Abstract
OBJECTIVE:
To determine whether racial differences exist in antibiotic prescribing among children treated by the same clinician.
METHODS:
Retrospective cohort study of 1 296 517 encounters by 208 015 children to 222 clinicians in 25 practices in 2009. Clinical, antibiotic prescribing, and demographic data were obtained from a shared electronic health record. We estimated within-clinician associations between patient race (black versus nonblack) and (1) antibiotic prescribing or (2) acute respiratory tract infection diagnosis after adjusting for potential patient-level confounders.
RESULTS:
Black children were less likely to receive an antibiotic prescription from the same clinician per acute visit (23.5% vs 29.0%, odds ratio [OR] 0.75; 95% confidence interval [CI]: 0.72–0.77) or per population (0.43 vs 0.67 prescriptions/child/year, incidence rate ratio 0.64; 95% CI 0.63–0.66), despite adjustment for age, gender, comorbid conditions, insurance, and stratification by practice. Black children were also less likely to receive diagnoses that justified antibiotic treatment, including acute otitis media (8.7% vs 10.7%, OR 0.79; 95% CI 0.75–0.82), acute sinusitis (3.6% vs 4.4%, OR 0.79; 95% CI 0.73–0.86), and group A streptococcal pharyngitis (2.3% vs 3.7%, OR 0.60; 95% CI 0.55–0.66). When an antibiotic was prescribed, black children were less likely to receive broad-spectrum antibiotics at any visit (34.0% vs 36.9%, OR 0.88; 95% CI 0.82–0.93) and for acute otitis media (31.7% vs 37.8%, OR 0.75; 95% CI 0.68–0.83).
CONCLUSIONS:
When treated by the same clinician, black children received fewer antibiotic prescriptions, fewer acute respiratory tract infection diagnoses, and a lower proportion of broad-spectrum antibiotic prescriptions than nonblack children. Reasons for these differences warrant further study.
Keywords: antimicrobials, children, outpatient, race, disparity
What’s Known on This Subject:
Racial disparities in health care have been reported in multiple settings, but not thoroughly examined at the clinician level. The frequent occurrence of respiratory tract infections allows the evaluation of differences in the management of children seen by the same clinician.
What This Study Adds:
Racial differences in the management of common pediatric infections occur among children treated by the same clinician. Given persistent concerns about nonjudicious antibiotic use, examining racial differences may inform our understanding of prescribing practices and identify opportunities for intervention.
Racial disparities in the delivery of health care and outcomes of disease have been identified in multiple clinical settings, 1 , 2 but have not been thoroughly examined in the primary care of children with acute respiratory tract infections (ARTI). Given persistent concerns about the nonjudicious use of antibiotics, examining racial differences may inform our understanding of community prescribing practices and suggest opportunities for targeted intervention.
ARTIs provide a unique opportunity to examine whether race affects the diagnosis and treatment decisions of individual providers. First, childhood ARTIs are common, have little variation in disease severity, and are generally managed within a single encounter by a clinician who manages hundreds of ARTIs per year. This contrasts with more complex conditions for which disparities have been identified, such as coronary artery disease 3 or pediatric leukemia, 4 which vary in disease severity and often are managed by several clinicians over multiple encounters. Second, the high volume of ARTI visits means that any disparity would affect a large population. Third, because diagnostic criteria are subjective and antibiotics are known to be overprescribed for pediatric ARTIs, 5 – 7 clinician practice is likely to vary. Fourth, although associations between race and antibiotic prescribing have been reported, 8 , 9 most studies simply report population statistics; lacking are studies that identify the association of race with prescribing patterns for individual clinicians. Finally, antibiotics are the most frequently prescribed medications to children, 10 and ARTIs account for the vast majority of antibiotic prescribing. 7
We therefore examined antibiotic prescribing patterns across a large, diverse network of pediatric primary care practices to characterize whether individual clinicians managed ARTIs differently based on patient race. Considering the many factors that could influence such differences, we explored this potential association at the practitioner level, afforded by the large number of clinicians linked via a comprehensive, shared electronic health record (EHR); the frequency of both antibiotic prescribing and ARTI diagnosis; and the racial diversity of the children served.
Methods
Data Source
Data were obtained from an ambulatory network of 222 clinicians from 25 primary care pediatric practices located across southeastern Pennsylvania and southern New Jersey. A common EHR (EpicCare; Epic Systems, Inc, Verona, WI), used exclusively by all practices for charting and medication prescribing for all office and telephone encounters since 2004, served as the data source.
Data Collection
Patient-level data extracted from the EHR included race (parent reported), gender, age, insurance type, and antibiotic allergies. Visit-level data included practice location; encounter month; encounter type (office, telephone, emergency department); encounter purpose (preventive, nonpreventive); provider type (physician, nurse practitioner, trainee), gender, and years in practice (provider race was not available); encounter and “problem list” ICD-9 (International Classification of Diseases, Ninth Edition) codes, and prescriptions. Patients were considered members of a practice if they had at least 2 encounters in 2009.
