Abstract
OBJECTIVE:
A high prevalence of nonconvulsive status epilepticus (NCSE) has been reported in critically ill adults and neonates. Recent prospective pediatric studies focus on critically ill children and show wide variability in the frequency of NCSE. This study examines prevalence of pediatric NCSE regardless of inpatient setting and retrospectively identifies risk factors indicating a need for urgent continuous EEG.
METHODS:
Medical records from patients aged 3 months to 21 years were identified either by (1) searching a clinical EEG database (n = 18) or (2) consecutive inpatient EEG referrals for NCSE over an 8-month period (n = 57).
RESULTS:
Seventy-five children, mean age of 7.8 years, were studied. NCSE was identified in 26 patients (35%) and in 8 of 57 (14%) patients referred for possible NCSE. More than half of the patients referred were outside of the ICU. A witnessed clinical seizure was observed in 24 of 26 (92%) patients with NCSE. Acute cortical neuroimaging abnormalities were significantly more frequent in patients with NCSE. The presence of clinical seizures and acute neuroimaging abnormality was associated with an 82% probability of NCSE. All but 1 patient with NCSE had electrographic or electroclinical seizures within the first hour of monitoring.
CONCLUSIONS:
A high prevalence of NCSE was observed, comparable to adult studies, but within a wider range of inpatient settings. Children with acute encephalopathy should undergo continuous EEG. This evaluation is more urgent if certain clinical risk factors are present. Optimal duration of monitoring and the effect of NCSE on prognosis should be studied.
Keywords: nonconvulsive status epilepticus, status epilepticus, pediatric epilepsy, diagnosis, MRI abnormal, risk factors
What’s Known on This Subject:
Nonconvulsive status epilepticus (NCSE) is the diagnosis for encephalopathy caused by continuous epileptic activity on EEG. It is a well-known cause of morbidity and mortality in critically ill adults and neonates. NCSE is increasingly reported in critically ill children.
What This Study Adds:
We show that NCSE is common in all inpatient settings, not only in the critically ill. Key risk factors that should dramatically increase suspicion of NCSE in clinical practice include a history of convulsive seizure and acute imaging abnormalities.
The International League Against Epilepsy defines status epilepticus (SE) as 30 minutes of continuous epileptic activity. 1 Nonconvulsive status epilepticus (NCSE) is a clinical diagnosis describing prolonged electrographic seizure activity resulting in nonconvulsive clinical symptoms. 2 In comatose adults and high-risk neonates, NCSE occurs frequently, and correlates with poor outcome. 3 – 5 Identification of nonconvulsive seizures and NCSE changes management, making pediatric NCSE an important problem. 6 , 7 Current pediatric literature is derived predominantly from the pediatric ICU. Estimates of frequency of nonconvulsive seizures and NCSE in critically ill children are highly variable from 7% to 46%. 8 , 9 This ICU-based approach risks ignoring the heterogeneity of pediatric epilepsy: in many specific childhood disorders, including metabolic disorders and chromosomal anomalies, NCSE may occur in the absence of an acute etiology. 10 , 11 Additionally, the difficulty of interpreting the neurologic exam in children with variable neurocognitive levels increases the decision-making dilemma: When is an urgent EEG or continuous EEG (cEEG) warranted?
The identification of NCSE is made by a combination of clinical suspicion and electrographic monitoring. Prolonged cEEG monitoring is established as an important test in comatose adults and high-risk neonates. 12 – 15 To address the literature deficiencies in the identification of nonneonatal pediatric NCSE, this study addressed (1) prevalence of NCSE when enough clinical suspicion existed to order an EEG and (2) identification of clinical risk factors increasing the odds of NCSE.
METHODS
This study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board. Patients aged 3 months to 21 years referred for EEG to evaluate for NCSE were identified using 2 methods: (1) the EEG database was searched by diagnosis for the term “NCSE” within the date range of January 1, 2005, through June 30, 2010. There were 25 354 EEGs performed during this period. Eighteen patients had sufficient chart information available to be included using this method. (2) From July 1, 2010, to April 30, 2011, all inpatient EEG referral documents were reviewed. Children with the following referring diagnoses were included: “NCSE,” “altered mental status,” “unresponsive,” “subclinical seizures,” “evaluation for SE.” Fifty-seven patients were identified through this method. To validate the prospective approach used to obtain NCSE patients based on referring diagnosis, the EEG database was searched, by using the same criteria as the prior search, from July 1, 2010, to April 30, 2011. Only 1 additional patient with NCSE was identified with a referring diagnosis of “seizure.” This patient was not included in the analysis.
