonia-related symptom (780.6 or 786.00–786.52 [except 786.1]) and a secondary diagnosis of pneumonia; or (3) primary diagnosis of pleural effusion (510.0, 510.9, 511.0, 511.1, and 511.9) and a secondary diagnosis of pneumonia.
Children were excluded for the following reasons: (1) interhospital transfer; (2) chronic comorbid condition predisposing to severe, recurrent, or health care–associated pneumonia (eg, cystic fibrosis, malignancy, sickle cell disease) as defined by a previously reported classification scheme 15 ; and (3) no antibiotic therapy on the first calendar day of hospitalization, suggesting that pneumonia was not present or diagnosed at the time of admission. To create a cohort of children presenting with nonsevere CAP, we additionally excluded children who were admitted to an ICU or died during the first calendar day of hospitalization. 1 We also excluded children who underwent a pleural drainage procedure. These were identified by using International Classification of Diseases, Ninth Revision, procedure codes for thoracentesis (34.91), chest tube placement (34.04), video-assisted thoracoscopic surgery (34.21), and thoracotomy (34.02 or 34.09).
Measured Exposures
The primary exposure of interest was the hospital availability of a CPG for CAP. The CPGs were classified by whether they addressed 6 diagnostic tests (complete blood count [CBC], serum electrolytes, blood culture, C-reactive protein, respiratory tract viral testing, and chest radiographs [CXRs]) and antibiotic therapy, specifically the use of penicillin/aminopenicillins or macrolides. The CPGs were grouped by whether each test or therapy was recommended for, recommended against, or not mentioned. Each CPG was reviewed by 2 investigators (M.I.N. and A.L.H.), by using a standardized template for data abstraction, and discrepancies were resolved by group consensus.
Measured Outcomes
The measured outcomes were hospital LOS, readmission within 14 days of index discharge, cost of hospitalization, performance of diagnostic testing, and antibiotic selection. Total cost was calculated for the index hospitalization as well as for the episode of illness, which included the index visit and any subsequent emergency department visits and hospitalizations within 14 days. Total hospital charges in the Pediatric Health Information System database were adjusted for hospital location by using the Centers for Medicare and Medicaid price/wage index. We used hospital-level cost-to-charge ratios to convert the charges to costs. Performance of diagnostic testing and antibiotic administration were assessed based on the presence or absence of specific recommendations pertaining to that test or antibiotic within the CPGs.
Statistical Analysis
Hospital level covariates were summarized by using median and interquartile range (IQR) values for continuous variables, and frequencies and percentages for categorical variables. We compared median LOS and median hospitalization and episode costs by using the Wilcoxon rank-sum test. We assessed the relationship between presence of CPG and of CPG recommendations and diagnostic testing by using χ2 statistics. We also assessed the relationship between the use of penicillin/aminopenicillins or macrolides and the presence of a CPG and of CPG recommendations. Furthermore, we compared rates of testing by the presence of a CPG and by CPG recommendations. To account for the clustering of patients within hospitals, we used generalized estimating equations to generate adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
All statistical analyses were performed by using the statistical software SAS (version 9.3, SAS Institute, Inc, Cary, NC), and P values <.05 were considered statistically significant.
Results
Availability of a CAP CPG
Forty-one (95%) of the 43 hospitals surveyed completed the questionnaire, and 13 (32%) of 41 reported having an institutional CPG for CAP. Of the 13 institutions with a CPG, the guideline was in the form of a written algorithm in 8 institutions, of which, only 4 provided supporting evidence for the recommendations provided. Five institutions used a clinical guideline in the form of electronic order entry; 2 of these institutions also had a supporting document that provided the evidence for the recommendations cited.