Cohort Assembly
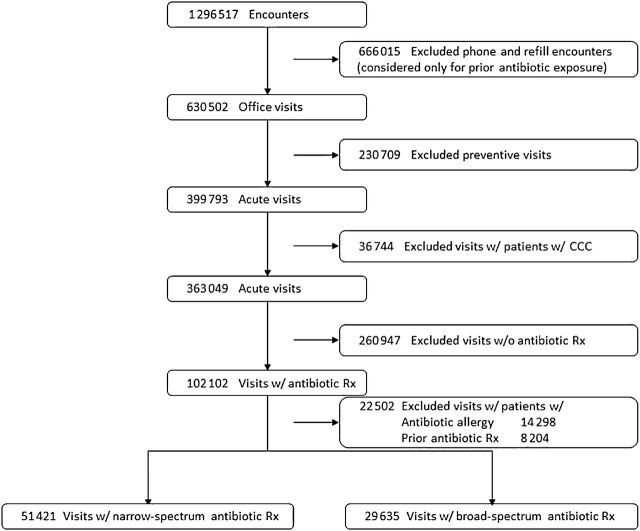
All primary encounters within the network from January 1 through December 31, 2009 by children ≤18 years were included in the initial cohort (Fig 1). For encounter-level analyses, the following were excluded: (1) preventive encounters, to focus on “acute visits” (at which the vast majority of antibiotics were prescribed); (2) encounters by children with complex chronic conditions, 11 to restrict the cohort to previously healthy children; and (3) when relevant, encounters by children with antibiotic allergies or by children who received an antibiotic prescription within the previous 3 months (including both office- and telephone-based prescribing), to avoid prescribing decisions influenced by allergies, recurrent disease, or the consideration of resistant pathogens from recent drug exposure. We also repeated the analyses after excluding children with asthma encounters, defined by encounter ICD-9 codes for asthma and wheezing, to address the potential for dilution of the denominator differentially by patient race. 12
FIGURE 1.
Description of the study cohort. CCC, complex chronic condition; Rx, prescription; w/, with; w/o, without.
Exposures
The primary exposure was patient race, self-reported and documented in the EHR as black or African American, white, Asian, American Indian/Eskimo/Alaskan, Native Hawaiian/Pacific Islander, other, or refused. Primary analyses compared black race with a composite of all other race categories (“nonblack”), which consisted of predominantly white children. Results of antibiotic prescribing and diagnosis analyses were not significantly changed by the presence or absence of other race designations in the nonblack category. Because more than 97% of children in this network were non-Hispanic, ethnicity was not addressed. Frequent office visits during a single “episode of care” might increase the perceived need for an antibiotic prescription. To address this potential explanatory factor, we repeated the analyses with the addition of a covariate measuring the frequency of visits immediately preceding (within 7 days of) an encounter in which an antibiotic was prescribed.
Outcomes
Main outcome measures included (1) antibiotic prescriptions, (2) broad-spectrum antibiotic prescriptions, and (3) encounter diagnoses. Antibiotic receipt was defined as a prescription associated with a specified nonpreventive office visit for an oral antibacterial agent, including penicillin, amoxicillin, amoxicillin-clavulanate, cephalosporins, macrolides, trimethoprim-sulfamethoxazole, and clindamycin. Amoxicillin-clavulanate, second- and third-generation cephalosporins, and azithromycin were considered broad-spectrum antibiotics, based on American Academy of Pediatrics prescribing guidelines for ARTIs. 13 Fluoroquinolones, tetracyclines, and linezolid were not included, as they were used rarely for ARTIs. Encounter diagnoses for ARTIs (acute otitis media, acute sinusitis, group A streptococcal pharyngitis, and pneumonia) and urinary tract infection (a non-ARTI, with more objective diagnostic criteria) were defined by ICD-9 codes (Appendix 1). Case definitions for ARTIs required (1) the specified ICD-9 code, (2) an associated antibiotic prescription, and (3) for streptococcal pharyngitis, a positive rapid (uniformly available at point of care at each practice) or culture positive laboratory test. Encounters with a diagnosis code for an additional bacterial infection were excluded (Appendix 2). Case definitions were validated through iterative, manual chart review of fields collected electronically and by examining free text fields (eg, physical examination, assessment/plan) in 100 charts per bacterial diagnosis treated with antibiotics; 200 charts for nonbacterial diagnoses (ie, viral infections) treated with antibiotics were randomly selected for review.