In all, 75 patients were included. Electronic medical records were reviewed. Baseline clinical characteristics, imaging reports, EEG duration and results, hospital course, and outcome were abstracted. Baseline characteristics obtained included presence or absence of history of epilepsy, convulsive seizure within 24 hours of EEG hookup, duration of seizure, and abnormal development. All patients underwent continuous video EEG monitoring; electrode placement was performed using the international 10-20 system. Qualified EEG technologists were available 24 hours a day to perform the studies. EEGs were routinely reviewed by the neurologist on call within 1 hour of start and were continued in consultation with the ordering physician when deemed appropriate. Interim EEG data were reviewed every 24 hours by the epileptologist on call and as needed via Internet connection from home. The decision to stop cEEG monitoring was ultimately made by the treatment team. Categorization of NCSE was based on the clinical EEG interpretation at the time of the study, performed by 7 fellowship trained pediatric epileptologists. Because of the retrospective nature of the study, interreader reliability could not be determined. In general, the conventional criteria for NCSE as described by Abend et al were used. 8 Specifically, NCSE was defined as a continuous 30-minute seizure or briefer seizures occurring consecutively comprising at least 30 minutes of any 1-hour period. Electrographic patterns identified demonstrated evolution in frequency, amplitude, and location. Although periodic spikes were not interpreted as seizures, periodic bursts of β activity, evolving in frequency, were interpreted as seizures in 2 patients.
Imaging findings were classified as (1) normal; (2) chronic abnormalities including sequelae of prior infarct, gyral malformations, and other chronic lesions; (3) acute abnormalities to include cortical, thalamic, or basal ganglia signal changes with gyral swelling and/or diffusion restriction, acute extra-axial collections, or hemorrhage.
Descriptive statistics are provided. Relationships between categorical variables were performed using the Fisher exact test. Statistical analysis using logistic regression modeling was performed to analyze the incidence of NCSE in the presence of several dichotomous variables, including presence of witnessed seizure and type of imaging abnormality; their second order interactions were also included. Backward elimination variable selection was used in the logistic regression models. None of the interaction terms were found to be significant in the model. Statistical analysis was conducted by using SAS statistical software version 9.2 (SAS Institute, Cary, NC).
RESULTS
Demographics and Referral Characteristics
Seventy-five pediatric patients, aged 3 months to 21 years (mean 7.8 years), were reviewed. Eighteen patients with NCSE were identified by EEG database. Specific referral diagnosis terms were not available for these patients. Of the remainder, 26 of 57 (46%) were referred for EEG with the diagnosis term “NCSE.” “Altered mental status” was the cause for referral in 10; “unresponsive,” “subclinical seizures,” and “evaluate for SE” each represented 6 patients. Other terms clearly indicating evaluation for NCSE were seen in 3 patients. Of the patients identified prospectively, 25 of 57 (44%) were in the ICU; the remaining 56% were hospitalized but not critically ill at the time of the study, including 18 from the neurology inpatient service, 9 from other inpatient services, and 5 from the emergency department.
Prevalence of NCSE
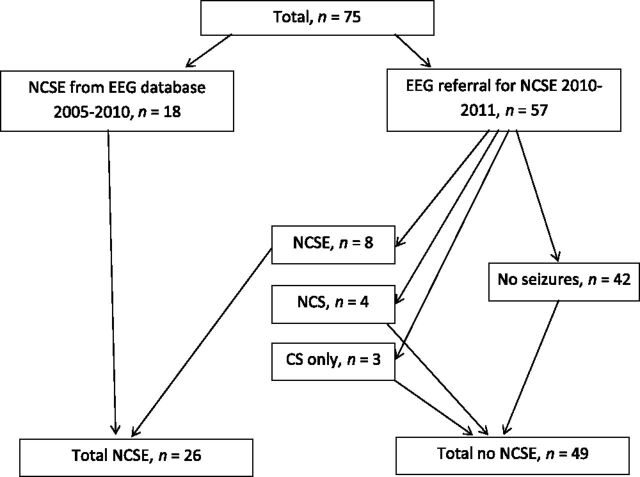
Including all 75 patients, 26 (35%) had NCSE. Ten of 26 (38%) patients with NCSE were in the ICU, 15 (57%) in the neurology inpatient service, and 1 (4%) in the neurosurgery inpatient service. Eight of the 57 (14%) patients identified through referral for EEG had NCSE (Fig 1). Four of these were in the ICU, and 4 were in the neurology inpatient service. Additionally, 4 had nonconvulsive seizures; because these children had daily seizures at baseline, detection of nonconvulsive seizures without NCSE did not change management. Three had convulsive seizures, 1 of which evolved into convulsive SE. Therefore, 15 of 57 (26%) had evidence of seizures on EEG (Fig 1). Two patients had nonepileptic spells identified, which were being treated as seizures before EEG monitoring. In these 2 patients, EEG clarified the diagnosis for paroxysmal movements, preventing further administration of intravenous benzodiazepines.
