Abstract
BACKGROUND AND OBJECTIVE:
Use of oral erythromycin in infants is associated with infantile hypertrophic pyloric stenosis (IHPS). The risk with azithromycin remains unknown. We evaluated the association between exposure to oral azithromycin and erythromycin and subsequent development of IHPS.
METHODS:
A retrospective cohort study of children born between 2001 and 2012 was performed utilizing the military health system database. Infants prescribed either oral erythromycin or azithromycin as outpatients in the first 90 days of life were evaluated for development of IHPS. Specific diagnostic and procedural codes were used to identify cases of IHPS.
RESULTS:
A total of 2466 of 1 074 236 children in the study period developed IHPS. Azithromycin exposure in the first 14 days of life demonstrated an increased risk of IHPS (adjusted odds ratio [aOR], 8.26; 95% confidence interval [CI], 2.62–26.0); exposure between 15 and 42 days had an aOR of 2.98 (95% CI, 1.24–7.20). An association between erythromycin and IHPS was also confirmed. Exposure to erythromycin in the first 14 days of life had an aOR of 13.3 (95% CI, 6.80–25.9), and 15 to 42 days of life, aOR 4.10 (95% CI, 1.69–9.91). There was no association with either macrolide between 43 and 90 days of life.
CONCLUSIONS:
Ingestion of oral azithromycin and erythromycin places young infants at increased risk of developing IHPS. This association is strongest if the exposure occurred in the first 2 weeks of life, but persists although to a lesser degree in children between 2 and 6 weeks of age.
Keywords: pyloric stenosis, azithromycin, erythromycin, macrolide
What’s Known on This Subject:
Exposure to oral erythromycin in the first few weeks of life has been associated with the development of pyloric stenosis. Although azithromycin has become an acceptable alternative, little is known on whether this medication increases the risk of pyloric stenosis.
What This Study Adds:
Exposure to oral azithromycin in the newborn period increases the risk of developing pyloric stenosis. Although this risk is highest if the exposure occurred in the first 2 weeks of life, the risk extends out to 6 weeks of age.
Infantile hypertrophic pyloric stenosis (IHPS) is a condition in young infants marked by elongation and thickening of the pylorus muscle leading to eventual gastric outlet obstruction. Although IHPS is more common in firstborn males, other genetic and environmental factors play a role in the development of this disease. 1 – 3 Oral administration of erythromycin, particularly in the first 2 weeks of life, has been shown to increase the risk of developing IHPS. 4 – 9 This association was described in 1999 in a cohort of nearly 200 infants who were given prophylaxis after an exposure to a health care worker infected with pertussis. In an effort to avoid exposure to erythromycin in young infants, many providers are turning to another macrolide, azithromycin, as an alternative. The risk associated with this antibiotic, however, remains unknown, and there has been only a single case series of IHPS developing in 2 infants exposed to azithromycin. 10 No population-based studies have evaluated early exposure to azithromycin and the risk of IHPS. We sought to evaluate the associated risk of exposure to oral azithromycin in the neonatal period and subsequent development of IHPS.
Methods
Design and Data Source
A retrospective cohort study of children born between June 1, 2001, and April 1, 2012, was performed by using the TRICARE Management Activity military health system (MHS) database. The TRICARE Management Activity oversees health care delivery for US uniformed services members and their families in the United States and abroad. The MHS database includes all outpatient and inpatient billing records and outpatient pharmacy utilization for all eligible uniformed services members and their dependents in both military and civilian facilities. TRICARE provides medication coverage for either no cost/copay at military pharmacies or for low copays at civilian pharmacies. The MHS database was queried for all children eligible for TRICARE health care starting at birth. To be included in the study, children must have had a medical record in the database demonstrating that they were followed within TRICARE since birth.