There was marked variability in the specific recommendations around diagnostic testing contained within the CPGs (Table 1). Some of the variability in testing recommendations was attributable to whether the testing was mentioned within the CPG (eg, CXR), whereas the performance of other tests varied based on the specific recommendation itself. For example, 6 institutions recommended obtaining a CBC, whereas 1 institution recommended against obtaining a CBC. Five institutions recommended obtaining a CBC only if the results would help to decide on or guide antimicrobial therapy (ie, obtain conditionally). CBC was not mentioned in 1 CPG.
TABLE 1.
Recommendations for Individual Diagnostic Testing by Institution (n = 13)
| Hospital | Diagnostic Test | |||||
|---|---|---|---|---|---|---|
| CBC | Serum Electrolytes | Blood Culture | C-Reactive Protein | Influenza/RSV Testing | CXR | |
| A | Obtain | Not mentioned | Obtain | Obtain | Not mentioned | Obtain |
| B | Obtain conditionally a | Not mentioned | Do not obtain | Do not obtain | Do not obtain | Not mentioned |
| C | Obtain | Not mentioned | Obtain | Obtain conditionally | Not mentioned | Obtain |
| D | Obtain conditionally a | Not mentioned | Do not obtain | Not mentioned | Obtain conditionally | Obtain |
| E | Obtain conditionally a | Not mentioned | Obtain | Not mentioned | Obtain conditionally | Obtain |
| F | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Obtain | Not mentioned |
| G | Obtain conditionally a | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| H | Obtain | Not mentioned | Obtain conditionally a | Not mentioned | Not mentioned | Not mentioned |
| I | Obtain | Obtain | Obtain | Obtain | Obtain | Obtain |
| J | Do not obtain | Not mentioned | Do not obtain | Not mentioned | Not mentioned | Obtain |
| K | Obtain conditionally a | Not mentioned | Do not obtain | Do not obtain | Not mentioned | Obtain |
| L | Obtain | Not mentioned | Obtain | Obtain conditionally | Obtain | Obtain |
| M | Obtain | Obtain | Obtain | Not mentioned | Not mentioned | Not mentioned |
| Summary of recommendations around diagnostic testing | Obtain (6) | Obtain (2) | Obtain (6) | Obtain (2) | Obtain (3) | Obtain (8) |
| Do not obtain (1) | Do not obtain (0) | Do not obtain (4) | Do not obtain (2) | Do not obtain (1) | Do not obtain (0) | |
| Conditional (5) | Conditional (0) | Conditional (1) | Conditional (2) | Conditional (2) | Conditional (0) | |
| Not mentioned (1) | Not mentioned (11) | Not mentioned (2) | Not mentioned (7) | Not mentioned (7) | Not mentioned (5) | |
RSV, respiratory syncytial virus.
Obtain if results of testing may help to identify or raise suspicion of bacterial etiology (for analyses, these classified as do not obtain).
Patient Characteristics
During the 2-year study period, 38 957 children were hospitalized at the participating institutions with a diagnosis of CAP. After selection criteria were applied (Fig 1), the final cohort included 19 710 children classified as having nonsevere CAP. Most children (70%) were between 1 and 5 years of age; children 6 to 12 years and 13 to 18 years accounted for 24% and 6% of patients, respectively. Approximately 52% of patients were male. Patient race included non-Hispanic white (45%), non-Hispanic black (25%), Hispanic (26%), Asian (4%), and other races (<1%). Hospitalizations were most common in the winter months (38%). Over the 2-year study period, the median number of hospitalizations at each institution was 395 (IQR, 303–682). Median hospital LOS was 2 days (IQR, 1–3 days).
FIGURE 1.