Statistical Analysis
To model the association between patient race and antibiotic prescription or ARTI diagnosis, we decomposed the within- and the across-clinician effects to avoid confounding by clinician. 14 – 16 The within-clinician parameter represents the estimated effect of patient race on the chances that a clinician will prescribe an antibiotic or diagnose a condition, assuming that a child, alternatively black or nonblack, has been evaluated (as opposed to an across-physician estimate, which measures the association between overall clinician prescribing/diagnosing and the racial composition of that clinician's practice). Thus, the within-clinician estimate controls for all physician characteristics, including the racial composition of children in their practice.
Because diagnosis is linked tightly with (and might be used to justify) antibiotic prescribing, we examined whether diagnosis of ARTIs varied by race within clinician. Because practitioners have a choice of antibiotic class when treating an ARTI, we asked whether the choice of broad-spectrum versus narrow-spectrum antibiotics differed within clinician according to patient race. We implemented logistic regression in the suite of programs for complex survey designs in Stata v 12.0 (Stata Corp, College Station, TX) to account for the stratification of clinicians within a practice and for the clustering of children within clinician. By using marginal standardization (predictive margins), we estimated probabilities of antibiotic prescribing and diagnosis adjusted for patient characteristics, including gender, age category (<1 year; 1–5 years; 6–10 years; >10 years), and insurance type (Medicaid/non-Medicaid) (Appendix 3). 17 To confirm these within-clinician estimates, we also performed fixed effects multivariable regression in which each clinician was a stratum, adjusted for all patient-level covariates, and created a simple table comparing effects by stratum.
An added concern was that any observed clinician effect might have been concentrated in a subset of the practices rather than diffused throughout the network. To investigate the potential for such effect modification by clinician and practice in the association of the child's race and outcome, we performed separate regressions by using the same decomposition parameterization for each of the 25 practices. This analysis estimated the site-level, within-clinician associations of patient race and outcome.
The Children’s Hospital of Philadelphia Committee for the Protection of Human Subjects approved this study.
Results
Study Population
Between January 1 and December 31, 2009, 208 015 children were seen by 222 clinicians across 25 practices (Table 1). Practices varied by number of clinicians (4 to 18), children served (4286 to 17 135), racial characteristics (1% to 96% black), and payer types (4% to 72% Medicaid insurance) of enrolled patients. Of 1 296 517 total encounters, 666 015 were non–office-based encounters (eg, telephone triage, medication refills). After excluding 230 709 preventive encounters, and 36 744 encounters by children with complex chronic conditions, 363 049 office-based nonpreventive encounters (“acute visits”) remained for the primary analyses (Fig 1).
TABLE 1.
Key Characteristics of Patients and Clinicians, by Practice
| Practice a | Clinicians b | Patients c | % Black Race | % Medicaid d | Office Visits | Acute Visits e |
|---|---|---|---|---|---|---|
| 1 | 9 | 10 197 | 1 | 8 | 32 059 | 21 508 |
| 2 | 4 | 4286 | 2 | 6 | 14 494 | 9570 |
| 3 | 10 | 11 013 | 53 | 20 | 29 797 | 17 072 |
| 4 | 5 | 5869 | 16 | 26 | 18 063 | 12 251 |
| 5 | 16 | 10 787 | 96 | 72 | 25 510 | 13 913 |
| 6 | 11 | 6615 | 51 | 16 | 19 326 | 11 524 |
| 7 | 7 | 5881 | 10 | 8 | 21 149 | 13 658 |
| 8 | 10 | 10 756 | 5 | 4 | 30 492 | 18 646 |
| 9 | 7 | 6109 | 3 | 9 | 22 273 | 14 990 |
| 10 | 7 | 6266 | 1 | 12 | 23 800 | 16 333 |
| 11 | 5 | 4821 | 7 | 24 | 14 668 | 9620 |
| 12 | 16 | 9950 | 87 | 72 | 24 504 | 13 629 |
| 13 | 7 | 9219 | 13 | 15 | 28 985 | 19 171 |
| 14 | 5 | 6644 | 2 | 11 | 20 283 | 12 207 |
| 15 | 5 | 5083 | 7 | 20 | 19 793 | 14 237 |
| 16 | 6 | 7277 | 3 | 8 | 25 385 | 16 162 |
| 17 | 6 | 4901 | 25 | 15 | 16 260 | 10 094 |
| 18 | 8 | 8497 | 39 | 19 | 28 648 | 19 784 |
| 19 | 9 | 11 513 | 3 | 7 | 40 441 | 29 984 |
| 20 | 16 | 9561 | 51 | 65 | 25 342 | 14 425 |
| 21 | 7 | 7305 | 11 | 19 | 23 818 | 14 927 |
| 22 | 15 | 17 135 | 21 | 25 | 49 375 | 30 677 |
| 23 | 18 | 11 319 | 89 | 71 | 26 048 | 12 591 |
| 24 | 6 | 9911 | 7 | 16 | 29 391 | 19 159 |
| 25 | 7 | 7110 | 3 | 15 | 20 598 | 13 661 |
| Total | 222 | 208 015 | 28 | 23 | 630 502 | 399 793 |
Twenty-five practices, serving 29 distinct practice sites, during calendar year 2009.