FIGURE 1.
Patient distribution. Eighteen patients with NCSE were identified by searching an EEG database and 57 by monitoring consecutive EEG referrals for NCSE. Eight of them had NCSE. Additionally, 4 had nonconvulsive seizures (NCS) and 3 had convulsive seizures (CS) only, demonstrated on video EEG.
Historical Factors Associated With NCSE
Age, history of epilepsy, and abnormal development were found equally in both NCSE and non-NCSE groups. A clinical seizure, before and within 24 hours of EEG, occurred in 24 of 26 (92%, P = .0015, Fisher exact test) of patients with NCSE; 28 of 49 (58%) without NCSE had a clinical seizure before EEG (Table 1).
TABLE 1.
Comparison of Clinical Factors and Outcome in Patients With and Without NCSE
| NCSE (n = 26) | No NCSE (n = 49) | |
|---|---|---|
| Age (y) | 7.4 | 8.2 |
| Prior epilepsy | 18 (67) | 32 (65) |
| Clinical seizure | 24 (92) P = .0004 | 28 (57) |
| Development abnormal | 18 (67) | 35 (71) |
| Acute etiology found (n = 31) | 10 (38) | 21 (43) |
| Extra-CNS infection (fever) | 3 (12) | 9 (18) |
| CNS Infection | 2 (8) | 5 (10) |
| Hypoxia | 3 (12) | 2 (4) |
| Toxic metabolic | — | 3 (6) |
| Other | 2 (8) | 2 (4) |
| Imaging (CT or MRI) | — | — |
| Acute cortical abnormality | 11a | 4 (8) |
| Acute noncortical | 1 (4) | 3 (6) |
| Chronic cortical | 5 (19) | 15 (31) |
| Chronic noncortical | 1 (4) | 2 (4) |
| Normal | 4 (15) | 11 (22) |
| No imaging | 4 (15) | 14 (29) |
| Outcome | — | — |
| Mortality | 4 (15) | 4 (8) |
| Neurologic morbidity | 8 (31) | 2 (4) |
Presence of witnessed clinical seizure was a statistically significant difference by using χ2 test. Patients with NCSE had a higher number of cortical abnormalities on neuroimaging, higher mortality, and higher incidence of neurologic morbidity, although the differences were not statistically significant. CNS, central nervous system.
Etiology
Only 31 of 75 (41%) had an acute etiology determined for their encephalopathy apart from clinical seizures, if present. The remaining children had apparently “unprovoked” encephalopathy, or unprovoked NCSE, usually occurring with a known history of epilepsy. The most common identified cause for encephalopathy was fever/infection of an extra–central nervous system source (n = 12). Other etiologies included CNS infection, hypoxia, and toxic-metabolic syndromes (Table 1).
EEG Characteristics
Twenty-six patients were identified with NCSE. Ictal abnormalities included generalized (n = 15), focal (n = 9), and multifocal (n = 2) distributions. A variety of EEG patterns were observed, including rhythmic δ (Fig 2), θ and β, generalized spike and wave, δ with interspersed spikes, and periodic bursts of β activity. All ictal EEG reports indicated an evolution in frequency, amplitude and distribution, meeting the definition of NCSE. SE was subclinical in all 26 cases described.
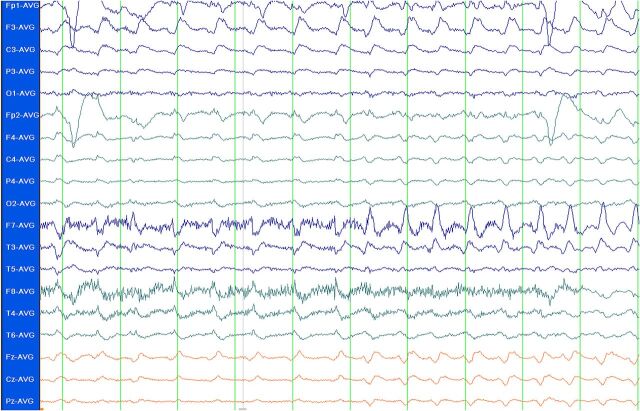
FIGURE 2.