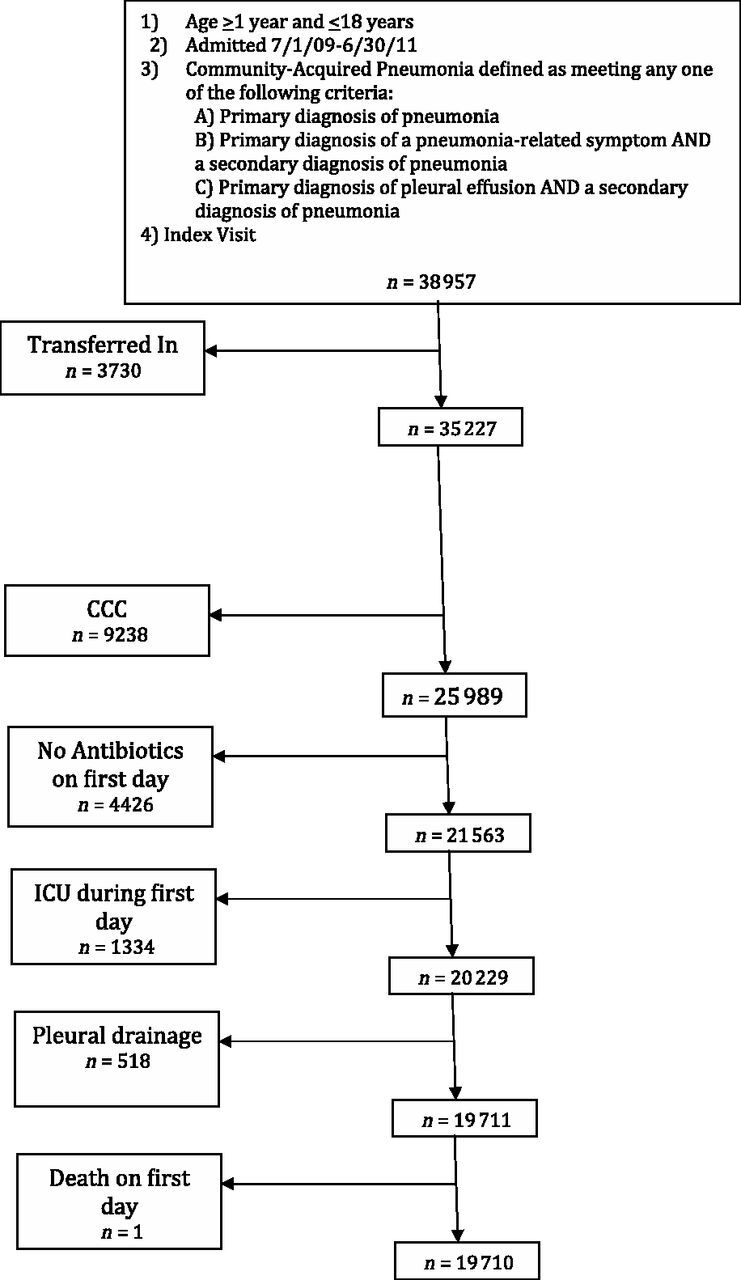
Flow diagram of study cohort. CCC, chronic comorbid condition.
Across all institutions, independent of the presence of a CPG, the use of laboratory testing was higher among older children, whereas viral testing was performed more commonly in younger children. Penicillin or aminopenicillins were used as first-line agents more often for children 1 to 5 years of age, and macrolides were more frequently used (either alone or in combination) in older children (Table 2).
TABLE 2.
Diagnostic Testing and Treatment of the Cohort Stratified by Age Group
| Age Category, y | ||||
|---|---|---|---|---|
| Overall, % | 1–5, % | 6–12, % | 13–18, % a | |
| n = 19 710 | n = 13 778 | n = 4842 | n = 1090 | |
| Diagnostic testing | ||||
| CBC | 9830 (50) | 6815 (50) | 2399 (50) | 616 (57) |
| Serum electrolytes | 5635 (29) | 3790 (28) | 1432 (30) | 413 (38) |
| Blood culture | 7853 (40) | 5595 (41) | 1826 (38) | 432 (40) |
| C-reactive protein | 3553 (18) | 2345 (17) | 944 (20) | 264 (24) |
| Viral testing (RSV, influenza) | 5251 (27) | 3900 (28) | 1113 (23) | 238 (22) |
| CXR | 12 358 (63) | 8770 (64) | 2953 (61) | 635 (58) |
| Initial antibiotic therapy | ||||
| Penicillin/Aminopenicillins | 5430 (28) | 4237 (31) | 1020 (21) | 173 (16) |
| Macrolide (alone or in combination) | 7364 (37) | 4080 (30) | 2610 (54) | 674 (62) |
RSV, respiratory syncytial virus.