Total clinicians, excluding trainees.
Patients were designated as members of a practice when having at least 2 encounters (office or telephone) at a practice in 2009.
Medicaid insurance was assigned to patients with any office encounter billed to Medicaid in 2009.
Acute visits were those without a preventive billing code.
Antibiotic Prescribing Rates
Multivariable analysis adjusting for age, gender, insurance type, clustering of patients within clinician, and stratifying by practice revealed that black children were less likely than nonblack children to receive any antibiotic prescription per acute visit (23.5% vs 29.0%, odds ratio [OR] 0.75; 95% confidence interval [CI]: 0.72–0.77) (Table 2). Neither the exclusion of asthma encounters 12 nor the addition of a variable measuring the frequency of visits immediately preceding an encounter in which antibiotics were received had appreciable influence on the estimates (OR 0.76 and 0.75, respectively). Black children also made fewer acute visits than nonblack children (1.2 vs 2.0 visits per year), despite attending the same number of preventive visits as nonblack children (1.1 visits per year per child). This difference was not compensated for by visits to the emergency department (0.57 visits per year for black children vs 0.63 visits per year for nonblack children). Given these differential rates of health care use by race, we performed a population-level analysis, revealing that black children received significantly fewer adjusted antibiotic prescriptions per child per year compared with nonblack children (0.43 vs 0.67, incidence rate ratio 0.64; 95% CI 0.63–0.66).
TABLE 2.
Within-Clinician Antibiotic Prescribing Rate by Patient Race
| Antibiotic Prescribing a (n) b | OR, Black versus Nonblack (95% CI) c | P Value | Standardized Probability % (95% CI) d | |
|---|---|---|---|---|
| Black | Nonblack | |||
| Overall (363 049) | 0.75 (0.72–0.77) | <.001 | 23.5 (22.5–24.5) | 29.0 (28.1–30.0) |
| Broad-spectrum (81 056) | 0.88 (0.82–0.93) | <.001 | 34.0 (31.5–36.5) | 36.9 (34.8–39.0) |
| Broad-spectrum, AOM (37 701) | 0.75 (0.68–0.83) | <.001 | 31.7 (28.6–34.8) | 37.8 (35.6–40.0) |
| Broad-spectrum, GAS (7964) | 0.89 (0.61–1.32) | .567 | 7.5 (4.6–10.4) | 8.3 (6.7–10.0) |
| Broad-spectrum, sinusitis (9863) | 0.97 (0.84–1.11) | .661 | 44.0 (38.5–49.4) | 44.7 (40.6–48.8) |
| Broad-spectrum, pneumonia (3038) | 1.00 (0.71–1.40) | .953 | 17.2 (12.3–22.1) | 17.1 (13.7–20.4) |
AOM, acute otitis media; GAS, group A streptococcal pharyngitis.
Excluding preventive encounters and encounters by children with complex chronic conditions; for analyses of broad-spectrum antibiotic prescribing, encounters by children with antibiotic allergies or antibiotic use within the prior 3 mo were additionally excluded.
Total visits used for the analysis.
ORs were adjusted and probabilities of antibiotic receipt were standardized for age, gender, age-gender interaction, and Medicaid insurance.
For example, standardized probabilities predict that 31.6% of black children diagnosed with AOM and given an antibiotic would receive a broad-spectrum antibiotic prescription versus 37.8% of nonblack children diagnosed with AOM by the same clinician.
Further, when an antibiotic was prescribed, even after exclusion of children with antibiotic allergies or recent antibiotic use, black children were less likely than nonblack children to receive a broad-spectrum antibiotic prescription at any visit (34.0% vs 36.9%, OR 0.88; 95% CI 0.82–0.93) or specifically for acute otitis media (31.7% vs 37.8%, OR 0.75; 95% CI 0.68–0.83) (Table 2).
Diagnosis Rates
After adjustment for age, gender, insurance type, clustering of patients within clinician, and stratifying by practice, black children were less likely to receive a diagnosis of acute otitis media (8.7% vs 10.7%, OR 0.79; 95% CI 0.75–0.82) than nonblack children. Similar differences were present for both acute sinusitis (3.6% vs 4.4%, OR 0.79; 95% CI 0.73–0.86) and group A streptococcal pharyngitis (2.3% vs 3.7%, OR 0.60; 95% CI 0.55–0.66) (Table 3). Rates of diagnosis of pneumonia and urinary tract infection, however, did not differ by patient race.
TABLE 3.