EEG showing SE. Standard 10-20 system EEG in average referential montage display, demonstrating rhythmic sharps and δ frequency ictal activity maximal in the left frontal region. Patient was a 2-year-old girl with cardiopulmonary arrest secondary to pulmonary hemorrhage. She became less responsive several days after her arrest. An MRI demonstrated multifocal cortical signal abnormalities consistent with cerebral edema. This EEG was obtained, and a diagnosis of NCSE was made. She was eventually stabilized but on follow-up had significant neurologic impairment.
Duration of EEG monitoring ranged from 1 hour to 12 days, with a median of 11.5 hours (mean 64 hours). In the 26 patients found to have NCSE, seizures were detected within the first hour in 25 of 26 (96%) of cases. The other patient, a 6-year-old girl with a history of tuberous sclerosis and epilepsy, had frequent focal spikes without seizures for the first 23 hours. She was previously monitored for 12 hours with no seizures recorded but had persistent obtundation. An MRI demonstrated focal abnormalities in multiple cortical tubers consistent with seizure-related edema. Because of high clinical suspicion, prolonged cEEG was initiated, and on the 24th hour, a pattern emerged consistent with NCSE.
Imaging Findings
Not all patients were imaged acutely; 57 of 75 had neuroimaging, with either head computed tomography (CT) or MRI, within 24 hours of EEG. Eighteen had normal findings. Of the remaining 39, 20 had acute abnormalities, 16 of which involved cerebral cortex, either because of the acute underlying cause or secondary excitotoxic edema (Table 2). The most common finding was multifocal signal abnormality consistent with diffuse cortical edema. Acute diffusion-weighted imaging restriction was also seen (Fig 3). There was a high prevalence of acute cortical abnormalities in the NCSE group: half (11 of 22) of the imaged patients with NCSE had these changes (Table 1). The other 4 acute imaging findings included acute hydrocephalus in 3 instances and extensive white matter edema in one child with a suspected mitochondrial disorder.
TABLE 2.
Clinical Information, EEG Pattern, and Outcome in 16 Patients With Acute Cortical Imaging Abnormalities
| Pt | Age, y | Etiology | Initial Imaging | Followup MRI (≥2 wk) | EEG | Outcome |
|---|---|---|---|---|---|---|
| 1 | 3 | Acute renal failure | CT diffuse cerebral edema | Bilateral hippocampal atrophy a | R temporal δ (NCSE) | Continued seizures, no deficits |
| 2 | 1 | Prematurity and perinatal IVH | MRI DWI restriction L hemisphere | Diffuse cortical necrosis L hemisphere a | L frontal α (NCSE) | R hemiparesis |
| 3 | 0.8 | Unknown | MRI R posterior FLAIR signal | Severe global brain atrophy a | L rhythmic δ/α (NCSE) | Developmental arrest |
| 4 | 0.5 | Systemic infection, hypoxia | MRI diffuse cerebral edema | None | Multifocal spike and wave (NCSE) | Death |
| 5 | 8 | Cardiopulmonary arrest | CT diffuse cerebral edema | None | R > L independent seizures (NCSE) | Death |
| 6 | 9 | Fever | MRI multifocal cortical signal | Increasing areas of signal abnormality | 1–2 Hz rhythmic δ (NCSE) | Progression of neurologic dysfunction |
| 7 | 3 | PRES | MRI multifocal cortical signal | Increasing periventricular signal | Rhythmic α/β (NCSE) | Death |
| 8 | 5 | No acute etiology, chromosomal anomaly | MRI FLAIR signal bilateral posterior | None | No seizures | No deficits |
| 9 | 21 | Fever | CT decreased gray-white differentiation occipital lobes | None | Diffuse rhythmic β (NCSE) | No deficits |
| 10 | 6 | Fever/known TS | MRI multifocal DWI change in cortical areas corresponding to known tubers | Diffuse cortical atrophy a | Bilateral posterior (NCSE) | Cognitive morbidity, motor disability |
| 11 | 2 | Anoxic injury | MRI diffuse cortical edema and white matter DWI restriction | None | L frontal temporal δ/θ (NCSE) | Severe neurologic impairment |
| 12 | 19 | Meningitis | MRI multifocal signal abnormality | MRI cortical atrophy a | No seizures | No deficits |
| 13 | 4 | Anoxic injury | CT diffuse cortical edema | None | No seizures | Death |
| 14 | 11 | Unknown inflammatory CNS process | MRI R cerebral FLAIR signal | None | R hemisphere slowing, no seizures | No deficits |
| 15 | 16 | Unknown, resected medulloblastoma | MRI bilateral temporal lobe signal | None | R posterior rhythmic θ | No deficits |
| 16 | 2 | Multiorgan failure | MRI multifocal cortical signal | None | No seizures | Death |
CNS, central nervous system; DWI, diffusion weighted imaging; FLAIR, fluid attenuated inversion recovery; IVH, intraventricular hemorrhage; PRES, posterior reversible leukoencephalopathy syndrome; TS, tuberous sclerosis.