P value <.001 for all comparisons across age categories.
CPGs and LOS, Readmissions, and Costs
There was no difference in the hospital LOS between those institutions with and without a CAP CPG (median 2.0 days [IQR, 1–3] in both groups; P = .269). The proportion of children readmitted within 14 days did not vary based on the availability of a CPG (2.3% vs 2.1%; P = .4). Additionally, the presence of a CAP CPG was not associated with either differences in the cost of hospitalization for the index visit, or the total cost for the episode of illness (including repeat visits with hospitalization within 14 days) (Table 3).
TABLE 3.
Outcomes of Patients Based on Presence of a Pneumonia CPG
| Group | Median [IQR] | P | Mean Difference (95% CI) | P a |
|---|---|---|---|---|
| Length of hospitalization, d | ||||
| No guideline | 2 [1–3] | .269 | Ref | |
| Guideline | 2 [1–3] | −0.05 (–0.29 to 0.20) | .745 | |
| Total cost, index hospitalization, $ | ||||
| No guideline | 10 015 [6304–15 931] | .773 | Ref | |
| Guideline | 9361 [6274–14 989] | 1667 (–1747 to 5081) | .339 | |
| Total cost, episode of illness, $ | ||||
| No guideline | 13 265 [6477–16 281] | .553 | Ref | |
| Guideline | 9478 [6332–15 292] | 1843 (–1861 to 5547) | .329 | |
| Group | Percent (95% CI) | P | OR (95% CI) | P b |
| Readmission rate, % | ||||
| No guideline | 2.3 (2.0–2.6) | .367 | Ref | |
| Guideline | 2.1 (1.7–2.5) | 0.89 (0.69 to 1.14) | .396 | |
P values comparing medians are unadjusted and aggregate (combining across hospitals).
P values are adjusted for hospital clustering.
CPGs and Use of Diagnostic Testing
Guideline recommendations for diagnostic testing were not significantly associated with care patterns for children hospitalized with pneumonia. Although blood testing (CBC, serum electrolytes, blood culture) was performed more often when specifically recommended in the guideline, the odds of obtaining these tests did not significantly differ from patients cared for at institutions where the guideline did not mention these specific diagnostic tests, or from institutions without a pneumonia guideline after adjustment for hospital clustering (Table 4). However, children cared for at institutions with a CPG that recommended viral testing, such as influenza or respiratory syncytial virus testing, were more likely to have this testing performed compared with children cared for at institutions without a CPG (38.7% vs 28.0%, respectively; OR 1.6, 95% CI 1.0–2.6). Children cared for at institutions that recommended obtaining a C-reactive protein level were also more likely to have this test performed (OR 4.8, 95% CI 2.3–10.2) compared with children cared for at institutions without a CPG. There was no difference in the rate of obtaining a CXR between patients cared for at institutions with and without a CAP CPG. We did not evaluate the performance of sputum Gram stain and culture, as they were rarely mentioned within the CPGs, and were performed in only 0.4% of patients in the study.
TABLE 4.