Within-Clinician Diagnosis Rate of Common Pediatric Conditions, by Patient Race
| Diagnosis a | OR, Black versus Nonblack (95% CI) b | P Value | Standardized Probability, % (95% CI) c | |
|---|---|---|---|---|
| Black | Nonblack | |||
| AOM | 0.79 (0.75–0.82) | <.001 | 8.7 (8.2–9.2) | 10.7 (10.3–11.2) |
| Sinusitis | 0.79 (0.73–0.86) | <.001 | 3.6 (3.1–4.0) | 4.4 (4.1–4.8) |
| GAS pharyngitis | 0.60 (0.55–0.66) | <.001 | 2.3 (2.1–2.5) | 3.7 (3.5–3.8) |
| Pneumonia | 1.0 (0.89–1.1) | .808 | 1.3 (1.1–1.4) | 1.3 (1.1–1.4) |
| UTI | 1.0 (0.93–1.1) | .725 | 1.7 (1.7–1.8) | 1.7 (1.6–1.8) |
AOM, acute otitis media; GAS, group A streptococcal; UTI, urinary tract infection.
Excluding preventive encounters and encounters by children with complex chronic conditions.
ORs were adjusted and probabilities of diagnosis were standardized for age, sex, age-sex interaction, and Medicaid.
For example, standardized probabilities predict that 11.7% of black children presenting for an acute visit would be diagnosed with AOM versus 14.5% of nonblack children presenting to the same clinician.
Additional Analyses
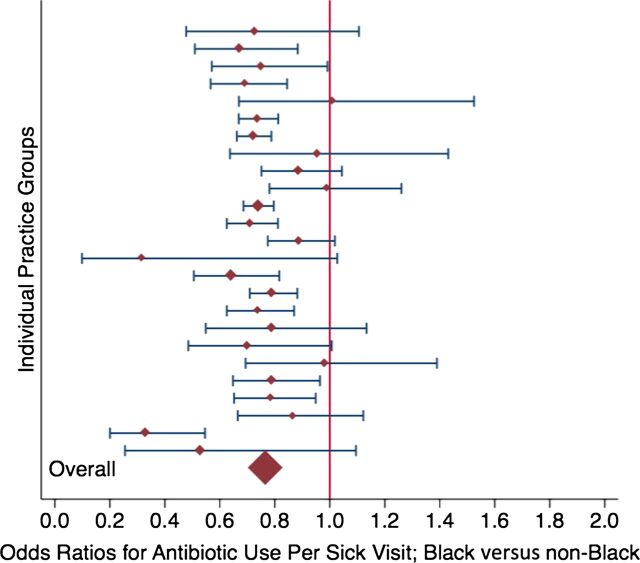
Because of concern of potential clustering of within-physician effects in a subset of practices, we repeated the analysis of antibiotic prescribing per acute visit by performing similar regressions for each individual practice. Figure 2 displays the ORs of antibiotic prescribing for black children compared with nonblack children for each practice; 24 of the 25 practices generated ORs <1, revealing a consistent pattern across settings. There was no evidence of effect modification by clinician gender or years of training (data not shown).
FIGURE 2.
Antibiotic prescribing by race across 25 practices. Associations of antibiotic prescription rates per acute visit for black children compared with nonblack children, excluding children with complex chronic conditions, controlling for age, gender, age-gender interaction, and Medicaid, for each of 25 practices. ORs (diamonds) and 95% CIs are presented. ORs <1.0 reflect lower odds of prescribing for black compared with similar nonblack children.
Discussion
Our data from a large, diverse group of pediatric primary care practices demonstrates that, for individual clinicians, rates of both antibiotic prescribing and ARTI diagnoses varied by patient race. Compared with nonblack children, clinicians were 25% less likely to prescribe an antibiotic to a black child at an acute visit, which equated to 36% fewer antibiotic prescriptions per child per year. Further, clinicians were significantly less likely to diagnose common ARTIs in black children. When black children were prescribed antibiotics, they were less likely to receive a broad-spectrum agent than nonblack children with the same diagnosis seen by the same provider. Exploration of the causes of these differences is critical to ensure the judicious use of antibiotics for ARTIs, which account for the vast majority of antibiotic use in ambulatory pediatrics. 7
Previous studies have identified differences in health care delivery by patient race in a variety of populations and settings, 1 , 2 including adult oncologic surgery, 18 cardiac care, 3 HIV treatment, 19 and emergency department prescribing, 20 as well as pediatric leukemia, 4 appendicitis, 21 asthma care, 22 and child abuse assessment. 23 , 24 Little is known, however, about disparities in diagnosis and treatment of simple and common pediatric conditions. Additionally, previous analyses were unable to isolate the impact of patient race on management decisions for illnesses of comparable severity within individual providers. Studies using national sampling data have reported lower rates of broad-spectrum antibiotic prescribing to black adults 8 and a similar (but not significant) trend in children 7 diagnosed with ARTIs in ambulatory settings, but these analysis were unable to compare prescribing by race within clinicians and had relatively limited clinical data available. Our study, however, isolates individual clinician decision-making, by comparing antibiotic prescribing and ARTI diagnosis rates between black and nonblack patients seen by the same clinician at the same practice in the same year, adjusted for patient-level factors that could be associated with the need for antibiotic use.