Evidence of resulting brain atrophy on follow-up MRI was seen in 5 patients.
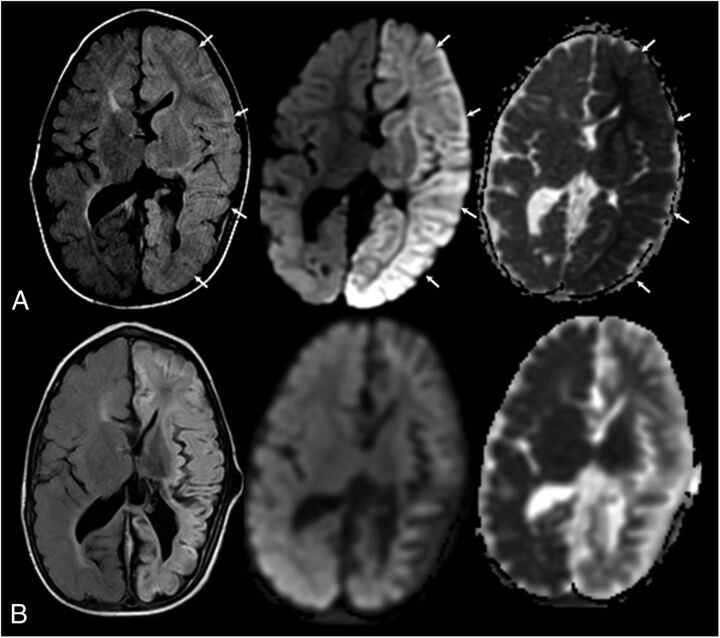
FIGURE 3.
A 2-year-old boy with new-onset seizures and sepsis. History of previous white matter injury of prematurity and intraventricular hemorrhage. Shunt dependent hydrocephalus. L-R: Axial fluid attenuated inversion recovery image, axial diffusion-weighted image, and apparent diffusion coefficient map. A, At acute clinical presentation. Note the extensive edema and gyral swelling throughout the left hemisphere on the fluid attenuated inversion recovery sequence (arrows). Increased signal is also noted involving the left thalami and basal ganglia. Trace diffusion-weighted image map demonstrates extensive increased signal throughout the left hemisphere. Apparent diffusion coefficient map demonstrates left hemispheric diffusion restriction involving both the cortex and white matter. Follow-up imaging (1 month later) demonstrates diffuse left hemispheric volume loss and evolution of diffusion changes. Clinically, he had acute flaccid right hemiplegia. At 6-month follow-up, he was improved but continued to have persistent spastic right hemiparesis. Language developmental milestones were normal.
Analysis of Risk for NCSE
Using logistic regression, a strong interaction between clinical seizure and acute cortical imaging abnormality was observed. With no witnessed clinical seizure before EEG and no acute cortical imaging findings, probability of NCSE was 4%. Presence of clinical seizure increased the probability of NCSE to 34%; acute cortical imaging findings increased the probability to 29%. When both of these variables were present, the probability of NCSE was 82%. This analysis had a high goodness of fit (Hosmer-Lemeshow P = .96).
Hospital Course and Outcome
Hospital follow-up in surviving patients ranged from 6 to 59 months, with a mean of 27 months. The clinical course and treatment approaches in patients with NCSE were variable. Although the majority (24 of 26, 92%) eventually presented with clinical seizures within 24 hours of the decision to perform an EEG, the initial presentation requiring admission did not include seizures in 7 of 26 (27%) of patients with eventual NCSE. In these 7 patients, the mean time from admission to EEG was 19 days. In the remaining patients with clinical seizures noted on admission, the mean time from admission to EEG was 7 days. Seven patients had NCSE identified within 24 hours of admission.
The diagnosis of NCSE changed management in 24 of 26 (92%) of patients, either causing administration of an anticonvulsant or increasing the dosage of an existing anticonvulsant. The most common first anticonvulsant administered after admission was lorazepam. Six patients had medically refractory NCSE requiring pharmacologic coma.
Among 26 patients with NCSE, 4 of 26 (15%) died. Two had cardiac arrest as the etiology of NCSE, and overall outcome was likely not attributable to seizures. In 1 case of a patient with known cryptogenic epilepsy, NCSE was aborted, but the patient developed a fatal complication from pentobarbital coma therapy. In another patient with cryptogenic epilepsy, NCSE remained uncontrolled until the decision was made to withdraw care. Eight of 26 (31%) patients with NCSE had significant neurologic morbidity on discharge. Of the 49 patients without NCSE, 4 of 49 (8%) died; 2 of 49 (4%) survived with neurologic morbidity.