Performance of Diagnostic Testing Based on CPG Specific Recommendations
| Group | Hospitals, n (%) | Patients, n | % Received | P a | OR (95% CI) | P b |
|---|---|---|---|---|---|---|
| CBC count | ||||||
| No CPG | 28 (68.3) | 13 265 | 50.3 | <.0001 | Ref | |
| CPG: obtain test | 6 (14.6) | 2660 | 57.2 | 1.3 (0.7–2.4) | .37 | |
| CPG: do not obtain | 6 (14.6) | 3340 | 39.4 | 0.6 (0.4–1.0) | .06 | |
| CPG: test not mentioned | 1 (2.4) | 445 | 72.1 | 2.6 (1.9–3.5) | <.001 | |
| Serum electrolytes | ||||||
| No CPG | 28 (68.3) | 13 265 | 28.8 | .46 | Ref | |
| CPG: obtain test | 2 (4.9) | 504 | 29.6 | 1 (0.5–2.1) | .92 | |
| CPG: do not obtain | 0 (0.0) | — | — | — | — | |
| CPG: test not mentioned | 11 (26.8) | 5941 | 28.0 | 1 (0.5–1.8) | .90 | |
| Blood culture | ||||||
| No CPG | 28 (68.3) | 13 265 | 41.8 | <.0001 | Ref | |
| CPG: obtain test | 6 (14.6) | 2818 | 40.9 | 1 (0.5–1.7) | .90 | |
| CPG: do not obtain | 5 (12.2) | 2916 | 24.9 | 0.5 (0.1–1.7) | .24 | |
| CPG: test not mentioned | 2 (4.9) | 711 | 60.9 | 2.2 (1.1–4.4) | .03 | |
| C-reactive protein | ||||||
| No CPG | 28 (68.3) | 13 265 | 17.6 | <.0001 | Ref | |
| CPG: obtain test | 2 (4.9) | 1235 | 50.6 | 4.8 (2.3–10.2) | <.0001 | |
| CPG: do not obtain | 4 (9.8) | 1869 | 8.2 | 0.4 (0.1–1.8) | .24 | |
| CPG: test not mentioned | 7 (17.1) | 3341 | 13.1 | 0.7 (0.3–1.9) | .50 | |
| Viral testing (RSV, influenza) | ||||||
| No CPG | 28 (68.3) | 13 265 | 28.0 | <.0001 | Ref | |
| CPG: obtain test | 3 (7.3) | 954 | 38.7 | 1.6 (1–2.6) | .04 | |
| CPG: do not obtain | 3 (7.3) | 2321 | 15.5 | 0.5 (0.2–1.5) | .19 | |
| CPG: test not mentioned | 7 (17.1) | 3170 | 25.5 | 0.9 (0.4–2.1) | .76 | |
| CXR | ||||||
| No CPG | 28 (68.3) | 13 265 | 63.6 | <.0001 | Ref | |
| CPG: obtain test | 8 (19.5) | 4374 | 60.5 | 0.9 (0.6–1.4) | .57 | |
| CPG: do not obtain | 0 (0.0) | — | — | — | — | |
| CPG: test not mentioned | 5 (12.2) | 2071 | 61.3 | 0.9 (0.6–1.4) | .66 | |
RSV, respiratory syncytial virus; —, no patients.
P values comparing differences in proportion of patients receiving testing based on specific mention in the CPG are unadjusted and aggregate (combining across hospitals).
P values are adjusted for hospital clustering.
CPGs and Antibiotic Selection
Antibiotic choice was associated with specific CPG recommendations. Penicillin or aminopenicillins were more likely to be used as first-line agents in children cared for at institutions where a CPG recommended the use of these antibiotics as first-line therapy compared with children evaluated at an institution without a CPG (46.3% vs 23.9%, adjusted OR 2.7; 95% CI 1.4–5.5) (Table 5). Macrolides were used less often among children older than 5 years of age when cared for at an institution using a CPG that did not specifically address criteria for use of a macrolide antibiotic compared with children cared for at institutions without CPGs (OR 0.6, 95% CI 0.3–0.9).
TABLE 5.