The difference in diagnosis rates of ARTIs and the associated need for antibiotics cannot be explained biologically. For example, although racial differences in the incidence of otitis media have been reported, 25 , 26 these findings were not based on within-clinician analyses. Similarly, the definitive, prospective study of otitis media revealed that, when objectively followed over time, black children developed otitis media at the same rate, if not more often, than white children. 27 In contrast, black children in our study were 21% less likely to receive a diagnosis of acute otitis media. Even more surprising, black children were 40% less likely to receive a diagnosis of streptococcal pharyngitis, a condition for which the case definition included laboratory testing. To our knowledge, there is neither a biological rationale nor epidemiologic evidence consistent with a lower incidence of streptococcal pharyngitis in black children.
We could not evaluate the drivers or effects of these differences. Although nonblack children attended more acute visits per year, we measured prescriptions and diagnoses per acute visit, which should adjust for this discrepancy. Also, black children did not have an increased rate of emergency department encounters. Finally, our population-based analysis revealed an even more profound difference in antibiotic prescribing, 36% fewer prescriptions per child per year, consistent with our findings that black children both attended fewer acute visits and, when presenting, received fewer antibiotics than nonblack children. Given the tight link between diagnosis and antibiotic prescribing, it is unclear from these data whether the diagnosis always drove antibiotic prescribing or, alternatively, whether the diagnosis of an ARTI was sometimes driven by the desire (and justification) for an antibiotic prescription.
The complex nature of the doctor-patient relationship might partially explain these observations. Differences in stated parental expectations (“My child needs antibiotics”), physician perception of parental expectations (“This parent is going to demand antibiotics”), or the use of shared decision-making (“Here are the options. Let’s decide together how to proceed.” 28 ) that correlate with patient race could account for some or most of the differential prescribing rates apparent in these analyses, consistent with previous reports. 9 , 29 Practitioner bias affecting medical decision-making by patient race and socioeconomic status has been described, 1 , 30 but its relevance to the diagnosis and treatment of ARTIs is unclear. Because overprescribing of antibiotics to children with ARTIs is common, 5 – 7 we hypothesize that this discrepancy reflects overprescribing, both for all antibiotics and for the relative proportion of broad-spectrum antibiotics, to nonblack patients, rather than underprescribing to black patients. Whether this represents a true health care disparity, however, remains uncertain, and future evaluation should include clinical outcomes, including treatment failure and adverse drug effects. The relatively subjective diagnosis (and subsequent treatment) and limited severity of illness of the ARTIs studied might facilitate such variable prescribing. Our study revealed no racial differences in diagnosis rates of urinary tract infection, a condition with more objective diagnostic criteria, or pneumonia, one with potentially more severe sequelae if left untreated.
This study had limitations. First, these results might not be generalizable. This network, however, included 222 clinicians serving 208 015 patients across urban, rural, and suburban settings in both academic and community practice environments, and results were consistent across these locations. Second, although data were extracted from an EHR by using ICD-codes to identify ARTIs, we used multifactorial case definitions (eg, streptococcal pharyngitis required an acute pharyngitis code, antibiotic receipt, and a positive laboratory test) to reduce misclassification, and case definitions were consistent across race categories. Third, we could not adjust for patient socioeconomic status, which is correlated with race; however, adjustment for insurance type did not affect the results. Fourth, although comparisons across individual practice groups revealed a consistent association between patient race and prescribing/diagnosis, we could not estimate variability in prescribing between individual practitioners because of a limitation in statistical power at this level of data. Last, unobserved factors might have influenced the relationship between patient race and antibiotic prescribing or diagnosis; however, selection differences were minimized by the within-clinician analyses, which controls for both physician characteristics and the racial composition of his or her practice, coupled with the broad scope of adjustments and exclusions.
In conclusion, compared with nonblack children managed by the same primary care pediatrician, black children received fewer overall antibiotic prescriptions, a lower rate of ARTI diagnoses, and, when receiving an antibiotic for an ARTI, a lower proportion of broad-spectrum antibiotic prescriptions. More research is needed to elucidate the factors that account for these differences, including parental preferences, provider perceptions of parental preferences, and implicit and unconscious biases of providers. A better understanding of these factors could inform interventions to help improve antibiotic use for all children.
APPENDIX TABLE 1.