Of the 7 patients with NCSE admitted for reasons that did not include seizures, prognosis was mostly poor. Mortality or significant morbidity was observed in 4 of 7 (57%). These patients generally had a longer time between admission and EEG monitoring (19 days). However, they were all critically ill with multiple life-threatening medical problems that contributed to development of NCSE and poor outcome.
Although not statistically significant, there was a trend toward an additive effect of acute cortical imaging findings and NCSE on prognosis. Eight of 11 (73%) patients with both of these findings died or had significant neurologic morbidity, whereas in their absence, only 4 of 11 (36%) cases had such a poor prognosis (Fisher exact test, P = .20). Fewer patients without a diagnosis of NCSE underwent neuroimaging: 15% vs 29% in the NCSE group. In the non-NCSE group, 4 of 49 (8%) had acute cortical imaging findings. Two of these patients died.
Discussion
Pediatric NCSE is relatively common. Our report highlights the need for continued vigilance for NCSE during hospitalization. In reviewing EEGs ordered for clinical suspicion of pediatric NCSE, we report a prevalence of NCSE of 14%, similar to prior pediatric studies focused on critically ill patients. 8 , 16 The difference in our sample was the heterogeneous acuity level: less than half of patients with NCSE were critically ill.
Children with both a convulsive seizure and acute cortical neuroimaging abnormalities had a very high probability of NCSE in our study. The usefulness of clinical factors to guide urgency of EEG is not well studied in children: a previous report in adults found a relationship between severe impairment in mental state and ocular movement abnormalities and NCSE. 17 Additionally, in 1 study, 14% of patients with terminated convulsive SE had NCSE. 18 Although children with terminated convulsive SE should probably undergo EEG routinely, our study indicates that other factors including imaging abnormalities be strongly considered.
Combining cEEG monitoring and child neurology expertise improves decision making. Kothare et al published a series of 32 emergency EEGs approved by the child neurologist on call. Six of 32 (19%) had NCSE. 19 In our study, the majority (15 of 26, 58%) of patients identified with NCSE were in the neurology service. Only 1 patient with NCSE was referred outside of the ICU or neurology service. In our report, correct diagnosis of nonepileptic paroxysmal movements prevented unnecessary anticonvulsant administration. In addition, the diagnosis of NCSE changed medical management in almost all patients, requiring ongoing cEEG monitoring and neurologist consultation.
Once the decision is made to obtain an EEG to evaluate for NCSE, determining duration of monitoring is important. In adults, at least 24 hours of monitoring is often recommended. In one study, 20% of comatose patients found to have NCSE did not have detectable seizures until the second day or later of monitoring. 20 In our population, the median duration of monitoring was 11.5 hours. However, there was a striking early capture of NCSE: 25 of 26 patients had findings consistent with electrographic seizures within 1 hour of monitoring, most of them at the beginning of the recording. This finding is somewhat incongruent with a recent prospective study of 100 critically ill children, showing only 50% of children with nonconvulsive seizures or NCSE were detected within the first hour of monitoring. 8 In this same study, 19% of children had NCSE (similar to our results), but they showed a higher percentage (46%) with nonconvulsive seizures.
Most children with acute encephalopathy undergo neuroimaging. Incorporating neuroimaging results with clinical information may help guide decisions regarding cEEG monitoring. Diffusion-weighted imaging changes on MRI, in particular, have a high concordance with EEG in partial SE. 21 – 23 These changes have only rarely been reported in NCSE. 24 There is increasing evidence to suggest that abnormal T2 and fluid attenuated inversion recovery signal, in both the hippocampus and neocortex, are commonly seen in convulsive SE and may correlate with sites of permanent injury. 25 – 27 In some cases, acute cortical imaging abnormalities may be the result of NCSE or demonstrate severity of injury from another etiology. Half of the imaged 22 children with NCSE in our study had acute cortical abnormalities on imaging, consistent with a previous report of 23 pediatric patients with NCSE in an ICU setting. 28
This study is limited by its retrospective design. EEG patterns considered to be NCSE are reader dependent and were not standardized in the analysis. There have been attempts to minimize interreader variability in NCSE by standardizing the diagnostic approach for rhythmic and periodic patterns on EEG. 29 – 31 However, patterns associated with NCSE in children are often atypical and would be excluded by algorithms derived from adults. For instance, in our report 2 children had periodic rhythmic β associated with NCSE. This pattern is not uncommon in children, but β presenting as SE is rare in adults. 32
There are several major limitations in this study. There is a potential selection bias, because the included patients presented with enough clinical indicators of NCSE to warrant EEG. It is unknown whether the prevalence of NCSE obtained in this report (14%) is reflective of the true prevalence for all acutely encephalopathic children. Many patients without conventional clinical indicators of NCSE, including witnessed seizure, or known risk factors including critical illness, anoxia, stroke, or sepsis, may have NCSE undetected in clinical practice. The high percentage of patients with clinical seizure before EEG may reflect a referral bias. However, more patients overall were referred without a history of clinical seizure, indicating that clinical seizure is an important risk factor.