Antimicrobial Use Based on CPG Specific Recommendations
| Group | Hospitals, n (%) | Patients, n | % Received | P a | OR (95% CI) | P b |
|---|---|---|---|---|---|---|
| Penicillin/Aminopenicillins | ||||||
| No CPG | 28 (68.3) | 13 265 | 23.9 | <.0001 | Ref | |
| CPG: recommend as first-line agent | 7 (17.1) | 3710 | 46.3 | 2.7 (1.4–5.5) | .005 | |
| CPG: not addressed | 6 (14.6) | 2735 | 19.8 | 0.8 (0.5–1.3) | .37 | |
| Macrolide (>5 y old) | ||||||
| No CPG | 28 (68.3) | 4013 | 55.2 | <.0001 | Ref | |
| CPG: recommends macrolide use for children ≥5 y | 10 (24.4) | 1633 | 58.2 | 1.1 (0.7–1.9) | .64 | |
| CPG: no recommendation for macrolide use | 3 (7.3) | 286 | 41.3 | 0.6 (0.3–0.9) | .026 | |
P values comparing differences in proportion of patients receiving antibiotics based on specific mention in the CPG are unadjusted and aggregate (combining across hospitals).
P values are adjusted for hospital clustering.
Discussion
We described institutional CAP CPGs from tertiary care children’s hospitals and evaluated their impact on hospitalized children. The presence, form, and content of CPGs for the management of hospitalized children with CAP varied widely. The presence of a CAP CPG itself was not associated with the hospital LOS, 14-day readmission rate, or total cost of hospitalization. With the exception of viral testing, the presence of a CPG was not associated with diagnostic testing. On the other hand, CPGs were associated with narrow-spectrum antibiotic use.
Our findings suggest that test-ordering patterns are difficult to influence with a guideline alone, and underscore the challenges inherent in changing clinical practice according to best evidence. A potential reason why testing and treatment of children with CAP guidelines did not match institutional guideline recommendations may lie in the fact that the evidence supporting such recommendations is weak. Clinical inertia, and lack of awareness regarding CPGs, may also reduce the impact of guidelines. 16 In contrast to diagnostic testing, recommendations for antibiotic selection were more readily followed in our study, a finding that is consistent with previous studies. 17 , 18 It is possible that once a decision is made to treat a patient with antimicrobial agents, the decision regarding specific antibiotic use may be easier to influence than the decision around whether to obtain a specific diagnostic test. Regardless, the formulation of evidence-based practice recommendations is only the first step in the process of implementing health care interventions, and institutional guideline development should be accompanied by the creation of effective dissemination, implementation, and evaluation strategies to ensure maximum impact.
Evidence-based practice guidelines have been developed to standardize the management of patients with a specific diagnosis. The Institute of Medicine published a reference guide that outlines the key elements for the development and updating of CPGs. 19 In this document, the Institute of Medicine recommends that for each recommendation listed on a CPG, an explanation of the reasoning underlying the recommendation should be provided. In our study, only half of the guidelines provided supporting evidence for the recommendations provided. Additionally, the recommendations provided within the CPGs varied among institutions, which supports the notion that a consensus on the best practices for the management of CAP is not standard among similar children’s hospitals. This is likely because of the variability in expert opinion around optimal management strategies for children hospitalized with CAP. 3 The recently published national consensus PIDS/IDSA guidelines summarize the evidence base for the key elements of diagnostic testing and treatment of the management of children with CAP, which may be helpful for future institutional guideline development. 10
A major goal of CPGs is to improve patient outcomes. The American Thoracic Society and IDSA developed guidelines for the management of adults with CAP independently in 1993 20 and 2001, 21 but in 2007 developed a joint guideline. 22 Guideline concordant therapy was associated with a reduction in mortality and LOS for adults with CAP. 23 In our study of children, the presence of institutional CPGs for management of CAP was not associated with differences in clinical outcomes, such as LOS, readmission, or cost. These measures may be too broad to detect small differences associated with CPG presence, and the varied nature of the recommendations within the CPGs also diminishes our ability to detect differences in patient outcomes. The recently published PIDS/IDSA pneumonia guidelines provide an easily accessible and common national set of recommendations for the diagnosis and management of children with CAP 10 ; however, future efforts must focus on guideline implementation and outcome measurement on a local level.