Variable Definitions
Outpatient prescription data were obtained through the MHS database. Children who were prescribed either oral erythromycin or azithromycin in the first 90 days of life were identified. Additionally, subjects prescribed cephalexin in this time frame were identified. The cephalexin exposure group was used as a reference for another commonly prescribed antibiotic in the neonatal period. The date of the prescription was counted as the day of life the prescription was dispensed from the pharmacy. For cases, erythromycin, azithromycin, or cephalexin exposure must have occurred before the date of pyloromyotomy to be counted. In cases where patients had multiple prescriptions, only the first exposure and exposure date were used for analysis. No subjects were excluded based on multiple exposures to macrolides or cephalexin. Age of macrolide or cephalexin exposure and age of pyloromyotomy were calculated in patients with IHPS. Gender and firstborn status were also obtained; a child was classified as firstborn if he or she was the first eligible dependent child of the uniformed member on record.
Children younger than 90 days were identified as having IHPS by using specific International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes for IHPS, to include 750.5 (congenital hypertrophic pyloric stenosis), 537.0 (acquired hypertrophic pyloric stenosis), or 537.81 (pylorospasm). In addition to having at least 1 of the above codes, children classified as IHPS were also required to have either a Current Procedural Terminology code of 43520, or an ICD-9-CM procedure code of 43.3 (pyloromyotomy), which is the corrective surgical procedure for IHPS. The age of diagnosis of IHPS was determined from the admission date that included the pyloromyotomy procedure.
Data Analysis
Univariate analyses were conducted to investigate normality of the data. We summarized skewed data with median and interquartile ranges (IQRs). The χ2 test was used to test differences of categorical variables. Wilcoxon Two-Sample test was used to compare continuous data between groups. Based on previous reports that early macrolide exposure may have higher risk, we created 2 categorical variables dividing both azithromycin and erythromycin into 4 categories based on the age in days at the time of exposure. The 4 categories were age of exposure at 0 to 14 days of life, 15 to 42 days of life, 43 to 90 days of life, and no prescription. Cephalexin was categorized similarly. Azithromycin, erythromycin, and cephalexin exposure time periods were evaluated in a single multivariable logistic regression model. The dependent variable was IHPS, and the independent variables were the categorical variables representing the time periods exposed to azithromycin, erythromycin, or cephalexin, firstborn status, gender, and the year of the birth. Two-way interactions between the independent variables were evaluated, and there were no interactions that were found to be significant or retained in the final model. For calculation of relative risk (RR) and adjusted odds ratios (aORs) for azithromycin, erythromycin, and cephalexin, the “no prescription” category was used as the reference category. The significance of trends for prescription across the study period for both azithromycin and erythromycin were tested utilizing the Cochran-Armitage test for trend. Statistical analyses were performed by using SAS version 9.3 (SAS Institute, Inc, Cary, NC), and all statistical tests were performed at a significance level of α = 0.05. The study was reviewed and approved by the responsible institutional review boards.
Results
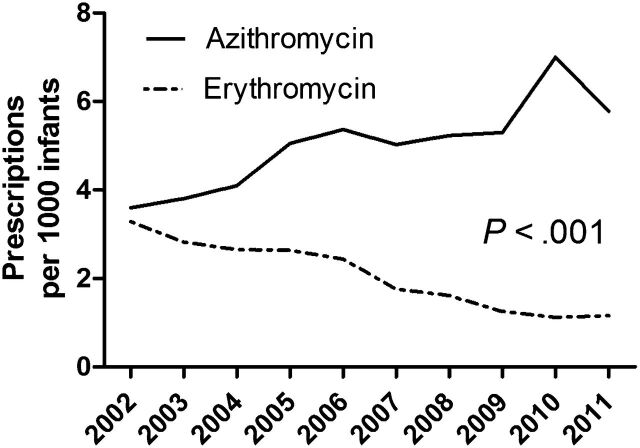
A total of 1 074 236 children were born between June 1, 2001, and April 1, 2012; 1902 of those children were prescribed oral erythromycin, and 4875 were prescribed oral azithromycin within the first 90 days of life. Over the 11-year study period, there was an increasing rate of azithromycin prescriptions, with a 15.8% annual increase per year, whereas there was an average 5.0% annual decrease of erythromycin prescriptions (Fig 1). Other demographic characteristics of infants prescribed macrolides are presented in Table 1.
FIGURE 1.
Prescriptions of oral erythromycin and azithromycin (per 1000 infants) in the first 90 days of life from 2002 to 2011.
TABLE 1.