Epilepsy and seizures are commonly comorbid in children with many acute and chronic conditions. 33 , 34 It would follow that NCSE is often comorbid as well, but this requires prospective study. Future prospective studies should be designed to determine the clinical relevance of nonconvulsive seizures and identify the optimal duration of cEEG in the pediatric patient for a given set of clinical risk factors. Developing an improved algorithm for urgent EEG referral, especially in places where EEG may be underused, such as the emergency department, will increase understanding of pediatric NCSE. 35
The impact of NCSE on neurologic outcome is unclear. Recent studies suggest that NCSE is an independent risk factor for brain atrophy in patients with posttraumatic brain injury. 36 In clinical practice, mortality in children with NCSE is thought to be related to the underlying cause of seizures, not the duration or presence of NCSE. Current evidence suggests that improvement is at least partly related to rapid and effective treatment of seizures. 37
CONCLUSIONS
Pediatric NCSE is an entity with heterogeneous presentation and prognosis, creating some obstacles to rigorous study. It is not rare. At our institution, we intend to investigate the role and timing of cEEG in children with encephalopathy prospectively and determine the effect of NCSE on neurologic outcome.
Glossary
- cEEG
continuous EEG
- CT
computed tomography
- NCSE
nonconvulsive status epilepticus
- SE
status epilepticus
Footnotes
Each author has contributed to the intellectual development of this study. As stipulated by this journal, each author has contributed in the following way to the submission: (1) conception, design or analysis of the data; (2) critical revision before submission; (3) final approval of the submitted version.
FUNDING: No external funding.
References
- 1. Shorvon S , Baulac M , Cross H , Trinka E , Walker M TaskForce on Status Epilepticus of the ILAE Commission for European Affairs . The drug treatment of status epilepticus in Europe: consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia. 2008;49(7):1277–1285 [DOI] [PubMed] [Google Scholar]
- 2. Walker M , Cross H , Smith S , et al. Nonconvulsive status epilepticus: Epilepsy Research Foundation workshop reports. Epileptic Disord. 2005;7(3):253–296 [PubMed] [Google Scholar]
- 3. Claassen J , Hirsch LJ , Frontera JA , et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4(2):103–112 [DOI] [PubMed] [Google Scholar]
- 4. Shneker BF , Fountain NB . Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61(8):1066–1073 [DOI] [PubMed] [Google Scholar]
- 5. Pisani F , Sisti L , Seri S . A scoring system for early prognostic assessment after neonatal seizures. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/4/e580 [DOI] [PubMed] [Google Scholar]
- 6. Hirsch LJ . Urgent continuous EEG (cEEG) monitoring leads to changes in treatment in half of cases. Epilepsy Curr. 2010;10(4):82–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kilbride RD , Costello DJ , Chiappa KH . How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66(6):723–728 [DOI] [PubMed] [Google Scholar]
- 8. Abend NS , Gutierrez-Colina AM , Topjian AA , et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shahwan A , Bailey C , Shekerdemian L , Harvey AS . The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia. 2010;51(7):1198–1204 [DOI] [PubMed] [Google Scholar]
- 10. Korff CM , Nordli DR Jr . Diagnosis and management of nonconvulsive status epilepticus in children. Nat Clin Pract Neurol. 2007;3(9):505–516 [DOI] [PubMed] [Google Scholar]
- 11. Maganti R , Gerber P , Drees C , Chung S . Nonconvulsive status epilepticus. Epilepsy Behav. 2008;12(4):572–586 [DOI] [PubMed] [Google Scholar]
- 12. Clancy RR , Sharif U , Ichord R , et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46(1):84–90 [DOI] [PubMed] [Google Scholar]
- 13. Pisani F , Cerminara C , Fusco C , Sisti L . Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69(23):2177–2185 [DOI] [PubMed] [Google Scholar]
- 14. Towne AR , Waterhouse EJ , Boggs JG , et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54(2):340–345 [DOI] [PubMed] [Google Scholar]