An important finding in this study was the greater use of narrow-spectrum antibiotics, such as penicillin and aminopenicillins, among patients admitted to hospitals with CPGs that recommended those antibiotics. Newman and colleagues 9 recently demonstrated that after an introduction of an antimicrobial stewardship program and a CAP CPG, the use of ampicillin increased by 34%, whereas ceftriaxone use decreased by 47%, without an increase in treatment failures. A particularly important finding was that more patients were subsequently discharged on amoxicillin after the implementation of the guideline. Although data on discharge medications cannot be ascertained from our current data set, it is likely that patients cared for in hospitals with guidelines also were discharged from the hospital with a narrower spectrum agent. Previous studies have shown that clinical outcomes among children treated with narrow-spectrum antibiotics for CAP are equivalent to those treated with broad-spectrum antibiotics, which underlies the current guideline recommendations from the IDSA. 2 , 24
This study had several limitations. First, our assessment of the presence of a CPG was based on responses received from the quality directors at each institution. Although we confirmed the presence of a CPG by manual review of the guidelines, we are unable to confirm the absence of a CPG for institutions reporting no guideline. We were also unable to determine the manner and extent to which the individual CPGs were disseminated within each institution, a factor that could influence its impact. Second, categorization of specific recommendations around diagnostic testing within the guidelines may be subject to reviewer bias, although we had 2 reviewers assess and categorize each recommendation contained within the guideline and final classification was based on reviewers’ consensus. Third, CPGs were in various formats, including algorithms, written recommendations, and order sets, making the evaluation and categorization of such guidelines difficult. Fourth, although our study was restricted to a relatively homogeneous group of patients without evidence of severe or complicated disease at admission, there remains the possibility of residual confounding by disease severity. We defined pneumonia by physician-assigned discharge diagnosis codes. It can be challenging to distinguish bacterial CAP from viral lower respiratory tract infection, and it is likely that some patients with viral lower respiratory tract infection were included; however, given the lack of a gold standard to diagnose bacterial pneumonia, this limitation is unavoidable and also mirrors clinical practice. We do not expect this limitation to meaningfully affect our results, as our intent was to determine the association between local CPGs and management of children suspected of having CAP by the treating physician. The accuracy of the diagnosis, although important clinically, is less critical to this study.
Conclusions
We observed wide variability in the presence, form, and content of CAP guidelines among children’s hospitals. This heterogeneity poses challenges in evaluating the impact of guidelines on resource utilization and outcomes. Although CAP guidelines were not significantly associated with diagnostic testing or clinical outcomes, they were associated with narrow-spectrum antibiotic use. The recent publication of consensus guidelines may help to standardize the approach to the care of the child with pneumonia. Strategies aimed at optimizing the implementation, dissemination, and evaluation of clinical guidelines will be needed to truly impact patient care.
Glossary
- CAP
community-acquired pneumonia
- CBC
complete blood count
- CI
confidence interval
- CPG
clinical practice guideline
- CXR
chest radiograph
- IDSA
Infectious Diseases Society of America
- IQR
interquartile range
- LOS
length of stay
- OR
odds ratio
- PIDS
Pediatric Infectious Diseases Society
Footnotes
Drs Neuman, Hersh, Brogan, Parikh, Newland, Blaschke, Williams, Grijalva, Tyler, and Shah conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted; Dr Hall conceptualized and designed the study, conducted data analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
This work was presented as a platform presentation at the annual meeting of the Pediatric Academic Societies; April 28 – May 1, 2012; Boston, MA.
FUNDING: No external funding.
COMPANION PAPER: A companion to this article can be found on page 941, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2012-2085.