ICD-9 Codes Used for Defining Encounter Diagnoses
| Condition | Inclusion |
|---|---|
| Acute otitis media | 382, 382.0, 382.00, 382.01, 382.02, 382.4, 382.9 |
| Acute sinusitis | 461.8, 461.9, 473.9, 473.2, 473.1, 473.0, 487.1 |
| Group A streptococcal pharyngitis | 034.0, 462, 463 |
| Pneumonia | 482.9, 486, 485, 483.8, 481, 519.8 |
| Urinary tract infection | 599, 599.0, 788.41, 788.1, 590.1, 590.10, 590.11, 590.80 |
APPENDIX TABLE 2.
ICD-9 Codes Used for Defining Non-ARTI Diagnoses Excluded From Analyses
| Otitis externa | 380.10, 380.11, 380.12, 380.13, 380.14, 380.15, 380.16 |
| Skin/soft tissue infection | 680, 680.0, 680.1, 680.2, 680.3, 680.4, 680.5, 680.6, 680.7, 680.8, 680.9, 681, 681.0, 681.00, 681.01, 681.02, 681.1, 681.10, 681.11, 681.9, 682, 682.0, 682.1, 682.2, 682.3, 682.4, 682.5, 682.6, 682.7, 682.8, 682.9 |
| Lyme disease | 088.81, 919.4, 711.80 |
| Acne | 706.1 |
| Chronic sinusitis, | 473.9 |
| Mycoplasma infection | 041.81, 483.0, 711.90 |
| Staphylococcal infection | 041.10, 041.11, 041.12, 041.19 |
| Bite wound | 879.8, 879.9, 959.9, E906.5, E906.0, E906.3, 891.0, 890.0, 884.0, 883.0, 882.0, 881.00 |
| Oropharyngeal infection (not group A streptococcal pharyngitis) | 522.5, 522.4, 528.3, 475, 478.24 |
| Streptococcal infection (without pharyngitis) | 034.1, 041, 041.00, 041.01, 041.1, 041.09, 390, 040.82, 566, 580.0, 686.9, 711.40 |
| Pertussis | 033.0, 033.9 |
| Sexually transmitted infection | 079.9, 079.88, 079.98, 614.9, 616.1, 616.10 |
| Bone/joint infection | 730.20, 730.21, 730.22, 730.23, 730.24, 730.25, 730.26, 730.27, 730.28, 730.29, 711.06, 711.05, 711.03, 711.00 |
| Bacterial gastroenteritis | 008.5, 008.43, 008.00, 004.9, 004.3, 003.9, 003.1, 003.0 |
APPENDIX TABLE 3.
Stata Code for Within-Clinician Analyses
| svyset a provnum b , strata(nsite2) c |
| sort provnum |
| by provnum: egen mrace = mean(afam) d |
| gen drace = (afam-mrace) e |
| svy: logit abx mrace drace i.age_cat sex i.age_cat_sex new_medicaid i.office_clust_cat, or f |
| estat effects, deff g |
| svy: logit abx mrace i.afam i.age_cat sex i.age_cat_sex new_medicaid i.office_clust_cat, or h |
| margins i.afam i |
| estat effects, deff |
Declares that the data will be analyzed by using survey design.
Declares that the primary sampling unit (or cluster) for the analysis will be the provider.
Declares that the analysis will be stratified by the practice.
The variable “mrace” represents the mean number of encounters with black patients who a given provider sees.
This is the between-clinician component of race (nonblack versus black).
The variable “drace” represents the within-clinician component of race (nonblack versus black).
The full survey logistic model adjusting for patient level factors and including both the between- and within-clinician components of the association of race (nonblack versus black). The primary outcome is antibiotic prescription.
Calculates the design effect of the model.
The full survey logistic model adjusting for patient level factors and including the between-clinician component of the association of race and race as a fixed effect. The primary outcome is antibiotic prescription.
Calculates the marginal probability of receipt of antibiotic by race (nonblack versus black).
Acknowledgments
We thank the network of primary care clinicians and their patients and families for their contributions to clinical research through the Pediatric Research Consortium at The Children’s Hospital of Philadelphia. In addition, we thank Jim Massey for his work on this project.
Glossary
- ARTI
acute respiratory tract infection
- CI
confidence interval
- EHR
electronic health record
- ICD-9
International Classification of Diseases, Ninth Revision
- OR
odds ratio
Footnotes
Dr Gerber conceptualized and designed the study and drafted the initial manuscript; Ms Prasad carried out the initial analyses and critically reviewed the manuscript; Dr Localio supervised all analyses and reviewed and revised the manuscript; Drs Fiks, Bell, Wasserman, Rubin, Keren, and Zaoutis conceptualized and designed the study, and reviewed and revised the manuscript; Dr Grundmeier designed the data collection instruments, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: Supported by the US Agency for Healthcare Research and Quality, contract no. HHSA2900200710013.