Characteristics of Infants <90 d Prescribed Macrolides
| Not Prescribed Macrolide | Prescribed Erythromycin | P | Prescribed Azithromycin | P | |
|---|---|---|---|---|---|
| Number | 1 067 459 | 1902 | — | 4875 | — |
| Male | 549 313 (51.1) | 1004 (52.9) | .21 | 2822 (58.2) | <.001 |
| Firstborn | 486 887 (45.8) | 812 (43.1) | .02 | 1842 (37.8) | <.001 |
Percentages are calculated based on nonmissing data within category. Data are presented as n (%) unless noted otherwise.
A total of 2466 of the 1 074 236 infants developed IHPS (incidence of 2.29 per 1000). Rates and unadjusted RRs of IHPS by demographics and exposure to macrolides are presented in Table 2. Of the 1902 infants prescribed erythromycin, 17 subsequently developed IHPS (8.94 per 1000), with 14 (82.4%) being boys and 5 (29.4%) firstborn. Of the 4875 infants prescribed azithromycin, 8 subsequently developed IHPS (1.64 per 1000). All 8 infants were boys and 3 (37.5%) were firstborn. Exposure to erythromycin in the first 90 days of life had a RR of 3.94 (95% confidence interval [CI], 2.44–6.36; P < .001) for developing IHPS, whereas exposure to azithromycin anytime during the first 90 days of life had a RR of 0.71 (95% CI, 0.36–1.43; P = .34), but did not achieve statistical significance.
TABLE 2.
Incidence, RR, and Multivariable Logistic Regression Results for IHPS
| Total in Group | Number With IHPS | Rate per 1000 | RR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|---|
| All infants | 1 074 236 | 2466 | 2.29 | — | |
| Male, n (%) | 553 139 (51.5) | 2037 (82.7) | 3.70 | 4.50 (4.05–4.99) | 4.49 (4.05–4.99) |
| Firstborn, n (%) | 486 887 (45.9) | 1239 (50.7) | 2.55 | 1.22 (1.12–1.32) | 1.21 (1.12–1.31) |
| Erythromycin prescription, d | 1902 | 17 | 8.94 | 3.94 (2.44–6.36) | |
| 0–14 | 291 | 9 | 30.9 | 13.9 (7.16–27.1) | 13.3 (6.80–25.9) |
| 15–42 | 535 | 5 | 9.35 | 4.11 (1.70–9.92) | 4.10 (1.69–9.91) |
| 43–90 | 1076 | 3 | 2.79 | 1.20 (0.39–3.78) | 1.19 (0.38–3.71) |
| Azithromycin prescription, d | 4875 | 8 | 1.64 | 0.71 (0.36–1.43) | |
| 0–14 | 148 | 3 | 20.3 | 9.00 (2.87–28.3) | 8.26 (2.62–26.0) |
| 15–42 | 729 | 5 | 6.86 | 3.01 (1.25–7.25) | 2.98 (1.24–7.20) |
| 43–90 | 3998 | 0 | — | — | — |
| Cephalexin prescription, d | 4338 | 6 | 1.38 | 0.60 (0.27–1.34) | |
| 0–14 | 453 | 1 | 2.21 | 0.96 (0.14–6.83) | 0.77 (0.11–5.48) |
| 15–42 | 1614 | 5 | 3.10 | 1.35 (0.56–3.25) | 1.14 (0.47–2.75) |
| 43–90 | 2271 | 0 | — | — | — |
The rate, RR, and aOR for the “Azithromycin prescription, 43–90 days” and “Cephalexin prescription, 43–90 days” categories could not be calculated because no cases of IHPS occurred in these groups.
The median (IQR) age at time of IHPS for all infants was 34 days (26–45). The median (IQR) age for infants prescribed erythromycin and azithromycin was 49 days (23–70) and 68 days (50–80; P < .001), respectively. The median number of days after exposure to erythromycin in which the 17 infants underwent pyloromyotomy was 13 days (range, 2–40; IQR, 8–25). The median number of days between exposure to azithromycin in the 8 infants and subsequent pyloromyotomy was longer than the interval for the erythromycin group at 29.5 days (range, 9–45; IQR 20.5–38.5; P = .08).