- 15. McBride MC , Laroia N , Guillet R . Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55(4):506–513 [DOI] [PubMed] [Google Scholar]
- 16. Jette N , Claassen J , Emerson RG , Hirsch LJ . Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63(12):1750–1755 [DOI] [PubMed] [Google Scholar]
- 17. Husain AM , Horn GJ , Jacobson MP . Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003;74(2):189–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeLorenzo RJ , Waterhouse EJ , Towne AR , et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39(8):833–840 [DOI] [PubMed] [Google Scholar]
- 19. Kothare SV , Khurana DS , Valencia I , Melvin JJ , Legido A . Use and value of ordering emergency electroencephalograms and videoelectroencephalographic monitoring after business hours in a children’s hospital: 1-year experience. J Child Neurol. 2005;20(5):416–419 [DOI] [PubMed] [Google Scholar]
- 20. Claassen J , Mayer SA , Kowalski RG , Emerson RG , Hirsch LJ . Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–1748 [DOI] [PubMed] [Google Scholar]
- 21. Di Bonaventura C , Bonini F , Fattouch J , et al. Diffusion-weighted magnetic resonance imaging in patients with partial status epilepticus. Epilepsia. 2009;50(suppl 1):45–52 [DOI] [PubMed] [Google Scholar]
- 22. Yu JT , Tan L . Diffusion-weighted magnetic resonance imaging demonstrates parenchymal pathophysiological changes in epilepsy. Brain Res Brain Res Rev. 2008;59(1):34–41 [DOI] [PubMed] [Google Scholar]
- 23. Katramados AM , Burdette D , Patel SC , Schultz LR , Gaddam S , Mitsias PD . Periictal diffusion abnormalities of the thalamus in partial status epilepticus. Epilepsia. 2009;50(2):265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu K , Kang DW , Kim JY , Chang KH , Lee SK . Diffusion-weighted magnetic resonance imaging in nonconvulsive status epilepticus. Arch Neurol. 2001;58(6):993–998 [DOI] [PubMed] [Google Scholar]
- 25. Milligan TA , Zamani A , Bromfield E . Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure. 2009;18(2):104–108 [DOI] [PubMed] [Google Scholar]
- 26. Bauer G , Gotwald T , Dobesberger J , et al. Transient and permanent magnetic resonance imaging abnormalities after complex partial status epilepticus. Epilepsy Behav. 2006;8(3):666–671 [DOI] [PubMed] [Google Scholar]
- 27. Tsuchida TN , Barkovich AJ , Bollen AW , Hart AP , Ferriero DM . Childhood status epilepticus and excitotoxic neuronal injury. Pediatr Neurol. 2007;36(4):253–257 [DOI] [PubMed] [Google Scholar]
- 28. Saengpattrachai M , Sharma R , Hunjan A , et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47(9):1510–1518 [DOI] [PubMed] [Google Scholar]
- 29. Jirsch J , Hirsch LJ . Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118(8):1660–1670 [DOI] [PubMed] [Google Scholar]
- 30. Chong DJ , Hirsch LJ . Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22(2):79–91 [DOI] [PubMed] [Google Scholar]
- 31. Hirsch LJ , Brenner RP , Drislane FW , et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22(2):128–135 [DOI] [PubMed] [Google Scholar]
- 32. Bonati LH , Naegelin Y , Wieser HG , Fuhr P , Ruegg S . Beta activity in status epilepticus. Epilepsia. 2006;47(1):207–210 [DOI] [PubMed] [Google Scholar]
- 33. Dhandayuthapani G , Chakrabarti S , Ranasinghe A , et al. Short-term outcome of infants presenting to pediatric intensive care unit with new cardiac diagnoses. Congenit Heart Dis. 2010;5(5):444–449 [DOI] [PubMed] [Google Scholar]
- 34. Gaggero R , Haupt R , Paola Fondelli M , et al. Intractable epilepsy secondary to cyclosporine toxicity in children undergoing allogeneic hematopoietic bone marrow transplantation. J Child Neurol. 2006;21(10):861–866 [DOI] [PubMed] [Google Scholar]
- 35. Alehan FK , Morton LD , Pellock JM . Utility of electroencephalography in the pediatric emergency department. J Child Neurol. 2001;16(7):484–487 [DOI] [PubMed] [Google Scholar]
- 36. Vespa PM , McArthur DL , Xu Y , et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75(9):792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arzimanoglou A . Outcome of status epilepticus in children. Epilepsia. 2007;48(suppl 8):91–93 [DOI] [PubMed] [Google Scholar]