References
- 1.Institute of Medicine. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment. Washington, DC: National Academies Press; 2003 [Google Scholar]
- 2. Flores G . Technical report: racial and ethnic disparities in the health and health care of children. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e979 [DOI] [PubMed] [Google Scholar]
- 3. Hernandez AF , Fonarow GC , Liang L , et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525–1532 [DOI] [PubMed] [Google Scholar]
- 4. Kadan-Lottick NS , Ness KK , Bhatia S , Gurney JG . Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–2014 [DOI] [PubMed] [Google Scholar]
- 5. Nyquist AC , Gonzales R , Steiner JF , Sande MA . Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279(11):875–877 [DOI] [PubMed] [Google Scholar]
- 6. Nash DR , Harman J , Wald ER , Kelleher KJ . Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(11):1114–1119 [DOI] [PubMed] [Google Scholar]
- 7. Hersh AL , Shapiro DJ , Pavia AT , Shah SS . Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061 [DOI] [PubMed] [Google Scholar]
- 8. Steinman MA , Landefeld CS , Gonzales R . Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719–725 [DOI] [PubMed] [Google Scholar]
- 9. Mangione-Smith R , Elliott MN , Stivers T , McDonald L , Heritage J , McGlynn EA . Racial/ethnic variation in parent expectations for antibiotics: implications for public health campaigns. Pediatrics. 2004;113(5). Available at: www.pediatrics.org/cgi/content/full/113/5/e385 [DOI] [PubMed] [Google Scholar]
- 10. Chai G , Governale L , McMahon AW , Trinidad JP , Staffa J , Murphy D . Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31 [DOI] [PubMed] [Google Scholar]
- 11. Feudtner C , Christakis DA , Connell FA . Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209 [PubMed] [Google Scholar]
- 12. Akinbami LJ , Rhodes JC , Lara M . Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115(5):1254–1260 [DOI] [PubMed] [Google Scholar]
- 13.Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009 [Google Scholar]
- 14. Neuhaus JM , Kalbfleisch JD . Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics. 1998;54(2):638–645 [PubMed] [Google Scholar]
- 15. Berlin JA , Kimmel SE , Ten Have TR , Sammel MD . An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999;55(2):470–476 [DOI] [PubMed] [Google Scholar]
- 16. Begg MD , Parides MK . Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591–2602 [DOI] [PubMed] [Google Scholar]
- 17.Korn EL, Graubard BI. Analysis of Health Surveys. Hoboken, NJ: John Wiley & Sons, Inc.; 1999
- 18. Bach PB , Cramer LD , Warren JL , Begg CB . Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205 [DOI] [PubMed] [Google Scholar]
- 19. Gebo KA , Fleishman JA , Conviser R , et al. HIV Research Network . Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103 [DOI] [PubMed] [Google Scholar]
- 20. Pletcher MJ , Kertesz SG , Kohn MA , Gonzales R . Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78 [DOI] [PubMed] [Google Scholar]
- 21. Kokoska ER , Bird TM , Robbins JM , Smith SD , Corsi JM , Campbell BT . Racial disparities in the management of pediatric appendicitis. J Surg Res. 2007;137(1):83–88 [DOI] [PubMed] [Google Scholar]
- 22. Lieu TA , Lozano P , Finkelstein JA , et al. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics. 2002;109(5):857–865 [DOI] [PubMed] [Google Scholar]
- 23. Lane WG , Rubin DM , Monteith R , Christian CW . Racial differences in the evaluation of pediatric fractures for physical abuse. JAMA. 2002;288(13):1603–1609 [DOI] [PubMed] [Google Scholar]
- 24. Wood JN , Hall M , Schilling S , Keren R , Mitra N , Rubin DM . Disparities in the evaluation and diagnosis of abuse among infants with traumatic brain injury. Pediatrics. 2010;126(3):408–414 [DOI] [PubMed] [Google Scholar]
- 25. Smith DF , Boss EF . Racial/ethnic and socioeconomic disparities in the prevalence and treatment of otitis media in children in the United States. Laryngoscope. 2010;120(11):2306–2312 [DOI] [PubMed] [Google Scholar]
- 26. Vakharia KT , Shapiro NL , Bhattacharyya N . Demographic disparities among children with frequent ear infections in the United States. Laryngoscope. 2010;120(8):1667–1670 [DOI] [PubMed] [Google Scholar]
- 27. Paradise JL , Rockette HE , Colborn DK , et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99(3):318–333 [DOI] [PubMed] [Google Scholar]
- 28. Merenstein D , Diener-West M , Krist A , Pinneger M , Cooper LA . An assessment of the shared-decision model in parents of children with acute otitis media. Pediatrics. 2005;116(6):1267–1275 [DOI] [PubMed] [Google Scholar]
- 29. Cox ED , Nackers KA , Young HN , Moreno MA , Levy JF , Mangione-Smith RM . Influence of race and socioeconomic status on engagement in pediatric primary care. Patient Educ Couns. 2012;87(3):319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Ryn M , Burke J . The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med. 2000;50(6):813–828 [DOI] [PubMed] [Google Scholar]