As presented above, the median age of prescriptions for both azithromycin and erythromycin was later than the median age of IHPS. Additionally, when stratifying by age at time of exposure, a higher rate, RRs, and aORs were found for early exposure to both erythromycin and azithromycin. The results of the multivariable logistic regression model, which included the stratified groups based on the age at the time of exposure to macrolide or cephalexin, are also shown in Table 2. Most notably, of the 148 infants exposed to azithromycin in the first 14 days of life, 3 (2.0%) developed IHPS (aOR, 8.26; 95% CI: 2.62–26.0, P < .001). Of the 729 infants aged 15 to 42 days at time of exposure to azithromycin, 5 subsequently developed IHPS (aOR, 2.98; 95% CI: 1.24–7.20, P = .015). There was no significant association between cephalexin and IHPS across all time periods. The number needed to harm in the 0- to 14-day azithromycin exposure group was 56, and the 0- to 14-day erythromycin exposure group was 35. The number needed to harm in the 15- to 42-day exposure group for azithromycin and erythromycin was 219 and 142, respectively.
Discussion
Using a large population of over 1 million infants, our study investigated the association between azithromycin and pyloric stenosis. The baseline rate of 2.29 cases of IHPS per 1000 live births in our study was consistent with previous estimates. 2 , 11 Our study demonstrates a male predominance in those affected by macrolide exposure, in that all of the 8 infants exposed to azithromycin who developed IHPS were boys, and 14 of the 17 infants exposed to erythromycin who developed IHPS were also boys.
Exposure to erythromycin in the first 90 days of life resulted in a RR of 3.94 for developing IHPS, whereas exposure to azithromycin resulted in a RR of 0.71, which was not statistically significant. When evaluating the RR over the combined 90 days, the discrepancy between an elevated RR for erythromycin and no significant risk for azithromycin is likely due to the clustering of prescriptions for the large majority of the azithromycin subjects (3998 of 4875) after the window of risk for the development of IHPS. Only upon grouping the infants according to age category (0–14, 15–42, 43–90 days of life) does the risk of IHPS after azithromycin exposure become evident in the younger infants. Among newborns who received azithromycin in the first 2 weeks of life, our study demonstrates a surprising eightfold increase in the odds of developing IHPS. Furthermore, this risk likewise persisted, although to a lesser extent with an aOR of 2.98, in the 15- to 42-day-old exposure age group. The risk of IHPS after azithromycin exposure was no different from baseline rates if the infant received the antibiotic after 42 days of life, likely representing infants maturing beyond the baseline window of risk for IHPS.
Our study identified a 13-fold increase in the odds of developing IHPS with early erythromycin exposure. This is similar to previous reports that demonstrated a 7-, 8-, or 10-fold increase in risk for developing IHPS after ingestion of oral erythromycin in the first 2 weeks of life. 7 – 9 Interestingly, however, this risk in our study persisted into the 15- to 42-day-old age group, although it had lessened to a fourfold increase in the odds. This finding has not been previously demonstrated. Similar to the older infants in the azithromycin group in our study, the risk of IHPS disappeared if erythromycin exposure occurred beyond 6 weeks of life. Thus, in our study, the increased risk of IHPS after macrolide use is limited to a narrow exposure window highest in the first 14 days of life and diminishing by 6 weeks of age.
Two key indications for the use of oral azithromycin in the newborn period include treatment of Chlamydia conjunctivitis and pneumonia and treatment/prophylaxis against Bordetella pertussis.