References
- 1. Brogan TV , Hall M , Williams DJ , et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia [published online ahead of print May 30, 2012]. Pediatr Infect Dis J. doi:10.1097/INF.0b013e31825f2b10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambroggio L , Tabb LP , O’Meara T , Sheffler-Collins S , McGowan KL , Shah SS . Influence of antibiotic susceptibility patterns on empiric antibiotic prescribing for children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(4):331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hersh AL , Shapiro DJ , Newland JG , Polgreen PM , Beekmann SE , Shah SS . Variability in pediatric infectious disease consultants’ recommendations for management of community-acquired pneumonia. PLoS ONE. 2011;6(5):e20325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conway PH , Keren R . Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789–796 [DOI] [PubMed] [Google Scholar]
- 5. Tieder JS , Robertson A , Garrison MM . Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124(6). Available at: www.pediatrics.org/cgi/content/full/124/6/ e1081 [DOI] [PubMed] [Google Scholar]
- 6. Willson DF , Horn SD , Hendley JO , Smout R , Gassaway J . Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness. Pediatrics. 2001;108(4):851–855 [DOI] [PubMed] [Google Scholar]
- 7. Bergman DA . Evidence-based guidelines and critical pathways for quality improvement. Pediatrics. 1999;103(1 suppl E):225–232 [PubMed] [Google Scholar]
- 8. Todd J , Bertoch D , Dolan S . Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156(11):1086–1090 [DOI] [PubMed] [Google Scholar]
- 9. Newman RE , Hedican EB , Herigon JC , Williams DD , Williams AR , Newland JG . Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/ e597 [DOI] [PubMed] [Google Scholar]
- 10. Bradley JS , Byington CL , Shah SS , et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project (H-CUP). Overview of the Kids’ Inpatient Database (KID). Available at: www.hcup-us.ahrq.gov/kidoverview.jsp. Accessed April 13, 2012
- 12. Owens P , Thompson J , Elixhauser A , Ryan K . Care of Children and Adolescents in US Hospitals. Rockville, MD: Agency for Healthcare Research and Quality; 2003. [Google Scholar]
- 13. Mongelluzzo J , Mohamad Z , Ten Have TR , Shah SS . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055 [DOI] [PubMed] [Google Scholar]
- 14. Shah SS , Hall M , Srivastava R , Subramony A , Levin JE . Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49(9):1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feudtner C , Hays RM , Haynes G , Geyer JR , Neff JM , Koepsell TD . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/ e99 [DOI] [PubMed] [Google Scholar]
- 16. Cabana MD , Rand CS , Powe NR , et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465 [DOI] [PubMed] [Google Scholar]
- 17. Coco A , Vernacchio L , Horst M , Anderson A . Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125(2):214–220 [DOI] [PubMed] [Google Scholar]
- 18. Shapiro DJ , Gonzales R , Cabana MD , Hersh AL . National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics. 2011;127(1):28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Clinical Practice Guidelines We Can Trust: Standards for Developing Trustworthy Clinical Practice Guidelines (CPGs). Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2011/Clinical-Practice-Guidelines-We-Can-Trust/Clinical%20Practice%20Guidelines%202011%20Insert.pdf. Accessed April 13, 2012
- 20. Niederman MS , Bass JB Jr , Campbell GD , et al. American Thoracic Society. Medical Section of the American Lung Association . Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am Rev Respir Dis. 1993;148(5):1418–1426 [DOI] [PubMed] [Google Scholar]
- 21. Niederman MS , Mandell LA , Anzueto A , et al. Guidelines for the management of adults with community-acquired pneumonia. Am J Respir Crit Care Med. 2001;163(7):1730–1754 [DOI] [PubMed] [Google Scholar]
- 22. Mandell LA , Wunderink RG , Anzueto A , et al. Infectious Diseases Society of America American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCabe C , Kirchner C , Zhang H , Daley J , Fisman DN . Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531 [DOI] [PubMed] [Google Scholar]
- 24. Kronman MP , Hersh AL , Feng R , Huang YS , Lee GE , Shah SS . Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011;127(3):411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]