The US Centers for Disease Control and Prevention has reported a rise in pertussis in the United States beginning in 2002, with over 25 000 cases for the years 2004, 2005, and 2010, and more than 48 000 cases in the year 2012 alone (National Notifiable Diseases Surveillance System and Supplemental Pertussis Surveillance System, US Centers for Disease Control and Prevention, unpublished data, 2013). The resurgence of pertussis may be a significant factor in the increase in prescriptions of azithromycin in neonates (Fig 1). The American Academy of Pediatrics continues to recommend azithromycin as the drug of choice for treatment or prophylaxis of pertussis in infants younger than 1 month of age because the risk of developing complications from pertussis outweighs the potential risk of IHPS. 12
Because of the advantages of once-daily dosing, a shorter required course, and the reported association between early exposure to oral erythromycin in neonates and the subsequent development of pyloric stenosis, azithromycin has seemingly replaced erythromycin as the macrolide of choice in the newborn period. Yet few data exist regarding the safety of this antibiotic in infants. One particular study, which provided 1 of the largest cohorts of young infants observed for side effects of azithromycin, involved 58 neonates from the nursery who received a 5-day course of azithromycin after potential exposure to a rotating medical student with confirmed pertussis. 13 The antibiotic was well tolerated, and none of these infants (mean age of 15 days; range, 3–24 days) subsequently developed IHPS. However, 58 infants might have been too small a number to detect a possible underlying association with IHPS. Our study demonstrated a number needed to harm of 56 just within the 0- to 14-day azithromycin prescription category. Additionally, most of these infants were older than 14 days, which we have identified as a key period of risk.
Only a single case series exists that suggests an association between azithromycin and IHPS. 10 The report describes how a set of triplets, who were born at 32 weeks’ gestation, were admitted at 7 weeks of life for paroxysmal cough and treated with azithromycin for 5 days after a laboratory-confirmed diagnosis of pertussis in 1 of the infants. The 2 female siblings were diagnosed with IHPS ∼6 weeks later. Although these infants were atypical for the classic case of IHPS (female and older age of onset), they had confounding factors associated with their clinical course, including previous transpyloric feeding and exposure to medications for gastroesophageal reflux disease, such as lansoprazole, bethanechol, ranitidine, and metoclopramide.
The association between azithromycin and IHPS can be biologically explained by the macrolide’s potent stimulation of gastrointestinal motility. Erythromycin has been shown to be a motilin receptor agonist, stimulating migrating motor complexes in the stomach. 14 , 15 Similarly, azithromycin acts as a motilin receptor agonist and causes long-lasting facilitation of cholinergic activity in experiments by using circular muscle of human gastric antrum. 16 Azithromycin has also been shown to stimulate antral activity and induce migrating motor complexes in the duodenum in patients with gastrointestinal dysmotility. 17 , 18
Our study has several limitations. As a retrospective study utilizing billing data, we were dependent on ICD-9-CM coding. We attempted to limit false diagnoses for IHPS, such as in the case of suspected IHPS, by using a definition that required both a diagnostic code for IHPS and a procedure code for pyloromyotomy. We understand that treatment of IHPS does not require surgery and an alternative includes transpyloric feeding. We opted to use more stringent classification criteria to minimize misclassification bias. We also considered medication exposure to be a dichotomous variable and did not assess other factors involving antibiotic prescription. These included the indication for the antibiotic, the per kilogram dosing of the antibiotics, length of treatment course, and compliance with antibiotics. We were also unable to examine inpatient prescription information. Strengths of our study include a large, representative, geographically, and demographically diverse newborn population across a variety of both military and civilian facilities. TRICARE is a universal health care system minimizing bias due to access to care. TRICARE’s no or low copay for medications maximizes capture of macrolide exposure within the data set.
Conclusions
Ingestion of oral azithromycin places young infants at increased risk of developing pyloric stenosis. This association is strongest if the exposure occurred in the first 2 weeks of life (eightfold increase in odds), but persists to a lesser degree in children between 3 and 6 weeks of age (threefold increase in odds). In an era when an increasing number of young infants are being considered for pertussis prophylaxis, practitioners must carefully weigh the risks and benefits when prescribing azithromycin, particularly to male infants in the first few weeks of life. These infants should be monitored for signs and symptoms of pyloric stenosis for 6 weeks after treatment with azithromycin.
Acknowledgments
We thank Kathleen Madden, PhD, and Rachel Sterett, BS, for their help in reviewing the manuscript.
Footnotes
Dr Eberly conceptualized and designed the study, interpreted analysis results, and drafted and edited the manuscript; Ms Eide collected and organized the data and revised the final manuscript; Dr Thompson contributed to the drafting of the manuscript; Dr Nylund conceptualized and designed the study, directed data analysis, and edited the manuscript; and all authors approved the final manuscript.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Air Force, Department of Defense, or the US Government. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States government work as “a work prepared by a military service member or employee of the United States government as part of that person’s official duties.” This work was prepared as part of our official duties.
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Contributor Information
Matthew D. Eberly, Department of Pediatrics, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
Matilda B. Eide, Department of Pediatrics, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
Jennifer L. Thompson, Department of Pediatrics, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
Cade M. Nylund, Department of Pediatrics, F. Edward Hebert School of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
References
- 1. Jedd MB , Melton LJ III , Griffin MR , et al. Factors associated with infantile hypertrophic pyloric stenosis. Am J Dis Child. 1988;142(3):334–337 [DOI] [PubMed] [Google Scholar]
- 2. Applegate MS , Druschel CM . The epidemiology of infantile hypertrophic pyloric stenosis in New York State, 1983 to 1990. Arch Pediatr Adolesc Med. 1995;149(10):1123–1129 [DOI] [PubMed] [Google Scholar]
- 3. Krogh C , Fischer TK , Skotte L , et al. Familial aggregation and heritability of pyloric stenosis. JAMA. 2010;303(23):2393–2399 [DOI] [PubMed] [Google Scholar]
- 4. SanFilippo A . Infantile hypertrophic pyloric stenosis related to ingestion of erythromycine estolate: a report of five cases. J Pediatr Surg. 1976;11(2):177–180 [DOI] [PubMed] [Google Scholar]
- 5. Stang H . Pyloric stenosis associated with erythromycin ingested through breastmilk. Minn Med. 1986;69(11):669–670, 682 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC) . Hypertrophic pyloric stenosis in infants following pertussis prophylaxis with erythromycin—Knoxville, Tennessee, 1999. MMWR Morb Mortal Wkly Rep. 1999;48(49):1117–1120 [PubMed] [Google Scholar]
- 7. Honein MA , Paulozzi LJ , Himelright IM , et al. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet. 1999;354(9196):2101–2105 [DOI] [PubMed] [Google Scholar]
- 8. Mahon BE , Rosenman MB , Kleiman MB . Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139(3):380–384 [DOI] [PubMed] [Google Scholar]
- 9. Cooper WO , Griffin MR , Arbogast P , Hickson GB , Gautam S , Ray WA . Very early exposure to erythromycin and infantile hypertrophic pyloric stenosis. Arch Pediatr Adolesc Med. 2002;156(7):647–650 [DOI] [PubMed] [Google Scholar]
- 10. Morrison W . Infantile hypertrophic pyloric stenosis in infants treated with azithromycin. Pediatr Infect Dis J. 2007;26(2):186–188 [DOI] [PubMed] [Google Scholar]
- 11. Schechter R , Torfs CP , Bateson TF . The epidemiology of infantile hypertrophic pyloric stenosis. Paediatr Perinat Epidemiol. 1997;11(4):407–427 [DOI] [PubMed] [Google Scholar]
- 12. American Academy of Pediatrics . Pertussis. In: Pickering LK , Baker CJ , Kimberlin DW , Long SS , eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:555 [Google Scholar]
- 13. Friedman DS , Curtis CR , Schauer SL , et al. Surveillance for transmission and antibiotic adverse events among neonates and adults exposed to a healthcare worker with pertussis. Infect Control Hosp Epidemiol. 2004;25(11):967–973 [DOI] [PubMed] [Google Scholar]
- 14. Peeters T , Matthijs G , Depoortere I , Cachet T , Hoogmartens J , Vantrappen G . Erythromycin is a motilin receptor agonist. Am J Physiol. 1989;257(3 pt 1):G470–G474 [DOI] [PubMed] [Google Scholar]
- 15. Ng E , Shah VS . Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst Rev. 2008;16(3):CD001815 [DOI] [PubMed] [Google Scholar]
- 16. Broad J , Sanger GJ . The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br J Pharmacol. 2013;168(8):1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moshiree B , McDonald R , Hou W , Toskes PP . Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis. Dig Dis Sci. 2010;55(3):675–683 [DOI] [PubMed] [Google Scholar]
- 18. Chini P , Toskes PP , Waseem S , Hou W , McDonald R , Moshiree B . Effect of azithromycin on small bowel motility in patients with gastrointestinal dysmotility. Scand J Gastroenterol. 2012;47(4):422–427 [DOI] [PubMed] [Google Scholar]